Abstract
The forkhead box A (FOXA) family of pioneer transcription factors is critical for the development of many endoderm-derived tissues. Their importance in regulating biological processes in the lung and liver is extensively characterized, though much less is known about their role in intestine. Here we investigate the contribution of FOXA2 to coordinating intestinal epithelial cell function using postconfluent Caco2 cells, differentiated into an enterocyte-like model. FOXA2 binding sites genome-wide were determined by ChIP-seq and direct targets of the factor were validated by ChIP-qPCR and siRNA-mediated depletion of FOXA1/2 followed by RT-qPCR. Peaks of FOXA2 occupancy were frequent at loci contributing to gene ontology pathways of regulation of cell migration, cell motion, and plasma membrane function. Depletion of both FOXA1 and FOXA2 led to a significant reduction in the expression of multiple transmembrane proteins including ion channels and transporters, which form a network that is essential for maintaining normal ion and solute transport. One of the targets was the adenosine A2B receptor, and reduced receptor mRNA levels were associated with a functional decrease in intracellular cyclic AMP. We also observed that 30% of FOXA2 binding sites contained a GATA motif and that FOXA1/A2 depletion reduced GATA-4, but not GATA-6 protein levels. These data show that FOXA2 plays a pivotal role in regulating intestinal epithelial cell function. Moreover, that the FOXA and GATA families of transcription factors may work cooperatively to regulate gene expression genome-wide in the intestinal epithelium.
Keywords: FOXA2, enterocyte function, ion and solute transport, GATA-4
Cis-regulatory elements recruit transcription factors, to coordinate gene expression genome-wide and integrate tissue-specific transcriptional programs. One such family of transcription factors is the forkhead box (FOX) proteins, which drive key pathways of differentiation and function in many cell types. The common DNA-binding domain in FOX factors, revealed by X-ray crystallography, forms a winged-helix structure that is similar to linker histones (13). Furthermore, FOXA proteins are able to remodel nucleosomes to create DNase I hypersensitive sites (DHS) in a SWI/SNF-independent manner (12). This observation led to their classification as pioneer factors, a special class of transcription factors, which remodel the chromatin environment at their binding sites and facilitate the recruitment of other transcription factors (59). FOXA1 and FOXA2 are coexpressed in the foregut endoderm (3, 37) and are critical for liver (41), hepatic (39), and neuronal (17, 43) development. Moreover, both factors also have overlapping roles in lung morphogenesis (52). The third family member, FOXA3, is also expressed in the embryonic endoderm; however, deletion of the gene has no morphological effects on endoderm-derived tissues such as the liver, pancreas, and gastrointestinal tract and only affects expression of some hepatic genes (34).
Another class of transcription factors that are important for development of endoderm-derived tissues is the GATA family. GATA factors contain cysteine-zinc-finger DNA binding domains and also display some chromatin remodeling activity (12). GATA-4, -5, and -6 are expressed in endoderm (5, 40) and are essential for foregut morphogenesis and development (7, 58). In the intestinal epithelium the three GATA proteins show cell type-specific expression patterns. GATA-4 and GATA-5 are important for the expression of terminal-differentiation genes, while GATA-6 is highly expressed in proliferating intestinal epithelial cells (21).
Our previous studies revealed that the FOXA1/A2 transcription factors were critical for expression of the cystic fibrosis transmembrane conductance regulator (CFTR) gene in intestinal epithelial cells (35, 36). Both FOXA1 and FOXA2 were recruited to DHS across the locus including a strong intestinal intronic enhancer. Moreover, siRNA-mediated depletion of both factors led to a 40% decrease in CFTR mRNA levels and was accompanied by changes in histone marks and occupancy of other critical transcription factors at multiple DHS (35, 36). Simultaneously, enhancer-promoter looping that is required for normal CFTR gene expression was repressed (35).
Earlier data demonstrated the importance of FOXA2 in foregut endoderm development (3, 53). These observations, together with its role in CFTR expression suggested that FOXA2 might function as a regulator of intestinal epithelial cell function, through a network of co-regulated genes. To test this hypothesis, we assayed genome-wide occupancy of FOXA2 in a colorectal adenocarcinoma cell line [Caco2 (18)] by chromatin immunoprecipitation followed by deep sequencing (ChIP-seq). In postconfluent monolayer culture, these cells become polarized and differentiate into a useful model of enterocyte function. About 24,300 FOXA2 ChIP-seq peaks were assigned to the nearest gene and the gene list subjected to a gene ontology (GO) process enrichment analysis. Many critical pathways of intestinal epithelial function were identified through this analysis. We validated FOXA2 binding to predicted targets in several of the enriched GO processes and further showed that depletion of FOXA1/A2 altered expression of relevant genes. Moreover, loss of FOXA1/A2 reduced intracellular adenosine 3′,5′-cyclic monophosphate (cAMP) levels in these cells, which is pivotal to driving multiple normal functions of enterocytes, including ion and solute transport.
MATERIALS AND METHODS
Cell culture.
Caco2 colorectal adenocarcinoma cells (18) were grown by standard methods in DMEM Low Glucose with 10% fetal bovine serum. For all experiments cells were harvested 2–3 days postconfluence to ensure consistent differentiation into an enterocyte-like model.
ChIP-seq.
Caco2 chromatin was prepared as described previously (8). ChIP-seq for FOXA2 was performed in 2 biological replicates by standard protocols (19). We used 10 μg of FOXA2 antibody [Santa Cruz Biotechnology (SCB) sc-6554x] and 10 × 106 cells, and libraries were prepared as described previously (19). Libraries were sequenced on a HiSeq 2000 machine and 33,140,000 and 18,020,000 reads were obtained for each replicate respectively. FASTQ files were aligned to the hg19 version of the human genome using Bowtie (38) and ChIP-seq peaks were identified using HOMER (26) with a false discovery rate of 0.1%. Peaks from both replicates were intersected using BEDTools and only the 24,345 sites found in both replicates were used for further analysis. The ChIP-seq data sets are available on GEO:GSE66218. Identification of transcription factor motifs in the data set and peak annotation based on the nearest gene were also performed using HOMER. GO terms enriched among the nearest genes in the ChIP-seq data were determined using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) (27, 28).
ChIP-quantitative PCR.
ChIP was performed as described previously (8, 19) using 10 μg of FOXA2 antibody (SCB sc-6554x) and 10 μg of Goat IgG (sc-2028) for the isotype control. SYBR Green quantitative (q)PCRs were run to validate binding at the selected targets using primers listed in Supplementary Table S5, which were all evaluated for efficiency before use.1
Transient siRNA knockdown experiments.
20 nM hFOXA1 (SCB sc-37930), hFOXA2 (SCB sc-35569), or 40 nM control (SCB sc-37007) siRNAs were reverse transfected in Caco2 cells as described previously (36), and cells were collected after 72 h.
Western blot analysis.
Standard protocols were used with antibodies against FOXA1 (Abcam ab-5089), FOXA2 (SCB sc-6554x), GATA-4 (SCB sc-1237), GATA-6 (SCB, sc-7244), and β-tubulin (Sigma-Aldrich T4026). Protein quantification was performed using ImageJ software (National Institutes of Health) (http://rsb.info.nih.gov/ij/).
RT-qPCR.
Total RNA was extracted with TRIzol (Life Technologies). mRNA levels for all the target genes were assayed using primers in different exons separated by introns. Primer sequences are listed in Supplementary Table S5.
Measurement of intracellular cAMP levels.
Intracellular cAMP levels were measured using the cAMP-Glo Assay (Promega). Briefly, the ATP not converted to ADP by cAMP is measured using a luciferase assay. Therefore, cAMP concentration is inversely proportional to luciferase activity. The cAMP assays were performed 72 h after depletion of FOXA1/A2. Luciferase levels were measured in 96-well plates using a luminometer.
RESULTS
FOXA2 binds to cis-regulatory elements for genes important for intestinal epithelial function.
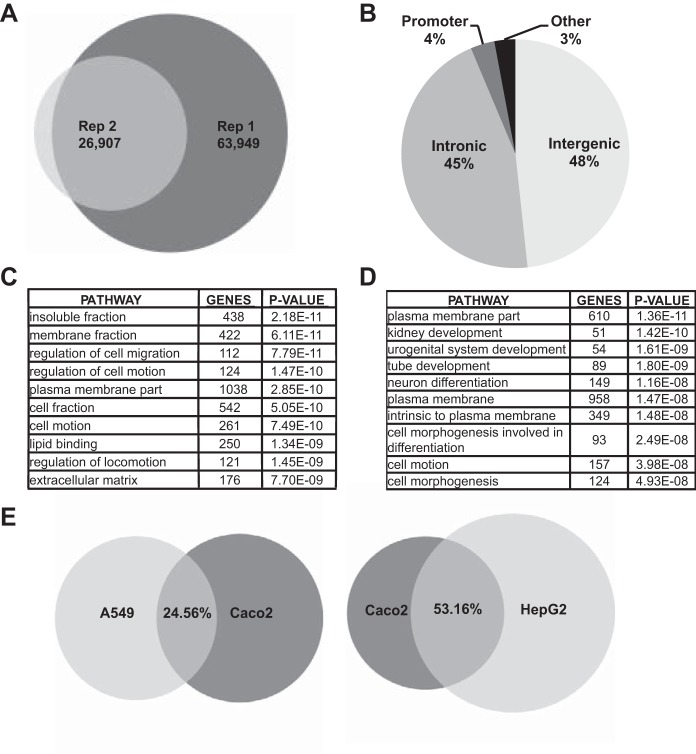
FOXA2 ChIP-seq performed in 3 days postconfluent Caco2 colon carcinoma cells identified 63,949 peaks in the first experiment and 26,907 in the replicate. Intersection of these two data sets revealed 24,345 shared peaks (Fig. 1A). Annotation of the genomic location of the 24,345 binding sites showed that 48.25% of FOXA2 peaks were intergenic, 45.4% intronic, 3.4% near promoters, and 2.95% in other regions (Fig. 1B). In the absence of more accurate experimental methods to assign cis-regulatory elements to their cognate genes, FOXA2 ChIP-seq peaks were assigned to the nearest locus. A GO process enrichment analysis by DAVID was then applied to the gene list. This revealed the enrichment of terms such as apical plasma membrane, plasma membrane part, cell morphogenesis, and extracellular matrix, which directly reflect the many secretory functions of the enterocyte (Fig. 1C, Supplementary Table S1). Individual genes contributing to each of these pathways are considered further below. Next, to identify genes and pathways that were regulated by FOXA2 uniquely in Caco2 cells, ChIP-seq peaks for this factor that were identified in HepG2 [hepatocellular carcinoma (1)] and A549 [lung carcinoma (22)] cells (16) were removed. This subtraction would be predicted to remove common pathways of epithelial/endoderm function together with some shared pathways of oncogenesis. After removal of the shared sites, 10,300 peaks remained that were unique to the Caco2 cells and these were again assigned to the nearest genes. GO analysis of these genes (Fig. 1D) revealed novel pathways with highly significant P values in addition to several of those seen in Fig. 1C. Plasma membrane part was the most significant pathway, while tube development, enzyme-linked protein receptor signaling, and regulation of cell morphogenesis and motion all showed P values < 5E-08. Each of these pathways is critical for the function of intestinal epithelial cells (Fig. 1D, Supplementary Table S2).
Fig. 1.
Forkhead box A (FOXA)2 chromatin immunoprecipitation followed by deep sequencing (ChIP-seq) in Caco2 intestinal epithelial cells. A: Venn diagram showing the overlap of the FOXA2 binding sites in 2 biological replicates of the ChIP-seq experiment. B: distribution of the combined FOXA2 peaks by genomic location. C: gene ontology (GO) pathways enriched in the combined FOXA2 binding sites dataset. D: GO terms enriched in FOXA2 dataset unique to Caco2 cells after removing peaks shared with HepG2 and A549. E: Venn diagrams showing the FOXA2 binding sites shared between A549 and Caco2 (left) and HepG2 and Caco2 (right).
Further analysis of individual gene targets in the plasma membrane part GO pathway identified many essential ion channels and solute transporters that are functionally integrated in the normal biology of the small intestine. In addition to the cAMP-activated CFTR chloride channel, which is a known target of FOXA2 (35, 36), voltage-gated chloride channels 3 and 5 (CLCN3, CLCN5), the solute carrier family 12 member 2 (SLC12A2), and aquaporin 9 (AQP9) all had FOXA2 ChIP-seq peaks either within or near the locus. Given the known role of Foxa2 in mouse foregut endoderm development (3, 25), it was of interest to identify the putative targets of FOXA2 within the GO pathways for tube development, cell morphogenesis, and cell motion. These include genes involved in developmental signaling pathways such as sonic hedgehog homolog (SHH), Indian hedgehog homolog (IHH), integrin alpha 2 (ITGA2), transforming growth factor beta 3 (TGFβ3), and transcription factors such as GATA-4 and GATA-6.
Next, the overlapping and unique FOXA2 binding sites in Caco2, A549, and HepG2 cells were examined. Caco2 and A549 cells revealed an overlap of 24.56% of FOXA2 peaks, while Caco2 and HepG2 showed a 53.16% overlap (Fig. 1E). Analysis of the GO pathways enriched in both the shared Caco2 and A549 peaks and the common Caco2 and HepG2 peaks revealed the regulation of RNA polymerase II transcribed genes as a highly significant process. The gene targets included transcription factors such as CDX2, GATA-6, histone deacetylases, and multiple chromatin remodeling proteins (Supplementary Tables S3, S4).
Genes involved in tube development and ion transport are directly regulated by FOXA2.
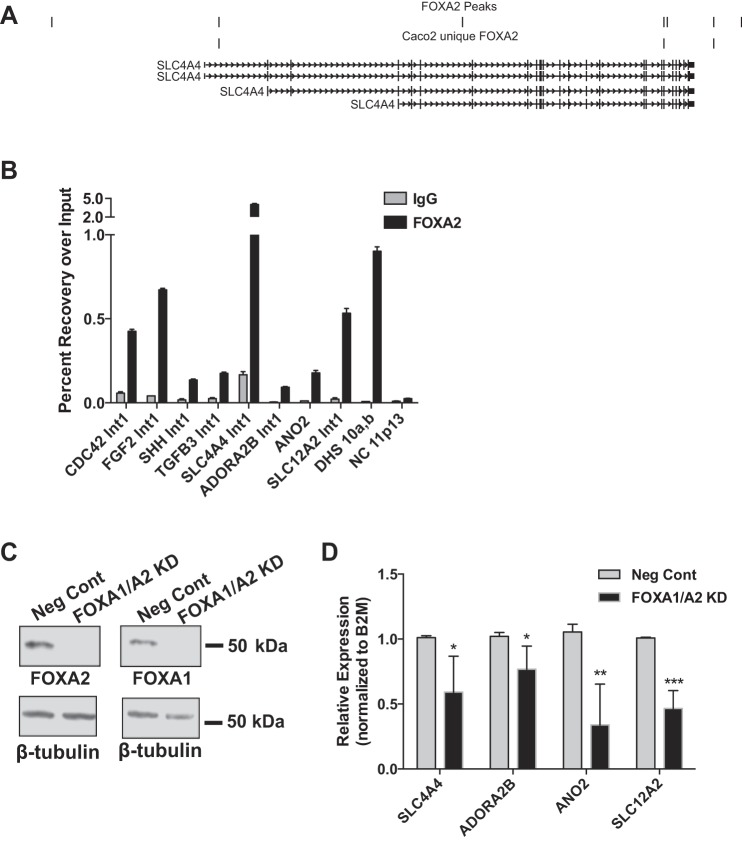
FOXA2 occupancy at ChIP-seq peaks identified near or within essential target genes in intestinal epithelium was first validated using ChIP-qPCR. Several loci in the highly significant tube development pathway were initially tested. These included fibroblast growth factor 2 (FGF2), SHH, TGFβ3 and the cell division control protein 42 (CDC42), a Rho GTPase that is important for epithelial morphogenesis (31). Next, several genes within the most significant GO pathways plasma membrane or plasma membrane part were analyzed. Among loci tested were the bicarbonate transporter solute carrier family 4 member 4 (SLC4A4) (Fig. 2A), the adenosine A2B receptor (ADORA2B), a calcium activated chloride ion channel, anoctamin-2 (ANO2), and the SLC12A2 gene. ChIP-qPCRs confirmed FOXA2 occupancy at ChIP-seq sites within introns of all of these genes (Fig. 2B), with a known FOXA2-binding cis-regulatory element in CFTR in intron 10 (36) providing a positive control (Fig. 2B; DHS 10a,b). Although levels of occupancy were variable at each site, all were significantly enriched over the background (IgG) and higher than a negative control region with no FOXA motifs (Fig. 2B, NC 11p13).
Fig. 2.
FOXA2 regulates gene targets identified by ChIP-seq. A: UCSC genome browser view of the SLC4A4 locus showing all FOXA2 peaks and those unique to Caco2 datasets. B: representative ChIP-qPCR validation of FOXA2 binding sites at targets enriched in the GO terms cell morphogenesis, tube development, and plasma membrane part. NC 11p13 is a negative control region with no FOXA motifs and no known regulatory function. C: representative Western blot showing Caco2 cells treated with either a negative control (Neg Cont) siRNA or siRNAs targeting both FOXA1/A2 [FOXA1/A2 knockdown (KD)]. β-Tubulin is shown below as a loading control. D: RT-quantitative (q)PCR analysis of selected target genes after targeting with NC or FOXA1/A2 siRNAs. All transcripts are normalized to β-2-microglobulin (B2M) levels and the data shown are from >3 biological replicates. *P < 0.05, **P < 0.01, ***P < 0.001.
FOXA1 and FOXA2 have very similar DNA binding motifs and cooperatively regulate lung and liver development (24). Caco2 cells express both factors, though FOXA2 is more abundant (35, 36); hence, it was necessary to consider each of them. Since depletion of FOXA2 might cause an increase in FOXA1 transcription, both were reduced in Caco2 cells by siRNA-mediated depletion (Fig. 2C). Loss of FOXA1/A2 led to significant decreases in expression of SLC4A4, ADORA2B, ANO2, and SLC12A2 (Fig. 2D).
Cooperation and co-regulation between the FOXA and GATA family of transcription factors.
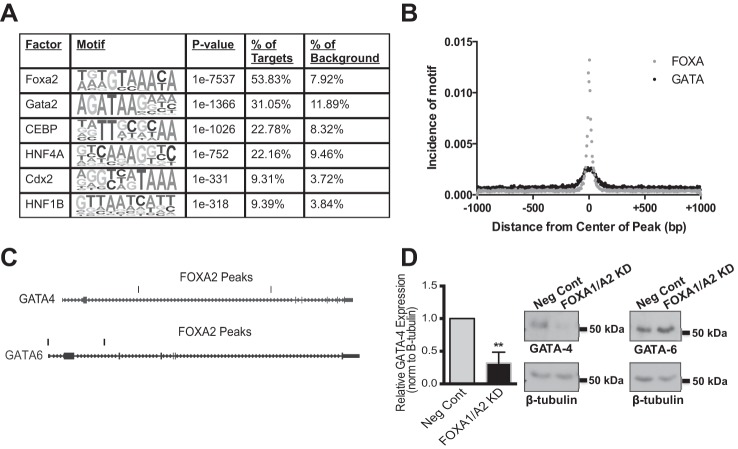
We previously identified a transcriptional network in intestinal epithelial cells that drives CFTR expression (36). Our data suggested that FOXA2 might be the initiating factor, followed by the recruitment of hepatocyte nuclear factor 1 (HNF1) and caudal type homeobox 2 (CDX2) to the same cis-elements (35). To look for other transcription factors that could co-occupy the FOXA2 ChIP-seq peaks, a de novo motif analysis was performed using HOMER. The most prominent motif identified was FOXA2 along with other FOX family members with similar binding matrices, which was present at 53.83% of the target sequences (Fig. 3A). HNF1 and CDX2 motifs were also present in ∼9% of sites. However, binding sites for GATA factors were enriched at 31.05% of the peaks with a P value of 1e−1366. This is particularly interesting as both FOXA and GATA family members are essential for endoderm development, specifically the foregut endoderm (37, 58). To determine the location and distribution of GATA motifs within the FOXA ChIP-seq peaks, their incidence was plotted against distance from the center of the peak (Fig. 3B). These data demonstrate that the GATA motif coincides with the FOXA motif and is present within 50 bp of the center of the peak.
Fig. 3.
Cooperation between the FOXA and GATA transcription factor families. A: de novo motif analysis of the combined FOXA2 peaks in Caco2 cells within a 200 bp window. B: incidence of GATA motifs measured as a function of distance from the center of the FOXA2 ChIP-seq peaks. C: UCSC genome browser views of the GATA-4 and GATA-6 loci showing FOXA2 binding sites in Caco2 cells. D: quantification of GATA-4 protein levels, from Western blots, in cells treated with a NC siRNA or FOXA1/A2 targeting siRNAs (left). **P < 0.01. Data are normalized to β-tubulin, as an endogenous loading control. On the right are representative Western blots of GATA-4 and GATA-6 respectively in cells treated with Neg Cont or FOXA1/A2 siRNAs. β-Tubulin is shown below.
Though the specific GATA factor predicted in the motif analysis is GATA-2, all GATA factors have a very similar binding motif. GATA-2 is generally restricted to cells of the hematopoietic lineage (56) and is not expressed in Caco2 cells, where GATA-4 and -6 are found. GATA-4 and GATA-6 were also among putative FOXA2 targets in GO pathways for tube development, cell morphogenesis, and cell motion. Visualization of the GATA-4 and GATA-6 genes on the UCSC genome browser reveals FOXA2 ChIP-seq peaks in both (Fig. 3C). To determine if these two transcription factors are targets of FOXA1/A2, Western blots were performed on Caco2 cells treated with negative control siRNAs or siRNAs targeting both FOXA1 and FOXA2. Loss of FOXA1/A2 led to a significant decrease in GATA-4 expression (Fig. 3D), but no change was observed in GATA-6 (Fig. 3D, representative Western blots). These data are consistent with previous observations that Foxa2 transcriptionally regulates mouse Gata-4 through a binding site in intron 2 of the gene (48).
FOXA2 depletion has a functional impact on intestinal epithelial cells.
Next, we investigated the biological outcome of FOXA1/A2 loss in intestinal epithelial cells. The gene expression data demonstrated a statistically significant decrease in ADORA2B mRNA levels after loss of FOXA1/A2 (Fig. 2D). ADORA2B is a membrane-bound G protein-coupled receptor (GPCR) that binds adenosine and activates adenylate cyclases. In turn adenylate cyclase converts adenosine triphosphate into cAMP and pyrophosphate, so we measured cAMP as a readout for ADORA2B levels. FOXA1/A2 were depleted in Caco2 cells and intracellular cAMP levels measured with luminescent substrate. Loss of both FOXA1/A2 led to a 40% reduction in intracellular cAMP levels compared with negative control siRNA-treated cells (Fig. 4A). This effect may be in part due to the decrease in ADORA2B expression, though other proteins that activate cAMP may also be altered by FOXA1/A2 depletion. Inspection of the FOXA2 ChIP-seq peak-associated gene list identified two candidates, adenylate cyclases (ADCY) 9 and 10, which are both expressed in Caco2 cells (Fig. 4B). Expression levels of these two genes after FOXA1/A2 depletion were measured by qRT-PCR. ADCY9 mRNA levels were unchanged, but ADCY10 mRNA increased (Fig. 4B). These observations make it more likely that ADORA2B is the adenylate cyclase that contributes significantly to the decrease in intracellular cAMP levels after FOXA1/A2 depletion. Other FOXA1/A2 target genes may also be contributing indirectly to the process.
Fig. 4.
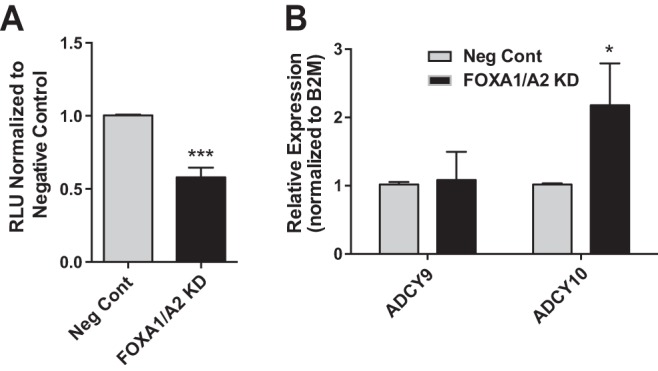
FOXA2 regulates a network of genes that are biologically important for intestinal epithelial function. A: luciferase-based cAMP assay showing the levels of intracellular cAMP in Caco2 cells treated with Neg Cont or FOXA1/A2 siRNAs. The level of cAMP in cells treated with Neg Cont siRNA has been set to 1. RLU, relative light units. ***P < 0.001. B: RT-qPCR analysis of ADCY9 and ADCY10, other putative FOXA2 targets that could alter the levels of intracellular cAMP in cells. *P < 0.05.
DISCUSSION
The role of the pioneer transcription factors FOXA1 and FOXA2 in development and differentiation of multiple endoderm-derived tissues was studied extensively in previous work (6, 32, 46, 51, 54, 55). However, their contribution to intestinal epithelial function has not yet been addressed on a genome-wide scale. FOXA2 was shown to regulate the expression of single genes within the intestine, including the apolipoprotein B locus, the gel-forming mucins, and the transcription factors Pax6 and HNF6 (4, 33, 42, 50, 57). Here we use genome-wide mapping of FOXA2 sites by ChIP-seq to reveal the biological processes regulated by this factor in a human enterocyte model. In postconfluent Caco2 cells, FOXA2 was recruited to 24,345 sites, which largely show an intronic and intergenic distribution consistent with other tissue-specific transcriptional regulators. FOXA2 appears to regulate many genes in diverse pathways contributing to intestinal function including tube development, regulation of cell morphogenesis and solute transport.
After subtracting FOXA2 ChIP-seq peaks that were common to other carcinoma cells of endodermal origin (airway epithelium and hepatocyte), sites unique to intestinal epithelial cells were examined. Genes associated with this enterocyte-restricted subset of FOXA2 binding sites are significantly enriched in gene ontology pathways for plasma membrane constituents, tube development, cell motion, and cell morphogenesis. A number of critical genes for normal intestinal epithelial differentiation and function were chosen for further analysis. Some of these were known to be regulated by FOXA2 in other cell types, though may be driven by different tissue-specific cis-elements recruiting FOXA2. Others were not previously associated with the FOXA2 network. Among genes in the GO plasma membrane and plasma membrane part pathways is CFTR. FOXA1/A2 were shown previously to regulate the expression of the CFTR gene in Caco2 cells (35). The CFTR protein is a cAMP-activated chloride ion channel located in the apical membrane of intestinal epithelial cells. It is a critical component of pathways promoting fluid secretion and maintaining a luminal environment that supports intestinal enzyme activity (20, 49). Chloride transport in the intestinal epithelium is also powered by other proteins in the plasma membrane part GO pathway. These include the voltage-gated chloride channels CLCN3 and CLCN5, which are both encoded by FOXA2-regulated genes, as is SLC12A2. This protein is a sodium, potassium, chloride cotransporter at the basolateral side of intestinal epithelial cells. SLC12A2 provides intracellular chloride ions for CFTR to secrete at the apical membrane (49). In support of our data are those from the sweat glands of Foxa1 null mice, which showed repressed sweating and downregulation of both the sodium, potassium, chloride transporter 1 (SLC12A1) and bestrophin-2 (BEST2) genes (14).
Another significant FOXA2-regulated “plasma membrane part” gene in normal intestinal epithelial function is AQP9, encoding aquaporin 9, a water transporter that is important in goblet cell function (47). Within the “tube development” GO pathway, SHH is critical in developmental signaling and requires FOXA2 for normal expression in the mouse ventral endoderm (25). Moreover, loss of SHH and IHH cause intestinal defects in mice and these factors are pivotal to the formation of the crypt-villus axis (44). Also within this pathway is CDC42, which regulates spindle orientation for appropriate apical positioning (31). Though not a previously known FOXA2 target CDC42 is involved in maintenance of cell-cell junctions and cell migration (29).
While validating ChIP-seq data we noticed that many (14.8%) of the intragenic FOXA2 binding sites genome-wide were located within the first intron of gene. These comprised ∼32.6% of all intronic FOXA2 binding sites. This is consistent with previous Foxa2 ChIP-seq in the adult mouse liver (55) and may reflect the recruitment of this pioneer factor close to the cognate gene promoter. Among the genes with FOXA2 occupancy at cis-elements in the first intron are several of ion transporters and GPCRs. mRNA levels of all the genes tested decreased after depletion of FOXA1/A2. To determine the functional outcome of FOXA1/A2 depletion we focused on ADORA2B, which is downregulated by loss of these factors. Intracellular cAMP levels fell after loss of FOXA1/A2, consistent with the observed reduction in ADORA2B mRNA levels. Other pathways that regulate cAMP may contribute to this effect, though, adenylate cyclases 9 and 10, which are expressed in Caco2 cells, did not participate (Fig. 4B). The upregulation of ADCY10 may be a secondary effect of changes in intracellular pH through altered expression of bicarbonate transporters such as SLC4A4, a mechanism known to regulate the activity of soluble adenylate cyclases (10). ATP-binding cassette transporters that export cAMP could also be involved (9) as could other FOXA2 targets that influence the process indirectly. Regardless of the mechanism, the decrease in cAMP levels after FOXA protein depletion (Fig. 4A) suggests this factor is critical to the function of intestinal epithelial cells. cAMP is an essential second messenger that is involved in many different signal transduction pathways. cAMP-dependent protein kinase A (PKA) phosphorylation of CFTR is required for activation of the channel (2, 11). cAMP levels also affect the expression of intestinal aquaporins (23, 30), suggesting several mechanisms by which cAMP regulates fluid secretion. Additionally, cAMP induces binding of the cAMP response element binding protein to regulatory sites in many important genes in various signaling pathways (45). These data on the biological outcome of FOXA1/A2 depletion in combination with the gene expression data reveal a network of genes regulated by FOXA2 in intestinal epithelia. This network is composed of ADORA2B, ANO2, and CFTR. Chloride transport and its associated fluid secretion are mediated by chloride channels such as CFTR and calcium-activated channels such as ANO2. Adenosine receptors such as ADORA2B regulate CFTR activity by modulating cAMP and PKA levels. Additionally, other genes regulated by FOXA2 are SLC4A4 and SLC12A2, which transport bicarbonate and chloride ions, respectively. Together this FOXA2-regulated network performs functions critical for maintaining an optimal luminal environment and a homeostatic intracellular pH permitting fluid secretion.
In this study we also investigate the potential cooperation between the FOXA and GATA family of transcription factors. Using de novo motif analysis, we demonstrate that the GATA binding site is present at the center of ∼30% of the FOXA2 peaks identified by ChIP-seq and its localization overlaps with the FOXA motif (Fig. 3B). Cooperative regulation by GATA and FOXA family members was demonstrated at individual loci such as the mucin 4 (33) and liver-enriched homeobox gene (15), but our data may be the first evidence of genome-wide co-regulation. Interestingly, unlike the FOXA2 targets, the peaks containing GATA motifs are not enriched at the first intron of genes and show a more random distribution for genomic location. Additionally, FOXA2 peaks were identified within both the GATA-4 and GATA-6 genes and GATA-4, but not GATA-6, protein levels decreased after FOXA1/A2 depletion. Regulation of GATA-4 by FOXA proteins was reported previously (48), though there is apparently no evidence of their regulation of GATA-6. These observations may reveal distinct roles for the GATA proteins in intestinal cells, which are difficult to decipher in silico due to their very similar DNA binding sequences.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant R01HD-068901 (PI: A. Harris) and the Cystic Fibrosis Foundation (Harris11G0; Harris14P0).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.G. and A.H. conception and design of research; N.G. and J.L.K. performed experiments; N.G., R.Y., and A.H. analyzed data; N.G. and A.H. interpreted results of experiments; N.G. prepared figures; N.G. and A.H. drafted manuscript; N.G. and A.H. edited and revised manuscript; N.G., R.Y., J.L.K., and A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Longtao Wu and Meredith Chase for assistance.
Current address for J. L. Kerschner: UNC Lineberger Comprehensive Cancer Center and Dept. of Biochemistry and Biophysics, Univ. of North Carolina School of Medicine, Chapel Hill, NC 27599-7260.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Aden DP, Fogel A, Plotkin S, Damjanov I, Knowles BB. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature 282: 615–616, 1979. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MP, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Generation of cAMP-activated chloride currents by expression of CFTR. Science 251: 679–682, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development 119: 1301–1315, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Antes TJ, Levy-Wilson B. HNF-3 beta, C/EBP beta, and HNF-4 act in synergy to enhance transcription of the human apolipoprotein B gene in intestinal cells. DNA Cell Biol 20: 67–74, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol 13: 2235–2246, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochkis IM, Schug J, Ye DZ, Kurinna S, Stratton SA, Barton MC, Kaestner KH. Genome-wide location analysis reveals distinct transcriptional circuitry by paralogous regulators Foxa1 and Foxa2. PLoS Genet 8: e1002770, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bossard P, Zaret KS. GATA transcription factors as potentiators of gut endoderm differentiation. Development 125: 4909–4917, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Browne JA, Harris A, Leir SH. An optimized protocol for isolating primary epithelial cell chromatin for ChIP. PLoS One 9: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheepala S, Hulot JS, Morgan JA, Sassi Y, Zhang W, Naren AP, Schuetz JD. Cyclic nucleotide compartmentalization: contributions of phosphodiesterases and ATP-binding cassette transporters. Ann Rev Pharmacol Toxicol 53: 231–253, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Cheng SH, Rich DP, Marshall J, Gregory RJ, Welsh MJ, Smith AE. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell 66: 1027–1036, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell 9: 279–289, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature 364: 412–420, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Cui CY, Childress V, Piao Y, Michel M, Johnson AA, Kunisada M, Ko MSH, Kaestner KH, Marmorstein AD, Schlessinger D. Forkhead transcription factor FoxA1 regulates sweat secretion through Bestrophin 2 anion channel and Na-K-Cl cotransporter 1. Proc Natl Acad Sci USA 109: 1199–1203, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denson LA, McClure MH, Bogue CW, Karpen SJ, Jacobs HC. HNF3beta and GATA-4 transactivate the liver-enriched homeobox gene, Hex. Gene 246: 311–320, 2000. [DOI] [PubMed] [Google Scholar]
- 16.ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489: 57–74, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferri ALM, Lin W, Mavromatakis YE, Wang JC, Sasaki H, Whitsett JA, Ang SL. Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development 134: 2761–2769, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Fogh J, Wright WC, Loveless JD. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst 58: 209–214, 1977. [DOI] [PubMed] [Google Scholar]
- 19.Fossum SL, Mutolo MJ, Yang R, Dang H, O'Neal WK, Knowles MR, Leir SH, Harris A. Ets homologous factor regulates pathways controlling response to injury in airway epithelial cells. Nucleic Acids Res 42: 13588–13598, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med 2: a009563, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Sedgwick T, Shi YB, Evans T. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol Cell Biol 18: 2901–2911, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, Parks WP. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst 51: 1417–1423, 1973. [DOI] [PubMed] [Google Scholar]
- 23.Hamabata T, Liu C, Takeda Y. Positive and negative regulation of water channel aquaporins in human small intestine by cholera toxin. Microb Pathog 32: 273–277, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet 10: 233–240, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrelson Z, Kaestner KH, Evans SM. Foxa2 mediates critical functions of prechordal plate in patterning and morphogenesis and is cell autonomously required for early ventral endoderm morphogenesis. Biol Open 1: 173–181, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38: 576–589, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, Lempicki RA. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res 35: W169–W175, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9: 846–859, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Itoh A, Tsujikawa T, Fujiyama Y, Bamba T. Enhancement of aquaporin-3 by vasoactive intestinal polypeptide in a human colonic epithelial cell line. J Gastroenterol Hepatol 18: 203–210, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol 183: 625–633, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin HJ, Zhao JC, Wu L, Kim J, Yu J. Cooperativity and equilibrium with FOXA1 define the androgen receptor transcriptional program. Nat Commun 5: 3972, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonckheere N, Vincent A, Perrais M, Ducourouble MP, Male AK, Aubert JP, Pigny P, Carraway KL, Freund JN, Renes IB, Van Seuningen I. The human mucin MUC4 is transcriptionally regulated by caudal-related homeobox, hepatocyte nuclear factors, forkhead box A, and GATA endodermal transcription factors in epithelial cancer cells. J Biol Chem 282: 22638–22650, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Kaestner KH, Hiemisch H, Schütz G. Targeted disruption of the gene encoding hepatocyte nuclear factor 3gamma results in reduced transcription of hepatocyte-specific genes. Mol Cell Biol 18: 4245–4251, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerschner JL, Gosalia N, Leir SH, Harris A. Chromatin remodeling mediated by the FOXA1/A2 transcription factors activates CFTR expression in intestinal epithelial cells. Epigenetics 9: 557–565, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerschner JL, Harris A. Transcriptional networks driving enhancer function in the CFTR gene. Biochem J 446: 203–212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai E, Prezioso VR, Smith E, Litvin O, Costa RH, Darnell JE. HNF-3A, a hepatocyte-enriched transcription factor of novel structure is regulated transcriptionally. Genes Dev 4: 1427–1436, 1990. [DOI] [PubMed] [Google Scholar]
- 38.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lantz KA, Kaestner KH. Winged-helix transcription factors and pancreatic development. Clin Sci (Lond) 108: 195–204, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Laverriere AC, MacNeill C, Mueller C, Poelmann RE, Burch JB, Evans T. GATA-4/5/6, a subfamily of three transcription factors transcribed in developing heart and gut. J Biol Chem 269: 23177–23184, 1994. [PubMed] [Google Scholar]
- 41.Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature 435: 944–947, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Lehner F, Kulik U, Klempnauer J, Borlak J. Inhibition of the liver enriched protein FOXA2 recovers HNF6 activity in human colon carcinoma and liver hepatoma cells. PLoS One 5: e13344, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin W, Metzakopian E, Mavromatakis YE, Gao N, Balaskas N, Sasaki H, Briscoe J, Whitsett JA, Goulding M, Kaestner KH, Ang SL. Foxa1 and Foxa2 function both upstream of and cooperatively with Lmx1a and Lmx1b in a feedforward loop promoting mesodiencephalic dopaminergic neuron development. Dev Biol 333: 386–396, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 132: 279–289, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2: 599–609, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Motallebipour M, Ameur A, Reddy Bysani MS, Patra K, Wallerman O, Mangion J, Barker MA, McKernan KJ, Komorowski J, Wadelius C. Differential binding and co-binding pattern of FOXA1 and FOXA3 and their relation to H3K4me3 in HepG2 cells revealed by ChIP-seq. Genome Biol 10: 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada S, Misaka T, Matsumoto I, Watanabe H, Abe K. Aquaporin-9 is expressed in a mucus-secreting goblet cell subset in the small intestine. FEBS Lett 540: 157–162, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Rojas A, Schachterle W, Xu SM, Martín F, Black BL. Direct transcriptional regulation of Gata4 during early endoderm specification is controlled by FoxA2 binding to an intronic enhancer. Dev Biol 346: 346–355, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thiagarajah JR, Verkman AS. CFTR pharmacology and its role in intestinal fluid secretion. Curr Opin Pharmacol 3: 594–599, 2003. [DOI] [PubMed] [Google Scholar]
- 50.van der Sluis M, Vincent A, Bouma J, Korteland-Van Male A, van Goudoever JB, Renes IB, Van Seuningen I. Forkhead box transcription factors Foxa1 and Foxa2 are important regulators of Muc2 mucin expression in intestinal epithelial cells. Biochem Biophys Res Commun 369: 1108–1113, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Wallerman O, Motallebipour M, Enroth S, Patra K, Bysani MSR, Komorowski J, Wadelius C. Molecular interactions between HNF4a, FOXA2 and GABP identified at regulatory DNA elements through ChIP-sequencing. Nucleic Acids Res 37: 7498–7508, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem 280: 13809–13816, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z, Dollé P, Cardoso WV, Niederreither K. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev Biol 297: 433–445, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Watts JA, Zhang C, Klein-Szanto AJ, Kormish JD, Fu J, Zhang MQ, Zaret KS. Study of FoxA pioneer factor at silent genes reveals Rfx-repressed enhancer at Cdx2 and a potential indicator of esophageal adenocarcinoma development. PLoS Genet 7: e1002277, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wederell ED, Bilenky M, Cullum R, Thiessen N, Dagpinar M, Delaney A, Varhol R, Zhao Y, Zeng T, Bernier B, Ingham M, Hirst M, Robertson G, Marra MA, Jones S, Hoodless PA. Global analysis of in vivo Foxa2-binding sites in mouse adult liver using massively parallel sequencing. Nucleic Acids Res 36: 4549–4564, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto M, Ko LJ, Leonard MW, Beug H, Orkin SH, Engel JD. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev 4: 1650–1662, 1990. [DOI] [PubMed] [Google Scholar]
- 57.Ye DZ, Kaestner KH. Foxa1 and Foxa2 control the differentiation of goblet and enteroendocrine L- and D-cells in mice. Gastroenterology 137: 2052–2062, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaret K. Developmental competence of the gut endoderm: genetic potentiation by GATA and HNF3/fork head proteins. Dev Biol 209: 1–10, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev 25: 2227–2241, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]