Abstract
Although previous studies reported the involvement of the TLR4-TRIF pathway in alcohol-induced liver injury, the role of TLR2 and TLR9 signaling in alcohol-mediated neutrophil infiltration and liver injury has not been elucidated. Since alcohol binge drinking is recognized to induce more severe form of alcohol liver disease, we used a chronic-binge ethanol-feeding model as a mouse model for early stage of alcoholic hepatitis. Whereas a chronic-binge ethanol feeding induced alcohol-mediated liver injury in wild-type mice, TLR2- and TLR9-deficient mice showed reduced liver injury. Induction of neutrophil-recruiting chemokines, including Cxcl1, Cxcl2, and Cxcl5, and hepatic neutrophil infiltration were increased in wild-type mice, but not in TLR2- and TLR9-deficient mice. In vivo depletion of Kupffer cells (KCs) by liposomal clodronate reduced liver injury and the expression of Il1b, but not Cxcl1, Cxcl2, and Cxcl5, suggesting that KCs are partly associated with liver injury, but not neutrophil recruitment, in a chronic-binge ethanol-feeding model. Notably, hepatocytes and hepatic stellate cells (HSCs) produce high amounts of CXCL1 in ethanol-treated mice. The treatment with TLR2 and TLR9 ligands synergistically upregulated CXCL1 expression in hepatocytes. Moreover, the inhibitors for CXCR2, a receptor for CXCL1, and MyD88 suppressed neutrophil infiltration and liver injury induced by chronic-binge ethanol treatment. Consistent with the above findings, hepatic CXCL1 expression was highly upregulated in patients with alcoholic hepatitis. In a chronic-binge ethanol-feeding model, the TLR2 and TLR9-dependent MyD88-dependent pathway mediates CXCL1 production in hepatocytes and HSCs; the CXCL1 then promotes neutrophil infiltration into the liver via CXCR2, resulting in the development of alcohol-mediated liver injury.
Keywords: ALD, MyD88, chemokine, AH, binge ethanol feeding
alcoholic liver disease (ALD) is a result of chronic consumption of excessive alcohol. The clinical spectrum of ALD includes alcoholic fatty liver, alcoholic hepatitis (AH), alcoholic cirrhosis, and hepatocellular carcinoma (12). Alcoholic fatty liver is considered a reversible and nonprogressive entity. AH, the most severe form of ALD, is associated with high mortality; up to 40% of severe AH patients die within 6 mo (22). Although AH shows such a high mortality, treatment of AH is still largely dependent on corticosteroids for controlling inflammation, which has not been improved in the past 40 years (14). Survived AH patients may have coexisting cirrhosis or it may progress to alcoholic liver cirrhosis, and 3–10% of cirrhotic patients ultimately develop hepatocellular carcinoma (HCC).
It has been shown that ethanol itself and its metabolite-induced cytotoxicity are directly related to ethanol-induced liver injury and additional immune responses can further augment hepatocyte injury and can lead to cirrhosis as well as HCC (21). Accumulating evidences demonstrate that Toll-like receptor (TLR)-mediated innate immune response is associated with liver damage, inflammation, fibrosis, and tissue remodeling. Chronic alcohol intake is known to increase LPS levels in systemic circulation owing to the increased intestinal permeability by the disruption of epithelial tight junction (41). Increased LPS in the liver subsequently activates Kupffer cells (KCs) and bone marrow-derived macrophages (BMDM) through the binding to TLR4, contributing to the pathogenesis of ALD (15, 17). Indeed, in mice, deficiency in TLR4, CD14, and LBP is protective against alcohol-induced liver injury (15, 17, 39, 43). Consistently, gut sterilization with oral administration of nonabsorbable antibiotics reduces plasma LPS levels and alcohol-induced liver inflammation and injury in mice (1). Although TLR4 activates both MyD88- and TRIF-dependent intracellular signaling pathways, the TRIF-IRF3 axis appears to be more critical than the MyD88-dependent pathway for the development of ALD (15, 29).
TLR2 and TLR9 recognize the surface structures of gram-positive bacteria (38) and the CpG containing DNA derived from virus and bacteria (2), respectively. We have previously reported that TLR2 and TLR9 signaling contribute to the development of nonalcoholic steatohepatitis (NASH) through activation of inflammasome and IL-1β signaling (27, 28). Since the common signaling pathways may be activated in both ALD and NASH, it is suggested that chronic alcohol consumption increases the hepatic and systemic levels of ligands for TLR2 and TLR9, which activate hepatic TLR2 and TLR9 signaling, resulting in induction of ALD. Indeed, serum levels of bacterial DNA are increased in patients with AH (5).
Neutrophil recruitment is strongly associated with the hepatocyte death in human AH (18, 45). Gao and colleagues (6) recently established a new mouse model of ALD as a chronic-binge ethanol-feeding model that closely mimics drinking patterns of human alcoholics and exhibits the characteristics of the early stage of AH. In contrast to chronic ethanol-containing Lieber-DeCarli diet model where liver macrophages play a role, the chronic-binge ethanol-feeding model induces hepatic neutrophil infiltration that recapitulates the feature of human AH. Depletion of neutrophils with anti-Ly-6G blocking antibody suppressed liver injury in a chronic-binge ethanol-feeding model, suggesting the crucial role of neutrophils in this model (6). Neutrophil infiltration promotes liver damage directly and/or indirectly through the generation of potent oxidants (hydrogen peroxide and chloramine) and serine proteases including proteinase-3 and elastase (30). Although the previous study has demonstrated that TLR2, 4, and 9 are involved in dysfunction of neutrophils in AH patients (35), the cellular and molecular mechanisms by which TLR2 and TLR9 signaling mediates the progression of AH and neutrophil infiltration have so far remained unclear.
In the present study, we investigated the functions of TLR2 and TLR9 signaling in hepatic neutrophil infiltration in the chronic-binge ethanol-feeding model and examined the responsible cell types for the production of neutrophil chemoattractant CXCL1. We also tested the potential of MyD88 blockade for the treatment of AH. Our results demonstrate that TLR2 and TLR9 signaling is required for CXCL1 production, neutrophil infiltration, and liver damage in the mouse model of early stage of AH.
MATERIALS AND METHODS
Study design.
C57BL/6 wild-type (WT) and TLR2-deficient mice were purchased from Jackson Laboratories (Bar Harbor, ME); TLR9-deficient mice were originally generated by Dr. Akira (Osaka University, Suita, Japan). All mice including WT mice were bred in the University of California San Diego vivarium. TLR2-deficient and TLR9-deficient mice were back-crossed at least 10 generations onto the C57BL/6 background and displayed a similar hepatic phenotype as WT mice under standard laboratory chow. Female mice of each genotype were divided into two groups at 8–10 wk of age: control diet and Lieber-DeCarli diet (Bio-Serv, Frenchtown, NJ) as followed by a previous study (6). Each experiment included 6–8 mice/group and the data were the averages from at least two independent experiments.
For induction of mouse ALD, we followed the protocol of chronic plus binge ethanol-feeding model as previously described (6) with slight modifications. Briefly, mice were fed with a control liquid diet ad libitum for the first 5 days as an acclimatization step and subsequently fed with a Lieber-DeCarli diet (Bio-Serv, Frenchtown, NJ) containing 6.3% ethanol (vol/vol) for 10 days. In the morning on the 11th day, mice were subjected to receive single dose of binge ethanol (5 g/kg body wt) and euthanized 9 h after the ethanol binge.
The mice received humane care according to National Institutes of Health recommendations outlined in the Guide for the Care and Use of Laboratory Animals. All animal experiments were approved by the University of California San Diego Institutional Animal Care and Use Committee.
In vivo treatment.
A MyD88 inhibitory peptide and a control peptide (100 μg/mouse, Novus, Littleton, CO) were injected intraperitoneally twice (12 h and 1 h prior to binge). A selective CXCR2 antagonist (SB225002, 25 mg/kg, Tocris, Minneapolis, MN) was administered orally 1 h before binge. To deplete Kupffer cells, ethanol-fed mice were given clodronate liposome injections (200 μl/mouse) intraperitoneally for 2 consecutive days (48 and 24 h prior to binge).
In vitro and ex vivo study.
Hepatocytes, KCs, and hepatic stellate cell (HSCs) were isolated from mice fed a control and an ethanol-containing diet as previously described (27). In some experiments using hepatocytes, 200 μl of liposomal clodronate was injected intravenously 1 day before isolation to exclude the KC contamination. Hepatocytes, KCs, and HSCs were cultured in serum-free M199, DMEM, and RPMI 1640 (GIBCO, Life Technologies, Grand Island, NY), respectively, for 16 h before treatment with the specific ligands. Pam3CSK4 (200 ng/ml, Invivogen, San Diego, CA) and ODN1668 (5 μg/ml, Invivogen, San Diego, CA) were used to stimulate liver cells.
Histological analysis.
Mouse liver tissues were collected, fixed in 10% neutral buffered formalin solution for 48 h, routinely processed, and then embedded in paraffin. Tissue sections (4 μm) were prepared by use of a microtome (HM-340E, Thermo Fisher Scientific, Waltham, MA) and placed on glass slides. Hematoxylin and eosin, TUNEL, and immunohistochemical staining for Ly-6G (eBiosciences, San Diego, CA), F4/80 (eBiosciences), and CXCL1 (LifeSpan Bioscience, Seattle, WA) were performed. F4/80- and CXCL1-positive areas were measured on at least eight random fields per slide and quantified by using Image J (1.46r, NIH, Bethesda, MD) software. The number of TUNEL or Ly-6G positive cells were counted on at least eight random fields per slide and expressed as cells per high-power field.
RNA isolation and quantitative RT-PCR analysis.
RNA was extracted from mouse liver tissues and cells by using TRIzol (Life Technologies, Grand Island, NY) plus column kit (NucleoSpin, Clontech, Mountain View, CA) and were treated with DNase I (Promega, Madison, WI). Extracted RNA was converted to complementary DNA by using a reverse transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Quantitative RT-PCR was then performed by using a CFX96 Real Time System (Bio-Rad Laboratories, Irvine, CA) with SYBR Green I as a double-strand DNA-specific binding dye. After the reaction was completed, specificity was verified by melting curve analysis. Quantification was performed by comparing Ct values of each sample with normalization to 18S RNA. Sequences of primers were summarized in Table 1.
Table 1.
Sequence of primers used for quantitative real-time polymerase chain reaction
| Gene | Forward | Reverse |
|---|---|---|
| 18S | AGTCCCTGCCCTTTGTACACA | CGATCCGAGGGCCTCACTA |
| Il1b | GGTCAAAGGTTTGGAAGCAG | TGTGAAATGCCACCTTTTGA |
| Il6 | ACCAGAGGAAATTTTCAATAGGC | TGATGCACTTGCAGAAAACA |
| Tnf | AGGGTCTGGGCCATAGAACT | CCACCACGCTCTTCTGTCTAC |
| Cxcl1 | TGCACCCAAACCGAAGTC | GTCAGAAGCCAGCGTTCACC |
| Cxcl2 | AAAGTTTGCCTTGACCCTGAA | CTCAGACAGCGAGGCACATC |
| Cxcl5 | TGATCCCTGCAGGTCCACA | CTGCGAGTGCATTCCGCTTA |
| Ly6g | TGCGTTGCTCTGGAGATAGA | CAGAGTAGTGGGGCAGATGG |
| F4/80 | ATCTCCCTGGTATGTCTTGCCTTG | AGCCGTCTGGTTGTCAGTCTTG |
| Elane | ACAACTGCTGAACGACATTGTGA | TGCACGTTGGCGTTAATGGTA |
| Ccl2 | ATTGGGATCATCTTGCTGGT | CCTGCTGTTCACAGTTGCC |
Measurement for ALT and CXCL1.
Serum alanine aminotransferase (ALT) levels were measured by Infinity ALT Reagent (Thermo, Waltham, MA). The levels of CXCL1 in serum and cell supernatant were analyzed by ELISA kits (R&D, Minneapolis, MN).
TG extraction and measurement.
Hepatic triglycerides (TG) were extracted as described (27). TG contents were measured by using a Triglyceride Measurement Kit (Pointe Scientific, Canton, MI) according to the manufacturer's instructions.
Human samples.
Unstained slides were obtained by using the paraffin-embedded human liver tissue blocks of patients with AH and control patients with normal liver histology who had a clinical indication of a liver biopsy. The study was approved by the University of California San Diego Institutional Review Board. Immunohistochemical staining was performed for CXCL1, and Ly-6G was used to assess neutrophil infiltration.
Statistical analysis.
All data were expressed as means ± standard error. Differences between two groups were compared by a two-tailed Student's t-test. Correlation was analyzed by Spearman correlation test. A P value <0.05 was considered statistically significant.
RESULTS
TLR2 and TLR9 are required for liver injury and expression of neutrophil-recruiting chemokines in mice after chronic-binge alcohol treatment.
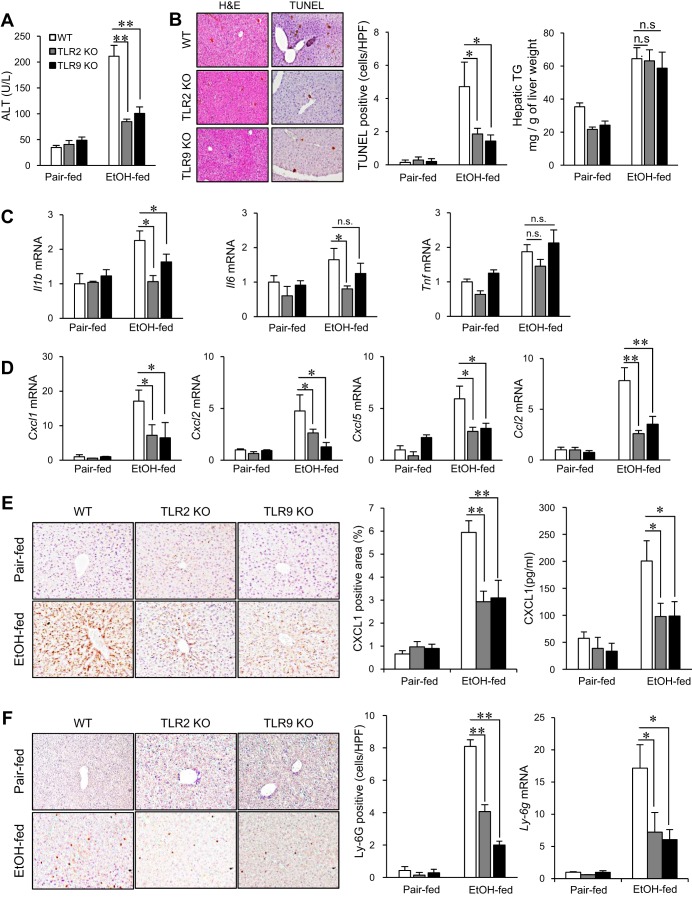
First, we investigated the role of TLR2 and TLR9 in the chronic-binge ethanol-feeding model using WT, TLR2-deficient, and TLR9-deficient mice. In WT mice, the early stage of AH was demonstrated by elevated serum ALT levels, hepatocyte death, neutrophil infiltration, and steatosis. In contrast, TLR2- and TLR9-deficient mice showed reduced serum ALT levels and decreased number of TUNEL-positive hepatocytes. However, degree of steatosis, as assessed by hepatic TG levels, was similar between WT and TLR2- or TLR9-deficient mice (Fig. 1, A and B). The levels of proinflammatory cytokines, such as Il1b, Il6, and Tnf, were elevated (1.5- to 2-fold) in WT livers whereas the hepatic levels of Il1b and Il6 were significantly reduced in TLR2-deficient mice and only Il1b was suppressed in TLR9-deficient mice after the chronic-binge ethanol feeding (Fig. 1C). Since a previous study showed the crucial role of neutrophils in the development of liver injury induced by chronic-binge ethanol feeding (6), we assessed neutrophil recruitment and neutrophil-recruiting chemokines. The expression of neutrophil-attracting chemokines, such as Cxcl1, Cxcl2, Cxcl5, and Ccl2, was markedly increased 6- to 15-fold in ethanol-treated WT livers whereas the expression of these chemokines were reduced in ethanol-treated TLR2- and TLR9-deficient mice (Fig. 1D). Increased hepatic protein expression of CXCL1 and serum level of CXCL1 in WT mice were significantly suppressed in TLR2- and TLR9-deficient mice (Fig. 1E). Accordingly, neutrophil infiltration was also suppressed in TLR2- and TLR9-deficient mice as assessed by the expression of Ly-6G (Fig. 1F). These results indicate that TLR2 and TLR9 signaling contribute to liver damage, production of neutrophil-recruiting chemokines and neutrophil infiltration in the chronic-binge ethanol-feeding model.
Fig. 1.
TLR2- and TLR9-deficient mice exhibit reduced liver injury and neutrophil-recruiting chemokine expression in chronic plus binge ethanol (EtOH)-induced alcoholic liver disease (ALD). A–E: wild-type (WT), TLR2-deficient, and TLR9-deficient female mice were subjected to control and Lieber-DeCarli diet (n = 10 per group for pair-fed, n = 14–16 per group for EtOH fed; each experiment was performed with 5–8 mice per group and repeated 2 times). A: liver injury was assessed by measuring serum alanine aminotransferase (ALT) levels. B: hepatocyte death was analyzed by TUNEL staining and TUNEL-positive cells were counted. Hepatic steatosis was determined by measuring hepatic triglyceride (TG) levels. The results are expressed as mg TG/g liver. HPF, high-power field. C: expression of proinflammatory cytokines (Il1b, Il6, and Tnf) was determined by quantitative real-time polymerase chain reaction (qRT-PCR) and shown as fold change compared with pair-fed WT mice. D: expression of neutrophil-recruiting chemokines (Cxcl1, Cxcl2, Cxcl5, and Ccl2) was determined by qRT-PCR and shown as fold change compared with pair-fed WT mice. E: hepatic expression of CXCL1 was assessed by immunohistochemistry and quantified by measuring CXCL1-positive area. Serum CXCL1 levels were measured by ELISA. KO, knockout. F: hepatic neutrophil infiltration was determined by immunohistochemistry for Ly-6G and the number of Ly-6G-positive cells was counted. Data are presented as means ± SE per group. *P < 0.05; n.s., not significant; n.d., not detected. Original magnification, ×100 [hematoxylin and eosin (H&E)], ×200 (TUNEL, CXCL1, and Ly-6G).
Hepatic expression of neutrophil-recruiting chemokines is independent of Kupffer cells in chronic-binge ethanol-feeding model.
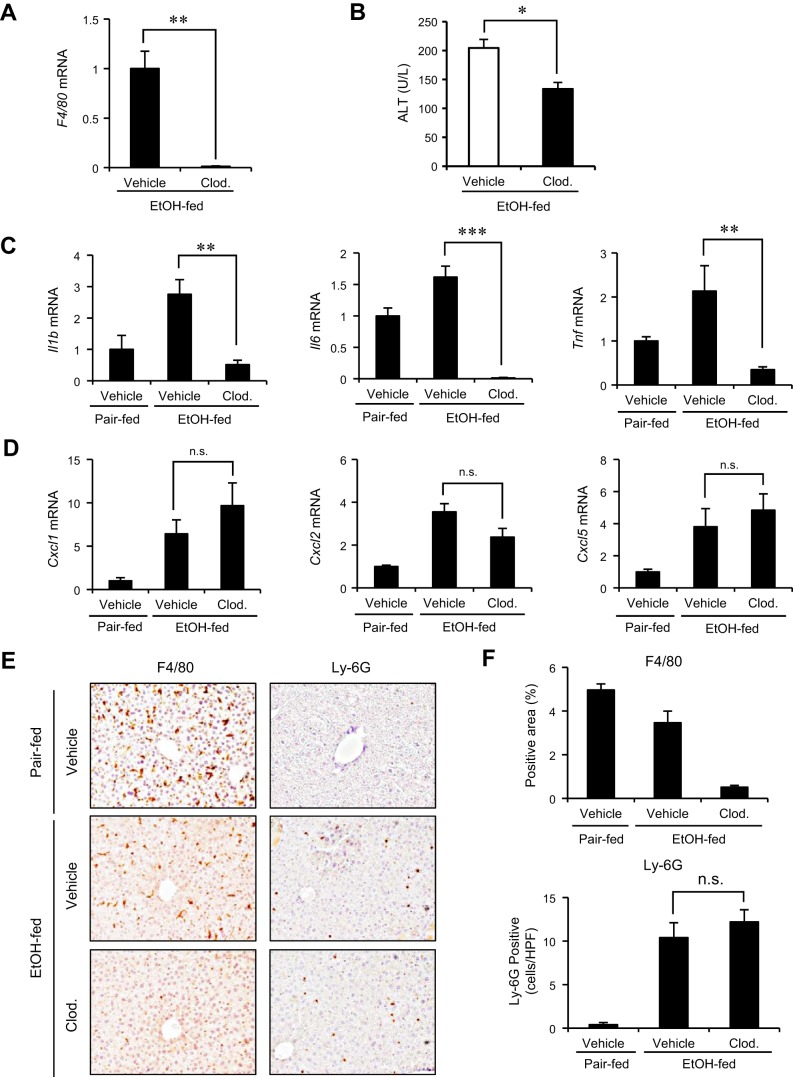
KC is the primary cell type to produce inflammatory cytokines involved in the pathogenesis of ALD (17, 34). We examined the role of KCs in neutrophil recruitment and liver injury using chronic-binge ethanol-feeding model. KCs were depleted by injection of liposomal clodronate and the mice were subjected to the chronic-binge ethanol feeding. The depletion of KCs was confirmed by blunted hepatic expression of F4/80 (Fig. 2, A and E). We observed the reduction of serum ALT levels accompanied by the decreased expression of proinflammatory cytokines, including Il1b, Il6, and Tnf in KC-depleted mice compared with vehicle-treated ones (Fig. 2, B and C). Intriguingly, the expression of neutrophil-recruiting chemokines was not decreased by KC depletion, suggesting that the source of neutrophil chemoattractants is the cells other than KCs in this model (Fig. 2D). Consistently, KC depletion did not alter the infiltration of Ly-6G-positive neutrophils (Fig. 2E). These results indicate that, in addition to neutrophils previously described, KCs partly contribute to the induction of liver injury but are not associated with production of neutrophil-recruiting chemokines in chronic-binge ethanol-feeding model.
Fig. 2.
Hepatic expression of neutrophil-recruiting chemokines induced by alcohol is independent of Kupffer cells (KCs). A–E: in vivo depletion of KCs was achieved by injection of clodronate liposome (Clod.; 200 μl/mouse) for 2 consecutive days (n = 6 per group). A: KC depletion was confirmed by hepatic expression of F4/80 by using qRT-PCR. B: liver injury was assessed by measuring serum ALT levels. C: expression of proinflammatory cytokines (Il1b, Il6, and Tnf) was determined by qRT-PCR and shown as fold change compared with vehicle-treated pair-fed mice. D: expression of neutrophil-recruiting chemokines (Cxcl1, Cxcl2, and Cxcl5) was determined by qRT-PCR and shown as fold change compared with vehicle-treated pair-fed mice. E: KCs and neutrophils were stained by immunohistochemistry for F4/80 and Ly-6G, respectively. F: quantification was done by measuring F4/80-positive area or counting Ly-6G-positive cells. Data are presented as means ± SE per group. *P < 0.05; **P < 0.01, ***P < 0.001; n.s., not significant. Original magnification, ×200 (F4/80 and Ly-6G).
Hepatocytes and hepatic stellate cells are responsible for producing CXCL1 in mouse ALD.
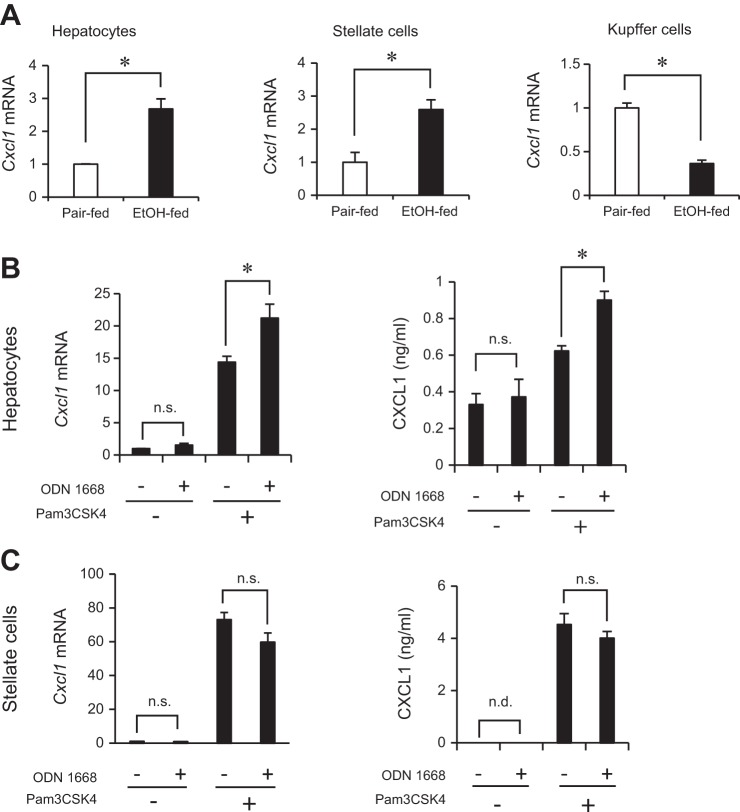
To identify the cellular sources of neutrophil-attracting chemokines, other than KCs, in chronic-binge ethanol-feeding model, we isolated different liver cell populations including hepatocytes, HSCs, and KCs. Hepatocytes and HSCs isolated from ethanol-fed mice had increased mRNA expression of Cxcl1 compared with cells from pair-fed animals. Interestingly, KCs from ethanol-fed mice showed reduced expression of Cxcl1 compared with KCs from pair-fed mice (Fig. 3A), which supports our data showing that KCs have minor roles in neutrophil infiltration induced by chronic-binge ethanol treatment (Fig. 2). To further investigate the mechanism underlying TLR2- and TLR9-dependent CXCL1 production, we treated primary cultured hepatocytes with a synthetic TLR2 ligand (Pam3CSK4) and TLR9 ligand (ODN1668). The ODN1668 alone could not induce Cxcl1 expression, but pretreatment with Pam3CSK4 enabled primary hepatocytes to produce CXCL1 in response to ODN1668 (Fig. 3B). Notably, the synergistic effect induced by TLR2 and TLR9 ligands was observed only in hepatocytes, but not in HSCs (Fig. 3C). These results indicate that TLR2 and TLR9 signaling has capacity to induce CXCL1 production in hepatocytes and HSCs.
Fig. 3.
Hepatocytes and hepatic stellate cells (HSCs) are the major cell types for the production of CXCL1. Each liver fraction (hepatocytes, HSCs, and KCs) was isolated from livers of EtOH- and pair-fed mice. Representative results are presented from 2 independent experiments (each isolation was assayed in triplicate). A: Cxcl1 expression in each fraction was determined by qRT-PCR and shown as fold change compared with those of pair-fed mice. B and C: hepatocytes and HSCs were pretreated with Pam3CSK4 (200 ng/ml), and then ODN1668 (5 μg/ml) was added for 30 min (qRT-PCR) and 6 h (ELISA). The Cxcl1 mRNA and CXCL1 protein levels in hepatocytes (B) and HSCs (C) were determined by qRT-PCR and ELISA, respectively. Data are presented as means ± SE per group. *P < 0.05, n.s., not significant.
A selective CXCR2 blockade inhibits neutrophil infiltration and alcohol-mediated liver injury.
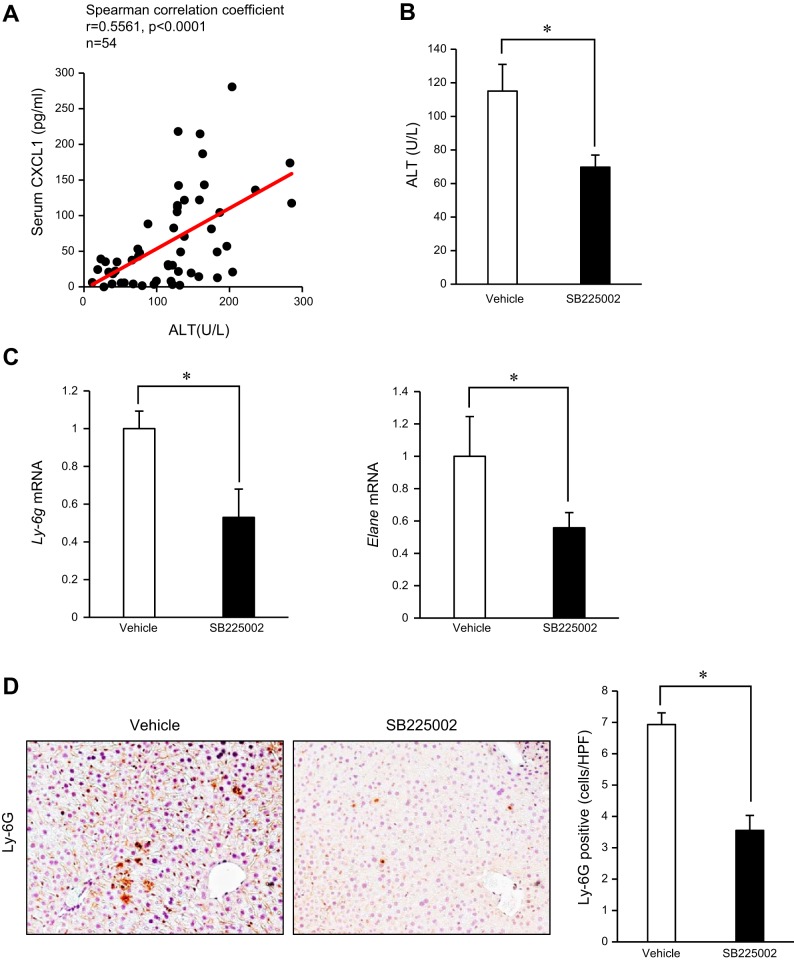
Since CXCL1 is a potent neutrophil-recruiting chemokines, we examined the correlation between liver injury and CXCL1 levels in mice with or without chronic-binge ethanol feeding. As illustrated in Fig. 4A, serum ALT levels positively correlate with serum CXCL1 levels in mice. These results prompted us to test whether systemic blockade of CXCR2, a receptor for CXCL1, has a therapeutic effect on liver injury through inhibiting neutrophil recruitment in chronic-binge ethanol-feeding model. The treatment with SB225002, a selective CXCR2 antagonist, suppressed liver injury as demonstrated by reduced serum ALT levels (Fig. 4B). The expression of neutrophil markers (Ly6g and Elane) and the infiltration of Ly-6G-positive neutrophils were also reduced in mice treated with SB225002 compared with vehicle-treated mice (Fig. 4, C and D). These results suggested that inhibition of the CXCL1-CXCR2 axis suppresses liver injury through suppressing neutrophil infiltration in chronic-binge ethanol-feeding model.
Fig. 4.
Treatment with a CXCR2 antagonist attenuates alcohol-induced neutrophil-mediated liver injury. A: correlation between serum CXCL1 and ALT levels in mice with or without ALD was analyzed by Spearman correlation test. B–D: CXCR2 blockade was accomplished by an oral administration of a selective antagonist, SB225002 (n = 16 per group; each experiment was performed with 8 mice per group and repeated 2 times). B: liver injury was assessed by measuring serum ALT levels. C: expression of neutrophil markers (Ly6g and Elane) was determined by qRT-PCR and shown as fold change compared with vehicle-treated mice. D: neutrophil infiltration was determined by immunohistochemistry for Ly-6G and its quantification was done by counting Ly-6G-positive cells. Data are presented as means ± SE per group. *P < 0.05. Original magnification, ×200 (Ly-6G).
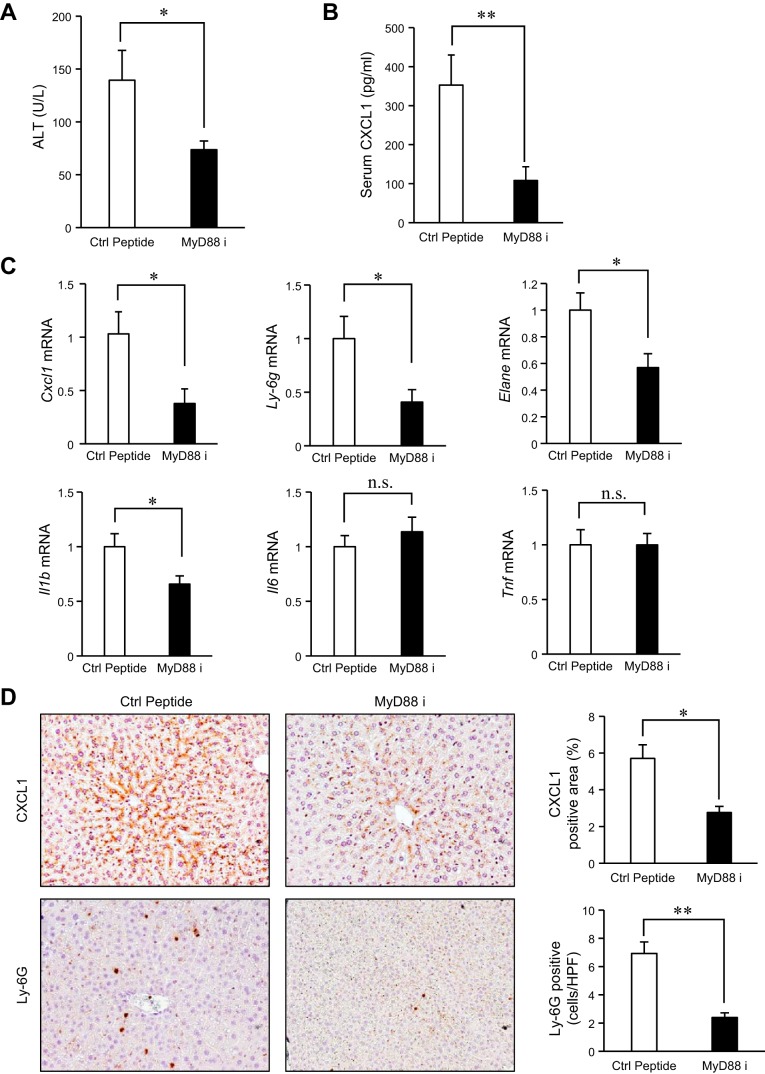
MyD88 inhibition attenuates CXCL1 production and liver injury in chronic-binge ethanol-feeding model.
Given that both TLR2- and TLR9-deficient mice were protected against alcohol insults (Fig. 1–4), MyD88, a common adaptor molecule for IL-1 receptor and all TLRs, except for TLR3, may play a crucial role in liver injury induced by chronic-binge ethanol treatment. To test the therapeutic potential of MyD88 inhibition, we treated mice with a MyD88 inhibitory peptide. Inhibiting MyD88 significantly suppressed liver injury (Fig. 5A) and serum CXCL1 levels in the chronic-binge ethanol-treated mice (Fig. 5B). These results were further supported by the reduction of CXCL1 and neutrophil markers in the liver (Fig. 5, C and D). The MyD88 inhibition also reduced the hepatic Il1b expression, but not Il6 and Tnf, which resembles the results from TLR2- and 9-deficient mice after the chronic-binge ethanol treatment (Fig. 1C). These results demonstrated that the MyD88 blockade could prevent liver damage through the suppression of CXCL1, IL-1β production, and neutrophil recruitment in an acute-on-chronic ethanol-feeding model.
Fig. 5.
Blocking MyD88 protects against alcohol-mediated CXCL1 production and liver injury. A–D: a MyD88 inhibitory peptide and a control peptide (100 μg/mouse) were administered with intraperitoneal injection to EtOH-fed WT mice (n = 16 per group; each experiment was performed with 8 mice per group and repeated 2 times). A: liver injury was assessed by measuring serum ALT levels. B: serum CXCL1 levels were measured by ELISA. C: expression of Cxcl1, neutrophil markers (Ly6g and Elane), and proinflammatory cytokines (Il1b, Il6, and Tnf) was determined by qRT-PCR and shown as fold change compared with vehicle-treated mice. D: hepatic expression of CXCL1 and neutrophil infiltration were determined by immunohistochemistry for CXCL1 and Ly-6G, respectively. Their quantification was performed. Ctrl Peptide, control peptide; MyD88 i, MyD88 inhibitory peptide. Data are presented as means ± SE per group. *P < 0.05, **P < 0.01; n.s., not significant. Original magnification, ×200 (CXCL1 and Ly-6G).
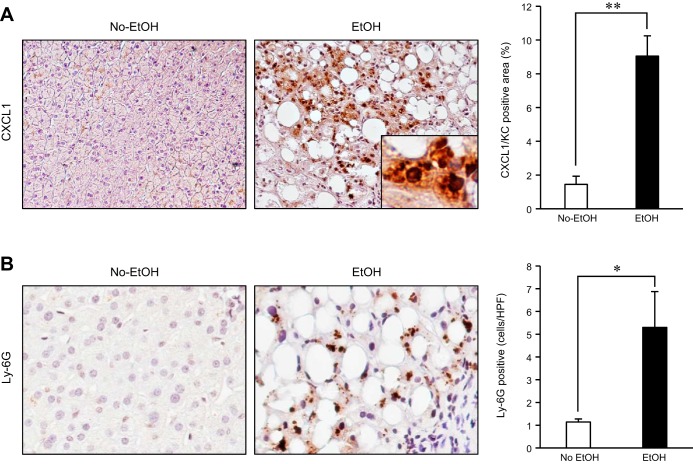
Hepatic CXCL1 levels are upregulated in patients with alcoholic hepatitis.
To understand the clinical relevance of CXCL1 in human disease, we analyzed hepatic CXCL1 expression in patients with AH. Consistent with the animal study, CXCL1 expression was strongly upregulated and neutrophil infiltration was increased in AH patients (Fig. 6, A and B). Our data suggest that the upregulation of hepatic CXCL1 and neutrophil recruitment are associated with the development of AH.
Fig. 6.
Hepatic CXCL1 expression is upregulated in patients with alcoholic hepatitis. A: hepatic CXCL1 expression was determined by immunohistochemistry on liver biopsy specimens from control individuals or patients with alcoholic hepatitis (AH; n = 5 for control, n = 10 for patient with AH). Quantification was done by measuring CXCL1-positive area. B: neutrophil infiltration was analyzed by immunohistochemistry for Ly-6G and its quantification on liver biopsy specimens of patients with AH. Data are presented as means ± SE per group. *P < 0.05; **P < 0.01. Original magnification, ×200 (CXCL1), ×400 (Ly-6G).
DISCUSSION
The present study clearly demonstrated that activation of TLR2 and TLR9 signaling contributes to hepatic production of CXCL1, recruiting neutrophils into the liver in the chronic-binge ethanol-feeding model, a mouse model of early stage AH. Our data also demonstrated that the biological relevant cellular source of CXCL1 is not KCs. Instead, we found that hepatocytes and HSCs are the major cellular sources of CXCL1. Notably, there is a synergistic effect of TLR2 and TLR9 signaling to induce CXCL1 production in hepatocytes, which induces the recruitment of neutrophils into the liver. Increased serum CXCL1 levels correlate with serum ALT in the current ethanol-feeding model. Furthermore, we demonstrated the therapeutic potential of CXCR2 blockade (a responsible receptor for CXCL1) and MyD88 inhibitor (a common adaptor molecule for all TLRs, except for TLR3, and IL-1 receptor) in the chronic-binge ethanol-feeding model. Finally, we demonstrated that CXCL1 expression was highly upregulated in patients with AH. Taken together, our data indicate that TLR2 and TLR9 signaling mediates CXCL1-mediated neutrophil infiltration and liver injury in the acute-on-chronic ethanol-feeding model.
TLR2 and TLR9 are the sensors for the cell wall components derived from gram-positive bacteria (38) and the unmethylated CpG-containing DNA derived from virus and bacteria (2), respectively. TLR2 also recognizes a number of endogenous ligands, such as saturated fatty acids, serum amyloid A, and oxidized low-density lipoprotein (ox-LDL) (13, 40, 42). On the other hand, denatured DNA produced from damaged cells and mitochondria can be recognized by TLR9 (16, 44). Of note, alcohol consumption has been reported to induce oxidization of LDL (3). These findings were further confirmed by the positive correlation of alcohol intake (10 g per day) with an increase of 2.4 U/l of circulating ox-LDL (31). Furthermore, ethanol treatment alters the permeability of mitochondrial membrane and promotes mitochondrial swelling through mitochondrial reactive oxygen species production, thereby leading to mitochondrial damage (23). Thus liver cells may be exposed to sufficient levels of TLR2 and TLR9 ligands during the excessive alcohol consumption.
The previous studies reported that chronic alcohol-induced liver damage and inflammation are prevented by TLR4 deficiency, but not MyD88 deficiency (15, 17). The TLR4-dependent TRIF-IRF3 axis mediates alcohol-induced liver injury through activation of proapoptotic Bax in hepatocytes (29). Thus the MyD88-dependent pathway has been underestimated in the pathogenesis of ALD. All TLRs, except for TLR3, and IL-1 receptor use MyD88 as an adaptor molecule, which activates NF-κB and MAPK pathways (37). These signaling cascades lead to the production of various cytokines and chemokines (7). In contrast to ALD, MyD88 has been highlighted as a critical regulator in various liver diseases including liver fibrosis, NASH, and HCC (32, 33). Recently, concerns about the experiments using whole-body MyD88-knockout (KO) mice have been discussed. Since MyD88 KO mice are severely immunocompromised and their commensal bacteria are often altered, functional compensation could occur, thereby masking or distortion of the phenotypes of these KO mice (25, 36). More careful and intensive investigation on the function of MyD88 in the pathogenesis of ALD should be facilitated. We will discuss in more detail below.
Neutrophil infiltration is a prominent feature of AH in patients and significantly correlates with the severity and the mortality of AH patients (18, 45). However, the mechanism of neutrophil infiltration in AH has not been fully elucidated. Our data demonstrated that TLR2 and TLR9, two TLRs that activate the MyD88-dependent pathway, are critical for neutrophil recruitment and its mediated pathogenesis in the chronic-binge ethanol-feeding model. These results were supported by the previous report showing that doxycycline-induced neutrophil infiltration is dependent on MyD88, TLR2, and TLR9, but independent of TRIF, TLR3, and TLR4 (20). Of note, CXCL1 production exclusively depends on the signaling through the MyD88-dependent pathway, but not TRIF. This may be explained by the fact that the CXCL1 promoter contains only NF-κB site, but not IRF site (9). Therefore, CXCL1 production is predominantly regulated by MyD88-dependent pathway.
KC is a major producer of proinflammatory cytokines to play crucial roles in the development of ALD and NASH (17, 34). In the chronic-binge ethanol-feeding model, in vivo depletion of KCs did not affect the production of neutrophil-recruiting chemokines and neutrophil infiltration in the liver, indicating that neutrophil-mediated alcohol-mediated liver injury is independent of KCs. The KC-mediated pathogenesis of liver injury may be mediated through production of inflammatory cytokines in the chronic-binge ethanol-feeding model. Other than KCs, our study highlighted hepatocytes and HSCs as the responsible cell types to produce neutrophil-attracting chemokines in ALD. During neutrophil migration and adhesion into the endothelial cell, they encounter immobilized chemokines from extravascular cells, which would give them further directional cues for migration and infiltration (11, 26). These extravascular cells could be hepatocytes and HSCs in the present model. Importantly, hepatocytes showed the synergistic effect induced by TLR2 and TLR9 ligands. Given that endocytosis is a prerequisite for proper endosomal TLR responses, hepatocytes generally do not respond to TLR9. Interestingly, pretreatment with a TLR2 ligand enables hepatocytes to synergistically respond to the TLR9 ligand to produce CXCL1. In same line with our present study, it has been reported that the cotreatment with TLR2 and TLR9 ligands induces the synergistic effect on immune responses upon bacterial infection (10). Moreover, accumulating evidences have suggested that TLR2 activation increases endocytotic activity through activation of Rab5 (4, 19). Thus the synergistic effects of TLR2 and TLR9 may be mediated by TLR2-induced enhancement of endocytosis of TLR9 ligand to hepatocytes.
The previous study reported by Hritz et al. (15) demonstrated the redundancy of TLR2 and MyD88 in a chronic feeding model of Lieber-DeCarli diet containing ethanol. In our study using the chronic-binge ethanol-feeding model, TLR2 and TLR9 signaling and MyD88 are required for the production of IL-1β and neutrophil-attracting chemokines and neutrophil recruitment, thereby inducing liver injury. In contrast, TLR2 and TLR9 signaling and MyD88 are dispensable for production of KC-derived inflammatory cytokines, such as IL-6 and TNF-α, which, in part, corroborates with the findings observed by Hritz et al. KC, a main producer of IL-6 and TNF-α, is a crucial cell type in the conventional chronic Lieber-DeCarli diet model (15), but KC does not play a major role in the recruitment of neutrophils in the chronic-binge ethanol-feeding model. Compared with the conventional chronic Lieber-DeCarli diet model, the chronic-binge ethanol-feeding model shows high degrees of liver injury and inflammation and neutrophil infiltration (6). The previous study showed that depletion of neutrophil with neutralizing antibody (anti-Ly-6G Ab) inhibits liver injury (6). Thus the chronic-binge ethanol-feeding model recapitulates neutrophil-mediated early stage of AH. In contrast, in the conventional Lieber-DeCarli diet model, neutrophil may not play a major role, and the pathogenesis may be mainly mediated by KCs.
Mortality and morbidity of AH is still very high (8). However, corticosteroids and pentoxifylline are the only drugs currently available for the treatment of AH (14). Therefore, we have to seek new and effective interventions for AH. Since AH is thought to be neutrophil dependent (18, 45) and the chronic-binge ethanol-feeding model causes liver injury driven by neutrophils (6), we took advantages of the chronic-binge ethanol-feeding model to test the new therapeutic agents. Our present study demonstrated that TLR2, TLR9, and CXCL1 are crucial factors for the development of acute-on-chronic mouse ALD and suggests that these molecules can be targets for the treatment of ALD. MyD88 is a common denominator for signaling of IL-1 signaling and all TLRs (except for TLR3) including TLR2 and TLR9 (37). We postulated that modulation of MyD88 could regulate the detrimental response by alcohol in the liver. To examine the protective effect of MyD88 inhibition on alcohol-induced liver injury, we administered a MyD88 inhibitory peptide to the chronic-binge ethanol-treated mice. The MyD88 inhibition successfully suppressed the production of neutrophil-recruiting chemokines and IL-1β, but not of IL-6 and TNF-α. MyD88 inhibitor may be insufficient for complete suppression of IL-6 and TNF-α while this inhibitor sufficiently reduced neutrophil recruitment and liver injury in the chronic-binge ethanol-feeding model. We also tested the preventive effect of CXCR2 blockade on the chronic-binge alcohol-feeding model. CXCR2, a responsible receptor for CXCL1, is highly expressed on neutrophils (24). CXCR2 blockade with a selective antagonist successfully inhibited neutrophil chemotaxis to the ethanol-treated livers. Collectively, the TLR-MyD88 signaling and the CXCL1-CXCR2 interaction can be attractive targets for the treatment of neutrophil-mediated early stage AH.
In conclusion, TLR2 and TLR9 promote CXCL1 production in hepatocytes and HSCs, which in turn induces neutrophil recruitment into the liver via CXCR2. This pathway plays a central role for the pathogenesis of alcohol-induced neutrophil-mediated liver injury. We also demonstrated that MyD88 inhibition and CXCR2 blockage attenuate alcohol-induced liver injury. Thus targeting TLR2, TLR9, MyD88, and CXCL1-CXCR2 to restrict neutrophil infiltration may become a novel, effective intervention for the early stage of AH.
GRANTS
This study was supported by National Institutes of Health Grants R01AA02172 (E. Seki) and R01DK085252 (E. Seki) and by the 2014 Congressman John Joseph Moakley Postdoctoral Research Fellowship from American Liver Foundation (Y. S. Roh). R. Loomba is supported in part by the American Gastroenterological Association (AGA) Foundation-Sucampo-ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award. Funding was provided by Atlantic Philanthropies, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.S.R. and E.S. conception and design of research; Y.S.R. and B.Z. performed experiments; Y.S.R., B.Z., and R.L. analyzed data; Y.S.R., R.L., and E.S. interpreted results of experiments; Y.S.R. prepared figures; Y.S.R. and E.S. drafted manuscript; Y.S.R., R.L., and E.S. edited and revised manuscript; E.S. approved final version of manuscript.
REFERENCES
- 1.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108: 218–224, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett 85: 85–95, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Alho H, Sillanaukee P, Kalela A, Jaakkola O, Laine S, Nikkari ST. Alcohol misuse increases serum antibodies to oxidized LDL and C-reactive protein. Alcohol Alcohol 39: 312–315, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Aoki MP, Carrera-Silva EA, Cuervo H, Fresno M, Girones N, Gea S. Nonimmune cells contribute to crosstalk between immune cells and inflammatory mediators in the innate response to Trypanosoma cruzi infection. J Parasitol Res 2012: 737324, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One 9: e96864, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology 58: 1814–1823, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dental Res 90: 417–427, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chedid A, Mendenhall CL, Gartside P, French SW, Chen T, Rabin L. Prognostic factors in alcoholic liver disease. VA Cooperative Study Group. Am J Gastroenterol 86: 210–216, 1991. [PubMed] [Google Scholar]
- 9.De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol 180: 4308–4315, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Duggan JM, You D, Cleaver JO, Larson DT, Garza RJ, Guzman Pruneda FA, Tuvim MJ, Zhang J, Dickey BF, Evans SE. Synergistic interactions of TLR2/6 and TLR9 induce a high level of resistance to lung infection in mice. J Immunol 186: 5916–5926, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med 187: 903–915, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572–1585, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He RL, Zhou J, Hanson CZ, Chen J, Cheng N, Ye RD. Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood 113: 429–437, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helman RA, Temko MH, Nye SW, Fallon HJ. Alcoholic hepatitis. Natural history and evaluation of prednisolone therapy. Ann Int Med 74: 311–321, 1971. [DOI] [PubMed] [Google Scholar]
- 15.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology 48: 1224–1231, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, Liao X, Billiar T, Xu J, Esmon CT, Tsung A. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology 54: 999–1008, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res 35: 1509–1518, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaeschke H. Neutrophil-mediated tissue injury in alcoholic hepatitis. Alcohol 27: 23–27, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Kochan T, Singla A, Tosi J, Kumar A. Toll-like receptor 2 ligand pretreatment attenuates retinal microglial inflammatory response but enhances phagocytic activity toward Staphylococcus aureus. Infect Immun 80: 2076–2088, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krysko DV, Kaczmarek A, Krysko O, Heyndrickx L, Woznicki J, Bogaert P, Cauwels A, Takahashi N, Magez S, Bachert C, Vandenabeele P. TLR-2 and TLR-9 are sensors of apoptosis in a mouse model of doxorubicin-induced acute inflammation. Cell Death Differ 18: 1316–1325, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loomba R, Yang HI, Su J, Brenner D, Barrett-Connor E, Iloeje U, Chen CJ. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol 177: 333–342, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 360: 2758–2769, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Manzo-Avalos S, Saavedra-Molina A. Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health 7: 4281–4304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19: 583–593, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA 101: 10679–10684, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 91: 385–395, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 139: 323–334.e7, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 57: 577–589, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrasek J, Iracheta-Vellve A, Csak T, Satishchandran A, Kodys K, Kurt-Jones EA, Fitzgerald KA, Szabo G. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci USA 110: 16544–16549, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol 35: 757–766, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Schroder H, Marrugat J, Fito M, Weinbrenner T, Covas MI. Alcohol consumption is directly associated with circulating oxidized low-density lipoprotein. Free Radic Biol Med 40: 1474–1481, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology 48: 322–335, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 13: 1324–1332, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Smith K. Liver disease: Kupffer cells regulate the progression of ALD and NAFLD. Nat Rev Gastroenterol Hepatol 10: 503, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Stadlbauer V, Mookerjee RP, Wright GA, Davies NA, Jurgens G, Hallstrom S, Jalan R. Role of Toll-like receptors 2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol 296: G15–G22, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J Clin Invest 123: 179–188, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeuchi O, Akira S. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr Topics Microbiol Immunol 270: 155–167, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11: 443–451, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gabele E, Isayama F, Thurman RG. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol 168: 2963–2969, 2002. [DOI] [PubMed] [Google Scholar]
- 40.West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 467: 972–976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 53: 96–105, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin J, Peng Y, Wu J, Wang Y, Yao L. Toll-like receptor 2/4 links to free fatty acid-induced inflammation and beta-cell dysfunction. J Leukocyt Biol 95: 47–52, 2014. [DOI] [PubMed] [Google Scholar]
- 43.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol 166: 4737–4742, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziol M, Tepper M, Lohez M, Arcangeli G, Ganne N, Christidis C, Trinchet JC, Beaugrand M, Guillet JG, Guettier C. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis. J Hepatol 34: 254–260, 2001. [DOI] [PubMed] [Google Scholar]