Abstract
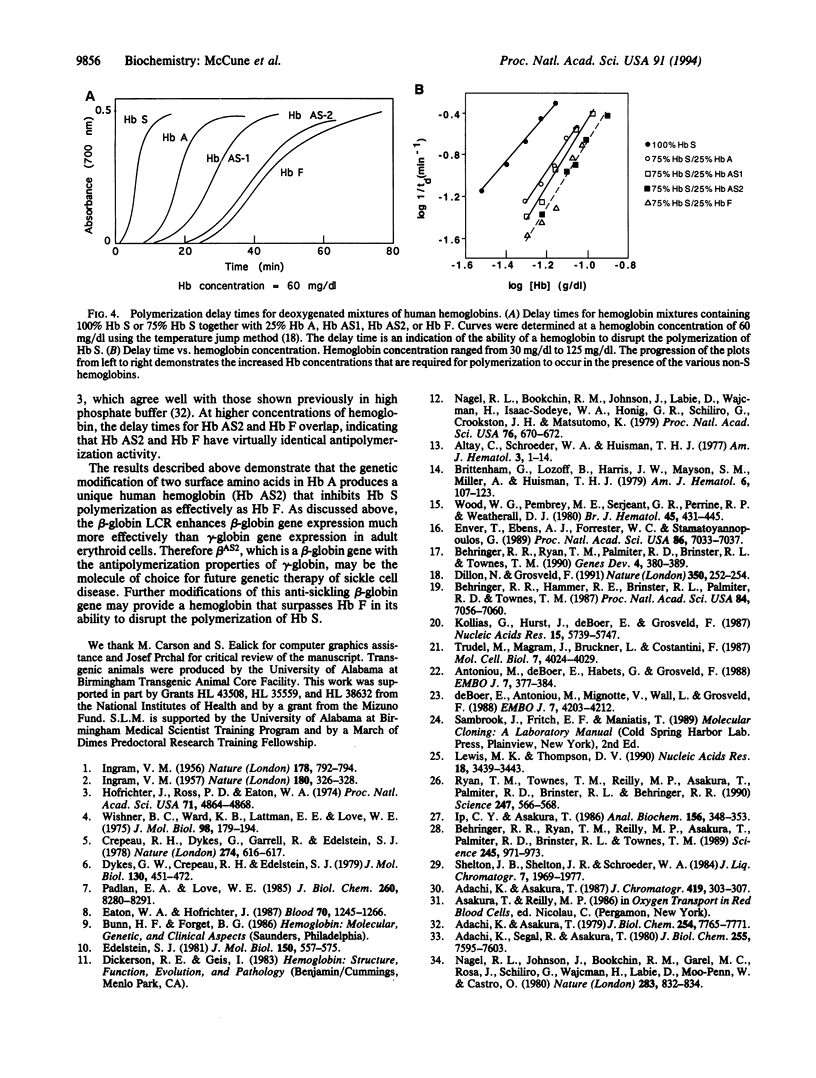
Two human hemoglobins designed to inhibit the polymerization of sickle hemoglobin (Hb S; alpha 2 beta S2) have been produced. Mutations that disrupt the ability of Hb S to form polymers were introduced into the normal human beta-globin gene by site-specific mutagenesis. These mutations affect the axial and lateral contacts in the sickle fiber. The recombinant hemoglobin designated anti-sickling hemoglobin 1 (Hb AS1) contains the mutations beta 22 glutamic acid to alanine and beta 80 asparagine to lysine. Hb AS2 has the same beta 22 glutamic acid to alanine mutation combined with beta 87 threonine to glutamine. Human alpha- and beta AS-globin genes were separately fused downstream of beta-globin locus control region sequences and these constructs were coinjected into fertilized mouse eggs. Transgenic mouse lines that synthesize high levels of each anti-sickling hemoglobin were established and anti-sickling hemoglobins were purified from hemolysates and characterized. Both AS hemoglobins bind oxygen cooperatively and the oxygen affinities of these molecules are in the normal range. Delay time experiments demonstrate that Hb AS2 is a potent inhibitor of Hb S polymerization; therefore, locus control region beta AS2-globin gene constructs may be suitable for future gene therapy of sickle cell disease.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi K., Asakura T. Nucleation-controlled aggregation of deoxyhemoglobin S. Possible difference in the size of nuclei in different phosphate concentrations. J Biol Chem. 1979 Aug 25;254(16):7765–7771. [PubMed] [Google Scholar]
- Adachi K., Asakura T. Separation of asymmetrical hybrid hemoglobins by hydrophobic interaction chromatography. J Chromatogr. 1987 Aug 7;419:303–307. doi: 10.1016/0378-4347(87)80291-8. [DOI] [PubMed] [Google Scholar]
- Adachi K., Segal R., Asakura T. Nucleation-controlled aggregation of deoxyhemoglobin S. Participation of hemoglobin F in the aggregation of deoxyhemoglobin S in concentrated phosphate buffer. J Biol Chem. 1980 Aug 25;255(16):7595–7603. [PubMed] [Google Scholar]
- Altay C., Schroeder W. A., Huisman T. H. The Ggamma deltabeta-thalassemia and Ggamma-betaO-hpfh conditions in combination with beta-thalassemia and Hb S. Am J Hematol. 1977;3:1–14. doi: 10.1002/ajh.2830030101. [DOI] [PubMed] [Google Scholar]
- Antoniou M., deBoer E., Habets G., Grosveld F. The human beta-globin gene contains multiple regulatory regions: identification of one promoter and two downstream enhancers. EMBO J. 1988 Feb;7(2):377–384. doi: 10.1002/j.1460-2075.1988.tb02824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer R. R., Hammer R. E., Brinster R. L., Palmiter R. D., Townes T. M. Two 3' sequences direct adult erythroid-specific expression of human beta-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7056–7060. doi: 10.1073/pnas.84.20.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer R. R., Ryan T. M., Palmiter R. D., Brinster R. L., Townes T. M. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990 Mar;4(3):380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Ryan T. M., Reilly M. P., Asakura T., Palmiter R. D., Brinster R. L., Townes T. M. Synthesis of functional human hemoglobin in transgenic mice. Science. 1989 Sep 1;245(4921):971–973. doi: 10.1126/science.2772649. [DOI] [PubMed] [Google Scholar]
- Brittenham G., Lozoff B., Harris J. W., Mayson S. M., Miller A., Huisman T. H. Sickle cell anemia and trait in southern India: further studies. Am J Hematol. 1979;6(2):107–123. doi: 10.1002/ajh.2830060203. [DOI] [PubMed] [Google Scholar]
- Crepeau R. H., Dykes G., Garrell R., Edelstein S. J. Diameter of haemoglobin S fibres in sickled cells. Nature. 1978 Aug 10;274(5671):616–617. doi: 10.1038/274616a0. [DOI] [PubMed] [Google Scholar]
- Dillon N., Grosveld F. Human gamma-globin genes silenced independently of other genes in the beta-globin locus. Nature. 1991 Mar 21;350(6315):252–254. doi: 10.1038/350252a0. [DOI] [PubMed] [Google Scholar]
- Dykes G. W., Crepeau R. H., Edelstein S. J. Three-dimensional reconstruction of the 14-filament fibers of hemoglobin S. J Mol Biol. 1979 Jun 5;130(4):451–472. doi: 10.1016/0022-2836(79)90434-0. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987 Nov;70(5):1245–1266. [PubMed] [Google Scholar]
- Edelstein S. J. Molecular topology in crystals and fibers of hemoglobin S. J Mol Biol. 1981 Aug 25;150(4):557–575. doi: 10.1016/0022-2836(81)90381-8. [DOI] [PubMed] [Google Scholar]
- Enver T., Ebens A. J., Forrester W. C., Stamatoyannopoulos G. The human beta-globin locus activation region alters the developmental fate of a human fetal globin gene in transgenic mice. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7033–7037. doi: 10.1073/pnas.86.18.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Kinetics and mechanism of deoxyhemoglobin S gelation: a new approach to understanding sickle cell disease. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4864–4868. doi: 10.1073/pnas.71.12.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM V. M. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956 Oct 13;178(4537):792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- INGRAM V. M. Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature. 1957 Aug 17;180(4581):326–328. doi: 10.1038/180326a0. [DOI] [PubMed] [Google Scholar]
- Ip C. Y., Asakura T. Separation of asymmetrical hybrid containing hemoglobin F by anaerobic anion-exchange high-performance liquid chromatography. Anal Biochem. 1986 Aug 1;156(2):348–353. doi: 10.1016/0003-2697(86)90264-2. [DOI] [PubMed] [Google Scholar]
- Kollias G., Hurst J., deBoer E., Grosveld F. The human beta-globin gene contains a downstream developmental specific enhancer. Nucleic Acids Res. 1987 Jul 24;15(14):5739–5747. doi: 10.1093/nar/15.14.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. K., Thompson D. V. Efficient site directed in vitro mutagenesis using ampicillin selection. Nucleic Acids Res. 1990 Jun 25;18(12):3439–3443. doi: 10.1093/nar/18.12.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R. L., Bookchin R. M., Johnson J., Labie D., Wajcman H., Isaac-Sodeye W. A., Honig G. R., Schilirò G., Crookston J. H., Matsutomo K. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A. 1979 Feb;76(2):670–672. doi: 10.1073/pnas.76.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R. L., Johnson J., Bookchin R. M., Garel M. C., Rosa J., Schiliro G., Wajcman H., Labie D., Moo-Penn W., Castro O. Beta-chain contact sites in the haemoglobin S polymer. Nature. 1980 Feb 28;283(5750):832–834. doi: 10.1038/283832a0. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Love W. E. Refined crystal structure of deoxyhemoglobin S. II. Molecular interactions in the crystal. J Biol Chem. 1985 Jul 15;260(14):8280–8291. [PubMed] [Google Scholar]
- Ryan T. M., Townes T. M., Reilly M. P., Asakura T., Palmiter R. D., Brinster R. L., Behringer R. R. Human sickle hemoglobin in transgenic mice. Science. 1990 Feb 2;247(4942):566–568. doi: 10.1126/science.2154033. [DOI] [PubMed] [Google Scholar]
- Trudel M., Magram J., Bruckner L., Costantini F. Upstream G gamma-globin and downstream beta-globin sequences required for stage-specific expression in transgenic mice. Mol Cell Biol. 1987 Nov;7(11):4024–4029. doi: 10.1128/mcb.7.11.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishner B. C., Ward K. B., Lattman E. E., Love W. E. Crystal structure of sickle-cell deoxyhemoglobin at 5 A resolution. J Mol Biol. 1975 Oct 15;98(1):179–194. doi: 10.1016/s0022-2836(75)80108-2. [DOI] [PubMed] [Google Scholar]
- Wood W. G., Pembrey M. E., Serjeant G. R., Perrine R. P., Weatherall D. J. Hb F synthesis in sickle cell anaemia: a comparison of Saudi Arab cases with those of African origin. Br J Haematol. 1980 Jul;45(3):431–445. doi: 10.1111/j.1365-2141.1980.tb07163.x. [DOI] [PubMed] [Google Scholar]
- deBoer E., Antoniou M., Mignotte V., Wall L., Grosveld F. The human beta-globin promoter; nuclear protein factors and erythroid specific induction of transcription. EMBO J. 1988 Dec 20;7(13):4203–4212. doi: 10.1002/j.1460-2075.1988.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]