Abstract
Objective
We evaluated family history as a predictor of incident and progressive coronary artery calcium (CAC) using data from the Multi-Ethnic Study of Atherosclerosis (MESA).
Background
MESA is a multi-center prospective study of 6,814 asymptomatic individuals. The relationship between family history of coronary heart disease (CHD) and CAC incidence or progression has not been described previously.
Methods
A total of 5,099 participants had detailed information about family history of CHD (late versus premature and parental versus sibling history). The mean time between CAC scans was 3.1 ± 1.3 years. The association of late versus premature family history was assessed against CAC change using multivariate regression model adjusted for demographics and cardiac risk factors.
Results
A family history of premature CHD was associated with an odds ratio (OR) of 1.55 (p < 0.01) for incident development of CAC after adjusting for risk factors and demographics. A premature family history was associated with 14.4 units (p < 0.01) greater volume scores compared to those with no family history in similarly adjusted models by median regression analysis. A combined parental and sibling family history was associated with the greatest incidence and progression in demographic-adjusted models. Caucasians demonstrated the most consistent predictive relationship between family history of premature CHD and incidence (p < 0.01) and progression (p < 0.05) of CAC, though no significant interaction with ethnicity was noted.
Conclusions
Family history of premature CHD is associated with enhanced development and progression of subclinical disease, independent of other risk factors, in a multiethnic, population-based study.
Keywords: Subclinical atherosclerosis, coronary calcium, family history
Introduction
Coronary artery calcification (CAC), measured using non-contrasted computed tomography gated to the cardiac cycle, is a well-validated metric for determining coronary heart disease (CHD) risk in asymptomatic patients. Across a variety of populations, increasing baseline measurements of CAC are associated with future CHD events, independent of traditional risk factors. Assimilation of CAC scores has been shown to improve established risk prediction models [1-5]. Clinical guideline statements have recommended that CAC scoring can be used in select groups of patients to further stratify CHD risk [6-8]. Currently the American Heart Association gives a Class IIa recommendation for measuring CAC in asymptomatic individuals at intermediate CHD risk, having a Framingham risk score (FRS) for 10-year CHD risk between 10% to 20%; there is also a IIa recommendation for testing asymptomatic diabetic patients over the age of 40 [9].
Repeat measurements of CAC enable non-invasive assessment of the development and progression of subclinical atherosclerosis over time. Currently, repeat CAC measurements are being studied for their potential in routine clinical practice in select patients [10]. Although not useful as a marker for near-term responsiveness to statin treatment [11-12], progression of CAC is predicative of CHD events as well as all-cause mortality [13-15]. There is increasing evidence indicating that both established as well as novel CHD risk factors are closely associated with CAC incidence and progression [16-19]. Family history of premature CHD is a recognized risk factor for development of CHD events and overall cardiovascular mortality [20-21]; however, it is not directly accounted for in traditional risk stratification methods, such as the Framingham risk score. Recent reports have shown that a family history of premature CHD is associated with a higher likelihood of presence of any CAC (>0) as well increased CAC scores among those with any detectable CAC [22-23]. Moreover, there is evidence that incorporating family history can improve prediction models for identifying individuals with higher burden of subclinical coronary atherosclerosis as measured by CAC scores [24]. However, to date the relationship between family history and the development and progression of CAC has not been described. In this study we sought to examine the association of family history of CHD with the development and progression of CAC in a multiethnic group of asymptomatic patients.
Methods
Study Participants
Details on the MESA study's prospective design and organization are available in previous reports (Bild et al) From July 2000 to September 2002, 6,814 adults from the general community were enrolled at six field centers in the United States (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota). Participants were aged 45-84 years at enrollment, free of clinical CVD, and identified themselves as white, black, Hispanic, or Chinese. The study was approved by the Institutional Review Boards at each site and all participants provided written informed consent. Medical history, anthropomorphic measurements, vital signs, laboratory data, and baseline imaging for coronary artery calcium score were collected during the baseline examination (performed from July 2000 to July 2002).
Collection of Family History Data
Detailed family history was obtained from 5,099 individuals (75% of all MESA participants) by questionnaire at the second evaluation (July 2002 to January 2004). Individuals were asked whether they had any first-degree relative who had experienced fatal or nonfatal myocardial infarction, received coronary angioplasty, and/or undergone coronary bypass surgery. Premature family history was defined as any of these events occurring before the age of 55 years in a male relative and before the age of 65 years in a female relative; events occurring after these respective age cutoffs were considered late-onset CHD. Individuals were excluded if they were not able to provide information regarding CHD events in first-degree relatives.
Measurement of CAC
Coronary artery calcium was determined by computed tomography scanning by either electron-beam tomography or multi-detector computed tomography, as described previously [26]. All patients received a baseline CT scan for CAC scoring at the initial examination from July 2000 to July 2002. Individuals were randomly selected to get repeat scanning at exam 2, between July 2002 and January 2004 (approximately 50% of participants), or exam 3, between January 2004 and July 2005. An additional subset of 25% of participants was randomly selected for a third CAC measurement during exam 4, between July 2005 and July 2007. Overall, the mean follow-up time was 3.1 years (± 1.3 years). All scans were read at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. Measurements of CAC were adjusted between the different centers and machines by using a standard calcium phantom of known density, which was scanned with each participant. The CAC volume was quantified using the volume scores, with any detectable calcium defined as a CAC score greater than 0. Each participant was scanned twice, and the mean volume score was calculated. This protocol has been described in detail [27]. Incident CAC was defined as a baseline CAC score of 0, with a follow-up score on repeat scan greater than 0. For individuals who had CAC > 0 at baseline scanning, progression of CAC was considered as the absolute difference in CAC score between the baseline exam and the follow-up score.
Multivariable Models and Statistical Analysis
The association of family history with incident CAC was analyzed by multivariable regression using a generalized linear model with logit link and binomial error distribution to determine odds ratios. A logistic regression was used in favor of relative risk regression in the analysis due to non-convergence in adjusted models. Progression of CAC among individuals with baseline CAC > 0 was evaluated by median regression analysis. For both incident CAC and CAC progression, we further examined associations between family history and CAC by race/ethnicity as well as source of premature family history, classified as parent, sibling, or both parent and sibling. Covariates were grouped into three models to adjust for demographic data and known CHD risk factors. In model 1, analyses were adjusted for age, gender, race/ethnicity, MESA site, and follow-up duration. Model 2 was adjusted age, gender, race/ethnicity, MESA site, follow-up duration, education, body mass index (BMI), hypertension, diabetes mellitus, systolic blood pressure, anti-hypertensive medications, lipid-lowering medications, cigarette smoking, low density lipoprotein cholesterol (LDL-C), and high density lipoprotein cholesterol (HDL-C). Model 3 differed between the analyses for incident CAC and CAC progression. When examining incident CAC, this model (referred to as model 3a) was adjusted for age, gender, race/ethnicity, MESA site, follow-up duration, and Framingham risk score (FRS). When analyzing progression of CAC, the covariates adjusted for were age, gender, race/ethnicity, MESA site, follow-up duration, education, BMI, hypertension, diabetes mellitus, systolic blood pressure, anti-hypertensive medications, lipid-lowering medications, cigarette smoking, LDL-C, HDL-C, and the logarithm of baseline CAC score (model 3b).
In order to correlate CAC change with CHD events, we performed a simple event analysis stratifying CHD events by the presence or absence of incident CAC or significant CAC progression. Significant CAC progression was defined as absolute CAC change ≥ 25 volume units with accompanying adjusted CAC percentage change ≥ 25% per 3 years. Indexing to 3 years was chosen because median time between scans was approximately 3 years. CHD events were defined as myocardial infarction, death from CHD, definite angina, probable angina followed by coronary revascularization, or resuscitated cardiac arrest. Events were expressed as occurrences per 1000 person-years. In order to rule out the coronary scan itself causing increased perceived symptomatology among patients or physicians, patients were notified whether they had above average, average, or below average CAC scores at the time of scanning. CHD events were analyzed 90 days post-scan, and no increased revascularizations or other events were noted; there were only 2 revascularizations within the 90-day time frame.
All statistical analyses were carried out by Stata 11.1 (StatCorp LP, College Station, Texas). A p value of less than 0.05 was considered significant.
Results
The baseline characteristics of the MESA study population are presented in Table 1. Statistical comparisons are made between those without a family history of CHD and those with histories of premature and late CHD. Within the total cohort, 47% of the population was male, with the majority of participants being Caucasian (n = 2166). Overall, 52% (n = 2633) of participants had a positive family history of CHD; 20% (n = 1002) of the individuals had a family history of premature CHD, of whom 456 reported the premature history in a parent only, 471 in a sibling only, and 75 in both parents and siblings. The group with a family history of premature CHD tended to be younger, had a higher percentage of participants who were women, African-Americans, current smokers, hypertensive, taking blood pressure and/or cholesterol-lowering medications, and had a lower 10 year CHD risk than other individuals. There was no significant difference among groups in the prevalence of diabetes mellitus or lipid profile.
Table 1. Baseline characteristics of the study population, grouped by family history.
| No FamHx | Late FamHx | Premature FamHx | p value | |
|---|---|---|---|---|
| Sample size (n) | 2466 | 1631 | 1002 | |
| Age (years) | 61 ± 10 | 62.5 ± 10 | 60 ± 10 | <0.001 |
| Gender (male) | 49% | 47% | 41% | <0.001 |
| Race | ||||
| White | 36% | 52% | 44% | |
| Black | 25% | 23% | 30% | |
| Hispanic | 24% | 19% | 22% | |
| Chinese | 15% | 7% | 4% | |
| BMI (kg/m2) | 28 | 29 | 29 | <0.001 |
| Smoking | ||||
| Never | 54% | 48% | 47% | |
| Former | 34% | 40% | 38% | |
| Current | 12% | 11% | 15% | |
| Diabetes Mellitus | 12% | 11% | 13% | 0.23 |
| Hypertension | 39% | 46% | 47% | < 0.001 |
| Systolic BP (mmHg) | 125 ± 21 | 127 ± 21 | 125 ± 20 | 0.002 |
| Diastolic BP (mmHg) | 72 ± 10 | 72 ± 10 | 72 ± 10 | 0.56 |
| BP-lowering med | 32% | 38% | 40% | <0.001 |
| ACE inhibitor | 11% | 13% | 15% | |
| ARB | 5% | 5% | 5% | |
| Thiazide | 9% | 12% | 14% | |
| Amlodipine | 4% | 6% | 5% | |
| Beta blocker | 8% | 10% | 9% | |
| LDL-C (mg/dl) | 117 ± 31 | 118 ± 31 | 118 ± 31 | 0.35 |
| HDL-C (mg/dl) | 50 ± 15 | 51 ± 15 | 51 ± 15 | 0.12 |
| Triglyceride (mg/dl) | 132 ± 89 | 133 ± 93 | 131 ± 75 | 0.81 |
| Lipid-lowering med | 14% | 19% | 20% | <0.001 |
| Statin | 12% | 17% | 18% | |
| Niacin | 1% | 1% | 1% | |
| Fibrates | 1% | 1% | 1% | |
| 10 yr CHD Risk | 7.5% | 8.2% | 7% | <0.001 |
Values expressed as percentage, mean ± standard deviation, or median [inter-quartile range] FamHx: Family history; BMI: Body mass index; BP: blood pressure; LDL-C: low-density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; CHD: coronary heart disease.
Family History and Incident CAC
Within the study group, 2645 individuals (52%) had no CAC at baseline. Among these, 527 (20%) patients developed detectable CAC on follow-up examination. There was a significant increase in incidence of CAC in patients with a premature family history of CHD (7.24 per 100 person-years) compared to those with no history (5.87 per 100 person-years) or a late family history (6.56 per 100 person-years) (p < 0.05) (Fig. 1A). For those with a premature family history, patients with a parental history had a significantly higher incidence in CAC compared to those with no family history (Figure 1B). Having both a parent and a sibling with premature CHD was associated with a higher incidence of CAC than either alone, but this trend did not reach statistical significance.
Figure 1. Incident CAC per 100 patient-years according to family history.
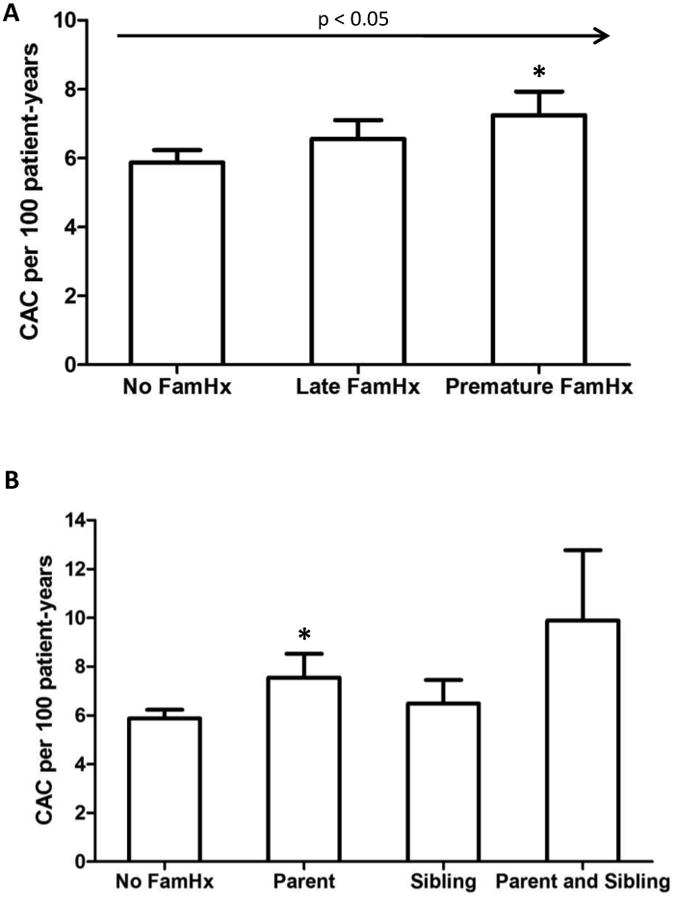
Panel A shows incidence of CAC according to whether patients had a late or premature family history (Fam Hx) of CHD. * indicates p < 0.05 when comparing incidence of CAC among those with a premature family history of CHD to patients with no family history. The p value for the overall trend is less than 0.05. Panel B expresses incidence of CAC according to source of premature family history of CHD. * indicates p < 0.05 when comparing incident CAC in the parental history of premature CHD group with no family history of CHD. Parental history is associated with faster development of CAC in comparison to sibling history, while a family history in both parents and siblings has the highest incidence of CAC.
Table 2 summarizes the odds ratios for development of incident CAC. Compared to those with no family history (taken as the reference group with OR of 1), individuals with a family history of premature CHD had significantly greater odds for developing CAC on follow-up (OR of 1.50) after adjusting for demographic factors, site, and follow-up duration (model 1); this association remained significant after adjusting for additional CHD risk factors and FRS in models 2 and 3, respectively (p < 0.05 for all models). In all three models, there was no significant increase in incident CAC among patients with a family history of late-onset CHD. Individual medications for blood pressure and lipid-lowering therapy were adjusted into model 2 and there was no change in incidence odds ratios. Ethnicity-specific analyses for CAC incidence are also detailed in Table 2. Whites with a premature family history of CHD had a consistently higher odds ratio for incident CAC (p < 0.01 for all three models), but heterogeneity across ethnic groups was not found to be statistically significant (p = 0.31). There was no statistically significant tendency for the development of CAC with a family history of late-onset CHD in any ethnic/racial group.
Table 2.
Odds ratio (OR) for incident development of CAC by family history and stratified by race/ethnicity.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Farm Hx Category | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value |
| Overall | ||||||
| Late | 1.10(0.87-1.39) | 0.42 | 1.06 (0.83-1.35) | 0.64 | 1.20 (0.93-1.53) | 0.16 |
| Premature | 1.50(1.16-1.95)* | 0.02 | 1.36 (1.04-1.78)* | 0.022 | 1.55(1.17-2.05)* | 0.002 |
| Whites | ||||||
| Late | 1.18(0.81-1.71) | 0.385 | 1.09 (0.74-1.61) | 0.67 | 1.18 (0.80-1.73) | 0.40 |
| Premature | 1.99 (1.31-3.03)* | 0.001 | 1.85 (1.20-2.87)* | 0.005 | 2.05(1.33-3.15)* | 0.001 |
| Chinese | ||||||
| Late | 1.31(0.54-3.11) | 0.55 | 1.16 (.48-2.82) | 0.73 | 1.42 (0.59-3.46) | 0.43 |
| Premature | 0.40(0.05-3.15) | 0.38 | 0.41 (0.05-3.64) | 0.42 | 0.47 (0.06-3.97) | 0.49 |
| African American | ||||||
| Late | 0.96 (0.62-1.48) | 0.85 | 1.02 (0.64-1.62) | 0.94 | 1.30(0.80-2.11) | 0.28 |
| Premature | 0.95 (0.58-1.56) | 0.85 | 0.97 (0.58-1.59) | 0.90 | 1.01 (0.57-1.81) | 0.96 |
| Hispanic | ||||||
| Late | 0.98 (0.59-1.63) | 0.94 | 0.97 (0.57-1.65) | 0.91 | 0.96 (0.55-1.67) | 0.89 |
| Premature | 1.76 (1.05-2.95)* | 0.03 | 1.55 (0.92-2.64) | 0.10 | 1.59 (0.90-2.79) | 0.10 |
indicates significance at the 0.05 level.
All analyses are in comparison to participants with no family history of CHD, which was the reference group. Model 1 adjusted for age, gender, race/ethnicity, MESA site, and follow-up duration. Model 2 adjusted age, gender, race/ethnicity, MESA site, follow-up duration, education, BMI, hypertension, diabetes mellitus, systolic blood pressure, anti-hypertensive medications, cigarette smoking, LDL-C, and HDL-C. Model 3 adjusted for age, gender, race/ethnicity, MESA site, follow-up duration, and Framingham risk score.
Table 3 stratifies the impact of premature family history of CHD by source (parent versus sibling). A parental history of premature disease was associated with incident CAC, after adjusting for demographics, CHD risk factors, and FRS, with odds ratios of 1.46 to 1.70 (p < 0.05 in all models). In contrast, premature CHD in siblings did not demonstrate a significant relation to incident CAC. A premature history of CHD in both parents and siblings did show a significant association with development of CAC when adjusted for demographics (OR of 2.58, p < 0.05) and demonstrated a trend towards greater odds of incident CAC when additionally adjusted for CHD risk factors. However, further adjustment for both risk factors and FRS attenuated the statistical significance of this relationship. Interaction testing by source of family history for CAC incidence was non-significant (p = 0.59).
Table 3. Odds ratio for incident CAC by source of premature family history.
| Source of Premature Family History | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| No Family History | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Parent | 1.70 (1.20-2.39)* | 0.003 | 1.46 (1.02-2.09)* | 0.04 | 1.70(1.18-2.46)* | 0.005 |
| Sibling | 1.21(0.84-1.76) | 0.31 | 1.16 (0.80-1.71) | 0.42 | 1.33 (0.89-1.98) | 0.17 |
| Parent and Sibling | 2.58 (1.13-5.87)* | 0.02 | 2.16 (0.96-4.88) | 0.07 | 2.21 (0.92-5.33) | 0.08 |
indicates significance at the 0.05 level.
All analyses are in comparison to participants with no family history of CHD, which was the reference group. Model 1 adjusted for age, gender, race/ethnicity, MESA site, and follow-up duration. Model 2 adjusted age, gender, race/ethnicity, MESA site, follow-up duration, education, BMI, hypertension, diabetes mellitus, systolic blood pressure, anti-hypertensive medications, cigarette smoking, LDL-C, and HDL-C. Model 3 adjusted for age, gender, race/ethnicity, MESA site, follow-up duration, and Framingham risk score.
Family History and Progression of CAC
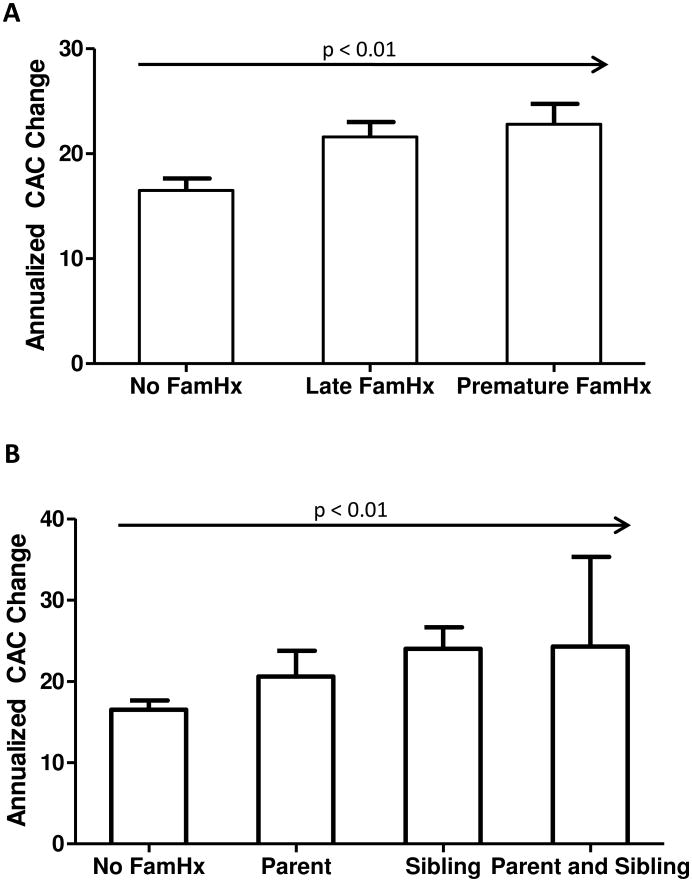
The association of family history with progression of CAC was examined in 2,454 MESA participants who had a CAC > 0 at baseline. No family history of CHD was observed in 1046 individuals (43%). Eight hundred ninety-four (36%) participants had a family history of late-onset CHD, and 514 (21%) had a history of premature CHD in a family member. The latter was associated with an unadjusted absolute median CAC progression of 57 volume units, compared to 42 in those without any family history of CHD (Fig. 2A); similarly, those with a family history of late CHD had an absolute median CAC increase of 59 (p < 0.01 for the trend). Those with a parental history of premature CHD had a median CAC volume that was 8 units higher than the group with no family history while those with a sibling history had a CAC score that was 22 units higher (Figure 2B). A combined sibling and parental history had incremental CAC score progression that was 32 units higher than the reference group. This trend of increased score progression was significant at p < 0.01.
Figure 2. Median CAC change according to family history of CHD for baseline CAC > 0.
Panel A demonstrates that participants with a positive family history (Fam Hx) of CHD had greater median CAC progression than participants with no family history. The p value for the trend is less than 0.01. Panel B stratifies CAC progression by source of premature family history; patients with both a parent and sibling with premature family history showed the greatest increase in CAC on follow-up examination followed by patients with a sibling history and parental history, respectively. The p value for the trend is less than 0.01.
Table 4 details the relationship of family history of CHD to progression of CAC. In analyzing CAC progression, we utilized median regression analysis, as opposed to logistic regression which was used for the incidence studies. This change was made because unlike incidence, which can be categorized as being “present” or “not present,” progression occurs on a continuum. Hence data analysis of this continuous variable is more amenable to median regression analysis. Individuals with a premature family history of CHD had 16.7 units greater progression when compared to those with no family history in a demographics-adjusted model (model 1, p < 0.001); this was over two-fold greater progression than the group with late CHD family history (8.17 units). There was some attenuation of this relationship after adjusting for CHD risk factors. Adjustment for both CHD risk factors as well as the logarithm of the baseline CAC score removed the significant difference for both family-history groups when compared with CAC progression in the negative family history group. Individual medications for blood pressure and lipid-lowering therapy were adjusted into model 2 and there was no change in progression data.
Table 4. Median CAC progression, expressed by CAC volume score, for late versus premature family history of CHD and stratified by race/ethnicity.
| Fam Hx Category | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| β (95% CI) CAC Score | p value | β (95% CI) CAC Score | p value | β (95% CI) CAC Score | p value | |
| No Family History | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Overall | ||||||
| Late | 8.17(1.12-15.22)* | 0.023 | 8.11(1.61-14.62)* | 0.01 | -1.45 (-8.93-6.03) | 0.70 |
| Premature | 16.69 (8.33-25.05)* | <0.001 | 14.44(6.13-22.42)* | <0.001 | 4.35 (-4.52-13.21) | 0.34 |
| Whites | ||||||
| Late | 8.20 (-3.47-21.11) | 0.16 | 10.98 (0.60-21.90) | 0.05 | -1.62(-12.95-9.71) | 0.78 |
| Premature | 27.76 (12.76-2.76)* | <0.001 | 21.32(7.84-34.81)* | 0.002 | 14.22(0.22-28.23)* | 0.046 |
| Chinese | ||||||
| Late | 7.35 (-13.84-28.54) | 0.50 | 23.22 (-5.04-51.49) | 0.11 | 13.19 (-14.62-41.01) | 0.35 |
| Premature | 29.38 (-0.28-59.04) | 0.05 | 38.99 (-1.47-79.44) | 0.06 | 30.80 (-6.95 - 68.54) | 0.11 |
| African American | ||||||
| Late | 6.93 (-10.49-24.35) | 0.44 | 9.72(-11.62-31.06) | 0.37 | 1.00(-14.52-16.52) | 0.9 |
| Premature | 0.04 (-18.57-18.65) | 0.997 | 7.75(-15.33-30.84) | 0.51 | -9.07(-25.68-7.55) | 0.28 |
| Hispanic | ||||||
| Late | 5.45 (-8.96 -19.86) | 0.46 | 0.52 (-14.43-15.47) | 0.95 | -17.24 (-35.39-0.90) | 0.06 |
| Premature | 5.48 (-11.35 - 22.30) | 0.52 | 4.01(-13.33-21.35) | 0.65 | -10.68 (-29.91-8.55) | 0.28 |
indicates significance at the 0.05 level.
All analyses are in comparison to participants with no family history of CHD, which was the reference group. Model 1 adjusted for age, gender, race/ethnicity, MESA site, and follow-up duration. Model 2 adjusted age, gender, race/ethnicity, MESA site, follow-up duration, education, BMI, hypertension, diabetes mellitus, systolic blood pressure, anti-hypertensive medications, cigarette smoking, LDL-C, and HDL-C. Model 3 adjusted for age, gender, race/ethnicity, MESA site, follow-up duration, education, BMI, hypertension, diabetes mellitus, systolic blood pressure, anti-hypertensive medications, cigarette smoking, LDL-C, HDL-C, and the logarithm of baseline CAC score.
Median regression analysis of CAC progression stratified by race/ethnicity demonstrated that whites with a premature family history of CHD showed significantly higher progression of median CAC of 21.3 volume units (p < 0.01) when compared to their counterparts with no CHD family history in a risk factor adjusted model (Table 4). African-Americans, Chinese, and Hispanics did not show a significant association between CAC progression and family history of CHD in this model, although Chinese participants with a premature family history of CHD did show a trend towards greater CAC progression compared to their no family history counterparts. The apparent heterogeneity by ethnic/racial group was however not found to be statistically significant (p = 0.73).
The association of CAC progression with family history of premature CHD was evaluated further through sub-group analysis by source of premature history. As listed in Table 5, a sibling history of premature CHD was associated with a CAC progression of 23.4 units greater volume when compared to individuals with no family history (model 1, p < 0.001), with only some attenuation of this relationship after adjusting for CHD risk factors (p < 0.01). A parental history only or a combined parent-sibling history of premature CHD was significantly associated with greater CAC progression in demographic-adjusted analyses, but this relationship became much weaker and did not reach statistical significance in the fully adjusted models (models 2 and 3). Additionally, there was no significant interaction between overall premature family history and source of premature history for CAC progression (p = 0.72).
Table 5. Median CAC progression, expressed as CAC volume score, by source of premature family history.
| Source of Premature Family History | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| β(95%CI) CAC Score | p value | β(95%CI) CAC Score | p value | β (95% CI) CAC Score | p value | |
| No Family History | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Parent | 16.86(6.10-27.62)* | 0.002 | 9.34 (-4.24-22.92) | 0.18 | 2.64 (-9.59-14.88) | 0.67 |
| Sibling | 23.39 (13.57-33.21)* | <0.001 | 16.98(4.74-29.22)* | 0.007 | 8.37 (-2.79-19.53) | 0.14 |
| Parent and Sibling | 37.17 (14.53-59.81)* | 0.001 | 26.24(-1.98-54.47) | 0.07 | 12.52 (-13.10-38.14) | 0.34 |
indicates significance at the 0.05 level.
All analyses are in comparison to participants with no family history of CHD, which was the reference group. Model 1 adjusted for age, gender, race/ethnicity, MESA site, and follow-up duration. Model 2 adjusted age, gender, race/ethnicity, MESA site, follow-up duration, education, BMI, hypertension, diabetes mellitus, systolic blood pressure, anti-hypertensive medications, cigarette smoking, LDL-C, and HDL-C. Model 3 adjusted for age, gender, race/ethnicity, MESA site, follow-up duration, education, BMI, hypertension, diabetes mellitus, systolic blood pressure, anti-hypertensive medications, cigarette smoking, LDL-C, HDL-C, and the logarithm of baseline CAC score.
To see if family history and CAC together correlate with a higher number of CHD events, we performed an event analysis of CHD events stratified by family history and the presence of CAC incidence/progression (Table 6). The presence of incident CAC and/or significant CAC progression was associated with great CHD events per 1000 person-years, although the analysis was limited by power when evaluating by family history group.
Table 6. CHD events in MESA participants by family history of coronary heart disease and aggregate incidence and progression of CAC.
| No Family Hx | Late Family Hx | Premature Family Hx | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Incidence/Progression? | Negative | Positive | Negative | Positive | Negative | Positive |
| Person-Years | 9099 | 4797 | 5206 | 3922 | 3192 | 2241 |
| CHD Events | 22 | 53 | 17 | 63 | 10 | 26 |
| Events per 1000 person-years | 2.4 | 11 | 3.3 | 16.1 | 3.3 | 10.7 |
| 95% CI | 1.6-3.7 | 8.3 - 14.5 | 2.0-5.3 | 12.5 - 20.6 | 1.7-5.8 | 7.3-15.6 |
Positive incidence/progression represents any CAC incidence or absolute CAC change ≥ 25 volume units with accompanying adjusted CAC percentage change ≥ 25% per 3 years (median time between scans). CHD: coronary heart disease
Discussion
In a population-based multiethnic cohort of asymptomatic, mostly low- and intermediate-risk men and women, a family history of CHD, particularly a combined parental and sibling history of premature CHD, is associated with CAC incidence and progression in MESA. The findings further support and reinforce the addition of family history of CHD in current methods of global risk assessment and practice guidelines.
Previous studies have indicated a relationship between premature family history and baseline CAC measured at a single time point [22-23, 28-31]. Those studies support the notion that family history has an important association with a static measure of coronary atherosclerosis. Our results now demonstrate that family history of premature CHD is associated with both initial development and progression of CAC. It is not entirely clear that the increased risk of coronary atherosclerosis progression among those with family history of premature CHD can be attributable entirely to additive genetic effects. It is important to note that atherosclerosis is influenced by a complex interplay among numerous environmental and genetic factors and family members share not share similar environmental factors such as diet and lifestyle pattern. In this regard determination of family history of premature CHD can serve as an excellent surrogate to identify cumulative effect of genetic and lifestyle factors that may have specific influence on CHD risk.
One important consideration when evaluating CAC progression is its relationship to baseline CAC. In the present study, we analyzed data for change in CAC utilizing models which included and excluded baseline CAC. Models that did not include baseline CAC as an adjustment parameter (models 1 and 2) revealed strong associations between family history and CAC progression, while adjusting for baseline CAC (model 3b) strongly attenuated the relationship and degree of progression. However, considering baseline CAC as a confounder for CAC progression is problematic since it ignores that progression occurs both before and after the initial observation of CAC is made. Baseline CAC is inherently a result of prior rates of progression of coronary calcium and is a known strong predictor of future CAC progression [18]. Thus, it would be reasonable to consider that prior progression, and hence baseline CAC, would influence future rates of change in calcifications. Baseline CAC can in fact be considered an integrative risk factor, taking into account several separate risk categories, including family history. Adjusting for baseline CAC may negate the environmental and genetic factors that interact with, rather than confound, CAC progression, as well as adjust out net effects of other risk factors including family history, leading to a smaller difference between groups.
This study examines the effect of a potent risk factor of CHD on the development and change in coronary calcification. It is also important to consider how CAC correlates with response to treatment. One area where this relationship is still being evaluated is statin effect and CAC. Although there is strong evidence indicating a beneficial impact of statin therapy on overall plaque stability and size by intravascular ultrasound [32-34], statin effect on the calcified component of the plaque is less clear. Data from observational and retrospective studies have suggested that statin therapy can be associated with a reduction in CAC progression [35-36]. Other studies have shown that in patients who develop an MI, CAC progression remains high and is unaffected by statin therapy [12, 37]. The largest prospective trial evaluating statin therapy on CAC was conducted in patients from the St. Francis Heart Study with CAC at or above the 80th percentile for age and gender [38]. No decrease in CAC progression was observed after a mean-time follow-up of 4.3 years, and the decrease in CHD events did not reach statistical significance. However, these studies were likely underpowered to detect a difference in CAC progression, and participants had very elevated baseline CAC scores, which makes interpretation of CAC progression more challenging. Interestingly, a recently completed post-hoc analysis of the St. Francis Heart Study found a significant reduction in CHD events with statin treatment for participants with a family history of premature CHD and elevated baseline CAC scores, although CAC score change was not examined in this analysis [39]. It would be interesting to see if individuals with varying degrees of risk factors, including extent of family history and CAC, demonstrate a risk-factor- and score-dependent relationship to statin response. In this context, our results provide compelling evidence to support the need for those future studies.
One difficulty associated with any measurement of family history is recall bias, since objective measurements, such as reviewing medical records for all family members, are often impractical or not achievable. Prior studies have shown that recall bias tends towards under-reporting CHD history in family members, resulting in a low sensitivity, although the specificity of a positive family history for a CHD event is variable [40-41]. Those prior studies also suggest some differences between recall of sibling versus parental history, with decreased recall of sibling history as well as a trend towards poorer recall of familial history with increasing age. The net effect of this is that computed CHD risks associated with family history may be falsely lowered, since the bias tends towards a negative family history. Misclassification can also be biased towards the null in general, since patients who are unaware of their relatives' medical history may report it as being negative. Alternatively, there is also the possibility of a bias away from the null as well; in this scenario, patients who have risk factors for CHD or a history of clinical events may more likely report relatives as having a history of CHD. This would lead to an over-estimation of risk posed by family history. One additional limitation of family history data collection in the MESA population is that family history information was obtained on the second evaluation rather than on the initial visit. Thus, family history data may be unavailable for participants who came to the initial visit and scan but who did not subsequently follow up. This could be a source of bias, particularly for those who had baseline CAC on that initial scan, for an association with no family history.
Studies have found that sibling histories of CHD are associated with a greater incidence and prevalence of cardiovascular events [42-44]. However, these studies looked at clinical cardiovascular events as their study end-points, rather than subclinical disease, which we evaluated through CAC. Prior studies have also suggested a stronger association between the prevalence of CAC and sibling history of premature CHD, as compared with parental history, after multivariate analysis adjusting for CHD risk factors in a cohort of MESA participants [23] and a cohort of physician-referred patients [30]. Intriguingly, we observed that a combined parental and sibling history of premature CHD was associated with the greatest incidence and progression in CAC. This suggests that, family history may have a “dose-dependent” effect, where individuals with more robust family histories of CHD are at greater risk, and hence may have a poorer prognosis. Further studies are needed to determine how source and extent of family history interacts with disease progression and the impact this has on vascular calcification as determined by CAC.
In analyzing the heterogeneity of the association of family history with coronary artery calcification, we observed that whites with a premature family history of CHD had a significant increase in incidence and progression of CAC. This data should be taken in light of prior reports showing a higher prevalence of CAC and greater baseline calcification scores in whites compared to other ethnic/racial groups [45-47]. However, in an analysis of clinical events, a study by Detrano and colleagues did not find a race/ethnicity-specific difference in the predicative value of CAC for coronary events, although the total number of observed events was low [48]. Additional studies are needed to better understand how the respective potential genetic loci and environmental factors entailed in family history and race/ethnicity interact with one another and how these affect clinically relevant endpoints. In this regard, one study has examined whole-blood gene expression profiles and CAC levels among white and African American women selected from MESA participants [49]. The study found concordantly decreased expression profiles of several genes in African Americans, particularly those involved in immunologic and inflammatory pathways, when compared to the overall multi-racial low CAC score group. It will be interesting to see how family history associates with these expression profiles to gain a more complete mechanistic understanding of the genetic influences on CAC progression, and whether such factors are ultimately associated with differences in clinical CHD events.
In summary, our study demonstrates that family history of premature CHD independently predicts incident CAC and is generally associated with increased progression of CAC in a large multiethnic cohort of asymptomatic individuals. The present results further support the notion that family history is an important determinant of cardiovascular risk, and suggest that this risk may be conferred by acceleration in subclinical atherosclerosis. More research is warranted to determine whether use of family history of CHD in conjunction with repeat CAC testing in intermediate term follow-up will better identify those subjects at higher risk who would be candidates for more aggressive primary prevention, including intensified diet and life style modifications as well as pharmacotherapy.
Acknowledgments
We thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of Interest Disclosures: Dr. Budoff is on the speaker's bureau for GE Healthcare.
References
- 1.Taylor AJ, Bindeman J, Feuerstein I, et al. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–814. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 2.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 3.Becker A, Leber A, Becker C, et al. Predictive value of coronary calcifications for future cardiac events in asymptomatic individuals. Am Heart J. 2008;155:154–160. doi: 10.1016/j.ahj.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Greenland P, LaBree L, Azen SP, et al. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 5.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Comput Tomogr. 2010;4:407 e401–433. doi: 10.1016/j.jcct.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 7.O'Rourke RA, Brundage BH, Froelicher VF, et al. American College of Cardiology/American Heart Association Expert Consensus Document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. J Am Coll Cardiol. 2000;36:326–340. doi: 10.1016/s0735-1097(00)00831-7. [DOI] [PubMed] [Google Scholar]
- 8.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Circulation. 2007;115:402–426. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 9.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 10.McEvoy JW, Blaha MJ, Defilippis AP, et al. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010;56:1613–1622. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Arad Y, Spadaro LA, Roth M, et al. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005;46:166–172. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 12.Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol. 2004;24:1272–1277. doi: 10.1161/01.ATV.0000127024.40516.ef. [DOI] [PubMed] [Google Scholar]
- 13.Arad Y, Goodman KJ, Roth M, et al. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–165. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 14.Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–1236. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Shemesh J, Apter S, Stolero D, et al. Annual progression of coronary artery calcium by spiral computed tomography in hypertensive patients without myocardial ischemia but with prominent atherosclerotic risk factors, in patients with previous angina pectoris or healed acute myocardial infarction, and in patients with coronary events during follow-up. Am J Cardiol. 2001;87:1395–1397. doi: 10.1016/s0002-9149(01)01561-2. [DOI] [PubMed] [Google Scholar]
- 16.DeFilippis AP, Kramer HJ, Katz R, et al. Association between coronary artery calcification progression and microalbuminuria: the MESA study. JACC Cardiovasc Imaging. 2010;3:595–604. doi: 10.1016/j.jcmg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaha MJ, DeFilippis AP, Rivera JJ, et al. The relationship between insulin resistance and incidence and progression of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2011;34:749–751. doi: 10.2337/dc10-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 19.Kretowski A, McFann K, Hokanson JE, et al. Polymorphisms of the renin-angiotensin system genes predict progression of subclinical coronary atherosclerosis. Diabetes. 2007;56:863–871. doi: 10.2337/db06-1321. [DOI] [PubMed] [Google Scholar]
- 20.Marenberg ME, Risch N, Berkman LF, et al. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Nam BH, D'Agostino RB, Sr, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291:2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 22.Philips B, de Lemos JA, Patel MJ, et al. Relation of family history of myocardial infarction and the presence of coronary arterial calcium in various age and risk factor groups. Am J Cardiol. 2007;99:825–829. doi: 10.1016/j.amjcard.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 23.Nasir K, Budoff MJ, Wong ND, et al. Family history of premature coronary heart disease and coronary artery calcification: Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;116:619–626. doi: 10.1161/CIRCULATIONAHA.107.688739. [DOI] [PubMed] [Google Scholar]
- 24.Scheuner MT, Setodji CM, Pankow JS, et al. General Cardiovascular Risk Profile identifies advanced coronary artery calcium and is improved by family history: the multiethnic study of atherosclerosis. Circ Cardiovasc Genet. 2010;3:97–105. doi: 10.1161/CIRCGENETICS.109.894527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 26.Detrano RC, Anderson M, Nelson J, et al. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility--MESA study. Radiology. 2005;236:477–484. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 27.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 28.Gidding SS, McMahan CA, McGill HC, et al. Prediction of coronary artery calcium in young adults using the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk score: the CARDIA study. Arch Intern Med. 2006;166:2341–2347. doi: 10.1001/archinte.166.21.2341. [DOI] [PubMed] [Google Scholar]
- 29.Michos ED, Nasir K, Rumberger JA, et al. Relation of family history of premature coronary heart disease and metabolic risk factors to risk of coronary arterial calcium in asymptomatic subjects. Am J Cardiol. 2005;95:655–657. doi: 10.1016/j.amjcard.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 30.Nasir K, Michos ED, Rumberger JA, et al. Coronary artery calcification and family history of premature coronary heart disease: sibling history is more strongly associated than parental history. Circulation. 2004;110:2150–2156. doi: 10.1161/01.CIR.0000144464.11080.14. [DOI] [PubMed] [Google Scholar]
- 31.Scheuner MT, Setodji CM, Pankow JS, et al. Relation of familial patterns of coronary heart disease, stroke, and diabetes to subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. Genet Med. 2008;10:879–887. doi: 10.1097/GIM.0b013e31818e639b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiro T, Kimura T, Morimoto T, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study) J Am Coll Cardiol. 2009;54:293–302. doi: 10.1016/j.jacc.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 34.Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 35.Budoff MJ, Lane KL, Bakhsheshi H, et al. Rates of progression of coronary calcium by electron beam tomography. Am J Cardiol. 2000;86:8–11. doi: 10.1016/s0002-9149(00)00820-1. [DOI] [PubMed] [Google Scholar]
- 36.Callister TQ, Raggi P, Cooil B, et al. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339:1972–1978. doi: 10.1056/NEJM199812313392703. [DOI] [PubMed] [Google Scholar]
- 37.Raggi P, Cooil B, Ratti C, et al. Progression of coronary artery calcium and occurrence of myocardial infarction in patients with and without diabetes mellitus. Hypertension. 2005;46:238–243. doi: 10.1161/01.HYP.0000164575.16609.02. [DOI] [PubMed] [Google Scholar]
- 38.Arad Y, Spadaro LA, Roth M, et al. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005;46:166–172. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 39.Mulders TA, Sivapalaratnam S, Stroes ES, et al. Asymptomatic individuals with a positive family history for premature coronary artery disease and elevated coronary calcium scores benefit from statin treatment: a post hoc analysis from the St. Francis Heart Study. JACC Cardiovasc Imaging. 2012;5:252–260. doi: 10.1016/j.jcmg.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Bensen JT, Liese AD, Rushing JT, et al. Accuracy of proband reported family history: the NHLBI Family Heart Study (FHS) Genet Epidemiol. 1999;17:141–150. doi: 10.1002/(SICI)1098-2272(1999)17:2<141::AID-GEPI4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 41.Silberberg JS, Wlodarczyk J, Fryer J, et al. Correction for biases in a population-based study of family history and coronary heart disease. The Newcastle Family History Study I. Am J Epidemiol. 1998;147:1123–1132. doi: 10.1093/oxfordjournals.aje.a009410. [DOI] [PubMed] [Google Scholar]
- 42.Friedlander Y, Siscovick DS, Weinmann S, et al. Family history as a risk factor for primary cardiac arrest. Circulation. 1998;97:155–160. doi: 10.1161/01.cir.97.2.155. [DOI] [PubMed] [Google Scholar]
- 43.Vaidya D, Yanek LR, Moy TF, et al. Incidence of coronary artery disease in siblings of patients with premature coronary artery disease: 10 years of follow-up. Am J Cardiol. 2007;100:1410–1415. doi: 10.1016/j.amjcard.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanez ND, Burke GL, Manolio T, et al. Sibling history of myocardial infarction or stroke and risk of cardiovascular disease in the elderly: the Cardiovascular Health Study. Ann Epidemiol. 2009;19:858–866. doi: 10.1016/j.annepidem.2009.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 46.McClelland RL, Chung H, Detrano R, et al. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 47.Fornage M, Lopez DS, Roseman JM, et al. Parental history of stroke and myocardial infarction predicts coronary artery calcification: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Eur J Cardiovasc Prev Rehabil. 2004;11:421–426. doi: 10.1097/01.hjr.0000129744.61087.b4. [DOI] [PubMed] [Google Scholar]
- 48.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 49.Huang CC, Lloyd-Jones DM, Guo X, et al. Gene expression variation between African Americans and whites is associated with coronary artery calcification: the multiethnic study of atherosclerosis. Physiol Genomics. 2011;43:836–843. doi: 10.1152/physiolgenomics.00243.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]