Abstract
Extracorporeal membrane oxygenation (ECMO) provides mechanical circulatory support for infants and children with postoperative cardiopulmonary failure. Nutritional support is mandatory during ECMO although specific actions for substrates on the heart have not been delineated. Prior work shows that enhancing pyruvate oxidation promotes successful weaning from ECMO. Accordingly, we tested the hypothesis that prolonged systemic pyruvate supplementation activates pyruvate oxidation in an immature swine model in vivo. Twelve male mixed-breed Yorkshire piglets (age 30–49 days) received systemic infusion of either normal saline (group C) or pyruvate (group P) during the final 6 h of 8 h of ECMO. Over the final hour, piglets received [2-13C] pyruvate, as a reference substrate for oxidation, and [13C6]-l-leucine, as an indicator for amino acid oxidation and protein synthesis. A significant increase in lactate and pyruvate concentrations occurred, along with an increase in the absolute concentration of the citric acid cycle intermediates. An increase in anaplerotic flux through pyruvate carboxylation in group P occurred compared with no change in pyruvate oxidation. Additionally, pyruvate promoted an increase in the phosphorylation state of several nutrient-sensitive enzymes, like AMP-activated protein kinase and acetyl CoA carboxylase, suggesting activation for fatty acid oxidation. Pyruvate also promoted O-GlcNAcylation through the hexosamine biosynthetic pathway. In conclusion, although prolonged pyruvate supplementation did not alter pyruvate oxidation, it did elicit changes in nutrient- and energy-sensitive pathways. Therefore, the observed results support the further study of pyruvate and its downstream effect on cardiac function.
Keywords: extracorporeal membrane oxygenation, leucine, pyruvate, metabolism
extracorporeal membrane oxygenation (ECMO) has been widely used in infants and children with life-threatening cardiopulmonary failure as both cardiac and respiratory support systems (16). Veno-arterial ECMO, especially, is frequently used as short-term extracorporeal life support for postoperative cardiopulmonary failure. However, unless adequate endogenous cardiac output and respiratory function ensue, patients are not able to be weaned from ECMO, and prolonged ECMO support can lead to higher morbidity and mortality (16, 46). Thus ongoing research is attempting to identify nutritional supplements, which can improve recovery and prevent maladaptive cardiac remodeling. These potential supplements could enhance eventual weaning from mechanical circulation. Our prior work shows that successful adaptation and weaning after ECMO requires some metabolic flexibility (22). In particular, although ECMO independently promotes fatty acid oxidation (23), cardiac function after additional insult, such as ischemia reperfusion, can be improved by promoting oxidative flux through pyruvate dehydrogenase (11).
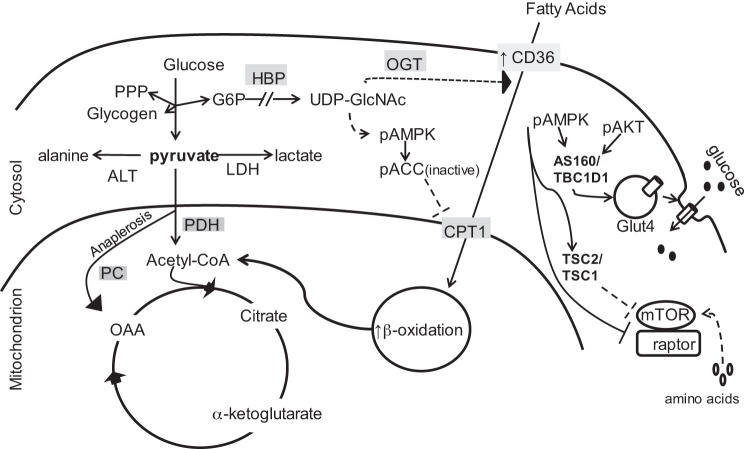
Pyruvate serves as an important branch point of substrate metabolism (Fig. 1). However, the role of pyruvate as a metabolic substrate and possible nutritional supplement during ECMO, particularly in immature myocardium, still requires further elucidation. Lactate through its dehydrogenase reaction provides a primary source for pyruvate to the heart in situ and is provided frequently in clinical scenarios. Pyruvate is not specifically available clinically but can be produced from lactate oxidation via this reaction. Cytosolic pyruvate can participate in multiple reactions including transamination to alanine. In the mitochondria, pyruvate enters the citric acid cycle (CAC) either as acetyl-CoA via decarboxylation by the pyruvate dehydrogenase complex or by carboxylation (anaplerosis) via malic enzyme or pyruvate carboxylase.
Fig. 1.
Schematic diagraming central role of pyruvate in glycolytic, citric acid cycle (CAC), and accessory pathways. PPP, pentose phosphate pathway; OGT, O-GlcNAc transferase; LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase; CPT1, carnitine palmitoyltransferase; PC, pyruvate carboxylase; ALT, alanine transaminase; OAA, oxaloacetate; HBP, hexosamine biosynthetic pathway; mTOR, mammalian target of rapamycin; TSC1/2, tuberous sclerosis complex; ACC, acetyl CoA carboxylase; AMPK, AMP activated protein kinase; AKT, serine/threonine kinase; Glut4, glucose transporter 4.
We previously examined pyruvate dehydrogenase (PDH) flux using lactate as the primary pyruvate source during ECMO (23). 13C-labeled lactate and free fatty acids were supplied over a 1-h period in either physiological or highly elevated doses but with similarly distributed coronary artery concentrations in separate experiments. This study showed that PDH flux relative to total free fatty acid flux did not change according to substrate concentration. Under those conditions, total anaplerosis was not influenced by substrate concentration, but we did not directly evaluate pyruvate carboxylation (PC). Both PDH and PC flux can influence oxidation of amino acids, as well as their entry into their own anaplerotic pathways and/or protein synthesis. Thus supplementation with specific substrates, such as pyruvate, might alter carbon contributions through these pathways. Effects on amino acid metabolism could alter protein synthesis or nutrient signaling with wide-ranging influence on myocardial remodeling during ventricular unloading by ECMO.
Although relationships between PC and decarboxylation have been closely evaluated in porcine heart in situ, virtually all these prior experimental models have involved only brief pyruvate infusion with some degree of ischemia with or without reperfusion (40) or normal (41) and high cardiac output work states (49). In the present study, we specifically examined the role of prolonged systemic pyruvate supplementation in modifying several metabolic parameters during the unique conditions of ventricular unloading provided by ECMO without prior cardiac ischemia/reperfusion injury. We investigated pyruvate entry into the CAC, as well as leucine oxidation and protein synthesis. Finally, we determined the role of pyruvate in modifying metabolic signaling and nutrient sensing under these unique physiological conditions, specifically the AMP-activated protein kinase (AMPK) pathway and the hexosamine biosynthesis pathway (HBP).
MATERIALS AND METHODS
All experimental procedures were approved by the Seattle Children's Research Institute Animal Care and Use Committee and adhered to both the American Physiological Society Guiding Principles in the Care and Use of Animals and the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Animal Models
The surgical preparation followed our methods described previously (22, 38, 42). We used 12 immature mixed-breed Yorkshire male piglets (body wt 8.4–16.3 kg, age 30–49 days) in this study. Animals were initially sedated with an intramuscular injection of ketamine (33 mg/kg) and xylazine (2 mg/kg). After intubation through surgical tracheostomy, the piglets were mechanically ventilated with oxygen (40–50%) and inhaled isoflurane (1–2%) mixture.
After median sternotomy, a flow probe was placed around the ascending aorta to measure cardiac output (TS420; Transonic Systems, Ithaca, NY). A 5-Fr high-fidelity micromanometer (Millar Instruments, Houston, TX) was used to measure left ventricular (LV) pressure. A cannula with an inflatable balloon cuff was placed into the coronary sinus via the right atrium, and the blood was returned to superior vena cava by shunt loop. A Transonic flow probe was placed around this shunt for continuous coronary venous return flow (CSF) (ml/min) monitoring. The hemiazygous vein, which drains systemic venous blood to the coronary sinus in swine, was ligated to avoid systemic contamination of the coronary venous blood. In all animals, we inserted a 24-G BD Saf-T-Infusion catheter (BD Biosciences, Franklin Lakes, NJ) into the left anterior descending artery in retrograde fashion starting just distal to the first major branch for intracoronary infusions. Myocardium was inspected to ascertain that there was no blanching indicative of ischemia distal to catheter insertion. A PowerLab 16/30 recorder (AD Instruments, Colorado Springs, CO) continuously recorded data in all cases.
The ECMO circuit consisted of the following: a roller peristaltic pump console (Sarn8000; Terumo, Tokyo, Japan) and a hollow fiber membrane oxygenator (CX-RX05RW; Terumo). The circuit was primed with dextran 40 in 0.9% sodium chloride, 5% dextrose, and 2,000 U heparin. The total prime volume was around 80 ml. A veno-arterial ECMO was established by central cannulation via the ascending aorta and right atrium. Anticoagulation was achieved with 120 U/kg of heparin as a bolus followed by continuous intravenous infusion of heparin. Management during ECMO kept pump flow rates of 80–100 ml·kg−1·min−1. We maintained a pH of 7.35 to 7.45, an arterial Pco2 of 35–45 mmHg, and a rectal temperature of 36.0–37.5°C. ECMO duration time was 8 h.
Protocols
Animals were divided into two groups, 8 h-ECMO alone (group C) and ECMO with pyruvate supplement (group P) (Fig. 2). Chemicals and 13C-labeled substrates were purchased from Sigma-Aldrich (St. Louis, MO). Group P received a continuous intravenous infusion of sodium pyruvate (11 mg·kg−1·min−1) during the final 6 h of the ECMO protocol (Fig. 2). Group C received an equivalent intravenous infusion of 0.9% sodium chloride.
Fig. 2.
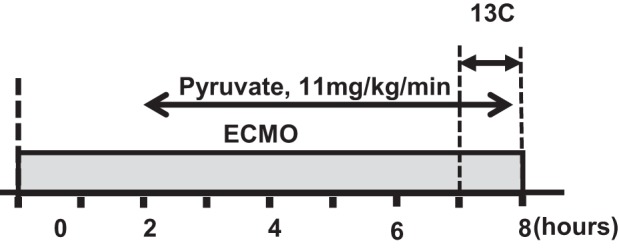
Diagram of experimental protocols. Baseline hemodynamics and plasma data were performed before time 0. The duration of labeled substrate was during the final 60 min. The extracorporeal membrane oxygenation (ECMO) duration was 8 h. Pyruvate supplementation was given intravenously in the pyruvate group (group P) and initiated at the 2-h time point.
[13C6,15N]-l-leucine (3.7 mM), as an indicator of protein synthesis and amino acid oxidation, was infused into coronary artery with [2-13C]-pyruvate (7.4 mM) as an alternate substrate for the final 60 min of the protocol as previously described (22, 42). The LV portions perfused by the 13C-labeled substrates were quickly freeze clamped and stored at −80°C for later extraction.
Metabolic Analyses
Preparation of myocardial extracts was performed with either methanol/chloroform or 1.2 M perchloric acid and neutralized with cold KOH to pH 7.4, as described previously (42).
Gas chromatography and mass spectrometry.
Gas chromatography and mass spectrometry (GC-MS) was performed to measure the absolute concentrations and 13C enrichment of leucine, pyruvate, lactate, and CAC intermediates (citrate, α-ketoglutarate, succinate, fumarate, and malate) in LV myocardial tissue samples, as previously described (19, 42, 43). Protein fractional synthesis rates (FSRs) were calculated using the methods of Jaleel et al. (19). The isotopic enrichment of [13C6,15N]-l-leucine in the proteins (Epro) and the free tissue fluid (Etf) were used with the following equation: FSR (%/h) = [(Epro × 100%)/(Etf × t)], where t is time of tracer incorporation, in hours (1 h for all groups).
Nuclear magnetic resonance.
Metabolic analyses by 13C-nuclear magnetic resonance (NMR) were performed on the swine heart as described previously for determination of specific carbon glutamate labeling (7). NMR spectra were acquired on a Varian Direct Drive (VNMRS) 600-MHz spectrometer (Varian, Palo Alto, CA) equipped with VNMRJ 3.2 on a Dell Precision T3500 workstation. Final spectra were obtained using a 45° excitation pulse (7.05 μs at 58 dB), with an acquisition time of 1.3 s, a recycle delay of 3.0 s, and a spectral width of 224.1 parts per million (ppm). All of the labeled carbon resonances of glutamate were analyzed by using the Lorentzian peak-fitting subroutine in the acquisition program (NUTS) and CAC analysis-fitting algorithm tcaCALC (kindly provided by the Advanced Imaging Research Center at the University of Texas, Southwestern). This allowed determination of relative fractional contribution (Fc) to glutamate labeling via acetyl-CoA between leucine and pyruvate, as well as the unlabeled substrate contribution.
1H-NMR was also performed on LV extracts using the Varian system to determine myocardial concentration of energy metabolites and amino acids, as previously described (23, 42). A standard one-dimensional proton NOESY with presaturation (TNNOESY) was collected on each sample with the following parameters: a nonselective 90° excitation pulse (∼7 μs at 53 dB), a mixing time of 100 ms, acquisition time of 4 s, a recycle delay of 1 s, a sweep width of 12 ppm, and temperature control set to 25°C. Collected spectra were analyzed using Chenomx software version 7.6 (Chenomx, Edmonton, Alberta, Canada).
Flux ratios.
Assessment of anaplerotic flux relative to oxidative flux was determined as described by Olson et al. (39). Oxidative flux, the pathway in which pyruvate enters the CAC via acetyl-CoA, is called pyruvate decarboxylation (PDC). Conversion of acetyl-CoA to citrate (CIT) by CIT synthase (CS) is the first step in the CAC. PDC flux rate relative to CS was assessed by the following formula: PDC/CS = (total MPE of CIT − total MPE of malate)/M1 of intracellular pyruvate, where MPE is the molar percent enrichment.
This ratio represents flux through PDC leading to CIT arising from all tissue pyruvate sources. Anaplerotic flux via PC as assessed using NMR peak areas for individual glutamate carbons as previously described (39) is as follows: PC/(PC + PDC) = (C2 + C3)/(C2 + C3 + C5).
Glycogen Assay
Samples were processed as detailed in the Glycogen Assay Kit manual (no. 700480; Cayman Chemical, Ann Arbor, MI). Pulverized LV tissue was homogenized in diluted assay buffer containing phosphatase and protein inhibitors (no. 78443; Life Technologies, Grand Island, NY), with the following steps as described in the manual.
Blood Analysis
Arterial and venous blood samples were collected at multiple time points: first, at baseline (before initiation of ECMO); second, 2 h into the ECMO protocol but before the supplemental pyruvate infusion; third, 7 h into the ECMO protocol but before infusion of C13-labeled substrates; and fourth, after completion of the labeled infusion. The samples were immediately centrifuged, and the plasma was stored at −80°C. Blood pH, Pco2, Po2, and hemoglobin were measured at regular intervals by a Radiometer ABL 800 (Radiometer America, Westlake, OH). Plasma from the 2- and 7-h time points were used for the following assays: plasma pyruvate levels were measured using the Pyruvate Assay Kit (no. 700470; Cayman Chemical,), and plasma free fatty acid levels were measured using the Free Fatty Acid Fluorometric Assay Kit (no. 700310; Cayman Chemical). Calculation for free fatty acid uptake = CSF × [arteriocoronary sinus concentration difference (μM/min)].
Western Blotting
Thirty micrograms of total protein extract from pig tissue were resolved using 10% SDS-PAGE and electroblotted onto PVDF membranes. Standard Western blotting protocols were followed. Horseradish peroxidase secondary antibodies were used and visualized with enhanced chemiluminescence upon exposure to Kodak BioMax Light ML-1 film. Membranes were stripped by washing them 2 × 15 min with 100 mM DTT, 2% (wt/vol) SDS, and 62.5 mM Tris·HCl, pH 6.7, at 70°C, followed by three 10-min washes with TBS.
The primary antibodies used in this study were serine/threonine protein kinase (AKT)1 (sc-1618) from Santa Cruz Biotechnology (Santa Cruz, CA), phospho-AKT (Ser 473, no. 9271S), PDH (no. 2784S), phospho-AMPK (Thr172, no. 2535S), AMPK (5831S), phospho-acetyl CoA carboxylase (ACC) (Ser 79, no. 3661S), ACC (no. 3676S), phospho-mammalian target of rapamycin (mTOR) (Ser 2488, no. 5536S), mTOR (no. 2983S) from Cell Signaling Technologies (Danvers, MA), phospho-PDH (ABS204) from EMD Millipore (Billerica, MA), and O-GlcNAc (ab2739) from Abcam (Cambridge, MA). Efficiency of protein transfer to PVDF membrane was determined using Pierce Reversible Protein Stain Kit for PVDF Membranes (no. 24585) (Thermo Scientific, Rockford, IL). Densitometry analyses were performed using Image J (48) and Quantity One (Bio-Rad, Hercules, CA). All Western blots were performed in duplicate.
Statistical Analysis
Statistical analyses were performed using Student's t-tests between groups. Criterion for significance was P < 0.05 for all comparisons. Error bars in figures, text, and tables indicate SE.
RESULTS
Cardiac Function
Table 1 shows the parameters of cardiac function measured at baseline and at the endpoint in each group. ECMO showed significantly reduced LV end-diastolic pressure compared with baseline (P < 0.05). Additionally, ECMO reduces aortic mean and pulse pressure, illustrating the effect of ventricular unloading. At the end point, hemoglobin and mean systemic blood pressure and LV end-diastolic pressure were similar among each of the two groups, indicating similar levels of ventricular unloading. Heart rate was increased in group P although this was not associated with an increase in myocardial oxygen consumption (Table 2).
Table 1.
Cardiac function parameters
| Group C (n = 6) |
Group P (n = 6) |
|||
|---|---|---|---|---|
| Baseline | Endpoint | Baseline | Endpoint | |
| Hemoglobin, g/dl | 9.8 ± 0.3 | 7.5 ± 0.3* | 9.5 ± 0.2 | 7.5 ± 0.3* |
| HR, beats/min | 99 ± 7 | 100 ± 8 | 108 ± 4 | 143 ± 12*† |
| Mean SBP, mmHg | 64 ± 2 | 50 ± 2* | 59 ± 2 | 50 ± 2* |
| LVEDP, mmHg | 10 ± 1 | 6 ± 1* | 8 ± 1 | 4 ± 1* |
| DP, SBP − DBP | 19.2 ± 1.7 | 3.8 ± 0.5* | 18.5 ± 3.4 | 7.33 ± 2.1* |
Values are means ± SE. Parameters of cardiac function at beginning (before initiating extracorporeal membrane oxygenation, ECMO) and end points (end of 8-h ECMO) for each group. HR, heart rate; SBP, systemic blood pressure; LVEDP, left ventricular end-diastolic pressure; DP, developed pressure = aortic systolic pressure − diastolic pressure).
P < 0.05 vs. baseline.
P < 0.05 group C vs. group P.
Table 2.
Myocardial free fatty acid data for pigs receiving systemic pyruvate infusion
| Hour 2 | Hour 7 | |
|---|---|---|
| (a-cs) FFA, mmol/l | 0.03 ± 0.03 | 0.23 ± 0.09 |
| CSF, ml/min | 24.0 ± 2.2 | 25.8 ± 3.17 |
| FFA uptake, μM/min | 0.91 ± 0.98 | 6.02 ± 2.61* |
| MVO2, μM·min−1·g−1 | 2.64 ± 0.03 | 2.58 ± 0.36 |
Values are means ± SE; n = 5. Hour 2 reflects duration of ECMO, just before initiation of pyruvate infusion. CSF, coronary sinus flow; (a-cs), arterio-coronary sinus substrate difference; FFA, free fatty acids; MVO2, myocardial oxygen consumption rate.
P < 0.01.
Serum Pyruvate Levels
Serum pyruvate levels were determined to confirm that the infusion of unlabeled sodium pyruvate was resulting in an increase in circulating pyruvate levels (Fig. 3). Group P shows a significant increase in serum pyruvate levels at 4 h postinitiation of pyruvate treatment compared with no difference in pyruvate levels for similar time points in group C.
Fig. 3.
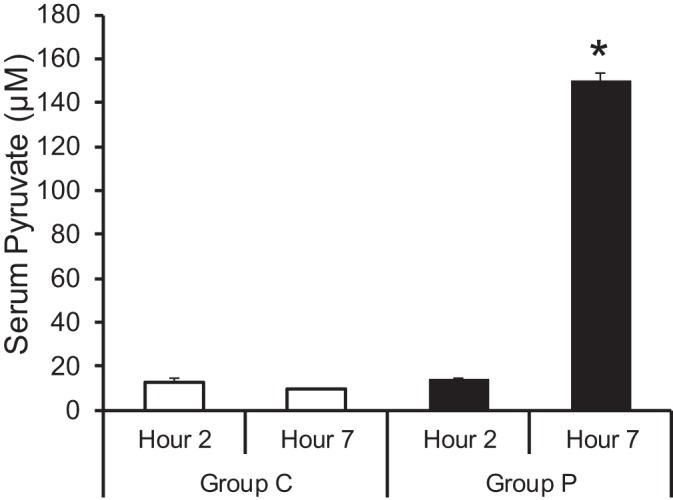
Arterial serum pyruvate concentrations. The absolute concentration of serum pyruvate significantly increased in group P at the 4-h time point compared with control (group C). Values are means ± SE; n = 6 per group. *P < 0.01 vs. group C.
CAC Intermediates
The long-term pyruvate infusion increased intracellular pyruvate and lactate pools (Fig. 4). The absolute concentrations of all the CAC intermediates examined were all significantly elevated in group P compared with group C. 1H-NMR confirmed the increased levels of myocardial lactate in group P compared with group C, as well as increases in alanine and glucose (Fig. 5), implying an increased pyruvate flux through lactate dehydrogenase and alanine transaminase.
Fig. 4.
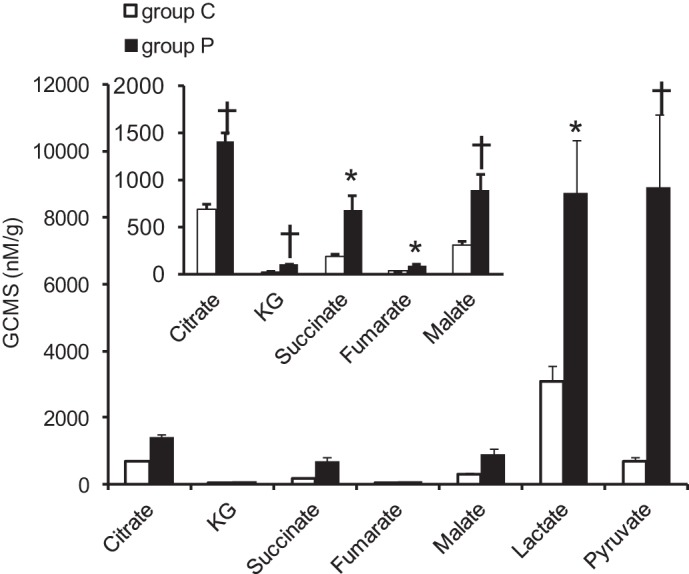
CAC intermediates by gas chromatography and mass spectrometry (GC-MS). The absolute concentration of CAC intermediates significantly increased with pyruvate compared with control. Values are means ± SE; n = 6 per group. *P < 0.05 vs. group C, †P < 0.01 vs. group C. KG, ketoglutarate.
Fig. 5.
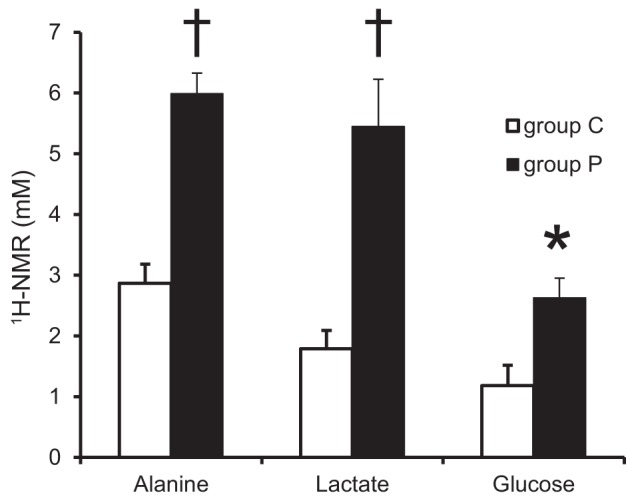
Concentrations of myocardial alanine, lactate and glucose by nuclear magnetic resonance (NMR). Absolute concentration of alanine, lactate, and glucose showed significant increase in group P. Values are means ± SE; n = 6 per group. *P < 0.05 vs. group C, †P < 0.01 vs. group C.
PDC/CS and PC/PDC Flux Ratio
Data from GC-MS and NMR as noted in materials and methods were used to estimate PDC/CIT and the anaplerotic PC pathway [PC/(PC + PDC)], respectively. No significant difference was observed between group C and group P for pyruvate oxidation (Fig. 6A); however, a significant increase in anaplerotic flux via PC was observed (Fig. 6B). These results suggest the CAC intermediate increases are not due to pyruvate oxidation by the PDC pathway but rather via PC.
Fig. 6.
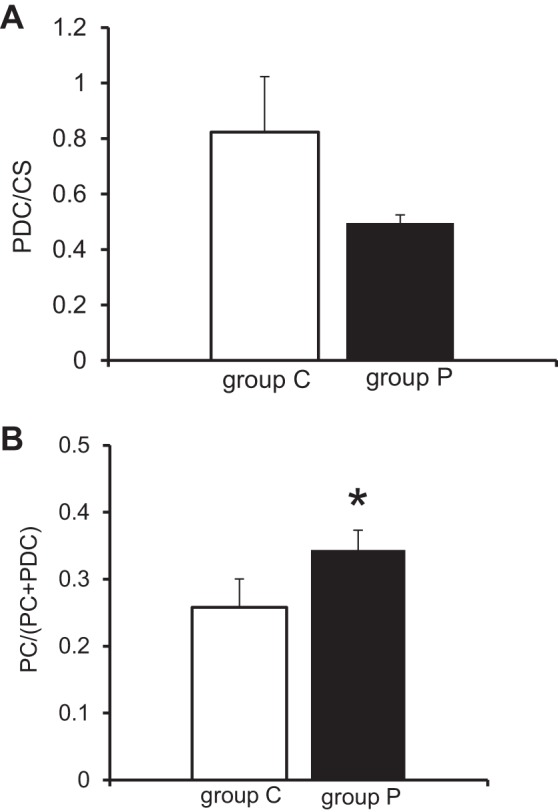
Flux Ratio. A: Pyruvate dehydrogenase complex (PDC) to citrate synthase (CS) flux ratio by GC-MS. No significant difference between group P and group C. B: PC to PDC flux ratio. Values are means ± SE; n = 6 per group. *P < 0.05 vs. group C.
Fractional Contributions of Acetyl-CoA to the CAC
13C-NMR data assessed the fractional contributions of acetyl-CoA to the CAC for the individual labeled substrates (pyruvate and leucine) and the unlabeled substrates (Fig. 7). The principal purpose of these fractional contribution analyses was to determine whether systemic pyruvate infusion altered amino acid (leucine oxidation) relative to total citric acid flux. Accordingly, we observed no significant change between groups during ECMO to the fractional contribution from 13C-labeled leucine (Fc-leucine). The Fc-pyruvate and Fc-unlabeled substrate showed a significant decrease and increase, respectively. However, this observed significant effect was probably due to dilution of the pyruvate pool from the unlabeled pyruvate provided during the extended infusion. Therefore, we did not use these data to interpret pyruvate fluxes.
Fig. 7.
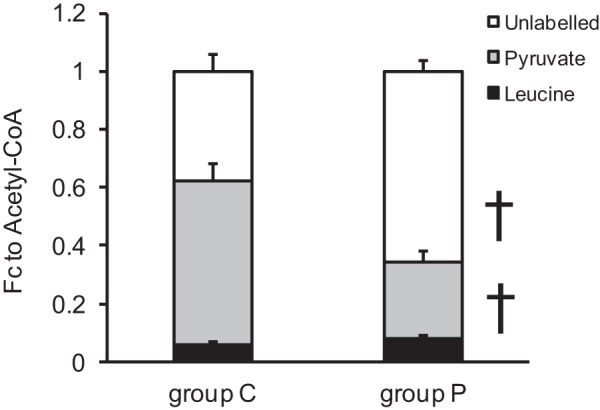
Fractional contributions of acetyl-CoA to the CAC by 13C-NMR. Fractional contribution (Fc) from [13C6,15N]-l-leucine did not change with pyruvate supplement during ECMO. Fc of [2-13C]-pyruvate was decreased in the unlabeled pyruvate infusion group, reflected by an increase in unlabeled substrate. Values are means ± SE; n = 6 per group, †P < 0.01.
Intracellular Free Leucine MPE and Global Protein FSR
No significant change was observed between group P and group C for FSR (Fig. 8A). However, the intracellular free leucine MPE as determined by GC-MS was significantly increased in group P (Fig. 8B). Interestingly, a previous study in our laboratory showed that pyruvate reduced the FSRs (42), suggesting that extended pyruvate infusion abrogated this short-term pyruvate effect.
Fig. 8.
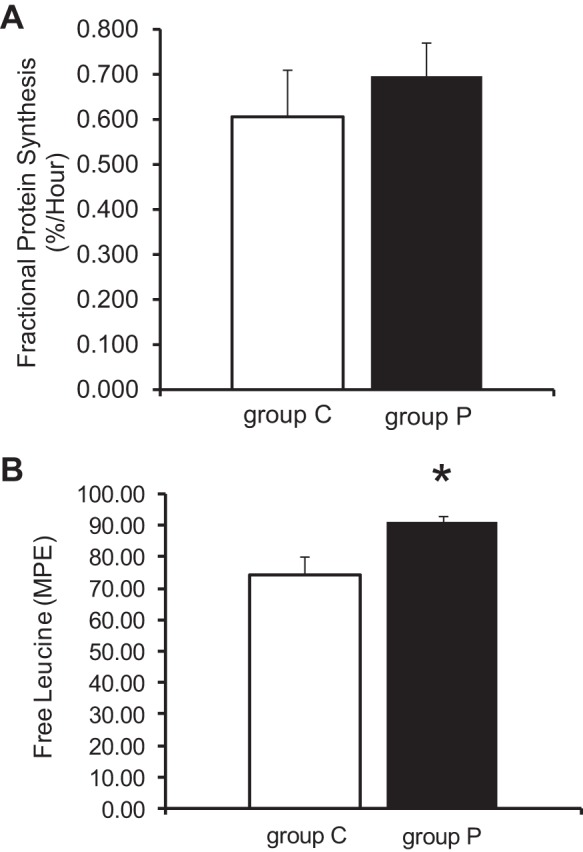
Intracellular free leucine and global fractional protein synthesis rates (FSRs) by GC-MS. A: FSRs were not significant different among the 2 groups. B: intracellular free leucine showed a significant increase in group P vs. group C. Values are means ± SE; n = 6 per group. *P < 0.05 vs. group C. MPE, molar percent enrichment.
Glycogen Stores and Free Fatty Acid Uptake
Glycogen stores were similar between the two groups, suggesting that the increased supply of pyruvate to the heart does not alter net glycogenolysis (Fig. 9). In contrast, we noted that systemic pyruvate infusion increased nonesterified fatty acid uptake over baseline (ECMO before pyruvate) (Table 2). Thus these data imply that fatty acids may represent a portion of the increased fractional contribution from unlabeled substrates with pyruvate infusion. Note that a change in myocardial oxygen consumption (MV̇o2) did not occur concurrent with the increase in free fatty acid uptake.
Fig. 9.
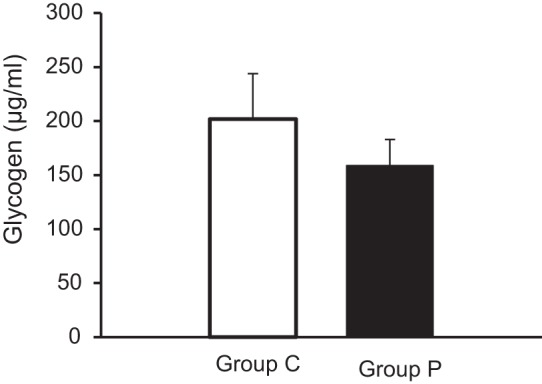
Glycogen levels in left ventricular tissue. No significant change was observed between group C and group P.
Protein Expression
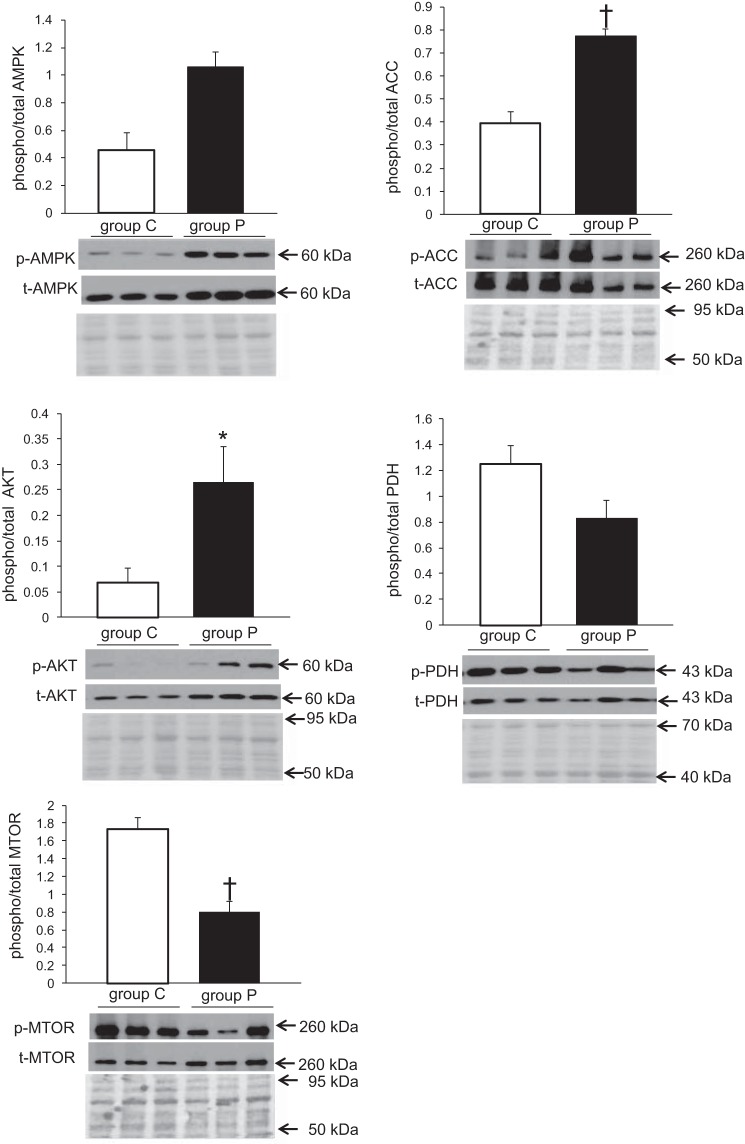
Five proteins known to be directly or indirectly involved in cellular energetics were analyzed (Fig. 10). AMPK, AKT, PDH, ACC, and mTOR. AMPK, a major regulator of cardiac energy substrate use, showed a twofold increase in phosphorylation at position Thr-172 in group P vs. group C (P < 0.01). ACC, which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, the rate-limiting step in fatty acid synthesis, is phosphorylated at Ser79, a direct target of AMPK, and also showed a twofold increase in group P vs. group C (P < 0.01). AKT, a negative regulator of AMPK activity in the heart (26), phosphorylated at Ser473, showed a fourfold increase in group P vs. group C (P < 0.05 total AMPK and AKT relative to total protein was also significantly greater in group P, graphs not shown). PDH catalyzes the decarboxylation of pyruvate to acetyl-CoA and showed a decreasing trend in group P compared with group C for phosphorylation at Ser 293; however, significance was not achieved (P = 0.07). mTOR functions as an ATP and amino acid sensor and plays numerous roles in protein synthesis. Examination of mTOR observed a significant decrease in group P compared with group C (P < 0.01). Of interest is that no change in fractional protein synthesis was observed although free leucine enrichment increased, suggesting that the enrichment is insufficient to counter the attenuation of mTOR phosphorylation.
Fig. 10.
Representative blots for expression profiles of proteins involved in regulation of nutrient or energy sensing. Phosphorylation levels normalized to total levels were assessed for AMPK, ACC, AKT, PDH, and mTOR. Total protein stains are shown below immunoblots to assess loading quantity. Values are means ± SE; n = 6 per group. All experiments were performed in duplicate, representative blot shown. *P < 0.05 vs. group C, †P < 0.01 vs. group C.
O-GlcNAcylation
O-GlcNAcylation is a posttranslational modification shown to alter protein activity in response to cellular stimuli. On the basis of the increase in glucose concentration detected by 1H-NMR and its use as a substrate in O-GlcNAcylation, O-GlcNAc levels were examined. Whole-cell O-GlcNAc levels were assessed and found to be significantly increased in group P compared with group C (P < 0.01) (Fig. 11).
Fig. 11.
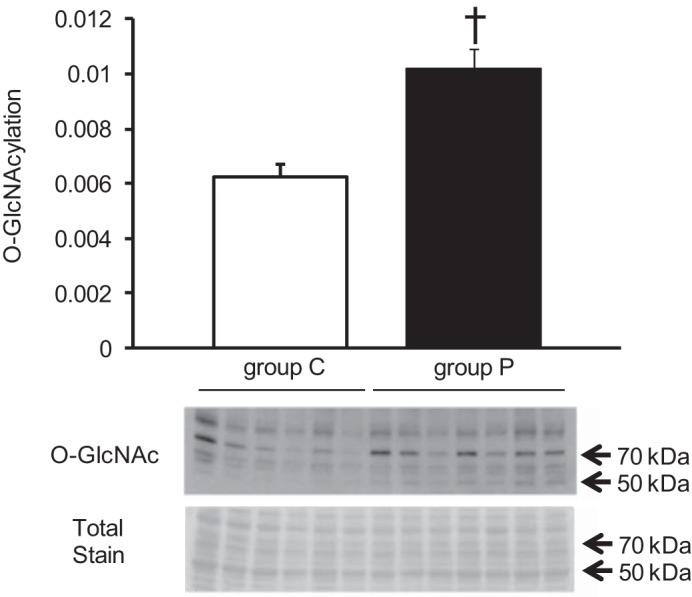
Representative immunoblots with densitometric analyses for O-GlcNAc proteins. O-GlcNAc proteins were normalized to total protein stain. Values are means ± SE; n = 3 per group. †P < 0.01 vs. group C.
DISCUSSION
ECMO produces physiological conditions that generally relieve cardiac workload but support systemic circulation. The overall influence of ECMO on cardiac remodeling still remains unclear. Unloading through ventricular assist devices (VAD) in adults appears to induce regression of hypertrophy associated with induction of fetal gene program and shifts in metabolic signaling (1, 14). However, ECMO remains unique and differs from ventricular assist in many aspects, including the goal of therapy. Whereas VAD usually serves as a bridge to transplant, ECMO at least for newborn and infant cardiac conditions commonly serves as a bridge to recovery. Nevertheless, several investigators have noted that some patients under VAD may eventually improve and achieve weaning. Thus it is imperative to clearly define processes that will control myocardial signaling and adaptation during ventricular unloading by mechanical circulation, whether by ECMO or VAD.
Nutritional regulation of these metabolic processes represents one such adaptive strategy, which previously has not been vigorously pursued in appropriate animal models. In the present study, we sought to determine whether nutritional supplementation with pyruvate could significantly alter metabolic signaling during these unique but clinically relevant conditions. First, we determined that pyruvate, presumably through mass-action kinetics, redirected pyruvate through several reactions. These include PC reactions, resulting in accumulation of CAC intermediates, lactate dehydrogenase producing lactate, and pyruvate transamination producing alanine. Prior studies have evaluated pyruvate partitioning between oxidation and anaplerosis but were not directed toward investigating pyruvate as a nutritional supplement (41). Those investigations used fairly brief equilibration periods for pyruvate infusions, as opposed to several hours as performed in our experiments. Accordingly, the investigators did not observe the substantial increases in tissue pyruvate and lactate concentration, which were achieved in our experiments, although steady-state pyruvate serum concentrations were similar. Thus the present study shows that, over several hours, steady influx of pyruvate promotes kinetics, which results in substantial accumulation of all the CAC intermediates. Our kinetic data from both GC-MS and NMR demonstrate that this accumulation likely occurs through PC, as no increase in PDC/CIT occurs. The specific influence on PC may relate to Km differences between enzymes regulating this anaplerosis and pyruvate dehydrogenase complex (25, 41).
There is close integration between the CAC intermediates and amino acids via oxidative and anaplerotic pathways. Utilization of 13C-labeled leucine to study the relationship between amino acid oxidation and protein synthesis has been previously detailed. Some experimental models, predominantly in vitro, use labeled phenylalanine to estimate fractional protein synthesis, as leucine can stimulate its own corporation into protein. However, several studies show that even long-term or repeated dosing with leucine does not stimulate protein synthesis in porcine heart in vivo (2, 10, 53). Furthermore, preliminary studies in our laboratory showed no significant differences between estimated fractional protein synthesis values in porcine heart in vivo provided by infusion of 13C- labeled phenylalanine and 13C-labeled leucine. In following, our observed alterations in concentrations of these intermediates did not alter fractional contribution of acetyl-CoA by leucine, our surrogate amino acid, to the CAC. Furthermore, we did not observe changes in fractional protein synthesis although leucine entry into the cell, estimated by mildly increased leucine MPE, did increase. Thus our expansion of the CAC intermediate pool occurred without substantial effect on amino acid oxidation or incorporation into protein.
Okere et al. (37) showed that expansion of the CAC intermediate pool with substrate supplementation does not necessarily improve cardiac function. However, in our study, accumulation of these metabolites occurs in conjunction with alterations in upstream metabolic signaling, suggesting operation of a feedback system. Signaling pathways coordinate enzyme activity of molecules controlling various aspects of substrate utilization; therefore, we assessed phosphorylation, a surrogate for activity of these key signaling proteins involved in metabolic regulation, which might be influenced by pyruvate supplementation in our experimental model. The AMPK is a serine-threonine kinase, which regulates cellular metabolism, senses the cellular energy status, and promotes ATP-generating pathways, such as glycolysis and fatty acid oxidation. AMPK activation enhances mitochondrial fatty acid uptake via carnitine palmitoyltransferase-1 (CPT-1) by the inhibitory phosphorylation of ACC. The inactivation of ACC results in downregulation of the malonyl CoA, a potent inhibitor of CPT-1, thereby allowing for an increase in fatty acyl-Co A transport into the mitochondria for β-oxidation (8, 9). Group P showed an increase in AMPK activation along with the reciprocal increase in deactivation of ACC, which suggests that pyruvate supplementation shifts oxidative substrate preference toward fatty acid oxidation, as opposed to pyruvate. This suggestion is further supported by our data showing an increase in free fatty acid uptake during pyruvate infusion. Of course, we recognize that uptake may not correspond with oxidation of the free fatty acids.
AMPK activation has also been shown to enhance translocation of GLUT4, a glucose transporter, to the sarcolemmal membrane (52) and the synthesis of pyruvate through glycolysis via the phosphorylation of phosphofructokinase 2 (35). GLUT4 translocation is also regulated by activation of AKT (24, 47), and in our study we observed an increase in AKT activation in the pyruvate group. AKT and AMPK phosphorylate the same substrate, AS160/TBC1D1, which leads to an increase in GLUT4 trafficking (45). We attempted to ascertain GLUT4 changes in this study; however, trials with two different GLUT4 antibodies yielded no usable data, most likely because of antibody cross-species incompatibility. We did note increased cytosolic glucose in pyruvate-supplemented hearts, suggesting that the increase in pyruvate either signals for increased glucose transmembrane transport as suggested by our observed changes in AMPK and AKT activation or decreased glycolysis.
Regardless of the precise mechanism, the glucose accumulation prompted us to further evaluate another nutrient-sensitive pathway involved in metabolic regulation. For this we examined the HBP, which shunts glucose toward synthesis of UDP-GlcNAc. O-GlcNAc transferase (OGT) uses UDP-GlcNAc as the monosaccharide donor to attach O-GlcNAc to target proteins as a posttranslational modification. OGT is highly sensitive to UDP-GlcNAc levels, making overall O-GlcNAc levels responsive to changes in HBP flux (27). The HBP demonstrates exquisite nutrient sensitivity, specifically responding to intracellular glucose concentration and/or transport rate. The hearts supplemented with pyruvate and displaying increased free intracellular glucose also demonstrated elevated levels of O-GlcNAcylated proteins by standard immunoblot methodology.
Another metabolite also implicated in regulation of HBP is glucosamine, which promoted flux through the HBP in isolated perfused rat heart (28). However, HBP modification by pyruvate has not been previously recognized. Like AMPK, final products of HBP have been shown to induce fatty acid oxidation (28, 33). The observed induction of fatty acid oxidation by glucosamine may relate to O-GlcNAc modification of the FAT/CD36 and, in particular, the membrane fraction of this transporter (28). Although employed by many investigators, the immunoblot method used to determine global O-GlcNAcylation does not identify specific protein targets. Thus further studies are required to identify proteins that undergo pyruvate-related modifications in HBP flux. Furthermore, it remains unclear whether an increase in protein O-GlcNAcylation represents a benefit or disadvantage in this model. O-GlcNAcylation is an intrinsic mechanism for cellular adaptation to acute stressful stimuli that is common across multiple cell types and to various adverse stimuli (4, 5, 51, 54). In the heart, acutely augmenting O-GlcNAc levels during ex vivo or in vivo ischemia is cardioprotective and improves functional recovery (4, 13, 21, 29–32, 36). Paradoxically, chronic elevation in O-GlcNAcylation yields cardiac dysfunction in certain animal models exposed to more prolonged stress (6, 12, 17). In particular, O-GlcNAcylation appears to cause dysfunction in a mouse model of diabetic cardiomyopathy (6, 12, 17).
Interestingly, OGT, the enzyme that catalyzes the transfer of O-GlcNAc to proteins, is phosphorylated by AMPK (3). Additionally, inhibition of O-GlcNAc decreased glucose-deprivation-induced activation of AMPK. Therefore, the altered activity expression in AMPK and O-GlcNAcylation may indicate a potential cooperative regulatory mechanism mediated by substrate availability.
The observed increase of leucine enrichment in group P prompted us to assess expression and activity for mTOR. This is a multidomain protein interacting with multiple protein partners and affecting a myriad of cellular processes, including protein synthesis. At least in vitro and in cell culture, mTOR signaling is regulated by amino acids and cellular energy status (34, 44, 50). Because protein synthesis requires a large amount of cellular energy, cross talk between AMPK and mTOR occurs. AMPK can modulate mTOR activation by its phosphorylation and activation of tuberous sclerosis complex 2, an inhibitor of mTOR (18). Additionally, AMPK phosphorylates Raptor, a binding partner of mTOR, resulting in the repression of mTOR activity (15). Conversely, amino acids upregulate mTOR, with leucine as the most effective stimulator (20). However, under the conditions used for group P, we observed a significant decrease in mTOR phosphorylation. This in conjunction with no change in fractional protein synthesis, even in the presence of a final infusion of leucine in the last hour of ECMO, suggests that increased leucine could not counter the deactivation of mTOR.
In conclusion, this study shows that pyruvate mediates several important processes in the heart, which may hold clinical relevance. Although prolonged pyruvate infusion does not activate oxidation via pyruvate dehydrogenase, it does appear to direct pyruvate toward other metabolic pathways. These include anaplerosis via PC, transamination to alanine, and reduction to lactate. Additionally, the results suggest that pyruvate activates fatty acid oxidation and redirects glucose to the HBP without a detriment to protein synthesis. The resulting increase in protein O-GlcNAcylation has potentially ubiquitous effects on numerous proteins including transcriptional factors. These modifications may be important for successful myocardial adaptation and successful weaning from the ECMO circuit following prolonged ventricular unloading. These experiments represent an initial phase of a continuing process to develop metabolic strategies for the unloaded heart during ECMO. In the present study, we did not evaluate the impact of these metabolic shifts and signaling patterns on cardiac function after weaning. Future studies will need to be performed that will specifically evaluate the functional impact of these processes on the heart during weaning.
There are some limitations of this study. Our 13C substrate-labeling pattern was directed at pyruvate and leucine metabolism and precluded simultaneous measurement of fatty acid contribution. Thus, although we identified mechanisms involved in the activation of the fatty acid oxidation pathway, we were not able to confirm an actual increase in relative fatty acid flux. Additionally, we performed these experiments under conditions emulating ventricular unloading during ECMO. Thus we could not evaluate the cardiac functional significance of these metabolic perturbations. Such experiments are planned for the future.
GRANTS
This work was supported by the National Institutes of Health (HL60666 to M. Portman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.R.L., M.K., C.M.O.P., N.I., I.R.F., and C.D.R. performed experiments; D.R.L., M.K., C.M.O.P., A.K.O., I.R.F., C.D.R., and M.A.P. analyzed data; D.R.L., M.K., C.M.O.P., A.K.O., C.D.R., and M.A.P. interpreted results of experiments; D.R.L. and M.K. prepared figures; D.R.L., M.K., A.K.O., C.D.R., and M.A.P. drafted manuscript; D.R.L., M.K., and M.A.P. edited and revised manuscript; M.K., C.M.O.P., and M.A.P. conception and design of research; M.A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Chun Xu for assistance with perfusion. A portion of the research was performed using Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the Department of Energy Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory.
REFERENCES
- 1.Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res 90: 234–242, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutry C, El-Kadi SW, Suryawan A, Wheatley SM, Orellana RA, Kimball SR, Nguyen HV, Davis TA. Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am J Physiol Endocrinol Metab 305: E620–E631, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullen JW, Balsbaugh JL, Chanda D, Shabanowitz J, Hunt DF, Neumann D, Hart GW. Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc transferase (OGT) and AMP-activated protein kinase (AMPK). J Biol Chem 289: 10592–10606, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol 294: C1509–C1520, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatham JC, Marchase RB. The role of protein O-linked beta-N-acetylglucosamine in mediating cardiac stress responses. Biochim Biophys Acta 1800: 57–66, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 278: 44230–44237, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Des Rosiers C, Lloyd S, Comte B, Chatham JC. A critical perspective of the use of (13)C-isotopomer analysis by GCMS and NMR as applied to cardiac metabolism. Metab Eng 6: 44–58, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Dyck JR, Kudo N, Barr AJ, Davies SP, Hardie DG, Lopaschuk GD. Phosphorylation control of cardiac acetyl-CoA carboxylase by cAMP-dependent protein kinase and 5′-AMP activated protein kinase. Eur J Biochem 262: 184–190, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol 574: 95–112, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab 290: E612–E621, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Files MD, Kajimoto M, O'Kelly Priddy CM, Ledee DR, Xu C, Des Rosiers C, Isern N, Portman MA. Triiodothyronine facilitates weaning from extracorporeal membrane oxygenation by improved mitochondrial substrate utilization. J Am Heart Assoc 3: e000680, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fricovsky ES, Suarez J, Ihm SH, Scott BT, Suarez-Ramirez JA, Banerjee I, Torres-Gonzalez M, Wang H, Ellrott I, Maya-Ramos L, Villarreal F, Dillmann WH. Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy. Am J Physiol Regul Integr Comp Physiol 303: R689–R699, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulop N, Zhang Z, Marchase RB, Chatham JC. Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol 292: H2227–H2236, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest 82: 2017–2025, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haines NM, Rycus PT, Zwischenberger JB, Bartlett RH, Undar A. Extracorporeal Life Support Registry Report 2008: neonatal and pediatric cardiac cases. ASAIO J 55: 111–116, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res 96: 1006–1013, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Jaleel A, Short KR, Asmann YW, Klaus KA, Morse DM, Ford GC, Nair KS. In vivo measurement of synthesis rate of individual skeletal muscle mitochondrial proteins. Am J Physiol Endocrinol Metab 295: E1255–E1268, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferson LS, Kimball SR. Amino acids as regulators of gene expression at the level of mRNA translation. J Nutr 133: 2046S–2051S, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117: 1172–1182, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Kajimoto M, O'Kelly Priddy CM, Ledee DR, Xu C, Isern N, Olson AK, Des Rosiers C, Portman MA. Myocardial reloading after extracorporeal membrane oxygenation alters substrate metabolism while promoting protein synthesis. J Am Heart Assoc 2: e000106, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kajimoto M, O'Kelly Priddy CM, Ledee DR, Xu C, Isern N, Olson AK, Portman MA. Extracorporeal membrane oxygenation promotes long chain fatty acid oxidation in the immature swine heart in vivo. J Mol Cell Cardiol 62: 144–152, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277: 22115–22118, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Khairallah M, Labarthe F, Bouchard B, Danialou G, Petrof BJ, Des Rosiers C. Profiling substrate fluxes in the isolated working mouse heart using 13C-labeled substrates: focusing on the origin and fate of pyruvate and citrate carbons. Am J Physiol Heart Circ Physiol 286: H1461–H1470, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem 278: 39422–39427, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem 274: 32015–32022, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Laczy B, Fulop N, Onay-Besikci A, Des Rosiers C, Chatham JC. Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation. PLoS One 6: e18417, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laczy B, Marsh SA, Brocks CA, Wittmann I, Chatham JC. Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner. Am J Physiol Heart Circ Physiol 299: H1715–H1727, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol 42: 177–185, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Marchase RB, Chatham JC. Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. Am J Physiol Heart Circ Physiol 293: H1391–H1399, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol 40: 303–312, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Luo B, Parker GJ, Cooksey RC, Soesanto Y, Evans M, Jones D, McClain DA. Chronic hexosamine flux stimulates fatty acid oxidation by activating AMP-activated protein kinase in adipocytes. J Biol Chem 282: 7172–7180, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10: 1247–1255, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Ngoh GA, Watson LJ, Facundo HT, Dillmann W, Jones SP. Non-canonical glycosyltransferase modulates posthypoxic cardiac myocyte death and mitochondrial permeability transition. J Mol Cell Cardiol 45: 313–325, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okere IC, McElfresh TA, Brunengraber DZ, Martini W, Sterk JP, Huang H, Chandler MP, Brunengraber H, Stanley WC. Differential effects of heptanoate and hexanoate on myocardial citric acid cycle intermediates following ischemia-reperfusion. J Appl Physiol 100: 76–82, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Olson AK, Bouchard B, Ning XH, Isern N, Rosiers CD, Portman MA. Triiodothyronine increases myocardial function and pyruvate entry into the citric acid cycle after reperfusion in a model of infant cardiopulmonary bypass. Am J Physiol Heart Circ Physiol 302: H1086–H1093, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson AK, Hyyti OM, Cohen GA, Ning XH, Sadilek M, Isern N, Portman MA. Superior cardiac function via anaplerotic pyruvate in the immature swine heart after cardiopulmonary bypass and reperfusion. Am J Physiol Heart Circ Physiol 295: H2315–H2320, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panchal AR, Comte B, Huang H, Dudar B, Roth B, Chandler M, Des Rosiers C, Brunengraber H, Stanley WC. Acute hibernation decreases myocardial pyruvate carboxylation and citrate release. Am J Physiol Heart Circ Physiol 281: H1613–H1620, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Panchal AR, Comte B, Huang H, Kerwin T, Darvish A, Des Rosiers C, Brunengraber H, Stanley WC. Partitioning of pyruvate between oxidation and anaplerosis in swine hearts. Am J Physiol Heart Circ Physiol 279: H2390–H2398, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Priddy CM, Kajimoto M, Ledee DR, Bouchard B, Isern N, Olson AK, Des Rosiers C, Portman MA. Myocardial oxidative metabolism and protein synthesis during mechanical circulatory support by extracorporeal membrane oxygenation. Am J Physiol Heart Circ Physiol 304: H406–H414, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riazi R, Khairallah M, Cameron JM, Pencharz PB, Des Rosiers C, Robinson BH. Probing pyruvate metabolism in normal and mutant fibroblast cell lines using 13C-labeled mass isotopomer analysis and mass spectrometry. Mol Genet Metab 98: 349–355, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Roccio M, Bos JL, Zwartkruis FJ. Regulation of the small GTPase Rheb by amino acids. Oncogene 25: 657–664, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab 295: E29–E37, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salvin JW, Laussen PC, Thiagarajan RR. Extracorporeal membrane oxygenation for postcardiotomy mechanical cardiovascular support in children with congenital heart disease. Paediatr Anaesth 18: 1157–1162, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma AB, Knott EM, Bi J, Martinez RR, Sun J, Mallet RT. Pyruvate improves cardiac electromechanical and metabolic recovery from cardiopulmonary arrest and resuscitation. Resuscitation 66: 71–81, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem 280: 18717–18727, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Sohn KC, Lee KY, Park JE, Do SI. OGT functions as a catalytic chaperone under heat stress response: a unique defense role of OGT in hyperthermia. Biochem Biophys Res Commun 322: 1045–1051, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Till M, Ouwens DM, Kessler A, Eckel J. Molecular mechanisms of contraction-regulated cardiac glucose transport. Biochem J 346: 841–847, 2000. [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson FA, Suryawan A, Orellana RA, Gazzaneo MC, Nguyen HV, Davis TA. Differential effects of long-term leucine infusion on tissue protein synthesis in neonatal pigs. Amino Acids 40: 157–165, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 279: 30133–30142, 2004. [DOI] [PubMed] [Google Scholar]