Abstract
Proper perfusion is vital for maintenance of neuronal homeostasis and brain function. Changes in the function and structure of cerebral parenchymal arterioles (PAs) could impair blood flow regulation and increase the risk of cerebrovascular diseases, including dementia and stroke. Hypertension alters the structure and function of large cerebral arteries, but its effects on PAs remain unknown. We hypothesized that hypertension increases myogenic tone and induces inward remodeling in PAs; we further proposed that antihypertensive therapy or mineralocorticoid receptor (MR) blockade would reverse the effects of hypertension. PAs from 18-wk-old stroke-prone spontaneously hypertensive rats (SHRSP) were isolated and cannulated in a pressure myograph. At 50-mmHg intraluminal pressure, PAs from SHRSP showed higher myogenic tone (%tone: 39.1 ± 1.9 vs. 28.7 ± 2.5%, P < 0.01) and smaller resting luminal diameter (34.7 ± 1.9 vs. 46.2 ± 2.4 μm, P < 0.01) than those from normotensive Wistar-Kyoto rats, through a mechanism that seems to require Ca2+ influx through L-type voltage-gated Ca2+ channels. PAs from SHRSP showed inward remodeling (luminal diameter at 60 mmHg: 55.2 ± 1.4 vs. 75.7 ± 5.1 μm, P < 0.01) and a paradoxical increase in distensibility and compliance. Treatment of SHRSP for 6 wk with antihypertensive therapy reduced PAs' myogenic tone, increased their resting luminal diameter, and prevented inward remodeling. In contrast, treatment of SHRSP for 6 wk with an MR antagonist did not reduce blood pressure or myogenic tone, but prevented inward remodeling. Thus, while hypertensive remodeling of PAs may involve the MR, myogenic tone seems to be independent of MR activity.
Keywords: hypertension, cerebral parenchymal arterioles, mineralocorticoid receptor, inward remodeling, myogenic tone
proper perfusion of the brain parenchyma is vital for neuronal homeostasis, since these cells have limited capacity to store energy and nutrients (9). Even small changes in cerebral perfusion pressure can have detrimental effects on neuronal function that lead to long-term consequences, such as small vessel disease and cognitive impairment (16). Parenchymal arterioles (PAs), also known as penetrating arterioles, are bottlenecks in the cerebral circulation (41), and they supply blood to discrete neuronal populations. PAs are high-resistance vessels, since they have a higher degree of myogenic tone than pial arteries (10). Importantly, dysfunction of PAs and alterations in their structure precede white matter lesions and may lead to development of vascular cognitive impairment (35).
Chronic hypertension is a major risk factor for cerebral vascular diseases, including small vessel disease, stroke (ischemic, hemorrhagic, and lacunar), and dementias (37). Hypertension causes functional and structural changes in large intracranial arteries (46, 48, 49), such as the middle cerebral artery (MCA), and smaller pial arteries and arterioles (3, 24, 46). These alterations include increased myogenic tone (32), a rightward shift in the autoregulatory curve (31), impaired endothelium-dependent dilation (62), and structural and mechanical changes (46). Hypertension increases intrinsic myogenic tone in MCAs from vasopressin-deficient rats (20) and stroke-prone spontaneously hypertensive rats (SHRSP) (54). The increase in myogenic tone may be linked to enhanced calcium influx into the vascular smooth muscle cells through L-type voltage-gated calcium channels (14, 34, 55, 58). Chronic hypertension also induces structural and mechanical changes in large pial arteries, including a reduced luminal diameter (inward remodeling) and increased wall thickness (wall hypertrophy) (47–49, 54). The structural alterations, in particular, are at least in part dependent on mineralocorticoid receptor (MR) activation, since antagonism of this receptor prevented (54) and reversed (53) remodeling of the MCA in SHRSP.
The PAs are an important branch of the cerebral vascular tree vital for neuronal function, yet the effect of hypertension on these arterioles has not been studied. It is important to note that the PAs and the pial arteries and arterioles are structurally very different. PAs have very few anastomoses (39), and having fewer branch points will theoretically reduce the regions of turbulent flow in the PAs. The innervation of the PAs and pial arteries is also different; pial arteries receive extrinsic innervation from the peripheral nervous system (26). In pial arteries, sympathetic nervous system activity is, at least in part, responsible for the hypertension associated wall hypertrophy normally observed in SHRSP (4, 27). PAs, on the other hand, are surrounded by astrocytic end feet (12), and the astrocytes are the target of most of the nerves associated with the PAs (13). Expression of neurotransmitter receptors also differs across the vascular tree. For example, α1-adrenoreceptor activation with norepinephrine causes MCA contraction (18, 30), but norepinephrine causes dilation in the PAs though activation of β-adrenoreceptors (38). For these reasons, we cannot assume that the PAs and the pial arteries will behave in a similar manner when challenged with an increase in blood pressures.
The goal of our study was to investigate the impact of chronic hypertension on PAs. We hypothesized that PAs from SHRSP will exhibit increased myogenic tone and inward hypertrophic remodeling compared with PAs from normotensive Wistar-Kyoto (WKY) rats. We further hypothesized that lowering blood pressure and blocking the MR would reduce myogenic tone and attenuate inward remodeling in PAs from hypertensive rats.
METHODS
Animals.
Twelve-week-old male SHRSP from the colony housed at Michigan State University were used for this study. Age-matched WKY rats were purchased from Harlan Sprague-Dawley, (Indianapolis, IN). Rats were maintained on a 12:12-h light-dark cycle, with tap water and regular chow ad libitum. At 18 wk of age, rats were anesthetized with 3% isoflurane, weighed, and euthanized by decapitation after exsanguination, and the brains were collected. The experimental protocol was approved by the Michigan State University Institutional Animal Care & Use Committee and was in accordance with the National Institutes of Health “Guiding Principles in the Care and Use of Animals.”
Antihypertensive therapy.
Twelve-week-old male SHRSP were treated with a combination of hydralazine (150 mg/l) + hydrochlorothiazide (50 mg/l) + reserpine (4 mg/l) in the drinking water for 6 wk [SHRSP + antihypertensive therapy (AhT)] (36, 42, 59). Age-matched vehicle-treated (distilled water) SHRSP were used as controls.
Eplerenone treatment.
Twelve-week-old male SHRSP were treated with eplerenone (EPL) (100 mg/kg) given orally suspended in peanut butter once daily; this treatment protocol has been used by our laboratory for other MR antagonists (53, 54). This dose of EPL was selected from studies in the literature (21, 63), and pilot studies in our laboratory show that it effectively reverses MCA remodeling in SHSRP, to an extent similar to that observed for spironolactone (53, 54). Vehicle-treated SHRSP received peanut butter daily and were used as controls for this study.
Measurement of arterial pressure.
Blood pressure was measured by tail-cuff plethysmography using a RTBP1001 tail-cuff blood pressure system (Kent Scientific, Torrington, CT), as described previously by our laboratory (47).
PA isolation and cannulation.
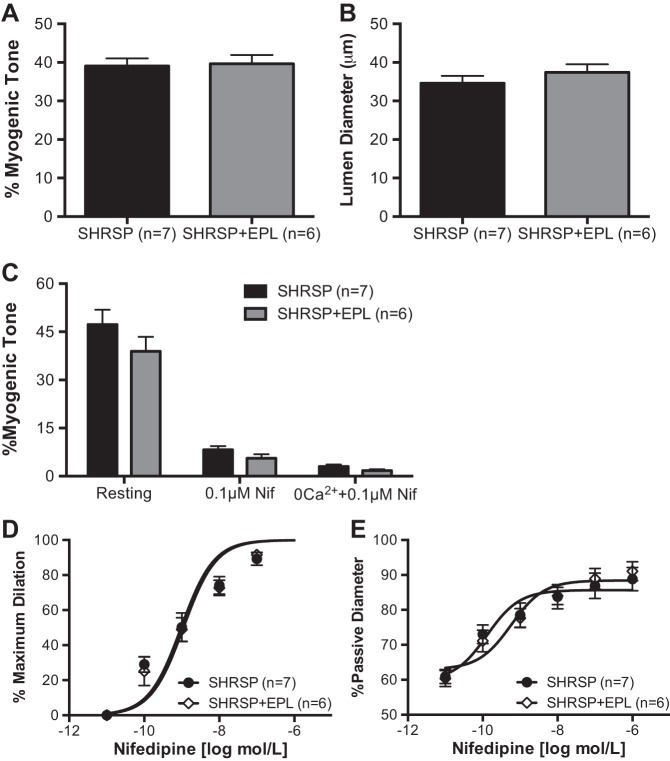
A 5 × 3-mm section of brain tissue containing the MCA was removed and placed in Ca2+-free physiological saline solution (PSS; in mM: 140 NaCl, 5 KCl, 1 MgCl2·7H2O, 10 HEPES, 10 dextrose) at 4°C, with 1% bovine serum albumin + 10 μM diltiazem + 10 μM sodium nitroprusside. The pia with the MCA was gently separated from the tissue, and PAs branching from the MCA were transferred to a custom-made cannulation chamber (61). PAs were then cannulated between glass pipettes, bathed in warm (37°C) PSS containing 1.8 mM Ca2+, and pressurized to 50 mmHg until development of intrinsic myogenic tone, which was calculated using the following formula: %myogenic tone = [1 − (active luminal diameter/passive luminal diameter)] × 100. PA outer and luminal diameters were constantly tracked and recorded using MyoView 2.0 software (Danish Myo Technology, Aarhus, Denmark). All reactivity experiments described below were performed after the diameter of the arteriole stabilized for at least 10 min.
Role of extracellular and intracellular Ca2+ in the maintenance of myogenic tone.
The dependence of myogenic tone on Ca2+ entry through voltage-gated Ca2+ channels and release of Ca2+ from stores was assessed by incubating pressurized PAs first with 0.1 μM nifedipine to block L-type voltage-gated Ca2+ channels, then with 0 Ca2+-PSS + 0.1 μM nifedipine, but without chelators, to assess the amount of myogenic tone remaining after removal of extracellular Ca2+.
PA reactivity to L-type voltage-gated Ca2+ channel blockade.
The sensitivity of PAs to L-type voltage-gated Ca2+ channel inhibition was assessed by incubating PAs with increasing concentrations of nifedipine (0.01 nM to 1 μM) in the bath. PAs were incubated with each concentration of nifedipine for 10 min, and the diameter was constantly recorded. Data are shown as percentage of the maximum dilation, and also as a percentage of passive diameter to normalize for structural alterations caused by the treatments.
PA structural and mechanical properties.
Structural and mechanical properties of the PAs were analyzed after incubation with 0 Ca2+-PSS supplemented with 2 mM EGTA and 10 μM sodium nitroprusside. Intraluminal pressure was increased from 3 to 180 mmHg in 20-mmHg increments, and arterioles were allowed to equilibrate for 5 min before measurements were made and intraluminal pressure was increased. PA outer and luminal diameters were constantly tracked and recorded, and the wall thickness was calculated as (outer diameter − luminal diameter). The wall-to-lumen ratio and circumferential wall stress were calculated as described previously (2), as was the passive distensibility (6). The elastic modulus (β-coefficient) was calculated from the stress/strain curves using an exponential model (y = aeβx), where y is circumferential stress, x is circumferential strain, a is intercept, and β is the slope of the exponential fit and is directly correlated to vascular stiffness.
Chemical and reagents.
Unless otherwise specified, all chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical analyses.
Myogenic tone and the β-coefficient data were analyzed by Student's t-test or a nonparametric alternative. PA reactivity to nifedipine and structural properties were analyzed by two-way ANOVA with a Sidak correction for multiple comparisons. Difference of means were considered significant when P < 0.05. All analyzes were performed using the GraphPad Prizm software (version 6.0c).
RESULTS
Physiological parameters.
The final body weights and blood pressures for the SHRSP used in this study are summarized in Table 1. AhT caused a significant reduction in blood pressure, whereas EPL slightly increased the blood pressure in the hypertensive rats. The AhT-treated rats weighed significantly less than the control or EPL-treated SHRSP. The weight loss could be a consequence of the reserpine treatment. Reserpine depletes monoamine neurotransmitters, serotonin, and catecholamines and has been shown to cause weight loss in rats (25).
Table 1.
Effect of treatments on systemic blood pressure in SHRSP
| SHRSP | SHRSP + AhT | SHRSP + EPL | |
|---|---|---|---|
| Systolic arterial pressure, mmHg | 214 ± 4 | 130 ± 6* | 237 ± 3* |
| Diastolic arterial pressure, mmHg | 170 ± 5 | 91 ± 6* | 203 ± 3* |
| Mean arterial pressure, mmHg | 181 ± 4 | 104 ± 6* | 214 ± 3* |
| Body weight, g | 339 ± 8 | 298 ± 9† | 343 ± 1 |
Values are means ± SE. SHRSP, stroke-prone spontaneously hypertensive rats (n = 8); AhT, antihypertensive treatment (n = 11); EPL, eplerenone (n = 5).
P < 0.05, different from SHRSP, one-way ANOVA with a Sidak correction for multiple comparisons.
P < 0.05 different from SHRSP and SHRSP + EPL, one-way ANOVA with Bonferroni posttest.
Chronic hypertension increases PA myogenic tone.
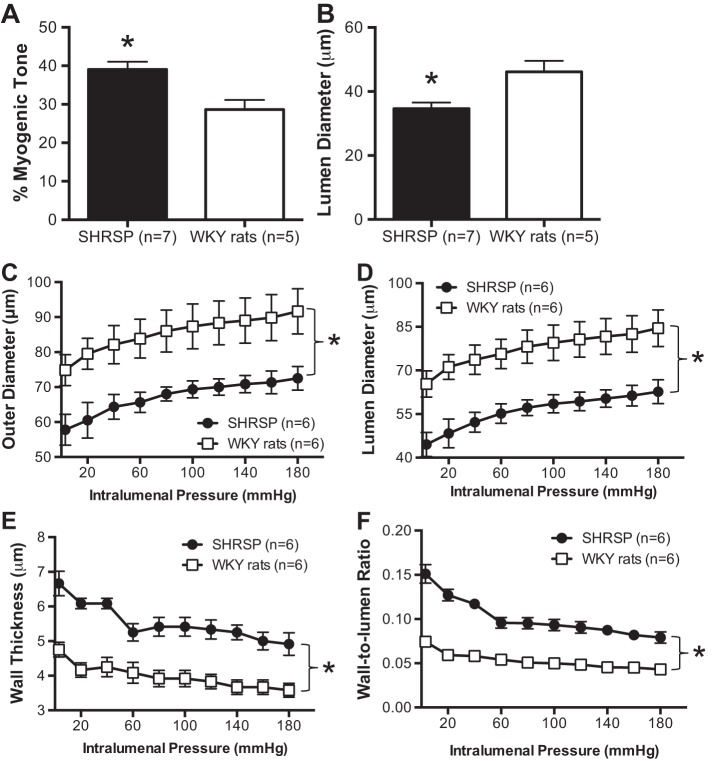
Tone was increased in PAs from SHRSP compared with WKY rats by ∼20% (Fig. 1A). As a consequence, the PA resting luminal diameter after tone generation was significantly smaller in PAs of SHRSP than WKY rats by ∼40% (Fig. 1B).
Fig. 1.
Chronic hypertension in stroke-prone spontaneously hypertensive rats (SHRSP) increases myogenic tone and is associated with inward hypertrophic remodeling in parenchymal arterioles (PAs). A: myogenic tone was increased in pressurized PAs from 18-wk-old SHRSP compared with age-matched normotensive Wistar-Kyoto (WKY) rats. B: PAs from SHRSP had smaller resting luminal diameter (diameter after myogenic tone generation). In addition, PAs from SHRSP exhibited a classical inward remodeling process, observed as a significant reduction in both the outer (C) and luminal diameter (D). Hypertrophic growth of the PA wall was also observed (E), which caused an increase in the PA wall-to-lumen ratio (F). For myogenic tone studies, PAs were incubated in Ca2+ physiological saline solution (PSS) and pressurized to 50 mmHg, then allowed to generate spontaneous myogenic tone. Passive structure was assessed after incubation of PA with 0 Ca2+ PSS supplemented with 100 μmol/l sodium nitroprusside (SNP) + 2 mmol/l EGTA. Values are means ± SE; n, no. of rats. *P < 0.05, SHRSP compared with WKY (Student's t-test).
PAs from SHRSP undergo inward remodeling.
Assessment of the passive structure of PAs from SHRSP showed a marked inward remodeling, characterized by a reduction in the outer (Fig. 1C) and luminal (Fig. 1D) diameters. In addition, the wall thickness was increased (Fig. 1E). As a consequence of this, the wall-to-lumen ratio of the PAs from SHRSP was reduced compared with that of the PAs from WKY rats (Fig. 1F).
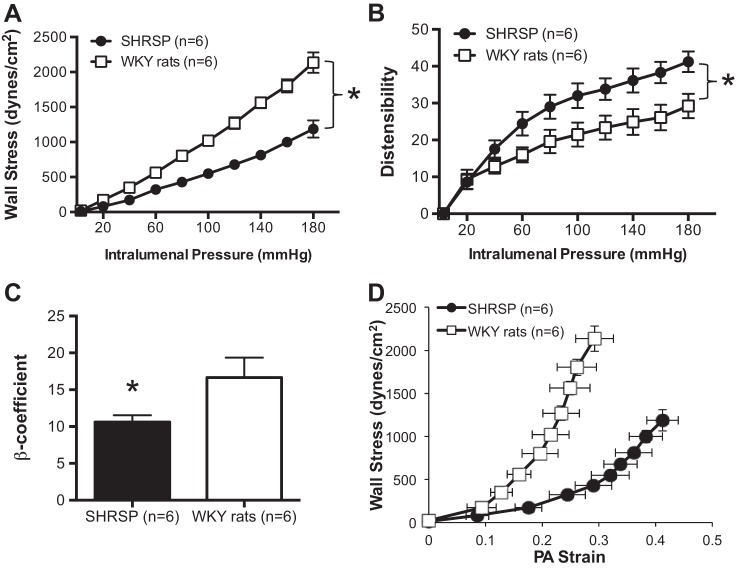
Stiffness is reduced and compliance and distensibility are increased in PAs from SHRSP.
Wall stress was lower in PAs from SHRSP compared with WKY rats (Fig. 2A), and distensibility was increased particularly at higher intraluminal pressures (Fig. 2B). We observed a reduction in the stiffness, calculated by the β-coefficient, of PAs from SHRSP (Fig. 2C), and an increase in compliance, evidenced by a rightward shift of the stress-strain curve (Fig. 2D).
Fig. 2.
Mechanical properties of PAs from SHRSP. A: there was an increase in wall stress in the PAs from SHRSP compared with PAs from normotensive WKY rats across the range of intraluminal pressures studied. PAs from SHRSP had increased distensibility than PAs from WKY rats (B), decreased stiffness as measured by β-coefficient (C), and increased compliance, observed as a leftward shift in the stress-strain relationship (D). Mechanical properties were assessed under passive conditions after inactivation of the smooth muscle cells by incubation with 0 Ca2+ PSS supplemented with 100 μmol/l SNP + 2 mmol/l EGTA. Values are means ± SE; n, no. of rats. *P < 0.05, SHRSP compared with WKY (Student's t-test).
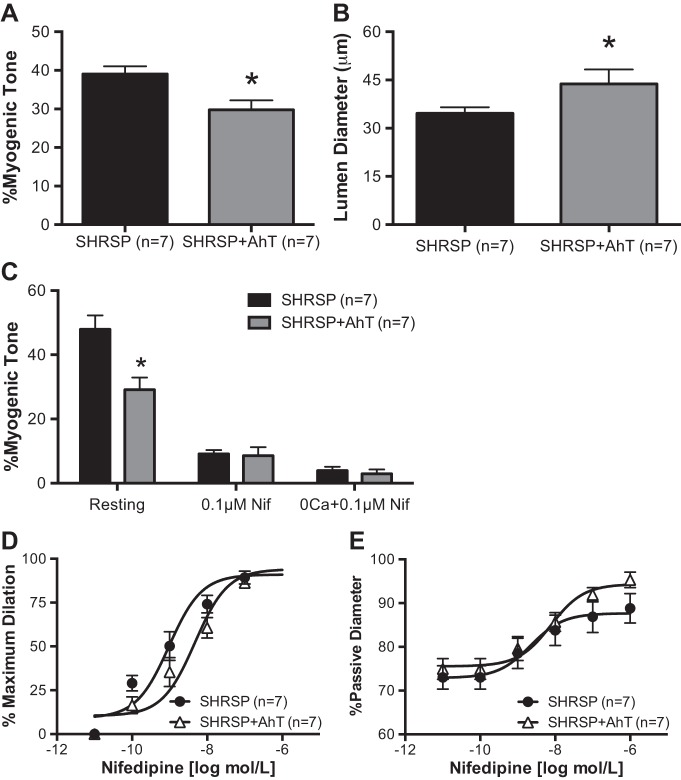
AhT reduced PA myogenic tone in SHRSP.
To test the hypothesis that chronic hypertension is associated with the increased myogenic tone in PAs, adult SHRSP were treated with AhT during the sustained phase of hypertension. AhT significantly reduced the blood pressure in SHRSP (Table 1). We observed a significant reduction in the PA intrinsic myogenic tone generation after AhT compared with placebo-treated SHRSP (Fig. 3A). In addition, the resting luminal diameter was significantly larger in PAs from SHRSP + AhT than from placebo-treated SHRSP (Fig. 3B).
Fig. 3.
Antihypertensive therapy (AhT) reduced PA myogenic tone and responsiveness to a L-type voltage-gated Ca2+ channel inhibitor. A: generation of spontaneous myogenic tone was reduced in PAs from SHRSP + AhT compared with PAs from SHRSP. B: in addition, PAs from SHRSP + AhT had a larger resting luminal diameter. C: blockade of L-type voltage-gated Ca2+ channels with nifedipine showed that the PAs from SHRSP + AhT had a smaller dilation than PAs from SHRSP, and elimination of extracellular Ca2+ caused a similar dilation in PAs from both groups. Furthermore, PAs from SHRSP + AhT showed a reduced sensitivity to increasing concentrations of the L-type voltage-gated Ca2+ channel inhibitor nifedipine, observed as a right shift in the concentration response curve (D), and a trend toward an increase in the %passive diameter of PAs from SHRSP + AhT after incubation to increasing concentrations of nifedipine (E). Values are means ± SE; n, no. of rats. *P < 0.05, SHRSP compared with WKY (Student's t-test).
Involvement of L-type voltage-gated Ca2+ channels in maintenance of PA myogenic tone.
To study if the increase in myogenic tone observed was associated with L-type voltage-gated Ca2+ channels, PAs were first incubated with the EC90 concentration of nifedipine and then 0 Ca2+ PSS + nifedipine. Incubation with 0.1 μmol/l nifedipine caused a more significant loss of myogenic tone in PAs from SHRSP than in PAs from SHRSP + AhT, to the point that differences were no longer observed in the %myogenic tone (Fig. 3C). Similarly, incubation with 0 Ca2+ PSS + 0.1 μmol/l nifedipine caused a similar loss of myogenic tone in PAs from SHRSP and SHRSP + AhT (Fig. 3C).
PAs from SHRSP + AhT are less sensitive to L-type voltage-gated Ca2+ channel blockade.
To assess the reactivity of PAs to calcium channel blockers, arterioles were allowed to generate myogenic tone and were then incubated with increasing concentrations of nifedipine. We observed a rightward shift in the percentage of maximum response curve to nifedipine in SHRSP + AhT (Fig. 3D), and a trend toward an increase (P = 0.058) after normalization of the data by the PA passive diameter (Fig. 3E).
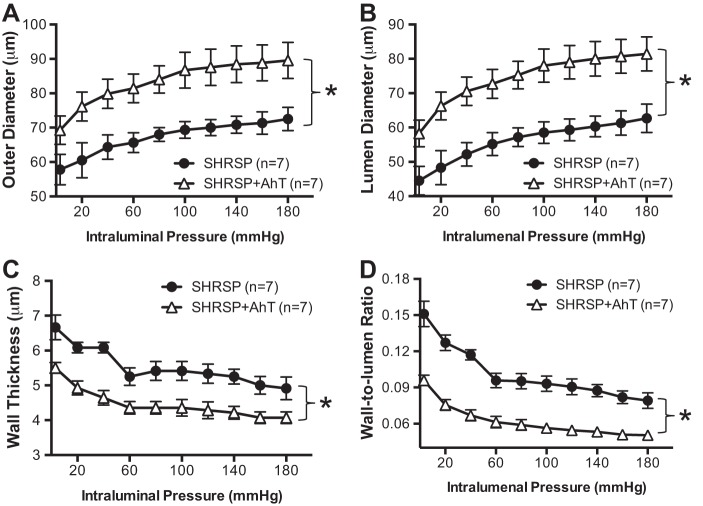
AhT reversed PA inward remodeling but did not alter mechanical properties.
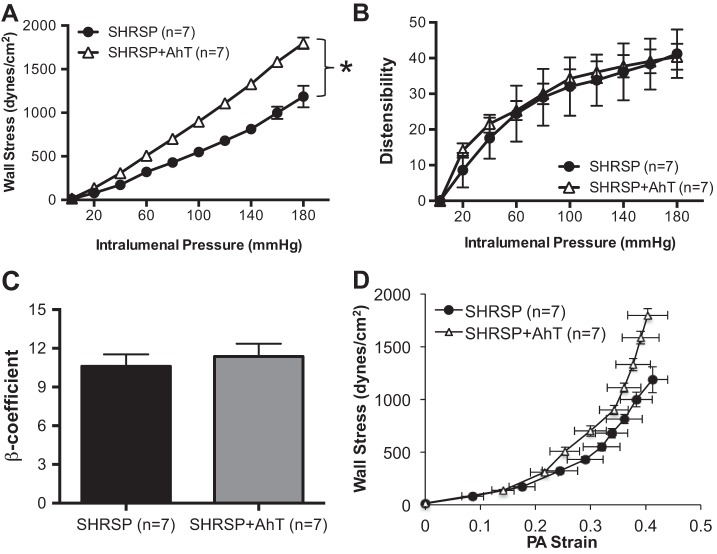
Lowering blood pressure in SHRSP resulted in an attenuation of the inward artery remodeling process, evidenced by an increase in the outer and luminal diameters of the PAs (Fig. 4, A and B, respectively). There was also a modest reduction in wall thickness and a significant reduction in the wall-to-lumen ratio after AhT in SHRSP (Fig. 4, C and D). PAs from SHRSP + Aht showed increased wall stress compared with PAs from placebo-treated SHRSP (Fig. 5A). However, AhT did not change the distensibility (Fig. 5B), stiffness (Fig. 5C), or compliance (Fig. 5D) of the PAs from SHRSP.
Fig. 4.
AhT prevented the inward hypertrophic remodeling of PAs from SHRSP. Reducing blood pressure in SHRSP caused an increase in PA outer (A) and luminal diameter (B), suggesting a prevention of the inward remodeling process. In addition, there was a decrease in wall thickness (C) and the wall-to-lumen ratio (D), showing that AhT prevented hypertrophic growth of the vascular wall in SHRSP. Passive structure was assessed after incubation of PAs with 0 Ca2+ PSS supplemented with 100 μmol/l SNP + 2 mmol/l EGTA. Values are means ± SE; n, no. of rats. *P < 0.05, SHRSP compared with WKY (Student's t-test).
Fig. 5.
AhT had no effect on PA mechanical properties. A: wall stress was higher in PAs from SHRSP + AhT compared with placebo-treated SHRSP. There were no differences in PA distensibility (B), stiffness, calculated as the β-coefficient (C), or in the stress-strain relationship (D) between groups. Passive structure was assessed after incubation of PAs with 0 Ca2+ PSS supplemented with 100 μmol/l SNP + 2 mmol/l EGTA. Values are means ± SE; n, no. of rats. *P < 0.05, SHRSP compared with WKY (Student's t-test).
EPL did not reduce PA myogenic tone.
Treatment of SHRSP with EPL for 6 wk during the sustained phase of hypertension did not reduce systemic blood pressure (Table 1). As a consequence, there were no differences in PA intrinsic myogenic tone generation (Fig. 6A) and in the resting luminal diameter (Fig. 6B).
Fig. 6.
Eplerenone (EPL) treatment did not alter PA myogenic tone and responsiveness to nifedipine. Spontaneous myogenic tone of pressurized PAs was not changed in SHRSP after treatment with EPL (A), and the PA resting diameter was not different between SHRSP + EPL and vehicle-treated SHRSP (B). C: blockade of L-type voltage-gated Ca2+ channels with nifedipine and removal of extracellular Ca2+ caused a similar dilation in PAs from SHRSP and SHRSP + EPL. The sensitivity of PAs to increasing concentrations of nifedipine, as seen as a %maximum dilation (D) and %passive diameter (E), was unchanged by EPL treatment. Values are means ± SE; n, no. of rats.
The role of extracellular Ca2+ in the maintenance of myogenic tone was not altered by EPL.
To assess whether EPL treatment altered the contribution of L-type voltage-gated Ca2+ channels in the development of PA myogenic tone, arterioles were incubated with nifedipine and 0 Ca2+ PSS, as described above. The loss of myogenic tone with nifedipine treatment was similar in the EPL-treated and placebo-treated SHRSP (Fig. 6C). Removal of extracellular Ca2+ had a similar effect on PAs from the placebo- and EPL-treated SHRSP, and the remaining myogenic tone was not different between groups (Fig. 6C).
L-type voltage-gated Ca2+ channel blockade.
There were no differences in the percent maximum response to nifedipine between PAs from SHRSP + placebo and SHRSP + EPL (Fig. 6D). Similarly, normalization of the change in diameter data by the passive diameter showed that the response to nifedipine was similar between groups (Fig. 6E).
EPL attenuated PAs inward remodeling and altered mechanical properties.
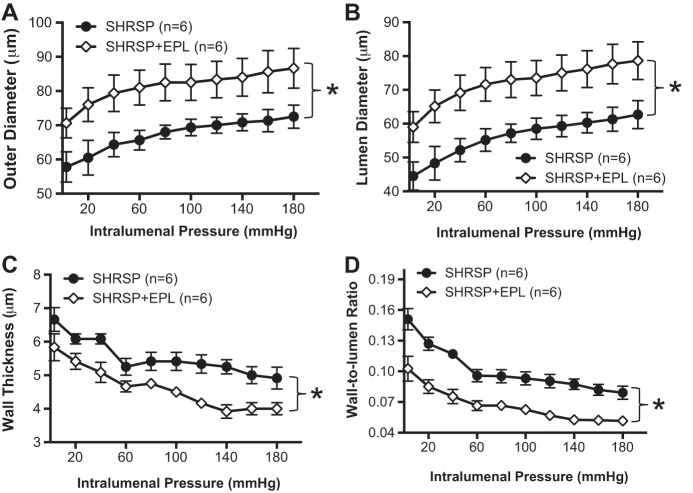
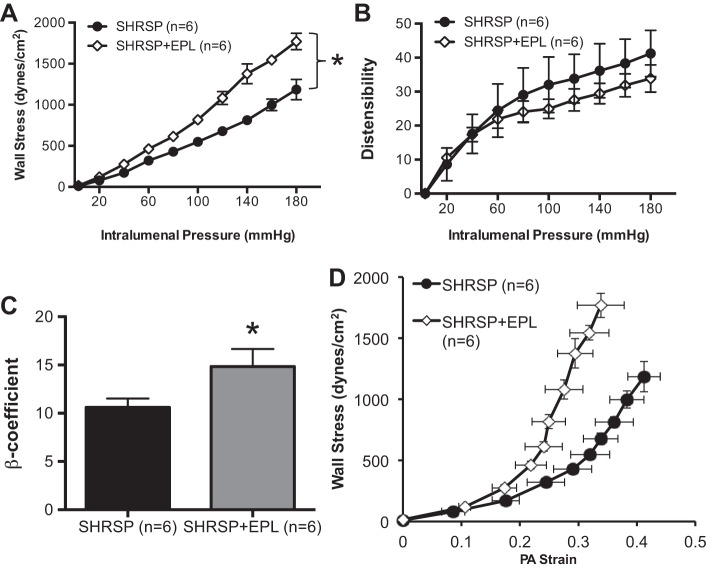
EPL treatment attenuated the inward artery remodeling process observed in PAs from SHRSP. The PAs from EPL-treated SHRSP had larger outer (Fig. 7A) and luminal (Fig. 7B) diameters over the entire range of intraluminal pressures studied. In addition, there was a significant decrease in wall thickness (Fig. 7C) and in the wall-to-lumen ratio (Fig. 7D), showing attenuation of the inward remodeling process in PAs from SHRSP. The increase in luminal diameter and reduction in wall thickness caused an expected increase in wall stress in PAs from SHRSP + EPL (Fig. 8A). PA stiffness was increased (Fig. 8C), and distensibility (Fig. 8B) and compliance were reduced (Fig. 8D).
Fig. 7.
EPL treatment prevented inward hypertrophic remodeling of PAs. Treatment of adult SHRSP with EPL caused an increased in PA outer (A) and luminal diameter (B). Wall thickness (C) and the wall-to-lumen ratio (D) were reduced in SHRSP + EPL compared with SHRSP + vehicle. Taken together, these data suggest that EPL treatment prevented the inward hypertrophic remodeling observed in PAs from SHRSP. Passive structure was assessed after incubation of PAs with 0 Ca2+ PSS supplemented with 100 μmol/l SNP + 2 mmol/l EGTA. Values are means ± SE; n, no. of rats. *P < 0.05, SHRSP compared with WKY (Student's t-test).
Fig. 8.
EPL altered stiffness of PAs from SHRSP. A: there was an increase in the wall stress in PAs from SHRSP + EPL compared with SHRSP treated with vehicle. Although distensibility was unchanged (B), PA from SHRSP + EPL showed increased stiffness, observed as an increased in the β-coefficient (C) and a rightward shift of the stress-strain relationship (D). Passive structure was assessed after incubation of PAs with 0 Ca2+ PSS supplemented with 100 μmol/l SNP + 2 mmol/l EGTA. Values are means ± SE; n, no. of rats. *P < 0.05, SHRSP compared with WKY (Student's t-test).
DISCUSSION
There are three major findings of this study. First, chronic hypertension increases myogenic tone in PAs and induces inward hypertrophic artery remodeling. Second, normalizing the blood pressure in SHRSP with AhT reduced myogenic tone and prevented the inward hypertrophic remodeling without affecting mechanical properties of PAs. Finally, EPL treatment did not lower blood pressure or myogenic tone, but it did prevent the inward hypertrophic remodeling. Together, these data suggest that the regulation of myogenic tone in PAs is likely linked to systemic blood pressure, whereas the remodeling process is more complex and involves circulating factors, MR activation, and blood pressure. To the best of our knowledge, this is the first study to demonstrate the effects of hypertension on the structure and function of the cerebral parenchymal microcirculation. It is important to note that, in these studies, the hypertension was allowed to develop completely before the drugs were administered. We propose that this treatment paradigm assesses the ability of MR antagonists and AhT to reverse the effects of hypertension on the cerebral vasculature; this increases the clinical relevance of the studies.
PAs connect the surface pial circulation to the deep cerebral microcirculation. They are considered bottlenecks of the cerebral circulation (41), since they have few branches. PAs perfuse discrete regions of the brain, and blood flow through them is tightly regulated by neurons and astrocytes through the process known as neurovascular coupling (19). PA dysfunction or structural remodeling could have detrimental effects on local perfusion, leading to a persistent hypoxic microenvironment and neuronal death. In fact, a recent study showed that thrombotic occlusion of a single PA results in a cylindrical infarct that was linked to cognitive decline (57). Despite the importance of PAs in the maintenance of cerebral function, little is known about their physiology, particularly in chronic pathological states, such as hypertension. As discussed in the Introduction, important differences between the pial circulation and the PAs prevent us from extrapolating the effects of drug treatments in the pial arteries to the PAs. Therefore, our study fills a gap in our understanding of the cerebral microcirculation.
The effects of hypertension on the regulation of myogenic tone in pial arteries are controversial. Some studies suggest that there is no difference in myogenic tone between posterior cerebral arteries of SHR and WKY rats (44). In contrast, another study showed that myogenic tone is higher in SHR than WKY rats (23, 33). The present study shows that myogenic tone is increased in PAs from SHRSP compared with WKY rats, and, consequently, the resting luminal diameter of PAs is smaller in hypertensive rats. Furthermore, PAs from SHRSP treated with AhT for 6 wk during the sustained phase of hypertension exhibited a reduction in myogenic tone compared with SHRSP + vehicle. These data suggest that systemic blood pressure may be the primary determinant of increased myogenic tone in PAs from hypertensive rats. This concept is supported by the finding that the resting luminal diameters of PAs from female SHR are smaller than those from WKY rats, possibly due to increased myogenic tone (7). This argument is strengthened by the fact that EPL treatment, which did not reduce systemic blood pressure, also did not change myogenic tone. It is unclear if the increased myogenic tone observed with hypertension is an adaptive response to increase segmental resistance in the cerebral vascular tree. Although plausible, this idea is controversial, because a previous study showed an increase in vascular resistance in upstream arteries, including the pial arteries and large intracranial arteries, which potentially normalizes perfusion pressure in downstream arterioles, including PAs (22). This hypothesis requires further investigation.
Regulation of myogenic tone ultimately relies on calcium influx into the smooth muscle cells and is modulated by the sensitivity of the contractile machinery to calcium (15, 29). PAs generate more myogenic tone than large intracranial arteries, such as the MCA, and this may be dependent on higher activity of voltage-dependent Ca2+ channels (11). Importantly, Ca2+ influx through voltage-gated Ca2+ channels seems to be increased during hypertension, which could further enhance myogenic tone (14, 34, 58). In fact, Ca2+ currents in vascular smooth muscle cells isolated from mesenteric arteries from SHR are larger than those from vascular myocytes isolated from WKY rats. This difference was linked to an increased open probability of the channel (43). These data suggest that hypertension changes the kinetics of L-type Ca2+ channels, rather than increasing the number of channels in the membrane. Increased channel opening increases whole cell amplitude of Ca2+ currents that may increase contractility, leading to higher myogenic tone. The present study adds to this picture, by showing that blockade of L-type Ca2+ channels with nifedipine caused a larger dilation in PAs from SHRSP + placebo and SHRSP + EPL, but not in SHRSP + AhT. These data suggest that the increased myogenic tone observed with high blood pressure may be dependent on Ca2+ influx through L-type Ca2+ channels, although a change in Ca2+ sensitivity could also play a role, as discussed below. One caveat in our study is that we did not study the biophysical characteristics of L-type Ca2+ channels in PAs from SHRSP; thus it is still possible that there is an increase in L-type Ca2+ channel number, in addition to an increase in their activity. Further, changes in kinetics of K+ channels could also cause the resting membrane potential of myocytes to be more depolarized, thus increasing myogenic constriction.
Alterations in Ca2+ sensitivity of smooth muscle cells, as a consequence of a phenotype switch, could also play a role in increased myogenic tone during chronic hypertension. Mechanical stretch induces the expression of contractile proteins in smooth muscle cells, namely smooth muscle myosin heavy chain-1 and -2 (52). An increase in these proteins could potentially increase contractility (51), thus accounting, at least in part, for the increased myogenic tone in PAs from SHRSP. In addition, the sensitivity of the contractile machinery to Ca2+ could be enhanced, a phenomenon that occurs in arterial smooth muscle cells from hypertensive rodents (28, 40). In particular, the activity of Rho-associated kinase, a protein that importantly modulates smooth muscle Ca2+ sensitivity (56), is increased by hypertension (8, 60). It remains undetermined if PAs from SHRSP have enhanced Ca2+ sensitivity as a consequence of a phenotype switch in the smooth muscle cells.
The link between hypertension and remodeling of large cerebral arteries is known, and it is clear that intraluminal pressure and circulating factors are involved in the process (46). However, the effects of hypertension on the PAs have not been widely studied. PAs from SHRSP showed a smaller passive outer and luminal diameter than PAs from normotensive WKY rats. There was also an increase in the wall thickness and wall-to-lumen ratio. Interestingly, the increased wall thickness was linked to lower stiffness, increased distensibility, and higher compliance in PAs from SHRSP. It is possible that the increased wall thickness is associated with thickening of the internal elastic lamina, which could account for increased distensibility. In fact, pial arteries of SHRSP show increased elastin content in their wall (5). Other studies have shown similar effects of hypertension on wall stiffness in the larger cerebral arteries (2, 17).
Reducing blood pressure attenuated the inward remodeling in PAs from SHRSP. SHRSP + AhT had PAs with larger outer and luminal diameters, and the wall thickness and wall-to-lumen ratios were also reduced. Attenuating the inward remodeling and reducing intrinsic myogenic tone may act together to increase the resting luminal diameter of PAs from SHRSP + AhT. This could have beneficial effects in vivo by preventing chronic mild local hypoperfusion, which has been linked to the development of small vessel disease and vascular cognitive impairment (64). In particular, the white matter is largely perfused by the PAs, and histological and in vivo studies show that arterioles within the white matter of patients with small vessel disease have narrowed lumens (1, 45). Thus AhT may delay the onset, if not prevent, the development of small vessel disease in hypertensive patients by increasing normal perfusion.
The MR receptor is involved in remodeling of large intracranial arteries independent of blood pressure. We showed previously that MCAs from SHRSP treated with spironolactone have larger luminal diameters than MCAs from control SHRSP (53, 54). Similarly, the present study shows that treatment of SHRSP with EPL attenuates inward PA remodeling, observed as an increase in the outer and luminal diameter, and a reduction in wall thickness and wall-to-lumen ratio. EPL also altered the mechanical properties of PAs, resulting in arterioles with increased stiffness, lower distensibility, and compliance. These changes occurred, despite there being a small but significant increase in blood pressure in the EPL-treated rats. The changes in mechanical properties observed may be age dependent. In young SHRSP, blockade of the MR receptor with spironolactone during the development phase of hypertension (6–12 wk of age) increases MCA compliance and reduces stiffness (54). However, when SHRSP are treated with spironolactone from 12 to 18 wk of age (the same treatment paradigm used in the present study), stiffness and compliance are unchanged (53). The differences in these previous findings and the ones reported here could be a consequence of the different segments of the cerebral vascular tree being studied. Furthermore, it is possible that the reduction in wall thickness observed in PAs from SHRSP + EPL may be a consequence of thinning of the internal elastic lamina, which would increase stiffness and reduce compliance and distensibility.
In summary, this study shows that chronic hypertension increases intrinsic myogenic tone and induces inward hypertrophic remodeling of PAs. The increased myogenic tone is dependent on the maintenance of high systemic blood pressure and Ca2+, either by increased influx through nifedipine-sensitive channels or enhanced Ca2+ sensitivity as a consequence of phenotype switch in smooth muscle cells. On the other hand, attenuation of the remodeling process occurs through blood pressure-dependent and -independent mechanisms.
Hypertension is a major modifiable risk factor for cerebral small vessel disease, which hastens the onset of vascular cognitive impairment, is linked to Alzheimer's disease, and increases the risk of ischemic stroke (50). Arterioles from patients with small vessel disease show narrowing of their lumen, suggesting that inward remodeling of PAs may be a major player in the development of small vessel disease (45). Thus therapies aimed at attenuation of remodeling, whether reducing systemic blood pressure or not, may be beneficial for patients at risk.
GRANTS
This work was funded by grants from the American Heart Association (13GRNT1721000 to A. M. Dorrance, and 12PRE8960019 to P. W. Pires) and the National Heart, Lung, and Blood Institute (PO1-HL-070687 to W. F. Jackson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.W.P., W.F.J., and A.M.D. conception and design of research; P.W.P. performed experiments; P.W.P. analyzed data; P.W.P., W.F.J., and A.M.D. interpreted results of experiments; P.W.P. prepared figures; P.W.P. drafted manuscript; P.W.P., W.F.J., and A.M.D. edited and revised manuscript; P.W.P., W.F.J., and A.M.D. approved final version of manuscript.
REFERENCES
- 1.Auriel E, Csiba L, Berenyi E, Varkonyi I, Mehes G, Kardos L, Karni A, Bornstein NM. Leukoaraiosis is associated with arterial wall thickness: a quantitative analysis. Neuropathology 32: 227–233, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension 21: 816–826, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension 13: 968–972, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Baumbach GL, Heistad DD, Siems JE. Effect of sympathetic nerves on composition and distensibility of cerebral arterioles in rats. J Physiol 416: 123–140, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumbach GL, Walmsley JG, Hart MN. Composition and mechanics of cerebral arterioles in hypertensive rats. Am J Pathol 133: 464–471, 1988. [PMC free article] [PubMed] [Google Scholar]
- 6.Chan SL, Chapman AC, Sweet JG, Gokina NI, Cipolla MJ. Effect of PPARgamma inhibition during pregnancy on posterior cerebral artery function and structure. Front Physiol 1: 130, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan SL, Sweet JG, Cipolla MJ. Treatment for cerebral small vessel disease: effect of relaxin on the function and structure of cerebral parenchymal arterioles during hypertension. FASEB J 27: 3917–3927, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chrissobolis S, Sobey CG. Evidence that Rho-kinase activity contributes to cerebral vascular tone in vivo and is enhanced during chronic hypertension: comparison with protein kinase C. Circ Res 88: 774–779, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Cipolla MJ. The Cerebral Circulation. San Rafael, CA: Morgan & Claypool Life Sciences, 2009. [PubMed] [Google Scholar]
- 10.Cipolla MJ, Li R, Vitullo L. Perivascular innervation of penetrating brain parenchymal arterioles. J Cardiovasc Pharmacol 44: 1–8, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Cipolla MJ, Sweet J, Chan SL, Tavares MJ, Gokina N, Brayden JE. Increased pressure-induced tone in rat parenchymal arterioles vs. middle cerebral arteries: role of ion channels and calcium sensitivity. J Appl Physiol 117: 53–59, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol 50: 335–362, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Cohen Z, Molinatti G, Hamel E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J Cereb Blood Flow Metab 17: 894–904, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Cox RH, Rusch NJ. New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure. Microcirculation 9: 243–257, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. [DOI] [PubMed] [Google Scholar]
- 16.de la Torre JC. Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer's disease. J Alzheimers Dis 32: 553–567, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Dorrance AM, Pollock DM, Romanko OP, Stepp DW. A high-potassium diet reduces infarct size and improves vascular structure in hypertensive rats. Am J Physiol Regul Integr Comp Physiol 292: R415–R422, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Duckworth JW, Wellman GC, Walters CL, Bevan JA. Aminergic histofluorescence and contractile responses to transmural electrical field stimulation and norepinephrine of human middle cerebral arteries obtained promptly after death. Circ Res 65: 316–324, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Dunn KM, Nelson MT. Neurovascular signaling in the brain and the pathological consequences of hypertension. Am J Physiol Heart Circ Physiol 306: H1–H14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn WR, Wallis SJ, Gardiner SM. Remodelling and enhanced myogenic tone in cerebral resistance arteries isolated from genetically hypertensive Brattleboro rats. J Vasc Res 35: 18–26, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Endemann DH, Touyz RM, Iglarz M, Savoia C, Schiffrin EL. Eplerenone prevents salt-induced vascular remodeling and cardiac fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 43: 1252–1257, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res 66: 8–17, 1990. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez JM, Somoza B, Conde MV, Fernandez-Alfonso MS, Gonzalez MC, Arribas SM. Hypertension increases middle cerebral artery resting tone in spontaneously hypertensive rats: role of tonic vasoactive factor availability. Clin Sci (Lond) 114: 651–659, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Hajdu MA, Baumbach GL. Mechanics of large and small cerebral arteries in chronic hypertension. Am J Physiol Heart Circ Physiol 266: H1027–H1033, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Halaris AE, Freedman DX. Loss of body weight as a predictor of reserpine-induced amine depletion. Eur J Pharmacol 32: 93–101, 1975. [DOI] [PubMed] [Google Scholar]
- 26.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 100: 1059–1064, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Hart MN, Heistad DD, Brody MJ. Effect of chronic hypertension and sympathetic denervation on wall/lumen ratio of cerebral vessels. Hypertension 2: 419–423, 1980. [DOI] [PubMed] [Google Scholar]
- 28.Hilgers RH, Todd J Jr, Webb RC. Increased PDZ-RhoGEF/RhoA/Rho kinase signaling in small mesenteric arteries of angiotensin II-induced hypertensive rats. J Hypertens 25: 1687–1697, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol 91: 973–983, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Hogestatt ED, Andersson KE. On the postjunctional alpha-adrenoreceptors in rat cerebral and mesenteric arteries. J Auton Pharmacol 4: 161–173, 1984. [DOI] [PubMed] [Google Scholar]
- 31.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab 7: 476–484, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim J, McGee A, Graham D, McGrath JC, Dominiczak AF. Sex-specific differences in cerebral arterial myogenic tone in hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol 290: H1081–H1089, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Izzard AS, Bund SJ, Heagerty AM. Myogenic tone in mesenteric arteries from spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 270: H1–H6, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Joseph BK, Thakali KM, Moore CL, Rhee SW. Ion channel remodeling in vascular smooth muscle during hypertension: implications for novel therapeutic approaches. Pharm Res 70: 126–138, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, Lemaire-Carrette B, Domenga V, Schedl A, Lacombe P, Hubner N. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest 120: 433–445, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang KT, Sullivan JC, Spradley FT, d'Uscio LV, Katusic ZS, Pollock JS. Antihypertensive therapy increases tetrahydrobiopterin levels and NO/cGMP signaling in small arteries of angiotensin II-infused hypertensive rats. Am J Physiol Heart Circ Physiol 300: H718–H724, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain Pathol 12: 358–370, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lincoln J. Innervation of cerebral arteries by nerves containing 5-hydroxytryptamine and noradrenaline. Pharmacol Ther 68: 473–501, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol 11: 431–439, 1990. [PMC free article] [PubMed] [Google Scholar]
- 40.Mukai Y, Shimokawa H, Matoba T, Kandabashi T, Satoh S, Hiroki J, Kaibuchi K, Takeshita A. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J 15: 1062–1064, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci U S A 104: 365–370, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Brien RC, Cooper ME, Jerums G, Doyle AE. The effects of perindopril and triple therapy in a normotensive model of diabetic nephropathy. Diabetes 42: 604–609, 1993. [DOI] [PubMed] [Google Scholar]
- 43.Ohya Y, Tsuchihashi T, Kagiyama S, Abe I, Fujishima M. Single L-type calcium channels in smooth muscle cells from resistance arteries of spontaneously hypertensive rats. Hypertension 31: 1125–1129, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Osol G, Halpern W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am J Physiol Heart Circ Physiol 249: H914–H921, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Pantoni L, Garcia JH. Cognitive impairment and cellular/vascular changes in the cerebral white matter. Ann N Y Acad Sci 826: 92–102, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol 304: H1598–H1614, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pires PW, Deutsch C, McClain JL, Rogers CT, Dorrance AM. Tempol, a superoxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats. Microvasc Res 80: 445–452, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pires PW, Girgla SS, McClain JL, Kaminski NE, van Rooijen N, Dorrance AM. Improvement in middle cerebral artery structure and endothelial function in stroke-prone spontaneously hypertensive rats after macrophage depletion. Microcirculation 20: 650–661, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pires PW, Rogers CT, McClain JL, Garver HS, Fink GD, Dorrance AM. Doxycycline, a matrix metalloprotease inhibitor, reduces vascular remodeling and damage after cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 301: H87–H97, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol 12: 267–277, 2015. [DOI] [PubMed] [Google Scholar]
- 51.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J 15: 100–108, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reusch P, Wagdy H, Reusch R, Wilson E, Ives HE. Mechanical strain increases smooth muscle and decreases nonmuscle myosin expression in rat vascular smooth muscle cells. Circ Res 79: 1046–1053, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Rigsby CS, Ergul A, Portik Dobos V, Pollock DM, Dorrance AM. Effects of spironolactone on cerebral vessel structure in rats with sustained hypertension. Am J Hypertens 24: 708–715, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res 73: 198–205, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rusch NJ, Hermsmeyer K. Calcium currents are altered in the vascular muscle cell membrane of spontaneously hypertensive rats. Circ Res 63: 997–1002, 1988. [DOI] [PubMed] [Google Scholar]
- 56.Schubert R, Lidington D, Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res 77: 8–18, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, Kleinfeld D. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci 16: 55–63, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol 44: 131–142, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Velasquez MT, Striffler JS, Abraham AA, Michaelis OE Scalbert E, Thibault N. Perindopril ameliorates glomerular and renal tubulointerstitial injury in the SHR/N-corpulent rat. Hypertension 30: 1232–1237, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Weber DS, Webb RC. Enhanced relaxation to the rho-kinase inhibitor Y-27632 in mesenteric arteries from mineralocorticoid hypertensive rats. Pharmacology 63: 129–133, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol 300: H1616–H1630, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang ST, Mayhan WG, Faraci FM, Heistad DD. Endothelium-dependent responses of cerebral blood vessels during chronic hypertension. Hypertension 17: 612–618, 1991. [DOI] [PubMed] [Google Scholar]
- 63.Zakrzeska A, Gromotowicz-Poplawska A, Szemraj J, Szoka P, Kisiel W, Purta T, Kasacka I, Chabielska E. Eplerenone reduces arterial thrombosis in diabetic rats. J Renin Angiotensin Aldosterone Syst. In press. [DOI] [PubMed] [Google Scholar]
- 64.Zhao Y, Gong CX. From chronic cerebral hypoperfusion to Alzheimer-like brain pathology and neurodegeneration. Cell Mol Neurobiol 35: 101–110, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]