Abstract
IL-6 signaling via the soluble IL-6 receptor (sIL-6r) has been shown to increase primary afferent responsiveness to noxious stimuli. This finding prompted us to test the hypothesis that IL-6 and sIL-6r would increase the exercise pressor reflex in decerebrate rats with freely perfused femoral arteries. We also tested the hypothesis that soluble glycoprotein (sgp)130, an inhibitor of IL-6/sIL-6r signaling, would decrease the exaggerated exercise pressor reflex that is found in decerebrate rats with ligated femoral arteries. In rats with freely perfused femoral arteries, coinjection of 50 ng of IL-6 and sIL-6r into the arterial supply of the hindlimb significantly increased the peak pressor response to static (control: 14 ± 3 mmHg and IL-6/sIL-6r: 17 ± 2 mmHg, P = 0.03) and intermittent isometric (control: 10 ± 2 mmHg and IL-6/sIL-6r: 15 ± 4 mmHg, P = 0.03) hindlimb muscle contraction. In rats with ligated femoral arteries, injection of 50 ng of sgp130 into the arterial supply of the hindlimb reduced the peak pressor response to static (control: 24 ± 2 mmHg and sgp130: 16 ± 3 mmHg, P = 0.01) and intermittent isometric (control: 16 ± 2 mmHg and sgp130: 13 ± 2 mmHg, P = 0.04) hindlimb muscle contraction, whereas there was no effect of sgp130 on the exercise pressor reflex in rats with freely perfused femoral arteries. We conclude that coinjection of exogenous IL-6 and sIL-6r increased the exercise pressor reflex in rats with freely perfused femoral arteries. More importantly, we also conclude that IL-6 and sIL-6r play an endogenous role in evoking the exercise pressor reflex in rats with ligated femoral arteries but not in rats with freely perfused femoral arteries.
Keywords: skeletal muscle afferents, sympathetic nervous system, cytokines
il-6 functions as both a proinflammatory cytokine released from macrophages and other tissues as well as an anti-inflammatory myokine released from skeletal muscle (16, 19, 29). IL-6 binds to a membrane-bound IL-6 receptor (IL-6r) when it is present and then associates with the secondary membrane-bound signaling protein glycoprotein (gp)130. Alternatively, IL-6 may bind to a soluble form of IL-6r (sIL-6r) and then associate with membrane-bound gp130. A soluble circulating form of gp130 (sgp130) acts as an inhibitor of the IL-6/sIL-6r complex (20). IL-6 has been linked to numerous physiological processes, including tissue regeneration (35), platelet aggregation (28), and metabolic control (23, 43). A large body of evidence has also demonstrated that IL-6 signaling via sIL-6r, which has been termed IL-6 “trans-signaling” (34), sensitizes primary afferents (8, 13, 27, 48). Obreja et al. (27) found, for example, that dermal application of IL-6 and sIL-6r via microdialysis, but not application of IL-6 alone, sensitized rat skin afferents to noxious heat and that the IL-6/sIL-6r-induced afferent sensitization was prevented by local application of sgp130.
Exercise elicits marked cardiovascular and autonomic adjustments that include increases in blood pressure and sympathetic nervous system activity. The exercise pressor reflex contributes importantly to the cardiovascular and autonomic adjustments that occur during exercise (21). The afferent arm of the exercise pressor reflex is comprised of thinly myelinated group III and unmyelinated group IV afferents, collectively known as thin fiber afferents, whose sensory endings are located within the interstitium of skeletal muscle and are activated by both mechanical and metabolic stimuli (22, 24). The exercise pressor reflex is activated during static exercise when blood flow and O2 supply to the contracting muscles change minimally (10, 24, 31, 39) as well as during dynamic exercise when blood flow and O2 supply to the contracting muscles are able to increase (1, 12).
Our laboratory has demonstrated that the exercise pressor reflex is larger in rats whose femoral artery was ligated 72 h before the experiment than it was in rats whose femoral arteries were freely perfused (41). Ligation of a femoral artery for 72 h in the rat does not impact hindlimb muscle blood flow at rest but reduces hindlimb muscle blood flow reserve capacity to only ∼20–30% of normal during exercise (33, 47). The hindlimb muscle blood flow patterns at rest and during exercise as well as the exaggerated exercise pressor reflex in rats with ligated femoral arteries closely resemble the leg blood flow patterns at rest and during exercise (4) and the exaggerated exercise pressor reflex that is found in patients with peripheral arterial disease (PAD) (4–6, 25). Moreover, at rest, PAD patients have increased levels of IL-6 and sIL-6r in their mixed venous blood compared with healthy age-matched subjects (3, 9, 17, 30, 36). During exercise, levels of IL-6 in the mixed venous blood increased to a greater extent in PAD patients than they did in healthy age-matched subjects (30, 36). The role played by IL-6 and sIL-6r on the exaggerated exercise pressor reflex in PAD patients or in rats with ligated femoral arteries, however, is unknown. The purpose of the present study was to investigate the role played by IL-6 and sIL-6r in evoking the exercise pressor reflex in decerebrate rats with freely perfused and ligated femoral arteries. We tested the hypotheses that 1) coinjection of IL-6 and sIL-6r into the arterial supply of the hindlimb of rats with freely perfused femoral arteries increases the exercise pressor reflex and 2) injection of sgp130 into the arterial supply of the hindlimb decreases the exercise pressor reflex in rats with a ligated femoral artery but not in rats with freely perfused femoral arteries.
MATERIALS AND METHODS
All procedures and protocols were approved by the Institutional Animal Care and Use Committee of the Penn State College of Medicine. Experiments were performed on adult male Sprague-Dawley rats (n = 51, body weight range: 343–510 g). In 14 rats, the left femoral artery was ligated 72 h before the experiment. Specifically, rats were anesthetized with 3–4% isoflurane (balance O2), and the left femoral artery was surgically exposed and ligated tightly (5-0 silk suture) ∼3 mm distal to the inguinal ligament. Three rats were subjected to sham surgery, which consisted of exposing the left femoral artery and then passing a suture under the artery without tying it. The experiments described below were completed in rats whose left femoral artery was ligated 72 h before the experiment (“ligated rats,” n = 14), rats subjected to sham surgery (“sham rats,” n = 3), or rats who were not subjected to any surgery and thus had patent femoral arteries (“freely perfused rats,” n = 34). See the text below and also Table 1 for details regarding the numbers of rats in each experimental group.
Table 1.
Baseline HRs and cardioaccelerator responses to muscle contraction
| Static Contraction |
Intermittent Isometric Contraction |
||||
|---|---|---|---|---|---|
| Number of Rats/Group | Baseline HR | ΔHR | Baseline HR | ΔHR | |
| Freely perfused rats | |||||
| Control | 8 | 380 ± 19 | 8 ± 1 | 371 ± 15 | 7 ± 2 |
| IL-6/sIL-6r (intra-arterial) | 373 ± 17 | 8 ± 1 | 371 ± 16 | 11 ± 2 | |
| Control | 5 | 383 ± 17 | 11 ± 4 | 380 ± 20 | 9 ± 5 |
| sgp130 + IL-6/sIL-6r (intra-arterial) | 344 ± 14* | 7 ± 2 | 337 ± 18* | 10 ± 4 | |
| Control | 6 | 358 ± 10 | 9 ± 1 | 342 ± 17 | 6 ± 1 |
| IL-6 (intra-arterial) | 323 ± 18 | 6 ± 1 | 322 ± 19 | 7 ± 2 | |
| Control | 6 | 352 ± 19 | 8 ± 2 | 346 ± 19 | 8 ± 4 |
| IL-6/sIL-6r (intravenous) | 339 ± 18 | 6 ± 3 | 351 ± 23 | 6 ± 2 | |
| Control | 6 | 316 ± 16 | 9 ± 2 | 309 ± 11 | 10 ± 2 |
| 50 ng sgp130 (intra-arterial) | 310 ± 17 | 7 ± 1 | 309 ± 17 | 9 ± 2 | |
| Control | 6 | 340 ± 28 | 10 ± 2 | 335 ± 25 | 9 ± 3 |
| 500 ng sgp130 (intra-arterial) | 325 ± 26 | 10 ± 5 | 334 ± 26 | 10 ± 1 | |
| Ligated rats | |||||
| Control | 7 | 363 ± 16 | 4 ± 1 | 369 ± 14 | 5 ± 1 |
| 50 ng sgp130 (intra-arterial) | 344 ± 22* | 6 ± 1 | 348 ± 20* | 4 ± 1 | |
| Control | 4 | 344 ± 12 | 7 ± 2 | 342 ± 16 | 7 ± 1 |
| 50 ng sgp130 (intravenous) | 334 ± 7 | 8 ± 2 | 339 ± 6 | 4 ± 1* | |
Values are means ± SE (in beats/min).
HR, heart rate; ΔHR, cardioaccelerator response to hindlimb muscle contraction; sIL-6r, soluble IL-6 receptor; sgp130, soluble glycoprotein 130.
P < 0.05 vs. the corresponding control value.
Surgical procedures.
On the day of the experiment, rats were anesthetized with 3–4% isoflurane (balance O2). The trachea was cannulated, and the lungs were mechanically ventilated (Harvard Apparatus) with the gaseous anesthetic until the decerebration procedure was completed. The right jugular vein and right carotid artery were cannulated with polyethylene (PE)-50 catheters for the injection of fluids and measurement of arterial blood pressure (P23 XL, Statham), respectively. Heart rate (HR) was calculated beat to beat from the arterial pressure pulse with a Gould Biotach. In freely perfused rats, the left carotid artery was cannulated with a PE-10 catheter whose tip was advanced to ∼3–4 mm above the bifurcation of the abdominal aorta. Reversible snares (2-0 silk suture) were placed around the right iliac artery and vein and the abdominal aorta and vena cava (above the tip of the PE-10 catheter). In ligated and sham rats, the left superficial epigastric artery was cannulated with a PE-8 catheter whose tip was placed near the junction of the femoral and superficial epigastric arteries. A reversible snare (2-0 silk suture) was placed around the left iliac artery and vein (i.e., proximal to the location of the catheter placed in the superficial epigastric artery). In all rats, the left sciatic nerve was surgically exposed. The left calcaneal bone was severed, the triceps surae (gastrocnemius, soleus, and plantaris) muscles were exposed, and the severed end of the calcaneal tendon was linked to a force transducer (model FT10, Grass Technologies, Warwick, RI), which was attached to a rack-and-pinion. In eight freely perfused rats in which IL-6 and sIL-6r were injected into the arterial supply of the hindlimb (see below), an ultrasonic flow probe (Transonic) was placed on the left popliteal artery (which supplies the triceps surae muscles) to measure blood flow. For all rats, arterial blood gases and pH were measured periodically throughout the experiment with a blood gas analyzer (ABL 80 FLEX, Radiometer) and maintained within normal limits (arterial Pco2: 35–45 mmHg, arterial Po2: ∼100 mmHg, and pH: 7.35–7.45) by the adjustment of ventilation and/or administration of intravenous sodium bicarbonate (8.5%) as appropriate. Core temperature was measured by a rectal probe and maintained at ∼37–38°C by a heating lamp.
Rats were placed in a Kopf customized stereotaxic frame and spinal unit with clamps placed around the pelvis and rostral lumbar vertebrae. Dexamethasone (0.2 mg iv) was injected to minimize brain stem edema. A precollicular decerebration procedure was performed, and all neural tissue rostral to the section was aspirated. After the decerebration was completed, anesthesia was terminated, and the lungs were ventilated with room air. Rats were given at least 60 min to recover and stabilize before the initiation of any experimental protocol. Experiments were performed in decerebrate, unanesthetized rats because anesthesia has been shown to prevent the exercise pressor reflex in this species (37).
Experimental procedures.
In eight freely perfused rats, we compared peak pressor responses to static and intermittent isometric hindlimb muscle contraction before and after coinjection of 50 ng of IL-6 and 50 ng of sIL-6r (50 ng of IL-6 and sIL-6r) into the arterial supply of the hindlimb. Baseline triceps surae muscle tension was set at ∼100 g, and the sciatic nerve was then stimulated for 30 s to contract the hindlimb muscles either statically (40 Hz, 0.01-ms pulse duration, ≤2 × motor threshold) or intermittently (40 Hz, 0.01-ms pulse duration, 500 ms train duration, ≤2 × motor threshold). Static and intermittent contraction periods were separated by ∼10–15 min and performed in random order. We then coinjected 50 ng of IL-6 and sIL-6r into the arterial supply of the left hindlimb via the catheter in the left carotid artery whose tip was located just above the bifurcation of the abdominal aorta. Before injection, the snares on the right iliac artery and vein and abdominal aorta and vena cava were tightened, which directed the injectate toward, and at least partially trapped the injectate within, the circulation of the left hindlimb. We have previously demonstrated that blue dye injected in this manner accessed the left hindlimb circulation (11). The snares were released 10 min after coinjection of IL-6 and sIL-6r, and the hindlimb was allowed to reperfuse for ∼50 min. Static and intermittent contraction protocols were then repeated in random order. The dose of IL-6 and sIL-6r and the timing of contractions relative to the injection were selected because Brenn et al. (8) found that coinjection of 20 ng of IL-6 and sIL-6r into the rat knee joint significantly increased primary afferent responsiveness to noxious stimuli after ∼60 min. We chose to coinject 50 ng of IL-6 and sIL-6r because coinjection into the arterial supply of the hindlimb likely resulted in its distribution throughout the entire hindlimb circulation.
In additional groups of freely perfused rats, we performed the same experimental protocol as that described above for coinjection of 50 ng of IL-6 and sIL-6r but instead injected either 1) 50 ng of sgp130 concurrent with 50 ng of IL-6 and sIL-6r into the arterial supply of the left hindlimb (n = 5), 2) 50 ng of IL-6 alone into the arterial supply of the left hindlimb (n = 6), or 3) 50 ng of IL-6 and sIL-6r into the jugular vein (n = 6). The intravenous injection experiments were performed to determine if systemic circulation of IL-6 and sIL-6r to central nervous system sites could explain the increased pressor responses to static and intermittent isometric hindlimb muscle contraction that we observed after injection of 50 ng of IL-6 and sIL-6r into the arterial supply of the hindlimb (see results). IL-6 and sIL-6r (Sigma-Aldrich) were dissolved in saline, which was also the vehicle for injections. sgp130 (Tocris) was reconstituted in 1% BSA in saline, which was also the vehicle for injections. The volume of injections across all experiments ranged from 0.1 to 0.3 ml.
To investigate whether IL-6 and sIL-6r play an endogenous role in the exercise pressor reflex, we injected sgp130 alone into the arterial supply of the left hindlimb of both freely perfused and ligated rats. In freely perfused rats, we compared the peak pressor response to static and intermittent hindlimb muscle contraction before and after injection of 50 ng (n = 6) or 500 ng (n = 6) of sgp130 into the arterial supply of the left hindlimb in the manner described above for coinjection of IL-6 and sIL-6r. In seven ligated rats and three sham rats, we compared the peak pressor response to static and intermittent isometric hindlimb muscle contraction before and after injection of 50 ng of sgp130 into the arterial supply of the left hindlimb via the catheter in the left superficial epigastric artery. The snare on the left iliac artery and vein was tightened just before injection and was released 10 min after injection. In three additional ligated rats, we compared the peak pressor response to static and intermittent contraction before and after injection of 0.1 ml of 1% BSA in saline, which was the vehicle for sgp130, into the arterial supply of the left hindlimb. In four additional ligated rats, we compared the peak pressor response to static and intermittent contraction before and after injection of 50 ng of sgp130 into the jugular vein. As described above, the intravenous injection experiments were performed to determine if systemic circulation of sgp130 to central nervous system sites could explain the decreased pressor response to static and intermittent contraction that we observed after injection of sgp130 in the arterial supply of the hindlimb of ligated rats (see results).
After all contraction protocols were completed, all rats were paralyzed with pancuronium bromide (0.5 mg/kg iv), and the sciatic nerve was stimulated with the same stimulation parameters as those used to induce muscle contraction to ensure that the pressor responses observed during contraction were not the result of electrical activation of the axons of thin fiber afferents in the sciatic nerve. Smith et al. (37) reported that that stimulation of the sciatic nerve at 40 Hz with a pulse duration of 0.025 ms at 2 × motor threshold produced marked pressor responses in decerebrate rats that had been paralyzed with pancuronium bromide. Based on that finding, we contracted the hindlimb muscles by stimulating the sciatic nerve at 40 Hz with a pulse duration of 0.01 ms at ≤2 × motor threshold. We have recently reported that stimulation of the sciatic or tibial nerves at those stimulation parameters does not induce a pressor response in paralyzed, decerebrate rats (11, 40).
Data analysis.
All physiological variables were measured and displayed continuously in real time with a Spike2 data-acquisition system (Cambridge Electronic Design). Data were recorded and stored on a computer hard drive (Dell) for future offline analysis. Baseline mean arterial pressure (MAP) and HR values were determined from the 30-s baseline period that preceded contraction. The peak pressor (increase in MAP) and cardioaccelerator (increase in HR) response to static and intermittent hindlimb muscle contraction were calculated as the difference between the peak MAP and HR value obtained during contraction and the baseline MAP and HR value. Tension-time indexes (TTIs; in kg·s) for static and intermittent contraction were calculated by subtracting the area under the tension signal trace for the 30-s baseline period from the area under the tension signal trace for the 30-s contraction periods (contraction baseline). In some experiments, the increase in the rate-pressure product (RPP = systolic blood pressure × heart rate) was calculated by subtracting the baseline RPP from the peak RPP during muscle contraction.
All data are expressed as means ± SE. Statistical comparisons were performed with paired or unpaired Student's t-tests as appropriate, and significance was accepted at P < 0.05.
RESULTS
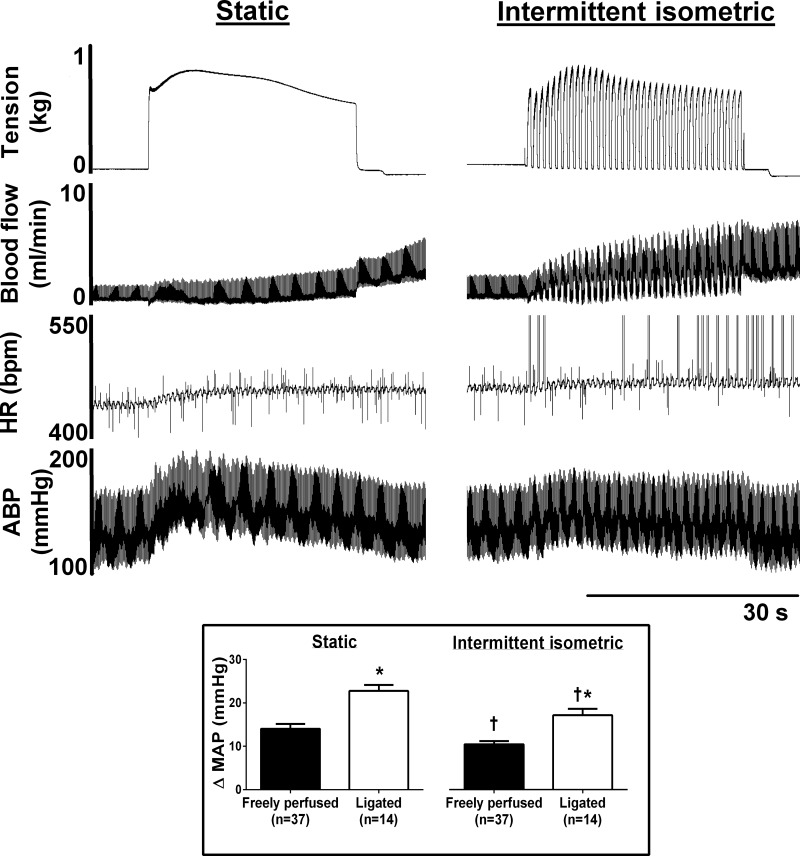
Both static and intermittent hindlimb muscle contraction markedly increased MAP, although in both freely perfused and ligated rats, the pressor response evoked by static contraction was significantly larger than the peak pressor response evoked by intermittent contraction. Peak pressor responses to static and intermittent contraction were significantly greater in ligated rats than in freely perfused rats (Fig. 1).
Fig. 1.
An example of original data from a freely perfused rat in which popliteal artery blood flow was measured during static and intermittent isometric hindlimb muscle contractions. Note the marked differences in the tension and blood flow profiles between the contraction bouts. HR, heart rate [in beats/min (bpm)]; ABP, arterial blood pressure. The large vertical spikes in the HR profile during intermittent contraction are artifacts. Inset: data from the control condition of all experiments in this investigation. Peak pressor responses to intermittent contraction were significantly lower than they were for static contraction (†P < 0.05 vs. static), and peak pressor responses to static and intermittent contraction were significantly greater in ligated rats compared with freely perfused rats (*P < 0.05 vs. freely perfused rats). ΔMAP, change in mean arterial pressure.
Effects of IL-6 and sIL-6r coinjection on the exercise pressor reflex.
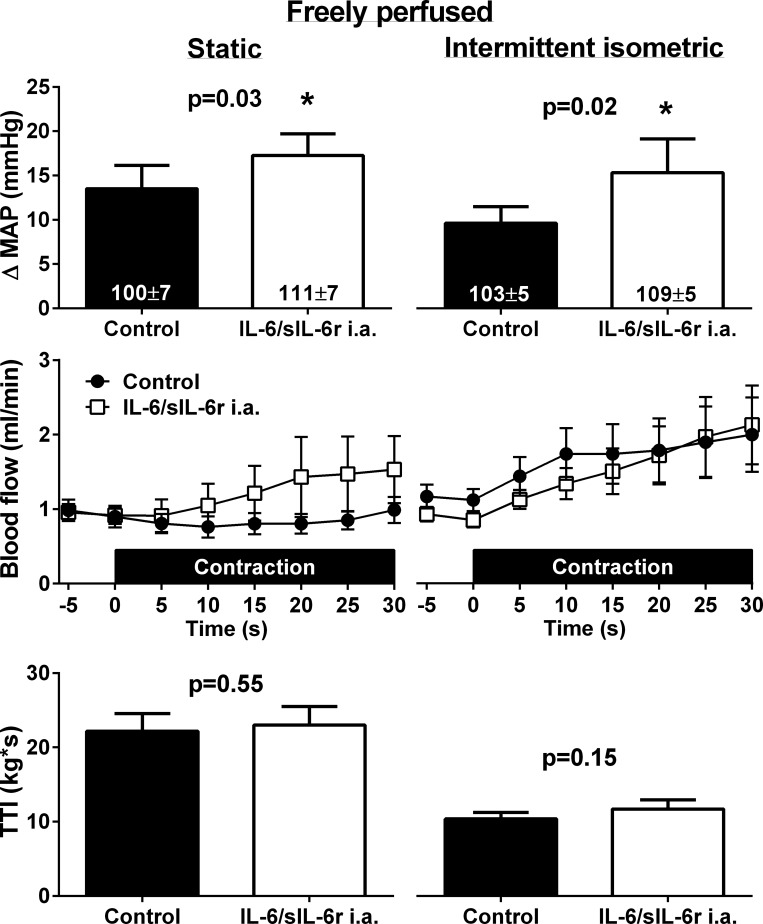
In eight freely perfused rats, coinjection of 50 ng of IL-6 and sIL-6r into the arterial supply of the hindlimb had no effect on baseline MAP, HR, or mean popliteal artery blood flow. Coinjection of 50 ng of IL-6 and sIL-6r, however, significantly increased the peak pressor response to static and intermittent hindlimb muscle contraction (Fig. 2). Coinjection of IL-6 and sIL-6r had no effect on either the cardioaccelerator (Table 1) or mean popliteal artery blood flow response (Fig. 2) to static or intermittent contraction. Consequent to the exaggerated peak pressor responses, coinjection of IL-6 and sIL-6r resulted in significantly greater increases in the RPP for static (control: 5,818 ± 1,218 mmHg·beats·min−1 and IL-6/sIL-6r: 7,705 ± 1,424 mmHg·beats·min−1, P = 0.04) and intermittent (control: 4,981 ± 819 mmHg·beats·min−1 and IL-6/sIL-6r: 8,237 ± 1,680 mmHg·beats·min−1, P = 0.02) contractions. TTIs were not different between control and IL-6/sIL-6r conditions for either contraction protocol.
Fig. 2.
In eight freely perfused rats, coinjection of 50 ng of IL-6 and soluble IL-6 receptor (sIL-6r) into the arterial supply (i.a.) of the hindlimb significantly increased the peak pressor response to static and intermittent isometric hindlimb muscle contraction. There were no effects of coinjection of IL-6 and sIL-6r on mean baseline popliteal artery blood flow or mean blood flow during static or intermittent isometric hindlimb muscle contractions (P > 0.05 between control and IL-6/sIL-6r at all time points). Numbers in columns indicate the corresponding baseline MAP values. TTI, tension-time index.
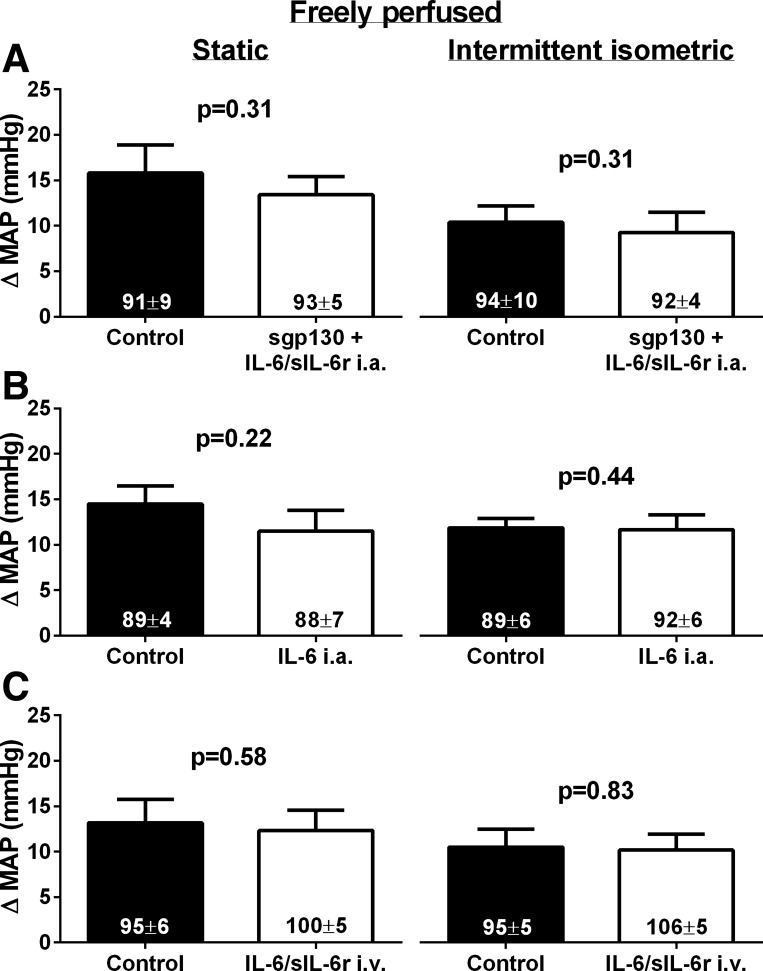
In five freely perfused rats, we found that the addition of 50 ng of sgp130 to the coinjection of 50 ng of IL-6 and sIL-6r (sgp130 + IL-6/sIL6r) into the arterial supply of the hindlimb prevented the increase in the peak pressor response to static and intermittent contraction that was induced by coinjection IL-6 and sIL-6r only (Fig. 3A). TTIs were not different between control and sgp130 + IL-6/sIL-6r for static (control: 18 ± 1 kg·s and sgp130 + IL-6/sIL-6r: 18 ± 2 kg·s, P > 0.05) or intermittent (control: 9 ± 1 kg·s and sgp130 + IL-6/sIL-6r: 9 ± 2 kg·s, P > 0.05) contraction. Furthermore, in six freely perfused rats, injection of 50 ng of IL-6 alone into the arterial supply of the hindlimb had no effect on the peak pressor response to static or intermittent contraction (Fig. 3B). TTIs were not different between control and IL-6 for static (control: 20 ± 3 kg·s and IL-6: 19 ± 1 kg·s, P > 0.05) or intermittent (control: 11 ± 2 kg·s and IL-6: 10 ± 1 kg·s, P > 0.05) contraction. In six additional freely perfused rats, coinjection of 50 ng of IL-6 and sIL-6r into the jugular vein had no effect on the peak pressor response to contraction (Fig. 3C). TTIs were not different between control and IL-6/sIL-6r for static (control: 20 ± 2 kg·s and IL-6: 19 ± 2 kg·s, P > 0.05) or intermittent (control: 9 ± 2 kg·s and IL-6: 9 ± 2 kg·s, P > 0.05) contraction. There was no effect of any of the injections on the cardioaccelerator response to static or intermittent hindlimb muscle contraction (Table 1).
Fig. 3.
A: in five freely perfused rats, injection of 50 ng of sgp130 in addition to 50 ng of IL-6 and sIL-6r into the arterial supply of the hindlimb had no effect on the peak pressor response to static or intermittent isometric hindlimb muscle contraction. B: in six freely perfused rats, injection of 50 ng of IL-6 alone into the arterial supply of the hindlimb had no effect on the peak pressor response to static or intermittent isometric hindlimb muscle contraction. C: in six freely perfused rats, injection of 50 ng of IL-6 and sIL-6r into the jugular vein (intravenous) had no effect on the peak pressor response to static or intermittent isometric hindlimb muscle contraction. TTIs were not different between control and IL-6/sIL-6r conditions for either contraction in all groups (see text). Numbers in columns indicate the corresponding baseline MAP values.
Effects of sgp130 injection on the exercise pressor reflex.
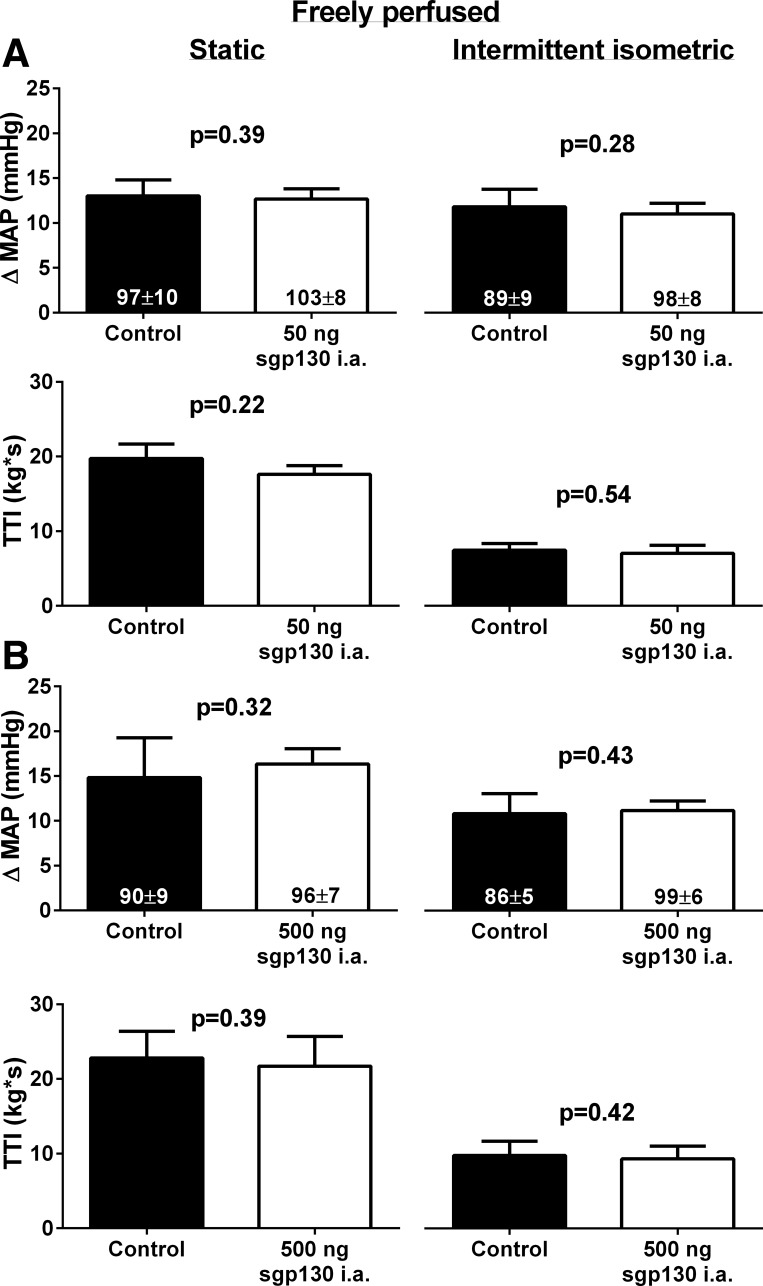
In freely perfused rats, there was no effect of injection of 50 ng (n = 6) or 500 ng (n = 6) of sgp130 into the arterial supply of the hindlimb on the peak pressor response to static or intermittent hindlimb muscle contraction (Fig. 4). In contrast, in seven ligated rats, injection of 50 ng of sgp130 into the arterial supply of the hindlimb significantly reduced the peak pressor response to static and intermittent contraction (Fig. 5). There were no effects of injection of sgp130 into the arterial supply of hindlimb on baseline MAP in either freely perfused or ligated rats. In three additional ligated rats, injection of 0.1 ml of 1% BSA, the vehicle for sgp130, into the arterial supply of the hindlimb had no effect on the peak pressor response to static (control: 23 ± 6 mmHg and 1% BSA: 23 ± 4 mmHg, P = 0.87) or intermittent (control: 14 ± 1 mmHg and BSA: 13 ± 1 mmHg, P = 0.63) contraction. Similarly, in three sham rats, injection of 50 ng of sgp130 had no effect on the peak pressor response to static (control: 15 ± 2 mmHg and sgp130: 14 ± 1 mmHg, P = 0.31) or intermittent (control: 10 ± 3 mmHg and sgp130: 11 ± 4 mmHg, P = 0.42) contraction. In four additional ligated rats, injection of 50 ng of sgp130 into the jugular vein had no effect on the peak pressor response to static (control: 21 ± 2 mmHg and sgp130: 25 ± 6 mmHg, P = 0.36) or intermittent (control: 22 ± 3 mmHg and sgp130: 23 ± 4 mmHg, P = 0.72) hindlimb muscle contraction. There was no effect of any sgp130 injection on the cardioaccelerator response to static or intermittent isometric contraction in freely perfused rats. In contrast, injection of sgp130 into the jugular vein slightly, but significantly, reduced the cardioaccelerator response to intermittent contraction (Table 1). TTIs for static and intermittent contraction were not different between control and sgp130 for any group of rats.
Fig. 4.
In freely perfused rats, injection of 50 ng (A; n = 6) or 500 ng (B; n = 6) of soluble glycoprotein (sgp)130 into the arterial supply of the hindlimb had no effect on the peak pressor response to static or intermittent isometric hindlimb muscle contraction. Numbers in columns indicate the corresponding baseline MAP values.
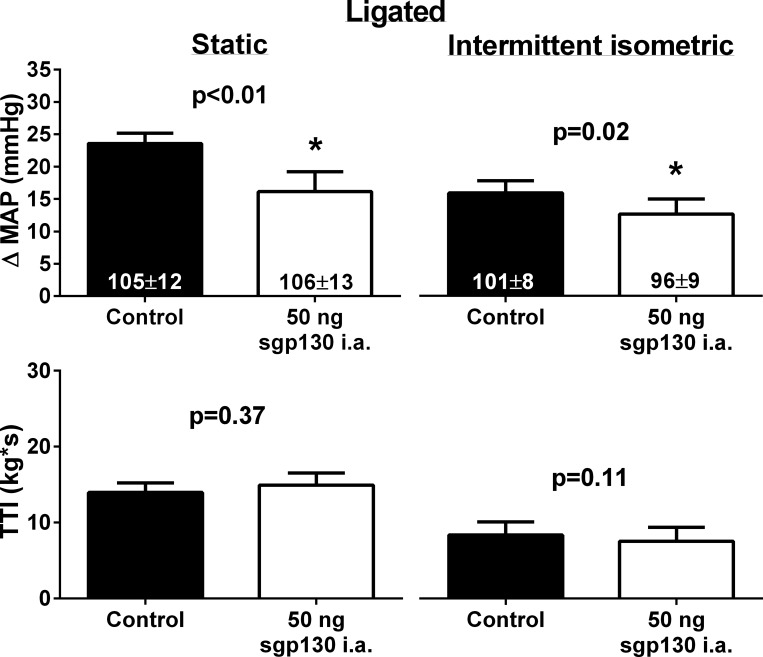
Fig. 5.
In seven ligated rats, injection of 50 ng of sgp130 into the arterial supply of the hindlimb significantly reduced the peak pressor response to static and intermittent isometric hindlimb muscle contraction. Numbers in columns indicate the corresponding baseline MAP values.
DISCUSSION
Consistent with our hypothesis, we found that coinjection of 50 ng of IL-6 and sIL-6r into the arterial supply of the hindlimb increased the exercise pressor reflex in freely perfused rats. Also consistent with our hypothesis, we found that injection of sgp130, an endogenous inhibitor of IL-6 and sIL-6r signaling, into the arterial supply of the hindlimb decreased the exaggerated exercise pressor reflex in rats with ligated femoral arteries but did not decrease the exercise pressor reflex in rats with freely perfused femoral arteries. Our findings indicate, therefore, that IL-6/sIL-6r signaling contributes to the exaggerated exercise pressor reflex that is found in rats with ligated femoral arteries but does not contribute to the exercise pressor reflex in rats with freely perfused femoral arteries.
Our finding that the coinjection of IL-6 and sIL-6r, but not the injection of IL-6 alone, into the arterial supply of the hindlimb increased the exercise pressor reflex in freely perfused rats is consistent with the previous reports (27, 42) indicating that IL-6 signaling via sIL-6r is required to sensitize sensory neurons. Brenn et al. (8), however, reported that the injection of IL-6 alone into the rat knee joint increased primary afferent responsiveness to noxious stimulation of the knee that was injected, but only at doses of IL-6 that were markedly higher than the dose of IL-6 and sIL-6r that increased primary afferent responsiveness. Whether the injection of doses of IL-6 that are >50 ng into the arterial supply of the hindlimb increase the exercise pressor reflex in rats with freely perfused femoral arteries is unknown.
We found that coinjection of IL-6 and sIL-6r into the arterial supply of the hindlimb of freely perfused rats had no effect on popliteal artery blood flow at rest or during static or intermittent isometric hindlimb muscle contraction. That was an important finding because IL-6 has been associated with impaired vascular function (15, 44), which may be attributable, at least in part, to thrombaxane A2-induced arteriolar vasoconstriction (7). If we had found that coinjection of IL-6 and sIL-6r reduced popliteal artery blood flow during contraction, the increased exercise pressor reflex could have been secondary to the increased build up of metabolites as opposed to primary afferent sensitization per se. Nevertheless, we cannot rule out the possibility that coinjection of IL-6 and sIL-6r impacted hindlimb muscle blood flow distribution and produced a mismatch between microvascular O2 supply and O2 demand in the face of unchanged mean popliteal artery blood flow. Tsuchimochi et al. (41), however, found that acute (i.e., 3 min) femoral artery ligation did not exaggerate the exercise pressor reflex in decerebrate rats. Based on that finding, even if coinjection of IL-6 and sIL-6r did impact hindlimb muscle blood flow distribution in our experiments, it would not have contributed to the increased exercise pressor reflex in our rat model. Moreover, the fact that injection of IL-6 and sIL-6r into the jugular vein did not increase peak pressor responses to contraction in the present investigation indicates that IL-6/sIL-6r signaling did not increase peripheral vasoconstriction in response to similar increases in sympathetic nervous activity during exercise. We believe, therefore, that our finding that coinjection of IL-6 and sIL-6r into the arterial supply of the hindlimb modestly, but significantly, increased the exercise pressor reflex in rats with freely perfused femoral arteries reflects the fact that IL-6 and sIL-6r sensitized the thin fiber afferents that evoke the exercise pressor reflex.
Injection of sgp130 concurrent with injection of IL-6 and sIL-6r into the arterial supply of the hindlimb prevented the ability of IL-6 and sIL-6r to increase the exercise pressor reflex in freely perfused rats. This finding is consistent with previous reports (8, 42, 45) that have found that the administration of sgp130 concurrent with IL-6 and sIL-6r prevented IL-6/sIL-6r-induced afferent sensitization in response to noxious stimuli. Injection of sgp130 with IL-6 and sIL-6r into the rat knee joint, for example, prevented IL-6- and sIL-6r-induced increases in primary afferent (8) and spinal interneuron (42) responsiveness to noxious twisting of the knee joint.
We also found that injection of sgp130 alone into the arterial supply of the hindlimb reduced the exaggerated exercise pressor reflex in ligated rats. In contrast to our finding in ligated rats, injection of sgp130 into the arterial supply of the hindlimb did not reduce the exercise pressor reflex in freely perfused rats, even at doses of sgp130 that were 10 times greater than the effective dose of sgp130 in ligated rats. Jostock et al. (20) reported that, in addition to inhibiting IL-6/sIL-6r signaling, sgp130 may also inhibit signaling of the IL-6-related cytokines leukemia inhibiting factor and oncostatin M. Importantly, however, sgp130 showed a markedly higher affinity for IL-6/sIL-6r than it did for leukemia inhibiting factor and oncostatin M (20). Our findings suggest, therefore, that injection of sgp130 at least partially reduced established endogenous IL-6/sIL-6r-induced sensitization of the primary afferents that evoke the exercise pressor reflex in ligated rats. In contrast, IL-6 and sIL-6 do play an endogenous role in evoking the exercise pressor reflex in freely perfused rats.
Our findings that coinjection of IL-6 and sIL-6r increased the exercise pressor reflex in freely perfused rats and that inhibition of IL-6 and sIL-6r signaling via injection of sgp130 reduced the exaggerated exercise pressor reflex in ligated rats raise the issue of the specific mechanisms through which IL-6/sIL-6r signaling sensitizes primary afferents. We did not specifically address that issue in this investigation, and, therefore, we can only offer our speculation based on the available literature. Previous studies (2, 26) have found that IL-6 and sIL-6r sensitize primary afferents by modulating the activity of transient receptor potential vallinoid (TRPV)1 channels. In ligated rats, however, blockade of TRPV1 channels with iodo-resiniferatoxin had no effect on the exercise pressor reflex (41). IL-6 and sIL-6r may also modulate the activity of endoperoxide (EP) receptors (14). Dina et al. (14) found, for example, that a single intramuscular injection of PGE2, which stimulates EP receptors, produced a marked hyperalgesia that was longer in duration when the muscle was pretreated with an injection of IL-6 compared when the muscle was pretreated with saline. Both EP3C and EP4 receptors have been shown specifically to produce PGE2-induced sensitization of dorsal root ganglion cells in vitro (38), but Yamauchi et al. (46) found that blockade of EP4 receptors, and not EP3 receptors, reduced the exercise pressor reflex in ligated rats. Collectively, therefore, the mechanisms of endogenous IL-6/sIL-6r-induced sensitization of the primary afferents that evoke the exaggerated exercise pressor reflex in ligated rats are likely to involve EP4 receptors, but not TRPV1 receptors, although other receptors may also be involved.
The rat model of unilateral femoral artery ligation followed by 72 h of recovery has been shown to replicate the limb blood flow patterns at rest and during exercise (33, 47) and the exaggerated exercise pressor reflex (41), which have been reported in PAD patients (4–6, 25). Previously, our laboratory (41) has found that the peak pressor response to static contraction was greater in ligated rats than in freely perfused rats. In the present investigation, we found that the peak pressor response to intermittent contraction was greater in ligated rats than in freely perfused rats. That finding is consistent with the increased pressor responses to during dynamic exercise found in PAD patients (4, 5, 25). Our results, therefore, may have important implications for PAD patients, especially given the fact that greater levels of mixed venous blood IL-6 and sIL-6r are found in PAD patients compared with healthy age-matched subjects (3, 9, 17, 30, 36). Our results may also have important implications for other disease states that have been associated with an exaggerated exercise pressor reflex and elevated levels of IL-6 and/or sIL-6r [e.g., heart failure (18, 32)]. Whether IL-6/sIL-6r signaling contributes specifically to the exaggerated exercise pressor reflex in PAD and/or heart failure patients, however, remains unknown.
In conclusion, we found that coinjection of IL-6 and sIL-6r into the arterial supply of the hindlimb increased the exercise pressor reflex in rats with freely perfused femoral arteries. We also found that injection of sgp130, an inhibitor of IL-6 and sIL-6r signaling, into the arterial supply of the hindlimb had no effect on the exercise pressor reflex in rats with freely perfused femoral arteries but reduced the exaggerated exercise pressor reflex in rats with ligated femoral arteries. Our results indicate that IL-6 and sIL-6r are capable of increasing the exercise pressor reflex in rats with freely perfused femoral arteries and that endogenous IL-6 and IL-6r contribute to the exaggerated exercise pressor that is evident in rats with a ligated femoral artery.
GRANTS
This work was supported by National Institutes of Health Grants HL-096570 and AR-059397.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.W.C., A.J.S., J.L., and M.P.K. conception and design of research; S.W.C. performed experiments; S.W.C. analyzed data; S.W.C., A.J.S., J.L., and M.P.K. interpreted results of experiments; S.W.C. prepared figures; S.W.C. drafted manuscript; S.W.C., A.J.S., J.L., and M.P.K. edited and revised manuscript; S.W.C., A.J.S., J.L., and M.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Joyce S. Kim for expert technical assistance.
REFERENCES
- 1.Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol 82: 1811–1817, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, Quarta S, Vogl C, Sailer CA, Uceyler N, Brockhaus J, Martini R, Sommer C, Zeilhofer HU, Muller W, Kuner R, Davis JB, Rose-John S, Kress M. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci 29: 13473–13483, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreozzi GM, Martini R, Cordova R, D'Eri A, Salmistraro G, Mussap M, Plebani M. Circulating levels of cytokines (IL-6 and IL-1β) in patients with intermittent claudication, at rest, after maximal exercise treadmill test and during restore phase. Could they be progression markers of the disease? Int Angiol 26: 245–252, 2007. [PubMed] [Google Scholar]
- 4.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33: 20–25, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bakke EF, Hisdal J, Kroese AJ, Jorgensen JJ, Stranden E. Blood pressure response to isometric exercise in patients with peripheral atherosclerotic disease. Clin Physiol Funct Imaging 27: 109–115, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Baudry N, Rasetti C, Vicaut E. Differences between cytokine effects in the microcirculation of the rat. Am J Physiol Heart Circ Physiol 271: H1186–H1192, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum 56: 351–359, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Chaparala RP, Orsi NM, Lindsey NJ, Girn RS, Homer-Vanniasinkam S. Inflammatory profiling of peripheral arterial disease. Ann Vasc Surg 23: 172–178, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copp SW, Stone AJ, Yamauchi K, Kaufman MP. Effects of peripheral and spinal κ-opioid receptor stimulation on the exercise pressor reflex in decerebrate rats. Am J Physiol Regul Integr Comp Physiol 307: R281–R289, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc 35: 221–228, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor α in the development of inflammatory hyperalgesia. Br J Pharmacol 107: 660–664, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience 152: 521–525, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esteve E, Castro A, Lopez-Bermejo A, Vendrell J, Ricart W, Fernandez-Real JM. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care 30: 939–945, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J 16: 1335–1347, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Fiotti N, Giansante C, Ponte E, Delbello C, Calabrese S, Zacchi T, Dobrina A, Guarnieri G. Atherosclerosis and inflammation. Patterns of cytokine regulation in patients with peripheral arterial disease. Atherosclerosis 145: 51–60, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Lommi J, Pulkki K, Koskinen P, Nameri H, Harkonen M, Kupari M. Hemodynamic, neuroendocrine and metabolic correlates of circulating cytokine concentrations in congestive heart failure. Eur Heart J 18: 1620–1625, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK. Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol 528: 157–163, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem 268: 160–167, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 10, p. 381–447. [Google Scholar]
- 22.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- 23.Matthews VB, Allen TL, Risis S, Chan MH, Henstridge DC, Watson N, Zaffino LA, Babb JR, Boon J, Meikle PJ, Jowett JB, Watt MJ, Jansson JO, Bruce CR, Febbraio MA. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53: 2431–2441, 2010. [DOI] [PubMed] [Google Scholar]
- 24.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller MD, Drew RC, Blaha CA, Mast JL, Cui J, Reed AB, Sinoway LI. Oxidative stress contributes to the augmented exercise pressor reflex in peripheral arterial disease patients. J Physiol 590: 6237–6246, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obreja O, Biasio W, Andratsch M, Lips KS, Rathee PK, Ludwig A, Rose-John S, Kress M. Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain 128: 1634–1641, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Obreja O, Schmelz M, Poole S, Kress M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain 96: 57–62, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Oleksowicz L, Mrowiec Z, Zuckerman D, Isaacs R, Dutcher J, Puszkin E. Platelet activation induced by interleukin-6: evidence for a mechanism involving arachidonic acid metabolism. Thromb Haemost 72: 302–308, 1994. [PubMed] [Google Scholar]
- 29.Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 508: 949–953, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer-Kazen U, Religa P, Wahlberg E. Exercise in patients with intermittent claudication elicits signs of inflammation and angiogenesis. Eur J Vasc Endovasc Surg 38: 689–696, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Gonzalez JF. Factors determining the blood pressure responses to isometric exercise. Circ Res 48: I-76–I-86, 1981. [PubMed] [Google Scholar]
- 32.Piepoli MF, Crisafulli A. Pathophysiology of human heart failure: importance of skeletal muscle myopathy and reflexes. Exp Physiol 99: 609–615, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci 8: 1237–1247, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schirmacher P, Peters M, Ciliberto G, Blessing M, Lotz J, Meyer zum Buschenfelde KH, Rose-John S. Hepatocellular hyperplasia, plasmacytoma formation, and extramedullary hematopoiesis in interleukin (IL)-6/soluble IL-6 receptor double-transgenic mice. Am J Pathol 153: 639–648, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Signorelli SS, Mazzarino MC, Di Pino L, Malaponte G, Porto C, Pennisi G, Marchese G, Costa MP, Digrandi D, Celotta G, Virgilio V. High circulating levels of cytokines (IL-6 and TNFα), adhesion molecules (VCAM-1 and ICAM-1) and selectins in patients with peripheral arterial disease at rest and after a treadmill test. Vasc Med 8: 15–19, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Southall MD, Vasko MR. Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J Biol Chem 276: 16083–16091, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Stebbins CL, Longhurst JC. Potentiation of the exercise pressor reflex by muscle ischemia. J Appl Physiol 66: 1046–1053, 1989. [DOI] [PubMed] [Google Scholar]
- 40.Stone AJ, Copp SW, Kaufman MP. Role of prostaglandins in spinal transmission of the exercise pressor reflex in decerebrated rats. Neuroscience 277C: 26–35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vazquez E, Kahlenbach J, Segond von Banchet G, Konig C, Schaible HG, Ebersberger A. Spinal interleukin-6 is an amplifier of arthritic pain in the rat. Arthritis Rheum 64: 2233–2242, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8: 75–79, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Weiner SD, Ahmed HN, Jin Z, Cushman M, Herrington DM, Nelson JC, Di Tullio MR, Homma S. Systemic inflammation and brachial artery endothelial function in the Multi-Ethnic Study of Atherosclerosis (MESA). Heart 100: 862–866, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine 70: 11–20, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Yamauchi K, Kim JS, Stone AJ, Ruiz-Velasco V, Kaufman MP. Endoperoxide 4 receptors play a role in evoking the exercise pressor reflex in rats with simulated peripheral artery disease. J Physiol 591: 2949–2962, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Zhong J, Dietzel ID, Wahle P, Kopf M, Heumann R. Sensory impairments and delayed regeneration of sensory axons in interleukin-6-deficient mice. J Neurosci 19: 4305–4313, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]