Abstract
The prevalence of many common respiratory disorders, including pneumonia, chronic obstructive lung disease, pulmonary fibrosis, and lung cancer, increases with age. Little is known of the host factors that may predispose individuals to such diseases. Macrophage migration inhibitory factor (MIF) is a potent upstream regulator of the immune system. MIF is encoded by variant alleles that occur commonly in the population. In addition to its role as a proinflammatory cytokine, a growing body of literature demonstrates that MIF influences diverse molecular processes important for the maintenance of cellular homeostasis and may influence the incidence or clinical manifestations of a variety of chronic lung diseases. This review highlights the biological properties of MIF and its implication in age-related lung disease.
Keywords: MIF, lung, innate immunity
the lungs are vulnerable to age and age-related diseases. Aging reduces ventilatory capacity and increases vulnerability to environmental stressors, including inhaled substances and microorganisms. Respiratory infections, acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), lung cancer, and interstitial lung disease all increase in prevalence with age. Because the number of Americans over age 65 is estimated to almost double within the next 20 years, there is an urgency to understand the fundamental biological mechanisms that predispose the aged lung to disease (140, 145).
With age, the capacity of cellular stress responses to maintain homeostasis in the face of environmental toxins, pathogens, and dysregulated intrinsic cellular processes is impaired (56). Perhaps nowhere is this age-related impairment more important than in the lung, which represents the largest interface between the internal and external environments. Here alveoli are continuously exposed to aerosolized pollutants, infectious agents, and oxidants (140). Both innate and adaptive immune responses are among the cellular stress responses that are compromised with age. Advanced age is associated with decreased neutrophil function, attenuated innate immune signaling, and a decreased repertoire of adaptive immune responses (39, 101, 127, 128). This aging immune response has been implicated in the increased prevalence of respiratory infections among the elderly. Yet, beyond its antimicrobial responses, the immune system is critical for sensing changes in the alveolar microenvironment and integrating with diverse cellular signaling pathways to maintain tissue homeostasis, which is critical for optimal respiratory function (28, 66, 70, 89, 102, 159, 161). The noninfectious consequences of age-related changes in immune function are only beginning to be understood.
Macrophage migration inhibitory factor (MIF) is a critical upstream regulator of the immune response (25). MIF induces the expression of cytokines and other inflammatory mediators including TNF-α IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-12, nitric oxide, specific matrix metalloproteases, and products of the arachidonic acid cascade (25). MIF also upregulates the canonical innate immune receptor Toll-like receptor 4 and specialized microbial sensors such as dectin-1 (33, 120). Highlighting the role of MIF in inflammation, diverse studies have shown that inhibition of MIF can mitigate deleterious inflammation in conditions such as ARDS, asthma, sepsis, and autoimmunity (8, 38, 86, 120, 146). In addition to its immunological function, MIF promotes cellular survival, antioxidant signaling, angiogenesis, and wound repair while mitigating cellular senescence (2, 4, 21, 29, 42, 47, 57, 80, 82, 87, 99, 100, 123, 160). This review will highlight the role of MIF in the pathogenesis of respiratory diseases that disproportionately afflict the elderly.
MIF: Structure and Function
MIF was first described and named in the 1950s as a lymphocyte-secreted molecule that arrested the migration of macrophages (37). Despite its historical namesake, MIF influences numerous cell lineages beyond the myeloid system and is ubiquitously expressed in the airway, alveolar, and resident immune cells of the lung. There is a single human MIF gene on chromosome 22 (22q11.23) and both the MIF primary sequence and three-dimensional structure are evolutionarily conserved (31, 77, 108, 135). The gene lies in close proximity to two theta-class glutathione S-transferases and D-dopachrome tautomerase (D-DT) (18, 91, 92, 114). D-DT is structurally homologous to MIF and shares many of its biological functions. Two polymorphisms in the promoter region of the MIF gene are commonly associated with disease. The first is a microsatellite repeat: −794 CATT5–8 (rs5844572), in which the number of CATT repeats directly regulates MIF expression. The second is a −173 G/C single nucleotide polymorphism (SNP) (rs755622) in which the C allele is in linkage disequilibrium with the high-expression −794 CATT7 allele. The functional −794 CATT5–8 microsatellite structural variant is not represented in the SNP-based chips used for genomewide association studies, and its impact on genetic susceptibility has only been uncovered by candidate gene studies. To date, MIF alleles and their haplotypes have been implicated in the incidence or clinical severity of various infectious and autoimmune diseases, as well as immune-related disorders such as autism, or cancers in which inflammation is considered to promote tumor growth (11, 20, 53, 98).
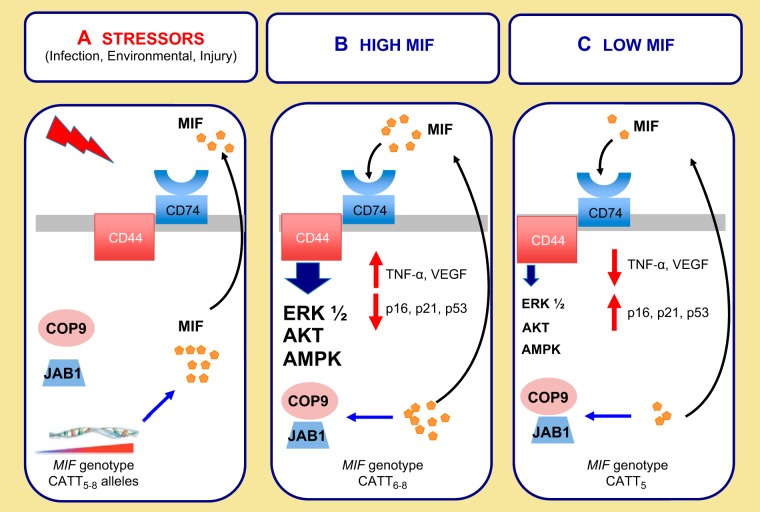
MIF exists preformed in cytoplasmic pools and is rapidly secreted in response to diverse stimuli including oxidative stress and bacterial products (16, 136). The MIF translation product lacks a NH2-terminal sequence for translocation into the endoplasmic reticulum, similar to other nonclassical secretory proteins, such as IL-1β or FGF-1 (48). In macrophages, a specialized pathway for MIF export exists that requires MIF interaction with the Golgi-associated protein, p115 (90). MIF secretion is markedly reduced following treatment with glyburide and probenacid, implicating the involvement of the ABCA1 transporter in MIF secretion (48). In the pituitary, MIF is secreted along with ACTH in response to stress (7, 149). Physiological concentrations of glucocorticoid regulate MIF secretion, and circulating MIF demonstrates a similar diurnal rhythm as glucocorticoids (23, 45, 110). Interestingly, MIF can override the effects of glucocorticoids via diverse signaling pathways that include 1) inhibition of glucocorticoid induction of IκBα (36), 2) inhibition of glucocorticoid-mediated transcription of MAP kinase phosphatase-1 (4, 117), and 3) autocrine and/or paracrine activation of a proinflammatory signaling cascade via binding to its receptor, CD74 (74) (Fig. 1).
Fig. 1.
A: in response to diverse biological stressors, macrophage migration inhibitory factor (MIF) is secreted from preformed intracytoplasmic pools. Subsequently there is an increase in MIF gene transcription. A CATT microsatellite repeat in the promoter region influences gene transcription. B: MIF can signal through its receptor CD74 and coreceptor CD44 to activate ERK 1/2 AKT and AMPK signaling pathways or via an intracellular JAB1 pathway. High MIF conditions result in the transcription of proinflammatory and angiogenic mediators such as TNF-α and VEGF. MIF is also a key cell cycle regulator and can suppress p53- and p16-mediated apoptosis and/or cellular senescence. High-expressing CATT6–8 alleles correspond to increased MIF gene expression. C: the CATT5 allele is associated with decreased transcription of MIF. Low MIF states result in relatively decreased transcription of proinflammatory and angiogenic mediators and a relatively increased susceptibility to apoptosis and cellular senescence.
CD74 is the cell surface expressed form of the MHC class II-invariant chain. It was initially described to play an important role in peptide loading onto MHC class II molecules. However, in 2003, Leng et al. (74) described CD74 to be the cell surface receptor for the MIF molecule. High-affinity binding of MIF to the CD74 ectodomain relies on structural features of MIF's vestigial tautomerase (44). In a healthy lung, CD74 has been described on macrophages and type II pneumocytes, but upon activation CD74 is upregulated in both epithelial and endothelial cells (14, 85, 124, 132). The intracellular domain of CD74 is phosphorylated upon MIF engagement, but it lacks canonical signaling motifs and recruits its coreceptor CD44 to initiate a Src kinase-dependent signaling cascade (129). Different downstream signaling pathways have been demonstrated in different cell types. MIF induces ERK1/2 and PI3K-AKT signaling and results in NF-κB-mediated transcription and activation of the arachidonic acid cascade. In addition to mediating MIF's involvement in host immunity and inflammation, these pathways are also implicated in MIF's role in cell survival and angiogenesis (79, 80, 93).
MIF promotes cellular growth and survival via multiple pathways. Critical to MIF's function is its central ability to protect cells from activation-induced apoptosis (24, 38, 47, 99, 120). The mechanism by which MIF suppresses p53 activation has been described to occur via multiple pathways. These include direct protein-protein interactions and CD74-mediated AKT activation (64, 65, 80). MIF has also been shown to mitigate p53-mediated apoptosis via its binding to c-Jun activation domain-binding protein-1 (JAB1) to modulate AP-1-mediated transcription factors (68, 104). JAB1 is a key component of the COP9 signalasome and the MIF-JAB1 interaction has been implicated in the regulation of integrins, cell-cycle regulatory proteins, and apoptosis (79, 103, 107). MIF can activate other signaling pathways that have been implicated in cell survival. Treatment of cardiomyocytes with exogenous MIF results in the phosphorylation of AMPK in a CD74-dependent manner (95). Paradoxically, MIF is a negative regulator of the AMPK signaling pathway in transformed human non-small cell lung cancer cell lines (19). Similarly, MIF mediates JNK and NO signaling pathways in a context-dependent manner (78, 113). In B cells, MIF initiates a specialized pathway of regulated intramembranous cleavage of CD74 to generate an intracytoplasmic CD74 peptide that activates RelA-dependent transcription (131).
There are other consequences of MIF signaling that are likely important in respiratory biology. MIF inhibits the expression of cellular senescence genes (106, 152). Cellular senescence is a hallmark of aging and is characterized by cell cycle arrest, either from repetitive cell division or from accumulated damage from oxidative stress (27, 116). Cellular senescence occurs in aging-related lung disease such as idiopathic pulmonary fibrosis, lung cancer, and COPD (22, 97). MIF inhibits the expression of cyclin-dependent kinase inhibitors including p21 and p16 (84, 106, 109, 123, 152). The pathological manifestations and clinical consequences of MIF's role in cellular senescence have not been thoroughly defined. MIF also has an important role in angiogenesis. Hypoxia is a potent inducer of angiogenesis via activation of hypoxia inducible factor (HIF-1) family of transcription factors. MIF and vascular endothelial growth factor (VEGF) are among its many target genes that promote angiogenesis via binding to their HIF1 promoter binding sites (12, 137). Reciprocally, MIF can help stabilize HIF-1α and enhance its transcription (55, 82, 155). MIF chemotactic function has been implicated in atherosclerotic plaque formation and the recruitment of cells important for neovascularization, such as endothelial progenitor cells (54, 75, 125, 130). Finally, MIF functions as a chemotactant for neutrophils, monocytes/macrophages, and lymphocytes to sites of injury. Although MIF does not possess the NH2-terminal cysteine motif of classically defined chemokines, it does possess a pseudo-(E)LR motif (Asp44-X-Arg11) that imparts its chemokine-like function by activation of the receptors CXCR2 and CXCR4 (17). These receptors can form heteromeric complexes with CD74. Notably, the other member of the MIF superfamily, D-DT, lacks this domain and has an attenuated ability to recruit neutrophils (69, 151).
MIF in Animal Models and Human Studies of Age-Related Lung Disease
MIF in respiratory infection, sepsis, and ARDS.
Respiratory infections are a leading cause of death in the elderly. Individuals older than 65 demonstrate an increased incidence of pneumonia compared with younger adults, and the highest incidence of pneumonia is among individuals >80 years of age (50). MIF is an important component of the antimicrobial response to infection. MIF is secreted into the alveolar space as a consequence of diverse pathological microorganisms and mediates inflammation and host defense. In certain contexts, this response may be maladaptive. Increased MIF is associated with increased pathogenicity of Pseudomonas pneumonia, a phenomenon that is supported by genetic studies in patients with cystic fibrosis (3). Similarly, MIF is increased in patients with Burkholderia pseudomallei and neutralization of MIF improves bacterial clearance of this pathogen in animal models of this disease (154). In sepsis, a systemic consequence of severe infection, intracellular MIF is significantly increased in immune cells and circulating concentrations of MIF correlate with clinical severity (7, 16, 26, 41). Similar associations have been demonstrated in ARDS (38, 51, 72). Mif-knockout mice or mice treated with systemic MIF inhibitors are protected against lethal doses of LPS or gram-positive exotoxins. Neutralization of MIF or Mif deficiency is associated with improved outcomes in multiple murine models of sepsis (26, 49, 118, 121).
However, the role of MIF in respiratory infection and sepsis is context dependent. Genetic variations consistent with low MIF expression are associated with increased risk for community-acquired pneumonia and recent studies have demonstrated that Mif-knockout mice have increased Streptococcus pneumoniae nasal carriage and decreased clearance of these bacteria (34, 158). MIF is critical for the transcription of the pattern recognition receptor dectin-1, which is important for mediating clearance of Mycobacterium tuberculosis (33). Mif knockout mice have impaired killing of gram-negative bacteria by macrophages and increased susceptibility to Klebsiella pneumonia (119). Similarly, there was significant enrichment of the low-expressing MIF allele among older individuals with gram-negative sepsis compared with healthy controls (35). It also has been shown that Mif-knockout mice have an impaired ability to clear secondary bacterial infections (111), and in a hyperoxic murine model of acute lung injury, both Mif- and Cd74-knockout mice have increased disease severity (124). Ultimately, MIF secretion in response to infection may have pathogenic consequences of overwhelming cytokine release and inflammation, but it likely plays an important role in routine clearance of potentially pathogenic organisms that are frequently involved in pneumonia in the elderly.
MIF and chronic obstructive pulmonary disease.
COPD is the third leading cause of death in the United States and occurs predominately among individuals greater than 45 years of age (112, 140). COPD is associated with an increase in cellular senescence, DNA damage, and oxidative stress (5, 144, 157, 161). Four studies have now evaluated circulating concentrations of MIF in COPD and have produced substantially similar findings, albeit with some nuanced differences. One study demonstrated that those with very severe disease, when compared with “never smokers,” had decreased levels of circulating MIF (42). Another study comparing never smokers, smokers without COPD, and smokers with COPD demonstrated that whereas plasma MIF was increased in “healthy” smokers compared with never smokers, plasma MIF was decreased in those with COPD compared with smokers without COPD (123). A third study suggested that peripheral blood mononuclear gene expression of MIF inversely correlated with disease severity as measured by percent emphysema and spirometric evidence of airway obstruction (9). Finally, a recent study, and the largest of its kind, has shown that, although MIF was increased in moderate disease (GOLD II) compared with healthy never, current, and former smokers, there was a significant decrease in circulating MIF among patients with severe and very severe disease (GOLD III, IV) (61). Overall these studies suggest that although MIF may be increased among healthy smokers or smokers with moderate disease, circulating levels of MIF fall as the disease progresses. Interestingly, such findings have been recapitulated in experimental animal models. Although 3 mo of cigarette smoke exposure in mice led to increased concentrations of MIF in the bronchoalveolar lavage (BAL) fluid, 6 mo of exposure, a time course consistent with the development of COPD in animal models, resulted in a decrease in total protein and BAL concentrations of MIF (123).
Histone deacetylase 2 (HDAC-2) function is diminished in COPD and potentially may account for decreased MIF gene expression in this disease (62, 81). Mif- and Cd74-knockout mice both develop spontaneous emphysema (123). Similarly, Mif-knockout mice demonstrate increased air space enlargement and apoptosis following exposure to chronic cigarette smoke (32, 42, 123). There are multiple MIF-related signaling factors that may contribute to this phenomenon. MIF may promote lung activation of NRF-2, which is a critical antioxidant transcription factor that is decreased in the lungs with age and in COPD (87). Since oxidative stress is a critical mediator of COPD, the blunting of antioxidants as a consequence of MIF deficiency may accelerate the progression of COPD. The BAL of Mif-knockout mice demonstrate increased markers of oxidative stress with age. Additionally, both the p16-RB and p53-21 cellular senescence pathways are suppressed by MIF and the lungs of Mif-knockout mice demonstrate increased markers of both cell senescence pathways with age (123). As cellular senescence is associated with the secretion of proinflammatory cytokines, this cellular senescence associated phenotype may contribute to ongoing inflammation and air space enlargement in the lungs of patients with COPD (59, 71, 143, 144, 148). Finally, diminished VEGF and vasculogenesis have been implicated in the pathogenesis of COPD (138, 147), and Mif-knockout mice show decreased VEGF signaling in the lung in response to oxidative stress (134).
It should be noted that an opposite relationship exists between MIF expression and clinical outcomes in asthma. In asthma, a low-expressing MIF genotype is associated with milder disease. Similarly, in the ovalbumin model of asthma, Mif-knockout mice show decreased airway hyperresponsiveness compared with control mice (100, 122). Because there is an overlap between COPD and asthma, it is interesting to consider the possibility that elevated MIF expression contributes to a certain proinflammatory airway subtype of COPD whereas decreased MIF expression contributes to cellular senescence, apoptosis, and vascular attrition, which are all considered to be part of the pathology of COPD.
MIF and non-small cell lung cancer.
Similar to other chronic respiratory diseases, the incidence of lung cancer increases with age. MIF's biological activity may contribute to the inflammatory pathogenesis of cancers by multiple mechanisms. MIF induces sustained ERK1/2 activation, which mimics oncogenic mutations in Ras, contributes to tumor growth and invasiveness, and upregulates VEGF leading to neovascularization. MIF can simulate mutations in two important tumor suppressors by suppressing p53-mediated growth arrest and apoptosis and disrupting the Rb-E2F signaling pathway (21, 30, 60, 99, 109). MIF also can inhibit T cell cytolytic responses (1). There is increased immunohistochemical staining of MIF and CD74 in most patients with non-small cell lung cancer, and MIF may even represent an early biomarker of disease (76, 88). Increased circulating MIF is associated with poorer outcomes and with increased circulating levels of angiocrine factors (67, 141, 153). No associations between MIF genotypes and lung malignancy have been reported to date, but in other cancers high-expressing MIF polymorphisms have been linked with incidence and/or invasiveness of disease (43, 94). Longevity studies in Mif-knockout mice demonstrated a marked increase in malignancies (with the notable exception of a marked decrease of hemangiosarcomas). These studies, together with data from experimental tumor models, suggest that anti-MIF therapy may be beneficial for the treatment of malignancies, and cancer is the first clinical indication for which humanized anti-MIF and anti-CD74 are being evaluated (52). However, critical unanswered questions remain. Does MIF contribute to a tumor-permissive microenvironment that allows the growth and replication of precancerous cells or does MIF's biological activity contribute to de novo mutations in the cellular genome? Investigations into these pathways suggest that MIF's role in the cell cycle is complex. A study comparing spontaneous mutations in p53-null mice with p53/Mif double-knockout mice demonstrated a shift in the spectrum of tumors with the single mutation p53-null mice demonstrating a decrease in T cell tumors but an increase in B cell lymphomas, hemangiosarcomas, and carcinomas compared with the double-knockout mice (103). MIF has been demonstrated to regulate cyclin-dependent kinases and E2F transcription binding via both JAB-1 and CD74 pathways that promote transition through various stages of the cell cycle and cell growth (46). In addition to being critical regulators of the cell cycle, cyclin-dependent kinases and E2F transcription factors play an important role in the DNA damage responses and can promote DNA repair in response to injury. Interestingly, we and others have identified increased markers of DNA damage in Mif-knockout mice suggestive of defective DNA repair (42, 124). This complex relationship between immune function, cell cycle regulation, DNA repair, and cancer requires further investigation.
MIF and lung fibrosis.
Lung fibrosis is associated with numerous hallmarks of aging including telomere shortening, oxidative stress, and aberrant extracellular matrix deposition (73). MIF is increased in the BAL of patients with idiopathic pulmonary fibrosis (IPF) (10, 83). Immunohistochemical analysis of lung tissue of patients with IPF demonstrated increased MIF in the epithelium and fibroblastic foci. The measurement of MIF in the BAL of mice following treatment with the fibrogenic agent bleomycin similarly demonstrated an increase in Mif expression. In one murine study, although treatment with anti-MIF antibody mitigated the acute effects of bleomycin-induced lung injury, there was no difference in hydroxyproline content or histopathological lung fibrosis scoring (139). Radiation-induced lung injury can often lead to pulmonary fibrosis in patients being treated for cancer (87). Aged, but not young, Mif-knockout mice appear to be sensitive to radiation-induced lung injury (87). This finding correlated with decreased antioxidant production in these Mif-knockout mice. A recent study has demonstrated that Mif-knockout mice were protected against hepatic fibrosis in multiple models of chronic liver injury and suggested the importance of MIF-CD74-AMPK signaling in mitigating PDGF activation of hepatic stellate cells (58). These data collectively suggest that the MIF signaling axis may be important in lung fibrosis, but further work is necessary to determine the exact role of MIF in the pathogenesis of fibrotic lung disease.
MIF and Longevity
Growing evidence suggests a role for MIF in aging and age-related lung diseases. In multiple aging rodent models, MIF secretion diminishes with age, including the lung (87, 123). Simultaneously, MIF was shown to be elevated in certain long-lived mouse breeds and following caloric restriction (96). Human studies have not shown a decline in MIF with age. However, in the setting of sepsis, individuals older than 65 with the low-expressing −794 CATT5 allele demonstrate diminished secretion of MIF (35). One study suggested that Mif-knockout mice live longer than control mice. This association persisted in calorically restricted mice as well (57). With the caveat that this study was performed in mice of mixed genetic backgrounds, it highlights the complex nature and important interaction between MIF and aging biology. Recent in vitro studies have also highlighted MIF's role in the modulation of glucose and fat metabolism, mitochondrial respiration, stem cell survival, ubiquitin-proteosome signaling, redox balance, cell cycle regulation, autophagy, and cellular senescence (13, 40, 46, 105, 115, 142, 156).
Therapeutic Opportunities
Beyond supportive observations in experimental models of disease, accruing data from human studies suggest a therapeutic rationale for a MIF-based treatment approach to age-related lung diseases. Pharmacological development in this area is advancing and has been facilitated by unique features of the MIF-CD74 interaction (126) and the recent discovery, using structure-based molecular design, of not only MIF antagonists but small molecule agonists that act to increase MIF's affinity and activation of CD74 (63). Whereas MIF antagonists may be utilized for those indications where excessive MIF production is a clinical feature, small molecule agonists may offer utility in chronic or age-related MIF deficiency (150). Ultimately, such therapies could provide precision-based medical approaches to age-related lung diseases in which genetic or acquired deficiencies in MIF expression are a pathogenic feature.
Conclusion
The involvement of MIF in the pathobiology of diverse age-related lung diseases suggests an important role for this protein in the maintenance of respiratory homeostasis. As summarized in Table 1, both human studies and animal models implicate MIF as a fundamental response to disease. Future studies will need to address which in vitro and murine models are the most relevant for human diseases and whether manipulation of the MIF signaling axis can be used as a therapeutic modality. The study of MIF also highlights the complex interaction between innate immunity and age-related lung biology. On one hand, homeostatic MIF expression promotes pathogenic clearance, antioxidant signaling, and DNA repair in the lung. However, excess MIF contributes to dysregulated inflammation in syndromes such as ARDS and sepsis. Furthermore, MIF may promote cellular growth, wound healing, and DNA repair at the cost of promoting cellular replication in cells with tumor potential. These complex tradeoffs may underscore the complexity of aging biology. Determining the role of innate immunity in age-related disease may help unravel subtypes of lung diseases that are in need of further refinement, such as COPD; this may lead to new and more precision-oriented approaches to disease prevention and therapy. As we acquire a closer understanding of the immunological changes that occur with aging, fundamental insights into the increased susceptibility of the elderly to lung disease and new opportunities for therapy will ensue.
Table 1.
Findings from both human studies and animal models of age-related lung disease
| Human Studies | Animal Studies | |
|---|---|---|
| Pneumonia | Low-expressing MIF allele is associated with increased mortality from CAP (158) | Anti-MIF antibody reduces cytokine production and increases survival in a mouse model of S. pneumoniae-induced pneumonia (25) |
| Low-expressing MIF allele is associated with decreased Pseudomonas colonization and milder disease in cystic fibrosis (3) | MIF promotes clearance of pneumococcal colonization (34) | |
| Acute respiratory distress syndrome | MIF is increased in the blood and BAL of ARDS and is associated with outcome (15, 38) | Mif-knockout mice are protected from ARDS/severe sepsis in various models including LPS, TSST, and Escherichia coli infection (7, 16, 26, 41, 72) |
| MIF expression is increased in endothelial cells and macrophages of patients with ARDS (72) | ||
| MIF neutralizations decreases TNF and IL-8 secretion in cultured alveolar macrophages from patients with ARDS (38) | Mif-knockout mice are susceptible to hyperoxic lung injury (124, 133, 134) | |
| Polymorphisms in the MIF gene are associated with outcomes in ARDS (51) | ||
| Chronic obstructive lung disease | MIF is increased in healthy smokers OR in moderate COPD (GOLD stage II) compared to control (61, 123) | Mif-knockout and Cd74-knockout mice are susceptible to cigarette smoke and the development of spontaneous emphysema (42, 123) |
| PBMC gene expression of MIF is directly associated with FEV1 percent predicted (9) | MIF is increased in the BAL of mice following short-term cigarette smoke (123) | |
| MIF is decreased in patients with severe COPD (GOLD III/IV) compared to milder disease (42, 61, 123) | MIF is decreased in the BAL and tissue of mice following long-term cigarette smoke (42, 123) | |
| Non-small cell lung cancer | MIF and CD74 are increased in NSCL cancer tissue compared to normal lung tissue (76, 88) | MIF promotes tumor cell growth in the context of lung injury (6) |
| MIF is associated with vessel density and risk of reoccurrence in lung cancer (67, 141, 153) | ||
| Idiopathic pulmonary fibrosis | MIF is increased in the BAL of patients with IPF (10, 83) | Aged Mif-knockout mice are susceptible to radiation-induced lung injury (87) |
| MIF is increased in epithelial cells and fibroblastic foci of patients with IPF (10) |
ARDS, acute respiratory distress syndrome; BAL, bronchoalveolar lavage; CAP, community-acquired pneumonia; COPD, chronic obstructive lung disease; FEV1, forced expiratory volume in 1 s; GOLD, Global Initiative for Obstructive Lung Disease; IL-8, interleukin-8; IPF, idiopathic pulmonary fibrosis; MIF, macrophage migration inhibitory factor; PBMC, peripheral blood mononuclear cells; SNP, single nucleotide polymorphism; TNF, tumor necrosis factor.
GRANTS
This research was funded by FAMRI CIA (Patty J. Lee 82384) and the National Institutes of Health (Richard Bucala A1042310).
DISCLOSURES
R. Bucala is listed as coinventor on a Yale University patent application describing the potential utility of MIF agonists and antagonists.
AUTHOR CONTRIBUTIONS
M.S. and P.J.L. conception and design of research; M.S. drafted manuscript; M.S., R.B., and P.J.L. edited and revised manuscript; M.S., R.B., and P.J.L. approved final version of manuscript.
REFERENCES
- 1.Abe R, Peng T, Sailors J, Bucala R, Metz CN. Regulation of the CTL response by macrophage migration inhibitory factor. J Immunol 166: 747–753, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Abe R, Shimizu T, Ohkawara A, Nishihira J. Enhancement of macrophage migration inhibitory factor (MIF) expression in injured epidermis and cultured fibroblasts. Biochim Biophys Acta 1500: 1–9, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Adamali H, Armstrong ME, McLaughlin AM, Cooke G, McKone E, Costello CM, Gallagher CG, Leng L, Baugh JA, Fingerle-Rowson G, Bucala RJ, McLoughlin P, Donnelly SC. Macrophage migration inhibitory factor enzymatic activity, lung inflammation, and cystic fibrosis. Am J Respir Crit Care Med 186: 162–169, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aeberli D, Yang Y, Mansell A, Santos L, Leech M, Morand EF. Endogenous macrophage migration inhibitory factor modulates glucocorticoid sensitivity in macrophages via effects on MAP kinase phosphatase-1 and p38 MAP kinase. FEBS Lett 580: 974–981, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Aoshiba K, Zhou F, Tsuji T, Nagai A. DNA damage as a molecular link in the pathogenesis of COPD in smokers. Eur Respir J 39: 1368–1376, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Arenberg D, Luckhardt TR, Carskadon S, Zhao L, Amin MA, Koch AE. Macrophage migration inhibitory factor promotes tumor growth in the context of lung injury and repair. Am J Respir Crit Care Med 182: 1030–1037, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacher M, Meinhardt A, Lan HY, Mu W, Metz CN, Chesney JA, Calandra T, Gemsa D, Donnelly T, Atkins RC, Bucala R. Migration inhibitory factor expression in experimentally induced endotoxemia. Am J Pathol 150: 235–246, 1997. [PMC free article] [PubMed] [Google Scholar]
- 8.Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci USA 93: 7849–7854, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahr TM, Hughes GJ, Armstrong M, Reisdorph R, Coldren CD, Edwards MG, Schnell C, Kedl R, LaFlamme DJ, Reisdorph N, Kechris KJ, Bowler RP. Peripheral blood mononuclear cell gene expression in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 49: 316–323, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bargagli E, Olivieri C, Nikiforakis N, Cintorino M, Magi B, Perari MG, Vagaggini C, Spina D, Prasse A, Rottoli P. Analysis of macrophage migration inhibitory factor (MIF) in patients with idiopathic pulmonary fibrosis. Respir Physiol Neurobiol 167: 261–267, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Baugh JA, Chitnis S, Donnelly SC, Monteiro J, Lin X, Plant BJ, Wolfe F, Gregersen PK, Bucala R. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun 3: 170–176, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Baugh JA, Gantier M, Li L, Byrne A, Buckley A, Donnelly SC. Dual regulation of macrophage migration inhibitory factor (MIF) expression in hypoxia by CREB and HIF-1. Biochem Biophys Res Commun 347: 895–903, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Baumann R, Casaulta C, Simon D, Conus S, Yousefi S, Simon HU. Macrophage migration inhibitory factor delays apoptosis in neutrophils by inhibiting the mitochondria-dependent death pathway. FASEB J 17: 2221–2230, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Becker-Herman S, Arie G, Medvedovsky H, Kerem A, Shachar I. CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol Biol Cell 16: 5061–5069, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beishuizen A, Thijs LG, Haanen C, Vermes I. Macrophage migration inhibitory factor and hypothalamo-pituitary-adrenal function during critical illness. J Clin Endocrinol Metab 86: 2811–2816, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, Manogue KR, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 365: 756–759, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 13: 587–596, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Blocki FA, Ellis LB, Wackett LP. MIF protein are theta-class glutathione S-transferase homologs. Protein Sci 2: 2095–2102, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brock SE, Rendon BE, Yaddanapudi K, Mitchell RA. Negative regulation of AMP-activated protein kinase (AMPK) activity by macrophage migration inhibitory factor (MIF) family members in non-small cell lung carcinomas. J Biol Chem 287: 37917–37925, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucala R. MIF, MIF alleles, and prospects for therapeutic intervention in autoimmunity. J Clin Immunol 33, Suppl 1: S72–S78, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucala R, Donnelly SC. Macrophage migration inhibitory factor: a probable link between inflammation and cancer. Immunity 26: 281–285, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM, Bardeesy N, Castrillon DH, Beach DH, Sharpless NE. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 152: 340–351, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377: 68–71, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Mannel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med 6: 164–170, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 3: 791–800, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calandra T, Spiegel LA, Metz CN, Bucala R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc Natl Acad Sci USA 95: 11383–11388, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol 75: 685–705, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatzinikolaou G, Karakasilioti I, Garinis GA. DNA damage and innate immunity: links and trade-offs. Trends Immunol 35: 429–435, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med 5: 181–191, 1999. [PMC free article] [PubMed] [Google Scholar]
- 30.Conroy H, Mawhinney L, Donnelly SC. Inflammation and cancer: macrophage migration inhibitory factor (MIF)—the potential missing link. QJM 103: 831–836, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crichlow GV, Lubetsky JB, Leng L, Bucala R, Lolis EJ. Structural and kinetic analyses of macrophage migration inhibitory factor active site interactions. Biochemistry 48: 132–139, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damico R, Simms T, Kim BS, Tekeste Z, Amankwan H, Damarla M, Hassoun PM. p53 mediates cigarette smoke-induced apoptosis of pulmonary endothelial cells: inhibitory effects of macrophage migration inhibitor factor. Am J Respir Cell Mol Biol 44: 323–332, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das R, Koo MS, Kim BH, Jacob ST, Subbian S, Yao J, Leng L, Levy R, Murchison C, Burman WJ, Moore CC, Scheld WM, David JR, Kaplan G, MacMicking JD, Bucala R. Macrophage migration inhibitory factor (MIF) is a critical mediator of the innate immune response to Mycobacterium tuberculosis. Proc Natl Acad Sci USA 110: E2997–E3006, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das R, LaRose MI, Hergott CB, Leng L, Bucala R, Weiser JN. Macrophage migration inhibitory factor promotes clearance of pneumococcal colonization. J Immunol 193: 764–772, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das R, Subrahmanyan L, Yang IV, van Duin D, Levy R, Piecychna M, Leng L, Montgomery RR, Shaw A, Schwartz DA, Bucala R. Functional polymorphisms in the gene encoding macrophage migration inhibitory factor are associated with Gram-negative bacteremia in older adults. J Infect Dis 209: 764–768, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daun JM, Cannon JG. Macrophage migration inhibitory factor antagonizes hydrocortisone-induced increases in cytosolic IκBα. Am J Physiol Regul Integr Comp Physiol 279: R1043–R1049, 2000. [DOI] [PubMed] [Google Scholar]
- 37.David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci USA 56: 72–77, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donnelly SC, Haslett C, Reid PT, Grant IS, Wallace WA, Metz CN, Bruce LJ, Bucala R. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med 3: 320–323, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Dorshkind K, Swain S. Age-associated declines in immune system development and function: causes, consequences, and reversal. Curr Opin Immunol 21: 404–407, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Bounkari O, Bernhagen J. MIF and autophagy: a novel link beyond “eating.” Cell Res 22: 950–953, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emonts M, Sweep FC, Grebenchtchikov N, Geurts-Moespot A, Knaup M, Chanson AL, Erard V, Renner P, Hermans PW, Hazelzet JA, Calandra T. Association between high levels of blood macrophage migration inhibitory factor, inappropriate adrenal response, and early death in patients with severe sepsis. Clin Infect Dis 44: 1321–1328, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Fallica J, Boyer L, Kim B, Serebreni L, Varela L, Hamdan O, Wang L, Simms T, Damarla M, Kolb TM, Bucala R, Mitzner W, Hassoun PM, Damico R. Macrophage migration inhibitory factor is a novel determinant of cigarette smoke-induced lung damage. Am J Respir Cell Mol Biol 51: 94–103, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fersching DM, Nagel D, Siegele B, Salat C, Heinemann V, Holdenrieder S, Stoetzer OJ. Apoptosis-related biomarkers sFAS, MIF, ICAM-1 and PAI-1 in serum of breast cancer patients undergoing neoadjuvant chemotherapy. Anticancer Res 32: 2047–2058, 2012. [PubMed] [Google Scholar]
- 44.Fingerle-Rowson G, Kaleswarapu DR, Schlander C, Kabgani N, Brocks T, Reinart N, Busch R, Schutz A, Lue H, Du X, Liu A, Xiong H, Chen Y, Nemajerova A, Hallek M, Bernhagen J, Leng L, Bucala R. A tautomerase-null macrophage migration-inhibitory factor (MIF) gene knock-in mouse model reveals that protein interactions and not enzymatic activity mediate MIF-dependent growth regulation. Mol Cell Biol 29: 1922–1932, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fingerle-Rowson G, Koch P, Bikoff R, Lin X, Metz CN, Dhabhar FS, Meinhardt A, Bucala R. Regulation of macrophage migration inhibitory factor expression by glucocorticoids in vivo. Am J Pathol 162: 47–56, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fingerle-Rowson G, Petrenko O. MIF coordinates the cell cycle with DNA damage checkpoints. Lessons from knockout mouse models. Cell Div 2: 22, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fingerle-Rowson G, Petrenko O, Metz CN, Forsthuber TG, Mitchell R, Huss R, Moll U, Muller W, Bucala R. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc Natl Acad Sci USA 100: 9354–9359, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flieger O, Engling A, Bucala R, Lue H, Nickel W, Bernhagen J. Regulated secretion of macrophage migration inhibitory factor is mediated by a non-classical pathway involving an ABC transporter. FEBS Lett 551: 78–86, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Froidevaux C, Roger T, Martin C, Glauser MP, Calandra T. Macrophage migration inhibitory factor and innate immune responses to bacterial infections. Crit Care Med 29: S13–S15, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA 294: 2712–2719, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Gao L, Flores C, Fan-Ma S, Miller EJ, Moitra J, Moreno L, Wadgaonkar R, Simon B, Brower R, Sevransky J, Tuder RM, Maloney JP, Moss M, Shanholtz C, Yates CR, Meduri GU, Ye SQ, Barnes KC, Garcia JG. Macrophage migration inhibitory factor in acute lung injury: expression, biomarker, and associations. Transl Res 150: 18–29, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Govindan SV, Cardillo TM, Sharkey RM, Tat F, Gold DV, Goldenberg DM. Milatuzumab-SN-38 conjugates for the treatment of CD74+ cancers. Mol Cancer Ther 12: 968–978, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Gregersen PK, Bucala R. Macrophage migration inhibitory factor, MIF alleles, and the genetics of inflammatory disorders: incorporating disease outcome into the definition of phenotype. Arthritis Rheum 48: 1171–1176, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Grieb G, Piatkowski A, Simons D, Hormann N, Dewor M, Steffens G, Bernhagen J, Pallua N. Macrophage migration inhibitory factor is a potential inducer of endothelial progenitor cell mobilization after flap operation. Surgery 151: 268–277.e1, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Hagemann T, Robinson SC, Thompson RG, Charles K, Kulbe H, Balkwill FR. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Cancer Ther 6: 1993–2002, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Haigis MC, Yankner BA. The aging stress response. Mol Cell 40: 333–344, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harper JM, Wilkinson JE, Miller RA. Macrophage migration inhibitory factor-knockout mice are long lived and respond to caloric restriction. FASEB J 24: 2436–2442, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heinrichs D, Knauel M, Offermanns C, Berres ML, Nellen A, Leng L, Schmitz P, Bucala R, Trautwein C, Weber C, Bernhagen J, Wasmuth HE. Macrophage migration inhibitory factor (MIF) exerts antifibrotic effects in experimental liver fibrosis via CD74. Proc Natl Acad Sci USA 108: 17444–17449, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14: 501–513, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med 190: 1375–1382, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Husebo GR, Bakke PS, Hardie JA, Ueland T, Gronseth R, Aukrust P, Eagan TM. Macrophage migration inhibitory factor (MIF), a biomarker in COPD? Eur Respir J 44: 1484, 2014. [Google Scholar]
- 62.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, Barnes PJ. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 352: 1967–1976, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Jorgensen WL, Gandavadi S, Du X, Hare AA, Trofimov A, Leng L, Bucala R. Receptor agonists of macrophage migration inhibitory factor. Bioorg Med Chem Lett 20: 7033–7036, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jung H, Seong HA, Ha H. Critical role of cysteine residue 81 of macrophage migration inhibitory factor (MIF) in MIF-induced inhibition of p53 activity. J Biol Chem 283: 20383–20396, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Jung H, Seong HA, Ha H. Direct interaction between NM23–H1 and macrophage migration inhibitory factor (MIF) is critical for alleviation of MIF-mediated suppression of p53 activity. J Biol Chem 283: 32669–32679, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Kabelitz D, Medzhitov R. Innate immunity—cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr Opin Immunol 19: 1–3, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Kamimura A, Kamachi M, Nishihira J, Ogura S, Isobe H, Dosaka-Akita H, Ogata A, Shindoh M, Ohbuchi T, Kawakami Y. Intracellular distribution of macrophage migration inhibitory factor predicts the prognosis of patients with adenocarcinoma of the lung. Cancer 89: 334–341, 2000. [PubMed] [Google Scholar]
- 68.Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, Johannes FJ, Roger T, Calandra T, Kapurniotu A, Grell M, Finkelmeier D, Brunner H, Bernhagen J. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature 408: 211–216, 2000. [DOI] [PubMed] [Google Scholar]
- 69.Kleemann R, Kapurniotu A, Frank RW, Gessner A, Mischke R, Flieger O, Juttner S, Brunner H, Bernhagen J. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J Mol Biol 280: 85–102, 1998. [DOI] [PubMed] [Google Scholar]
- 70.Koff JL, Shao MX, Ueki IF, Nadel JA. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am J Physiol Lung Cell Mol Physiol 294: L1068–L1075, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Kumar M, Seeger W, Voswinckel R. Senescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 51: 323–333, 2014. [DOI] [PubMed] [Google Scholar]
- 72.Lai KN, Leung JC, Metz CN, Lai FM, Bucala R, Lan HY. Role for macrophage migration inhibitory factor in acute respiratory distress syndrome. J Pathol 199: 496–508, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Lee J, Reddy R, Barsky L, Scholes J, Chen H, Shi W, Driscoll B. Lung alveolar integrity is compromised by telomere shortening in telomerase-null mice. Am J Physiol Lung Cell Mol Physiol 296: L57–L70, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF signal transduction initiated by binding to CD74. J Exp Med 197: 1467–1476, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liehn EA, Kanzler I, Konschalla S, Kroh A, Simsekyilmaz S, Sonmez TT, Bucala R, Bernhagen J, Weber C. Compartmentalized protective and detrimental effects of endogenous macrophage migration-inhibitory factor mediated by CXCR2 in a mouse model of myocardial ischemia/reperfusion. Arterioscler Thromb Vasc Biol 33: 2180–2186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Q, Yang H, Zhang SF. [Expression and significance of MIF and CD147 in non-small cell lung cancer]. Sichuan Da Xue Xue Bao Yi Xue Ban 41: 85–90, 2010. [PubMed] [Google Scholar]
- 77.Lolis E, Bucala R. Macrophage migration inhibitory factor. Expert Opin Ther Targets 7: 153–164, 2003. [DOI] [PubMed] [Google Scholar]
- 78.Lue H, Dewor M, Leng L, Bucala R, Bernhagen J. Activation of the JNK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on CXCR4 and CD74. Cell Signal 23: 135–144, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lue H, Kapurniotu A, Fingerle-Rowson G, Roger T, Leng L, Thiele M, Calandra T, Bucala R, Bernhagen J. Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell Signal 18: 688–703, 2006. [DOI] [PubMed] [Google Scholar]
- 80.Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Luscher B, Bernhagen J. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene 26: 5046–5059, 2007. [DOI] [PubMed] [Google Scholar]
- 81.Lugrin J, Ding XC, Le Roy D, Chanson AL, Sweep FC, Calandra T, Roger T. Histone deacetylase inhibitors repress macrophage migration inhibitory factor (MIF) expression by targeting MIF gene transcription through a local chromatin deacetylation. Biochim Biophys Acta 1793: 1749–1758, 2009. [DOI] [PubMed] [Google Scholar]
- 82.Ma H, Wang J, Thomas DP, Tong C, Leng L, Wang W, Merk M, Zierow S, Bernhagen J, Ren J, Bucala R, Li J. Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation 122: 282–292, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Magi B, Bini L, Perari MG, Fossi A, Sanchez JC, Hochstrasser D, Paesano S, Raggiaschi R, Santucci A, Pallini V, Rottoli P. Bronchoalveolar lavage fluid protein composition in patients with sarcoidosis and idiopathic pulmonary fibrosis: a two-dimensional electrophoretic study. Electrophoresis 23: 3434–3444, 2002. [DOI] [PubMed] [Google Scholar]
- 84.Maity A, Koumenis C. HIF and MIF—a nifty way to delay senescence? Genes Dev 20: 3337–3341, 2006. [DOI] [PubMed] [Google Scholar]
- 85.Marsh LM, Cakarova L, Kwapiszewska G, von Wulffen W, Herold S, Seeger W, Lohmeyer J. Surface expression of CD74 by type II alveolar epithelial cells: a potential mechanism for macrophage migration inhibitory factor-induced epithelial repair. Am J Physiol Lung Cell Mol Physiol 296: L442–L452, 2009. [DOI] [PubMed] [Google Scholar]
- 86.Martin TR. MIF mediation of sepsis. Nat Med 6: 140–141, 2000. [DOI] [PubMed] [Google Scholar]
- 87.Mathew B, Jacobson JR, Siegler JH, Moitra J, Blasco M, Xie L, Unzueta C, Zhou T, Evenoski C, Al-Sakka M, Sharma R, Huey B, Bulent A, Smith B, Jayaraman S, Reddy NM, Reddy SP, Fingerle-Rowson G, Bucala R, Dudek SM, Natarajan V, Weichselbaum RR, Garcia JG. Role of migratory inhibition factor in age-related susceptibility to radiation lung injury via NF-E2-related factor-2 and antioxidant regulation. Am J Respir Cell Mol Biol 49: 269–278, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McClelland M, Zhao L, Carskadon S, Arenberg D. Expression of CD74, the receptor for macrophage migration inhibitory factor, in non-small cell lung cancer. Am J Pathol 174: 638–646, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med 343: 338–344, 2000. [DOI] [PubMed] [Google Scholar]
- 90.Merk M, Baugh J, Zierow S, Leng L, Pal U, Lee SJ, Ebert AD, Mizue Y, Trent JO, Mitchell R, Nickel W, Kavathas PB, Bernhagen J, Bucala R. The Golgi-associated protein p115 mediates the secretion of macrophage migration inhibitory factor. J Immunol 182: 6896–6906, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Merk M, Mitchell RA, Endres S, Bucala R. D-dopachrome tautomerase (D-DT or MIF-2): doubling the MIF cytokine family. Cytokine 59: 10–17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Merk M, Zierow S, Leng L, Das R, Du X, Schulte W, Fan J, Lue H, Chen Y, Xiong H, Chagnon F, Bernhagen J, Lolis E, Mor G, Lesur O, Bucala R. The D-dopachrome tautomerase (DDT) gene product is a cytokine and functional homolog of macrophage migration inhibitory factor (MIF). Proc Natl Acad Sci USA 108: E577–E585, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol 177: 8730–8739, 2006. [DOI] [PubMed] [Google Scholar]
- 94.Meyer-Siegler KL, Vera PL, Iczkowski KA, Bifulco C, Lee A, Gregersen PK, Leng L, Bucala R. Macrophage migration inhibitory factor (MIF) gene polymorphisms are associated with increased prostate cancer incidence. Genes Immun 8: 646–652, 2007. [DOI] [PubMed] [Google Scholar]
- 95.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 451: 578–582, 2008. [DOI] [PubMed] [Google Scholar]
- 96.Miller RA, Chang Y, Galecki AT, Al-Regaiey K, Kopchick JJ, Bartke A. Gene expression patterns in calorically restricted mice: partial overlap with long-lived mutant mice. Mol Endocrinol 16: 2657–2666, 2002. [DOI] [PubMed] [Google Scholar]
- 97.Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L391–L401, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitchell R, Bacher M, Bernhagen J, Pushkarskaya T, Seldin MF, Bucala R. Cloning and characterization of the gene for mouse macrophage migration inhibitory factor (MIF). J Immunol 154: 3863–3870, 1995. [PubMed] [Google Scholar]
- 99.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA 99: 345–350, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mizue Y, Ghani S, Leng L, McDonald C, Kong P, Baugh J, Lane SJ, Craft J, Nishihira J, Donnelly SC, Zhu Z, Bucala R. Role for macrophage migration inhibitory factor in asthma. Proc Natl Acad Sci USA 102: 14410–14415, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest 123: 958–965, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol 94: 1167–1184, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nemajerova A, Mena P, Fingerle-Rowson G, Moll UM, Petrenko O. Impaired DNA damage checkpoint response in MIF-deficient mice. EMBO J 26: 987–997, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nguyen MT, Lue H, Kleemann R, Thiele M, Tolle G, Finkelmeier D, Wagner E, Braun A, Bernhagen J. The cytokine macrophage migration inhibitory factor reduces pro-oxidative stress-induced apoptosis. J Immunol 170: 3337–3347, 2003. [DOI] [PubMed] [Google Scholar]
- 105.Ohta S, Misawa A, Fukaya R, Inoue S, Kanemura Y, Okano H, Kawakami Y, Toda M. Macrophage migration inhibitory factor (MIF) promotes cell survival and proliferation of neural stem/progenitor cells. J Cell Sci 125: 3210–3220, 2012. [DOI] [PubMed] [Google Scholar]
- 106.Palumbo S, Tsai TL, Li WJ. Macrophage migration inhibitory factor regulates AKT signaling in hypoxic culture to modulate senescence of human mesenchymal stem cells. Stem Cells Dev 23: 852–865, 2014. [DOI] [PubMed] [Google Scholar]
- 107.Pan Y, Yang H, Claret FX. Emerging roles of Jab1/CSN5 in DNA damage response, DNA repair, and cancer. Cancer Biol Ther 15: 256–262, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paralkar V, Wistow G. Cloning the human gene for macrophage migration inhibitory factor (MIF). Genomics 19: 48–51, 1994. [DOI] [PubMed] [Google Scholar]
- 109.Petrenko O, Moll UM. Macrophage migration inhibitory factor MIF interferes with the Rb-E2F pathway. Mol Cell 17: 225–236, 2005. [DOI] [PubMed] [Google Scholar]
- 110.Petrovsky N, Socha L, Silva D, Grossman AB, Metz C, Bucala R. Macrophage migration inhibitory factor exhibits a pronounced circadian rhythm relevant to its role as a glucocorticoid counter-regulator. Immunol Cell Biol 81: 137–143, 2003. [DOI] [PubMed] [Google Scholar]
- 111.Pollak N, Sterns T, Echtenacher B, Mannel DN. Improved resistance to bacterial superinfection in mice by treatment with macrophage migration inhibitory factor. Infect Immun 73: 6488–6492, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schunemann H, Wedzicha W, MacDonald R, Shekelle P; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 155: 179–191, 2011. [DOI] [PubMed] [Google Scholar]
- 113.Qi D, Hu X, Wu X, Merk M, Leng L, Bucala R, Young LH. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. J Clin Invest 119: 3807–3816, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rajasekaran D, Zierow S, Syed M, Bucala R, Bhandari V, Lolis EJ. Targeting distinct tautomerase sites of d-dt and MIF with a single molecule for inhibition of neutrophil lung recruitment. FASEB J 28: 4961–4971, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rendon BE, Willer SS, Zundel W, Mitchell RA. Mechanisms of macrophage migration inhibitory factor (MIF)-dependent tumor microenvironmental adaptation. Exp Mol Pathol 86: 180–185, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 192: 547–556, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roger T, Chanson AL, Knaup-Reymond M, Calandra T. Macrophage migration inhibitory factor promotes innate immune responses by suppressing glucocorticoid-induced expression of mitogen-activated protein kinase phosphatase-1. Eur J Immunol 35: 3405–3413, 2005. [DOI] [PubMed] [Google Scholar]
- 118.Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 414: 920–924, 2001. [DOI] [PubMed] [Google Scholar]
- 119.Roger T, Delaloye J, Chanson AL, Giddey M, Le Roy D, Calandra T. Macrophage migration inhibitory factor deficiency is associated with impaired killing of gram-negative bacteria by macrophages and increased susceptibility to Klebsiella pneumoniae sepsis. J Infect Dis 207: 331–339, 2013. [DOI] [PubMed] [Google Scholar]
- 120.Roger T, Froidevaux C, Martin C, Calandra T. Macrophage migration inhibitory factor (MIF) regulates host responses to endotoxin through modulation of Toll-like receptor 4 (TLR4). J Endotoxin Res 9: 119–123, 2003. [DOI] [PubMed] [Google Scholar]
- 121.Roger T, Glauser MP, Calandra T. Macrophage migration inhibitory factor (MIF) modulates innate immune responses induced by endotoxin and Gram-negative bacteria. J Endotoxin Res 7: 456–460, 2001. [PubMed] [Google Scholar]
- 122.Rossi AG, Haslett C, Hirani N, Greening AP, Rahman I, Metz CN, Bucala R, Donnelly SC. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role in asthma. J Clin Invest 101: 2869–2874, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sauler M, Leng L, Trentalange M, Haslip M, Shan P, Piecychna M, Zhang Y, Andrews N, Mannam P, Allore H, Fried T, Bucala R, Lee PJ. Macrophage migration inhibitory factor deficiency in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 306: L487–L496, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sauler M, Zhang Y, Min JN, Leng L, Shan P, Roberts S, Jorgensen WL, Bucala R, Lee PJ. Endothelial CD74 mediates macrophage migration inhibitory factor protection in hyperoxic lung injury. FASEB J 29: 1940–1949, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwartz V, Lue H, Kraemer S, Korbiel J, Krohn R, Ohl K, Bucala R, Weber C, Bernhagen J. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lett 583: 2749–2757, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Senter PD, Al-Abed Y, Metz CN, Benigni F, Mitchell RA, Chesney J, Han J, Gartner CG, Nelson SD, Todaro GJ, Bucala R. Inhibition of macrophage migration inhibitory factor (MIF) tautomerase and biological activities by acetaminophen metabolites. Proc Natl Acad Sci USA 99: 144–149, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol 13: 875–887, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol 22: 507–513, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shi X, Leng L, Wang T, Wang W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E, Noble P, Knudson W, Bucala R. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 25: 595–606, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Simons D, Grieb G, Hristov M, Pallua N, Weber C, Bernhagen J, Steffens G. Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment. J Cell Mol Med 15: 668–678, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Starlets D, Gore Y, Binsky I, Haran M, Harpaz N, Shvidel L, Becker-Herman S, Berrebi A, Shachar I. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood 107: 4807–4816, 2006. [DOI] [PubMed] [Google Scholar]
- 132.Stein R, Mattes MJ, Cardillo TM, Hansen HJ, Chang CH, Burton J, Govindan S, Goldenberg DM. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin Cancer Res 13: 5556s–5563s, 2007. [DOI] [PubMed] [Google Scholar]
- 133.Sun H, Choo-Wing R, Fan J, Leng L, Syed MA, Hare AA, Jorgensen WL, Bucala R, Bhandari V. Small molecular modulation of macrophage migration inhibitory factor in the hyperoxia-induced mouse model of bronchopulmonary dysplasia. Respir Res 14: 27, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun H, Choo-Wing R, Sureshbabu A, Fan J, Leng L, Yu S, Jiang D, Noble P, Homer RJ, Bucala R, Bhandari V. A critical regulatory role for macrophage migration inhibitory factor in hyperoxia-induced injury in the developing murine lung. PLoS One 8: e60560, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun HW, Bernhagen J, Bucala R, Lolis E. Crystal structure at 2.6-A resolution of human macrophage migration inhibitory factor. Proc Natl Acad Sci USA 93: 5191–5196, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takahashi M, Nishihira J, Shimpo M, Mizue Y, Ueno S, Mano H, Kobayashi E, Ikeda U, Shimada K. Macrophage migration inhibitory factor as a redox-sensitive cytokine in cardiac myocytes. Cardiovasc Res 52: 438–445, 2001. [DOI] [PubMed] [Google Scholar]
- 137.Takahashi N, Nishihira J, Sato Y, Kondo M, Ogawa H, Ohshima T, Une Y, Todo S. Involvement of macrophage migration inhibitory factor (MIF) in the mechanism of tumor cell growth. Mol Med 4: 707–714, 1998. [PMC free article] [PubMed] [Google Scholar]
- 138.Tang K, Rossiter HB, Wagner PD, Breen EC. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol 97: 1559–1566; discussion 1549, 2004. [DOI] [PubMed] [Google Scholar]
- 139.Tanino Y, Makita H, Miyamoto K, Betsuyaku T, Ohtsuka Y, Nishihira J, Nishimura M. Role of macrophage migration inhibitory factor in bleomycin-induced lung injury and fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 283: L156–L162, 2002. [DOI] [PubMed] [Google Scholar]
- 140.Thannickal VJ, Murthy M, Balch WE, Chandel NS, Meiners S, Eickelberg O, Selman M, Pardo A, White ES, Levy BD, Busse PJ, Tuder RM, Antony VB, Sznajder JI, Budinger GR. Blue journal conference. Aging and susceptibility to lung disease. Am J Respir Crit Care Med 191: 261–269, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tomiyasu M, Yoshino I, Suemitsu R, Okamoto T, Sugimachi K. Quantification of macrophage migration inhibitory factor mRNA expression in non-small cell lung cancer tissues and its clinical significance. Clin Cancer Res 8: 3755–3760, 2002. [PubMed] [Google Scholar]
- 142.Toso C, Emamaullee JA, Merani S, Shapiro AM. The role of macrophage migration inhibitory factor on glucose metabolism and diabetes. Diabetologia 51: 1937–1946, 2008. [DOI] [PubMed] [Google Scholar]
- 143.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med 174: 886–893, 2006. [DOI] [PubMed] [Google Scholar]
- 144.Tuder RM, Kern JA, Miller YE. Senescence in chronic obstructive pulmonary disease. Proc Am Thorac Soc 9: 62–63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vincent GK, Velkoff VA, Census Bureau US. The Next Four Decades: The Older Population in the United States: 2010 to 2050. Washington, DC: U. S. Dept. of Commerce, Economics and Statistics Administration, U. S. Census Bureau, 2010, p. 14. [Google Scholar]
- 146.Vivarelli M, D'Urbano LE, Insalaco A, Lunt M, Jury F, Tozzi AE, Ravelli A, Martini A, Donn R, De Benedetti F. Macrophage migration inhibitory factor (MIF) and oligoarticular juvenile idiopathic arthritis (o-JIA): association of MIF promoter polymorphisms with response to intra-articular glucocorticoids. Clin Exp Rheumatol 25: 775–781, 2007. [PubMed] [Google Scholar]
- 147.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 290: L209–L221, 2006. [DOI] [PubMed] [Google Scholar]
- 148.Volonte D, Kahkonen B, Shapiro S, Di Y, Galbiati F. Caveolin-1 expression is required for the development of pulmonary emphysema through activation of the ATM-p53-p21 pathway. J Biol Chem 284: 5462–5466, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Waeber G, Thompson N, Chautard T, Steinmann M, Nicod P, Pralong FP, Calandra T, Gaillard RC. Transcriptional activation of the macrophage migration-inhibitory factor gene by the corticotropin-releasing factor is mediated by the cyclic adenosine 3′,5′-monophosphate responsive element-binding protein CREB in pituitary cells. Mol Endocrinol 12: 698–705, 1998. [DOI] [PubMed] [Google Scholar]
- 150.Wang J, Tong C, Yan X, Yeung E, Gandavadi S, Hare AA, Du X, Chen Y, Xiong H, Ma C, Leng L, Young LH, Jorgensen WL, Li J, Bucala R. Limiting cardiac ischemic injury by pharmacological augmentation of macrophage migration inhibitory factor-AMP-activated protein kinase signal transduction. Circulation 128: 225–236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weber C, Kraemer S, Drechsler M, Lue H, Koenen RR, Kapurniotu A, Zernecke A, Bernhagen J. Structural determinants of MIF functions in CXCR2-mediated inflammatory and atherogenic leukocyte recruitment. Proc Natl Acad Sci USA 105: 16278–16283, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Welford SM, Bedogni B, Gradin K, Poellinger L, Broome Powell M, Giaccia AJ. HIF1alpha delays premature senescence through the activation of MIF. Genes Dev 20: 3366–3371, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, Orringer MB, Arenberg DA. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res 9: 853–860, 2003. [PubMed] [Google Scholar]
- 154.Wiersinga WJ, Calandra T, Kager LM, van der Windt GJ, Roger T, le Roy D, Florquin S, Peacock SJ, Sweep FC, van der Poll T. Expression and function of macrophage migration inhibitory factor (MIF) in melioidosis. PLoS Negl Trop Dis 4: e605, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Winner M, Koong AC, Rendon BE, Zundel W, Mitchell RA. Amplification of tumor hypoxic responses by macrophage migration inhibitory factor-dependent hypoxia-inducible factor stabilization. Cancer Res 67: 186–193, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu X, Ren J. Macrophage migration inhibitory factor (MIF) knockout preserves cardiac homeostasis through alleviating Akt-mediated myocardial autophagy suppression in high-fat diet-induced obesity. Int J Obes (Lond) 9: 387–396, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yao H, Rahman I. Role of histone deacetylase 2 in epigenetics and cellular senescence: implications in lung inflammaging and COPD. Am J Physiol Lung Cell Mol Physiol 303: L557–L566, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yende S, Angus DC, Kong L, Kellum JA, Weissfeld L, Ferrell R, Finegold D, Carter M, Leng L, Peng ZY, Bucala R. The influence of macrophage migration inhibitory factor gene polymorphisms on outcome from community-acquired pneumonia. FASEB J 23: 2403–2411, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest 116: 3050–3059, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao Y, Shimizu T, Nishihira J, Koyama Y, Kushibiki T, Honda A, Watanabe H, Abe R, Tabata Y, Shimizu H. Tissue regeneration using macrophage migration inhibitory factor-impregnated gelatin microbeads in cutaneous wounds. Am J Pathol 167: 1519–1529, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zuo L, He F, Sergakis GG, Koozehchian MS, Stimpfl JN, Rong Y, Diaz PT, Best TM. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am J Physiol Lung Cell Mol Physiol 307: L205–L218, 2014. [DOI] [PubMed] [Google Scholar]