Abstract
As a member of the Eph family of receptor tyrosine kinases, EphA7 plays an important role in cancer. However, the expression and significance of Eph receptors in esophageal squamous cell carcinoma (ESCC) remain unclear. Here, we detected the expression of EphA7 by immunohistochemistry in a sample of 352 patients with ESCC, and aimed to investigate the expression status of EphA7 in ESCC and its impact on prognosis. The results showed that low EphA7 expression significantly correlated with lymph node metastases (N0: 29%; N1: 64%. p<0.001), poor degree of tumor differentiation (G1: 31%; G2: 49%; G3: 58%. p=0.009) and pTNM staging (I+II: 33%; III+IV: 58%. p<0.001). Furthermore, in a combined analysis, patients with low EphA7-expressing tumors showed a shorter overall survival than those with high expression, resulting in a five-year overall survival rate of 47.4% vs. 52.6%, respectively (p=0.016). Consequently, patients with a low EphA7 expression have poorer prognosis in ESCC compared with those manifesting high expression.
Keywords: EphA7, esophageal squamous cell carcinoma, lymph mode metastasis, immunohistochemistry, prognosis
I. Introduction
Esophageal squamous cell carcinoma (ESCC) is the most common malignant tumor of the upper aerodigestive tract [15]. The most difficult aspect of treating ESCC is its propensity for local invasion and metastasis, which is the leading cause of death in cancer patients [20]. Ephrin receptors (Ephs) named after the erythropoietin-producing hepatocellular carcinoma cell line from which their cDNA was derived, are over-expressed in numerous human tumors, with prognostic implications [24]. Eph receptors are the largest of receptor tyrosine kinases, and activated by binding of Ephrins [16, 17, 20]. According to their structural features and their preference for different Ephs, Eph receptors have been divided into 2 groups, designated EphA (A1–A8) and EphB (B1–B6). EphA receptors preferentially bind to glycosylphosphatidylinositol (GPI)-anchored ligands such as ephrin-A (A1–A5), whereas EphB (B1–B6) receptors preferentially bind to transmembrane ligands such as ephrin-B (B1–B3) [6]. Those are phosphorylated after Eph/ephrin interaction [4]. The amino-terminal “Ephrin-binding” domain contains a high-affinity binding site that mediates receptor-Ephrin interaction between cells [9]. These activities depend on the Eph receptors. Ephrins activate complex bidirectional signaling networks and other signaling systems in physiology and disease [21, 23]. Eph receptors and ephrins are involved not only in early developmental processes, but also in adult physiology and the ability to modulate molecular signaling pathways is associated with important medical applications [10].
EphA7 (formerly known as Mdk1/Ebk/Ehk) is highly conserved in vertebrates from fish to humans [26]. It is widely expressed in embryonic tissues, especially in developing central nervous system [5]. Recent studies have demonstrated EphA7 expression in human breast cancer [8], prostate cancer [12], colorectal cancer [29], gastric carcinoma [30] and glioblastoma multiforme [31]. However, the relationship between EphA7 expression and tumor invasiveness, metastasis and prognosis in patients with ESCC is still unclear.
We conducted an immunohistochemical (IHC) analysis of EphA7 protein expression to determine the relationship between EphA7 expression and clinicopathological factors in ESCC.
II. Materials and Methods
Patients and tissue specimens
ESCC specimens from 352 patients with primary esophageal cancer and adjacent normal esophageal epithelium from 36 of these patients, as well as another 17 cases of low-grade dysplasia tissues and 13 cases of high-grade dysplasia tissues from these patients, were obtained during surgical resection at the Department of Pathology of Shantou Central Hospital from 2003 to 2013. No patients had received radiotherapy or chemotherapy before the surgical resection. Of the 352 patients, 239 were male and 113 were female (ratio, 2.1: 1), with ages ranging from 37 to 81 years (median, 59 years). We used the revised TNM stage criteria by AJCC in 2009 [7]: stage I (n=33), II (n=161), III (n=158), IV (n=0). Evaluation of tumor differentiation was based on histological criteria of the World Health Organization guidelines [3]: complete (53 cases), moderate (270 cases) and poor differentiation (29 cases). All cases had complete follow-up records. The follow-up after esophageal resection was continued until death, and only patients who died of ESCC were included in the tumor-related deaths. Patients who had severe postoperative complications or other tumors, or those who died of other causes were excluded. This study was conducted under the regulations of the Institutional Review Board of Shantou Central Hospital. Informed consent was obtained from all the enrolled patients prior to surgery.
Tissue microarray (TMA) construction
TMA construction of esophageal carcinoma tissue has been described previously [33]. Briefly, TMA for immunohistochemistry were based on samples with adequate tissue available for persistent correlative studies. Representative tissue areas were demarcated from hematoxylin and eosin-stained sections on individual paraffin blocks. At least two tissue cores were acquired from each specimen, measuring 1.8 mm in diameter and 1.0 to 3.0 mm in length depending on the depth of tissue in the donor block. Each core was precisely arrayed into a new paraffin block. These microarrays were serially sectioned (4 μm) and stained with hematoxylin and eosin to ensure tissue sampling and completeness. The unstained sections were baked overnight at 56°C in preparation for immunohistochemistry staining.
Immunohistochemical analysis
The sections were dewaxed in xylene and rehydrated in a graded series of alcohols. Subsequently, slides were immersed in a peroxidase quenching solution containing one part of 30% hydrogen peroxide in nine parts of absolute methanol, for 10 min. After rinsing in PBS, antigen retrieval from the tissue was carried out by autoclaving in 0.01 M sodium citrate buffer (pH 6.0) at 120°C for 3 min. Next, sections were blocked in 10% normal goat serum for 10 min at room temperature and then incubated overnight at 4°C with rabbit anti-EphA7 monoclonal antibody (Abcam, Cambridge, UK; product number ab5400) against amino acids 26–41 of human Eph A7, at a dilution of 1:50. The sections were then subjected to immunostaining with the PV-9000 2-step plus Poly-HRP Anti-Mouse/Rabbit IgG Detection System (ZSGB-BIO, Beijing, China) and the Liquid DAB Substrate Kit (Invitrogen, San Francisco, CA). Samples were rinsed with distilled water. Subsequently, slides were counterstained with Mayer’s Hematoxylin, dehydrated, and mounted.
Evaluation of immunostained samples
The immunostained samples were assigned a mean score based on the staining intensity and the proportion of tumor cells showing unequivocal positive reaction. Each section was independently assessed by two pathologists blinded to patients’ data. Positive reactions were defined as those showing brown signals in the cell cytoplasm. For EphA7, a staining index (values 0–12) was determined by multiplying the staining intensity score with the positive reaction score. The intensity was scored as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. The frequency of positive cells was graded as follows: 0, less than 5%; 1, 5% to 25%; 2, 26% to 50%; 3, 51% to 75%; and 4, greater than 75%. Heterogeneous staining was scored for each component independently and the results were aggregated. For example, a specimen containing 75% tumor cells with moderate intensity (3 × 2 = 6), and another 25% tumor cells with weak intensity (1 × 1 = 1) received a final score of 6 + 1 = 7. For statistical analyses, scores of 0 to 7 were considered low expression whereas scores of 8 to 12 were deemed high [34].
Statistical analysis
SPSS 13.0 for Windows (SPSS Inc, IL, USA) was used for statistical analysis. The relationship between EphA7 expression and other clinicopathological characteristics including age, gender, tumor size, tumor location, tumor differentiation grade, invasive depth, lymph nodes metastasis, and TNM stage, was analyzed using Pearson’s Chi-Square test. Kaplan–Meier survival analysis (Log-Rank test) was used to evaluate differences in survival between patient subgroups. In addition, a Cox proportional hazards regression model was used to determine potential prognostic factors of postoperative survival. P<0.05 was considered statistically significant.
III. Results
Expression of EphA7 protein in normal esophageal tissue, esophageal dysplasia and ESCC
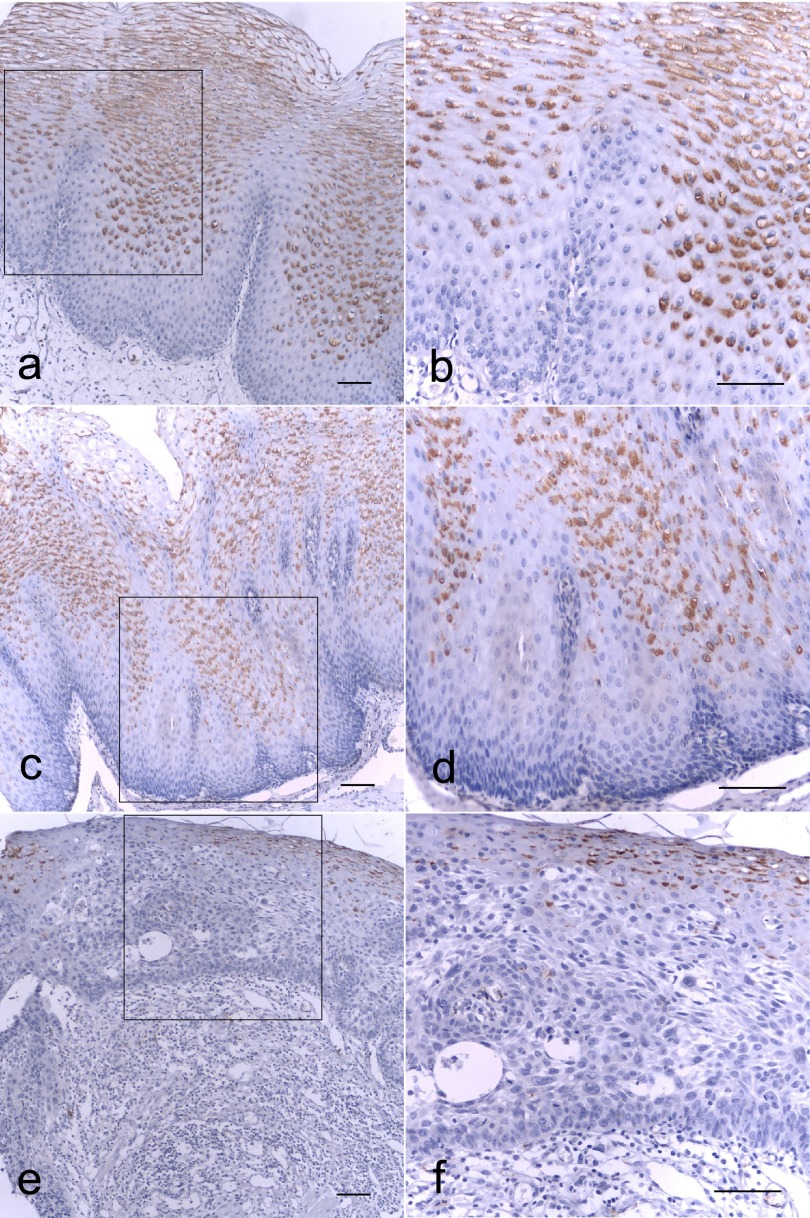
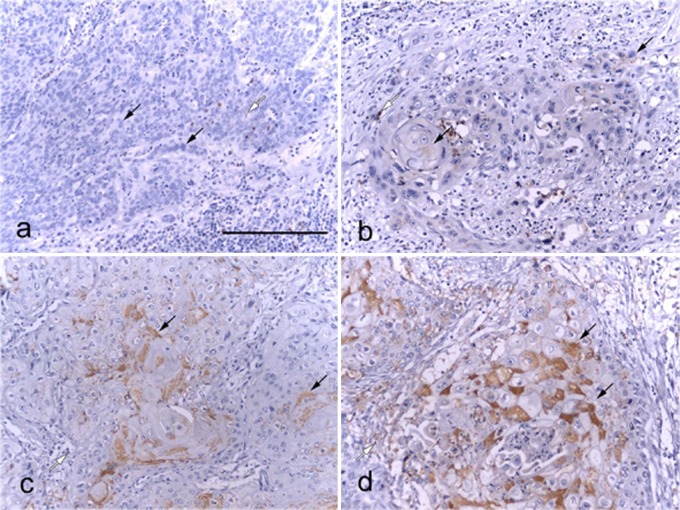
EphA7 expression in ESCC was investigated by immunohistochemical analysis of formalin-fixed, paraffin-embedded specimens using an EphA7-specific MAb. In normal esophageal tissue, EphA7 immunostaining appeared in the cytoplasm and cell membrane of the differentiated zones (Fig. 1a and b). Immunostaining of EphA7 was also seen in the cytoplasm and cell membrane of low-grade and high-grade dysplasia, particularly in cells located in the differentiated zones (Fig. 1c–f). However, the cytoplasm and cell membrane of the atypical cell layer cells in the high-grade dysplasia were EphA7-negative (Fig. 1e and f). In addition, weak, moderate or strong immunostaining of EphA7 was found in the cytoplasm of cancer cell nests with abundant keratin pearl cells (Fig. 2b–d). However, complete loss of EphA7 expression was found in the cytoplasm of cancer cell nests without formation of abundant keratin pearl cells (Fig. 2a). These data indicate that EphA7 expression may be related to tumor differentiation.
Fig. 1. .
Immunohistochemical analysis of EphA7 expression in the progression from normal esophageal mucosa (a, b), low-grade (c, d), to high-grade (e, f) dysplasia. EphA7 located in the cytoplasm and cell membrane of the differentiated zones (a and b). Immunostaining of EphA7 was also seen in the cytoplasm and cell membrane of low-grade (c and d) and high-grade dysplasia (e and f) but EphA7 was negative in the cytoplasm and cell membrane of high-grade dysplasia involving atypical cell layer (e and f). Bars=200 μm.
Fig. 2. .
Immunostaining analysis of EphA7 in ESCC. Representative photographs of EphA7 expression status. Black arrowheads show EphA7-positive cytoplasm in ESCC. White arrowheads show EphA7-positive regions in the cytoplasm of inflammatory cells. a, negative staining; b, weak staining; c, moderate staining; and d, strong staining. Bars=200 μm.
EphA7 expression correlated with various clinicopathologic characteristics of ESCC
To elucidate the clinical significance of EphA7 expression in ESCC, we sought to determine possible correlation between EphA7 expression and various clinicopathologic characteristics of 352 ESCC cases (Table 1). Significant association between low cytoplasmic EphA7 expression and lymph node metastasis was observed (rs=0.344; p<0.001). In the 183 cases of ESCC with lymph node metastasis, 66 patients (66/183; 36.1%) presented high cytoplasmic EphA7 expression, whereas 119 (119/169; 70.4%) of the 169 cases of ESCC without lymph node metastases exhibited high cytoplasmic EphA7 expression, suggesting that the ESCC cases without lymph node metastasis were more likely to show high cytoplasmic EphA7 expression. In addition, low expression of EphA7 was correlated with a poor degree of tumor differentiation (rs=0.134; p=0.009). Furthermore, in the 160 cases of ESCC with pTNM staging (III+IV), 56 cases (56/160; 35.0%) presented high cytoplasmic EphA7 expression, whereas 129 (129/192; 67.2%) of the 192 cases of ESCC with pTNM staging (I+II) exhibited high cytoplasmic EphA7 expression, suggesting that low EphA7 expression was correlated with TNM Classification (rs=0.321; p<0.001). However, there was no significant association with age, gender, tumor size, tumor location or depth of tumor invasion.
Table 1 .
Association between A7 expression and parameters in esophageal squamous cell carcinoma
| Clinical Parameters | Low expression | High expression | X2 | p-value | |
|---|---|---|---|---|---|
| Age | <59 | 88 | 94 | 0.019 | 0.749 |
| ≥59 | 79 | 91 | |||
| Gender | Male | 109 | 130 | 0.053 | 0.361 |
| Female | 58 | 55 | |||
| Tumor size | ≤3 cm | 44 | 42 | ||
| 3–5 cm | 70 | 90 | 0.002 | 0.985 | |
| >5 cm | 52 | 52 | |||
| Tumor location | Upper | 7 | 14 | ||
| Middle | 68 | 78 | 0.059 | 0.266 | |
| Lower | 92 | 93 | |||
| Invasive depth | T1+T2 | 39 | 43 | 0.001 | 1.000 |
| T3+T4 | 128 | 142 | |||
| Differentiation | G1 | 16 | 35 | ||
| G2 | 133 | 137 | 0.134 | 0.009 | |
| G3 | 18 | 13 | |||
| Lymph node metastasis | N0 | 50 | 119 | 0.344 | <0.001 |
| N1 | 117 | 66 | |||
| pTNM staging | I+II | 63 | 129 | 0.321 | <0.001 |
| III+IV | 104 | 56 |
Low expression (≤2), High expression (>2).
Correlations between EphA7 expression and prognosis of : patients with ESCC
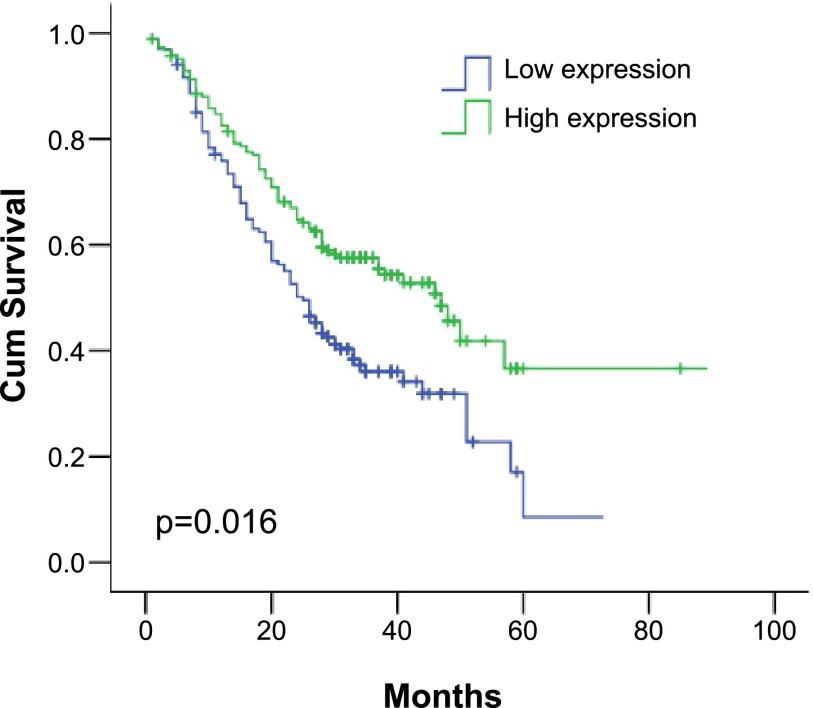
The survival rate of patients with low EphA7 expression was significantly lower than in patients with high EphA7 expression (5-year survival rate 52.6% vs 47.4%, respectively; p=0.016; Fig. 3). These results indicate that EphA7 might be a potential prognostic marker of ESCC.
Fig. 3. .
Postoperative overall survival related to EphA7 expression. Patients with high EphA7 expression showed a significantly more favorable prognosis than those with low EphA7 expression (5-year survival rate: high expression, 44%; low expression, 56%; p=0.016).
Further, a multivariate analysis of gender, age, tumor size, tumor location, invasive depth, differentiation, pTNM staging and lymph node metastasis was conducted. The results showed that EphA7 expression (risk=0.874; 95% CI≈0.613–1.248; p=0.459) was not an independent prognostic factor (Table 2).
Table 2 .
Multivariate cox regression analysis of clinicopathologic factors for risk prediction in 352 patients with esophageal squamous cell carcinoma
| Factor | Risk | 95% CI | p-value |
|---|---|---|---|
| Age | 1.393 | 1.009–1.923 | 0.044 |
| Gender | 0.811 | 0.557–1.179 | 0.273 |
| Tumor size | 0.659 | 0.414–1.049 | 0.079 |
| Tumor location | 0.903 | 0.646–1.262 | 0.549 |
| Invasive depth | 1.101 | 0.700–1.734 | 0.677 |
| Differentiation | 0.547 | 0.335–0.894 | 0.016 |
| pTNM staging | 2.022 | 1.462–2.796 | <0.001 |
| Lymph node metastasis | 2.113 | 1.519–2.940 | <0.001 |
| A7 high expression | 0.874 | 0.613–1.248 | 0.459 |
IV. Discussion
ESCC is a lethal malignancy, and development of biomarkers for predicting prognosis is a clinical imperative. The Eph family of proteins is a potential candidate for predictive biomarkers. It has been reported that high EphA2 expression results in poor survival in ESCC [22]. EphA3, another member of Eph family, plays a tumor suppressor role in several tumors [28]. Previous studies have shown that EphA7 plays an important role in embryonic development of animals and humans [2, 25, 27]. Contrasting EphA7 levels of expression were observed in different tumors. For instance, the expression of EphA7 was upregulated in breast cancer [8] and gallbladder adenocarcinoma [19], but was downregulated in human gastric carcinoma, colorectal cancer and prostate cancer [12, 29, 30]. Therefore, EphA7 plays diverse roles in carcinogenesis. In our study, we first evaluated EphA7 expression in normal esophageal tissue and esophageal dysplasia by IHC analysis. We found that EphA7 was predominantly expressed in the cytoplasm and cell membrane of differentiated zones, whereas atypical cell layer cells did not show EphA7 immunoreactivity. Similar results were found in different tissues [13], but the Eph7 was immunostained in the cytoplasm of cancer cells, which was confirmed in our study. Similar expression was also found in lung squamous cell carcinoma and adenocarcinoma [11]. We speculate that the result was attributed to truncated EphA7 protein expression in esophageal cancer. In addition, survival analysis showed that low EphA7 expression correlated with poor survival of ESCC.
Tumor metastasis is the main cause of death in most cancer patients. In particular, lymph node metastasis is associated with a poor prognosis in ESCC [1]. Previous studies demonstrated that high EphA7 expression was closely related to carcinogenesis, progression, clinical biological behaviors, and prognosis of glioblastoma multiforme [31] and gallbladder adenocarcinoma [19]. In contrast, we found that low expression of EphA7 protein was correlated with tumor differentiation, lymph node metastases and TNM Classification in ESCC. These results suggest that EphA7 may play a pivotal role in ESCC progression.
Previous studies suggested that EphA7 resembles other members of Eph family structurally, including a cysteine-rich region and tandem fibronectin type-III domains in extracellular portion of EphA7 [14]. Tyrosine phosphorylation of Eph/Eprin system promotes cellular transformation, invasion, proliferation, and also inhibits cellular spread or migration [18, 24, 32] mediated via JAK2, PI3K or ILK signal transduction pathways. However, downregulation of EphA7 resulting from methylation in human colorectal cancer leads to biological and histopathological effects associated with carcinogenesis and differentiation [12, 29]. These studies suggested that downregulation of EphA7 may also play an important role in carcinogenesis and differentiation of ESCC. However, specific signal transduction pathways of EphA7 mediating ESCC carcinogenesis have yet to be elucidated.
In conclusion, our results demonstrate that low EphA7 expression is involved in the differentiation and lymph node metastases of ESCC, suggesting that EphA7 may be associated with the progression of ESCC, and play an important role in the prognosis of ESCC patients.
V. Acknowledgments
This work was supported by grants from the National Basic Research Program (973 Program No. 2012CB526608), the Inner Mongolia Autonomous Region Natural Science Foundation (No. 2013MS1113 to Yu-Qin Bai) and the Natural Science Foundation of China (No. 81201844 to Jun-Yi Zhang; No. 81360331 to Chun-Ying Bai), scientific research and innovation team building program of Chifeng University.
VI. References
- 1.Altorki N. and Skinner D. (2001) Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann. Surg. 234; 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo M. and Nieto M. A. (1997) The expression of chick EphA7 during segmentation of the central and peripheral nervous system. Mech. Dev. 68; 173–177. [DOI] [PubMed] [Google Scholar]
- 3.Bosman, F. T., Carneiro, F., Hruban, R. H. and Theise, N. D. (2010) WHO Classification of Tumours of the Digestive System. 4th ed. World Health Organization, Lyon. [Google Scholar]
- 4.Bruckner K., Pasquale E. B. and Klein R. (1997) Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science 275; 1640–1643. [DOI] [PubMed] [Google Scholar]
- 5.Ciossek T., Millauer B. and Ulrich A. (1995) Identification of alternatively spliced encoding variants of MDKs, a novel receptor tyrosine kinase expressed in the murine nervous system. Oncogene 10; 97–108. [PubMed] [Google Scholar]
- 6.Committee Eph Nomenclature. (1997) Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell 90; 403–404. [DOI] [PubMed] [Google Scholar]
- 7.Edge, S., Byrd, D. R., Compton, C. C., Fritz, A. G., Greene, F. L. and Trotti, A. (2010) AJCC Cancer Staging Manual. 7th ed. Springer, New York. [Google Scholar]
- 8.Fox B. P. and Kandpal R. P. (2004) Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem. Biophys. Res. Commun. 318; 882–892. [DOI] [PubMed] [Google Scholar]
- 9.Gale N. W. and Yancopoulos G. D. (1999) Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, Angiopoietins, and ephrins in vascular development. Genes Dev. 13; 1055–1066. [DOI] [PubMed] [Google Scholar]
- 10.Genander M. and Frisen J. (2010) Ephrins and Eph receptors in stem cells and cancer. Curr. Opin. Cell Biol. 22; 611–616. [DOI] [PubMed] [Google Scholar]
- 11.Giaginis C., Tsoukalas N., Bournakis E., Alexandrou P., Kavantzas N., Patsouris E. and Theocharis S. (2014) Ephrin (Eph) receptor A1, A4, A5 and A7 expression in human non-small cell lung carcinoma: associations withclinicopathological parameters, tumor proliferative capacity and patients’ survival. BMC Clin. Pathol. 14; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan M., Xu C., Zhang F. and Ye C. (2009) Aberrant methylation of EphA7 in human prostate cancer and its relation to clinicopathologic features. Int. J. Cancer 124; 88–94. [DOI] [PubMed] [Google Scholar]
- 13.Hafner C., Schmitz G., Meyer S., Bataille. F., Hau P., Langmann T., Dietmaier W., Landthaler M. and Vogt T. (2004) Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin. Chem. 50; 490–499. [DOI] [PubMed] [Google Scholar]
- 14.Hirai H., Maru Y., Hagiwara K., Nishida J. and Takaku F. (1987) A novel putative tyrosine kinase receptor encoded by the eph gene. Science 238; 1717–1720. [DOI] [PubMed] [Google Scholar]
- 15.Kamangar F., Dores G. M. and Anderson W. F. (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 24; 2137–2150. [DOI] [PubMed] [Google Scholar]
- 16.Kullander K. and Klein R. (2002) Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 3; 475–486. [DOI] [PubMed] [Google Scholar]
- 17.Lemke G. (1997) A coherent nomenclature for Eph receptors and their ligands. Mol. Cell. Neurosci. 9; 331–332. [DOI] [PubMed] [Google Scholar]
- 18.Lisabeth E. M., Falivelli G. and Pasquale E. B. (2013) Eph receptor signaling and ephrins. Cold Spring Harb. Perspect. Biol. 5; a009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D. C. and Yang Z. L. (2013) MTDH and EphA7 are markers for metastasis and poor prognosis of gallbladder adenocarcinoma. Diagn. Cytopathol. 41; 199–205. [DOI] [PubMed] [Google Scholar]
- 20.Mariette C., Balon J. M., Piessen G., Fabre S., Van Seuningen I. and Triboulet J. P. (2003) Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 97; 1616–1623. [DOI] [PubMed] [Google Scholar]
- 21.Merlos-Suárez A. and Batlle E. (2008) Eph–ephrin signalling in adult tissues and cancer. Curr. Opin. Cell Biol. 20; 194–200. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki T., Kato H., Fukuchi M., Nakajima M. and Kuwano H. (2003) EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int. Cancer 103; 657–663. [DOI] [PubMed] [Google Scholar]
- 23.Pasquale E. B. (2008) Eph-ephrin bidirectional signaling in physiology and disease. Cell 133; 38–52. [DOI] [PubMed] [Google Scholar]
- 24.Pasquale E. B. (2010) Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer 10; 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers J. H., Ciossek T., Ullrich A., West E., Hoare M. and Muir E. M. (1999) Distribution of the receptor EphA7 and its ligands in development of the mouse nervous system. Brain Res. Mol. Brain Res. 74; 225–230. [DOI] [PubMed] [Google Scholar]
- 26.Taneja R., Thisse B., Rijli F. M., Thisse C., Bouillet P. and Chambon P. (1996) The expression patter of the mouse receptor tyrosine kinase gene MDK1 is conserved through evolution and requires Hoxa-2 for rhombomere-specific expression in mouse embryos. Dev Biol. 177; 397–412. [DOI] [PubMed] [Google Scholar]
- 27.Traylor R. N., Fan Z., Hudson B., Rosenfeld J. A., Shaffer L. G., Torchia B. S. and Ballif B. C. (2009) Microdeletion of 6q16.1 encompassing EPHA7 in a child with mild neurological abnormalities and dysmorphic features: case report. Mol. Cytogenet. 2; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vail M. E., Murone C., Tan A., Hii L., Abebe D., Janes P. W., Lee F. T., Baer M., Palath V., Bebbington C., Yarranton G., Llerena C., Garic S., Abramson D., Cartwright G., Scott A. M. and Lackmann M. (2014) Targeting EphA3 inhibits cancer growth by disrupting the tumor stromal microenvironment. Cancer Res. 74; 4470–4481. [DOI] [PubMed] [Google Scholar]
- 29.Wang J., Kataoka H., Suzuki M., Sato N., Nakamura R., Tao H., Maruyama K., Isogaki J., Kanaoka S., Ihara M., Tanaka M., Kanamori M., Nakamura T., Shinmura K. and Sugimura H. (2005) Downregulation of EphA7 by hypermethylation in colorectal cancer. Oncogene 24; 5637–5647. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Li G., Ma H., Bao Y., Wang X., Zhou H., Sheng Z., Sugimura H., Jin J. and Zhou X. (2007) Differential expression of EphA7 receptor tyrosine kinase in gastric carcinoma. Hum. Pathol. 38; 1649–1656. [DOI] [PubMed] [Google Scholar]
- 31.Wang L. F., Fokas E., Juricko J., You A., Rose F., Pagenstecher A., Engenhart-Cabillic R. and An H. X. (2008) Increased expression of EphA7 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. BMC Cancer 8; 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xi H. Q., Wu X. S., Wei B. and Chen L. (2012) Eph receptors and ephrins as targets for cancer therapy. J. Cell. Mol. Med. 16; 2894–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F. R., Tao L. H., Shen Z. Y., Lv Z., Xu L. Y. and Li E. M. (2008) Fascin Expression in human embryonic, fetal, and normal adult tissue. J. Histochem. Cytochem. 56; 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y. F., Xu Q. X., Liao L. D., Xu X. E., Wu J. Y., Shen J., Wu Z. Y., Shen J. H., Li E. M. and Xu L. Y. (2013) κ-Opioid receptor in the nucleus is a novel prognostic factor of esophageal squamous cell carcinoma. Hum. Pathol. 44; 1756–1765. [DOI] [PubMed] [Google Scholar]