Abstract
Asthma development and pathogenesis are influenced by the interactions of airway epithelial cells and innate and adaptive immune cells in response to allergens. Oxidative stress is an important mediator of asthmatic phenotypes in these cell types. Nuclear erythroid 2-related factor 2 (Nrf2) is a redox-sensitive transcription factor that is the key regulator of the response to oxidative and environmental stress. We previously demonstrated that Nrf2-deficient mice have heightened susceptibility to asthma, including elevated oxidative stress, inflammation, mucus, and airway hyperresponsiveness (AHR) (Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. J Exp Med 202: 47–59, 2005). Here we dissected the role of Nrf2 in lung epithelial cells and tested whether genetic or pharmacological activation of Nrf2 reduces allergic asthma in mice. Cell-specific activation of Nrf2 in club cells of the airway epithelium significantly reduced allergen-induced AHR, inflammation, mucus, Th2 cytokine secretion, oxidative stress, and airway leakiness and increased airway levels of tight junction proteins zonula occludens-1 and E-cadherin. In isolated airway epithelial cells, Nrf2 enhanced epithelial barrier function and increased localization of zonula occludens-1 to the cell surface. Pharmacological activation of Nrf2 by 2-trifluoromethyl-2′-methoxychalone during the allergen challenge was sufficient to reduce allergic inflammation and AHR. New therapeutic options are needed for asthma, and this study demonstrates that activation of Nrf2 in lung epithelial cells is a novel potential therapeutic target to reduce asthma susceptibility.
Keywords: ovalbumin, oxidative stress, inflammation, airway hyperresponsiveness, Th2
asthma is a complex airway disorder characterized by reversible airflow obstruction, airway hyperresponsiveness (AHR), airway inflammation, excessive mucus production, and elevated levels of IgE and T helper type 2 (Th2) cytokines (9, 44, 58). Asthmatic symptoms are often triggered by inhaled exposure to allergens or other environmental factors, including airborne pollutants, infections, or chemicals, that cause wheezing, chest tightness, shortness of breath, and coughing. Inhaled agents first encounter the airway epithelium, which provides a first line of defense to suppress activation of innate and adaptive immune cells. Despite substantial progress in understanding of the mechanisms responsible for asthma pathogenesis, prevalence of asthma in the US has continued to rise over the past several decades, with over 8% of the US population (25.7 million individuals) currently having a diagnosis of asthma (1). Despite the availability of medications to control the disease, there are 2.1 million emergency department visits, 480,000 hospitalizations, and over 3,300 deaths from asthma in the US each year (34).
The transcription factor nuclear erythroid 2-related factor 2 (Nrf2) is a key regulator of cytoprotective proteins, including antioxidants, xenobiotic detoxification enzymes, and proteins in the proteasomal pathway (17, 54). Under nonstressed conditions, Nrf2 persists at low levels in the cytoplasm where it is bound to its inhibitor Kelch ECH associating protein 1 (Keap1), which facilitates its ubiquitination and proteolytic degradation (18). However, in the presence of environmental stress, Nrf2 releases from Keap1, translocates to the nucleus, and binds to the antioxidant response element found in the promoter of all Nrf2-dependent genes, resulting in activation of an adaptive cytoprotective response that detoxifies environmental stressors and exhibits numerous immuno-modulatory effects (20). This Nrf2-dependent response consists of 650 direct inducible target genes that coordinate to attenuate environmental and oxidative stress in multiple ways (30), such as 1) providing direct antioxidants (35, 36); 2) encoding enzymes that directly inactivate oxidants (15); 3) increasing levels of glutathione synthesis and regeneration (33); 4) stimulating NADPH synthesis (14, 54); 5) enhancing toxin export via the multidrug response transporters (14); 6) inhibiting cytokine-mediated inflammation (37); and 7) enhancing the recognition, repair, and removal of damaged proteins (27).
Using an ovalbumin (OVA) allergen-induced asthma model, our laboratory previously reported that Nrf2-deficient mice exhibit increased AHR, eosinophilic airway inflammation, oxidative stress, mucus hyperplasia, and Th2 cytokine secretion, compared with wild-type controls (39). Additionally, dendritic cells from Nrf2-deficient mice display enhanced surface expression of activation markers, increased oxidative stress, and heightened Th2 responses, compared with dendritic cells from wild-type mice after ex vivo stimulation with allergen (29, 40, 57). Furthermore, Nrf2 protein levels and Nrf2-regulated antioxidant responses are markedly reduced in airway smooth muscle cells from severe asthmatic subjects, compared with normal subjects, suggesting that the Nrf2 response may be impaired in asthmatic subjects (32). Taken together, this evidence suggests that Nrf2 signaling is a major determinant of susceptibility to allergic asthma, and augmenting Nrf2 signaling could be a promising approach for treatment of allergic asthma.
The airway epithelium is an important determinant of asthma susceptibility, as it provides several key functions, including maintenance of the epithelial barrier, mucociliary clearance, wound repair, and secretion of cytokines, antioxidants, surfactants, antiproteases, and antibacterial agents. Emerging evidence suggests that the allergic inflammatory responses that are typical of asthma are driven by altered epithelial cell function, and asthmatic subjects exhibit impaired barrier function (52), defective antioxidant pathways (45), aberrant repair (11), and enhanced epithelial-derived cytokine secretion (16). Nrf2 has been implicated in several of these phenotypes, and targeting Nrf2 in airway epithelium could play a vital role in modulating the asthmatic response.
The goals of our present study were to determine whether activation of Nrf2 attenuates OVA-induced asthmatic phenotypes in mice and to further elucidate the role of Nrf2 in the airway epithelium after allergen exposure. Here, we demonstrated that activation of Nrf2, via either genetic deletion of Keap1 or pharmacological activation of the pathway, suppresses OVA-induced asthma, and we showed that Nrf2 increases cytoprotective responses in the airway epithelium to reduce allergic asthma.
MATERIALS AND METHODS
Animals.
Male C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD). Keap1-floxed (Keap1fl/fl) mice on a C57BL/6 background were generated as previously described (4, 25). Briefly, LoxP sites were inserted into the Keap1 gene, flanking exons 2 and 3. The Keap1 transcript lacking exons 2 and 3 codes for a truncated nonfunctional Keap1 protein that contains the NH2-terminal BTB domain essential for Keap1 dimerization but lacks the redox-sensitive IVR domain and the Nrf2-binding Kelch domains. Tamoxifen-inducible CMVCre-Keap1fl/fl mice were generated by crossing Keap1fl/fl mice with CAG-CreERT2+ mice. To induce Cre-mediated deletion of Keap1, tamoxifen-inducible CMVCre-Keap1fl/fl mice and Keap1fl/fl mice were treated with tamoxifen (1 mg·mouse−1·day−1; ip injection). Deletion of Keap1 was determined as previously described (23), and activation of Nrf2 was confirmed by measuring expression of its downstream target NADPH quinone oxidoreductase 1 (Nqo1) by quantitative PCR (TaqMan, Applied Biosystems). CC10-Keap1−/− mice, in which cre is controlled by the club cell-specific 10-kDa protein (CC10) promoter, were generated as previously described (4). Briefly, Keap1fl/fl mice were bred with CCtCre+ transgenic mice that express cre under the control of the CC10 promoter. Mice were further crossed to produce Keap1Δ2−3/Δ2−3; CCtCre+ mice (CC10-Keap1−/−). These mice did not display any gross structural alterations in the lungs in the absence of stress. CCtCre+ transgenic mice were developed and characterized previously and show specific activation of cre in the airway epithelium, with no effect on endogenous CC10 expression (46). All mice were housed under controlled conditions for temperature and humidity, using a 12:12-h light-dark cycle. All experimental protocols were performed in accordance with the standards established by the US Animal Welfare Acts, as set forth in National Institutes of Health guidelines and in the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee. All procedures were approved by the Johns Hopkins University Animal Care and Use Committee.
Animal exposures.
Mice were sensitized to Grade V OVA (Sigma Aldrich) via intraperitoneal injection of 20 μg OVA plus 2 mg Alum (Imject Alum, Thermo) in 200 μl PBS. A second sensitization was performed on day 14 using 100 μg OVA plus 2 mg Alum. Mice were challenged every 48 h starting at day 21 with intratracheal administration of either 100 μg OVA in 50 μl PBS or PBS alone. Mice were harvested 48 h after the fourth challenge. For tamoxifen-inducible deletion of Keap1, mice were treated with tamoxifen for 5 consecutive days before the first sensitization. 2-Trifluoromethyl-2′-methoxychalone (TMC) was synthesized as previously described (26). Mice were treated with TMC or vehicle by gavage 4 h before each challenge.
AHR.
Mice were anesthetized with ketamine-xylazine, intubated, and paralyzed with 0.21 mg intramuscular succinylcholine chloride (Hospira). Mice were ventilated at 150 breaths/min at a tidal volume of 0.2 ml. AHR was induced by a 10-s inhalation of 30 mg/ml acetyl-β-methylcholine (Sigma Aldrich), and dynamic airway pressure (cmH2O·s) was followed for 5 min.
Inflammation and cytokine secretion.
Inflammatory cells were quantified in bronchoalveolar lavage (BAL) fluid, as previously described (49). Differential cell counts were quantified on cytospin preps stained with Diff-Quik stain (Siemens). IL-4, IL-5, and IL-13 were measured in BAL fluid by ELISA (R&D Systems).
Mucus secretion.
Left lungs from OVA-exposed Keap1fl/fl and CC10-Keap1−/− mice were inflated to a pressure of 25 cmH2O with 0.6% melted agarose, cooled, cut into four pieces, fixed in 10% formalin, embedded in paraffin, sectioned, and stained with periodic acid Schiff (PAS) kit (Sigma Aldrich) without counterstain. The sections were blinded and scored by an independent observer.
Oxidative stress.
Lipid peroxidation was measured in lung homogenates after mixing with SDS lysis buffer (100 mM NaCl, 500 mM Tris, pH 8.0, 10% SDS) at a ratio of 1:1, followed by addition of thiobarbituric acid in 10% acetic acid. Samples were boiled and then mixed with n-butanol at a ratio of 1:1, and absorbance was measured at 532 nm. Malondialdehyde content was compared with a standard and normalized to protein content. Protein carbonyls were measured in lung homogenates that were treated with 1% streptomycin sulfate followed by dinitrophenylhydrazine in 2 N HCl. After 1 h, 20% cold trichloroacetic acid was added at a ratio of 1:1. Precipitated protein was resuspended in 6 M guanidine hydrochloride, and protein carbonyls were determined from the absorbance at 370 nm using the molar absorption coefficient of 22,000 M−1·cm−1.
Epithelial barrier function.
Tracheal epithelial cells were isolated as previously described (28) and plated on 12-well inserts (Falcon). Paracellular permeability was assessed via addition of FITC-coupled dextran beads (4 kDa, 10 mg/ml; Calbiochem) to the upper chamber, and fluorescence in the basal media was determined at 20 min. Transepithelial electrical resistance (TEER) was assessed by an epithelial voltohmmeter (World Precision Instruments). Zonula occludens-1 (Zo-1) immunofluorescence was measured after staining epithelial monolayers or paraffin lung sections with an anti-Zo-1 rabbit polyclonal antibody (61–7300, Life Technologies). E-cadherin immunofluorescence was performed using a rabbit polyclonal E-cadherin antibody (H-108 Santa Cruz Biotechnology).
Statistical analyses.
The Student's unpaired t-test was used to determine statistical significance between each group. One-way ANOVA followed by post hoc analysis using Tukey test was used for comparisons of multiple groups. Values are presented as means ± SE.
RESULTS
Genetic activation of Nrf2 reduces OVA-induced asthma.
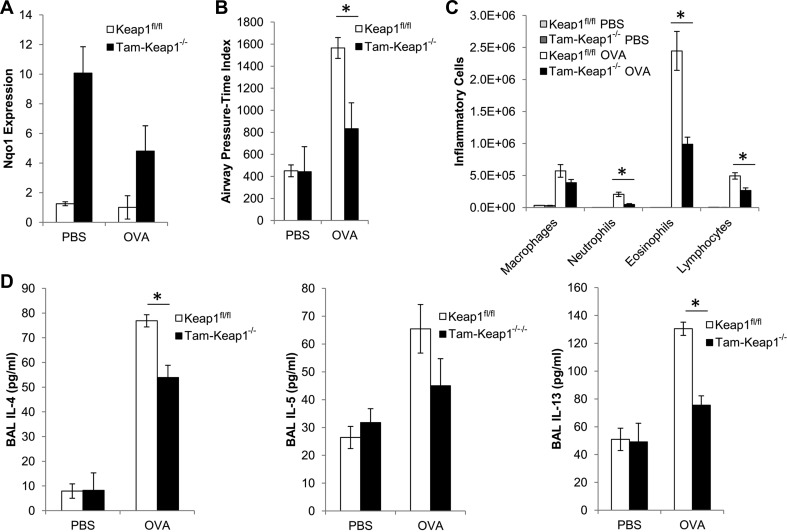
As a result of our previous study demonstrating that Nrf2-deficient mice have increased asthmatic phenotypes (39), we presently examined whether activation of Nrf2 attenuates asthma. Keap1-deficient mice are postnatally lethal. Therefore, to genetically activate Nrf2, we generated Keap1-floxed mice (Keap1fl/fl) that contain a tamoxifen-inducible CMV-cre recombinase. Tamoxifen treatment resulted in increased expression of Nqo1, an Nrf2-dependent gene that serves as a marker for Nrf2 activation, in lungs shortly after treatment (data not shown), and this elevated expression was sustained at 36 days after treatment (Fig. 1A). Mice that were sensitized and challenged with OVA exhibited elevated methacholine-induced airway pressure-time index, which is a measure of AHR, increased eosinophilic inflammation, and increased secretion of Th2 cytokines (IL-4 and IL-13) and the eosinophilic chemokine IL-5 (Fig. 1). Tam-Keap1−/− mice showed significant reductions in OVA-induced AHR (Fig. 1B), eosinophilic inflammation (Fig. 1C), and secretion of IL-4 and IL-13 in BAL (Fig. 1D), compared with Keap1fl/fl controls. We also observed a trend toward reduced IL-5 in Tam-Keap1−/− mice, but this was not significant by ANOVA. Additionally, we observed mild infiltration of neutrophils and lymphocytes in the Keap1fl/fl mice, which were significantly reduced in Tam-Keap1−/− mice (Fig. 1C). Thus genetic activation of Nrf2 attenuates asthmatic phenotypes.
Fig. 1.
Genetic activation of nuclear erythroid 2-related factor 2 (Nrf2) reduces ovalbumin (OVA)-induced asthmatic phenotypes. A: NADPH quinone oxidoreductase 1 (Nqo1) gene expression, as a marker of Nrf2 activity, was measured in lungs of Kelch ECH associating protein 1-floxed (Keap1fl/fl) and Tam-Keap1−/− mice that were treated with tamoxifen (Tam) before sensitization. These same mice were used in B–D. B: airway hyperresponsiveness (AHR) was assessed by airway pressure-time index (APTI) (cmH2O·s) after inhalation of methacholine. Inflammation (C) and cytokines (D) were quantified in the bronchoalveolar lavage (BAL). Values are means ± SE; N = 6–9 mice per group. *P < 0.05 by ANOVA.
Pharmacological activation of Nrf2 reduces the asthmatic response.
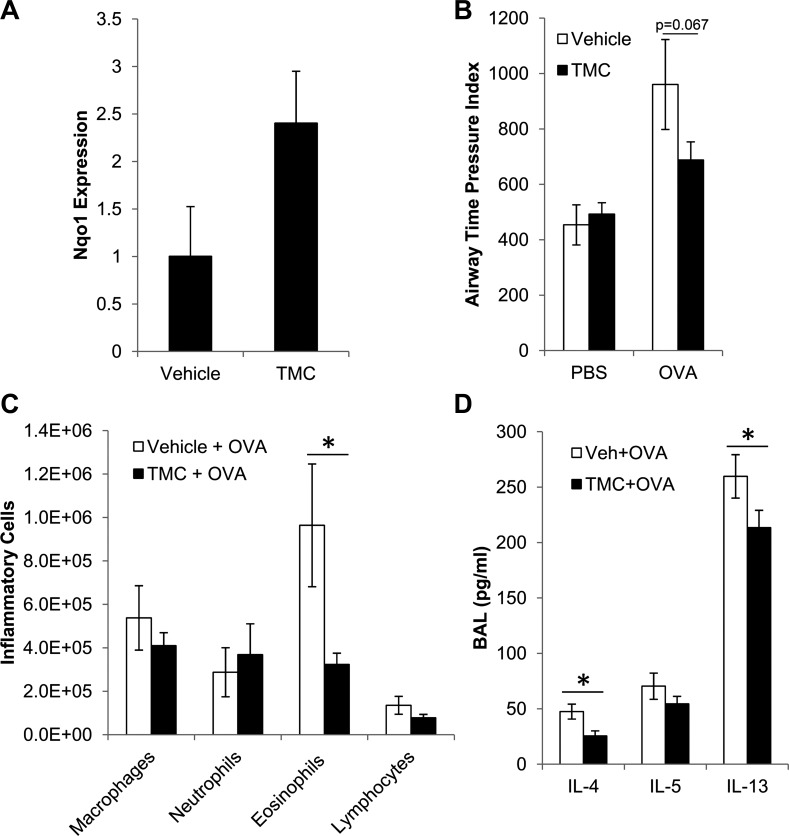
To further identify the role of Nrf2 in reducing asthmatic phenotypes, we treated mice with the Nrf2 activator, TMC, before OVA challenges. Based on previous characterization of TMC (26) and our own preliminary dosing experiments (not shown), 400 mg/kg TMC via gavage gave maximal induction of Nrf2 target gene expression at 6 h after either a single treatment or multiple daily treatments, with no obvious toxicity. Expression returned to baseline within 24 h. To test whether TMC decreased susceptibility to our asthma model, mice were given TMC/vehicle 4 h before each OVA challenge. Lungs were harvested in a small subset of mice (N = 3) at 2 h after OVA challenge (6 h after vehicle/TMC treatment) to confirm that treatment with TMC by gavage increased expression of the Nrf2 target gene Nqo1 in our experimental mice (Fig. 2A). This increase in Nqo1 expression was not statistically significant (P = 0.10), but the fold induction was similar to our preliminary dosing experiments. Treatment with TMC 4 h before each OVA challenge decreased OVA-induced AHR (Fig. 2B), although this decrease did not quite reach statistical significance. TMC also significantly decreased OVA-induced eosinophilic inflammation (Fig. 2C) and secretion of IL-4 and IL-13 (Fig. 2D). Secretion of IL-5 was decreased slightly, but not significantly. Although genetic activation of Nrf2 (Fig. 1) resulted in greater induction of Nqo1 and stronger reduction of asthmatic phenotypes, compared with pharmacological activation of Nrf2, these data still demonstrate that pharmacological activation of Nrf2 during the challenge phase reduces asthmatic phenotypes and suggests that Nrf2 can suppress the susceptibility to allergen challenges.
Fig. 2.
Pharmacological activation of Nrf2 during the challenge phase reduces OVA-induced asthmatic phenotypes. A: Nqo1 expression was measured by quantitative PCR in lungs at 6 h after treatment with 2-trifluoromethyl-2′-methoxychalone (TMC) or vehicle by gavage (N = 3). AHR (B), inflammation (C), and cytokines (D) were measured in BAL fluid of C57BL/6 mice that were treated with 400 mg/kg TMC or vehicle by gavage 4 h before each challenge. Values are means ± SE; N ≥ 5 mice per group. *P < 0.05 by Student's t-test.
Activation of Nrf2 in airway epithelium attenuates asthma.
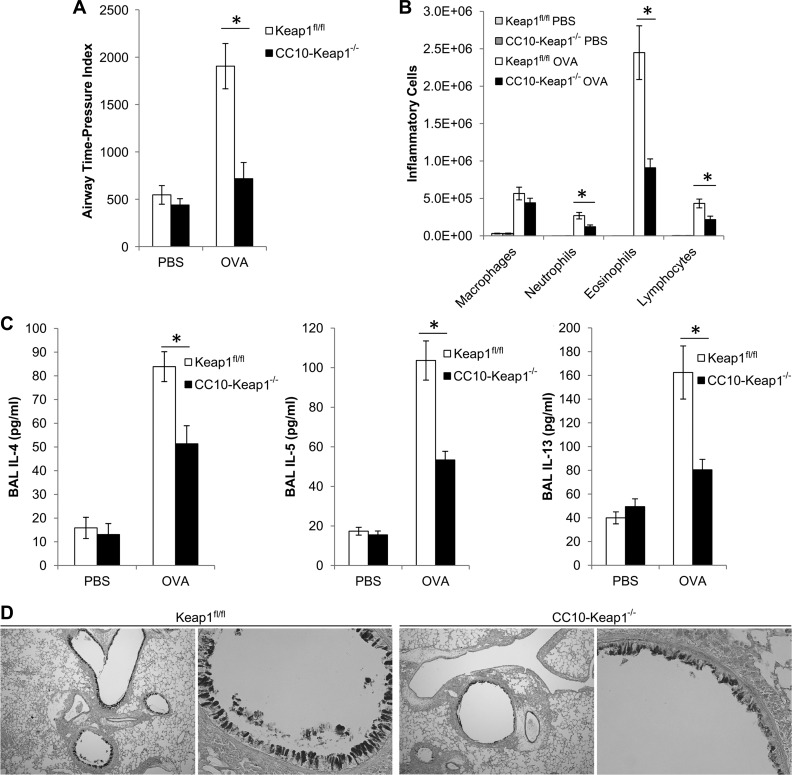
While asthma has traditionally been considered a disease of exaggerated allergic pathways, an emerging paradigm proposes that asthma is primarily an epithelial disorder, and that its origin and clinical manifestations have more to do with altered epithelial physical and functional barrier properties (16). Our laboratory previously generated mice that contain activated Nrf2 specifically in club cells of the airway epithelium (CC10-Keap1−/−), and we demonstrated that these mice exhibit reduced oxidative stress and inflammation in the lungs after exposure to cigarette smoke (4). Thus we assessed the role of epithelial-derived Nrf2 activation on asthma responses. CC10-Keap1−/− mice exhibited significant reductions in OVA-induced AHR (Fig. 3A), eosinophilic inflammation (Fig. 3B), and secretion of IL-4, IL-5, and IL-13 (Fig. 3C), compared with Keap1fl/fl controls. These reductions in asthmatic features were similar in magnitude to the reductions observed in the Tam-Keap1−/− mice (Fig. 1). Additionally, mucus secretion was assessed by PAS staining of paraffin sections from lungs of Keap1fl/fl and CC10-Keap1−/− mice after OVA sensitization and challenge (N = 5). Staining was ranked and scored by a blinded observer, and representative images presented in Fig. 3D depict the median-scored sample from each genotype. Airways from CC10-Keap1−/− mice showed decreased PAS staining compared with that from Keap1fl/fl mice (Fig. 3D). Thus Nrf2 regulates club cell function to reduce asthma in mice.
Fig. 3.
Activation of Nrf2 in club cells attenuates OVA-induced asthmatic phenotypes. A: AHR was assessed by APTI (cmH2O·s) in Keap1fl/fl and club cell-specific 10-kDa protein (CC10)-Keap1−/− mice in response to inhalation of methacholine. Inflammation (B) and cytokines (C) were quantified in the BAL. Values are means ± SE; N = 11–12 mice per group. *P < 0.05 by ANOVA. D: mucus secretion was assessed by periodic acid Schiff staining of airways from OVA-exposed Keap1fl/fl and CC10-Keap1−/− mice (N = 5 mice per group). Representative images were captured at ×40 (left) and ×200 (right) magnification.
Nrf2 regulates club cell function.
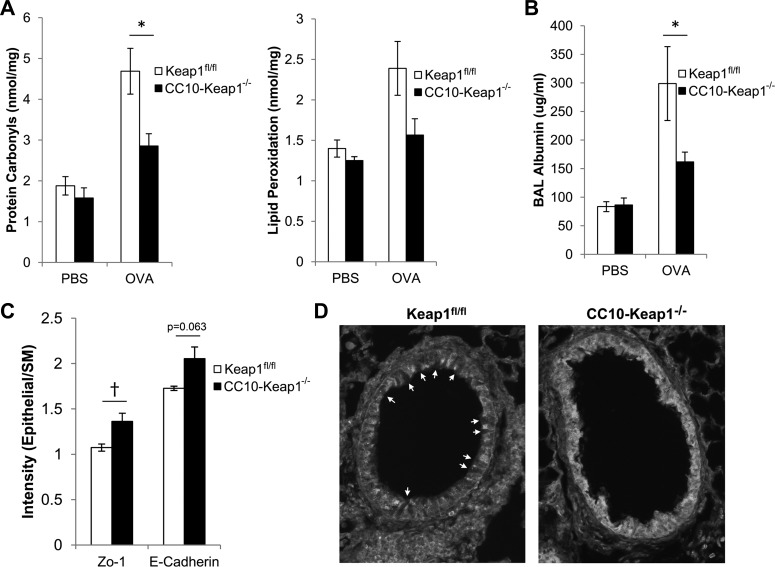
Club cells are nonciliated secretory cells that are a major cell type of the airway epithelium. Club cells have an array of functions, including formation of the epithelial barrier, detoxification of oxidative stress, and secretion of epithelial-derived cytokines (16). These functions are important determinants of asthma susceptibility, and we investigated the role of Nrf2 in club cell function. Mice that were challenged with OVA exhibited oxidative stress in their lungs, as indicated by elevated levels of protein carbonyls and lipid peroxidation (Fig. 4A). However, protein carbonyls were significantly reduced in lungs of CC10-Keap1−/− mice, compared with Keap1fl/fl mice after OVA challenges (Fig. 4A), and lipid peroxidation showed a trend toward reduction in CC10-Keap1−/− mice. We also measured albumin concentration in the BAL fluid, which is an indicator of protein permeability across the airway epithelium. Airway challenges with OVA increased albumin levels in the cell-free BAL fluid, and this concentration was significantly decreased in CC10-Keap1−/− mice compared with Keap1fl/fl mice (Fig. 4B), suggesting that epithelial barrier function is enhanced by Nrf2 after allergen challenge. Thus our data demonstrate that Nrf2 regulates oxidative stress and barrier function in airway epithelial cells.
Fig. 4.
Nrf2 regulates club cell function. A: oxidative damage to macromolecules was measured via quantification of protein carbonyls and lipid peroxidation in lung homogenates of CC10-Keap1−/− and Keap1fl/fl mice. B: airway leakiness was assessed via quantification of albumin in the cell-free BAL fluid by the Albumin Blue Fluorescent Assay Kit (Active Motif). Values are means ± SE; N = 7–8 mice per group. C: Zonula occludens-1 (Zo-1) and E-cadherin intensity were quantified in the airway epithelium of OVA-sensitized/challenged Keap1fl/fl and CC10-Keap1−/− mice, and normalized to background levels using Image J. Quantification was performed on a minimum of 4 airways per mouse. *P < 0.05 by ANOVA. †P < 0.05 by Student's t-test. D: representative ×200 images of airways from OVA-sensitized/challenged Keap1fl/fl and CC10-Keap1−/− mice immunostained for Zo-1. Arrows indicate epithelial cells that do not express Zo-1. SM, smooth muscle.
Asthmatic subjects have reduced expression of the tight junction proteins Zo-1 and E-cadherin, leading to impaired epithelial barrier function. To determine whether tight junction formation is regulated by Nrf2, we quantified Zo-1 and E-cadherin in airways of OVA-challenged Keap1fl/fl and CC10-Keap1−/− mice. CC10-Keap1−/− mice showed increased levels of Zo-1 in the airways compared with background levels in the adjacent smooth muscle, which was significantly higher than in Keap1fl/fl mice (Fig. 4C). Notably, airways from Keap1fl/fl mice contained uneven protein expression of Zo-1, with many cells that were nearly absent for Zo-1 (Fig. 4D, arrows), whereas airways from CC10-Keap1−/− mice showed greater overall Zo-1 levels and more consistent staining within the airways. Additionally, E-cadherin was elevated slightly in airways of CC10-Keap1−/− mice, which was nearly significant (Fig. 4C). Thus Nrf2 enhances tight junction formation in airways of mice.
Nrf2 enhances airway barrier function.
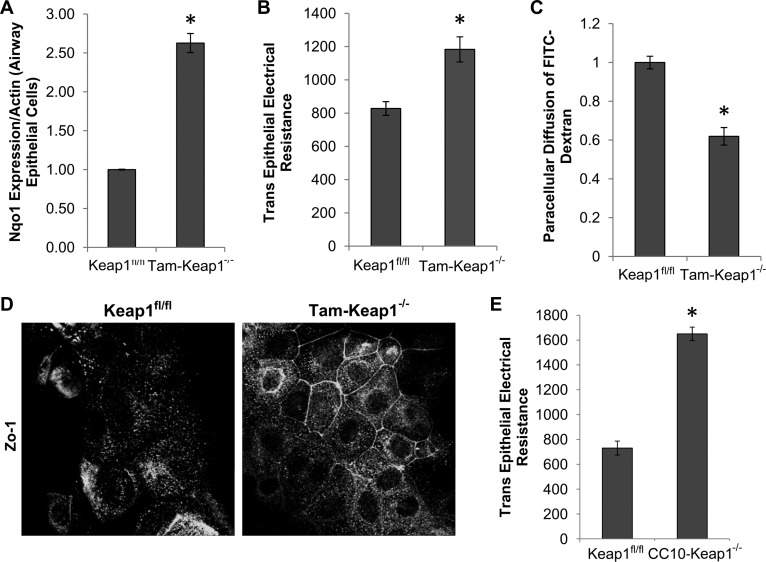
To further examine the role of Nrf2 in airway epithelial barrier function, tracheal epithelial cells from Keap1fl/fl and Tam-Keap1−/− mice were cultured on transwell inserts to form a complete monolayer. Barrier function was assessed by quantification of TEER and paracellular diffusion of FITC-dextran across the monolayer. Confirmation of Nrf2 activation in Tam-Keap1−/− mice was verified by elevated expression of Nqo1 (Fig. 5A). Epithelial monolayers from Tam-Keap1−/− mice exhibited enhanced TEER (Fig. 5B) and decreased FITC-dextran diffusion (Fig. 5C) compared with Keap1fl/fl control mice, indicating increased barrier function. The epithelial barrier is mediated by surface expression of tight junction proteins. We stained epithelial cells for the tight junction protein, Zo-1, and observed increased localization of Zo-1 at the cell surface of Tam-Keap1−/− cells compared with Keap1fl/fl cells (Fig. 5D). However, Zo-1 gene expression was not dependent on Nrf2 (data not shown). We also observed increased barrier function (TEER) in CC10-Keap1−/− epithelial cells, compared with Keap1fl/fl (Fig. 5E). Therefore, activation of Nrf2 in epithelial cells enhances airway epithelial barrier function.
Fig. 5.
Nrf2 enhances barrier function of airway epithelial cells. A: Nqo1 gene expression, as a marker of Nrf2 activity, was measured in epithelial cell monolayers of Tam-Keap1−/− and Keap1fl/fl mice that were treated with Tam before harvesting of tracheas. B: after cells formed a confluent monolayer [confirmed by stable transepithelial electrical resistance (TEER) readings], TEER was measured across the monolayer daily for 5 days, and the average of all days was represented as a single replicate. C: FITC-dextran was added to the apical side of the confluent monolayer, and paracellular diffusion was quantified after 20 min. D: Zo-1 localization was measured by immunofluorescence. E: TEER was measured in Keap1fl/fl and CC10-Keap1−/− epithelial monolayers. Values are means ± SE; N = 6 mice per group. *P < 0.05 by Student's t-test.
DISCUSSION
Our present study extends our previous findings to demonstrate that activation of Nrf2 reduces asthmatic phenotypes in mice. We utilized multiple approaches to activate Nrf2, including genetic activation, via tamoxifen-induced deletion of Keap1, and pharmacological activation, via treatment with the Nrf2 activator TMC. Previous studies demonstrate that patients with severe asthma, chronic obstructive pulmonary disease (COPD), or idiopathic pulmonary fibrosis contain deficient activity of Nrf2 (3, 12, 32, 51), which suggests that the normal Nrf2 response is not maintained in chronic pulmonary diseases. Thus targeting the Nrf2 response may be a viable potential therapy for asthma and other chronic conditions. Indeed, activation of Nrf2 has shown beneficial effects in a variety of disease models, including emphysema (49), bacterial exacerbations of COPD (13), viral infection (5), chronic kidney disease (2), sepsis (55), and radiation injury (23). Additionally, dimethyl fumarate, which is an activator of Nrf2, is currently used as a therapy for multiple sclerosis. However, cancer cells often hijack this pathway to confer resistance to chemotherapy and radiotherapy (47), while Nrf2-deficient mice are protected from atherosclerosis (48). Thus there is tremendous therapeutic potential for Nrf2 activators, but there are potential consequences that must be explored further.
Sensitization and progression of asthma are determined by a balance between the functions of epithelial cells, innate immune cells, and adaptive immune cells. We demonstrated that activation of Nrf2 after sensitization was sufficient to reduce allergic inflammation. While this does not preclude Nrf2 from having a role during sensitization, these data indicate that Nrf2-mediated reduction in asthma occurs at the challenge phase, which may be due to acute cytoprotective responses. Other studies demonstrate that Nrf2 alters dendritic cell function and skews the Th2 response (29, 57), suggesting that Nrf2 may also function during sensitization. We explored the role of Nrf2 in airway epithelial cell function, which is an important component of asthma susceptibility, and we showed that Nrf2 has a strong protective role in the airway epithelium of sensitized and challenged mice. Emerging evidence suggests that damage to the airway epithelium is a driving force of asthma susceptibility, and our data shows that Nrf2 plays a prominent protective role in this tissue.
Club cells are a major cell type of the airway epithelium, and their primary functions include maintenance of the epithelial barrier, epithelial wound repair, and secretion of antioxidants, antibacterial compounds, and cytokines in response to cellular stress (16). The role of Nrf2 as a critical mediator of the antioxidant response has been well studied. Previous studies demonstrate that Nrf2 deficiency exacerbates inflammation and oxidative stress in a variety of disease models, including emphysema (38), COPD exacerbations (13), acute lung injury (31, 42), pulmonary fibrosis (6, 22), and sepsis (53). Alternatively, activation of Nrf2 in these disease models reduces oxidative stress and inflammation (24, 43, 49). In our present study, intratracheal OVA challenges triggered oxidative stress in wild-type mice; however, protein carbonyls, a marker of oxidative stress, was significantly reduced in CC10-Keap1−/− mice. This finding demonstrates that activation of Nrf2 in club cells is sufficient to alter the redox status of the lung to substantially limit OVA-induced oxidative damage, which is consistent with the role of Nrf2 that has been established in other disease models. However, this study does not preclude other cell types from having an Nrf2-dependent effect on asthma susceptibility. In fact, previous studies suggest that Nrf2 activity in dendritic cells and smooth muscle may also alter asthma susceptibility (32, 57).
Oxidative stress has been shown to play a significant role in the pathogenesis of asthma. Markers of oxidative stress are elevated in asthmatic subjects, compared with healthy subjects, while antioxidant levels, including superoxide dismutase and glutathione peroxidase, are reduced in airway epithelium of asthmatic subjects (7, 19). Replenishing antioxidants by exogenous administration of N-acetylcysteine reduces asthmatic inflammation in Nrf2−/− mice, suggesting that oxidative stress is an important contributor (39). Oxidative stress also plays a role in contraction of airway smooth muscle (50). Furthermore, oxidative stress can cause damage to airway epithelium and disrupt the physical barrier that normally precludes entry of inhaled allergens into the submucosa. While antioxidant therapies may have some benefit in attenuating asthmatic phenotypes, activation of Nrf2 is likely to be a more effective therapy. Unlike antioxidant-based therapies that stoichometrically scavenge individual oxidants, Nrf2 targets hundreds of genes to mount a coordinated and effective response against inhaled stressors.
The epithelial cell layer of the conducting airways acts as a physical barrier to exclude inhaled antigens from penetrating into the airway wall. We demonstrated that Nrf2 reduces airway leakiness in vivo. Both in vitro and in vivo studies have shown that the airway epithelium of individuals with asthma is leakier than in normal subjects (21, 59). To further address the role of Nrf2 in barrier function, we performed ex vivo experiments using tracheal epithelial cells from Tam-Keap1−/− and CC10-Keap1−/− mice and demonstrated that activation of Nrf2 in epithelial cells enhances epithelial barrier function and causes an increase in surface expression of the tight junction protein Zo-1. We also demonstrated that Nrf2 increases Zo-1 staining in airways of OVA-challenged mice. Epithelial cells from asthmatic subjects have reduced expression of tight junction proteins, including Zo-1 and E-cadherin (8, 56), and our study demonstrates that activation of Nrf2 may restore these levels after allergen challenge. It remains unclear whether this Nrf2-dependent enhanced barrier function is directly caused by suppression of oxidative damage. Oxidative stress causes a rapid increase in phosphorylation of tight junction proteins, including Zo-1 and E-cadherin, leading to a redistribution of the Zo-1-occludin and E-cadherin-β-catenin complexes from the intercellular junctions and decreased TEER (41). This is consistent with our observations of Nrf2-induced increases in Zo-1 surface expression, which correlate with decreased oxidative stress. We did not observe Nrf2-dependent changes in mRNA expression of Zo-1 in isolated tracheal epithelial cells (data not shown), which suggests that Zo-1 is regulated at the protein level. Furthermore, the antioxidant glutathione was shown to increase tight junction protein levels in a rat transgenic model of human immunodeficiency virus (10), suggesting that suppression of oxidative stress is an important component of the Nrf2-dependent increase in epithelial barrier integrity. Since tight junction proteins are expressed by numerous cell types in addition to club cells, it is possible that Nrf2 activation alters epithelial barrier integrity in a variety of tissues.
In conclusion, Nrf2 reduces asthmatic features in mice, and activation of Nrf2 in airway epithelial cells is sufficient to mediate this protective response. The airway epithelium is easily accessible from the standpoint of therapeutic interventions, and this presents a viable therapeutic target for reduction of asthma symptoms. We showed that pharmacological activation of Nrf2, via oral TMC treatment, reduces AHR and eosinophilic inflammation in the airways after allergen challenges, but further research is warranted to determine whether Nrf2 reduces asthmatic phenotypes in response to other pollutants or infections. Additionally, one limitation of this study was that TMC was administered before allergen challenges, as opposed to after onset of asthmatic symptoms. Thus this strategy would mimic a controller medication rather than a rescue medication. We did not investigate whether TMC was effective after challenge with antigen. Recently, the Nrf2 activator dimethyl fumarate received Food and Drug Administration approval for treatment of multiple sclerosis, and the Nrf2 activator sulforaphane was shown to be safe in a recent phase II clinical trial for COPD. Future studies are needed to assess the effectiveness of these and other Nrf2 activating compounds in patients with asthma.
GRANTS
T. E. Sussan is supported in part by a Young Clinical Scientist Award from the Flight Attendant Medical Research Institute. S. Biswal is supported by National Institute of Environmental Health Sciences Grants P50-ES-015903 and P01-ES-018176.
DISCLOSURES
S. Biswal and the Johns Hopkins University hold intellectual property on the development of TMC. Cureveda LLC has licensed this intellectual property. S. Biswal is cofounder of, has equity in, and serves as a scientific consultant for Cureveda LLC. These potential individual and institutional conflicts of interest have been reviewed and managed by the Johns Hopkins University School of Public Health.
AUTHOR CONTRIBUTIONS
Author contributions: T.E.S., P.N.B., G.B.D., V.K.S., and S.B. conception and design of research; T.E.S., S.G., S.C., P.M., S.M., K.S., and S.K. performed experiments; T.E.S., S.C., P.M., S.M., and K.S. analyzed data; T.E.S., S.C., V.K.S., and S.B. interpreted results of experiments; T.E.S. prepared figures; T.E.S. drafted manuscript; T.E.S., S.G., S.C., P.M., S.M., K.S., S.K., P.N.B., G.B.D., V.K.S., and S.B. approved final version of manuscript; V.K.S. and S.B. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Stephane Lajoie for assistance with analysis of the asthma mouse models, Allen Myers for assistance with the immunofluorescence experiments, and Sarai Arrozena for assistance with isolation of tracheal epithelial cells. We also thank Rajesh Thimmulappa for comments on the manuscript.
REFERENCES
- 1.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 94: 1–8, 2012. [PubMed] [Google Scholar]
- 2.Aminzadeh MA, Reisman SA, Vaziri ND, Shelkovnikov S, Farzaneh SH, Khazaeli M, Meyer CJ. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores endothelial function impaired by reduced Nrf2 activity in chronic kidney disease. Redox Biol 1: 527–531, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artaud-Macari E, Goven D, Brayer S, Hamimi A, Besnard V, Marchal-Somme J, Ali ZE, Crestani B, Kerdine-Romer S, Boutten A, Bonay M. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal 18: 66–79, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Blake DJ, Singh A, Kombairaju P, Malhotra D, Mariani TJ, Tuder RM, Gabrielson E, Biswal S. Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. Am J Respir Cell Mol Biol 42: 524–536, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, Polack FP, Kleeberger SR. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am J Respir Crit Care Med 179: 138–150, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J 18: 1258–1260, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Comhair SA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet 355: 624, 2000. [DOI] [PubMed] [Google Scholar]
- 8.de Boer WI, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, Braunstahl GJ. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol 86: 105–112, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest 111: 291–297, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan X, Staitieh BS, Jensen JS, Mould KJ, Greenberg JA, Joshi PC, Koval M, Guidot DM. Activating the Nrf2-mediated antioxidant response element restores barrier function in the alveolar epithelium of HIV-1 transgenic rats. Am J Physiol Lung Cell Mol Physiol 305: L267–L277, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedorov IA, Wilson SJ, Davies DE, Holgate ST. Epithelial stress and structural remodelling in childhood asthma. Thorax 60: 389–394, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goven D, Boutten A, Lecon-Malas V, Marchal-Somme J, Amara N, Crestani B, Fournier M, Leseche G, Soler P, Boczkowski J, Bonay M. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax 63: 916–924, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, Feller-Kopman D, Wise R, Biswal S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med 3: 78ra32, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun 310: 824–829, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol 45: 51–88, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev 242: 205–219, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76–86, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet 354: 482–483, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Khor YH, Teoh AK, Lam SM, Mo DC, Weston S, Reid DW, Walters EH. Increased vascular permeability precedes cellular inflammation as asthma control deteriorates. Clin Exp Allergy 39: 1659–1667, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi N, Ishii Y, Morishima Y, Yageta Y, Haraguchi N, Itoh K, Yamamoto M, Hizawa N. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir Res 11: 31, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JH, Thimmulappa RK, Kumar V, Cui W, Kumar S, Kombairaju P, Zhang H, Margolick J, Matsui W, Macvittie T, Malhotra SV, Biswal S. NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J Clin Invest 124: 730–741, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong X, Thimmulappa R, Craciun F, Harvey C, Singh A, Kombairaju P, Reddy SP, Remick D, Biswal S. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med 184: 928–938, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong X, Thimmulappa R, Craciun F, Harvey C, Singh A, Kombairaju P, Reddy SP, Remick D, Biswal S. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med 184: 928–938, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar V, Kumar S, Hassan M, Wu H, Thimmulappa RK, Kumar A, Sharma SK, Parmar VS, Biswal S, Malhotra SV. Novel chalcone derivatives as potent Nrf2 activators in mice and human lung epithelial cells. J Med Chem 54: 4147–4159, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol 23: 8786–8794, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam HC, Choi AM, Ryter SW. Isolation of mouse respiratory epithelial cells and exposure to experimental cigarette smoke at air liquid interface. J Vis Exp 48: 2513, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li N, Wang M, Barajas B, Sioutas C, Williams MA, Nel AE. Nrf2 deficiency in dendritic cells enhances the adjuvant effect of ambient ultrafine particles on allergic sensitization. J Innate Immun 5: 543–554, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 38: 5718–5734, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGrath-Morrow S, Lauer T, Yee M, Neptune E, Podowski M, Thimmulappa RK, O'Reilly M, Biswal S. Nrf2 increases survival and attenuates alveolar growth inhibition in neonatal mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 296: L565–L573, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michaeloudes C, Chang PJ, Petrou M, Chung KF. Transforming growth factor-beta and nuclear factor E2-related factor 2 regulate antioxidant responses in airway smooth muscle cells: role in asthma. Am J Respir Crit Care Med 184: 894–903, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moinova HR, Mulcahy RT. Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem Biophys Res Commun 261: 661–668, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Moorman J, Akinbami L, Bailey C, Zahran H, King M, Johnson C, Liu X. National Surveillance of Asthma: United States, 2001–2010: National Center for Health Statistics. Vital Health Stat 35: 1–67, 2012. [PubMed] [Google Scholar]
- 35.Prestera T, Talalay P, Alam J, Ahn YI, Lee PJ, Choi AM. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: regulation by upstream antioxidant-responsive elements (ARE). Mol Med 1: 827–837, 1995. [PMC free article] [PubMed] [Google Scholar]
- 36.Primiano T, Kensler TW, Kuppusamy P, Zweier JL, Sutter TR. Induction of hepatic heme oxygenase-1 and ferritin in rats by cancer chemopreventive dithiolethiones. Carcinogenesis 17: 2291–2296, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Primiano T, Li Y, Kensler TW, Trush MA, Sutter TR. Identification of dithiolethione-inducible gene-1 as a leukotriene B4 12-hydroxydehydrogenase: implications for chemoprevention. Carcinogenesis 19: 999–1005, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114: 1248–1259, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202: 47–59, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rangasamy T, Williams MA, Bauer S, Trush MA, Emo J, Georas SN, Biswal S. Nuclear erythroid 2 p45-related factor 2 inhibits the maturation of murine dendritic cells by ragweed extract. Am J Respir Cell Mol Biol 43: 276–285, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao RK, Basuroy S, Rao VU, Karnaky KJ Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368: 471–481, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, Reddy SP. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol 182: 7264–7271, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy NM, Suryanaraya V, Yates MS, Kleeberger SR, Hassoun PM, Yamamoto M, Liby KT, Sporn MB, Kensler TW, Reddy SP. The triterpenoid CDDO-imidazolide confers potent protection against hyperoxic acute lung injury in mice. Am J Respir Crit Care Med 180: 867–874, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol 54: 577–589, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sackesen C, Ercan H, Dizdar E, Soyer O, Gumus P, Tosun BN, Buyuktuncer Z, Karabulut E, Besler T, Kalayci O. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J Allergy Clin Immunol 122: 78–85, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Tsai LW, Ingenito EP, Gonzalez F, Shapiro SD, Mariani TJ. Epithelial cell PPAR[gamma] contributes to normal lung maturation. FASEB J 20: 1507–1509, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med 3: e420, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sussan TE, Jun J, Thimmulappa R, Bedja D, Antero M, Gabrielson KL, Polotsky VY, Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLos One 3: e3791, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, Sporn MB, Gabrielson KL, Champion HC, Tuder RM, Kensler TW, Biswal S. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci U S A 106: 250–255, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutcliffe A, Hollins F, Gomez E, Saunders R, Doe C, Cooke M, Challiss RA, Brightling CE. Increased nicotinamide adenine dinucleotide phosphate oxidase 4 expression mediates intrinsic airway smooth muscle hypercontractility in asthma. Am J Respir Crit Care Med 185: 267–274, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Downregulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and COPD patients. Am J Respir Cell Mol Biol 39: 673–682, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Swindle EJ, Collins JE, Davies DE. Breakdown in epithelial barrier function in patients with asthma: identification of novel therapeutic approaches. J Allergy Clin Immunol 124: 23–34; quiz 35-26, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest 116: 984–995, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62: 5196–5203, 2002. [PubMed] [Google Scholar]
- 55.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-imidazolide. Biochem Biophys Res Commun 351: 883–889, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trautmann A, Kruger K, Akdis M, Muller-Wening D, Akkaya A, Brocker EB, Blaser K, Akdis CA. Apoptosis and loss of adhesion of bronchial epithelial cells in asthma. Int Arch Allergy Immunol 138: 142–150, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Williams MA, Rangasamy T, Bauer SM, Killedar S, Karp M, Kensler TW, Yamamoto M, Breysse P, Biswal S, Georas SN. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol 181: 4545–4559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 17: 255–281, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D, Bedke N, Cremin C, Sones J, Djukanovic R, Howarth PH, Collins JE, Holgate ST, Monk P, Davies DE. Defective epithelial barrier function in asthma. J Allergy Clin Immunol 128: 549–556, 2011. [DOI] [PubMed] [Google Scholar]