Abstract
Estrogen facilitates higher cognitive functions by exerting effects on brain regions such as the prefrontal cortex and hippocampus. Estrogen induces spinogenesis and synaptogenesis in these two brain regions and also initiates a complex set of signal transduction pathways via estrogen receptors (ERs). Along with the classical genomic effects mediated by activation of ER α and ER β, there are membrane-bound ER α, ER β, and G protein-coupled estrogen receptor 1 (GPER1) that can mediate rapid nongenomic effects. All key ERs present throughout the body are also present in synapses of the hippocampus and prefrontal cortex. This review summarizes estrogen actions in the brain from the standpoint of their effects on synapse structure and function, noting also the synergistic role of progesterone. We first begin with a review of ER subtypes in the brain and how their abundance and distributions are altered with aging and estrogen loss (e.g., ovariectomy or menopause) in the rodent, monkey, and human brain. As there is much evidence that estrogen loss induced by menopause can exacerbate the effects of aging on cognitive functions, we then review the clinical trials of hormone replacement therapies and their effectiveness on cognitive symptoms experienced by women. Finally, we summarize studies carried out in nonhuman primate models of age- and menopause-related cognitive decline that are highly relevant for developing effective interventions for menopausal women. Together, we highlight a new understanding of how estrogen affects higher cognitive functions and synaptic health that go well beyond its effects on reproduction.
I. INTRODUCTION
Estrogen receptors were first identified by radioautography in the rodent hypothalamus after the introduction of tritium-labeled steroid hormones and the discovery of the nuclear receptor mechanism of steroid hormone action (reviewed in Refs. 90, 169, 252). These receptors were tied to the regulation of reproductive behavior and neuroendocrine function. Nuclear estrogen receptors were only sparsely present outside of the hypothalamus, yet there were many actions of estrogens on brain systems and behaviors unrelated to reproduction and neuroendocrine regulation (reviewed in Ref. 133). Resolution of this inconsistency came via the identification of rapid nonnuclear actions of estrogens and identification of membrane-associated estrogen receptors, first in nonneural cells and subsequently in the brain (reviewed in Ref. 101). The hippocampal formation was the first brain region in which these membrane-associated, nonnuclear forms of the classical estrogen receptor were identified along with signaling pathways (reviewed in Ref. 134). These findings were prompted by the discovery that ovarian hormones modulate and mediate the density of spine synapses in the hippocampus of rodents and later of rhesus monkeys. Subsequent investigations revealed the presence of estrogen receptors in many, if not all, brain regions with subcellular localizations in synaptic terminals and dendritic spines, dendritic shafts, axons, mitochondria, and glial cell processes. Cell nuclear labeling in cortical areas is often in inhibitory interneurons. Further investigations of androgen and progestin receptors have revealed both nuclear and nonnuclear forms and locations of classical receptors (reviewed in Ref. 134).
The discovery that ovarian hormones regulate the turnover of a subset of synapses in the hippocampus, along with regulating signaling pathways and neurotransmitter release and actions, has triggered much further investigation showing, among other findings, that the hormonal regulation of synapse turnover is not limited to the hippocampus (reviewed in Ref. 133). Furthermore, the presence of a multiplicity of signaling mechanisms of estrogens at the cellular level has important implications for basic and applied neuroscience (reviewed in Ref. 134). This includes a new understanding of how peripheral hormones affect higher cognitive functions and other neural processes beyond reproduction, including mood, motor coordination, pain sensitivity, cognition, and neuroprotection from stroke as well as Parkinson's and Alzheimer's diseases (reviewed in Refs. 33, 133). This review considers estrogen actions in the brain from the standpoint of their effects on synapse structure and function across age and sex. We begin with a review of estrogen receptor subtypes in the brain and what is known about their changes with aging in rodent, primate, and human brain. These sections are followed by discussion on the menopausal transition and changes in structure and function of the aging brain.
II. NUCLEAR and EXTRANUCLEAR STEROID RECEPTORS IN THE BRAIN
A. Discovery of Estrogen Receptors Outside of the Hypothalamus in Nuclear and Extranuclear Sites
Here, we review briefly the discovery and current knowledge that classical estrogen and other steroid hormone receptors exist in both nuclear and nonnuclear sites in the brain as well as in other cell types throughout the body. Early understanding of the important roles of estrogen receptors in brain regions outside the hypothalamus was stimulated by the discovery of estrogen-induced spine synapse formation in the hippocampus (42, 58, 235) (Figure 1). Prior to that time, a number of studies had identified nuclear estrogen receptors by light microscopy in the hippocampus and other brain regions but at much lower density than in the hypothalamus (32, 121). The demonstration of estradiol effects on hippocampal spine density was followed by light and electron microscopy studies showing the presence of steroid hormone receptors in the synapses throughout the brain.
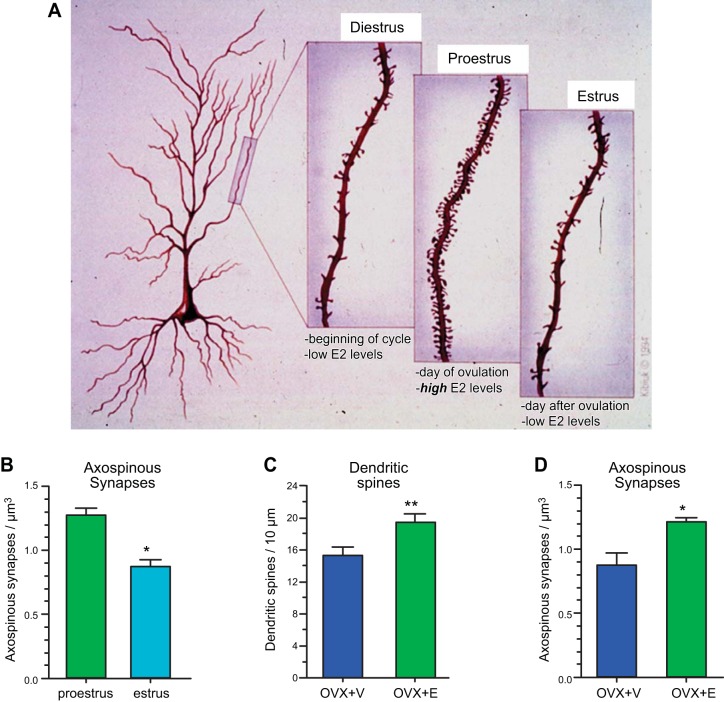
FIGURE 1.
Estradiol regulates spine synapses on hippocampal pyramidal neurons, which are sites of excitatory neurotransmission important for learning and memory. A: in the diestrus phase, which is the beginning of the estrus cycle when estradiol levels are low, spine densities are also low. During proestrus when ovulation occurs, estrogen levels peak and spine densities increase in parallel. In the estrus phase, the day after proestrus, the system begins to reset itself for the next cycle and spine densities return to baseline. [Image adapted from McEwen and Schmeck (134a) with permission.] B: axospinous synapse density is higher in the proestrus phase compared with the estrus phase. C and D: in ovariectomized (OVX) rats, estradiol (E) treatment increases dendritic spine density (C) as well as axospinous synapse density (D). V, vehicle-treated. Histograms (B–D) are plotted from data originally presented in Gould et al. (58) and Woolley and McEwen (236).
Since the discovery of estrogen receptors (ERs) (91), and the subsequent cloning of ER α (59), the discovery of new steroid hormone receptors has continued at a rapid pace. This timeline suggests that new steroid receptors remain to be discovered along with new steroid analogs, both endogenous steroids and exogenous steroids including environmental and botanical derived agents. During this same time, the understanding of the roles of steroid receptors has expanded from its nuclear or genomic actions to a number of nonnuclear, nongenomic functions. Estrogen receptors are members of the nuclear receptor superfamily and DNA binding transcription factors. Despite extensive homology, ER α and ER β diverge in their expression and actions (see review in Ref. 29). ER α and β are differentially expressed throughout the brain (192) and are also differentially regulated by estradiol (114, 163). ER α and β are able to heterodimerize (158), suggesting that different responses are generated in the presence of both receptors (63, 64). Shifts in the ratio of ER α to ER β regulate estrogen-mediated gene transcription and memory during aging (41, 66). That many of these interactions occur outside the nucleus and connect ERs to other cellular systems, particularly second messenger systems (201), ultimately contributes to the multifaceted actions of estrogen and other steroid receptors.
B. ER α
ER α was the first steroid receptor investigated for nonnuclear actions. In regions that mediate learning and memory, mainly the prefrontal cortex (PFC) and hippocampus, nuclear estrogen receptors are sparse; however, extranuclear ER α is found throughout the cell, in both cytoplasmic and membrane locations including within dendritic spines and at the synapse (Figure 2).
FIGURE 2.
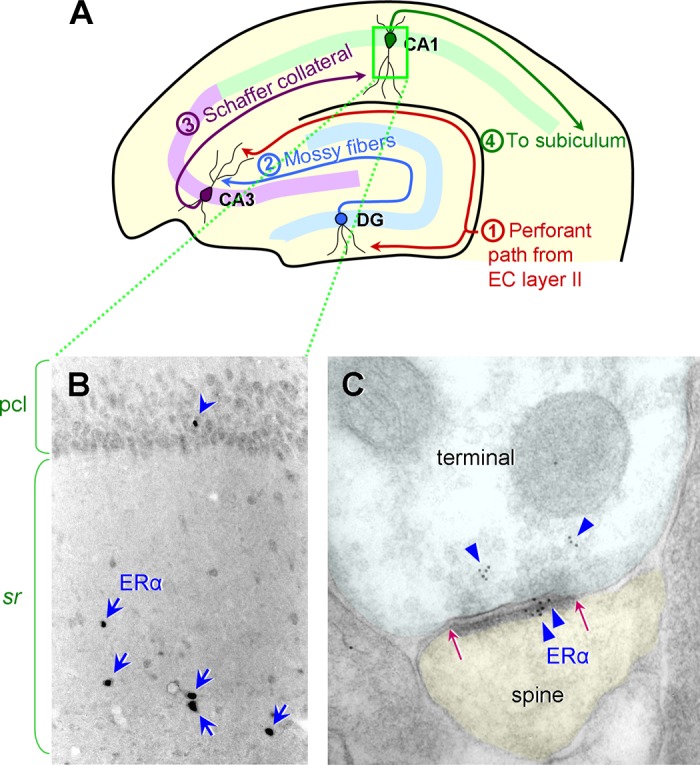
ER α expression in the rat and monkey hippocampal CA1. A: a schematic diagram illustrating the hippocampal circuit and the major inputs to and outputs from the dentate gyrus (DG), CA3, and CA1. The perforant path from the entorhinal cortex (EC) layer II projects to the DG middle/outer molecular layers and the CA3 stratum lacunosum/moleculare. The DG granule cells project to the CA3 stratum lucidum via the mossy fiber tract. The CA3 pyramidal neurons project to the CA1 stratum radiatum and oriens via the Schaffer collaterals. The CA1 pyramidal neurons project to the subiculum. [Diagram modified from Hara and Morrison (68).] B: in the rat CA1, a few interneurons with cell nuclear ER α are found primarily in the stratum radiatum (sr), indicated by blue arrows. Nuclear ER α is less frequently found in the pyramidal cell layer (pcl; blue arrowhead). [Adapted from Milner et al. (140).] C: similar to rodents, rhesus monkeys express abundant ER α in the CA1. ER α immunogold particles (blue triangles) are present in both the presynaptic terminal and the postsynaptic spine. Postsynaptic ER α labeling is concentrated within the postsynaptic density (PSD), the extent of which is indicated by red arrows. [Electron micrograph adapted from Wang et al. (223).]
1. Primates young versus aged
ER α mRNA and protein are widespread in the young adult human and monkey telencephalon.
In human adults, ER α mRNA is detected at multiple sites in the human telencephalon including the PFC and the hippocampal formation, especially the dentate gyrus, by microarray punch studies and in situ hybridization (161). It is yet to be determined whether ER α mRNA levels or distributions are altered with aging or are different between males and females in humans. An immunocytochemical study performed on human fetal (n = 38) and adult (n = 8) brains has revealed that protein expression of ER α is first detected in proliferating zones and the cortical plate at 9 gestational weeks, much before ER β is expressed (57). In the human hippocampus, ER α is first detected at 15 gestational weeks in the nuclei of pyramidal cells of the Ammon's horn. During prenatal life, ER α protein is found exclusively in nuclei of cortical and hippocampal cells, while in adulthood, ER α expression is maintained but appears in both the cytoplasm and nuclei of pyramidal neurons (57). An equal number of brains from male and female adults (n = 4 for each sex; age range, 17-52 yr old) were included in this study, and no significant sex differences were observed in the cell number, immunolabeling intensity, or distributions of ER α in the cortex and hippocampus (57). As the samples were obtained from young to middle-aged adults, it is unknown whether ER α protein expression is altered in elderly people.
The distribution patterns of ER α mRNA in macaque monkeys is similar to what is observed in humans (162). ER α mRNA in monkeys is detected in many areas that lack prominent nuclear labeling (164, 177), and this difference is reconciled by the understanding that not only does ER α shuttle between the cytoplasm and nucleus, it also resides and acts at extranuclear locations (16). Nuclear ER α immunoreactivity is sparse in the hippocampus and cortex, although it is located both pre- and postsynaptically in the PFC of young and aged monkeys (143) (Figure 2). A recent study demonstrated that the hypothalamus in aged rhesus monkeys retained youthful expression levels of ER α, GPER1, and progestin receptor (PR) protein and neuronal levels of these receptors were responsive to estradiol treatment in both young and aged monkeys (151). In ovariectomized female monkeys, estradiol treatment along with the presence of postsynaptic ER α improved cognitive performance (222), supporting a role of ER α in tasks mediated by the PFC. Nonneuronal expression of ER α may also contribute to estrogen actions, including injury-sensitive expression of ER α in glial cells (15).
2. Rodents young versus aged
Expression of ER α mRNA is widespread throughout the rodent telencephalon (192) (Allen Brain Atlas). Within the hippocampus, adult rats and mice show similar patterns of ER α protein expression, although the levels of nuclear and extranuclear receptors vary (140, 142). Nuclear ER α is concentrated in the hilus of the dentate gyrus and CA1 stratum radiatum in both males and females (229). In dorsal hippocampus, ER α-labeled nuclei are found predominantly in interneurons (150), although in ventral hippocampus, nuclear ER α is also found in pyramidal cells (75).
Extranuclear ER α is found in most cellular compartments in young adult female and male rat hippocampus and neocortex (140, 147). ER α is found in both neurons and glial cells in the hippocampus (140, 142, 195). Pyramidal neurons in the hippocampal CA1 region have spine synapses that contain ER α (2, 147); these neurons also receive inputs from cholinergic terminals that contain ER α (215).
ER α expression in the hippocampus varies across the estrous cycle and is mostly conserved between young adult female rats and mice. In young adult mice, extranuclear ER α levels in the hippocampus peak when circulating estrogen levels are lowest either during diestrus or after ovariectomy (140, 142). However, ER α expression in rat hippocampal dendritic spines is significantly higher in proestrus females with high estrogen levels compared with males and diestrus females (182). Estradiol has no effect on the frequency of ER α-containing synapses in young or aged females, but this frequency decreases with age (2) (Figure 3). In addition, young ovariectomized rats receiving estrogen replacement have less ER α in nonsynaptic compartments within presynaptic terminals and postsynaptic spines compared with vehicle-treated rats (Figure 3). In male rats, the density of ER α-expressing hypothalamic cells is retained in middle-aged compared with young rats, but the density decreases in response to circulating testosterone in both young and middle-aged rats (240).
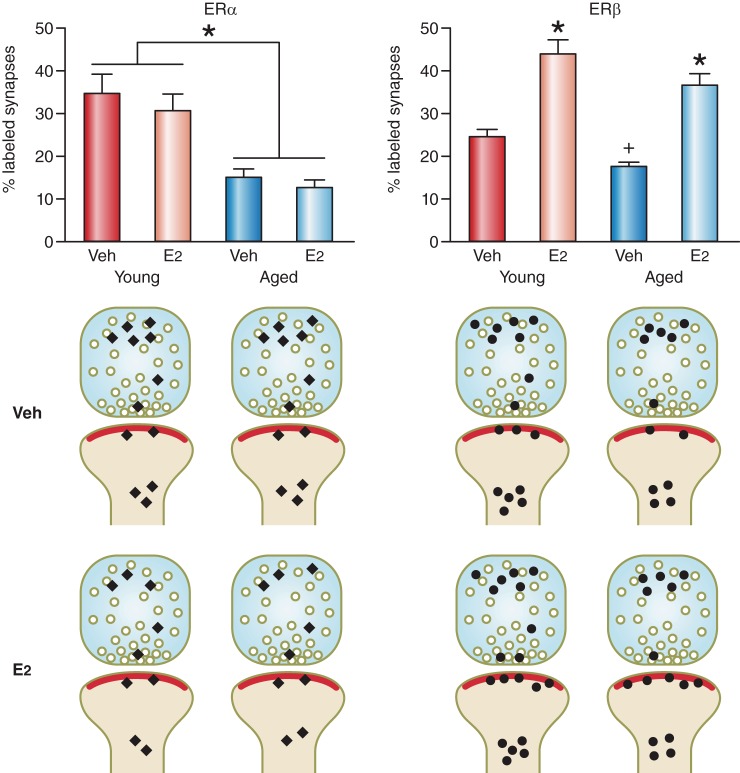
FIGURE 3.
Age and estradiol interact to differentially regulate the number of ER-labeled synapses and ER α and ER β within the synapses in the hippocampus of ovariectomized female rats receiving estradiol for 2 days. Top left: the percentage of ER α-labeled synapses decreases with age (*P < 0.0001); however, there is no effect of estradiol (E2) treatment in either group on the number of synapses containing ER α (both P values > 0.31). Top right: the percentage of ER β-labeled synapses decreases with age (+P < 0.02). Estradiol treatment increases the percentage of ER β-labeled synapses in both age groups (*P < 0.0001). Bottom left: in young and aged females, estradiol affects the distribution of ER α labeling in the pre- and postsynaptic compartments most distal from the synaptic cleft. Bottom right: estradiol but not age affects ER β distribution within the synapse. In both groups, estradiol increases ER β within the postsynaptic density. Histograms and diagrams are plotted and drawn, respectively, from data originally presented in Adams et al. (2) and Waters et al. (227).
In mice, ER α protein is also expressed in the cortex of young adults but decreases with age (22). This is consistent with the high mRNA levels seen early in development that decline with age (see review in Ref. 232).
C. ER β
ER β mRNA is transcribed from a different gene than ER α (217). First cloned in 1996, ER β has ligand binding properties similar to ER α (109, 110); however, ER β may have both ligand-dependent and -independent actions (216). Splice variants that alter the DNA-binding domain (27, 144) and NH2 terminus (12) are also detected.
1. Primates young versus aged
ER β mRNA is expressed in human hippocampus and neocortex, but distribution patterns are distinct from those of ER α (76, 161). For example, ER α mRNA levels are highest in the amygdala and hypothalamus, while ER β mRNA is most abundant in the hippocampal formation, claustrum, thalamus, and cerebral cortex (161, 162). In deeper cortical lamina, ER β mRNA is found in layers V–VI, while ERα is localized specifically to layer V (161). In humans, ER β protein is first detected at 15 gestational weeks in proliferative zones, 6 wk later than ER α expression begins (57). At 17 gestational weeks, ER β is expressed in the nuclei of cortical plate cells, pyramidal cells of the Ammon's horn, and dentate gyrus granule cells (57). At 25 gestational weeks, ER β immunostaining is widely detected in cortical neurons, with intense labeling present in the nuclei and weaker labeling in the cytoplasm. At birth and into adulthood, much of the nuclear labeling is lost and ER β is found predominantly in the cytoplasm of cortical and hippocampal pyramidal neurons (57). As with ER α, ER β levels and distributions are comparable across males and females. It is currently unknown whether ER β protein expression is altered in elderly people.
In monkeys, mRNA expression of ER β is present in the hippocampus, PFC, and the hypothalamus, among other brain regions (164, 177). Due to the small sample sizes in non-human primate studies, there was limited power to detect age-related effects or sex differences in ER β transcripts in the monkey. In addition, age- and sex-related differences in ER β protein expression and subcellular distribution have not yet been defined in the monkey model.
2. Rodents young versus aged
ER β distribution in rodents parallels but does not completely overlap with ER α (192). No nuclear ER β is detected in hippocampal primary cells or interneurons in young adult rats (139), although it does occur in newly born neurons in the hippocampal dentate gyrus (DG) of neonatal rats (82). In mice, sparse nuclear ER β immunoreactivity is found in the hippocampus (142).
ER β is found in pre- and postsynaptic compartments in both male and female rat (139) and mouse hippocampus (142). In the hippocampal CA1 region, postsynaptic ER β predominates, although it is also found presynaptically. There is a reduction in synaptic ER β during middle age; however, age does not affect the ability of estradiol to increase synaptic ER β labeling, particularly near the synaptic cleft (227) (Figure 3).
Estrogen regulates ER β in young adult rats and mice, in that levels are highest during estrus and diestrus (142). ER β expression decreases with age in the hippocampus but persists in the cortex (243). Estrogen regulation of ER β mRNA declines in middle age (231); however, postsynaptic protein expression does not decline in aged female rats and retains the ability to be upregulated by estradiol replacement (227). In mice, ER β protein expression in the cortex also decreases with age (22, 188).
The degree to which ER α and ER β are colocalized remains unclear. The similar actions of ER α and ER β agonists on hippocampal targets suggest that they may be present in the same cells if not the same cellular compartments (224), but direct protein colocalization has not been established. In the diencephalon, the Bed nucleus of the stria terminalis, medial amygdala, and preoptic area contain neurons that coexpress ER α and ER β mRNA. In contrast, in the arcuate nucleus, cortical amygdala, and ventromedial nucleus, both receptors are expressed but rarely colocalized (193).
D. Membrane ERs
Rapid nongenomic estrogen actions can occur through extranuclear ER α and β as well as new classes of membrane ERs. G protein-coupled estrogen receptor 1 (GPER1) is a membrane ER, identified in cancer studies but is also shown to have widespread expression throughout the body and central nervous system (160, 172). It is linked to rapid membrane-initiated estrogen actions (39). GPER1 expression is regulated by insulin in cancer cells (31); however, it is currently unknown whether similar effects are seen in the brain.
In monkeys, GPER1 mRNA is present in the forebrain as well as in luteinizing hormone-releasing hormone (LHRH) neurons where it mediates fast release (156). Human GPER1 mRNA expression is also detected in the hippocampus and cortex (available from http://human.brain-map.org) (76). In the monkey hypothalamus, GPER1 is localized to cell processes and perikarya, and the number of GPER1-containing cells in the periventricular area increases with aging but is unaltered with estradiol treatment (151). A study examining the effects of aging and estradiol replacement on GPER1 distribution in monkey PFC synapses is currently underway.
In the rodent, GPER1 is described in rat and mouse hippocampus and cortex (65, 77, 87, 225). It is present on the plasma membrane of hippocampal pyramidal cells (44, 128) and medial PFC neurons (6). Electron microscopic studies have revealed that GPER1 is localized to neuronal perikarya, endoplasmic reticulum, dendritic shafts, synaptic and extrasynaptic sites on dendritic spines, and axon terminals in the mouse hippocampus (225). The overall abundance of GPER1 in hippocampal subregions (CA1, CA3, and dentate gyrus) is comparable between male and female mice. However, estrous cycle phase influences GPER1 distribution such that proestrous females with elevated estrogen levels have more GPER1-containing axonal profiles than estrous females (225). In rat hippocampal dendritic spines, GPER1 binds to the synaptic scaffolding protein postsynaptic density-95 (PSD-95) (5). GPER1 interactions with another synapse-associated protein, SAP97, have also been observed in mouse hippocampal synapses (225).
E. PRs
Estrogen-sensitive genomic and nongenomic PRs are present in the hippocampus, largely in nonnuclear locations. In mouse and rat, genomic PR expression changes across development such that in adults it is found largely in extranuclear sites (142, 226). Nuclear labeling detected early in postnatal development in rats (173) does persist in a small number of interneurons in adult mice (142). Extranuclear PR expression found in pre- and postsynaptic compartments of excitatory neurons and glial cells is inducible by estrogen (142, 226). Expression of PR mRNA is found in monkeys and humans (76), but protein localization of extranuclear sites remains to be identified.
Nongenomic membrane PRs discovered in the last two decades are classified into two families. The first is the class II progestin and adipoQ receptor (PAQR) family called membrane progestin receptors (mPRs) and includes the following: mPRα (PAQR7), mPRβ (PAQR8), mPRγ (PAQR5), mPRδ (PAQR6), and mPRε (PAQR9) (see review in Ref. 167). The second is the b5-like heme/steroid-binding protein family that includes progesterone receptor membrane component 1 (PGRMC1), PGRMC2, neudesin, and neuferricin. Receptors from the PGRMC1/S2R family and mPRs are present in forebrain structures important for neuroendocrine regulation and other nongenomic effects of progesterone in rodents (136, 167). Estrogen regulates membrane PR expression in the hypothalamus (118, 254) and hippocampus (9). PGRMC expression is also detected in human and monkey cortex and hippocampus (76).
F. Steroid Receptors Beyond ER and PR
ER and PR belong to a superfamily of nuclear receptors that include other steroid receptors, including androgen, glucocorticoid, mineralocorticoid, vitemin D, retinoic acid, thyroid hormone, and orphan receptors/transcription factors (see reviews in Refs. 24, 218). Evidence supports actions of these receptors in hippocampal function. androgens regulate hippocampal synaptic plasticity through dihydrotestosterone (DHT)-sensitive mechanisms in male rodent hippocampus (117, 199) and androgen receptors are present in rodent (135, 137), non-human primate (1, 26, 183), and human (185) forebrain. Corticosterone acts via glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs) to exert genomic and nongenomic actions (see review in Ref. 148). While translocation of cytosolic MRs and GRs to the nucleus mediates gene transcription (179), nongenomic, rapid actions can be mediated by synaptic GR and MR in the hippocampus and amygdala (95, 171, 247) that modulate synaptic plasticity (93, 247). Glucocorticoids also regulate vitemin D receptor transcription in mice (83). Vitemin D receptors present in the rat hippocampus (112) have been attributed to regulation of plasticity (92) and steroid-mediated outcomes (see reviews in Refs. 38, 47).
III. ESTROGEN and PROGESTIN ACTIONS ON RAPID SIGNALING THAT AFFECT SYNAPTIC FUNCTION
A. Rapid Nongenomic Actions of Estrogens and Other Steroid Hormones
There is now substantial evidence for estrogen activation of signaling pathways at extranuclear sites that may also have indirect effects on gene expression in addition to the rapid effects on many aspects of cell function. Beginning with work on rapid effects induced by iontophoretic application of 17β-estradiol (102), and continuing with work on neural and nonneural systems (101), it is now evident estradiol has rapid actions via receptors that in many cases are posttranslationally modified forms of the classical ER that reside in parts of the cell outside of the nucleus, including mitochondria (155), presynaptic terminals (115, 214), postsynaptic spines (2, 140), and glial cell processes (142). Caveoli associated with, but not part of the cell membrane, are involved as a reservoir of classical ERs that are activated by estradiol near the cell surface (18). An exception to the classical ERs is the GPER1 (111, 208), which associates with PSD-95 in the dendritic spine and appears to couple estrogen action to membrane progestin receptors, 5HT1A receptors, and corticotrophin releasing hormone (CRH1) receptors (35). Finally, it should be noted that rapid, nongenomic actions are recognized along with classical cell nuclear actions for all classes of steroid hormones: progestins (142, 230), androgens (209), glucocorticoids (210, 211), mineralocorticoids (98), and vitemin D (86).
B. Estrogen Signaling and Synapse Formation
Rapid estrogen signaling leads to axospinous synapse formation in the hippocampus through at least two pathways (Figure 4). The first concerns estrogen stimulation of filopodia formation in neurons via a pathway involving estrogen-stimulated LIMK-1 phosphorylation and cofilin phosphorylation, which releases actin from cofilin inhibition so that it can polymerize and facilitate filopodia formation that may develop into mature spines (250). The second involves rapid stimulation of translation of the synaptic scaffolding protein, PSD-95, before any transcription of PSD-95 mRNA occurs; this is mediated by phosphatidylinositol 3-kinase activation of Akt, leading to phosphorylation and disinhibition of a protein translation regulator, 4E-BP1 (4, 253).
FIGURE 4.
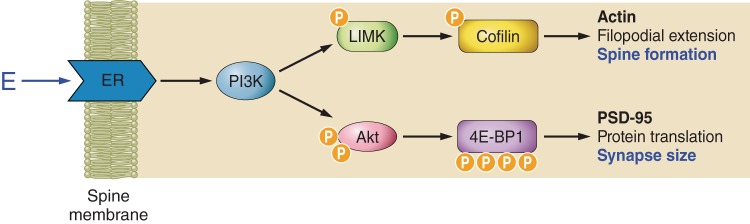
Nongenomic estrogen-initiated signal transduction leading to spinogenesis and changes in synapse size. Estrogen initiates a complex set of signal transduction pathways in the hippocampal neuron via membrane-bound estrogen receptors. Rapid activation of Akt (protein kinase B) via PI3K is mediated by ER α. Subsequently, activated Akt initiates translation of PSD-95 by disinhibiting the initiation factor 4E-binding protein 1 (4E-BP1). Estradiol-mediated phosphorylation of cofilin occurs via activation of LIMK. Cofilin depolymerizes actin and is inactivated by phosphorylation. Therefore, in the presence of estrogen, cofilin repression of actin polymerization is removed, resulting in an increase in filopodial density. The signal transduction pathways illustrated here are an oversimplification of a large body of work performed in an in vitro cell line.
Before these pathways are engaged, estrogen treatment increases glutamate NMDA receptor binding and expression (40, 228) and increases NMDA receptor-mediated synaptic input (239). In addition, blocking NMDA receptors during estrogen treatment in ovariectomized rats prevents the estrogen-induced spine synapse formation in the CA1 region of the hippocampus (237). The upregulation of NMDA receptors in the CA1 region of female rat hippocampus by estradiol is indirect, in that it is blocked by M2 muscarinic receptor antagonists and mimicked, bypassing estradiol, by elevating cholinergic activity (30), and this appears to involve disinhibition of the inhibitory GABAergic input to CA1 pyramidal neurons (184). In this connection, it is important to note that estrogens upregulate the activity of choline acetyltransferase (123), the enzyme responsible for acetylcholine synthesis, as well as potassium-stimulated acetylcholine release (54), and the latter may be mediated by nongenomic ER α in cholinergic terminals (214).
C. Rapid Estrogen Actions on Excitability
Estrogen induces spinogenesis and synaptogenesis upon the enhancement of fast glutamatergic transmission and long-term potentiation (LTP). Actin dynamics within the synaptic terminal also play important roles in the structural plasticity of the postsynaptic spine. In hippocampal slices, estrogen increases spine concentrations of filamentous actin and strongly enhances actin polymerization in association with LTP, and it does so by activating the small GTPase RhoA and phosphorylates and thus inactivates cofilin, a downstream target of RhoA (108). In this study, an antagonist of RhoA kinase (ROCK) blocked the effects of estrogen on synapses. According to these authors (108), estrogen does not, however, strongly affect a second LTP-related pathway, involving the GTPases Rac and Cdc42 and their effector p21-activated kinase, which may explain why its acute effects are reversible. Finally, these authors note that “. . . . .ovariectomy depressed RhoA activity, spine cytoskeletal plasticity, and LTP, whereas brief infusions of estrogen rescued plasticity, suggesting that the deficits in plasticity arise from acute, as well as genomic, consequences of hormone loss” (108).
D. Estrogen Actions on Synapse Formation Across Species
While the seminal studies on spine induction in the hippocampus were performed in rats, there have been more recent studies showing that related processes occur in mice as well as in monkeys. In mice, we have seen that estrogen and estrogen-inducible progestin receptors are found in the hippocampus in the same nongenomic and genomic sites as in the rat (142). A Golgi impregnation study showed that estrogen treatment had no overall effect on spine synapse density in the CA1 region of the dorsal mouse hippocampus, but caused an increase in the number of spines with mushroom shapes that are associated with high synaptic efficacy and strength (116). Radioimmunocytochemistry and silver-enhanced immunocytochemistry were used to show that the immunoreactivity for postsynaptic markers (PSD-95 and spinophilin) and a presynaptic marker (syntaxin) was enhanced by estrogen treatment throughout all fields of the dorsal hippocampus. Moreover, estrogen treatment enhanced performance on the object-placement test, a measure of spatial episodic memory that is dependent on the hippocampus (116). Taken together, the morphological and radioimmunocytochemistry results suggest a previously uncharacterized role of estrogen in synaptic structural plasticity that, consistent with the literature in rat hippocampus, may be interpreted as a facilitation of the spine-maturation process and may be associated with enhancement of hippocampus-dependent memory.
The same signaling pathways activated by estrogen in the rat hippocampus are also triggered in the female mouse hippocampus. In female C57BL/6 mice, activation of Akt, LIMK, and the tropomyosin-related kinase B receptor (TrkB) neurotrophin receptor is increased in dorsal hippocampus during proestrus when estradiol levels peak (204). Estrus cycle stage also modulates expression of the pre- and postsynaptic markers synaptophysin and PSD-95. Despite these changes in synaptic markers, cycle phase does not regulate spatial memory performance in the object placement test. Interestingly, this type of spatial memory is sensitive to the complete absence of ovarian hormones. Together these results suggest that ovarian estradiol and progesterone through activation of specific signaling pathways modulate actin remodeling, synapse formation, and ultimately cell excitability.
Because the TrkB receptor that binds to the brain-derived neurotrophic factor (BDNF) is important for the synaptic plasticity underlying hippocampal-dependent learning and memory, the precise location of TrkB activation is likely important for its specific actions. An electron microscopic study revealed that phosphorylated-TrkB (pTrkB), the activated form of TrkB, is localized primarily to axons and axon terminals, but is also present in dendritic shafts and spines of hippocampal neurons (202). There was also abundant pTrkB in microglial profiles identified using immunofluorescence and confocal microscopy. In high estradiol states in proestrus females, pTrkB in axons and glial cells are more abundant in the hippocampal CA1 stratum radiatum than in low estradiol states (estrus and diestrus females and males). Thus it would appear that presynaptic TrkB is positioned to modulate estradiol-mediated and BDNF-dependent synaptic plasticity, and they also suggest that there may be a novel role for TrkB in microglial function in the neuroimmune system.
E. Interactive Effects of Progestin on Synapse Formation and Withdrawal
Progestin can interact with the effects of estrogen to exert a biphasic effect on synapse number in the hippocampal CA1. After estrogen increases spine density, subsequent progestin administration further boosts spine density for 6 h, but this increase is followed by a rapid reduction in spines (238). By 18 h after progestin administration, spine density is decreased to levels observed after ovariectomy. Similar to estrogen, progestin activates mitogen-activated protein (MAP) kinase and Akt signaling (62, 138, 198). However, work in a mammary tumor cell line suggested that a direct interaction between PR and ER may be necessary for progestin to induce MAP kinase signaling (138). These receptor interactions may underlie the initial increase in synapse number detected after progestin treatment. After a prolonged period following progestin administration, ER levels drop and other PR actions can facilitate the withdrawal of spines and reduction in synapse number. Together, estrogen and progesterone act together to promote both the induction and ultimate retraction of spines through nongenomic mechanisms as well as genomic actions leading to PR expression. While the mechanism for synapse downregulation is presently unknown, it is likely to involve novel nongenomic actions based on the predominant nonnuclear localization of PR in rodents.
F. Roles of Different Estrogen Receptor Types
There are two primary nuclear/nonnuclear estrogen receptors, ER α and ER β, and they both contribute to signaling and synaptic plasticity. ER α and β knockout mice and wild-type littermates were used to investigate their respective roles in estradiol effects on hippocampal plasticity and learning (201). After 6 h of estradiol administration, there was increased immunoreactivity for phosphorylated Akt throughout the hippocampal formation, and after 48 h of estradiol treatment, there was increased immunoreactivity for the pTrkB receptor. Estradiol administration in ER α and ER β knockout mice did not result in increased phosphorylation of Akt and TrkB. However, there were different effects of estradiol in ER α and β knockout mice upon PSD-95 protein levels and BDNF mRNA. Overall, it thus appears that estradiol acts through both ER α and β, albeit somewhat differently, throughout the hippocampal formation, and the different actions of 6 and 48 h of estradiol suggest that multiple estrogen signaling mechanisms participate in hippocampal synaptic structural and functional plasticity.
One of the advantages of using mouse models is the possibility to study alleles of common genes, such as is the case with the BDNF polymorphism and the influence of this polymorphism on estrogen actions related to cognitive function and affective behavior. The effects of the common human variant BDNF Val66Met were examined in cycling female mice (203). Mice homozygous for the BDNF Met variant had increased anxiety behavior, impaired memory, and increased expression of BDNF and its receptor TrkB in the hippocampal formation. The magnitude of fluctuation in spatial memory, hippocampal Akt phosphorylation, and PSD-95 protein expression across the estrous cycle was also significantly altered in BDNF Met homozygous mice. The BDNF Val66Met variant should therefore be considered as one of the candidates that mediates genetic differences in ovarian steroid-related behavioral changes and disorders.
IV. INTERACTIVE EFFECTS OF ESTROGEN and AGING
A. Introduction
The findings reviewed above provide ample support for a prominent role for estradiol and multiple ERs in the synaptic underpinnings of cognitive functions mediated by the hippocampus and PFC. In addition, as discussed above, there are age-related alterations in ERs that vary across regions and species. Thus there is a critical convergence of endocrinology and brain aging with respect to the role played by estradiol and ERs in cognitive functions such as memory and learning. It is widely recognized that cognitive functions mediated by the medial temporal lobe and PFC are highly vulnerable to aging in rodents, monkeys, and humans (145), yet the cause of the heightened vulnerability of these regions is not well understood. It is of particular importance to women's health to determine the degree to which alterations in circulating estradiol and other endocrine events associated with menopause mediate age-related decline, since women in the developed world can expect to live one-third of their lives after the menopausal transition. The aged female rhesus monkey has proven to be a highly valuable model for the investigation of the synaptic events that are sensitive to both estradiol and aging in an effort to tease out the particular role of estradiol in age-related synaptic alterations that affect cognitive performance. Below, we review these findings and their translational relevance to cognitive aging in women.
B. Menopause in Women and Its Association With Cognitive Functions
Menopause is characterized by the permanent cessation of menstrual cycles and the loss of ovarian hormone production, signaling the end of a woman's reproductive phase. The menopausal transition is accompanied by changes in hormonal levels, including ovarian ones. While widely variable across women, estradiol starts to decrease 2 yr prior to the final menstrual period and reaches barely detectable levels 2 yr after the final period (21). Women undergoing the menopausal transition often report memory problems, among other symptoms. It has become increasingly evident that an interaction exists between age-related cognitive decline and endocrine changes (67, 78).
Menopause can occur naturally, or in cases of medical necessity, can also be induced surgically. Removal of ovaries in women, referred to as oophorectomy, is associated with an increased risk in cognitive impairment and dementia later in life (181, 190). Indeed, estrogen replacement in these women can improve cognitive functions (79, 170). In contrast, natural menopause in women has a smaller magnitude of effect on cognitive skills. A cross-sectional study involving 326 women found no significant differences across different menopausal statuses (early and late menopausal transition, and early and late postmenopause) in episodic verbal memory as measured by a supraspan word list recall task, although their performance was not compared directly with that of premenopausal women (80). In the Study of Women's Health Across the Nation (SWAN) study, 2,362 women (42–52 yr old) were enrolled and their cognitive performance was followed for 4 yr (60). While verbal memory (East Boston Memory Test scores) improved with time in premenopausal and postmenopausal women, this enhancement was not observed in women undergoing early or late perimenopause, suggesting that the ability to learn may be temporarily compromised during the menopausal transition (60). Mild deficits in concentration and processing speed are also observed in women undergoing the menopausal transition (106). More recently, the effect of menopause on verbal memory was assessed in a 14-yr, longitudinal, population-based study involving 403 women from the Penn Ovarian Aging Study cohort (36). As all women were recruited when they were premenopausal, verbal memory scores (from the Buschke Selective Reminding Test) could be precisely followed through, and compared across, individual phases of the menopausal transition. Independent of the effects of normal aging, delayed verbal recall declined early in the menopausal transition while immediate recall declined late in the transition, although the magnitudes of change for both were modest (36). Collectively, it appears the abrupt loss of estrogen with oophorectomy has more severe consequences to cognitive functions than the gradual decline of estrogen seen in natural menopause (21).
Menopause induced surgically can also have different physiological consequences from that occurring naturally. In women, serum levels of estradiol and testosterone are lower in oophorectomized women compared with those who have undergone natural menopause (107). In addition, recent work has shown that ER β expression in the monkey hippocampal subiculum is upregulated in natural menopause, while ovariectomy is associated with increased aromatase (enzyme required for estrogen synthesis) expression without any changes in ER β levels (84). These studies and others suggest that the morphological and molecular consequences of menopause may vary depending on the different trajectories of endocrine and physiological changes that occur with natural versus surgical menopause.
C. Clinical Trials Examining the Effects of Hormone Replacement Therapy on Cognition in Postmenopausal Women
Clinical studies that tested the efficacy of hormone replacement therapy in protecting cognitive functions have produced inconsistent findings compared with those from animal models (8, 191). The largest trial ever undertaken was the Women's Health Initiative Memory Study (WHIMS), a randomized, double-blind, controlled clinical trial, which tested the effects of estrogen plus progestin on the incidence of dementia and mild cognitive impairment in 4,532 postmenopausal women (194). This trial concluded that not only did hormone therapy fail to improve cognitive function, but it increased the risk for probable dementia in postmenopausal women aged 65 years and older (194).
This WHIMS study has a major caveat that may partly explain its findings that are contrasting from other trials in women and from work in animal models. The women enrolled in the WHIMS clinical trial were on average 72 yr old at the time of hormone therapy initiation (194), ∼15 yr after menopause. This study and other work in animal models have led to a “critical period” hypothesis, which proposes that there is a narrow window of opportunity in which hormone therapy can be initiated for it to be beneficial (53, 125, 191, 251). Indeed, initiation of hormone replacement therapy during perimenopause or shortly after menopause can have beneficial effects on cognitive function. In a cross-sectional study involving 428 subjects, women receiving hormone replacement therapy before the age of 56 scored higher on a test of global cognitive function (the Mini-Mental State Examination; MMSE) and had better attention compared with those who started therapy after 56 yr of age (124). This same study revealed that women who started therapy after the age of 56 performed worse on the MMSE compared with those who never used hormone therapy (124), suggesting that late initiation of hormone therapy can have detrimental effects. In another study, women who initiated hormone replacement therapy at a younger age (mean age of 48.8) and terminated treatment after an average of 5.2 yr, scored higher on the MMSE and exhibited better mental flexibility compared with those who never received hormone replacement or those who continued treatment for a longer duration (average of 14.30 yr) (130).
With regard to more specific cognitive functions, verbal memory has received particular attention and has been assessed in postmenopausal women receiving hormone replacement therapy. A longitudinal study that enrolled 727 elderly women showed that a subgroup of 81 women who had initiated hormone therapy around the time of menopause scored higher than untreated women on tests of verbal memory and fluency (88). When follow-up tests were administered a few years later, verbal scores had improved in the past-hormone-users, while declined in the never-users (88). This improvement in verbal memory with hormone replacement therapy has also been observed in women who underwent oophorectomy and received estrogen treatment soon after the surgery (219).
It is important to note that other studies in women have found inconclusive or negative effects of hormone replacement on cognitive function, even when the treatment was initiated soon after menopause (97, 126). Factors other than the timing of treatment initiation, such as the hormone formulation, treatment schedule, and administration route (oral versus transdermal), likely also contributed to and confounded the clinical outcomes. One critical consideration is the effect of the synthetic progestin (medroxyprogesterone acetate; MPA) that was often given in combination with estrogen, such as in the WHIMS. Unlike endogenous progesterone, MPA can antagonize the neuroprotective effects of estradiol in hippocampal cell cultures (153). Potential detrimental effects of the combination formulation (conjugated equine estrogens plus MPA) on verbal memory have been observed in postmenopausal women, even when treatment was started shortly after menopause (average age, 52 yr old) (126).
A greater consensus is seen in conclusions derived from reports where hormone replacement was initiated later in life relative to the age of menopause. Many of these show that hormone therapy is ineffective or detrimental to cognitive function (37, 89, 178, 194, 241), further supporting the “critical period” hypothesis for its effectiveness.
In addition to the wide-ranging effects exerted by different hormone treatments, the brain itself has the capacity to synthesize small amounts of estradiol “on demand” through aromatase, which is present in cortical and hippocampal neurons (85, 242). Evidence from models of ischemia suggests that traumatic challenges can activate the aromatase system, promoting extragonadal estradiol synthesis that is critical for neuroprotection (132). This same capacity of local synthesis of estrogen in the brain is likely called into play during menopause when circulating estrogen levels fall dramatically. Finally, there may be endocrine factors other than circulating estradiol that impact the symptoms associated with the menopausal transition, including effects on cognition. Recent data from both women and rhesus monkeys highlight the importance of adrenal steroids during the menopausal transition, which can vary greatly across individuals, have estrogenic bioactivity, and are also responsive to hormone treatments (28, 113, 131). Whether changes in circulating adrenal steroid levels in the face of decreasing estradiol affect synaptic health or cognition is not known at this time, but it warrants investigation.
D. Nonhuman Primates as a Model of Menopause
Many factors can influence cognitive outcomes of hormone replacement therapy in women. Other than the various formulations, schedules, and routes of the therapy regimen, participants may have had variable levels of educational attainment, socioeconomic status, and diet; some may also have had undiagnosed medical conditions or prodromal neurodegenerative disorders. The use of an animal model allows investigation of the effects of hormone treatment on cognitive function while controlling for, or avoiding altogether, some of these complex variables. Female macaque monkeys are valuable models of menopause, because their reproductive physiology and patterns of endocrine senescence closely resemble those of women (56, 129, 152, 221, 234). For example, premenopausal monkeys have 28-day menstrual cycles with ovarian hormone fluctuations similar to those of women (55). This is in contrast to the 4-day estrous cycles rodents experience. and most importantly, monkeys undergo a low-estrogen menopause in a manner similar to women (55, 152, 221).
In addition to these similarities in reproductive physiology, macaques are also ideal for studying menopause-related cognitive symptoms. Their brain anatomy, neuronal structures, and neuronal gene expression closely resemble those of women (72, 120, 168). Furthermore, their cognitive functions can be assessed using the same or similar tests that assess the rich repertoire of cognitive capacities in humans (149, 207). In contrast to humans, macaques do not develop the neuropathological symptoms of Alzheimer's disease (48, 105, 165). Therefore, menopause-related cognitive symptoms can be assessed in macaques without the confounding factors inherent to neurodegenerative diseases.
E. Effects of Menopause in the Monkey Hippocampus
The menopause-related cognitive impairment seen in women is also evident in female nonhuman primates. Compared with premenopausal monkeys, naturally peri/postmenopausal female monkeys exhibit deficits in recognition memory (70, 180), a function reliant on the integrity of medial temporal lobe structures that include the hippocampal formation and perirhinal cortex (141, 175, 206). This menopause-related impairment is significant even after accounting for the effect of chronological age (70, 180). In surgically menopausal aged ovariectomized monkeys, cyclic estradiol replacement consisting of a single injection every 3 wk, a regimen closely mimicking the natural fluctuations of estrogen, modestly improves recognition memory scores (176).
The dentate gyrus (DG) is a subregion of the hippocampus that has received the most attention with regards to memory decline in normal aging. The DG receives perforant path input from the entorhinal cortex (Figure 2), and this projection is now thought to be one of the most vulnerable to the effects of normal, nonpathological aging (146, 233, 246). Indeed, energy metabolism in the DG, but not the other hippocampal subregions, declines with normal aging and is correlated with worse recognition memory scores (200).
In the rhesus monkey, hippocampal volume and neuronal numbers remain stable with chronological age (23, 104, 127, 166, 174, 186, 187). However, in the monkey DG, natural menopause results in a lower density of perforated synapse spines, which is correlated with worse recognition memory (70). The menopause-related decrease in perforated synapse spines is significant after accounting for the effect of age, and no other synaptic parameters examined, including total synapse density or synapse size are different across groups designated by age or menses status (70). Perforated synapses are an important class of synapses for memory functions (50, 51) and are morphologically characterized by a discontinuity in the postsynaptic density (a hole, slit, or complete segmentation in the postsynaptic density plate) (52). These synapses are often present on large mushroom spines and are proposed to be a structural correlate of synaptic efficacy (52, 61, 213). In fact, they have an exceptionally high level of glutamate AMPA receptors (45, 71), a correlate of synaptic strength (81, 103). This study suggests that the decrease in estrogen that accompanies menopause may impact memory, in part, by the selective loss of this highly efficient and exceptionally strong synaptic subtype. Interestingly, the synaptic dynamics in PFC are quite different from the hippocampus, with the highly plastic thin spines representing the primary target of estradiol.
In a related study in the monkey DG, types of synaptic connections have been compared between young and aged, premenopausal and naturally postmenopausal monkeys (69). Normal aging resulted in an increase in boutons making no synaptic contact (nonsynaptic boutons; NSBs) and a loss of boutons forming multiple synaptic contacts (MSBs) (Figure 5A). MSBs are presynaptic boutons that are synaptically connected to more than one dendritic shaft or spine and can act as a morphological substrate to enhance coupling between presynaptic and postsynaptic neurons (49, 74, 212, 245). While natural menopause did not cause a further increase in NSBs or a further decline in total MSBs, it was associated with a loss of a distinct class of synapses. Compared with aged premenopausal monkeys, postmenopausal monkeys exhibited a lower incidence of MSBs forming perforated synapses (69) (Figure 5A). MSBs forming perforated synapses may represent a special class of synaptic connection that possesses the strongest synaptic strength and the ability to simultaneously depolarize multiple postsynaptic neurons. Indeed, the prevalence of this class of synapses positively correlates with recognition memory scores in monkeys (69) (Figure 5B). Together, these studies suggest that one way estrogen exerts its procognitive effects may be via preservation of this special class of DG synapses that are associated with better memory.
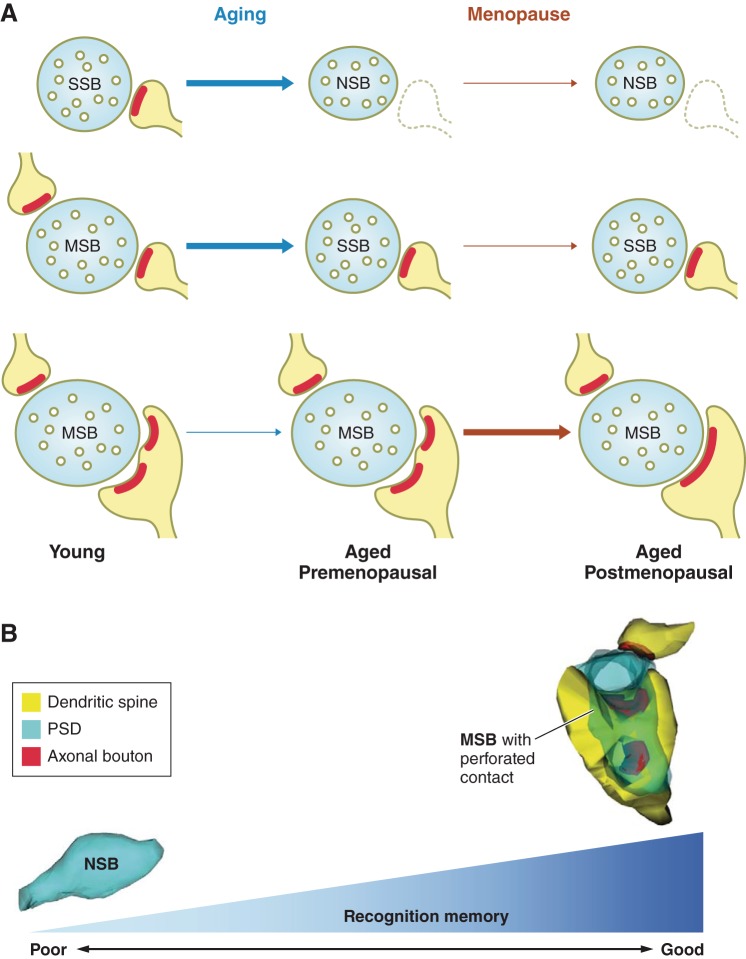
FIGURE 5.
Natural menopause is associated with a selective loss of complex and strong synaptic connections in the monkey hippocampus. A: schematic diagrams illustrating shifts in the types of synaptic connections in the monkey dentate gyrus with aging and menopause. Aging results in an increase in nonsynaptic boutons (NSB) and a decrease in multiple-synaptic boutons (MSB). While menopause does not result in changes in the proportions of NSBs or MSBs, it is associated with a selective decrease in MSBs forming one or more perforated synapses, a type of connection that possesses strong synaptic strength and the ability to simultaneously activate multiple postsynaptic neurons. Arrows indicate potential shifts in bouton subtypes with aging and menopause. Blue, axonal bouton; red, postsynaptic density; yellow, dendritic spine. B: a diagram illustrating the relationships between types of synaptic connections in the monkey dentate gyrus and recognition memory performance. Recognition memory scores positively correlate with the incidence of MSBs forming one or more perforated synapses and inversely correlate with NSB frequency. Serial sections from the monkey dentate gyrus were reconstructed using the free software developed by Dr Kristen Harris and her laboratory. Reconstruct, version 1.1.0.0; http://synapses.clm.utexas.edu. Diagrams and 3-dimensional reconstructions were made based on findings from Hara et al. (69).
F. Effects of Aging and Estrogen in the Monkey PFC
1. Nonhuman primate models mimicking natural estrogen cycles
The prefrontal cortex is critical for executive functions, including working memory and cognitive set-shifting (7, 43, 72). The causes of menopause-associated cognitive symptoms in women are likely to be multifactorial; however, estrogen deficiency is believed to be one of the culprits for deficits in cognitive functions subserved by the PFC (100). In fact, postmenopausal women receiving hormone replacement therapy outperform women receiving no treatment on tests of executive functioning (100). Some of these PFC-reliant cognitive functions are also restored by hormone replacement therapy in nonhuman primates. For example, in aged ovariectomized female monkeys, the cyclic estradiol regimen that closely mimics the natural fluctuations of estrogen in premenopausal monkeys reverses the age-related impairment in working memory (176). Estrogen replacement also improves executive functions in middle-aged ovariectomized monkeys, as measured by performance on a set-shifting task (220).
Improvements in cognitive function with cyclic estradiol treatment are accompanied by alterations in neuronal morphology in dorsolateral PFC area 46, a cortical region strongly implicated in working memory (43, 122). The effects of estrogen on area 46 pyramidal neurons were experimentally tested in a cohort of young and aged ovariectomized female monkeys receiving cyclic unopposed estradiol or vehicle injections (67). In the delayed response test of visuospatial working memory, young monkeys receiving cyclic estradiol or vehicle, and aged monkeys receiving cyclic estradiol perform equally well (67, 176). Reconstructions of pyramidal neurons in PFC area 46 revealed that cyclic estradiol increases the density of the highly plastic small thin spines in both young and aged monkeys (67) (Figure 6). Although young vehicle-treated monkeys exhibit lower spine density, their total dendritic length and branch numbers are increased such that the total number of spines per neuron is equivalent to the young monkeys treated with estradiol (67) (Figure 6). This dendritic expansion may be a compensatory mechanism that protects cognitive function in young monkeys experiencing an abrupt loss of estrogen. In contrast, only vehicle-treated aged ovariectomized monkeys exhibit significant working memory impairment, and this deficit occurs concomitantly with a dramatic decrease in area 46 spine density in the absence of a compensatory extension of dendritic arborization (67) (Figure 6). Indeed, the combined effects of aging and estrogen deprivation result not only in decreased spine density, but also a significant shift towards larger spines such that aged ovariectomized vehicle-treated monkeys are left with a mere one-third of small plastic thin dendritic spines compared with what is observed in young estradiol-treated monkeys (67, 99) (Figure 6).
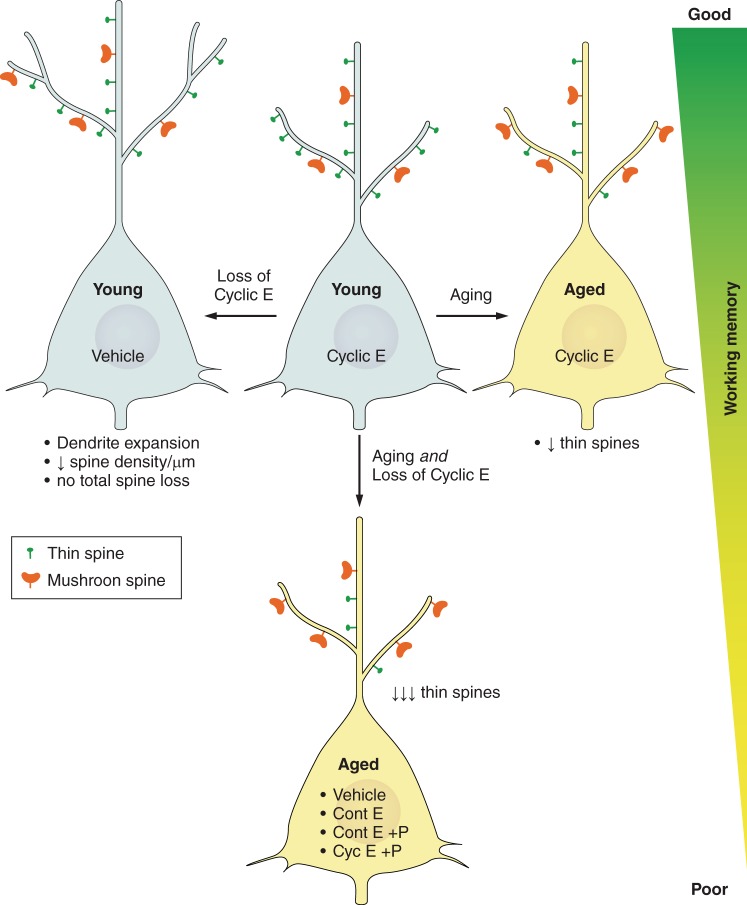
FIGURE 6.
Schematic diagrams illustrating the neuronal and spine morphology of monkey area 46 in response to aging, surgical menopause (ovariectomy), and hormonal manipulations and how these relate to working memory performance. Young ovariectomized monkeys treated with cyclic estradiol (E) or vehicle and aged ovariectomized monkeys treated with cyclic estradiol perform equally well on the delayed response test of working memory. Compared with young treated with cyclic E, the young receiving vehicle have lower spine density, but longer dendritic length; consequently, these two young groups have comparable numbers of spines per neuron. Aging results in decreased spine density, a selective loss of small thin spines, and a relative increase in mushroom spines. Aging combined with loss of cyclic E results in a further loss of thin spines such that these monkeys only have one-third the density of thin spines compared with the young cyclic E-treated subjects. Treatment regimens that included continuous E alone (Cont E), continuous E with cyclic or continuous progesterone (Cont E +P), or cyclic E with cyclic P (Cyc E +P) resulted in spine densities and morphologies that were comparable to vehicle treatment in aged ovariectomized monkeys. None of these treatments in aged ovariectomized monkeys restored working memory. Diagrams were drawn based on findings from Hao et al. (67) and Ohm et al. (159) and updated from Hara and Morrison (68). Only apical dendrites are drawn for simplicity, but similar changes occur in basal dendrites and throughout distal higher order dendrites.
As discussed above, estrogen has many sites of action, and there are likely to be multiple mechanisms and pathways that underlie its beneficial effects on cognition in aged monkeys. One such example was explored in the same behaviorally characterized young and aged ovariectomized monkeys. Specifically, the synaptic distribution of ER α in area 46 has been examined with electron microscopy (222). While ER α levels remain stable across age and hormone treatment groups, the abundance of ER α within the spine postsynaptic density (PSD) correlates with working memory performance, exclusively in aged monkeys receiving cyclic estradiol (222) (Figure 7). Hence, the cognitive benefits observed in aged monkeys with cyclic estradiol may be mediated, in part, by the activation of ER α in a synaptic domain coupled to signaling cascades involved in spine/synapse formation and stabilization (205).
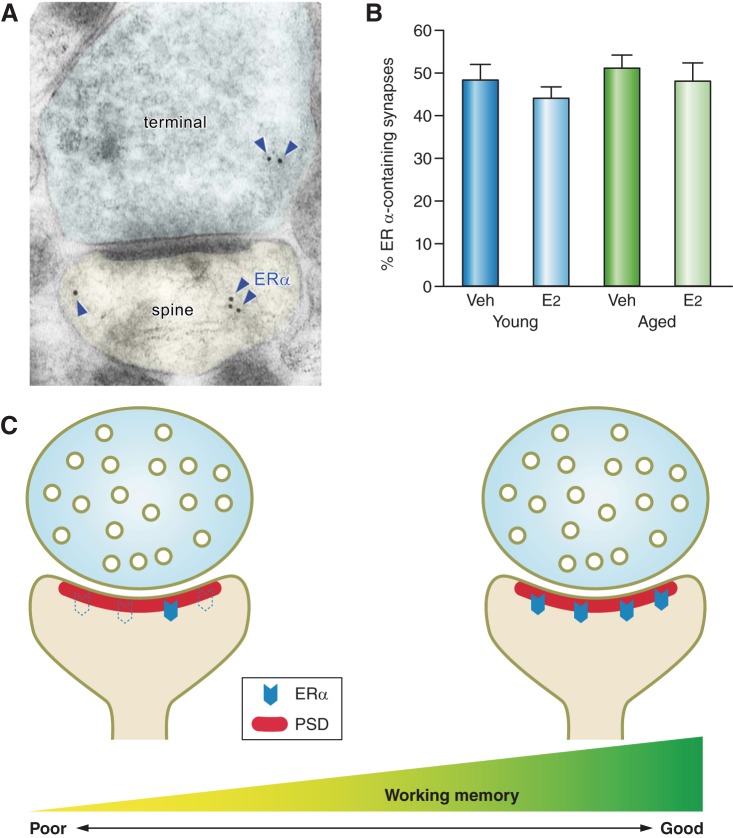
FIGURE 7.
ER α expression in the monkey dorsolateral PFC and its relationship to working memory. A: an electron micrograph shows ER α immunogold particles (blue arrowheads) in pre- and postsynaptic compartments. [Micrograph modified from Wang et al. (222).] B: ER α is abundantly expressed in monkey PFC synapses, and the proportion of ER α-containing synapses is not significantly altered with aging or estradiol (E2) treatment. Veh, vehicle-treated. [Histogram replotted from data presented in Wang et al. (222).] C: schematic diagrams illustrating that in aged E2-treated monkeys, the abundance of ER α in the postsynaptic density (PSD) correlates with working memory performance. [Diagrams drawn based on findings from Wang et al. (222).]
2. Nonhuman primate models mimicking clinically relevant hormone treatments
In contrast to the unopposed cyclic estradiol regimen that produced procognitive effects in rhesus monkeys, the hormone replacement therapy used in women in the WHIMS trial consisted of estrogenic compounds combined with a progestin, in a continuous (daily) regimen. Therefore, hormone treatments modeled after those used in clinical settings were tested in aged ovariectomized monkeys. Treatments that included continuous unopposed estrogen, continuous estrogen opposed with cyclic or continuous progesterone, or cyclic estrogen opposed by cyclic progesterone, all failed to improve PFC-reliant working memory in aged ovariectomized monkeys (11). Consistent with these findings, these same treatments also failed to restore dendritic spine synapses on area 46 neurons that had been observed with cyclic unopposed estrogen treatment (159) (Figure 6). Young ovariectomized monkeys also showed parallel results; continuous estrogen with or without progesterone resulted in area 46 spine density and morphology that were indistinguishable from those receiving vehicle alone (249). Homeostatic mechanisms likely take effect with chronic estrogen administration, during which its levels remain high, leading to desensitization of mechanisms involved in spinogenesis. Indeed, there is evidence in the rodent literature that continuous treatment of estrogen can lead to decreased levels of ER mRNA and protein in several brain areas (14, 17, 20, 196). Different formulations of hormone treatment also differentially affect circulating adrenal steroids that have estrogenic activity through ERs, and these effects are age dependent. For example, both unopposed estrogen and estrogen combined with progesterone decrease circulating dehydroepiandrosterone sulfate (DHEAS) in young ovariectomized monkeys (28). In aged monkeys, however, unopposed estrogen has no effect on DHEAS levels and combined estrogen plus progesterone increases DHEAS (28).
Together, these studies suggest that the specific formulation of hormones (estrogen alone versus a combination of estrogen and progesterone) and the treatment schedule (continuous versus cyclic) are both key factors for estrogen to exert its positive effects on cognitive and synaptic health. These data provide evidence that the standard forms of hormone replacement therapy commonly prescribed to women may not provide the same cognitive and synaptic benefits as formulations that mimic the natural menstrual cycle.
G. Estrogen Effects on Metabolism and Mitochondria: Implications for Cognition and Menopause
Among the many functions estrogen has on the brain, it also improves cerebral metabolic rate and blood flow (34, 189). In a double-blind trial that enrolled 52 peri- and postmenopausal women, estrogen therapy increased PFC blood oxygenation levels during tests of verbal and spatial working memory, and this increase was associated with fewer errors (94).
In addition, estrogen exerts many antioxidant effects. Estradiol increases the expression of the antiapoptotic protein Bcl-2 (46, 154). This estrogen-induced increase in Bcl-2 enhances the capacity for mitochondria to sequester cytosolic calcium, imparting more resistance to neurons from the harmful effects of glutamate-induced excitotoxicity (19, 154). Furthermore, a single injection of estradiol in adult ovariectomized rats upregulates mitochondrial proteins that include key metabolic enzymes such as pyruvate dehydrogenase, aconitase, and ATP-synthase (155). These increased mitochondrial protein expressions translate to enhanced mitochondrial efficiency as measured by increased respiratory control and improved enzymatic activities (155). Importantly, estradiol reduces formation of reactive oxygen species (ROS) (155), which damage DNA, RNA, proteins, and lipids, and can induce cell death. These antioxidant effects of estrogen are noteworthy in light of strong evidence that mitochondrial dysfunction and damage accrued from oxidative stress precede, and may be causative for, neurodegenerative disorders such as Alzheimer's disease (96).
Some of the estrogen effects on metabolism are likely mediated by ER β. Estradiol can bind to ER β that are localized to the mitochondrial matrix (244). It can then impact the expression of mitochondrial DNA genes that encode 13 proteins that are components of the electron transport chain and oxidative phosphorylation (197). Both ER α and ER β also regulate many nuclear genes involved in mitochondrial electron transport and ROS pathways (157).
Working memory reliant on the dorsolateral PFC area 46 (in humans and nonhuman primates) is susceptible to decline with aging and menopause (10, 43, 176, 180), and this may be partly due to the high energy requirement to sustain this function. Mitochondria supply the energy required for neurons, and often accumulate at synapses, where energy demands are especially high (13, 25). Mitochondria are also structurally dynamic and continually undergo fusion and fission events (248) (Figure 8). A recent study in rhesus monkeys revealed that estrogen treatment alters mitochondrial morphology in area 46 (73). Specifically, cyclic estradiol treatment in aged ovariectomized monkeys decreases the incidence of presynaptic donut-shaped mitochondria (73), a form that is associated with oxidative stress and ROS production (3, 119) (Figure 8). Notably, presynaptic boutons harboring donut-shaped mitochondria are inversely correlated with working memory performance in monkeys and form abnormally small synaptic contacts (73) (Figure 8). Collectively, these studies suggest that cyclic estrogen treatment after surgical menopause may restore working memory, in part, by promoting mitochondrial and synaptic health in Area 46.
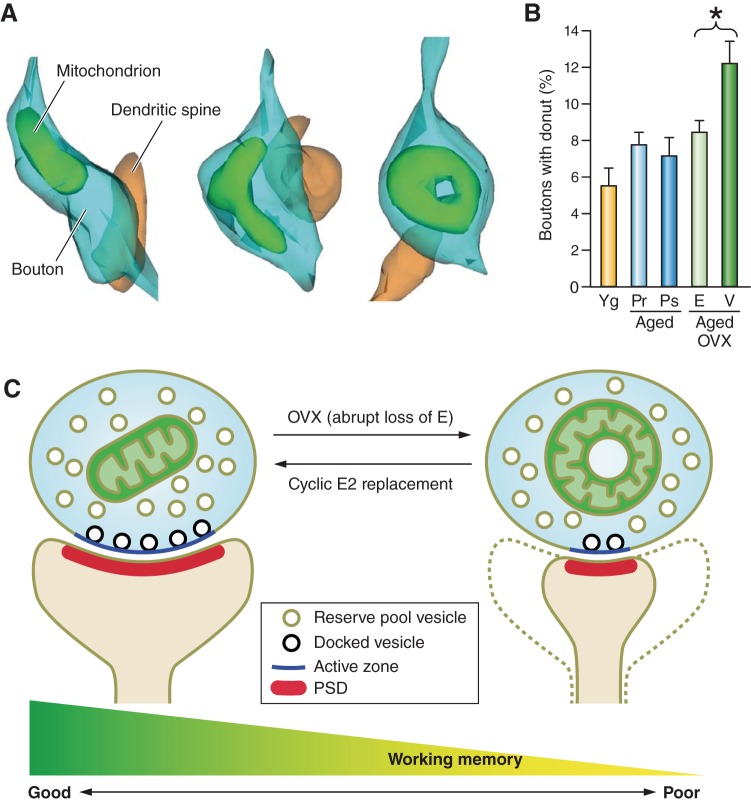
FIGURE 8.
Estradiol improves working memory and presynaptic mitochondrial health in the monkey dorsolateral PFC. A: presynaptic mitochondria in the monkey dorsolateral PFC come in different shapes. Three-dimensional reconstructions were performed using the free software developed by Dr Kristen Harris and her laboratory: Reconstruct, version 1.1.0.0; http://synapses.clm.utexas.edu. B: surgical menopause induced by ovariectomy (OVX) induces a significant increase in the incidence of malformed donut-shaped mitochondria, while cyclic unopposed estradiol (E) treatment reverses this increase to levels comparable to young (Yg) and aged naturally pre- (Pr) and postmenopausal (Ps) menopausal monkeys. [Histogram adapted from Hara et al. (73).] C: schematic diagrams demonstrating the effects of estradiol on presynaptic mitochondria. Abrupt loss of estrogen in aged monkeys results in a higher incidence of donut-shaped mitochondria, a mitochondrial shape associated with oxidative stress. These boutons harboring donut-shaped mitochondria form smaller synaptic contacts with fewer docked synaptic vesicles and are associated with poor working memory performance. Cyclic unopposed estradiol treatment in aged OVXed monkeys decreases the incidence of these pathological mitochondria while restoring working memory. [Diagrams drawn based on findings from Hara et al. (73).]
V. CONCLUSIONS and FUTURE DIRECTIONS
Over the last two decades, it has become clear that estradiol exerts effects on the brain that go well beyond the regulation of reproductive behaviors mediated by the hypothalamus. In fact, brain regions such as the hippocampus and PFC contain all of the key ERs present throughout the body and are highly responsive to estradiol, as are the cognitive behaviors these regions mediate. In addition, along with the classical genomic effects mediated by activation of ER α and ER β, there are membrane-bound ER α, ER β, and GPER1 that can mediate rapid nongenomic effects, and all three receptors are present in excitatory synapses in the hippocampus and PFC.
While we know less about progesterone and its effects on these cortical regions, it is clear that it also has rapid, nongenomic effects and the cerebral cortex is a key target. In addition, adrenal steroids can activate ERs, particularly when circulating estradiol is low and adrenal steroids increase such as during the menopausal transition (28), yet we know virtually nothing about how such interactions might affect hippocampal or neocortical synaptic transmission. Finally, cortical neurons can synthesize their own estradiol and it is synaptically active, yet the role of this system and its relationship to circulating estradiol remains poorly understood. Clearly, we must now incorporate the actions of this critically important sex steroid into our conceptual frameworks for the synaptic organization of cerebral cortex and cognitive behavior along with its direct role in the regulation of female reproductive behavior.
In addition, male rodents and primates also express ER α, ER β, and GPER1, but little is known about potential sex differences in the abundance and distributions of these receptors. Most studies that attempted to explore sex effects in these receptors found no significant differences between males and females, but it is unclear whether these negative findings are due to limited sample size or statistical power. It is also worth noting here that androgens can exert their effects on androgen receptors present in the forebrain of rodents and primates. There is currently limited knowledge on how androgens such as testosterone may interact with the effects of estrogen on synaptic plasticity. Furthermore, only a few studies thus far have explored sex differences in cognitive aging altogether. Therefore, understanding the roles of these hormones, their receptors, and their interactions would shed light on potential sex-related differences in the trajectories of cognitive decline.
Estradiol is a powerful mediator of multiple signaling cascades in the brain related to synaptic signaling, actin polymerization with respect to spine dynamics, protein synthesis, calcium balance, energy metabolism, and neuronal survival. These actions are presumably mediated by synaptic and nuclear ER α, ER β, and GPER1. These receptors have different subcellular localization, have various actions (genomic and/or nongenomic), and are linked to unique second messenger pathways that can oppose one another. It is only recently that studies have emerged showing estrogen-induced neuroprotective effects that are mediated selectively by distinct estrogen receptors. Given the wide-ranging effects estrogen has on the body, including effects on female reproductive cancers, it is critical to delineate the actions of each of these receptors and their interactions. Such information will be critical for development of better interventions for cognitive symptoms experienced in menopause that are devoid of adverse effects on peripheral organs. This objective is further complicated by the fact that synaptic ERs and responses to estrogen are altered with age and such alterations vary across brain regions and species. While it seems clear that estrogen depletion can affect these brain regions and the cognitive processes they mediate, such effects are highly variable in women and we do not know the source of such variability. The hormone treatments that have been used thus far for women concentrate largely on circulating estrogen and progesterone levels rather than on receptor dynamics and synaptic regulation, and this focus must shift if we are to develop more successful treatments.
One clear reflection of this problem has emerged from the nonhuman primate model; while cyclical unopposed estrogen treatments led to better synaptic health and cognitive performance in ovariectomized females, chronic estrogen or a combination of estrogen with progesterone did not. Our current hypothesis is that this reflects the important role of cyclical estrogen with respect to receptor dynamics that would be disrupted by chronic levels of estrogen or estrogen combined with progesterone.
Another issue that requires further attention is the differential effects of natural versus surgical menopause. Recent literature suggests that the abrupt loss of estrogens induced by oophorectomy/ovariectomy in humans and nonhuman primates produces a more severe consequence in cognitive and synaptic health than what are seen with the gradual loss of estrogens with natural menopause. In addition, differences in estrogen receptor and aromatase levels have been observed in naturally versus surgically menopausal monkeys. These differences warrant investigation of distinct treatment regimens that are most efficacious for each condition.
In summary, a more detailed appreciation of the signaling cascades activated by estrogen, the distinct role of classical and nonclassical actions for each receptor, and age- and menopause-related alterations of such processes will be required to develop interventions for menopausal women that are both more effective and more easily tailored to individual variations.
GRANTS
This work was supported by National Institute on Aging Grants R37 AG06647 and P01 AG16765 (to J. H. Morrison) and P01 AG16765 (to B. S. McEwen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Y. Hara and E. M. Waters contributed equally to this manuscript.
Address for reprint requests and other correspondence: B. S. McEwen, Harold and Margaret Milliken Hatch Laboratory of Neuroendocrinology, The Rockefeller University, 1230 York Ave., New York, NY 10065 (e-mail: mcewen@mail.rockefeller.edu).
REFERENCES
- 1.Abdelgadir SE, Roselli CE, Choate JV, Resko JA. androgen receptor messenger ribonucleic acid in brains and pituitaries of male rhesus monkeys: studies on distribution, hormonal control, and relationship to luteinizing hormone secretion. Biol Reprod 60: 1251–1256, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci 22: 3608–3614, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad T, Aggarwal K, Pattnaik B, Mukherjee S, Sethi T, Tiwari BK, Kumar M, Micheal A, Mabalirajan U, Ghosh B, Sinha Roy S, Agrawal A. Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis 4: e461, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci 23: 2333–2339, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akama KT, Thompson LI, Milner TA, McEwen BS. Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J Biol Chem 288: 6438–6450, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almey A, Cannell E, Bertram K, Filardo E, Milner TA, Brake WG. Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology 155: 4422–4432, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76: 223–239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey ME, Wang AC, Hao J, Janssen WG, Hara Y, Dumitriu D, Hof PR, Morrison JH. Interactive effects of age and estrogen on cortical neurons: implications for cognitive aging. Neuroscience 191: 148–158, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bali N, Arimoto JM, Iwata N, Lin SW, Zhao L, Brinton RD, Morgan TE, Finch CE. Differential responses of progesterone receptor membrane component-1 (Pgrmc1) and the classical progesterone receptor (Pgr) to 17beta-estradiol and progesterone in hippocampal subregions that support synaptic remodeling and neurogenesis. Endocrinology 153: 759–769, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartus RT, Fleming D, Johnson HR. Aging in the rhesus monkey: debilitating effects on short-term memory. J Gerontol 33: 858–871, 1978. [DOI] [PubMed] [Google Scholar]
- 11.Baxter MG, Roberts MT, Gee NA, Lasley BL, Morrison JH, Rapp PR. Multiple clinically relevant hormone therapy regimens fail to improve cognitive function in aged ovariectomized rhesus monkeys. Neurobiol Aging 34: 1882–1890, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhat RA, Harnish DC, Stevis PE, Lyttle CR, Komm BS. A novel human estrogen receptor beta: identification and functional analysis of additional N-terminal amino acids. J Steroid Biochem Mol Biol 67: 233–240, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci 22: 5840–5847, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaustein JD. Estrogen receptor immunoreactivity in rat brain: rapid effects of estradiol injection. Endocrinology 132: 1218–1224, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Blurton-Jones M, Tuszynski MH. Reactive astrocytes express estrogen receptors in the injured primate brain. J Comp Neurol 433: 115–123, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Blurton-Jones MM, Roberts JA, Tuszynski MH. Estrogen receptor immunoreactivity in the adult primate brain: neuronal distribution and association with p75, trkA, and choline acetyltransferase. J Comp Neurol 405: 529–542, 1999. [PubMed] [Google Scholar]
- 17.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci 29: 15323–15330, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci 27: 9941–9950, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewer GJ, Reichensperger JD, Brinton RD. Prevention of age-related dysregulation of calcium dynamics by estrogen in neurons. Neurobiol Aging 27: 306–317, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Brown TJ, Scherz B, Hochberg RB, MacLusky NJ. Regulation of estrogen receptor concentrations in the rat brain: effects of sustained androgen and estrogen exposure. Neuroendocrinology 63: 53–60, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 84: 4025–4030, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Cai M, Ma YL, Qin P, Li Y, Zhang LX, Nie H, Peng Z, Dong H, Dong HL, Hou WG, Xiong LZ. The loss of estrogen efficacy against cerebral ischemia in aged postmenopausal female mice. Neurosci Lett 558: 115–119, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Calhoun ME, Mao Y, Roberts JA, Rapp PR. Reduction in hippocampal cholinergic innervation is unrelated to recognition memory impairment in aged rhesus monkeys. J Comp Neurol 475: 238–246, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Carson-Jurica MA, Schrader WT, O'Malley BW. Steroid receptor family: structure and functions. Endocr Rev 11: 201–220, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci 26: 7035–7045, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choate JV, Slayden OD, Resko JA. Immunocytochemical localization of androgen receptors in brains of developing and adult male rhesus monkeys. Endocrine 8: 51–60, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Chu S, Fuller PJ. Identification of a splice variant of the rat estrogen receptor beta gene. Mol Cell Endocrinol 132: 195–199, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Conley AJ, Stanczyk FZ, Morrison JH, Borowicz P, Benirschke K, Gee NA, Lasley BL. Modulation of higher-primate adrenal androgen secretion with estrogen-alone or estrogen-plus-progesterone intervention. Menopause 20: 322–328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20: 358–417, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci 21: 6949–6956, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Marco P, Romeo E, Vivacqua A, Malaguarnera R, Abonante S, Romeo F, Pezzi V, Belfiore A, Maggiolini M. GPER1 is regulated by insulin in cancer cells and cancer-associated fibroblasts. Endocr Relat Cancer 21: 739–753, 2014. [DOI] [PubMed] [Google Scholar]
- 32.DonCarlos LL, Monroy E, Morrell JI. Distribution of estrogen receptor-immunoreactive cells in the forebrain of the female guinea pig. J Comp Neurol 305: 591–612, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Ann NY Acad Sci 1204: 104–112, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eberling JL, Reed BR, Coleman JE, Jagust WJ. Effect of estrogen on cerebral glucose metabolism in postmenopausal women. Neurology 55: 875–877, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus 22: 82–91, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab 98: 3829–3838, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA 291: 2959–2968, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Eyles DW, Burne TH, McGrath JJ. Vitemin D, effects on brain development, adult brain function and the links between low levels of vitemin D and neuropsychiatric disease. Front Neuroendocrinol 34: 47–64, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 148: 3236–3245, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Fischer AK, von Rosenstiel P, Fuchs E, Goula D, Almeida OF, Czeh B. The prototypic mineralocorticoid receptor agonist aldosterone influences neurogenesis in the dentate gyrus of the adrenalectomized rat. Brain Res 947: 290–293, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus 22: 656–669, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frankfurt M, Gould E, Wolley C, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology 51: 530–535, 1990. [DOI] [PubMed] [Google Scholar]
- 43.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun 346: 904–910, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience 125: 615–623, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Segura LM, Cardona-Gomez P, Naftolin F, Chowen JA. Estradiol upregulates Bcl-2 expression in adult brain neurons. Neuroreport 9: 593–597, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitemin D functions in the nervous system. Trends Endocrinol Metab 13: 100–105, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Gearing M, Tigges J, Mori H, Mirra SS. A beta40 is a major form of beta-amyloid in nonhuman primates. Neurobiol Aging 17: 903–908, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J Neurosci 21: 5568–5573, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geinisman Y, de Toledo-Morrell L, Morrell F. Aged rats need a preserved complement of perforated axospinous synapses per hippocampal neuron to maintain good spatial memory. Brain Res 398: 266–275, 1986. [DOI] [PubMed] [Google Scholar]
- 51.Geinisman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc Natl Acad Sci USA 83: 3027–3031, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Structural synaptic plasticity associated with the induction of long-term potentiation is preserved in the dentate gyrus of aged rats. Hippocampus 2: 445–456, 1992. [DOI] [PubMed] [Google Scholar]
- 53.Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res 74: 637–643, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Gibbs RB, Hashash A, Johnson DA. Effect of estrogen on potassium-stimulated acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Res 749: 143–146, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod 57: 335–340, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Gill S, Sharpless JL, Rado K, Hall JE. Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab 87: 2290–2296, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez M, Cabrera-Socorro A, Perez-Garcia CG, Fraser JD, Lopez FJ, Alonso R, Meyer G. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol 503: 790–802, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Gould E, Woolley C, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 10: 1286–1291, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green S, Walter P, Greene G, Krust A, Goffin C, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human oestrogen receptor cDNA. J Steroid Biochem 24: 77–83, 1986. [DOI] [PubMed] [Google Scholar]
- 60.Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 72: 1850–1857, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greenough WT, West RW, DeVoogd TJ. Subsynaptic plate perforations: changes with age and experience in the rat. Science 202: 1096–1098, 1978. [DOI] [PubMed] [Google Scholar]
- 62.Guerra-Araiza C, Amorim MA, Pinto-Almazan R, Gonzalez-Arenas A, Campos MG, Garcia-Segura LM. Regulation of the phosphoinositide-3 kinase and mitogen-activated protein kinase signaling pathways by progesterone and its reduced metabolites in the rat brain. J Neurosci Res 87: 470–481, 2009. [DOI] [PubMed] [Google Scholar]
- 63.Gustafsson JA. Estrogen receptor beta–a new dimension in estrogen mechanism of action. J Endocrinol 163: 379–383, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140: 5566–5578, 1999. [DOI] [PubMed] [Google Scholar]
- 65.Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology 36: 182–192, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han X, Aenlle KK, Bean LA, Rani A, Semple-Rowland SL, Kumar A, Foster TC. Role of estrogen receptor alpha and beta in preserving hippocampal function during aging. J Neurosci 33: 2671–2683, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA 104: 11465–11470, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hara Y, Morrison JH. Synaptic correlates of aging and cognitive decline. In: The Synapse: Structure and Function, edited by Pickel VM and Segal M. Amsterdam: Elsevier, 2014, p. 301–342. [Google Scholar]
- 69.Hara Y, Park CS, Janssen WG, Punsoni M, Rapp PR, Morrison JH. Synaptic characteristics of dentate gyrus axonal boutons and their relationships with aging, menopause, and memory in female rhesus monkeys. J Neurosci 31: 7737–7744, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hara Y, Park CS, Janssen WG, Roberts MT, Morrison JH, Rapp PR. Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol Aging 33: 417–428, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hara Y, Punsoni M, Yuk F, Park CS, Janssen WG, Rapp PR, Morrison JH. Synaptic distributions of GluA2 and PKMzeta in the monkey dentate gyrus and their relationships with aging and memory. J Neurosci 32: 7336–7344, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hara Y, Rapp PR, Morrison JH. Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age 34: 1051–1073, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci USA 111: 486–491, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harris KM. How multiple-synapse boutons could preserve input specificity during an interneuronal spread of LTP. Trends Neurosci 18: 365–369, 1995. [DOI] [PubMed] [Google Scholar]
- 75.Hart SA, Patton JD, Woolley CS. Quantitative analysis of ER alpha and GAD colocalization in the hippocampus of the adult female rat. J Comp Neurol 440: 144–155, 2001. [DOI] [PubMed] [Google Scholar]
- 76.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, Abajian C, Beckmann CF, Bernard A, Bertagnolli D, Boe AF, Cartagena PM, Chakravarty MM, Chapin M, Chong J, Dalley RA, Daly BD, Dang C, Datta S, Dee N, Dolbeare TA, Faber V, Feng D, Fowler DR, Goldy J, Gregor BW, Haradon Z, Haynor DR, Hohmann JG, Horvath S, Howard RE, Jeromin A, Jochim JM, Kinnunen M, Lau C, Lazarz ET, Lee C, Lemon TA, Li L, Li Y, Morris JA, Overly CC, Parker PD, Parry SE, Reding M, Royall JJ, Schulkin J, Sequeira PA, Slaughterbeck CR, Smith SC, Sodt AJ, Sunkin SM, Swanson BE, Vawter MP, Williams D, Wohnoutka P, Zielke HR, Geschwind DH, Hof PR, Smith SM, Koch C, Grant SG, Jones AR. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489: 391–399, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hazell GG, Yao ST, Roper JA, Prossnitz ER, O'Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol 202: 223–236, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henderson VW. Cognitive changes after menopause: influence of estrogen. Clin Obstet Gynecol 51: 618–626, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer's disease risk and therapy. Prog Brain Res 182: 77–96, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henderson VW, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Estrogen exposures and memory at midlife: a population-based study of women. Neurology 60: 1369–1371, 2003. [DOI] [PubMed] [Google Scholar]
- 81.Henley JM, Barker EA, Glebov OO. Routes, destinations and delays: recent advances in AMPA receptor trafficking. Trends Neurosci 34: 258–268, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herrick SP, Waters EM, Drake CT, McEwen BS, Milner TA. Extranuclear estrogen receptor beta immunoreactivity is on doublecortin-containing cells in the adult and neonatal rat dentate gyrus. Brain Res 1121: 46–58, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Hidalgo AA, Trump DL, Johnson CS. Glucocorticoid regulation of the vitemin D receptor. J Steroid Biochem Mol Biol 121: 372–375, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Higaki S, Takumi K, Itoh M, Watanabe G, Taya K, Shimizu K, Hayashi M, Oishi T. Response of ERbeta and aromatase expression in the monkey hippocampal formation to ovariectomy and menopause. Neurosci Res 72: 148–154, 2012. [DOI] [PubMed] [Google Scholar]
- 85.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA 101: 865–870, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitemin D receptor is present in caveolae-enriched plasma membranes and binds 1α,25(OH)2-vitemin D3 in vivo and in vitro. Mol Endocrinol 18: 2660–2671, 2004. [DOI] [PubMed] [Google Scholar]
- 87.Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 150: 1722–1730, 2009. [DOI] [PubMed] [Google Scholar]
- 88.Jacobs DM, Tang MX, Stern Y, Sano M, Marder K, Bell KL, Schofield P, Dooneief G, Gurland B, Mayeux R. Cognitive function in nondemented older women who took estrogen after menopause. Neurology 50: 368–373, 1998. [DOI] [PubMed] [Google Scholar]
- 89.Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci 12: 407–414, 2000. [DOI] [PubMed] [Google Scholar]
- 90.Jensen E, Jacobson H. Basic guides to the mechanism of estrogen action. Rec Prog Horm Res 18: 387–408, 1962. [Google Scholar]
- 91.Jensen EV, Suzuki T, Kawashima T, Stumpf WE, Jungblut PW, DeSombre ER. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci USA 59: 632–638, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ji LL, Tong L, Peng JB, Jin XH, Wei D, Xu BK, Wang ZY. Changes in the expression of the vitemin D receptor and LVSCCA1C in the rat hippocampus submitted to single prolonged stress. Mol Med Rep 9: 1165–1170, 2014. [DOI] [PubMed] [Google Scholar]
- 93.Joels M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev 64: 901–938, 2012. [DOI] [PubMed] [Google Scholar]
- 94.Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 13: 411–422, 2006. [DOI] [PubMed] [Google Scholar]
- 95.Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience 136: 289–299, 2005. [DOI] [PubMed] [Google Scholar]
- 96.Johri A, Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther 342: 619–630, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang JH, Grodstein F. Postmenopausal hormone therapy, timing of initiation, APOE and cognitive decline. Neurobiol Aging 33: 1129–1137, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102: 19204–19207, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci 26: 360–368, 2003. [DOI] [PubMed] [Google Scholar]
- 100.Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen's effect on cognition. Psychoneuroendocrinology 26: 577–590, 2001. [DOI] [PubMed] [Google Scholar]
- 101.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab 12: 152–156, 2001. [DOI] [PubMed] [Google Scholar]
- 102.Kelly MJ, Moss RL, Dudley CA, Fawcett CP. The specificity of the response of preoptic-septal area neurons to estrogen: 17α-estradiol versus 17β-estradiol and the response of extrahypothalamic neurons. Exp Brain Res 30: 43–52, 1977. [DOI] [PubMed] [Google Scholar]
- 103.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron 61: 340–350, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keuker JI, Luiten PG, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging 24: 157–165, 2003. [DOI] [PubMed] [Google Scholar]
- 105.Kimura N, Tanemura K, Nakamura S, Takashima A, Ono F, Sakakibara I, Ishii Y, Kyuwa S, Yoshikawa Y. Age-related changes of Alzheimer's disease-associated proteins in cynomolgus monkey brains. Biochem Biophys Res Commun 310: 303–311, 2003. [DOI] [PubMed] [Google Scholar]
- 106.Kok HS, Kuh D, Cooper R, van der Schouw YT, Grobbee DE, Wadsworth ME, Richards M. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause 13: 19–27, 2006. [DOI] [PubMed] [Google Scholar]
- 107.Korse CM, Bonfrer JM, van Beurden M, Verheijen RH, Rookus MA. Estradiol and testosterone levels are lower after oophorectomy than after natural menopause. Tumour Biol 30: 37–42, 2009. [DOI] [PubMed] [Google Scholar]
- 108.Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. J Neurosci 29: 12982–12993, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138: 863–870, 1997. [DOI] [PubMed] [Google Scholar]
- 110.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93: 5925–5930, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. A critical review of fundamental controversies in the field of GPR30 research. Steroids 75: 603–610, 2010. [DOI] [PubMed] [Google Scholar]
- 112.Langub MC, Herman JP, Malluche HH, Koszewski NJ. Evidence of functional vitemin D receptors in rat hippocampus. Neuroscience 104: 49–56, 2001. [DOI] [PubMed] [Google Scholar]
- 113.Lasley BL, Chen J, Stanczyk FZ, El Khoudary SR, Gee NA, Crawford S, McConnell DS. androstenediol complements estrogenic bioactivity during the menopausal transition. Menopause 19: 650–657, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lauber AH, Mobbs CV, Muramatsu M, Pfaff DW. Estrogen receptor messenger RNA expression in rat hypothalamus as a function of genetic sex and estrogen dose. Endocrinology 129: 3180–3186, 1991. [DOI] [PubMed] [Google Scholar]
- 115.Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J Neurosci 29: 1457–1468, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li C, Brake WG, Romeo RC, Dunlop JC, Gordon m Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen treatment alters hippocampal dendritic spine shape, enhances synaptic protein immunoreactivity and performance in a spatial working memory task in female C57Bl/6J mice. Proc Natl Acad Sci USA 101: 2185–2190, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li S, Kang L, Zhang C, Xie G, Li N, Zhang Y, Du J, Cui H. Effects of dihydrotestosterone on synaptic plasticity of hippocampus in male SAMP8 mice. Exp Gerontol 48: 778–785, 2013. [DOI] [PubMed] [Google Scholar]
- 118.Liu B, Arbogast LA. Gene expression profiles of intracellular and membrane progesterone receptor isoforms in the mediobasal hypothalamus during pro-oestrus. J Neuroendocrinol 21: 993–1000, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu X, Hajnoczky G. Altered fusion dynamics underlie unique morphological changes in mitochondria during hypoxia-reoxygenation stress. Cell Death Differ 18: 1561–1572, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Loerch PM, Lu T, Dakin KA, Vann JM, Isaacs A, Geula C, Wang J, Pan Y, Gabuzda DH, Li C, Prolla TA, Yankner BA. Evolution of the aging brain transcriptome and synaptic regulation. PLoS One 3: e3329, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Loy R, Gerlach J, McEwen BS. Autoradiographic localization of estradiol-binding neurons in rat hippocampal formation and entorhinal cortex. Dev Brain Res 39: 245–251, 1988. [DOI] [PubMed] [Google Scholar]
- 122.Luebke J, Barbas H, Peters A. Effects of normal aging on prefrontal area 46 in the rhesus monkey. Brain Res Rev 62: 212–232, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Luine VN, Khylchevcskaya RI, McEwen BS. Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain Res 86: 293–306, 1975. [DOI] [PubMed] [Google Scholar]
- 124.MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, Stocks NP, Taylor AW. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause 13: 28–36, 2006. [DOI] [PubMed] [Google Scholar]
- 125.Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause 20: 695–709, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology 69: 1322–1330, 2007. [DOI] [PubMed] [Google Scholar]
- 127.Makris N, Kennedy DN, Boriel DL, Rosene DL. Methods of MRI-based structural imaging in the aging monkey. Methods 50: 166–177, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matsuda K, Sakamoto H, Mori H, Hosokawa K, Kawamura A, Itose M, Nishi M, Prossnitz ER, Kawata M. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett 441: 94–99, 2008. [DOI] [PubMed] [Google Scholar]
- 129.Matt DW, Kauma SW, Pincus SM, Veldhuis JD, Evans WS. Characteristics of luteinizing hormone secretion in younger versus older premenopausal women. Am J Obstet Gynecol 178: 504–510, 1998. [DOI] [PubMed] [Google Scholar]
- 130.Matthews K, Cauley J, Yaffe K, Zmuda JM. Estrogen replacement therapy and cognitive decline in older community women. J Am Geriatr Soc 47: 518–523, 1999. [DOI] [PubMed] [Google Scholar]
- 131.McConnell DS, Stanczyk FZ, Sowers MR, Randolph JF Jr, Lasley BL. Menopausal transition stage-specific changes in circulating adrenal androgens. Menopause 19: 658–663, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci 23: 8701–8705, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev 20: 279–307, 1999. [DOI] [PubMed] [Google Scholar]
- 134.McEwen BS, Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev 55: 343–355, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134a.McEwen BS, Schmeck HM Jr. The Hostage Brain. New York: Rockefeller Univ. Press, 1994. [Google Scholar]
- 135.McLachlan RI, Tempel BL, Miller MA, Bicknell JN, Bremner WJ, Dorsa DM. androgen receptor gene expression in the rat central nervous system: evidence for two mRNA transcripts. Mol Cell Neurosci 2: 117–122, 1991. [DOI] [PubMed] [Google Scholar]
- 136.Meffre D, Labombarda F, Delespierre B, Chastre A, De Nicola AF, Stein DG, Schumacher M, Guennoun R. Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience 231: 111–124, 2013. [DOI] [PubMed] [Google Scholar]
- 137.Meyer K, Korz V. Age dependent differences in the regulation of hippocampal steroid hormones and receptor genes: relations to motivation and cognition in male rats. Horm Behav 63: 376–384, 2013. [DOI] [PubMed] [Google Scholar]
- 138.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J 17: 2008–2018, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol 491: 81–95, 2005. [DOI] [PubMed] [Google Scholar]
- 140.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol 429: 355–371, 2001. [PubMed] [Google Scholar]
- 141.Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature 273: 297–298, 1978. [DOI] [PubMed] [Google Scholar]
- 142.Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol 518: 2729–2743, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Montague D, Weickert CS, Tomaskovic-Crook E, Rothmond DA, Kleinman JE, Rubinow DR. Oestrogen receptor alpha localisation in the prefrontal cortex of three mammalian species. J Neuroendocrinol 20: 893–903, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, Su JL, Kliewer SA, Lehmann JM, Willson TM. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun 247: 75–78, 1998. [DOI] [PubMed] [Google Scholar]
- 145.Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci 13: 240–250, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science 278: 412–419, 1997. [DOI] [PubMed] [Google Scholar]
- 147.Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, Morrison JH, Janssen WG, Rose JA, Chambon P, Kato S, Izumi S, Yamazaki T, Kimoto T, Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem 100: 950–967, 2007. [DOI] [PubMed] [Google Scholar]
- 148.Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front Neuroendocrinol 35: 180–196, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nagahara AH, Bernot T, Tuszynski MH. Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiol Aging 31: 1020–1031, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nakamura NH, McEwen BS. Changes in interneuronal phenotypes regulated by estradiol in the adult rat hippocampus: a potential role for neuropeptide Y. Neuroscience 136: 357–369, 2005. [DOI] [PubMed] [Google Scholar]
- 151.Naugle MM, Nguyen LT, Merceron TK, Filardo E, Janssen WG, Morrison JH, Rapp PR, Gore AC. G-protein coupled estrogen receptor, estrogen receptor alpha, and progesterone receptor immunohistochemistry in the hypothalamus of aging female rhesus macaques given long-term estradiol treatment. J Exp Zool A Ecol Genet Physiol 321: 399–414, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM, Kubisch HM. Ovarian senescence in the rhesus monkey (Macaca mulatta). Hum Reprod 20: 79–83, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci USA 100: 10506–10511, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nilsen J, Diaz Brinton R. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci USA 100: 2842–2847, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nilsen J, Irwin RW, Gallaher TK, Diaz Brinton R. Estradiol in vivo regulation of brain mitochondrial proteome. J Neurosci 27: 14069–14077, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol 23: 349–359, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.O'Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol 21: 1281–1296, 2007. [DOI] [PubMed] [Google Scholar]
- 158.Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem Biophys Res Commun 243: 122–126, 1998. [DOI] [PubMed] [Google Scholar]
- 159.Ohm DT, Bloss EB, Janssen WG, Dietz KC, Wadsworth S, Lou W, Gee NA, Lasley BL, Rapp PR, Morrison JH. Clinically relevant hormone treatments fail to induce spinogenesis in prefrontal cortex of aged female rhesus monkeys. J Neurosci 32: 11700–11705, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Olde B, Leeb-Lundberg LM. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab 20: 409–416, 2009. [DOI] [PubMed] [Google Scholar]
- 161.Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocrinol Metab 85: 3840–3846, 2000. [DOI] [PubMed] [Google Scholar]
- 162.Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience 95: 333–342, 2000. [DOI] [PubMed] [Google Scholar]
- 163.Patisaul HB, Whitten PL, Young LJ. Regulation of estrogen receptor beta mRNA in the brain: opposite effects of 17beta-estradiol and the phytoestrogen, coumestrol. Brain Res 67: 165–171, 1999. [DOI] [PubMed] [Google Scholar]
- 164.Pau CY, Pau KY, Spies HG. Putative estrogen receptor beta and alpha mRNA expression in male and female rhesus macaques. Mol Cell Endocrinol 146: 59–68, 1998. [DOI] [PubMed] [Google Scholar]
- 165.Peters A, Jones EG, Morrison JH. Cerebral Cortex: Neurodegenerative and Age-Related Changes in Structure and Function of Cerebral Cortex. New York: Springer, 1999, p. 796. [Google Scholar]
- 166.Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol 55: 861–874, 1996. [DOI] [PubMed] [Google Scholar]
- 167.Petersen SL, Intlekofer KA, Moura-Conlon PJ, Brewer DN, Del Pino Sans J, Lopez JA. Novel progesterone receptors: neural localization and possible functions. Front Neurosci 7: 164, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci 11: 1011–1036, 1999. [DOI] [PubMed] [Google Scholar]
- 169.Pfaff DW. Estrogens and Brain Function. New York: Springer-Verlag, 1980. [Google Scholar]
- 170.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology 17: 485–495, 1992. [DOI] [PubMed] [Google Scholar]
- 171.Prager EM, Brielmaier J, Bergstrom HC, McGuire J, Johnson LR. Localization of mineralocorticoid receptors at mammalian synapses. PLoS One 5: e14344, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Prossnitz ER, Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. Mol Cell Endocrinol 389: 71–83, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Quadros PS, Pfau JL, Wagner CK. Distribution of progesterone receptor immunoreactivity in the fetal and neonatal rat forebrain. J Comp Neurol 504: 42–56, 2007. [DOI] [PubMed] [Google Scholar]
- 174.Rapp PR. Emotion, Memory and Behavior: Studies on Human and Nonhuman Primates. Tokyo: Japan Scientific Societies Press, 1995. [Google Scholar]
- 175.Rapp PR, Amaral DG. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol Aging 12: 481–486, 1991. [DOI] [PubMed] [Google Scholar]
- 176.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized Rhesus monkeys. J Neurosci 23: 5708–5714, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Register TC, Shively CA, Lewis CE. Expression of estrogen receptor alpha and beta transcripts in female monkey hippocampus and hypothalamus. Brain Res 788: 320–322, 1998. [DOI] [PubMed] [Google Scholar]
- 178.Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab 91: 1802–1810, 2006. [DOI] [PubMed] [Google Scholar]
- 179.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117: 2505–2511, 1985. [DOI] [PubMed] [Google Scholar]
- 180.Roberts JA, Gilardi KV, Lasley B, Rapp PR. Reproductive senescence predicts cognitive decline in aged female monkeys. Neuroreport 8: 2047–2051, 1997. [DOI] [PubMed] [Google Scholar]
- 181.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de andrade M, Melton LJ 3rd. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69: 1074–1083, 2007. [DOI] [PubMed] [Google Scholar]
- 182.Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced N-methyl-d-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrinology 81: 391–399, 2005. [DOI] [PubMed] [Google Scholar]
- 183.Roselli CE, Klosterman S, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J Comp Neurol 439: 208–223, 2001. [DOI] [PubMed] [Google Scholar]
- 184.Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci 23: 4479–4490, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sarrieau A, Mitchell JB, Lal S, Olivier A, Quirion R, Meaney MJ. androgen binding sites in human temporal cortex. Neuroendocrinology 51: 713–716, 1990. [DOI] [PubMed] [Google Scholar]
- 186.Shamy JL, Buonocore MH, Makaron LM, Amaral DG, Barnes CA, Rapp PR. Hippocampal volume is preserved and fails to predict recognition memory impairment in aged rhesus monkeys (Macaca mulatta). Neurobiol Aging 27: 1405–1415, 2006. [DOI] [PubMed] [Google Scholar]
- 187.Shamy JL, Habeck C, Hof PR, Amaral DG, Fong SG, Buonocore MH, Stern Y, Barnes CA, Rapp PR. Volumetric correlates of spatiotemporal working and recognition memory impairment in aged rhesus monkeys. Cereb Cortex 21: 1559–1573, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sharma PK, Thakur MK. Expression of estrogen receptor (ER) alpha and beta in mouse cerebral cortex: effect of age, sex and gonadal steroids. Neurobiol Aging 27: 880–887, 2006. [DOI] [PubMed] [Google Scholar]
- 189.Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA 281: 1197–1202, 1999. [DOI] [PubMed] [Google Scholar]
- 190.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology 13: 345–357, 1988. [DOI] [PubMed] [Google Scholar]
- 191.Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol 5: 620–627, 2009. [DOI] [PubMed] [Google Scholar]
- 192.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388: 507–525, 1997. [DOI] [PubMed] [Google Scholar]
- 193.Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology 139: 5267–5270, 1998. [DOI] [PubMed] [Google Scholar]
- 194.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN 3rd Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289: 2651–2662, 2003. [DOI] [PubMed] [Google Scholar]
- 195.Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia 56: 659–674, 2008. [DOI] [PubMed] [Google Scholar]
- 196.Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribonucleic acid in rat hypothalamus by sex steroid hormones. Mol Endocrinol 5: 424–432, 1991. [DOI] [PubMed] [Google Scholar]
- 197.Simpkins JW, Yang SH, Sarkar SN, Pearce V. Estrogen actions on mitochondria–physiological and pathological implications. Mol Cell Endocrinol 290: 51–59, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine 14: 407–415, 2001. [DOI] [PubMed] [Google Scholar]
- 199.Skucas VA, Duffy AM, Harte-Hargrove LC, Magagna-Poveda A, Radman T, Chakraborty G, Schroeder CE, MacLusky NJ, Scharfman HE. Testosterone depletion in adult male rats increases mossy fiber transmission, LTP, and sprouting in area CA3 of hippocampus. J Neurosci 33: 2338–2355, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci USA 101: 7181–7186, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience 202: 131–146, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Spencer-Segal JL, Waters EM, Bath KG, Chao MV, McEwen BS, Milner TA. Distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. J Neurosci 31: 6780–6790, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Spencer JL, Waters EM, Milner TA, Lee FS, McEwen BS. BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc Natl Acad Sci USA 107: 4395–4400, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience 155: 1106–1119, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol 29: 219–237, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science 253: 1380–1386, 1991. [DOI] [PubMed] [Google Scholar]
- 207.Squire LR, Zola-Morgan S, Chen KS. Human amnesia and animal models of amnesia: performance of amnesic patients on tests designed for the monkey. Behav Neurosci 102: 210–221, 1988. [DOI] [PubMed] [Google Scholar]
- 208.Srivastava DP, Evans PD. G-protein oestrogen receptor 1: trials and tribulations of a membrane oestrogen receptor. J Neuroendocrinol 25: 1219–1230, 2013. [DOI] [PubMed] [Google Scholar]
- 209.Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience 130: 151–163, 2005. [DOI] [PubMed] [Google Scholar]
- 210.Tang AC, Reeb-Sutherland BC, Yang Z, Romeo RD, McEwen BS. Neonatal novelty-induced persistent enhancement in offspring spatial memory and the modulatory role of maternal self-stress regulation. J Neurosci 31: 5348–5352, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tasker JG, Di S, Malcher-Lopes R. Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology 147: 5549–5556, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402: 421–425, 1999. [DOI] [PubMed] [Google Scholar]
- 213.Toni N, Buchs PA, Nikonenko I, Povilaitite P, Parisi L, Muller D. Remodeling of synaptic membranes after induction of long-term potentiation. J Neurosci 21: 6245–6251, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor-α in the dorsal hippocampus. J Comp Neurol 463: 390–401, 2003. [DOI] [PubMed] [Google Scholar]
- 215.Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor-alpha in the dorsal hippocampus. J Comp Neurol 463: 390–401, 2003. [DOI] [PubMed] [Google Scholar]
- 216.Tremblay A, Tremblay GB, Labrie F, Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell 3: 513–519, 1999. [DOI] [PubMed] [Google Scholar]
- 217.Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol 11: 353–365, 1997. [DOI] [PubMed] [Google Scholar]
- 218.Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63: 451–486, 1994. [DOI] [PubMed] [Google Scholar]
- 219.Verghese J, Kuslansky G, Katz MJ, Sliwinski M, Crystal HA, Buschke H, Lipton RB. Cognitive performance in surgically menopausal women on estrogen. Neurology 55: 872–874, 2000. [DOI] [PubMed] [Google Scholar]
- 220.Voytko ML, Murray R, Higgs CJ. Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J Neurosci 29: 10362–10370, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Walker ML, Herndon JG. Menopause in nonhuman primates? Biol Reprod 79: 398–406, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci 30: 12770–12776, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang AC, Janssen WG, Morrison JH. Aged female rhesus monkeys retain synaptic estrogen receptor-α (ER-α) in hippocampus equal to that of young monkeys. Abstr Soc Neurosci 2008. [Google Scholar]
- 224.Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res 1290: 1–11, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, Clegg DJ, Gorecka J, Akama KT, McEwen BS, Milner TA. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci 35: 2384–2397, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Waters EM, Torres-Reveron A, McEwen BS, Milner TA. Ultrastructural localization of extranuclear progestin receptors in the rat hippocampal formation. J Comp Neurol 511: 34–46, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res 1379: 86–97, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-d-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology 131: 662–668, 1992. [DOI] [PubMed] [Google Scholar]
- 229.Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol 388: 603–612, 1997. [DOI] [PubMed] [Google Scholar]
- 230.Williams TJ, Mitterling KL, Thompson LI, Torres-Reveron A, Waters EM, McEwen BS, Gore AC, Milner TA. Age- and hormone-regulation of opioid peptides and synaptic proteins in the rat dorsal hippocampal formation. Brain Res 1379: 71–85, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev 123: 593–601, 2002. [DOI] [PubMed] [Google Scholar]
- 232.Wilson ME, Westberry JM, Trout AL. Estrogen receptor-alpha gene expression in the cortex: sex differences during development and in adulthood. Horm Behav 59: 353–357, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Witter MP, Van Hoesen GW, Amaral DG. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci 9: 216–228, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Woller MJ, Everson-Binotto G, Nichols E, Acheson A, Keen KL, Bowers CY, Terasawa E. Aging-related changes in release of growth hormone and luteinizing hormone in female rhesus monkeys. J Clin Endocrinol Metab 87: 5160–5167, 2002. [DOI] [PubMed] [Google Scholar]
- 235.Woolley C, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci 10: 4035–4039, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Woolley C, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci 12: 2549–2554, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Woolley C, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-d-aspartate receptor dependent mechanism. J Neurosci 14: 7680–7687, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336: 293–306, 1993. [DOI] [PubMed] [Google Scholar]
- 239.Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci 17: 1848–1859, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wu D, Gore AC. Changes in androgen receptor, estrogen receptor alpha, and sexual behavior with aging and testosterone in male rats. Horm Behav 58: 306–316, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yaffe K, Vittinghoff E, Ensrud KE, Johnson KC, Diem S, Hanes V, Grady D. Effects of ultra-low-dose transdermal estradiol on cognition and health-related quality of life. Arch Neurol 63: 945–950, 2006. [DOI] [PubMed] [Google Scholar]
- 242.Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, Morrison JH. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res 1209: 115–127, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-beta mRNA in the female rat brain. Brain Res 1155: 34–41, 2007. [DOI] [PubMed] [Google Scholar]
- 244.Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci USA 101: 4130–4135, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci USA 98: 3525–3530, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci USA 107: 12687–12691, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yoshiya M, Komatsuzaki Y, Hojo Y, Ikeda M, Mukai H, Hatanaka Y, Murakami G, Kawata M, Kimoto T, Kawato S. Corticosterone rapidly increases thorns of CA3 neurons via synaptic/extranuclear glucocorticoid receptor in rat hippocampus. Front Neural Circuits 7: 191, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, stress. Science 337: 1062–1065, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Young ME, Ohm DT, Janssen WG, Gee NA, Lasley BL, Morrison JH. Continuously delivered ovarian steroids do not alter dendritic spine density or morphology in macaque dorsolateral prefrontal cortical neurons. Neuroscience 255: 219–225, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yuen GS, McEwen BS, Akama KT. LIM kinase mediates estrogen action on the actin depolymerization factor Cofilin. Brain Res 1379: 44–52, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, Brann DW. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci USA 108: E617–624, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zigmond RE, McEwen BS. Selective retention of oestradiol by cell nuclei in specific brain regions of the ovariectomized rat. J Neurochem 17: 889–899, 1970. [DOI] [PubMed] [Google Scholar]
- 253.Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal Ca1 dendrites. J Neurosci 23: 2340–2347, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zuloaga DG, Yahn SL, Pang Y, Quihuis AM, Oyola MG, Reyna A, Thomas P, Handa RJ, Mani SK. Distribution and estrogen regulation of membrane progesterone receptor-beta in the female rat brain. Endocrinology 153: 4432–4443, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]