Abstract
Increased levels of physical activity are associated with reduced cardiovascular disease (CVD) risk and mortality in obesity and diabetes. Available evidence suggests that local factors, including local hemodynamics, account for a significant portion of this CVD protection, and numerous studies have interrogated the therapeutic benefit of physical activity/exercise training in CVD. Less well established is whether basal differences in endothelial cell phenotype between/among vasculatures related to muscle recruitment patterns during activity may account for reports of nonuniform development of endothelial dysfunction in obesity. This is the focus of this review. We highlight recent work exploring the vulnerability of two distinct vasculatures with established differences in endothelial cell phenotype. Specifically, based largely on dramatic differences in underlying hemodynamics, arteries perfusing soleus muscle (slow-twitch muscle fibers) and those perfusing gastrocnemius muscle (fast-twitch muscle fibers) in the rat exhibit an exercise training-like versus an untrained endothelial cell phenotype, respectively. In the context of obesity, therefore, arteries to soleus muscle exhibit protection from endothelial dysfunction compared with vulnerable arteries to gastrocnemius muscle. This disparate vulnerability is consistent with numerous animal and human studies, demonstrating increased skeletal muscle blood flow heterogeneity in obesity coincident with reduced muscle function and exercise intolerance. Mechanistically, we highlight emerging areas of inquiry exploring novel aspects of hemodynamic-sensitive signaling in endothelial cells and the time course of physical activity-associated endothelial adaptations. Lastly, further exploration needs to consider the impact of endothelial heterogeneity on the development of endothelial dysfunction because endothelial dysfunction independently predicts CVD events.
Keywords: hemodynamics, exercise, diabetes, muscle recruitment, fiber type
this article is part of a collection on 1st PanAmerican Congress of Physiological Sciences: Physiology Without Borders. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Physical Activity Reduces Cardiovascular Risk
Epidemiological evidence demonstrates that higher levels of physical activity are associated with reduced cardiovascular disease in healthy and diseased populations (30, 41, 45, 53). Interestingly, this inverse association is not entirely explained by activity-associated reductions of established or emerging cardiovascular risk factors (inflammatory markers, blood pressure, etc.) since ∼40% of this relationship is unaccounted for after adjustment for these factors (41). Furthermore, physical activity is associated with reduced all-cause (39, 55) and cardiovascular mortality (49) across sex, age, and race/ethnicity. Lastly, in the context of disease, long-term, follow-up studies have reported this inverse relationship across a range of body mass indexes, including patients with obesity (11) and in patients with type 2 diabetes (30, 52), highlighting an indispensable role of physical activity to reduce cardiovascular risk.
The mechanism(s) underlying the substantive risk reduction by physical activity not accounted for by the improvement in traditional systemic risk factors has garnered much attention. In general, these investigations have focused on local mechanisms including hemodynamics/shear stress, stem cell mobilization, arterial stiffening/remodeling, and collateral vessel growth, among others [see reviews (37, 47)]. Indeed, local hemodynamic forces during exercise have been shown to promote a healthy endothelial cell phenotype and improve vascular function [see reviews (36, 37, 44)]. While this concept has been well established experimentally, a less often-discussed concept is whether basal local hemodynamics confer differential protection or vulnerability of the endothelium to dysfunction and disease. Thus this conceptually focused review will specifically address 1) evidence that skeletal muscle recruitment patterns and resulting local hemodynamics during activity/exercise dictate differential endothelial cell phenotypes among muscle vasculatures (2, 3, 36) and 2) whether these differential phenotypes contribute to the nonuniform development of endothelial dysfunction within and among skeletal muscles and ultimately impaired muscle perfusion and exercise intolerance in obesity. It should be noted that the latter concept, while based on experimentally derived evidence, remains somewhat speculative, and thus we propose a conceptual framework intended to reconcile currently available information.
Muscle Recruitment and Hemodynamics During Activity/Exercise
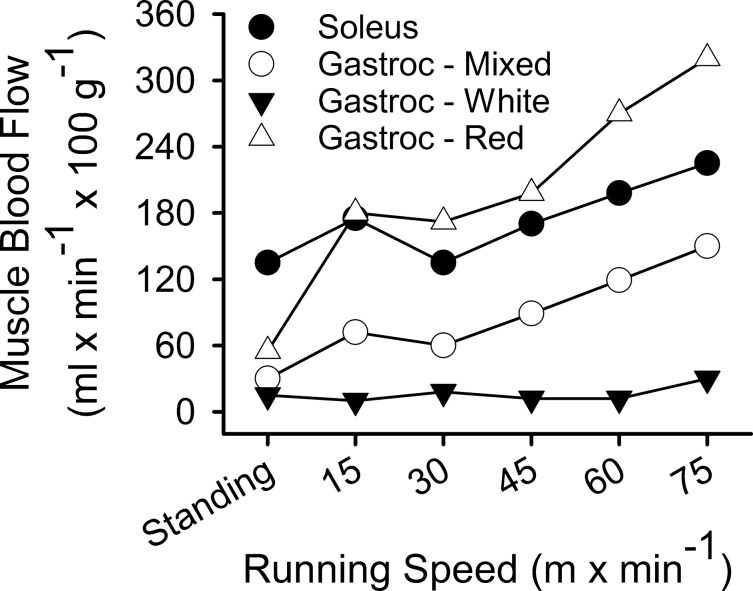
Classically, activity/exercise-induced muscle recruitment with attendant elevations in skeletal muscle blood flow follows a characteristic pattern. Specifically, slow-twitch, oxidative muscle fibers are activated at lower intensities with increasing recruitment of fast-twitch, glycolytic fibers as intensity increases to maximal oxygen consumption. The heterogeneous fiber-type composition of mammalian skeletal muscle thereby results in heterogeneous blood flows within skeletal muscle at rest and during activity/exercise. An exception to this occurs in the rat that has more extreme skeletal muscle fiber-type stratification, allowing examination of fiber type-specific effects on blood flow and vascular function (2, 3). In particular, a comparison of the soleus and gastrocnemius muscle vasculatures offer such insight with regard to slow-twitch, oxidative (soleus) and fast-twitch, glycolytic (gastrocnemius) fiber types. The rat soleus muscle is 80–90% slow-twitch fibers, whereas the rat gastrocnemius is >75% fast-twitch fibers (3). Determination of skeletal muscle blood flow during standing and increasing exercise intensities in the rat has been previously performed using the microsphere technique (Fig. 1) (35). These data demonstrated that during standing blood flow to the primarily slow-twitch, soleus muscle is two- to fourfold greater than that to the fast-twitch, gastrocnemius muscle (35). During treadmill exercise, soleus blood flow increased ∼60%, whereas gastrocnemius muscle blood flow increased ∼400% (35). These blood flow patterns reflect the integration of muscle-fiber recruitment patterns and fiber-type makeup of each muscle in that the soleus is near maximally recruited as a postural muscle during standing, whereas the gastrocnemius is heavily recruited during increasing exercise intensities. In rats exposed only to ambulatory-caged activity, arteries perfusing the soleus muscle therefore experience regular increases in blood flow and shear stress. Conversely, arteries perfusing the gastrocnemius muscle experience chronic exposure to relatively low blood flow and shear stress. Thus the vasculatures perfusing these muscles experience dramatically different hemodynamic environments in response to daily ambulatory activity.
Fig. 1.
Blood flows to soleus and gastrocnemius (Gastroc) muscles in the rat at rest (standing) and during treadmill exercise. Blood flows to the mixed slow-twitch/fast-twitch, the predominately fast-twitch white, and predominately fast-twitch red portions of the gastrocnemius were determined individually. Adapted from Laughlin and Armstrong (35).
Further studies have established that the soleus, but not gastrocnemius, vasculature exhibits an exercise training-like endothelial phenotype under basal conditions in the rat. Specifically, the soleus feed artery (SFA) did not exhibit enhanced endothelium-dependent vasodilation or endothelial nitric oxide (NO) synthase (eNOS) expression in response to exercise training (27). Prevention of soleus recruitment by hindlimb unweighting or reduced activity in aging, however, resulted in reduced endothelium-dependent vasodilation and eNOS expression (58, 59). This is in opposition to the gastrocnemius feed artery (GFA) that exhibits increased eNOS expression following exercise training and no change in endothelium-dependent vasodilation or eNOS expression following hindlimb unweighting (38, 59). Thus the GFA appears to exhibit an untrained endothelial phenotype. Together, these data demonstrate that the SFA and GFA exhibit fundamentally different endothelial cell phenotypes in healthy animals and that these phenotypes are dictated, in part, by the prevailing local hemodynamic environments induced by daily activity and muscle-fiber recruitment/activity.
Local Variation in Vulnerability to Obesity-Related Endothelial Dysfunction
A number of studies have reported nonuniform development of endothelial dysfunction in various animal models of obesity, insulin resistance, and diabetes (8, 26, 42, 54, 64). Assuming that all vascular endothelium is exposed similarly to the prevailing comorbidities associated with these disease states (i.e., blood concentrations of glucose, inflammatory markers, etc.), the differential onset of endothelial dysfunction likely involves disparate influences of local factors such as the local hemodynamic environment, as discussed above. While microvascular dysfunction is a known early defect in obesity-related cardiovascular disease (56), these differences in onset of dysfunction have been reported in both conduit vessels and the microcirculation. For instance, the Otsuka Long-Evans Tokushima fatty (OLETF) rat model of insulin resistance exhibits delayed onset of endothelial dysfunction in the carotid artery compared with the aorta (64). In the Zucker obese (ZO) rat model of insulin resistance at 17 to 18 wk of age, penile arterioles develop endothelial dysfunction not present in coronary arterioles (54). Coronary arteriolar endothelial dysfunction does ultimately occur in older ZO rats, however, demonstrating a limit to the reduced vulnerability of this vasculature (7, 42). A role for fundamental differences in endothelial phenotype between the vascular beds interrogated in these particular studies is unclear, and whether any potential difference in phenotype contributed to the disparate development of dysfunction was not examined. The evidence and thorough characterization of differences in endothelial phenotype discussed above regarding the rat SFA and GFA, however, present a novel experiment in nature whereby these differences can be exploited to examine their role in modifying endothelial vulnerability to dysfunction. Furthermore, examination of skeletal muscle feed artery function in disease is relevant to downstream muscle perfusion and function since the feed artery is an important contributor to muscle blood flow regulation (57).
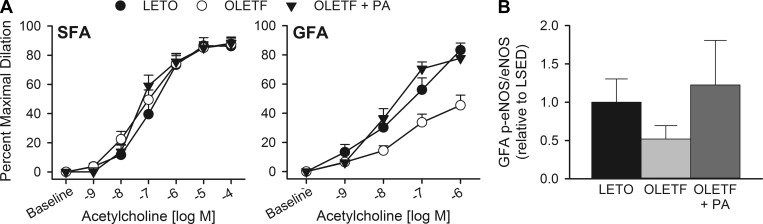
We recently addressed this question of whether the fundamental difference in endothelial phenotype between the SFA and GFA might lead to differential vulnerability of these vessels to obesity-related endothelial dysfunction (8). Specifically, we hypothesized that the SFA, not the GFA, would be protected from dysfunction based on its apparent exercise training-like phenotype. If so, we further explored whether increased physical activity via access to a running wheel would prevent dysfunction in the GFA. This question was explored in SFA and GFA from OLETF rats, a model of obesity and type 2 diabetes, compared with Long-Evans Tokushima Otsuka (LETO) lean control rats. Overall, our results supported this hypothesis in that the GFA, but not the SFA, from OLETF rats exhibited impaired endothelium-dependent vasodilation (Fig. 2A) and enhanced constriction to the endothelium-derived vasoconstrictor endothelin-1 (8). Interestingly, whereas endothelium-dependent vasodilation to acetylcholine (ACh) was not impaired in the SFA from obese rats, our data suggest that the mechanism of this vasodilation is altered by obesity. Specifically, the NO synthase (NOS)-dependent component of SFA dilation to ACh, assessed by NOS blockade with NG-nitro-l-arginine methyl ester, was increased in OLETF rats due, in part, to increased smooth muscle NO sensitivity, assessed by vasodilation to the NO donor sodium nitroprusside (8). This contrasts the GFA in which NOS blockade eliminated differences between OLETF and LETO with no difference in sodium nitroprusside vasodilation but a potential decrease in eNOS Ser1177 phosphorylation (Fig. 2B), indicating an obesity-associated reduction of NO bioavailability in this vessel. It remains unclear whether the mechanistic shift underlying endothelium-dependent vasodilation in the SFA represents an early compensated vascular dysfunction. Nonetheless, these data demonstrate a fundamental difference in the vulnerability of these vessels to develop obesity-related endothelial dysfunction.
Fig. 2.
Endothelium-dependent vasodilation to acetylcholine (A) of isolated, pressurized soleus (SFA) and gastrocnemius (GFA) feed arteries from the lean Long-Evans Tokushima Otsuka (LETO), obese Otsuka Long-Evans Tokushima fatty (OLETF), and obese OLETF rat following elevated physical activity (PA). B: ratio of phospho (p)-Ser1177 endothelial nitric oxide synthase (eNOS) to total eNOS in gastrocnemius feed arteries. LSED, sedentary LETO. Adapted from Bender et al. (8).
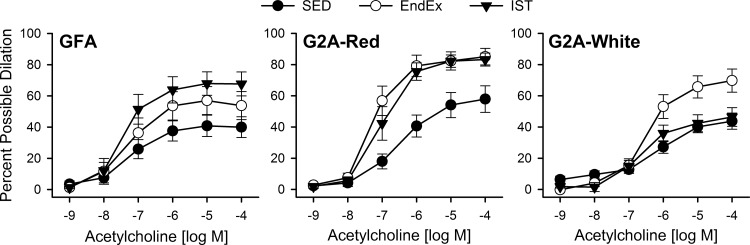
In light of this differential vulnerability, we explored whether spontaneous activity on running wheels to elicit recruitment of the fast-twitch, gastrocnemius muscle would prevent the development of GFA dysfunction in the OLETF rat. The OLETF rat is a unique model of obesity and diabetes, compared with other similar animal models, in that it displays high levels of spontaneous activity on running wheels (50). Indeed, in our study, OLETF rats ran ∼8 km/day for an average of ∼4 h/day of activity, and this activity prevented endothelial dysfunction in the GFA (Fig. 2A) (8). Recruitment of the gastrocnemius by wheel running was confirmed by a 32% increase of citrate synthase activity in the red portion of this muscle (8, 10). Similarly, a follow-up study demonstrated that endurance and interval sprint training also improved endothelium-dependent vasodilation in the GFA as well as downstream arterioles, but not in the SFA (Fig. 3) (40). Thus wheel running and exercise training with resultant recruitment of the gastrocnemius muscle are sufficient to prevent obesity-associated endothelial dysfunction in this vasculature, further supporting the importance of physical activity and, in particular, the resulting local hemodynamic environment in skeletal muscle as a mediator of endothelial health, as reflected in measures of endothelium-dependent vasodilation. In support of a protective role for local hemodynamics, a recent study in healthy subjects and patients with type 2 diabetes demonstrated that increased brachial artery blood flow and shear rate (induced by forearm heating) are sufficient to prevent acute hyperglycemia-induced attenuation of endothelium-dependent, flow-mediated vasodilation (24).
Fig. 3.
Endothelium-dependent vasodilation to acetylcholine in isolated, pressurized GFAs, second-order arterioles from the fast-twitch red portion of the gastrocnemius (G2A-Red), and second-order arterioles from the fast-twitch white portion of the gastrocnemius (G2A-White) from sedentary (SED), endurance exercise trained (EndEx), and interval sprint trained (IST) obese OLETF rats. Adapted from Martin et al. (40).
Recent evidence in humans and animals has shed further insight into the regulation of endothelial phenotype by physical activity. In particular, several studies have delineated the time course of the endothelial benefit of physical activity and agree that even a short (i.e., several day) hiatus from adequate physical activity leads to impaired endothelial function and a likely reduction in endothelial protection. Specifically, in rats, endothelial function was improved for ∼2 days following a single bout of acute exercise, and this improvement was prolonged to ∼4 days following 6 wk of exercise training (25). This is consistent with recent evidence in healthy human subjects, demonstrating that transitioning from high (aerobic exercise 3+ days/wk and 10,000 steps/day) to low (no planned exercise and <5,000 steps/day) physical activity for 5 days reduced flow-mediated (i.e., endothelium dependent) dilation of the popliteal, but not brachial, artery by more than half (9). Taken together, physical activity-induced improvements in endothelial phenotype are relatively short lived in healthy subjects, and a certain level of physical activity is necessary to maintain an improved endothelial function and protected endothelial phenotype. These data add strong support to the American College of Cardiology/American Heart Association recommendation of 40 min of physical activity 3 to 4 days/wk (16) to reduce cardiovascular risk. Therefore, endothelial phenotype appears to be dynamically regulated and is sensitive to levels of physical activity.
Recent Advances in Understanding Differential Endothelial Vulnerability
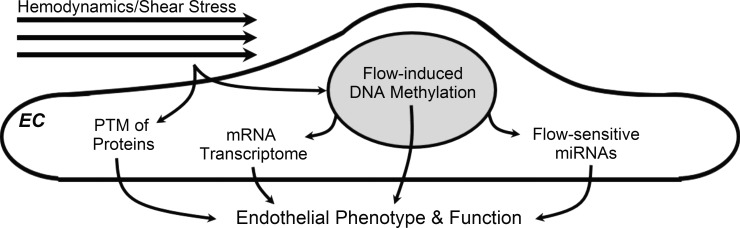
The mechanisms underlying endothelial cell heterogeneity have been a topic of significant focus for several decades. Indeed, recommendations by a National Heart, Lung, and Blood Institute workshop discussing areas needing further exploration focused largely on issues related to better understanding the origins of endothelial heterogeneity in vivo and “the contribution of endothelial cell phenotypes to . . . the susceptibility to site-specific disease” (51). Advances in the understanding of vascular development, discrete local hemodynamics, and molecular technologies (i.e., microarrays) since that time have yielded a broad picture of the regulation of endothelial phenotype beyond hemodynamics-related posttranslational modification of endothelial proteins (Fig. 4). A similar convergence of techniques has been exploited to explore the focal nature of atherogenesis (12, 14). In regard to endothelial phenotype, these areas have converged most recently with evidence of flow-mediated regulation of the endothelial epigenome via DNA methylation [see review (13)] in addition to flow-sensitive endothelial microRNAs [see review (33)]. Furthermore, while still unclear but heavily investigated, differences in the developmental origin of tissue-specific endothelial cells are likely an important contributor to endothelial heterogeneity (15, 22). Thus, while we believe that available evidence confirms a primary role for local hemodynamics in modulating endothelial phenotype and susceptibility to dysfunction, these important contributory factors likely modify endothelial function and phenotype and therefore warrant further investigation in health and disease.
Fig. 4.
Emerging molecular mechanisms contributing to hemodynamic modulation of endothelial phenotype and function. EC, endothelial cell; PTM, posttranslational modifications; miRNA. microRNA.
Many studies have explored the mechanism(s) underlying the beneficial effects of physical activity and local hemodynamics on vascular function using cellular and molecular techniques (36, 37). While these techniques have garnered useful data, the zoomed-in view they provide is limited particularly when dealing with robust physiological stressors such as obesity and activity/exercise. With regard to the fundamental differences observed between the SFA and GFA, next-generation RNA sequencing was recently employed in the OLETF rat model to acquire a zoomed-out view of the transcriptomic signatures of these vessels in health, obesity, and following exercise training (28, 43).
Transcriptomic analysis of SFA and GFA from lean LETO rats revealed fundamental differences in gene expression that may be representative of either developmental differences or the local hemodynamic environments of each vessel (28). Examination of the impact of obesity on gene expression of these important vessels revealed that, overall, the GFA exhibits a relatively greater prooxidant, dysfunctional gene expression profile than the SFA despite the SFA exhibiting a greater number of differentially expressed genes (415 genes) than the GFA (240 genes) compared with the same vessel from LETO controls (28). Several gene pathways characteristic of a dysfunctional vascular phenotype were differentially expressed in the SFA; however, lending support to the concept that local hemodynamics act to protect this vessel from dysfunction despite external insult of systemic risk factors in this model of obesity. Increased transcriptional plasticity was also observed in the SFA in response to exercise training in that the number of genes differentially expressed following endurance and interval sprint training was greater in the SFA compared with the GFA in obese rats (43). Given previous evidence that the SFA experiences a relatively lower increase in blood flow than the GFA during exercise (35), these data suggest an important dissociation between local hemodynamics and global vascular gene expression. Together, these data support a role for fundamental differences in gene expression between the SFA and GFA likely due, in part, to local hemodynamics as an underlying contributor to the differential vulnerability to dysfunction. Exercise-induced transcriptomic changes and associated changes in vessel function, however, appear to involve signals beyond local hemodynamics likely residing in the nexus of vascular development and genomics that warrant further examination.
Functional Consequence of Differential Endothelial Vulnerability in Obesity
Humans and animals with obesity exhibit impaired skeletal muscle perfusion with an associated limitation of exercise capacity (1, 4–6, 17, 29, 31, 61, 63). Whether differential vascular dysfunction in skeletal muscle plays a role in this limitation of perfusion and performance in obesity remains unclear. This is a particularly difficult issue to resolve in humans given the mixed fiber type makeup of human muscle resulting in pronounced blood flow heterogeneity within the muscle as a function of the differing oxygen demands of fiber types and their recruitment patterns (32). Recent evidence in humans demonstrates impaired exercise hyperemia and oxygen consumption rate in both the soleus and gastrocnemius muscle in patients with diabetes using noncontrast magnetic resonance imaging (63). In addition, reduced soleus blood flow, assessed by positron emission tomography, was recently reported in fasted patients with type 2 diabetes (23). In light of these data collected in long-term diabetes, it may be that the concept of differential vascular vulnerability in skeletal muscle represents an early event in the progression of diabetes-associated vascular disease. These findings may also result from inherent differences between human and rodent muscle composition and recruitment patterns. Preclinical animal models allow for closer examination of early events in disease pathogenesis, offering useful insight into the etiology of obesity/diabetes-related vascular dysfunction, thus allowing speculation as to how these events may transpire in the progression of human disease.
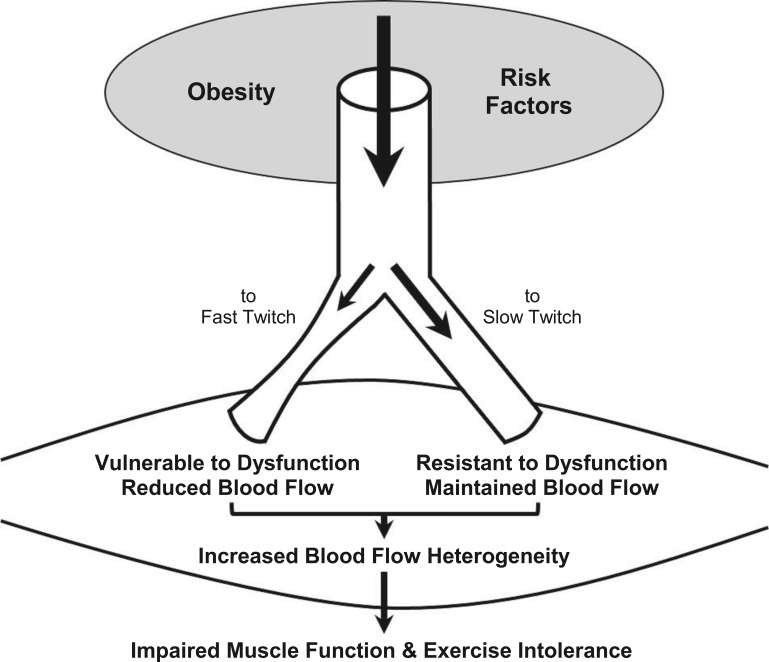
Available evidence demonstrates that skeletal muscle blood flow heterogeneity is increased in obesity and that this may result from differential vascular dysfunction within the skeletal muscle microcirculation, ultimately resulting in impaired muscle function. In healthy human subjects, blood flow heterogeneity within the quadriceps femoris muscle is reduced during exercise (34). We posit that the reduced flow heterogeneity during exercise results, in part, from recruitment of fast-twitch fibers, resulting in more homogenous flow to slow- and fast-twitch fibers alike in mixed fiber-type muscle. Resting perfusion heterogeneity in human muscle is consistent with longitudinal differences in perfusion distribution reported throughout the cremaster muscle microcirculation of the lean Zucker rat (21). No study, to date, has examined the impact of obesity or diabetes on perfusion heterogeneity in human muscle; however, elegant studies in the ZO rat have revealed increased perfusion heterogeneity in the cremaster and gastrocnemius muscle (21). The fiber-type makeup of these muscles in the rat includes a large percentage of fast-twitch fibers (3, 46) that, based on the concept covered in this review, demonstrate an increased likelihood of endothelial dysfunction with subsequent impairment of flow control to these fibers when recruited (i.e., perfusion heterogeneity). In the ZO rat, the increase in perfusion heterogeneity and associated alteration in capillary flow distribution (60) is a primary contributor to perfusion-demand mismatching, resulting in impaired skeletal muscle functional hyperemia (62), attenuated muscle oxygen uptake, and reduced muscle force output (Fig. 5) (18). Unlike muscle in healthy humans, cremaster blood flow heterogeneity in the ZO rat is not reduced and remains elevated during increased metabolic demand (i.e., muscle contraction) (19). Importantly, pharmacological interventions to improve endothelial function, namely, antioxidant treatment or prostaglandin/thromboxane receptor antagonism, are able to reduce perfusion heterogeneity during cremaster muscle contraction (19). The acute improvement of endothelial function with these treatments did not reduce heterogeneity at rest. These data suggest an important role for endothelium-dependent flow regulation as a mediator of high-resolution flow distribution within skeletal muscle during muscle contraction (19). Overall, these studies suggest that in mixed muscle, endothelial dysfunction of vessels perfusing fast-twitch fibers may contribute to increased flow heterogeneity and an impairment in flow recruitment to these fibers during contraction, thereby maintaining increased flow heterogeneity, attenuating oxygen delivery to fast-twitch fibers, and compromising muscle function. This concept requires further preclinical and clinical evaluation particularly with regard to the relationship between flow heterogeneity and fiber-type recruitment in health and disease.
Fig. 5.
Schematic representation of the effect of obesity and associated risk factors to induce endothelial dysfunction of vessels perfusing fast, but not slow, twitch muscle fibers, thereby leading to increased blood flow heterogeneity in mixed fiber-type muscle (predominant in humans) with subsequent impairment of muscle function and exercise tolerance.
The concept on which this review is focused goes one step further in suggesting that recruitment of fast-twitch fibers during exercise/physical activity is able to reduce the likelihood of endothelial dysfunction in vessels perfusing these fibers. Consistent with this hypothesis, in the ZO rat, exercise training improved functional vasodilation in response to contraction in the spinotrapezius and cremaster muscles (48). Further examination revealed that this improvement involved reduced endothelium-dependent vasoconstriction (i.e., prostaglandin/thromboxane), indicating improved endothelial function (48). Lastly, exercise training also improved contraction-induced increases in gastrocnemius blood flow in the ZO rat involving increased NO bioavailability (20). Taken together, these data provide robust evidence for a role of fiber-type recruitment patterns as a contributor to differential endothelial vulnerability to dysfunction ultimately leading to impaired skeletal muscle hyperemia and perfusion-demand mismatching in obesity and diabetes. Future studies are needed both in obese humans, using high resolution technologies like positron emission tomography imaging, and in preclinical animal models to more carefully tease apart the mechanistic intricacies of this issue. In particular, these studies should extend as far as review of the exercise paradigms prescribed to obese patients to ensure that adequate exercise intensity is recommended to facilitate recruitment of those fiber types most predisposed to endothelial and vascular dysfunction.
Conclusions
Heterogeneity of endothelial cell phenotype throughout the vasculature, including conduit arteries and arterioles, is clearly established in the literature. Available evidence supports an important, perhaps even a primary, role of local hemodynamics in dictating basal endothelial phenotype. Indeed, beyond important developmental differences among vasculatures, an emerging understanding of endothelial phenotypic modulation converges largely on hemodynamic forces as primary underlying stimuli. In skeletal muscle, available evidence suggests that local hemodynamics dictate differential endothelial phenotypes in vessels perfusing slow-twitch versus fast-twitch muscle fibers related to the unique recruitment patterns of these fiber types. In the context of obesity, arteries to soleus muscle display a marked resistance to development of endothelial dysfunction compared with arteries perfusing the gastrocnemius muscle. The resultant nonuniform development of endothelial dysfunction seems to be a likely candidate underlying increased skeletal muscle blood flow heterogeneity, impaired perfusion-demand matching, and exercise intolerance, particularly in mixed fiber-type muscle.
Overall, as highlighted over a decade ago by a National Heart, Lung, and Blood Institute work group (51), continued study into the origins of endothelial cell heterogeneity is critical for a better understanding of basic endothelial biology but also as a basis for the differential and focal impact of disease states on endothelial function and vascular disease. The application of emerging technologies to these questions holds great promise in discriminating vascular bed-specific differences in endothelial phenotype and the impact of disease. Indeed, our data reveal a dramatic difference in transcriptomic plasticity (i.e., larger number of differentially expressed transcripts) in endothelium exhibiting reduced vulnerability to dysfunction in obesity. Whether this represents inherent differences in cellular biology, the impact of local hemodynamics, or both is a multifaceted question requiring a multifaceted experimental approach. For application to clinical practice, future studies are necessary to elucidate appropriate exercise modalities designed to exploit muscle recruitment patterns that will benefit vulnerable endothelium and whether this improves muscle blood flow heterogeneity, perfusion, and exercise tolerance.
GRANTS
This work funded by the National Institutes of Health Grant HL36088 (to M. H. Laughlin) and the Department of Veterans Affairs Biomedical Laboratory Research and Development Grant CDA2 IK2 BX002030 (to S. B. Bender). This work was also supported by resources and the use of facilities at the Harry S. Truman Memorial Veterans' Hospital in Columbia, MO.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.B.B. and M.H.L. conception and design of research; S.B.B. prepared figures; S.B.B. drafted manuscript; S.B.B. and M.H.L. edited and revised manuscript; S.B.B. and M.H.L. approved final version of manuscript.
REFERENCES
- 1.Amarteifio E, Wormsbecher S, Demirel S, Krix M, Braun S, Rehnitz C, Delorme S, Kauczor HU, Weber MA. Assessment of skeletal muscle microcirculation in type 2 diabetes mellitus using dynamic contrast-enhanced ultrasound: a pilot study. Diab Vasc Dis Res 10: 468–470, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Distribution of blood flow in muscles of miniature swine during exercise. J Appl Physiol 62: 1285–1298, 1987. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol 344: 189–208, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltgalvis KA, White K, Li W, Claypool MD, Lang W, Alcantara R, Singh BK, Friera AM, McLaughlin J, Hansen D, McCaughey K, Nguyen H, Smith IJ, Godinez G, Shaw SJ, Goff D, Singh R, Markovtsov V, Sun TQ, Jenkins Y, Uy G, Li Y, Pan A, Gururaja T, Lau D, Park G, Hitoshi Y, Payan DG, Kinsella TM. Exercise performance and peripheral vascular insufficiency improve with AMPK activation in high-fat diet-fed mice. Am J Physiol Heart Circ Physiol 306: H1128–H1145, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care 30: 2880–2885, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Behnke BJ, Kindig CA, McDonough P, Poole DC, Sexton WL. Dynamics of microvascular oxygen pressure during rest-contraction transition in skeletal muscle of diabetic rats. Am J Physiol Heart Circ Physiol 283: H926–H932, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bender SB, DeMarco VG, Padilla J, Jenkins NT, Habibi J, Garro M, Pulakat L, Aroor AR, Jaffe IZ, Sowers JR. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension 65: 1082–1088, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender SB, Newcomer SC, Laughlin MH. Differential vulnerability of skeletal muscle feed arteries to dysfunction in insulin resistance: impact of fiber type and daily activity. Am J Physiol Heart Circ Physiol 300: H1434–H1441, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol 115: 1519–1525, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunker AK, Arce-Esquivel AA, Rector RS, Booth FW, Ibdah JA, Laughlin MH. Physical activity maintains aortic endothelium-dependent relaxation in the obese type 2 diabetic OLETF rat. Am J Physiol Heart Circ Physiol 298: H1889–H1901, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlsson AC,Ärnlöv J, Sundström J, Michaëlsson K, Byberg L, Lind L. Physical activity, obesity and risk of cardiovascular disease in middle-aged men during a median of 30 years of follow-up. Eur J Prev Cardiol 2015. January 20 pii: 2047487314568034. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 99: 315–327, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies PF, Manduchi E, Stoeckert CJ, Jiménez JM, Jiang YZ. Emerging topic: Flow-related epigenetic regulation of endothelial phenotype through DNA methylation. Vasc Pharmacol 62: 88–93, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies PF, Polacek DC, Shi C, Helmke BP. The convergence of haemodynamics, genomics, and endothelial structure in studies of the focal origin of atherosclerosis. Biorheology 39: 299–306, 2002. [PMC free article] [PubMed] [Google Scholar]
- 15.Domigan CK, Iruela-Arispe ML. Recent advances in vascular development. Curr Opin Hematol 19: 176–183, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckel RH, Jakicic JM, Ard JD, Hubbard VS, de Jesus JM, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Miller NH, Nonas CA, Sacks FM, Smith SC, Svetkey LP, Wadden TW, Yanovski SZ. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129: S76–S99, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Frisbee JC. Impaired skeletal muscle perfusion in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 285: R1124–R1134, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Frisbee JC, Goodwill AG, Butcher JT, Olfert IM. Divergence between arterial perfusion and fatigue resistance in skeletal muscle in the metabolic syndrome. Exp Physiol 96: 369–383, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frisbee JC, Hollander JM, Brock RW, Yu HG, Boegehold MA. Integration of skeletal muscle resistance arteriolar reactivity for perfusion responses in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 296: R1771–R1782, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ Physiol 291: H2483–H2492, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Frisbee JC, Wu F, Goodwill AG, Butcher JT, Beard DA. Spatial heterogeneity in skeletal muscle microvascular blood flow distribution is increased in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 301: R975–R986, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia MD, Larina I. Vascular development and hemodynamic force in the mouse yolk sac. Front Physiol 5: 308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodpaster BH, Bertoldo A, Ng JM, Azuma K, Pencek RR, Kelley C, Price JC, Cobelli C, Kelley DE. Interactions among glucose delivery, transport, and phosphorylation that underlie skeletal muscle insulin resistance in obesity and type 2 diabetes: studies with dynamic PET imaging. Diabetes 63: 1058–1068, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greyling A, Schreuder THA, Landman T, Draijer R, Verheggen RJ, Hopman MT, Thijssen DH. Elevation in blood flow and shear rate prevents hyperglycemia-induced endothelial dysfunction in healthy subjects and those with type 2 diabetes. J Appl Physiol 118: 579–585, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Haram PM, Adams V, Kemi OJ, Brubakk AO, Hambrecht R, Ellingsen O, Wisloff U. Time-course of endothelial adaptation following acute and regular exercise. Eur J Cardiovasc Prev Rehabil 13: 585–591, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Heinonen I, Rinne P, Ruohonen ST, Ruohonen S, Ahotupa M, Savontaus E. The effects of equal caloric high fat and western diet on metabolic syndrome, oxidative stress and vascular endothelial function in mice. Acta Physiologica 211: 515–527, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Jasperse JL, Laughlin MH. Vasomotor responses of soleus feed arteries from sedentary and exercise-trained rats. J Appl Physiol 86: 441–449, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins NT, Padilla J, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. Transcriptome-wide RNA sequencing analysis of rat skeletal muscle feed arteries. I. Impact of obesity. J Appl Physiol 116: 1017–1032, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi D, Shiwalkar A, Cross MR, Sharma SK, Vachhani A, Dutt C. Continuous, non-invasive measurement of the haemodynamic response to submaximal exercise in patients with diabetes mellitus: evidence of impaired cardiac reserve and peripheral vascular response. Heart 96: 36–41, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karjalainen JJ, Kiviniemi AM, Hautala AJ, Piira OP, Lepojärvi ES, Perkiömäki JS, Junttila MJ, Huikuri HV, Tulppo MP. Effects of physical activity and exercise training on cardiovascular risk in coronary artery disease patients with and without type 2 diabetes. Diabetes Care 38: 706–715, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care 26: 899–904, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Koga S, Rossiter HB, Heinonen I, Musch TI, Poole DC. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med Sci Sports Exerc 46: 860–876, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Kim CW, Simmons RD, Jo H. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs. Arterioscler Thromb Vasc Biol 34: 2206–2216, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laaksonen MS, Kalliokoski KK, Kyröläinen H, Kemppainen J, Teräs M, Sipilä H, Nuutila P, Knuuti J. Skeletal muscle blood flow and flow heterogeneity during dynamic and isometric exercise in humans. Am J Physiol Heart Circ Physiol 284: H979–H986, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol 243: H296–H306, 1982. [DOI] [PubMed] [Google Scholar]
- 36.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Comp Physiol 2: 321–447, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol 104: 588–600, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol 96: 233–244, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc 33: S546–S471, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Martin JS, Padilla J, Jenkins NT, Crissey JM, Bender SB, Rector RS, Thyfault JP, Laughlin MH. Functional adaptations in the skeletal muscle microvasculature to endurance and interval sprint training in the type 2 diabetic OLETF rat. J Appl Physiol 113: 1223–1232, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 116: 2110–2118, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 291: H1780–H1787, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. Transcriptome-wide RNA sequencing analysis of rat skeletal muscle feed arteries. II. Impact of exercise training in obesity. J Appl Physiol 116: 1033–1047, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology 26: 132–145, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson CR, Kriska AM, Lantz PM, Hayward RA. Physical activity and mortality across cardiovascular disease risk groups. Med Sci Sports Exerc 36: 1923–1929, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Sarelius IH, Maxwell LC, Gray SD, Duling BR. Capillarity and fiber types in the cremaster muscle of rat and hamster. Am J Physiol Heart Circ Physiol 245: H368–H374, 1983. [DOI] [PubMed] [Google Scholar]
- 47.Schuler G, Adams V, Goto Y. Role of exercise in the prevention of cardiovascular disease: results, mechanisms, and new perspectives. Eur Heart J 34: 1790–1799, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Sebai M, Lu S, Xiang L, Hester RL. Improved functional vasodilation in obese Zucker rats following exercise training. Am J Physiol Heart Circ Physiol 301: H1090–H1096, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation 122: 743–752, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Stern JS, Johnson PR. Spontaneous activity and adipose cellularity in the genetically obese Zucker rat (fafa). Metabolism 26: 371–380, 1977. [DOI] [PubMed] [Google Scholar]
- 51.Stevens T, Rosenberg R, Aird W, Quertermous T, Johnson FL, Garcia JG, Hebbel RP, Tuder RM, Garfinkel S. NHLBI workshop report: endothelial cell phenotypes in heart, lung, and blood diseases. Am J Physiol Cell Physiol 281: C1422–C1433, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation 107: 2435–2439, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Vanhees L, De Sutter J, Gelada SN, Doyle F, Prescott E, Cornelissen V, Kouidi E, Dugmore D, Vanuzzo D, Borjesson M, Doherty P. Importance of characteristics and modalities of physical activity and exercise in defining the benefits to cardiovascular health within the general population: recommendations from the EACPR (Part I). Eur J Prev Cardiol 19: 670–686, 2012. [DOI] [PubMed] [Google Scholar]
- 54.Villalba N, Martínez P, Bríones AM, Sánchez A, Salaíces M, García-Sacristán A, Hernández M, Benedito S, Prieto D. Differential structural and functional changes in penile and coronary arteries from obese Zucker rats. Am J Physiol Heart Circ Physiol 297: H696–H707, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 378: 1244–1253, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Wiernsperger N, Nivoit P, De Aguiar LG, Bouskela E. Microcirculation and the metabolic syndrome. Microcirculation 14: 403–438, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. J Physiol 463: 631–646, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 93: 1685–1690, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Woodman CR, Schrage WG, Rush JW, Ray CA, Price EM, Hasser EM, Laughlin MH. Hindlimb unweighting decreases endothelium-dependent dilation and eNOS expression in soleus not gastrocnemius. J Appl Physiol 91: 1091–1098, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Wu F, Beard DA, Frisbee JC. Computational analyses of intravascular tracer washout reveal altered capillary-level flow distributions in obese Zucker rats. J Physiol 589: 4527–4543, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol 294: H1658–H1666, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Xiang L, Naik J, Hester RL. Exercise-induced increase in skeletal muscle vasodilatory responses in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 288: R987–R991, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Zheng J, Hasting MK, Zhang X, Coggan A, An H, Snozek D, Curci J, Mueller MJ. A pilot study of regional perfusion and oxygenation in calf muscles of individuals with diabetes with a noninvasive measure. J Vasc Surg 59: 419–426, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhong MF, Shen WL, Tabuchi M, Nakamura K, Chen YC, Qiao CZ, He J, Yang J, Zhang C, Kamenov Z, Higashino H, Chen H. Differential changes of aorta and carotid vasodilation in type 2 diabetic GK and OLETF rats: paradoxical roles of hyperglycemia and insulin. Exp Diabetes Res 2012: 429020, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]