Abstract
Acute lung injury (ALI) and the more severe acute respiratory distress syndrome are common responses to a variety of infectious and noninfectious insults. We used a mouse model of ALI induced by intratracheal administration of sterile bacterial wall lipopolysaccharide (LPS) to investigate the changes in innate lung microbiota and study microbial community reaction to lung inflammation and barrier dysfunction induced by endotoxin insult. One group of C57BL/6J mice received LPS via intratracheal injection (n = 6), and another received sterile water (n = 7). Bronchoalveolar lavage (BAL) was performed at 72 h after treatment. Bacterial DNA was extracted and used for qPCR and 16S rRNA gene-tag (V3–V4) sequencing (Illumina). The bacterial load in BAL from ALI mice was increased fivefold (P = 0.03). The community complexity remained unchanged (Simpson index, P = 0.7); the Shannon diversity index indicated the increase of community evenness in response to ALI (P = 0.07). Principal coordinate analysis and analysis of similarity (ANOSIM) test (P = 0.005) revealed a significant difference between microbiota of control and ALI groups. Bacteria from families Xanthomonadaceae and Brucellaceae increased their abundance in the ALI group as determined by Metastats test (P < 0.02). In concordance with the 16s-tag data, Stenotrohomonas maltophilia (Xanthomonadaceae) and Ochrobactrum anthropi (Brucellaceae) were isolated from lungs of mice from both groups. Metabolic profiling of BAL detected the presence of bacterial substrates suitable for both isolates. Additionally, microbiota from LPS-treated mice intensified IL-6-induced lung inflammation in naive mice. We conclude that the morbid transformation of ALI microbiota was attributed to the set of inborn opportunistic pathogens thriving in the environment of inflamed lung, rather than the external infectious agents.
Keywords: acute lung injury, LPS, microbiota, metabolic profiling
acute lung injury (ALI) and the more severe acute respiratory distress syndrome (ARDS) are common responses to a variety of infectious and noninfectious insults. Continuing vascular leak and uncontrolled inflammation are the major pathological events defining ventilation-perfusion mismatch, lung dysfunction, and overall severity of ALI/ARDS. Until now, few effective therapeutic approaches exist to confront this devastating illness with unacceptably high mortality rates (30–40%) (31, 39); thus studies aimed at better understanding the pathogenic processes that develop in the injured lung are keenly awaited. Current clinical strategies for ALI management, such as anti-inflammatory corticosteroids and β-agonist treatment, have failed to reduce case mortality, suggesting that an essential element of pathological process was escaping treatment (30).
Recently, the human microbiome, a vast and complex polymicrobial community that coexists with humans, has been identified as playing a significant role in altering the development and function of the host organism. Previous studies have shown that the host microbiota may program immune cell differentiation (24, 27, 34, 43, 56). Deregulation of the microbiome has been associated with altered immunity, causing human disease (25, 41). However, since the environment of the healthy lung was thought to be sterile, the pulmonary microbiome remains largely unexplored. The assumption of a sterile lung also diverted the attention of researchers from the pulmonary microbiota reaction to ALI and the potential role of innate pulmonary microbiota in ARDS. In this study we used a mouse model of ALI induced by intratracheal administration of sterile bacterial wall lipopolysaccharide (LPS) to investigate the changes in innate lung microbiota and to study microbial community reaction to lung inflammation and barrier dysfunction induced by endotoxin insult.
METHODS
Animal studies.
All animal care and treatment procedures were approved by the University of Chicago Institutional Animal Care and Use Committee. Animals were handled according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained in certified Helicobacter pylori-free facility, by using ventilated nonsterile cages upon arrival. Water and food were provided ad libitum: water via central line, food from factory-sealed bags. Bacterial LPS (0.63 mg/kg body wt; Escherichia coli O55:B5) or sterile water was injected intratracheally in a small volume (20–30 μl) via a 20-gauge catheter (Exelint International, Los Angeles, CA), as previously described (6, 7), to yield the following experimental groups: control (LPS−) (n = 8) and treatment (LPS+) (n = 8). Three days after LPS challenge, mice underwent bronchial airway lavage (BAL) of both lungs with 0.5 ml phosphate-buffered saline (PBS) and gently aspirated the fluid. The procedure was performed three times and the recovered BAL fluid was used to measure total protein according to the manufacturer's manual (BCA Protein Assay Kit; Bio-Rad). Cell pellets were examined for total cell count by using a hemocytometer and for differential cell count by cytocentrifugation and Diff-Quik staining (Dade Diagnostics, Deerfield, IL). The BAL supernatants after low-speed cytocentrifugation were further collected and used for isolation of bacterial material. For analysis of LPS-induced lung vascular leak, Evans blue dye (30 ml/kg) was injected into the external jugular vein 2 h before termination of the experiment. Measurement of Evans blue accumulation in the lung tissue was performed by spectrofluorimetric analysis of lung tissue lysates according to the protocol described previously (6, 7).
In additional experiments we tested the potential impact of microbiota generated during LPS-induced lung inflammation on IL-6-induced inflammation in naive mice. For this purpose, we collected BAL from control mice and mice after 3 days of LPS injection. After low-speed centrifugation (800 g, 5 min) to sediment cells and mucus, the remaining supernatant was subjected to high-speed centrifugation (15,000 g, 30 min) to pellet bacterial particles. After removal of supernatant, the pellets were resuspended in 50 μl of sterile saline and injected intratracheally, simultaneously with IL-6 (5 mg/kg), to naive mice. Analysis of BAL cell count and protein concentration was performed as described above.
Lung histology.
For histopathological lung analysis, separate groups of mice were treated with vehicle or LPS without further lavage. Lungs were agarose inflated in situ and fixed with 10% buffered formalin overnight, followed by embedding in paraffin. Lung microsections were used for histological evaluation by hematoxylin and eosin staining. Cellular infiltration in treated lungs was examined by light microscopy.
Metabolic profile of BAL.
BAL samples (0.5 ml), free of mucus and host cells, of animals from LPS− (n = 3) and LPS+ (n = 3) groups were evaporated at −60°C under vacuum with and derivatized with 50 μl methoxyamine hydrochloride (40 mg/ml in pyridine) for 60 min at 50°C followed by 50 μl N-methyl-N-(trimethylsilyl) trifluoroacetamide at 70°C for 120 min and 2-h incubation at room temperature. Five microliters (5 μl) of the internal standard (hentriacontanoic acid, 10 mg/ml) were added to each sample prior to derivatization. Samples were processed on a gas chromatography mass spectrometry (GC/MS) system (Agilent) consisting of an Agilent 7890 gas chromatograph, an Agilent 5975 mass selective detector, and a HP 7683B autosampler. Gas chromatography was performed on a ZB-5MS (60 m × 0.32 mm ID and 0.25-μm film thickness) capillary column (Phenomenex). The inlet and MS interface temperatures were 250°C, and the ion source temperature was adjusted to 230°C. An aliquot of 1 μl was injected with the split ratio of 7:1. The helium carrier gas was kept at a constant flow rate of 2 ml/min. The temperature program was 5-min isothermal heating at 70°C, followed by an oven temperature increase of 5°C/min to 310°C and a final 10 min at 310°C. The mass spectrometer was operated in positive electron impact mode at 69.9 eV ionization energy at m/z 30–800 scan range. The spectra of all chromatogram peaks were compared with electron impact mass spectrum libraries NIST08 (NIST), W8N08 (Palisade), and a custom-built database (460 unique metabolites). All known artificial peaks were identified and removed. To allow comparison between samples, all data were normalized to the internal standard in each chromatogram and the initial sample volume: Ni = Xi × X−1IS × initial sample volume−1. The spectra of all chromatogram peaks were evaluated by use of the AMDIS 2.71 (NIST) program. The instrument variability was 5%, which is within the standard acceptance limit. Chemometric models were obtained by using log-transformed and autoscaled data with the SIMCA-P+ (12.0.0.0) program (Umea, Sweden; www.umetrics.com/simca).
Preparation of DNA and analysis of 16S rRNA tags.
DNA was isolated from BAL collected from LPS− (n = 7) and LPS+ (n = 6) groups by use of a BiOstic Bacteremia DNA Isolation Kit (MoBio). DNA samples were used for bacterial quantification by means of qPCR assay as described in Palmer et al. (36). The same DNA samples were used for PCR amplification of the 16S rRNA gene hypervariable regions. We utilized the Illumina MiSeq platform and the set of barcoded primers (9) to generate 16S RNA-tag libraries. Sequences were processed by using the Mothur suite of programs (44, 45) according to MiSeq SOP (28). We conducted pair-end splicing and quality check, sequence chimera removal, and alpha diversity calculation for each sample. Sample distribution was visualized via principal coordinate analysis (PCoA). Metastats (55) was used to determine differentially abundant genera between experimental groups. The significance of group-related sample aggregation was assessed by analysis of similarity (ANOSIM) test.
Microbial culture isolation and characterization.
Aliquots of BAL specimens were inoculated on trypticase soy agar with 5% sheep blood (BAP) and chocolate II agar plates (CHOC) (BD Microbiology Systems, Sparks, MD) to assess the growth of aerobic and fastidious bacteria, respectively, and Brucella agar with 5% sheep blood, hemin, and vitamin K1 (BRU) and chopped meat carbohydrate broth, PR II (CMB) (BD Microbiology Systems), to assess the growth of anaerobic bacteria. A single drop of the specimen was dispensed into CMB broth and each plate. Plates were streaked in four quadrants. BAP, CHOC, and CMB were incubated in 5% CO2 atmosphere at 37°C and BRU were incubated in anaerobic conditions. Plates were analyzed for growth at 24, 48, 72, and 96 h and CMB daily for 14 days. Single colonies of distinct phenotype were selected for identification by MALDI-TOF MS Vitek-MS RUO (bioMerieux, Marcy l'Etoile, France) as previously described (10). Bacterial isolates were then characterized by using phenotype array according to manufacturer's instruction (Biolog). Plates PM1 and PM2 were used to study carbon source specificity, plate PM9 resistance to osmolytes, and plate PM10 resistance to pH. Dye A was used with Stenotrohomonas maltophilia and dye H with Ochrobactrum anthropi.
RESULTS
Analysis of LPS-induced lung dysfunction.
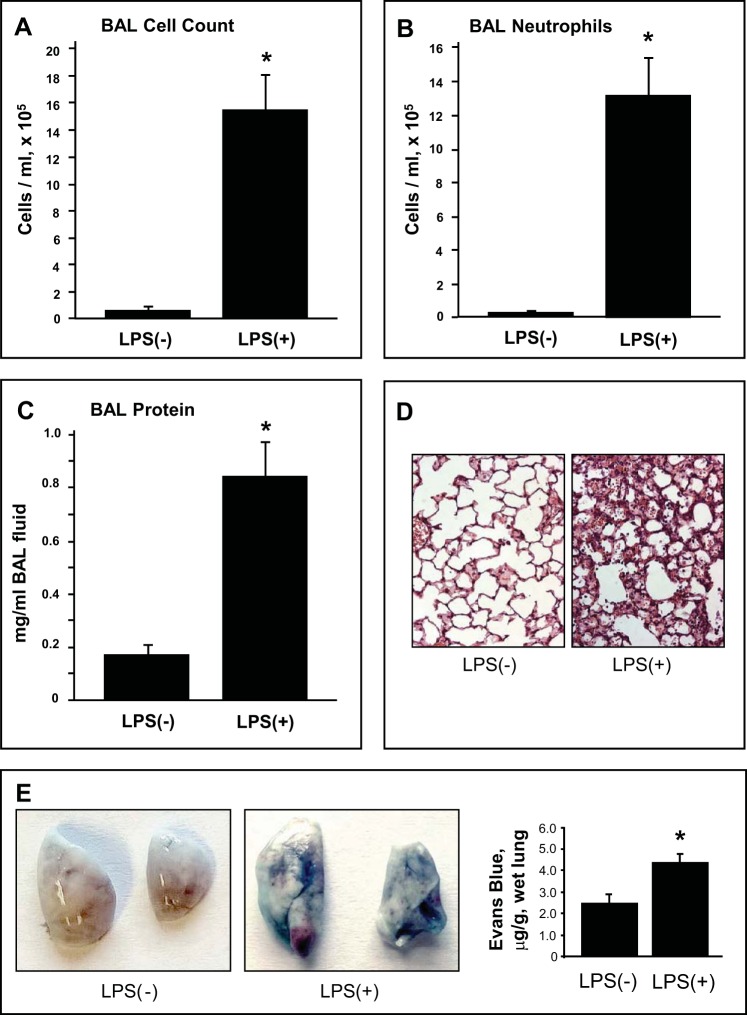
Evaluation of the BAL in mice 72 h after intratracheal LPS administration revealed a significant increase in BAL total cell count (Fig. 1A) and protein concentration (Fig. 1C) compared with the vehicle-treated control mice. LPS exposure induced pronounced leukocyte recruitment to the BAL with predominant presence of neutrophils in the mix (Fig. 1B). Analysis of lung histological changes revealed LPS-induced accumulation of protein-rich fluid in alveolar compartments and inflammatory cell infiltration (predominantly neutrophils and mononuclear cells) in both lung interstitium and alveolar compartments (Fig. 1D). LPS challenge also induced lung vascular leak that was evaluated by measurements of Evans blue extravasation into the lung tissue (Fig. 1E).
Fig. 1.
Analysis of LPS-induced lung injury and barrier dysfunction. C57BL/6J mice were challenged by single intratracheal injection of LPS (0.63 mg/kg). Control animals were treated with sterile saline solution. Total cell count (A), differential cell count (B), and protein concentration (C) were measured in bronchoalveolar lavage (BAL) fluid collected 72 h after the LPS challenge. Data are expressed as means ± SD, n = 6–10 per condition; *P < 0.05, compared with vehicle control. D: histological analysis of lung tissue (×40 magnification). Whole lung (4 to 6 animals from each experimental group) were agarose-inflated in situ, fixed with 10% formalin, and used for histological evaluation by hematoxylin and eosin staining. E: Evans blue dye (30 ml/kg iv) was injected 2 h before termination of the experiment. Photographs depict lung vascular permeability assessed by Evans blue accumulation in the lung tissue. Bar graph shows quantitative analysis of Evans blue extracted from the lung tissue samples; n = 4 per condition; *P < 0.05.
Chemical composition of BAL.
BAL was collected from six representative animals from LPS− (n = 3) and LPS+ (n = 3) groups and analyzed by GC/MS to study chemical environment of the lungs experiencing LPS-induced ALI. Partial least squares discriminant analysis of log-transformed and autoscaled data GC/MS identified six metabolites (putrescine, desmosterol, tetracosanoic acid, uric acid, 3-hydroxycholestane, lactic acid) significantly (P < 0.01) elevated in samples from injured lungs. To address the problem of multiple comparisons, an additional false discovery rate analysis was performed with QVALUE plug-in for R (http://genomics.princeton.edu/storeylab/qvalue). The elevated metabolite putrescine, recently determined as a metabolic marker in bacterial vaginosis study (57), indirectly indicated bacterial growth in ALI lung.
Microbial community profiling.
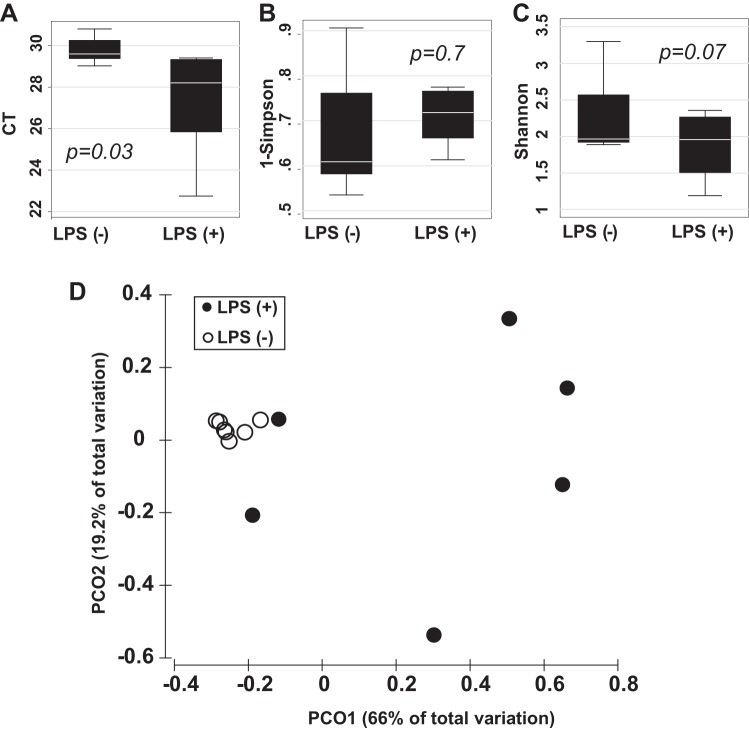
DNA was extracted from BAL and used in a PCR reaction with universal primers targeting regions of the 16S rRNA gene. PCR amplicons were sequenced, assembled in operational taxonomic units, taxonomically annotated, and used to assess lung microbial community membership and structure. On average 55,130 ± 10,598 sequences per sample were collected, but to facilitate the analysis a smaller subset of 7,000 amplicons per sample was used. Good's coverage estimation (18) indicated that sequencing of 7,000 amplicons per sample provides near complete (>99.9%) community coverage. The annotated sequences were attributed to 17 bacterial families with abundance level above 1% in LPS− and LPS+ groups, of them 15 and 5 were present in LPS− and LPS+ groups, respectively (Table 1). The lungs of mice from the LPS− group harbored microbiota dominated by bacteria from family Alicyclobacillaceae, which alone accounted for 55% of sequences. The remaining 14 families were present at relative abundances between 1 and 5%; of them, 7 families fell between 5 and 2% of abundance (Moraxellaceae, Enterobacteriaceae, Xanthomonadaceae, unclassified Burkholderiales, Staphylococcaceae, Carnobacteriaceae, Brucellaceae) and 7 more between 2 and 1%. qPCR analysis was performed (36) to compare the amount of bacterial DNA in the BAL of LPS− and LPS+ mice. The extracted DNA was normalized to 1 ml of BAL fluid prior to reaction. On average we detected 2.5 PCR cycles difference between mean Ct values from LPS-treated and control groups (P = 0.03) (Fig. 2A). These data indicate an average fivefold increase of bacterial load in BAL samples from the experimental ALI mice. The analysis of 16S RNA tags indicated that the community complexity, measured by Gini-Simpson (1-λ) diversity index, remained unchanged after LPS treatment (P = 0.7) (Fig. 2B), whereas Shannon diversity index shift indicated the tendency to increase the community evenness in response to ALI (P = 0.07) (Fig. 2C). Although the effect of ALI on both measures was statistically insignificant, the community evenness exhibited stronger change. Thus the analysis suggested that the observed bacterial growth is attributed to species existing within the lung ecosystem before the ALI rather than new species acquired posttrauma. A matrix based on Yue and Clayton measures of dissimilarity between the community structures was used to differentiate samples by PCoA (Fig. 2D). The analysis indicated that 66% of total variation in the dates could be explained by the principal coordinate PC1. In our study the PC1 was collinear with LPS treatment and experimental ALI. ANOSIM test confirmed significant separation between control and ALI groups at the level of P = 0.005. The Metastats-based comparison of LPS− and LPS+ groups revealed significant (P < 0.01) changes in the abundance of five bacterial families. The families Alicyclobacillaceae, Bradyrhizobiaceae, and undetermined family from order Burkholderiales decreased relative abundance in response to ALI. In contrast, families Brucellaceae (class Alfaproteobacteria) and Xanthomonadaceae (class Gammaproteobacteria) were blooming in response to lung injury (Table 1). In summary, the major trend in microbial community reaction to LPS-induced ALI was attributed to the loss of Firmicutes, represented by family Alicyclobacillaceae [from 55 to 19% (P < 0.001)], and bloom of Proteobacteria, represented by families Brucellaceae and Xanthomonadaceae [from 2 to 20% (P < 0.016) and 4 to 19% (P < 0.017) or 10-fold and 5-fold, respectively].
Table 1.
Most abundant bacterial families and their reaction on LPS treatment
| Phylum | Family | LPS− | LPS+ | P Value |
|---|---|---|---|---|
| Proteobacteria | Bradyrhizobiaceae* | 0.02 ± 0.003 | 0.01 ± 0.002 | 0.003 |
| Brucellaceae† | 0.02 ± 0.004 | 0.2 ± 0.076 | 0.016 | |
| Methylobacteriaceae | 0.01 ± 0.007 | 0.01 ± 0.003 | 0.25 | |
| Sphingomonadaceae | 0.02 ± 0.002 | 0.01 ± 0.005 | 0.241 | |
| Comamonadaceae | 0.02 ± 0.003 | 0.02 ± 0.009 | 0.929 | |
| unclassified Burkholderiales* | 0.03 ± 0.009 | 0.01 ± 0.003 | 0.013 | |
| Enterobacteriaceae | 0.04 ± 0.008 | 0.1 ± 0.077 | 0.632 | |
| Xanthomonadaceae† | 0.04 ± 0.012 | 0.19 ± 0.065 | 0.017 | |
| Moraxellaceae | 0.05 ± 0.007 | 0.16 ± 0.073 | 0.11 | |
| Firmicutes | Alicyclobacillaceae* | 0.55 ± 0.055 | 0.19 ± 0.088 | 0.001 |
| unclassified Bacillales | 0.01 ± 0.005 | 0 ± 0.001 | 0.041 | |
| Paenibacillaceae_1 | 0.01 ± 0.006 | 0 ± 0.002 | 0.083 | |
| Staphylococcaceae | 0.03 ± 0.006 | 0.01 ± 0.007 | 0.135 | |
| Carnobacteriaceae | 0.02 ± 0.012 | 0 ± 0.001 | 0.047 | |
| Lachnospiraceae | 0.005 ± 0.002 | 0.01 ± 0.008 | 0.544 | |
| Actinobacteria | Nocardiaceae | 0.01 ± 0.002 | 0.02 ± 0.012 | 0.513 |
| Bacteroidetes | Flavobacteriaceae | 0.02 ± 0.005 | 0.02 ± 0.005 | 0.901 |
LPS− n = 7, LPS+ n = 6; table displays median relative abundance ± SE.
Negative response on LPS treatment;
positive response on LPS treatment.
Fig. 2.
Microbiota reaction to the LPS-induced acute lung injury. A: qPCR quantification (Ct count) indicates the increase of bacterial DNA presence in BAL after LPS treatment. B: Gini-Simpson (1-Simpson) diversity index remained unchanged after LPS treatment. C: Shannon diversity index shift indicated the tendency to increase the community evenness in response to acute lung injury (ALI). D: principal coordinate (PCo) analysis plot depicts separation of samples between control and LPS-treated group. (P values are given for 2-sided t-test.)
Microbial culture isolation and characterization.
BAL samples of animals from LPS− and LPS+ groups were sent to the Clinical Microbiology Laboratory at The University of Chicago in attempt to isolate cultivable bacteria. After plating and incubation, BAL samples from control and ALI lungs were positive for bacterial growth. Two types of phenotypically distinct colonies were observed. Colonies were identified, by MALDI-TOF MS, as Stenotrophomonas maltophilia and Ochrobactrum anthropi bacteria from families Xanthomonadaceae and Brucellaceae, respectively. Identification concurred with reported 16S RNA gene sequencing data.
Pure isolates of O. anthropi and S. maltophilia were subsequently examined by using phenotype microarray plates (Biolog) to describe their biochemical phenotypes. Of 190 carbon sources tested, 87 were metabolized by either microorganism, 71 were specific for O. anthropi, 1 (gelatin) was specific for S. maltophilia, and 15 were shared between both stains; therefore the metabolic niche of O. anthropi appeared broader compared with fastidious S. maltophilia. O. anthropi was also more salt tolerant (NaCl, KCl, sodium lactate) than S. maltophilia, but S. maltophilia revealed higher tolerance to sodium formate and ammonium sulfate pH 8.0. Table 2 presents the list of 35 lung metabolites, identified by GS/MS, that were proven as suitable substrates for O. anthropi and S. maltophilia. In addition, both pathobionts were resistant to similar concentrations of sodium sulfate (up to 5%), ethylene glycol (up to 15%), sodium phosphate pH 7.0 (up to 200 mM), and sodium nitrate (up to 80 mM) and grew in a similar pH range (pH 5.5–10).
Table 2.
Substrates of Ochrobactrum anthropi and Stenotrohomonas maltophilia found among bronchoalveolar lavage metabolites
| Substrate | Elevated in LPS+* | O.a. | S.m. | Chemical Class |
|---|---|---|---|---|
| Alanine | + | Amino acid | ||
| β-Alanine | + | Amino acid | ||
| Aspartic acid | + | Amino acid | ||
| Glutamic acid | + | Amino acid | ||
| Glutamine | + | Amino acid | ||
| Glycine | + | Amino acid | ||
| Isoleucine | + | Amino acid | ||
| Ornithine | + | Amino acid | ||
| Proline | + | Amino acid | ||
| Serine | + | Amino acid | ||
| Threonine | + | Amino acid | ||
| Valine | + | Amino acid | ||
| Arabitol | + | Alcohol | ||
| Glycerol | + | Alcohol | ||
| Inositol | + | Alcohol | ||
| Sorbitol | + | Alcohol | ||
| Fructose | + | + | Carbohydrate | |
| Galactose | + | Carbohydrate | ||
| Glucose | + | + | Carbohydrate | |
| Maltose | + | + | Carbohydrate | |
| Mannose | + | + | Carbohydrate | |
| Ribose-5-p | + | Carbohydrate | ||
| Sucrose | + | Carbohydrate | ||
| Trehalose | + | Carbohydrate | ||
| Xylose | + | Carbohydrate | ||
| Inosine | + | + | Nucleotide/nucleoside | |
| Uridine | + | + | Nucleotide/nucleoside | |
| Citric acid | + | + | Organic acid | |
| Fumaric acid | + | Organic acid | ||
| Gluconic acid | + | Organic acid | ||
| Glycolic acid | + | Organic acid | ||
| Lactic acid | P < 0.01 | + | + | Organic acid |
| Malic acid | + | Organic acid | ||
| Pyruvic acid | + | Organic acid | ||
| Succinic acid | + | Organic acid |
LPS− n = 3, LPS+ n = 3. +Positive test on Phenotype Array (Biolog Inc.).
O.a., Ochrobactrum anthropi; S.m., Stenotrohomonas maltophilia.
Morbid transformation of lung microbiota exacerbate experimental lung inflammation.
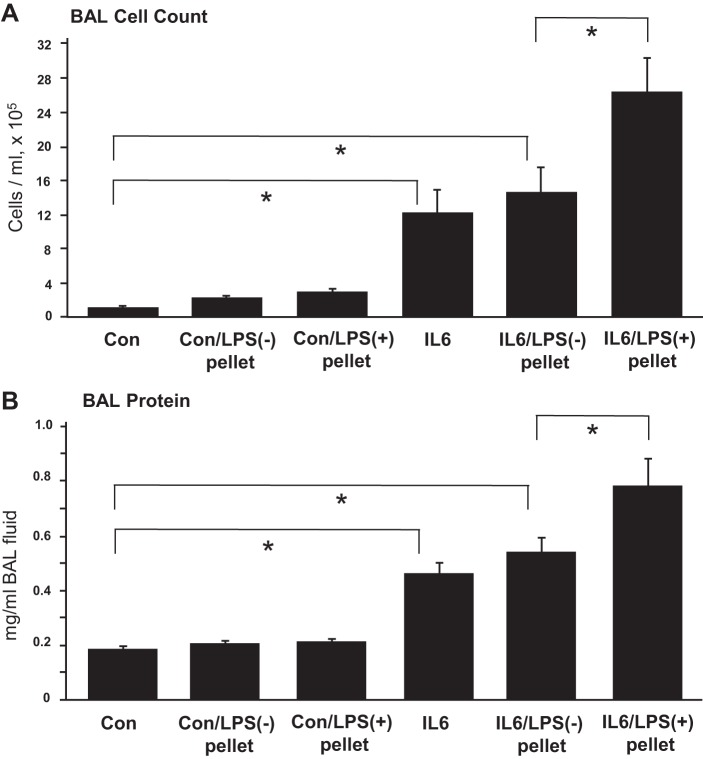
Our data demonstrate that LPS-induced lung injury was associated with significant alterations in microbiota profile (Fig. 2) and growth of known pathogens O. anthropi and S. maltophilia (2, 13, 37, 40, 42, 48, 51). To test the impact of microbiota generated during LPS-induced lung inflammation, in the setting of a repeated insult we performed an additional experiment using mice model of IL-6-induced lung injury. IL-6 is a proinflammatory cytokine that appears in BAL during lung injury. We obtained high-speed centrifugation pellets carrying bacterial particles from BAL samples of control and LPS-treated mice. The pellets were resuspended in sterile saline and administered simultaneously with IL-6 to naive mice. Intratracheal injection of IL-6 alone increases protein content and stimulates neutrophil accumulation in BAL (5). Coinjection of pellets from control mice did not affect BAL protein levels and cell counts in IL-6-treated mice. However, coinjection of pellets from LPS-treated mice significantly increased BAL protein content and cell count, suggesting exacerbation of IL-6-induced lung inflammation (Fig. 3). Interestingly, intratracheal injection of BAL-derived bacterial pellets from LPS-treated mice into naive mice without IL-6 challenge did not cause considerable inflammation, suggesting that the increased inflammation seen in mice challenged with the bacterial pellets from the BAL of LPS-treated mice and IL-6 was the result of modulated IL-6 signaling by the bacterial flora and not by inflammatory activation induced by materials that came along with the pellet, e.g., cytokines or other inflammatory molecules.
Fig. 3.
Effect of microbiota from ALI-related lungs on IL-6 induced lung injury. C57BL/6J mice were challenged by single intratracheal injection of IL-6 (5 mg/kg) alone, with bacterial pellet obtained from BAL of LPS-treated and control (Con) mice, or with combination of IL-6 and BAL-derived bacterial pellets from control and LPS-treated mice. The parameters of inflammation: total cell count (A) and protein concentration (B) were measured in BAL fluid collected 72 h after the challenge. Data are expressed as means ± SD, n = 4 per condition; *P < 0.05.
DISCUSSION
This study assessed the microbial community in healthy and inflamed mouse lungs as a first step toward comparative analysis and identification of common features in microbiome changes reported in patients with lung dysfunction. The majority of bacterial sequences in the control group were attributed to phylum Firmicutes and Alicyclobacillaceae family. Alicyclobacillaceae are scarcely reported among the human or mice microbiota. Currently, only one report mentions that bacteria of the Alicyclobacillus genus have been cultured from human blood specimen (22). In contrast, the bacterial growth in the diseased lungs is a well-known clinical observation and a frequent complication among patients receiving mechanical ventilation support in the intensive care unit (ICU). The set of microorganisms routinely cultured from the lung of patients with ALI includes gram-negative bacilli, which indicates significantly worse prognosis than the presence of gram-positive pathogens (11). The results of this study support the previously discovered association of Proteobacteria species with lung trauma. Both Stenotrophomonas and Ochrobactrum have had a history of pulmonary presentation. Stenotrophomonas is frequently found in lungs of ICU patients (42), individuals immunocompromised due to posttransplant treatment (8, 20), and cancer patients (17). This genus has been reported among other bacteria in traumatized lung and was associated with “tsunami lung” syndrome (26). The controversial role of Stenotrophomonas in cystic fibrosis (47, 52, 53) was recently resolved in the study of Di Bonaventura et al. (14) demonstrating that Stenotrophomonas directly contributes to the inflammatory process and compromises respiratory function. Lung infection with Stenotrophomonas has been experimentally achieved in mice (40, 58). The severity of infection was equally dependent on the host and bacterial factors. Infected mice responded by elevated level of proinflammatory cytokines further confirming the relevance of Stenotrophomonas growth for ALI progression. The less acknowledged Ochrobactrum is an emerging opportunistic pathogen associated with catheter or intravenous line infections (3, 16, 32, 46). Ochrobactrum was found in 11 cases of extrinsic allergic alveolitis, as reported by Dawkins et al. (13), and one case of pneumonia (33), which confirms its involvement in lung inflammatory processes.
The bacteria from genus Stenotrophomonas are gram-negative bacilli that are common in various aquatic habitats. In a hospital, S. maltophilia is frequently found in different fluids (irrigation solutions) and patient secretions: respiratory secretions, wound exudates, and urine (2). Similarly, Ochrobactrum species, widely distributed in the environment (12, 49, 54), are found in hospital and environmental water sources (4, 21). Therefore, the question arises which environmental features of injured lungs allow two “aquatic” bacteria to rise.
The acute phase of septic lung injury is characterized by increased vascular permeability; expression of adhesive surface molecules such as ICAM-1, VCAM1, E-selectin; activated endothelial cells that promote leukocyte adhesion and recruitment to the lung; impaired fluid balance; accumulation of protein-rich fluid in the air spaces; and accumulation of inflammatory cytokines and activated neutrophils, with active H+ production (35), in the lung tissue (15, 23). We hypothesize that these mechanisms cause severe lung inflammation and pulmonary edema, which, in a way, prove advantageous to both Proteobacterial pathogens.
It is known that microorganisms co-occur in the same habitat if their metabolic demands are similar (29). The data of phenotype array demonstrated that all but one carbon sources, metabolized by S. maltophilia, were also metabolized by O. anthropi and that the optimal pH range of both species is similar and lies between 5.5 and 10. This range of pH widely exceeds the pH of healthy or inflamed lungs. Therefore ecological niches of both bacteria are extensively overlapped, signifying their ability to occupy and share same habitat. In our study eight BAL metabolites, determined by GC/MS, were also shared by two pathogens. The shared nutritional niche consists of four carbohydrates, two nucleotide/nucleoside compounds, and two organic acids, citrate and lactate (Table 2). This finding poses the alveolar fluid as a habitat suitable for both species. Furthermore, the fact that lactic acid (lactate) was among several metabolites elevated in BAL from LPS+ lungs suggests a possible mechanistic link between sterile inflammation and bacterial growth. Lactate is known as a key marker of inflammatory sites under hypoxia (19). Excess of cellular lactate is normally excreted or becomes recirculated via conversion to glucose. In sufficiently oxygenated cells lactate reacts with intracellular NAD+ and turns to pyruvate. However, in poorly perfused tissues the lactate production escapes from the homeostatic control and causes lactate accumulation and excretion (19). Hypoxia occurs during the inflammatory process as a result of blood vessel occlusion or vasoconstriction that leads to lactate overproduction. It is highly plausible that LPS-induced sterile inflammation follows the same pattern: it causes functional vessel vasoconstriction and shunting from the poorly aerated regions leading to tissue ischemia and lactate accumulation in alveoli. In turn, the increased availability of lactate may induce bacterial growth. Further investigations are warranted to precisely elucidate the relationship between tissue-produced lactate and growth of Proteobacteria pathogens.
In addition, the proteolytic properties of S. maltophilia, detected by gelatin consumption test, suggest that this bacterium can get an exclusive benefit from protein-rich fluid accumulated in ALI alveoli. It is worth mentioning that O. anthropi is unable to digest proteins (gelatin test negative) but can metabolize a wide range of free amino acids, which presents a basis for speculation that O. anthropi could metabolically complement S. maltophilia in the protein degradation network, analogs to a network of polysaccharide utilization in gut ecosystem (38), even though the existence of cooperative microbial phenotypes still remains disputable (19).
BAL of animals with LPS-induced ALI has not been studied before for bacterial content, perhaps because an LPS-induced inflammation in mice model is characterized by full resolution without significant long-term consequences (1). However, the experiment with IL-6-induced lung inflammation demonstrated that change in lung microbiota induced by ALI in fact results in increased susceptibility to lung injury in the setting of a repeated insult. Therefore this study not only described a reaction of innate lung microbiota on experimental ALI and proposed possible explanation for this reaction by metabolic and phenotype array analysis but also demonstrated a clinical role of altered microbial community in exacerbation of lung injury in the setting of repeated insult.
We originally hypothesized that inflammatory host response alters the lung ecosystem and enhances growth of bacteria by supplying substrates for bacterial consumption. With careful consideration of bacterial ecology, we inferred conditions capable of promoting bacterial growth. We now speculate that since the postinjury lung environment creates an ecological niche favorable for the bloom of previously dormant microbial species, the control of lung microenvironment can prevent bacterial growth, decrease severity of ALI, and ameliorate the risk of ARDS. The example of such ecology-driven approach was demonstrated by Tsaknis et al. (50). That group found that, in the mouse model, metformin, the drug of choice for the treatment of Type 2 diabetes and normalization of glucose levels, effectively reduced the severity of ventilator-induced lung injury.
In conclusion, this study demonstrated that LPS treatment induces clinically relevant alteration of the microbial community, causing the spontaneous bloom of endogenous Proteobacteria. Thus the mouse model of LPS-induced ALI can be a useful tool for the development of treatment strategies aimed at control of morbid transformation of innate lung microbiota.
GRANTS
This research was supported in part by National Institutes of Health Grants 1R21AI099713-01, HL087823, and HL107920.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
V.P. and K.G.B. conception and design of research; V.P., F.M., A.M., T.A., A.U., E.S., and A.A.B. performed experiments; V.P., A.U., and A.A.B. analyzed data; V.P., A.U., V.T., and K.G.B. interpreted results of experiments; V.P., O.L., A.A.B., and K.G.B. prepared figures; V.P. drafted manuscript; V.P., A.U., and K.G.B. edited and revised manuscript; V.P. and K.G.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge help provided by Biolog (Hayward), Matthew Perisin, and Dr. Joy Bergelson.
REFERENCES
- 1.Aggarwal NR, D'Alessio FR, Tsushima K, Sidhaye VK, Cheadle C, Grigoryev DN, Barnes KC, King LS. Regulatory T cell-mediated resolution of lung injury: identification of potential target genes via expression profiling. Physiol Genomics 41: 109–119, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araoka H, Baba M, Yoneyama A. Risk factors for mortality among patients with Stenotrophomonas maltophilia bacteremia in Tokyo, Japan, 1996–2009. Eur J Clin Microbiol Infect Dis 29: 605–608, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Arora U, Kaur S, Devi P. Ochrobactrum anthropi septicaemia. Indian J Med Microbiol 26: 81–83, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Barson WJ, Cromer BA, Marcon MJ. Puncture wound osteochondritis of the foot caused by CDC group Vd. J Clin Microbiol 25: 2014–2016, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birukova AA, Tian Y, Meliton AY, Leff AR, Wu T, Birukov KG. Stimulation of Rho signaling by pathologic mechanical stretch is a “second hit” to Rho-independent lung injury induced by IL-6. Am J Physiol Lung Cell Mol Physiol 302: L965–L975, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birukova AA, Wu T, Tian Y, Meliton A, Sarich N, Tian X, Leff A, Birukov KG. Iloprost improves endothelial barrier function in lipopolysaccharide-induced lung injury. Eur Respir J 41: 165–176, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birukova AA, Xing J, Fu P, Yakubov B, Dubrovskyi O, Fortune JA, Klibanov AM, Birukov KG. Atrial natriuretic peptide attenuates LPS-induced lung vascular leak: role of PAK1. Am J Physiol Lung Cell Mol Physiol 299: L652–L663, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonatti H, Pruett TL, Brandacher G, Hagspiel KD, Housseini AM, Sifri CD, Sawyer RG. Pneumonia in solid organ recipients: spectrum of pathogens in 217 episodes. Transplant Proc 41: 371–374, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6: 1621–1624, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charnot-Katsikas A, Tesic V, Boonlayangoor S, Bethel C, Frank KM. Prospective evaluation of the VITEK MS for the routine identification of bacteria and yeast in the clinical microbiology laboratory: assessment of accuracy of identification and turnaround time. J Med Microbiol 63: 235–241, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 165: 867–903, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Chester B, Cooper LH. Achromobacter species (CDC group Vd): morphological and biochemical characterization. J Clin Microbiol 9: 425–436, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawkins P, Robertson A, Robertson W, Moore V, Reynolds J, Langman G, Robinson E, Harris-Roberts J, Crook B, Burge S. An outbreak of extrinsic alveolitis at a car engine plant. Occup Med (Lond) 56: 559–565, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Di Bonaventura G, Pompilio A, Zappacosta R, Petrucci F, Fiscarelli E, Rossi C, Piccolomini R. Role of excessive inflammatory response to Stenotrophomonas maltophilia lung infection in DBA/2 mice and implications for cystic fibrosis. Infect Immun 78: 2466–2476, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dreyfuss D, Ricard JD. Acute lung injury and bacterial infection. Clin Chest Med 26: 105–112, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Duran R, Vatansever U, Acunas B, Basaran UN. Ochrobactrum anthropi bacteremia in a preterm infant with meconium peritonitis. Int J Infect Dis 13: e61–e63, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Elsner HA, Duhen U, Hollwitz B, Kaulfers PM, Hossfeld DK. Fatal pulmonary hemorrhage in patients with acute leukemia and fulminant pneumonia caused by Stenotrophomonas maltophilia. Ann Hematol 74: 155–161, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Esty WW. The efficiency of Good's nonparametric coverage estimator. Ann Stat 14: 1257–1260, 1986. [Google Scholar]
- 19.Foster KR, Bell T. Competition, not cooperation, dominates interactions among culturable microbial species. Curr Biol 22: 1845–1850, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Gasparetto EL, Bertholdo DB, Davaus T, Marchiori E, Escuissato DL. Stenotrophomonas maltophilia pneumonia after bone marrow transplantation: case report with emphasis on the high-resolution CT findings. Br J Radiol 80: e19–e20, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Gill MV, Ly H, Mueenuddin M, Schoch PE, Cunha BA. Intravenous line infection due to Ochrobactrum anthropi (CDC group Vd) in a normal host. Heart Lung 26: 335–336, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Glaeser SP, Falsen E, Martin K, Kampfer P. Alicyclobacillus consociatus sp. nov., isolated from a human clinical specimen. Int J Syst Evol Microbiol 63: 3623–3627, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev 14: 523–535, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Hansel TT, Johnston SL, Openshaw PJ. Microbes and mucosal immune responses in asthma. Lancet 381: 861–873, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Hansen CH, Nielsen DS, Kverka M, Zakostelska Z, Klimesova K, Hudcovic T, Tlaskalova-Hogenova H, Hansen AK. Patterns of early gut colonization shape future immune responses of the host. PLoS One 7: e34043, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue Y, Fujino Y, Onodera M, Kikuchi S, Shozushima T, Ogino N, Mori K, Oikawa H, Koeda Y, Ueda H, Takahashi T, Terui K, Nakadate T, Aoki H, Endo S. Tsunami lung. J Anesth 26: 246–249, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Konstantinov SR, van der Woude CJ, Peppelenbosch MP. Do pregnancy-related changes in the microbiome stimulate innate immunity? Trends Mol Med 19: 454–459, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79: 5112–5120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy R, Borenstein E. Metabolic modeling of species interaction in the human microbiome elucidates community-level assembly rules. Proc Natl Acad Sci USA 110: 12804–12809, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacCallum NS, Evans TW. Epidemiology of acute lung injury. Curr Opin Crit Care 11: 43–49, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 167: 1027–1035, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Mattos FB, Saraiva FP, Angotti-Neto H, Passos AF. Outbreak of Ochrobactrum anthropi endophthalmitis following cataract surgery. J Hosp Infect 83: 337–340, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Naik C, Kulkarni H, Darabi A, Bhanot N. Ochrobactrum anthropi: a rare cause of pneumonia. J Infect Chemother 19: 162–165, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Nakata K, Yamamoto M, Inagawa H, Soma G. Effects of interactions between intestinal microbiota and intestinal macrophages on health. Anticancer Res 33: 2849–2853, 2013. [PubMed] [Google Scholar]
- 35.Ng AW, Bidani A, Heming TA. Innate host defense of the lung: effects of lung-lining fluid pH. Lung 182: 297–317, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol 5: e177, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pompilio A, Pomponio S, Crocetta V, Gherardi G, Verginelli F, Fiscarelli E, Dicuonzo G, Savini V, D'Antonio D, Di Bonaventura G. Phenotypic and genotypic characterization of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis: genome diversity, biofilm formation, and virulence. BMC Microbiol 11: 159, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rakoff-Nahoum S, Coyne MJ, Comstock LE. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr Biol 24: 40–49, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Eur Respir J Suppl 42: 2s–9s, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Rouf R, Karaba SM, Dao J, Cianciotto NP. Stenotrophomonas maltophilia strains replicate and persist in the murine lung, but to significantly different degrees. Microbiology 157: 2133–2142, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, Gill N, Blanchet MR, Mohn WW, McNagny KM, Finlay BB. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 13: 440–447, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saugel B, Eschermann K, Hoffmann R, Hapfelmeier A, Schultheiss C, Phillip V, Eyer F, Laugwitz KL, Schmid RM, Huber W. Stenotrophomonas maltophilia in the respiratory tract of medical intensive care unit patients. Eur J Clin Microbiol Infect Dis 31: 1419–1428, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Schachtschneider KM, Yeoman CJ, Isaacson RE, White BA, Schook LB, Pieters M. Modulation of systemic immune responses through commensal gastrointestinal microbiota. PLoS One 8: e53969, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One 6: e27310, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75: 7537–7541, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siti Rohani AH, Tzar MN. Ochrobactrum anthropi catheter-related bloodstream infection: the first case report in Malaysia. Med J Malaysia 68: 267–268, 2013. [PubMed] [Google Scholar]
- 47.Stanojevic S, Ratjen F, Stephens D, Lu A, Yau Y, Tullis E, Waters V. Factors influencing the acquisition of Stenotrophomonas maltophilia infection in cystic fibrosis patients. J Cyst Fibros 12: 575–583, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Tada K, Kurosawa S, Hiramoto N, Okinaka K, Ueno N, Asakura Y, Kim SW, Yamashita T, Mori SI, Heike Y, Maeshima AM, Tanosaki R, Tobinai K, Fukuda T. Stenotrophomonas maltophilia infection in hematopoietic SCT recipients: high mortality due to pulmonary hemorrhage. Bone Marrow Transplant 48: 74–79, 2013. [DOI] [PubMed] [Google Scholar]
- 49.Tatum HW, Ewing W, Weaver RE. Miscellaneous gram-negative bacteria. In: Manual of Clinical Microbiology (2nd ed), edited by Lennette EH, Spaulding EH, Truant JP. Washington, DC: American Society for Microbiology, 1974, p. 270–294. [Google Scholar]
- 50.Tsaknis G, Siempos II, Kopterides P, Maniatis NA, Magkou C, Kardara M, Panoutsou S, Kotanidou A, Roussos C, Armaganidis A. Metformin attenuates ventilator-induced lung injury. Crit Care 16: R134, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waters V, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients. J Cyst Fibros 12: 482–486, 2013. [DOI] [PubMed] [Google Scholar]
- 52.Waters V, Atenafu EG, Salazar JG, Lu A, Yau Y, Matukas L, Tullis E, Ratjen F. Chronic Stenotrophomonas maltophilia infection and exacerbation outcomes in cystic fibrosis. J Cyst Fibros 11: 8–13, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Waters V, Yau Y, Prasad S, Lu A, Atenafu E, Crandall I, Tom S, Tullis E, Ratjen F. Stenotrophomonas maltophilia in cystic fibrosis: serologic response and effect on lung disease. Am J Respir Crit Care Med 183: 635–640, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Weaver RE, Tatum HW, Hollis DG. The Identification of Unusual Pathogenic Gram-Negative Bacteria. (Elizabeth O. King). Atlanta, GA: Center for Disease Control, 1972. [Google Scholar]
- 55.White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol 5: e1000352, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winter SE, Lopez CA, Baumler AJ. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep 14: 319–327, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeoman CJ, Thomas SM, Miller ME, Ulanov AV, Torralba M, Lucas S, Gillis M, Cregger M, Gomez A, Ho M, Leigh SR, Stumpf R, Creedon DJ, Smith MA, Weisbaum JS, Nelson KE, Wilson BA, White BA. A multi-omic systems-based approach reveals metabolic markers of bacterial vaginosis and insight into the disease. PLoS One 8: e56111, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zgair AK, Chhibber S. Stenotrophomonas maltophilia flagellin induces a compartmentalized innate immune response in mouse lung. J Med Microbiol 59: 913–919, 2010. [DOI] [PubMed] [Google Scholar]