Abstract
Age-related degeneration of the musculoskeletal system, accelerated by menopause, is further complicated by increased systemic and muscular adiposity. The purpose of this study was to identify at the molecular, cellular, and tissue levels the impact of ovariectomy on adiposity and satellite cell populations in mice and whether mechanical signals could influence any outcomes. Eight-week-old C57BL/6 mice were ovariectomized, with one half subjected to low-intensity vibration (LIV; 0.3 g/90 Hz, 15 min/day, 5 day/wk; n = 10) for 6 wk and the others sham vibrated (OVX; n = 10). Data are compared with age-matched, intact controls (AC; n = 10). In vivo μCT analysis showed that OVX mice gained 43% total (P < 0.001) and 125% visceral adiposity (P < 0.001) compared with their baseline after 6 wk, whereas LIV gained only 21% total (P = 0.01) and 70% visceral adiposity (P < 0.01). Relative to AC, expression of adipogenic genes (PPARγ, FABP4, PPARδ, and FoxO1) was upregulated in OVX muscle (P < 0.05), whereas LIV reduced these levels (P < 0.05). Adipogenic gene expression was inversely related to the percentage of total and reserve satellite cell populations in the muscle, with both declining in OVX compared with AC (−21 and −28%, respectively, P < 0.01). LIV mitigated these declines (−11 and −17%, respectively). These results provide further evidence of the negative consequences of estrogen depletion and demonstrate that mechanical signals have the potential to interrupt subsequent adipogenic gene expression and satellite cell suppression, emphasizing the importance of physical signals in protecting musculoskeletal integrity and slowing the fat phenotype.
Keywords: muscle stem cells, myogenesis, skeletal muscle, vibration
menopause, the age-associated loss of ovarian hormones (estrogen, activin, inhibin, follistatin, etc.), accelerates degeneration of the musculoskeletal system, leading to osteoporosis (35), lower muscle mass (52), reduced strength (48), and altered tissue composition (15). Reduced bone mass increases fracture risk (31) whereas concurrent weakening of the muscle increases the risk of falls (66), in aggregate, increasing the vulnerability of the musculoskeletal system. Although sarcopenia (diminishing muscle mass) inherently plays a role in the deterioration of muscle quality in postmenopausal women (65), the progressive decline of estradiol (E2), independent of aging, is believed to have direct consequences on skeletal muscle (21, 55). The absence of this hormone can have detrimental effects on muscle strength, which are demonstrated by decreased force-generating capacity in the muscle of ovariectomized mice (22).
In parallel with a diminished muscle phenotype, menopause is related to an accumulation of abdominal adiposity, particularly in the visceral compartment (37), leading to a higher incidence of obesity in postmenopausal women (49). Increased fat (15) and lipid content (34) have been observed in skeletal muscle after menopause, an adipose burden that has the potential to negatively affect muscle quality. The changes in muscle composition that occur after menopause can be significant, as women between the ages of 65 and 80 have approximately twice the amount of noncontractile muscle tissue than women aged 23-57 (29). Significant adipocyte accumulation has also been observed in other conditions where muscle integrity is compromised (1, 20, 64). Additionally, ovariectomy has resulted in impaired recovery capabilities of atrophied muscle mass in rats (59), which may be related to fat encroachment and a subsequent disruption of muscle homeostasis and stem cell health.
The ability of skeletal muscle to regenerate following injury is highly dependent on the activation of satellite cells (30) and related gene expression (46) in the muscle tissue. Satellite cells are skeletal muscle stem cells that lie above the sarcolemma and below the basal lamina of muscle fibers (16). As the frontrunners of skeletal muscle repair mechanisms, they have the ability to activate from a quiescent state in healthy tissue (11) and proliferate and/or differentiate into myoblasts during regeneration. Satellite cell activity is affected by circulating estrogen levels. Enns and Tiidus (12) showed increased satellite cell activation and proliferation following downhill running with estrogen supplementation in ovariectomized rats. Therefore, it is plausible that the lack of estrogen experienced during menopause or following ovariectomy will hamper satellite cell activation and/or proliferation, impairing the overall stem cell pool. Because the source of intramuscular adipocytes is variable [e.g., mesenchymal progenitors (9), fibro/adipogenic progenitors (28)], it has been proposed that satellite cells also possess the ability to commit to an adipogenic lineage (8, 53, 58), and thus it is suggested that the increased adiposity resulting from ovariectomy may in part be a result of biased stem cell differentiation toward an adipogenic fate. Retaining satellite cells may be achieved by suppressing the accumulation of intramuscular fat, ultimately helping to maintain muscle homeostasis and regenerative potential.
Because it is well accepted that exercise in general, and mechanical signals in particular, are anabolic to muscle (63) and catabolic to fat (3), it represents a nondrug means to address susceptibility to both obesity and compromised muscle following menopause. Because fat encroachment into muscle may arise in part by stem cell populations committing to adipogenesis rather than myogenesis, mechanically biasing stem cell fate toward muscle and away from fat may aid in retaining muscle quality. As a potential adjunct to exercise, mechanical stimulation in the form of low-intensity vibration (LIV; <0.4 g, where g is earth's gravitational field at 9.81 m/s2) has been shown to provide a signal that is both anabolic to muscle (68) and biases stem cell differentiation away from adipogenesis (40). As proposed, LIV may be beneficial in protecting muscle homeostasis after menopause by reducing intramuscular adipogenic gene expression and maintaining satellite cell populations that are likely to be negatively affected by the lack of E2 and/or secondary complications of ovariectomy.
This study tested the hypothesis that ovariectomy will compromise skeletal muscle satellite cell populations and increase abdominal adiposity and muscular adipogenic gene expression, whereas daily bouts of LIV would protect the muscle from increased adipogenic gene expression and muscle stem cell impairment. Using an ovariectomized (OVX) C57BL/6 murine model, we identified the effects of ovariectomy on satellite cell populations and adipogenic and myogenic gene expression within the skeletal muscle and determined whether LIV could mitigate this deterioration.
MATERIALS AND METHODS
Experimental design.
All experimental procedures were reviewed and approved by Stony Brook University's Institutional Animal Care and Use Committee. Eight-week-old female C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) received an ovariectomy (n = 20) or sham surgery (n = 10). Following 2 wk of recovery and acclimation, a weight-matched, randomized MATLAB script was used to divide the ovariectomized mice into two groups, OVX (n = 10) and OVX + vibration treatment (LIV; n = 10). Animals receiving a sham surgery were grouped as age-matched controls (AC; n = 10). Experimental treatment and baseline measurements began 2 wk postsurgery at 10 wk of age; this time point is considered the start of the experimental protocol (t = 0 wk). Six weeks following time zero, all 30 mice were anesthetized with isoflurane and euthanized via cervical dislocation (8 wk postsurgery, 16 wk of age; Fig. 1). End-point in vivo measurements were taken 2 days prior to euthanasia. Mice were single-housed at 21°C and fed a standard rodent chow diet (LabDiet Prolab RMH 3000; Purina Mills, St. Louis, MO), with ad libitum access to food and water.
Fig. 1.
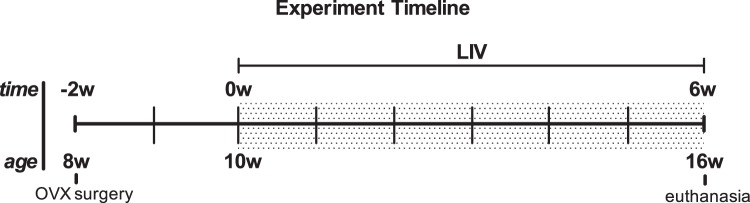
Experimental timeline of mice ovariectomized (OVX) at 8 wk of age with low-intensity vibration (LIV) treatment beginning 2 wk postsurgery, at 10 wk of age, the start of the experimental protocol (t = 0 wk). All mice were euthanized 6 wk following the start of the experiment (8 wk postsurgery) at 16 wk of age.
Mechanical stimulation protocol.
Animals receiving mechanical stimulation treatment were subject to low-magnitude, high-frequency, vertically oscillating vibrations (0.3 g at 90 Hz). LIV was administered for 15 min/day, 5 day/wk for 6 wk. Mice were placed into a partitioned box that was centered on the vibration plate. AC and OVX were sham handled and placed in the same box, but on an inactive device. The last vibration treatment was administered the day prior to euthanasia.
In vivo microcomputed tomography measurements.
Total abdominal adiposity was quantified in vivo at both baseline (t = 0 wk) and endpoint (t = 6 wk) using X-ray microcomputed tomography (μCT) (vivaCT 40; Scanco Medical, Brüttisellen, Switzerland). Animals were anesthetized with 2% isoflurane inhalation and stabilized in a custom-made, foam scanning bed. Scans of the abdomen (76 μm isotropic resolution, 45 kV intensity, 133 μA current) were evaluated from the L1–L5 vertebrae (∼20-mm region). Visceral and subcutaneous adipose tissues in this region were segregated using a well-established segmentation script (38). Parameters that included visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and total adipose tissue (TAT; subcutaneous + visceral) were reported by volume (mm3).
Serum biochemical markers.
Biochemical markers were quantified in serum (stored at −80°C until analysis) from blood collected at euthanasia. Serum leptin and adiponectin levels were evaluated with commercial ELISA kits (Mouse Leptin ELISA and Mouse Adiponectin ELISA; EMD Millipore, St. Charles, MO), and standard curves were determined according to the manufacturer's protocols.
Quantitative polymerase chain reaction of skeletal muscle gene expression.
Following euthanasia, the soleus muscle was excised and stored in RNAlater at 4°C and then moved to −20°C for long-term storage. RNA was isolated using a commercial spin kit for skeletal muscle (RNeasy Fibrous Tissue Mini Kit; Qiagen, Austin, TX). RNA purity was quantified using 1 μl of mRNA and a NanoDrop 1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). cDNA conversion was performed using a high-capacity reverse transcription kit (Applied Biosystems, Foster City, CA), and amplification was conducted using TaqMan Gene Expression Assays and TaqMan Gene Expression Master Mix (Applied Biosystems). Expression levels of genes critical to adipogenesis, including peroxisome proliferator-activated receptor (PPAR)γ, fatty acid-binding protein 4 (FABP4), PPARδ, forkhead box protein O1 (FoxO1), adiponectin (Adipoq), and those critical to myogenesis, including paired box protein 7 (Pax7), myoblast determination protein (MyoD), myogenic factor 5 (Myf5), insulin-like growth factor 1 (IGF-1), and myostatin (Mstn), were analyzed and compared with the housekeeping gene β-actin. Relative expression was compared against intact control animals using the ΔΔCT method of analysis.
Flow cytometric analysis of satellite cells.
To evaluate satellite cell status in skeletal muscle, the left gastrocnemius and quadriceps muscles were harvested immediately following euthanasia, minced, pooled for each animal, and stored in Dulbecco's modified essential medium (DMEM; Gibco, Carlsbad, CA) with 1% penicillin-streptomycin. Satellite cell isolation was adapted from a previous protocol (25), with changes as noted. Tissue fragments were digested with 2 mg/ml collagenase type II, 1.2 U/ml trypsin, and 2 mM CaCl2 in PBS. After trituration, samples were neutralized with DMEM supplemented with 1% penicillin-streptomycin and 15% horse serum (Thermo Scientific). Mononuclear cells were filtered with 40 μm nylon cell strainers (BD Biosciences, San Diego, CA). Samples were centrifuged at 2,000 rpm for 5 min and resuspended in 1× lysis buffer (Pharm Lyse; BD Biosciences) for 5 min at room temperature. Cells were again centrifuged, resuspended in DMEM supplemented with 1% penicillin-streptomycin, and counted; 1 × 106 cells were removed from each sample for staining. Samples were incubated for 45 min in the dark at room temperature with the following antibodies: FITC-conjugated CD34 (eBioscience, San Diego, CA), APC-conjugated CD45, CD31, Sca-1 (eBioscience), and PE-conjugated integrin α7 (R & D Systems, Minneapolis, MN). Satellite cells were defined as CD45−/CD31−/Sca-1−/integrin α7+, with the reserve population as CD34+, and have previously been demonstrated to yield a pure satellite cell population (25). Flow cytometric analysis was conducted using the FACSCalibur system (BD, San Jose, CA) at 200,000 events/sample. Data were analyzed with FlowJo version 7.2.5 (TreeStar, San Carlos, CA).
Statistical analyses.
Comparisons between groups were made using a one-way analysis of variance (ANOVA) and Tukey post hoc test, whereas comparisons between time points were made with a paired Student's t-test. Correlations were evaluated using a two-tailed Pearson correlation. All presented data show means ± SD. All data were considered significant when P ≤ 0.05, and analyses were conducted with SPSS 14.0 software (SPSS, Chicago, IL).
RESULTS
Ovariectomy effects on body mass, tissue mass, and food intake.
Ovariectomy-induced menopause was confirmed at euthanasia by a significant reduction in uterine mass (data not shown). As expected, ovariectomy resulted in an increase in body weight. Two weeks following surgery and immediately prior to LIV treatment, ovariectomized animals (both OVX and LIV) were 10–12% (P < 0.05) heavier than AC (Fig. 2A). Eight weeks following surgery and after 6 wk of LIV, the differences in body weight between AC and OVX remained at 12%, whereas LIV mice were not significantly different from OVX, indicating that these mechanical signals had no impact on body weight (Fig. 2A). No differences in food consumption were detected between the three groups, suggesting that the weight gain resulting from ovariectomy was driven by metabolic and/or hormonal factors. The soleus muscle and gonadal fat pad extracted and weighed at euthanasia had greater masses in both the OVX and LIV groups compared with AC (OVX soleus +19%, LIV soleus +18%, both P < 0.01; OVX gonadal fat pad +83%, LIV gonadal fat pad +58%, both P < 0.05; Fig. 2, B and C).
Fig. 2.
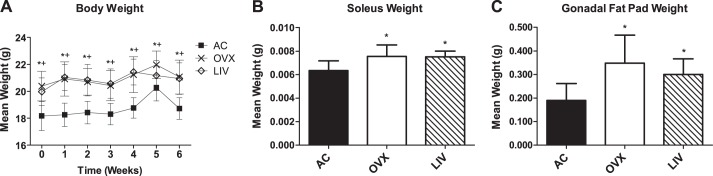
A: body weights from week 0 (2 wk postovariectomy) through week 6. At time 0, differences in weight had already occurred due to the ovariectomy surgery 2 wk prior to study baseline. *P < 0.05, OVX compared with age-matched controls (AC); +P < 0.05 LIV compared with AC. B: soleus wet weights measured at euthanasia. *P < 0.05 compared with AC. C: gonadal fat pad wet weights measured at euthanasia. *P < 0.05 compared with AC.
Ovariectomy increases serum adipokine concentrations.
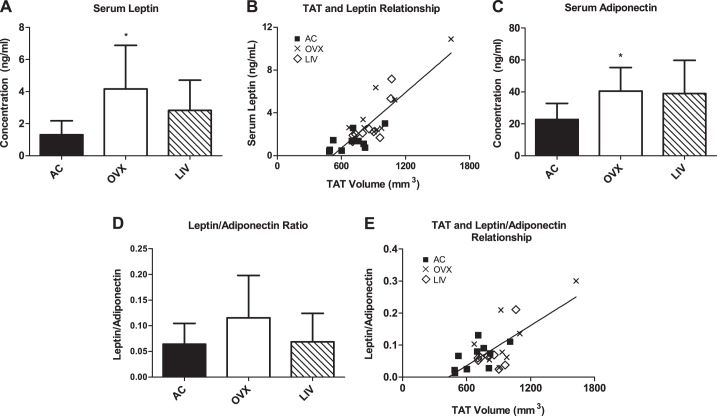
At euthanization, adipokine levels were elevated in OVX, with serum leptin up by +220% (P < 0.01) compared with AC, whereas LIV showed no significant differences from AC (+117%, P > 0.05) (Fig. 3A). Leptin levels were positively correlated to TAT volume across all groups at the time of euthanasia (P < 0.01, r = 0.848; Fig. 3B). Similarly, serum adiponectin was elevated in OVX animals compared with AC (+79%, P < 0.05), whereas the increase in LIV mice was not significantly different from AC (+72%, P > 0.05; Fig. 3C). No differences were detected between groups in the leptin/adiponectin ratio (LAR) (P > 0.05; Fig. 3D), but these values were positively correlated to TAT volume (P < 0.01, r = 0.718; Fig. 3E).
Fig. 3.
Serum adipokine levels measured at euthanasia. A: leptin levels measured with ELISA. B: leptin levels correlated to total adipose tissue (TAT) volume. Linear regression shown. C: adiponectin levels measured with ELISA. D: leptin/adiponectin ratio (LAR). E: LAR correlated to TAT volume. Linear regression shown. *P < 0.05 compared with AC.
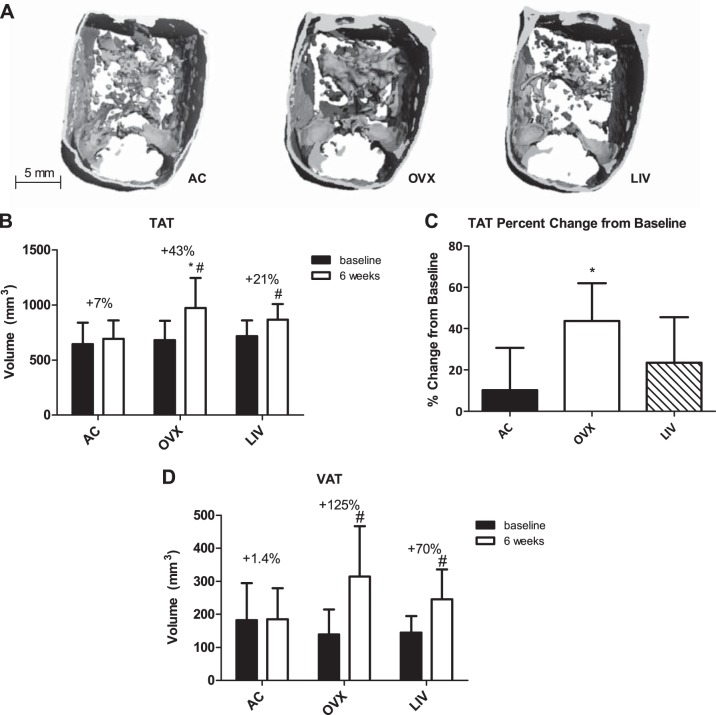
Abdominal adiposity is increased by OVX, whereas LIV trends toward mitigated adipose accretion.
At the study baseline, 2 wk postovariectomy, and immediately before LIV, adipose distributions in both OVX and LIV groups were significantly different from AC, whereas there was no difference in TAT at this point in time (Fig. 4B). VAT represented 19% of TAT in both OVX and LIV groups at baseline compared with 26% in AC (P ≤ 0.05), whereas SAT represented 81% of TAT in both OVX and LIV compared with 74% in AC (P ≤ 0.05).
Fig. 4.
A: In vivo microcomputed tomography 3-dimensional reconstructions (76-μm resolution) of transverse abdominal sections depicting end-point (after 6 wk of LIV treatment) TAT. B: TAT volume; %increases shown are the differences between the group's average TAT volume at baseline vs. the group's end-point volume. C: TAT volume %change was calculated for each individual animal from its own baseline value, all of which were used to obtain a group average. D: VAT volume. *P < 0.05 compared with AC at the corresponding time point. #P < 0.05 compared with baseline measurement.
Over the course of the study, OVX animals gained 43% in TAT (P < 0.001), whereas LIV gained 21% (P = 0.01) and AC +7% (Fig. 4B). The TAT percent change from baseline for each individual animal in OVX mice was 44% greater than AC (P < 0.05), whereas the 23% increase in mice subject to LIV was not significantly different from AC (Fig. 4C). After 6 wk, both OVX and LIV animals had greater subcutaneous fat volumes (658 ± 124 and 621 ± 58 mm3, respectively) compared with AC (507 ± 93 mm3, P < 0.01 and P < 0.05, respectively). OVX animals indicated a trend toward greater VAT volume compared with AC, with a 70% increase by the study conclusion (P = 0.06), whereas LIV animals had only a 33% increase (Fig. 4D). Compared with baseline, VAT accumulation increased in OVX animals 125% (P < 0.001) compared with 70% (P < 0.01) with LIV, whereas control animals gained 1.4% VAT over this same period of time (Fig. 4D).
Ovariectomy increases adipogenic gene activity in muscle.
PPARδ and PPARγ increased 120 and 78%, respectively, in the soleus muscle of OVX compared with AC (P ≤ 0.001; Fig. 5). In contrast, expression levels of PPARδ increased by 27% and PPARγ decreased by 15% in LIV compared with AC (P > 0.05), representing a differential expression of LIV relative to OVX of −42 and −52%, respectively (P ≤ 0.001). Compared with AC, FABP4 was upregulated in OVX by 93% (P < 0.001) and downregulated in LIV by 46% compared with OVX (P < 0.001). Similarly, FoxO1 was upregulated by 113% in OVX compared with AC (P < 0.001), whereas LIV reduced this expression by 59% compared with OVX (P < 0.001). No differences in Adipoq expression levels were detected.
Fig. 5.
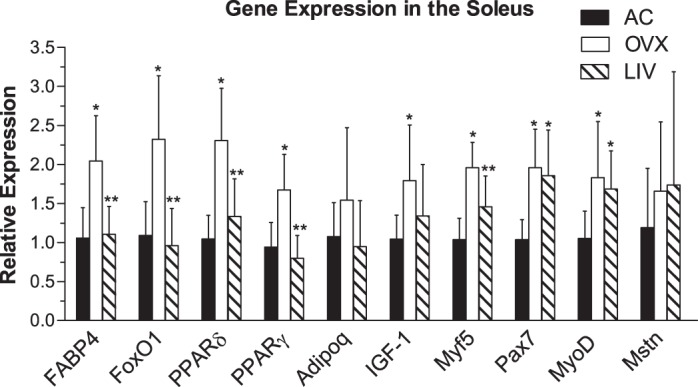
Gene expression analysis in the soleus muscle. Adipogenic [fatty acid-binding protein 4 (FABP4), forkhead box protein O1 (FoxO1), peroxisome proliferator-activated receptor (PPAR)δ, PPARγ, and adiponectin (Adipoq)] and myogenic [insulin-like growth factor 1 (IGF-1), myogenic factor 5 (Myf5), paired box protein 7 (Pax7), myoblast determination protein (MyoD), and myostatin (Mstn)] genes were measured. Analyses were conducted using real-time PCR and the ΔΔCT method of analysis. *P < 0.05 compared with AC; **P < 0.05 compared with OVX.
OVX modulates myogenic gene expression in the soleus.
Pax7 and MyoD were upregulated in both the OVX and LIV mice compared with AC, with Pax7 up 89 (P < 0.001) and 70% (P < 0.01) and MyoD elevated by 74 (P < 0.01) and 60% (P < 0.05), respectively (Fig. 5). Expression levels of Myf5 were elevated in OVX compared with AC by 89% (P < 0.001), whereas LIV was 26% lower than OVX (P < 0.05). IGF-I was upregulated in OVX by 72% (P < 0.05) compared with AC, whereas changes in LIV (+28%, P > 0.05) were not significantly different from AC. Lastly, no differences were detected in Mstn expression levels.
OVX compromises satellite cell populations.
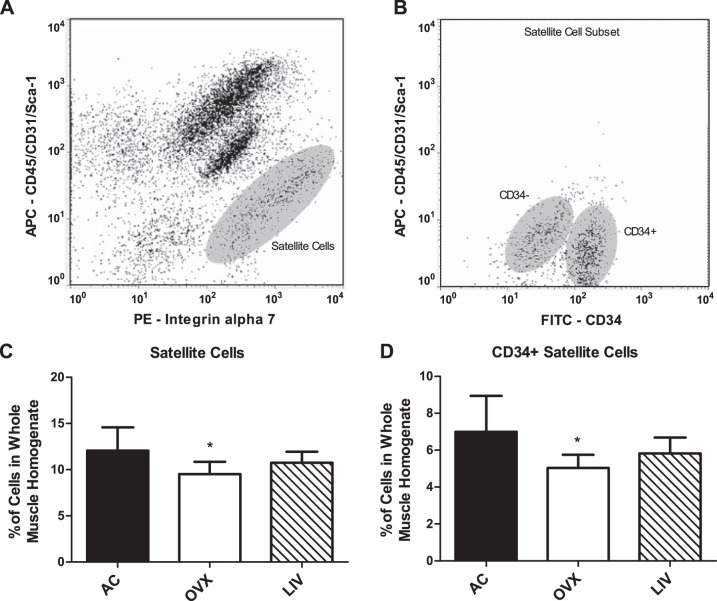
Satellite cell populations measured in the pooled gastrocnemius and quadriceps muscles (Fig. 6, A and B), positive for integrin α7 and negative for markers CD31, CD45, and Sca-1 (25), were 21% lower in OVX compared with AC (P < 0.01; Fig. 6C). In contrast, there were no significant differences between AC and LIV (−11%, P > 0.05). This relationship is consistent in the proportion of reserve satellite cells in the muscle tissue, as indicated by the positive marker CD34 (25). OVX mice had a 28% reduction (P < 0.01) in percent CD34+ satellite cells compared with AC, whereas LIV mice had proportions reduced by 17% (P > 0.05; Fig. 6D). LIV was not different from OVX in either cell population. No differences were measured in the number of CD34− cells (data not shown). Additionally, total cell number was not different between any of the three groups, indicating true changes in satellite cell populations (data not shown).
Fig. 6.
Satellite cell quantification in the gastrocnemius and quadriceps muscles quantified via flow cytometry. A: flow cytogram representing the isolated satellite cell population negative for allophycocyanin (APC) (CD45/CD31/Sca-1) and positive for phycoerythrin (PE) (integrin α7). B: flow cytogram showing a satellite cell subset depicting both FITC (CD34)-positive and -negative populations. C: %satellite cells (CD45−/CD31−/Sca-1−/integrin α7+) in the hindlimbs. No differences were measured in total cell number. D: the proportion of reserve satellite cells in the hindlimbs, positive for CD34. *P < 0.01 compared with AC.
DISCUSSION
Ovariectomy in the mouse was used to model phenotypic changes that follow menopause in the human, with a specific focus on the rapid escalation of systemic adiposity and changes in muscular composition that parallel estrogen deficiency. It is well established that ovariectomy in mice increases susceptibility to obesity (44, 51), and the data reported here indicate that the transformation in body habitus occurs within 2 wk following ovariectomy. Whereas estrogen depletion resulted in a higher average body mass of 11% compared with controls only 2 wk postsurgery, the following 6 wk resulted only in an additional 1.5% increase relative to controls. Certainly, when considering the consequences of ovariectomy on the mouse, the reality that this response happens so rapidly must be recognized. In retrospect, the introduction of the LIV signal, or any intervention, to this model beginning 2 wk postsurgery rather than immediately after surgery would appear to constitute more of a test of “reversal of” rather than “protection from” the impact of OVX.
LIV has previously been shown to suppress adipogenesis in murine models of diet-induced obesity (40, 54), and the OVX data presented here indicate trends toward this mitigation. However, when comparing gonadal fat pad weights and abdominal adiposity as quantified by μCT, the data suggest that LIV continues to suppress adiposity despite no effects on total body mass. Whereas both OVX and LIV mice had significant increases in adiposity from baseline (2 wk following OVX), TAT and VAT volume increases in LIV mice at the conclusion of 6 wk were approximately one-half of that measured in the OVX group. And certainly, the challenges of catabolizing extant adipose tissue that existed after 2 wk are different from that of suppressing the formation of fat.
The idea that adipogenesis is being mitigated by these low-level mechanical signals is further supported by differences in serum adipokine levels measured at the end of protocol. Circulating leptin levels were 220% higher in OVX relative to age-matched controls, similar to previously published trends (26), and may reflect metabolic abnormalities related to excessive adipose tissue. In some contrast, no significant differences in leptin levels were measured in LIV mice compared with controls. Circulating adiponectin concentrations showed similar trends. Expressing leptin and adiponectin as a ratio can be indicative of insulin resistance and may be a better indication of adipocyte or metabolic health than solely leptin or adiponectin (7, 13, 45, 56). However, the data reported here show no differences in LAR despite a relatively strong correlation to TAT. These data indicate that mechanical signals represent a reasonable means to control adipokine levels even in the face of systemic pressures to become imbalanced.
The systemic increase in adipose burden that followed ovariectomy, measured as increased fat depots in the subcutaneous and visceral compartments, was also realized locally in the musculature, as reflected by the elevation of adipogenic gene expression measured in the soleus. Regulators of adipogenesis, including PPARγ, PPARδ, FABP4, and FoxO1, were significantly upregulated in muscle from OVX mice, suggesting that despite a conservation of mass, the quality of the muscle was deteriorating. Increases in the PPAR genes indicate alterations in fatty acid metabolism (39), an outcome supported by the upregulation of FABP4. The measured increases in PPARγ expression, a major regulator of adipocyte differentiation, are likely linked to increased fatty acid transport and binding in the muscle tissue. Fatty acids bind with high affinity to fatty acid-binding proteins such as FABP4, which are upregulated as a result of both fatty acid transport and elevated PPARγ expression. PPARδ is also activated by fatty acids and plays a role in preadipocyte proliferation (27), which activates PPARγ and may ultimately contribute to an overall increase in adipocyte encroachment into the muscle. Transcription factor FoxO1 is downstream of PPARδ and plays a role in the mediation of oxidative metabolism and preadipocyte differentiation. Paired regulation of PPARδ and FoxO1 expression levels have been reported in the OVX model, although both genes were downregulated (50). Differences in expression trends are possibly due to specific muscle selection (soleus vs. quadriceps) and differing fiber compositions. No differences were measured in Adipoq, an antiadipogenic gene that is associated with decreased skeletal muscle triglyceride content in mice (69) and increases fatty acid oxidation in skeletal muscle cells (71).
That the marked elevations in circulating adipokine concentrations following ovariectomy were mirrored by elevated adipogenic gene expression in the muscle tissue implicates the depletion of ovarian hormones in the decline of multiple tissue systems. Whether the noted increase in intramuscular adiposity is a result of adipocyte encroachment or adipogenic differentiation of preexisting stem cells within the muscle tissue requires further investigation. Furthermore, although these genes are known to participate in adipogenesis, all are involved in other skeletal muscle metabolic processes such as muscular mitochondrial biogenesis and glucose metabolism (43); changes in expression may have additional influences on the muscle tissue.
High levels of intramuscular adiposity are associated with both aging (6) and obesity (19); however, an “athlete's paradox” that demonstrates similarly elevated skeletal muscle adiposity in well-trained endurance athletes exists (18). Fat is a key regulator of many physiological processes (2, 4, 60) and increased intramuscular fat may not translate to decreased function. In this particular case, a buildup of fat in the muscle due to ovarian hormone depletion is likely to put muscle function at risk (62), including a reduction in muscular strength (17), which intrinsically plays a role in functional performance and contractile properties (47). In some contrast to the adipogenic bias measured in the muscle of OVX, there was a significant suppression (>40%) of those parameters in mice subjected to LIV, suggesting that these mechanical signals provided some form of a protective mechanism against adipogenesis that may ultimately lead to a reduction in the number of intramuscular adipocytes and the protection of muscle quality. Because exercise is known to reduce adiposity and is often prescribed to postmenopausal women to help maintain muscular integrity, it is possible that the mechanical signals derived from LIV serve as a form of exercise surrogate to provide similar salutary benefit. Considering that LIV has been shown in humans to protect postural stability (42) and reduce falls in the elderly (36), it is possible that these clinical outcomes are achieved by retaining both quality and neuromuscular control of the muscle (41).
Increases in intramuscular adiposity will invariably disrupt the muscle microniche, ultimately encroaching upon satellite cell populations and thus impacting the ability for repair and regeneration of muscle tissue. Indeed, in parallel with the increased adipogenic activity of muscle in OVX, there was a significant decrease in the proportions of satellite cells compared with total cells in the muscle. It has been noted that CD34 expression on satellite cells is a reversible state of activation that influences stem cell quiescence. CD34+ satellite cells have been identified as a reserve population of satellite cells that divide early on in response to injury. These cells will eventually become CD34− for later proliferation involved in the reparative process (25). The proportion of CD34+ cells in OVX was reduced compared with controls, which may suggest that the immediate ability of the muscle to respond to injury or demands for myogenesis is limited. The simultaneous increase in local adipogenic gene expression may be what is driving the reduction of satellite cells and/or CD34+ satellite cells through biased stem cell differentiation or disrupted muscle homeostasis.
Although LIV did not have higher satellite cell proportions compared with OVX, no differences in overall satellite cell proportion or CD34+ satellite cells were observed in LIV compared with age-matched controls, suggesting that these mechanical signals served to protect these progenitors. Preserving the CD34+ reserve population of satellite cells may be critical in maintaining muscle quality and quantity during a progressive loss of ovarian hormone. Mechanical signals delivered in the form of exercise or, when that is not possible, perhaps as LIV may play a critical role in the regeneration, rehabilitation, and restoration of function of muscle tissue.
Myogenic gene expression influences the proliferation and differentiation of satellite cells. Proliferating satellite cells simultaneously express Pax7 and MyoD (23), both of which were upregulated in OVX. Whereas satellite cell proliferation and differentiation genetic markers were increased, the number of satellite cells in the OVX group was lower than that of controls. Although the actual proportion of satellite cells in the muscle is compromised, it appears that OVX is responding to injury by upregulating myogenic gene expression to repair overall muscle homeostasis. MyoD, a gene also expressed by myocytes, was also upregulated, possibly because of the initiation of proliferating muscle cells. Myf5 promotes satellite cell renewal and myoblast differentiation and was again upregulated in OVX animals, perhaps as another effort to repair and regenerate damaged muscle. IGF-I was also upregulated in the OVX group. Overexpression of IGF-I stimulates muscle hypertrophy (5) through satellite cell activation and the upregulation of protein synthesis (24) during active postnatal muscle development or adult regeneration (57). Both OVX and LIV groups had heavier soleus weights at euthanasia, consistent with heavier muscle weights found in other OVX murine models (10, 14, 61). There were no changes in the expression of Mstn, which is responsible for preventing muscle growth (32).
LIV mice also had elevated levels of Pax7 and MyoD, an indication that despite the exercise surrogate, the muscle tissue was still signaling the process of repair. Although mechanical stimulation is generally found to increase the expression of myogenic factors (67, 70), there was a significant decrease in Myf5 expression in LIV relative to OVX. Myf5 is a one of the earliest markers of active satellite cell commitment to the myogenic lineage. The noted changes of Myf5 in the LIV group may be a result of the transition of stem cells into a myoblast differentiation phase where Myf5 is consequently downregulated (33). Overall, LIV resulted in less of an impact on myogenic markers than those related to adipogenesis, which suggests that the primary influence of LIV is associated with suppressing adiposity rather than influencing myogenesis.
Our gene expression profiles consisted of chiefly anabolic genes, which we saw respond toward an adipogenic profile. Certainly, a concurrent assessment of catabolic genes would give a more complete analysis of the effects of LIV on OVX skeletal muscle tissue. Although two catabolic genes in this study were assessed (adiponectin and myostatin), no differences in their expression profile were detected between any groups. Clearly, the systemic insult of OVX is significant, and the mechanisms involved in the consequences, and the mechanical protection, are certain to be complex. Ultimately, a more comprehensive transcriptional profile must be performed before a more complete picture is possible.
The reported changes in gene expression due to OVX and vibration treatment cannot be attributed solely to one cell population and rather represent variations across the muscular environment. Changes in the stem cell niche may be due to a number of cell types involved in skeletal muscle regeneration and maintenance, e.g., fibroblasts, endothelial cells, fibro/adipogenic progenitors, telocytes, motor neurons, and mesenchymal and hematopoietic stem cells and precursors, and are likely to influence satellite cell activity. Additionally, although all muscles used for this study were extracted from the hindlimbs, muscle groups differed among assays due to assay cell number and tissue mass requirements. Variations in gene expression and satellite cell numbers may occur, depending on anatomic location. Further investigation into the mechanism behind OVX's impact and LIV's salutary influence on adipogenesis and mygoenesis, in addition to local variations in the skeletal muscle, is necessary. It is also important to note that future studies must include the assessment of muscular composition on the phenotypic level (lipid content, adipocyte infiltration, etc.) and functional testing to demonstrate changes in muscular strength and quality.
The work presented here provides evidence for the rapid and marked consequences of ovariectomy on a number of physiological systems. In particular, this work shows that the removal of ovarian hormones increases adipogenic gene expression in muscle, reducing its quality, and simultaneously suppresses the number of satellite cells available for muscle regeneration and repair. These data also indicate that extremely low-level mechanical signals, introduced for brief periods each day using LIV, reduce levels of total adiposity and suppress muscular adipogenic gene expression while protecting the number of satellite cells.
Exercise, representing the primal mechanical stimulus, is a critical modality to slow the range of complications that arise from menopause, including the deterioration of muscle quality and strength. Ironically, however, the menopause and its sequelae, such as the rapid decline of the musculoskeletal system, can erode the safety, ease, and compliance required of exercise. Although there is no true substitute for exercise, the data presented here suggest that LIV may have the potential to serve as a surrogate to exercise for the injured or impaired. Ultimately, alternatives to exercise, either chemical or physical, that help retain the progenitor population and its microniche sooner rather than later may help preserve the integrity of a range of physiological systems.
GRANTS
This work was supported by the National Institutes of Health through National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-43498 and National Institute of Biomedical Imaging and Bioengineering grant EB-14351.
DISCLOSURES
C. T. Rubin is a founder of Marodyne Medical. All other authors declare no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
D.M.F., D.K., M.E.C., and C.T.R. conception and design of research; D.M.F. and D.K. performed experiments; D.M.F., D.K., B.J.A., M.E.C., and C.T.R. analyzed data; D.M.F., D.K., B.J.A., M.E.C., and C.T.R. interpreted results of experiments; D.M.F. prepared figures; D.M.F. drafted manuscript; D.M.F. and C.T.R. edited and revised manuscript; C.T.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alyssa Tuthill, Andrea Trinward, Danielle Green, Gabriel Pagnotti, Gunes Uzer, and James Lennon for technical assistance related to this project.
REFERENCES
- 1.Banker BQ, Engel AG. Basic reactions of muscle. Myology 1: 832–888, 1994. [Google Scholar]
- 2.Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 328: 238–244, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P, Gautier JF. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Eur J Endocrinol 149: 421–424, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Chang CY, Ke DS, Chen JY. Essential fatty acids and human brain. Acta Neurol Taiwan 18: 231–241, 2009. [PubMed] [Google Scholar]
- 5.Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270: 12109–12116, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, Urban R, Wolfe RR. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab 89: 3864–3871, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Cristina Landi Masquio D, De Piano Ganen A, Munhoz da Silveira Campos R, De Lima Sanches P, Campos Corgosinho F, Caranti D, Tock L, De Mello MT, Tufik S, Dâmaso AR. Cut-Off Values of Waist Circumference to Predict Metabolic Syndrome in Obese Adolescents. Nutr Hosp 31: 1540–1550, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Csete M, Walikonis J, Slawny N, Wei YW, Korsnes S, Doyle JC, Wold B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J Cell Physiol 189: 189–196, 2001. [DOI] [PubMed] [Google Scholar]
- 9.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119: 2204–2213, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Dagdeviren S, Kandilci HB, Uysal B, Zeybek ND, Korkusuz P, Gumusel B, Korkusuz F. Tumor necrosis factor-alpha antagonist administration recovers skeletal muscle dysfunction in ovariectomized rats. J Orthop Res 29: 275–280, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol 15: 666–673, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol 104: 347–353, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Finucane FM, Luan J, Wareham NJ, Sharp SJ, O'Rahilly S, Balkau B, Flyvbjerg A, Walker M, Højlund K, Nolan JJ; European Group for the Study of Insulin Resistance: Relationship between Insulin Sensitivity and Cardiovascular Disease Risk Study Group, Savage DB. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia 52: 2345–2349, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher JS, Hasser EM, Brown M. Effects of ovariectomy and hindlimb unloading on skeletal muscle. J Appl Physiol (1985) 85: 1316–1321, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Forsberg AM, Nilsson E, Werneman J, Bergstrom J, Hultman E. Muscle composition in relation to age and sex. Clin Sci (Lond) 81: 249–256, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Fukada S, Ma Y, Ohtani T, Watanabe Y, Murakami S, Yamaguchi M. Isolation, characterization, and molecular regulation of muscle stem cells. Front Physiol 4: 317, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 90: 2157–2165, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 49: 467–472, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Wolf D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr Diabetes 5: 219–226, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Greeves JP, Cable NT, Reilly T, Kingsland C. Changes in muscle strength in women following the menopause: a longitudinal assessment of the efficacy of hormone replacement therapy. Clin Sci (Lond) 97: 79–84, 1999. [PubMed] [Google Scholar]
- 22.Greising SM, Baltgalvis KA, Kosir AM, Moran AL, Warren GL, Lowe DA. Estradiol's beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol (1985) 110: 109–115, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halevy O, Piestun Y, Allouh MZ, Rosser BW, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn 231: 489–502, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Hill M, Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549: 409–418, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ieronimakis N, Balasundaram G, Rainey S, Srirangam K, Yablonka-Reuveni Z, Reyes M. Absence of CD34 on murine skeletal muscle satellite cells marks a reversible state of activation during acute injury. PLoS One 5: e10920, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwasa T, Matsuzaki T, Kinouchi R, Gereltsetseg G, Murakami M, Nakazawa H, Fujisawa S, Yamamoto S, Kuwahara A, Yasui T, Irahara M. Effect of immune stress on body weight regulation is altered by ovariectomy in female rats. J Reprod Immunol 91: 41–47, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Jehl-Pietri C, Bastie C, Gillot I, Luquet S, Grimaldi PA. Peroxisome-proliferator-activated receptor delta mediates the effects of long-chain fatty acids on post-confluent cell proliferation. Biochem J 350: 93–98, 2000. [PMC free article] [PubMed] [Google Scholar]
- 28.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153–163, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jubrias SA, Odderson IR, Esselman PC, Conley KE. Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflugers Archiv 434: 246–253, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Kang JS, Krauss RS. Muscle stem cells in developmental and regenerative myogenesis. Curr Opin Clin Nutr Metab Care 13: 243–248, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 4: 368–381, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Kirk S, Oldham J, Kambadur R, Sharma M, Dobbie P, Bass J. Myostatin regulation during skeletal muscle regeneration. J Cell Physiol 184: 356–363, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ, Fernandez A. The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J Cell Biol 142: 1447–1459, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leite RD, Prestes J, Bernardes CF, Shiguemoto GE, Pereira GB, Duarte JO, Domingos MM, Baldissera V, de Andrade Perez SE. Effects of ovariectomy and resistance training on lipid content in skeletal muscle, liver, and heart; fat depots; and lipid profile. Appl Physiol Nutr Metab 34: 1079–1086, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Lerner UH. Bone remodeling in post-menopausal osteoporosis. J Dental Res 85: 584–595, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Leung KS, Li CY, Tse YK, Choy TK, Leung PC, Hung VW, Chan SY, Leung AH, Cheung WH. Effects of 18-month low-magnitude high-frequency vibration on fall rate and fracture risks in 710 community elderly—a cluster-randomized controlled trial. Osteoporos Int 25: 1785–1795, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 32: 949–958, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lublinsky S, Luu YK, Rubin CT, Judex S. Automated separation of visceral and subcutaneous adiposity in in vivo microcomputed tomographies of mice. J Digit Imaging 22: 222–231, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luquet S, Gaudel C, Holst D, Lopez-Soriano J, Jehl-Pietri C, Fredenrich A, Grimaldi PA. Roles of PPAR delta in lipid absorption and metabolism: a new target for the treatment of type 2 diabetes. Biochim Biophys Acta 1740: 313–317, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res 24: 50–61, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mettlach G, Polo-Parada L, Peca L, Rubin CT, Plattner F, Bibb JA. Enhancement of neuromuscular dynamics and strength behavior using extremely low magnitude mechanical signals in mice. J Biomech 47: 162–167, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muir J, Judex S, Qin YX, Rubin C. Postural instability caused by extended bed rest is alleviated by brief daily exposure to low magnitude mechanical signals. Gait Posture 33: 429–435, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res 53: 124–144, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Nunez NP, Carpenter CL, Perkins SN, Berrigan D, Jaque SV, Ingles SA, Bernstein L, Forman MR, Barrett JC, Hursting SD. Extreme obesity reduces bone mineral density: complementary evidence from mice and women. Obesity (Silver Spring) 15: 1980–1987, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Oda N, Imamura S, Fujita T, Uchida Y, Inagaki K, Kakizawa H, Hayakawa N, Suzuki A, Takeda J, Horikawa Y, Itoh M. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metabolism 57: 268–273, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Partridge TA. Muscle satellite cell structure, proliferation and fusion. In: eLS. Chichester, UK: John Wiley & Sons, 2012. [The software is available at http://onlinelibrary.wiley.com/doi/10.1002/9780470015902.a0022530/pdf] [Google Scholar]
- 47.Peterson MD, Liu D, Gordish-Dressman H, Hubal MJ, Pistilli E, Angelopoulos TJ, Clarkson PM, Moyna NM, Pescatello LS, Seip RL, Visich PS, Zoeller RF, Thompson PD, Devaney JM, Hoffman EP, Gordon PM. Adiposity attenuates muscle quality and the adaptive response to resistance exercise in non-obese, healthy adults. Int J Obes (Lond) 35: 1095–1103, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 84: 95–98, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Rappelli A. Hypertension and obesity after the menopause. J Hypertens Suppl 20: S26–S28, 2002. [PubMed] [Google Scholar]
- 50.Rogers NH, Perfield JW 2nd, Strissel KJ, Obin MS, Greenberg AS. Loss of ovarian function in mice results in abrogated skeletal muscle PPARdelta and FoxO1-mediated gene expression. Biochem Biophys Res Commun 392: 1–3, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogers NH, Perfield JW 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150: 2161–2168, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rolland YM, Perry HM 3rd, Patrick P, Banks WA, Morley JE. Loss of appendicular muscle mass and loss of muscle strength in young postmenopausal women. J Gerontol A Biol Sci Med Sci 62: 330–335, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Rossi CA, Pozzobon M, Ditadi A, Archacka K, Gastaldello A, Sanna M, Franzin C, Malerba A, Milan G, Cananzi M, Schiaffino S, Campanella M, Vettor R, De Coppi P. Clonal characterization of rat muscle satellite cells: proliferation, metabolism and differentiation define an intrinsic heterogeneity. PLoS One 5: e8523, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci USA 104: 17879–17884, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samson MM, Meeuwsen IB, Crowe A, Dessens JA, Duursma SA, Verhaar HJ. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing 29: 235–242, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Satoh N, Naruse M, Usui T, Tagami T, Suganami T, Yamada K, Kuzuya H, Shimatsu A, Ogawa Y. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care 27: 2488–2490, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Shavlakadze T, Chai J, Maley K, Cozens G, Grounds G, Winn N, Rosenthal N, Grounds MD. A growth stimulus is needed for IGF-1 to induce skeletal muscle hypertrophy in vivo. J Cell Sci 123: 960–971, 2010. [DOI] [PubMed] [Google Scholar]
- 58.Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci 117: 5393–5404, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol (1985) 100: 286–293, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 60: 329–339, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Tsai WJ, McCormick KM, Brazeau DA, Brazeau GA. Estrogen effects on skeletal muscle insulin-like growth factor 1 and myostatin in ovariectomized rats. Exp Biol Med (Maywood) 232: 1314–1325, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Tuttle LJ, Sinacore DR, Mueller MJ. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J Aging Res 2012: 172957, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring) 19: 312–318, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 60: 324–333, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Walsh MC, Hunter GR, Livingstone MB. Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos Int 17: 61–67, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Wickham C, Cooper C, Margetts BM, Barker DJ. Muscle strength, activity, housing and the risk of falls in elderly people. Age Ageing 18: 47–51, 1989. [DOI] [PubMed] [Google Scholar]
- 67.Wilborn CD, Taylor LW, Greenwood M, Kreider RB, Willoughby DS. Effects of different intensities of resistance exercise on regulators of myogenesis. J Strength Cond Res 23: 2179–2187, 2009. [DOI] [PubMed] [Google Scholar]
- 68.Xie LQ, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol 104: 1056–1062, 2008. [DOI] [PubMed] [Google Scholar]
- 69.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 98: 1745–1752, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 55: 2562–2570, 2006. [DOI] [PubMed] [Google Scholar]