Abstract
The process of informed consent remains a constant challenge in clinical research. The aim of the present study was to evaluate the understanding of researchers and members of Institutional Review Boards (IRBs) regarding the essential elements of an Informed Consent Form (ICF) as required by internationally recognized regulations. Using eight case studies to illustrate basic ethical elements, the study involved 107 participants, mainly from the Asia Pacific and African regions. The results showed that most of the participants had general knowledge regarding the essential elements required in an ICF. However, the issues of confidentiality of data and payment for study participation proved to be problematic for some participants, accounting for 35% and 28% of all incorrect answers respectively. This suggests that participants’ understanding of the underlying concepts of the required ICF elements is limited. Ethical training of researchers and IRB members, particularly in the Asia Pacific and African regions, concerning valid informed consent is still needed.
Keywords: Informed Consent, Institutional Review Board, IRB, Ethics Committee, Research Ethics
Introduction
The process of informed consent is widely recognized as a necessary step in health-related research involving human subjects. It is a process that is emphasized in the Nuremberg Code [1] in response to the horrific and unethical experiments committed during World War II. The Code stresses the importance of voluntary participation of human subjects in research as well as the ethical responsibilities of investigators. In 1979, the Belmont Report [2] defined three basic ethical principles for biomedical research. “Respect for Persons” is one of the principles mentioned and addresses the topic of informed consent. The report further identifies three elements that are required for consent to be valid: (1) sufficient relevant information for decision making, (2) comprehension, and (3) voluntariness. In other words, for a participant’s consent to be valid, the participant should have received sufficient relevant information, should adequately understand the information provided, and should arrive at a decision without being subjected to coercion or undue influence. In 1996, the International Conference on Harmonization (ICH) of Good Clinical Practice (GCP) [3] was published as a collaborative effort between Japan, the EU and the US in an attempt to globally harmonize good clinical practice. The ICH GCP sets out 20 basic essential elements that should be included in an informed consent form (ICF) (see Table 1) as well as how the informed consent should be documented.
Table 1.
Elements required in an informed consent form based on ICH GCP and US Regulation
| ICH GCP E6 (4.8.10) [3] | US Regulation (45 CRF 46.116) [7] |
|---|---|
| Non-study-specific required elements – Recognition that the trial involves research. – The compensation of the subject if any trial-related injury occurs. – The anticipated payment as prorated, if any, to the subject for trial participation. – The voluntariness of the subject’s participation, including the right of the subject to refuse to participate and right of the subject to withdraw from the trial, at any time. – The confidentiality of records identifying the subject. – The limits of the confidentiality of records: the monitor(s), the auditor(s), the IRB/IEC and the regulatory authority(ies) will be granted direct access to the records for verification of the study procedures. – The contact person(s) for further information. |
Non-study-specific basic elements required – A statement that the study involves research. – An explanation of the extent of the confidentiality of records identifying the subject. – An explanation of any compensation and any available treatments if injury occurs during study participation. – An explanation of appropriate contact persons for answering pertinent questions about the study. – A statement that the subject’s study participation is voluntary: (1) right of the subject to refuse from study participation and (2) right of the subject to discontinue from study participation at any time. |
| Study-specific elements – The purpose of the trial. – The trial treatment(s). – The trial procedures to be followed. – The subject’s responsibilities. – Identification of any aspects of the trial which are experimental. – The foreseeable risks or inconveniences to the subject. – The reasonably expected benefits. – The available alternative procedure(s) or course(s) of treatment. – The anticipated expenses, if any, to the subject for trial participation. – Provision of that the new available findings that may be relevant to the subject’s willingness to continue participation in the trial will be informed to the subject. – The foreseeable circumstances and/or reasons under which the subject’s participation may be terminated without the subject’s consent. – The expected duration of the subject’s participation in the trial. – The approximate number of subjects involved in the trial. |
Study-specific basic elements required – An explanation of the purposes of the study and the expected duration of the subject’s participation. – A description of the procedures to be followed and identification of any procedures which are experimental. – A description of any foreseeable risks or discomforts to the subject. – A description of any direct and/or indirect benefits to the subject. – A disclosure of alternative procedures or courses of treatment, if any. Study-specific additional elements required – A statement of possibly unforeseeable risks to the subject or to the embryo or fetus. – An explanation of any anticipated circumstances under which the subject’s participation may be terminated by the investigator without regard to the subject’s consent. – A description of the anticipated expenses, if any, to the subject for study participation. – An explanation of the consequences of withdrawal from study participation by the subject. – A statement that, if any, significant new information that may be relevant to the subject’s willingness to continue participation will be provided during the study. – The approximate number of subjects involved in the study. |
ICH GCP, International Conference on Harmonization of Good Clinical Practice; IRB, Institutional Review Board; IEC, Independent Ethics Committee.
Although the informed consent process is recognized as an essential requirement in clinical research, in practice it is still far from fully realizing the principle of Respect for Persons [4–6]. The ICH GCP indicates that it is the responsibility of researchers to develop an ICF which includes sufficient relevant information for a potential research participant’s decision making, while it is the role of the Institutional Review Board (IRB) or Independent Ethics Committee (IEC) to review such an ICF and the informed consent process to ensure the obtainment of valid consent [3]. As such it becomes essential, in the pursuit of valid consent, that there is a good understanding of the concepts underlying the essential ICF elements required by internationally recognized guidelines and regulations.
The aim of the present study was to evaluate the understanding of researchers and IRB/IEC members concerning the essential elements required in an ICF in order to identify issues that need to be addressed, as well as to improve the competency of researchers and IRB/IEC members in obtaining valid consent. Although the internationally recognized sources mentioned above have contributed to the discussion of inform consent, this paper will mainly focus on the elements of an ICF provided by the ICH GCP [3] and the US Regulation (45 CFR 46.116) [7], because the majority of clinical trials currently being conducted are governed by these two documents (see Table 1).
Methods
Eight case studies and questions were developed based on non-study-specific elements required by the ICH GCP [3] and the US Regulation [7] (see Table 1). The case studies were designed so that each case would illustrate one of eight basic ethical issues in clinical research. Case study one focuses on the experimental nature of the study, case study two on the right of research subjects to refuse to participate in the study, three on subjects’ right to withdraw from the study, four on the confidentiality of data, five on the limits of confidentiality, six on the payment for study participation, seven on the compensation in case of any injury incurred during study participation, and case eight on the contact person providing further information during the study (see Table 2).
Table 2.
The questionnaire containing eight short case studies: each case study followed by a question with four multiple-choice answers
| Ethical elements | Short case studies and answer choices | |
|---|---|---|
| Recognition that the study involves research | Mr. Cahill is invited to participate in Phase I Clinical Study. This study aims to test a new drug in healthy volunteers. The new drug has never been used in humans. Which of the following choices is true? | |
| A. | This study is a research study to test a new drug for the first time in humans. | |
| B. | This study is part of a teaching course for medical students to learn how to give drugs to patients. | |
| C. | This study is part of medical treatment that is expected to treat Mr. Cahill’s underlying disease. | |
| D. | This study is part of hospital regulation to test drugs used in the hospital once a year. | |
| CORRECT ANSWER: A | ||
| Right of the subject to refuse to participate in research | Ms. Jones is a secretary in the hospital. She is being invited to participate in a research study. This study will test a new drug in healthy volunteers. She worries about adverse effects from the new drug, so she does not want to take part in this study. What can she do? | |
| A. | She has the right to refuse to participate in this study. | |
| B. | She must participate in this study because it is the duty of healthy people to contribute to the development of new drugs for patients. | |
| C. | She cannot refuse to participate in the study because she works in this hospital. If she refuses, her boss may not be pleased. | |
| D. | She should not worry about adverse effects from the study drug since the research team specializes in related research. She will be closely observed. | |
| CORRECT ANSWER: A | ||
| Right of the subject to withdraw from the study | Mr. Knight was invited to participate in a research study. After being informed of all aspects of the research study, he was willing to participate in the study and signed the consent form. However, when he went home, his wife did not agree with him regarding his participation in such a study. Can he withdraw from study participation? | |
| A. | He cannot because he signed the consent form. | |
| B. | He can tell the research team and withdraw from the study. | |
| C. | He can but he has to pay some penalty because he signed the consent form. | |
| D. | He can but he has to find another volunteer to participate in this study in his place. | |
| CORRECT ANSWER: B | ||
| The confidentiality of data | Ms. Christine decided to participate in a research study. She is 30 years old. During the study, the research team collected her personal information and blood samples for research purposes. At the end of the study, her father would like to see her health data from this study due to safety concerns for his daughter. Can her father see her data from this study? | |
| A. | Yes, he can because he is her father. In general, parents have the right to see their son/daughter’s data. | |
| B. | Yes, he can because it was one of the aims of the study to distribute data from the study to the public. | |
| C. | No, he cannot because he is not an academic person. Only academic persons will be allowed to access the data for academic purpose. | |
| D. | No, he cannot because the data from the research study are kept confidential. | |
| CORRECT ANSWER: D | ||
| Authority of persons who can access the data | Dr. John is an auditor from XXX company, which sponsors the Phase I Clinical Trial. After the study finishes, he would like to get access to the data for verification. Can he see the data in this study? | |
| A. | No, he cannot because the data will be kept confidential. No one but the research team can see these data. | |
| B. | No, he cannot because only the representatives from the National Authority for the drug used and Institutional Review Board (IRB) members can see these data. | |
| C. | Yes, he can because he is a representative of the sponsor who has a responsibility to ensure the accuracy of the data. | |
| D. | Yes, he can because the study aims to distribute the data obtained from study to public. Therefore, everyone can see these data. | |
| CORRECT ANSWER: C | ||
| Prorated payment for study participation | Ms. Catherine decided to participate in a research study. She must make four visits in total to see the research team. She will receive payment for study participation. When will she receive this payment? | |
| A. | After signing the informed consent form. | |
| B. | After her personal information and blood samples have been collected at the screening visit. | |
| C. | After finishing study participation at the end of the study. | |
| D. | Every visit that she comes as prorated payment | |
| CORRECT ANSWER: D | ||
| Compensation in case of any injury directly resulting from study participation | Mr. Adam participated in Phase I Clinical Trial. Two days after taking an investigational drug, he had diarrhea many times. He was admitted to the hospital for two days. The cost of treatment was 500 US dollars. Who will pay for this treatment? | |
| A. | Mr. Adam | |
| B. | Mr. Adam and the researcher | |
| C. | The sponsor of the study | |
| D. | The doctor who treated his illness | |
| CORRECT ANSWER: C | ||
| Research contact person | Ms. Caroline decided to take part in Phase I Clinical Trial. The research staff told her that the study will start next Monday. By Monday, she has a high fever. She is not sure whether she can take any medication or not. Which of the following choices is the best choice for her? | |
| A. | She should call the contact person named on the information sheet of the study and ask for suggestions. | |
| B. | She must not take any treatment since the information sheet states clearly that she cannot take any medication one week prior to the start of her participation in the study. | |
| C. | She should use warm towels to absorb the heat from her body and ask the research team on Monday about what to do. | |
| D. | She should ask another participant who has experience in study participation. | |
| CORRECT ANSWER: A | ||
Each question was designed to reflect the area we considered of most concern regarding that particular element. Following each scenario, there is a question with four multiple-choice answers intended to test the participants’ understanding of the underlying ethical issues presented in the given scenario. The developed case studies and answer choices were reviewed by two independent experts whose comments were then integrated into the final version of the case studies and questions (see Table 2). The assessment of knowledge on the above-mentioned basic ethical issues was performed in three groups of participants on three separate occasions. The first group consisted of researchers in the field of product research and development who attended the 7th International Diploma Course of Research and Development of Products to Meet Public Health Needs (October 29th–November 9th, 2013) (n = 26). The second group was the participants who attended the 13th International Annual FERCAP (the Forum for Ethical Review Committees in Asia and the Western Pacific) Conference in Bali, Indonesia (November 18th–20th, 2013) (n = 69). The last group was PhD candidates or researchers at the Institute of Tropical Medicine (NEKKEN), Nagasaki University in 2013 (n = 12). For the first and second groups of participants, the questionnaires were distributed prior to the training course on informed consent. The questionnaires were answered in an anonymous manner and collected by a qualified individual who had no teacher-student/trainer-trainee relationship with the respondents. Each correct answer was worth 1 point, making the perfect score a total of 8 points.
The data were analyzed using SPSS version 16.0. The distribution of data was tested using Kolmogorov-Smirnov statistics. The frequency of incorrect answers in each question was identified and presented as a percentage of total incorrect answers.
Results
A total of 107 individuals from 20 countries participated in the study. The majority came from Asia and the Western Pacific region (China, India, Indonesia, Japan, Nepal, the Philippines, Sri Lanka, Thailand and Vietnam). Most of the remaining participants came from the African region (Algeria, Botswana, Ethiopia, Guinea, Kenya, Nigeria, Sudan and Uganda), and only four participants came from American and South American regions (Bolivia, Guatemala and USA). Each participant came from one of four backgrounds: researcher, medical doctor, PhD candidate, or IRB/IEC member.
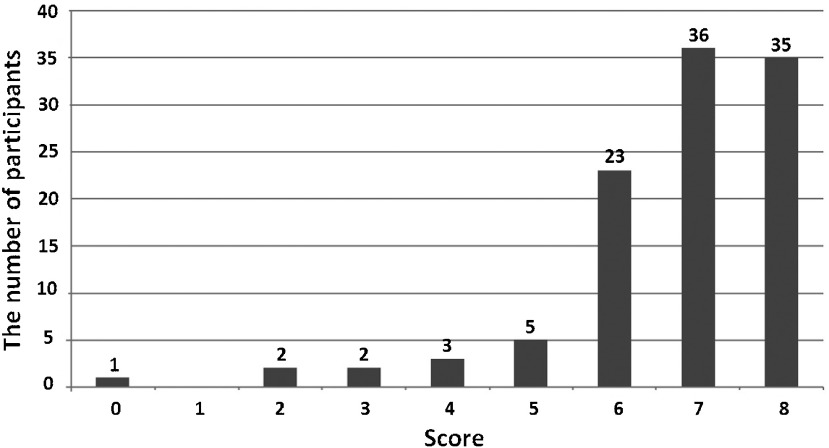
The collected data were not normally distributed, thus the results were presented as a median (Fig. 1). The median score was 7 (interquartile, 6–8). Thirty-five participants (32.7%) had a perfect score (score = 8). Five participants (4.7%) had a score of less than 4. No significant difference of score was observed among the three groups of participants.
Fig. 1.
The data distribution of the participants’ scores. Of 107 participants, the median score was 7 (interquartile, 6–8).
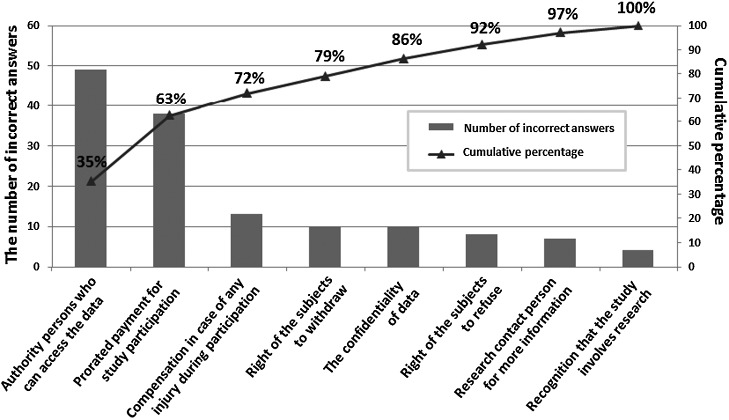
There were a total of 139 incorrect answers from the 107 participants; the frequency of incorrect answers for each question is presented in Figure 2. The most common incorrect answer was in case study 5, which describes the authority of research-related persons to access the data. This question accounted for 35% of all the incorrect answers (49/139). The second most common mistake was in case study 6, describing the need for prorated payment, which accounted for 28% of all the incorrect answers (38/139). Most of the participants who made mistakes on case study 6 chose the answer that suggests that payment should be made at the end of the study.
Fig. 2.
The frequency of incorrect answers for each question. Of the 139 incorrect answers from 107 participants, the two most common incorrect answers were on the ethical issues of (1) authority of persons who can access the data and (2) the need for prorated payment, which accounted for 35% (49/139) and 28% (38/139) of all the incorrect answers, respectively. The participants made a relatively small number of mistakes on the remaining six ethical issues: compensation in case of any injury during participation (13/139), right of the subjects to withdraw (10/139), confidentiality of the data (10/139), right of the subjects to refuse (8/139), contact person for more information during the study (7/139), and experimental nature of the study (4/139).
Discussion
The aim of the present study was to assess the knowledge of researchers and IRB/IEC members regarding the essential ICF elements required by internationally recognized guidelines and regulations. The majority of participants generally understood the elements (median score = 7 and one-third of participants got a perfect score). However, there were two elements which caused confusion for a significant number of participants. The first is that concerning a person’s authority to access confidential data. Among the 107 participants, 49 failed to recognize that certain individuals with the responsibility to ensure the quality and validity of the collected data must have direct access to the identity and collected health data of research subjects [3]. The duty of the investigator team is to respect the wishes of research subjects who grant the team access to their information. Thus, it is important to specify in the ICF who else, other than the investigator team, will have access to the subjects’ confidential information. This piece of information is important in obtaining valid consent, as research subjects may not feel comfortable participating in a particular study if other people can see their health information. If this detail is not disclosed, research subjects may incorrectly assume that no one but the investigator team can get access to their confidential data. In this context, an issue arises when the investigator team allows the monitoring and/or overseeing team access to the confidential information of the subjects. Based on the subject’s assumption, this would be considered a breach of the confidentiality agreement, in violation of the ethical principle of Respect for Persons.
The second common mistake made by over one-third of the participants (38 out of 107) was the requirement of prorated payment, if any, for research participation. Many thought that payment should be made at the end of the study. The ICH GCP [3] requires the sponsor or investigator team to make a payment, if any, to the subjects in a prorated manner so as to ensure the voluntariness of the subject’s study participation. In other words, the method of payment could affect the voluntariness of research subjects. If the payment is made at the end of the study, it could suppress the desire to withdraw from the study, thereby decreasing the voluntariness of research participation. Since voluntariness is one of the three components emphasized in the Belmont Report [2] as necessary for the validity of informed consent, the informed consent will become invalid if the component of voluntariness is missing.
To our knowledge, this is the first empirical study to evaluate researchers’ and IRB/IEC members’ understanding of the essential basic elements required in an ICF. The two major misunderstandings of the study participants, who were mainly researchers or IRB/IEC members in Asia, the Western Pacific and Africa region, raise concern about the validity of informed consent in research practice in tropical countries. In order to improve human subject protection in research in these regions, there is a need to address these weaknesses. It is the role of researchers as well as IRB/IEC members to review the validity of an ICF. As such, proper training in research ethics and clinical studies that emphasize the application of ethical principles underlying each required ICF element is recommended. The researchers and IRB/IEC members should understand and appreciate the importance of each required element.
Our study has some limitations. First, there was the potential impact of selection bias, that is, the study participants might not be representative of researchers and IRB/IEC members throughout the Asia Pacific and African regions. The majority of the study participants were attendees of the Forum of Ethical Review Committees and so may have been more likely to be interested in and knowledgeable about the issue of informed consent, compared to IRB/IEC members/researchers who do not participate in the forum. Second, since the questionnaire used in this survey was in English while the participants came from several countries in the Asia Pacific and African regions, some of the participants might have had a limited grasp of English as it is neither their mother tongue nor an official language in their countries. Due to the language barrier, the score of some participants might be compromised. Five participants whose scores were less than 4 could be resulted from their obstacle to understand the contents of the questionnaire.
In conclusion, the results of this study suggest that there is a need to improve training regarding informed consent among researchers and IRB/IEC members, especially in the Asia Pacific and African regions. The two common significant mistakes fall under the category of relevant information for decision making (i.e. persons who are required by regulation to have access to the confidential data) and factors affecting voluntariness of research participants (i.e. prorated payment for research participation). The objective of ethical training should not only detail the required elements and documentation of an ICF, but also emphasize the importance of valid consent.
Acknowledgement
We would like to thank all the participants for their time in answering the questionnaires. We appreciate the collaboration of the experts from FERCAP who reviewed the case studies used in this study. We thank Professor Cristina Torres and Dr. Qi Lu for coordinating the assessment of the case studies and the conduct of this study. We thank Professor Kenji Hirayama for making comments on this study.
Conflict of Interest
We declare no conflicts of interest.
References
- 1.The Nuremberg Code. Trials of War Criminals before the Nuremberg Military Tribunals under Control Council Law No. 10: Nuremberg October 1946–April 1949. Washington: U.S. Government Printing Office (n.d.); 1949. pp 181–182. [Google Scholar]
- 2.The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research: the Superintendent of Documents. Washington, D.C.: U.S. Government Printing Office; 20402; 1979. [Google Scholar]
- 3.International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use: Good Clinical Practice (E6). Washington, D.C.: European Medicines Agency (EMEA); 1996. [Google Scholar]
- 4.Cox AC, Fallowfield LJ, Jenkins VA. Communication and informed consent in phase 1 trials: a review of the literature. Support Care Cancer 2006; 14: 303–309. [DOI] [PubMed] [Google Scholar]
- 5.Kass NE, Chaisson L, Taylor HA, et al. Length and complexity of US and international HIV consent forms from federal HIV network trials. J Gen Intern Med 2011; 26: 1324–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutta ZA. Beyond informed consent. Bull World Health Organ 2004; 82: 771–777. [PMC free article] [PubMed] [Google Scholar]
- 7.Title 45 Code of Federal Regulations, Part 46 Protection of Human Subjects (45 CFR 46), revised January 15, 2009. Washington, D.C.: U.S. Department of Health and Human Services. [PubMed] [Google Scholar]