Abstract
Over the past five decades, liver cirrhosis has become an increasingly prevalent disease and one that will often require considerable medical intervention. However, current treatment options have demonstrated severe problems that have prompted research to provide a suitable alternative. These treatments are scarcely available, very expensive and present at a huge cost to the patient's quality of life. The introduction of stem cell therapy into liver disease has been heralded as the future of personalized medicine and may be the alternative that the healthcare system desperately seeks.
To truly determine the scientific basis surrounding this excitement, a literature search was carried out in January 2013 to determine all the data that was present in this topic area. All articles also underwent full cross-referencing to ensure no data was missed.
11 clinical trials were found to meet this criteria and trials were included in both English and non-English languages. The sporadic nature of the data across the trials, with various methods and stem cell types, made comparisons difficult.
The basic trends from the data were positive and the majority deemed the use of stem cells safe and feasible in patients presenting with cirrhotic liver disease. However, there is a clear requirement for more research, not only to determine the most efficacious technique and stem cell type but also to further understand stem cells to enhance progress. There may also be a requirement for a framework that future stem cell trials can be based on, which would allow future data to be comparative and allow valid conclusions to be drawn which may propel this therapy into standard clinical practice.
Keywords: liver cirrhosis, stem cell therapy, haematopoetic stem cells, mesenchymal stem cells, hepatic progenitor cells
Abbreviations: HSC, Hematopoietic stem cell; MSC, Mesenchymal Stem cell; hHPC, Human Hepatic Progenitor cell; MNC, Mononuclear Stem cell; G-CSF, Granulocyte colony stimulating factor
In the past 50 years there has been a marked increase in the incidence and mortality of liver cirrhosis. As with any complex and advanced disease state, the incidence of severe associated complications has also risen. This has been attributed to an increase in alcohol abuse and non-alcoholic fatty liver disease in western culture and primarily be attributed to viral infection in eastern societies. Consequently, in 2011, liver cirrhosis accounted for over 33,500 deaths each year in the USA.1 Liver cirrhosis is also a major risk factor for Hepatocellular Carcinoma, which accounted for 30,000 new cases with 22,000 deaths in USA in the past year.2 The shocking nature of these figures shows the scale of the problem at hand and the requirement for a solution.
From a pathophysiological point of view, liver cirrhosis occurs as a progression of liver fibrosis, when the initial injury continues to persist.3 Liver fibrosis is defined as the initial distortion of hepatic architecture. The accumulation of excessive collagen and extracellular matrix proteins is the primary cause of this change.4
Hepatocytes within a cirrhotic liver still have the ability to regenerate but this mismatch of regeneration and fibrosis is responsible for the clinical and biochemical dysfunction of the liver. It is questionable whether increasing the number of hepatocytes alone would have a positive benefit to the patients. This would not address the problem of the altered architecture and fibrotic tissue will still be present in vast quantities. A potential hypothesis is that a fully functioning compartment will be created through the proliferation of the infused stem cells allowing the return of liver function. This sounds promising to allow the medical community to consider pursuing this novel treatment option.
This review aims to highlight all current available evidence regarding the use of stem cell therapy in the treatment of liver cirrhosis and determine whether there is any factual basis for this excitement surrounding their potential.
Current treatment
Currently the only proven, effective and therefore recommended treatment of end stage liver disease is liver transplantation5 which would require the donation of a healthy organ from either a living or cadaveric donor.
This treatment option presents with its own set of problems; firstly, it is expensive6 ‘estimated at $150,000 or more during the first year following transplantationʼ. The most critical issue however is the marked shortage of donor organs available. This problem has been experienced globally and has led to high patient mortality.7 Post-operatively, lifelong immunosuppression therapy is required to reduce the risk of rejection, compromising the patients’ immune system. Long-term renal, cardiovascular and infective complications can occur as well as post-transplant lympho-proliferative diseases.8 This expansive list of problems of the therapy highlights the advantageous nature of an alternative. The problems aren't just limited to the transplantation itself, as a knock on effect of the long waiting list and the critical condition of many patients, there may be a requirement for intensive supportive care and treatment to be maintained, either as palliative care or as bridging therapy to transplantation. These treatments come at a huge cost, not only to the health systems but also to the individuals.
With a niche in the market so blatantly visible, a surge in research is into alternative treatment for liver disease makes logical sense. The alternative treatment should not only be less invasive but also not generate the immune response commonly associated with transplantation.9 To fully counteract the massive problems associated with current therapy, the requirement for the treatment to be readily available and economically affordable is one that must ideally be addressed. Hence, in theory, the use of stem cell therapy may be a viable future option and if fully harnessed could change the whole face of the treatment of liver disease.
Stem cells
Over the years of research, numerous types of stem cells have been identified. Each cell presents with a unique list o merits and capabilities but the disadvantages have been the main driving factor in determining the frequency of their use. As a broad entity, stem cells are defined as clonogenic undifferentiated cells, which cannot only self-renew indefinitely but can also differentiate into a variety of cell lineages.10 These properties have raised excitement within the medical community, stem cells have been heralded as the future of personalized medicine and biological insurance for humans.
A stem cell's capabilities are mainly classified by their differentiative potential. Totipotent stem cells have the greatest range of differentiative capability, being able to transform into any cell type as well as forming the trophoblast.11 However, it isn't possible nor necessary to extract a cell with such capabilities. Pluripotent stem cells are unable to form the trophoblast but are able to form any cell type from all three blastodermic layers12 and these types of cells are available, if not commonly used. Multipotent stem cells are most commonly found in adult humans. However, their differentiative capabilities are limited to one germ line and are primarily used to replace damaged tissue within the human body.8 The different types of stem cells that have currently been discovered for use are listed in Table 1.
Table 1.
Different Types of Stem Cells and their Differentiation Potential.
| Type of stem cell | Source | Differentiation potential |
|---|---|---|
| Embryonic | Human embryos | Pluripotent |
| Induced pluripotent | Reprogramming human somatic cells | Pluripotent |
| Hematopoietic | Bone marrow | Multipotent |
| Mesenchymal | Bone marrow | Multipotent |
| Hepatic progenitor | Human umbilical cord blood | Multipotent |
| Endothelial progenitor | Bone marrow | Multipotent |
As previously mentioned, the pitfalls of some of these stem cells is so severe that their use cannot be licensed and consequently only a handful of the stem cell types mentioned have been permitted for human use:
Hematopoietic stem cells (HSC) have been most routinely used in investigative stem cell therapy trials. While classically derived from bone marrow, it has been shown that these cells can be obtained from both umbilical and cytokine-mobilized blood.9 These use of these cells is not a novel approach, they have been used in the treatment of blood disorders for almost three decades.13
Even though these cells are thought to be limited to cell lines within the hematopoietic system, the results of animal trials has highlighted their ability to differentiate into other lines, most relevantly into hepatocytes.14–16 This strong pre-clinical evidence base has allowed the approval of these cells in cirrhotic patients.
Mesenchymal stem cells (MSC) are an alternative, multipotent, adult stem cell source that were initially derived from bone marrow stroma. As research progressed, menstrual blood and endometrium were found to be newer, potentially less invasive sources.17 Russo and Parola18 describe them as ʻplate-adhering, fibroblast-like cells possessing self-renewal ability with the capacity to differentiate into multiple mesenchymal cell lineagesʼ. To allow their use in liver disease patients, in vitro evidence has been presented showing the cells ability to differentiate into hepatocyte-resembling cells.19
Hepatic Progenitor cells (HPC) are found naturally and replicate specifically in the liver. However, the mechanism of this action is not fully understood20 but it has been proven that the cell population increases proportionally to the severity of the disease progression.21 Since these cells can be derived from human umbilical cord blood, its transplantation has been suggested as a treatment option to counteract liver failure.22 A major pitfall appears as evidence shows that excessive HPC replication can lead to Hepatocellular Carcinoma21 making its use unethical. The argument presented against this case is that, in severe liver injury, the natural HPC population is insufficient to allow regeneration and consequently, infusion of these cells may allow a greater degree of regeneration to occur. These theories need to be tested in both animal and human models before they can be taken further.
Methods
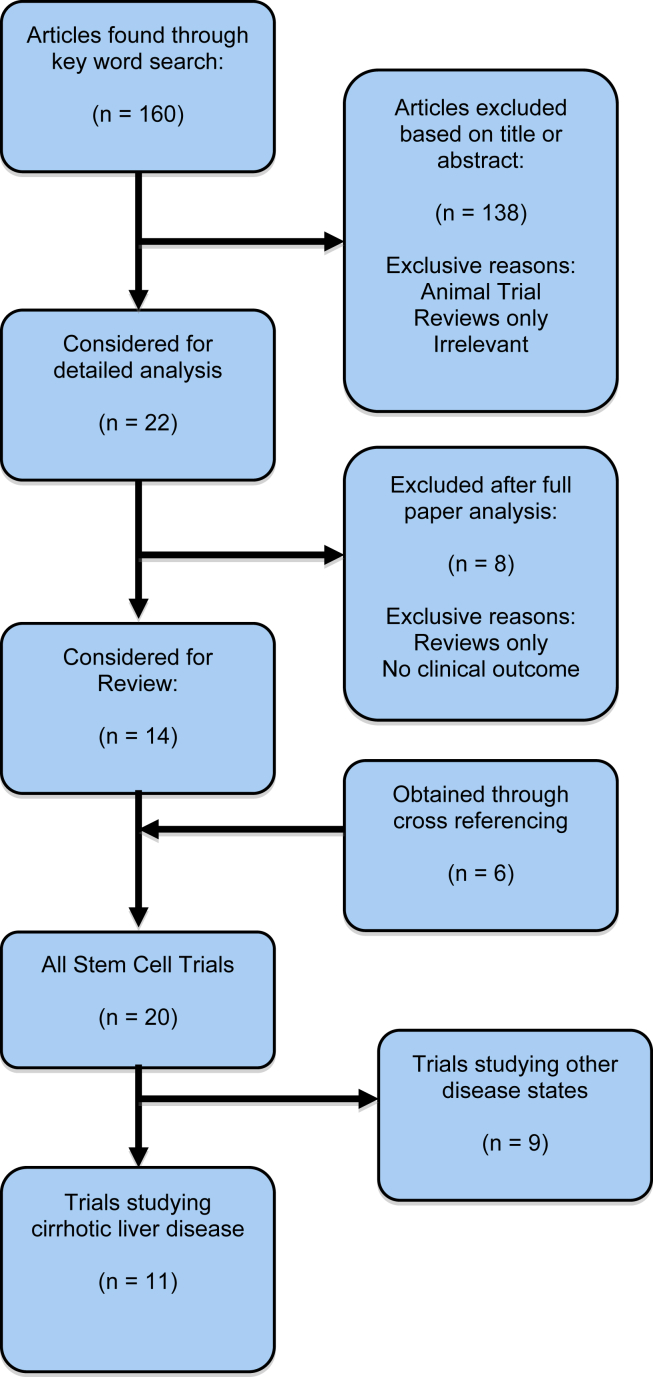
To obtain primary data, a literature search was carried out on PubMed in January 2013. All articles obtained were cross-referenced and studies in both English and non-English languages were included. An initial search was carried out to determine all evidence of stem cell therapy in the treatment of liver disease and this was later narrowed to only those studies focusing on Cirrhotic Liver disease (Figure 1).
Figure 1.
Search strategy.
Results
11 clinical trials3,23–32 were found to investigate the effects on stem cell therapy in the treatment of liver cirrhosis. Not all trials studied the same cirrhotic etiologies with some applying more strict inclusion criteria. There was also great variation in the methodology applied and the type of stem cell used across the studies. The list of the studies is shown in Table 2 highlighting the various different approaches taken by the authors.
Table 2.
Clinical Trials Assessing Cirrhotic Liver Disease.
| Study | Author year | Sample size | Methodology | Stem cell | Author conclusions |
|---|---|---|---|---|---|
| 1 | Gaia et al,23 2006 | Active Treatment: 8 (5 male) Healthy donors: 40 |
Mobilization using G-CSF | HSC | Safe and feasible. Increase in cell count with potential regeneration |
| 2 | Khan et al,29 2010 | Active Treatment: 4 | Infusion of human fetal stem cells | hHPC | Significance decrease in patient MELD score. may be used as supportive treatment |
| 3 | Terai et al,24 2006 | Active Treatment: 9 (8 male) | Infusion of BM taken from ileum | MNC | Improvement of liver function |
| 4 | Kharaziha et al,28 2009 | Active Treatment: 8 (4 male) | Infusion of BM taken from iliac spine | MSC | No increase in morbidity or mortality. May improve liver function |
| 5 | Mohamadnejad et al,25 2007 | Active Treatment: 4 (1 male) | Infusion of BM taken from iliac crest | MSC | Safe and feasible. Some improvement in liver function |
| 6 | Mohamadnejad et al,26 2007 | Active Treatment: 4 (2 male) | Infusion of BM taken from iliac crest | HSC | Infusion not safe through hepatic artery due to side effects |
| 7 | Nikeghbalian et al,31 2011 | HSC Treatment: 3 (1 male) MNC Treatment: 3 (2 male) |
Infusion of BM taken from iliac crest | HSC MNC |
Safe and feasible. No significant difference between HSC and MNC |
| 8 | Lin et al,32 2012 | Active Treatment: 38 Placebo Treatment: 16 |
Infusion of cells derived from umbilical cord | MSC | Safe and has potential to improve quality of life of patient |
| 9 | Zhang et al,3 2012 | Active Treatment: 30 Placebo Treatment: 15 | Infusion of cells derived from umbilical cord | MSC | Safe and feasible. Can improve liver function |
| 10 | Salama et al,30 2010 | Active Treatment: 90 (78 male) Placebo Treatment: 50 (38 male) |
Mobilization using G-CSF followed by infusion from iliac crest | HSC | Safe and tolerated. HSC transplantation can be used as supportive treatment. |
| 11 | Pai et al,27 2008 | Active Treatment: 9 (6 male) | Mobilization using G-CSF followed by infusion | HSC | Safe and feasible. Improvement in liver function |
Only one trial23 mobilized HSCs using Granulocyte colony stimulating factor (G-CSF). Two furthers trials27,30 also mobilized the bone marrow stem cells using G-CSF, extracted the stem cells from the patient before re-infusing into the hepatic system. The most adhered method (Five trials24–26,28,31) employed was the extraction of stem cells from the ileum without any prior manipulation prior to re-infusion into the hepatic system. There were two instances3,32 of MSC extraction directly from umbilical cord blood prior to re-infusion. Only one trial29 extracted human HPC cells from aborted fetuses before transplanting them into cirrhotic patients.
Discussion
Compared to other therapies, 11 clinical trials would not be classified as a strong evidence base. However, due to the very small amount of primary data in the utilization of stem cell therapy in the treatment of liver, comparatively the evidence concerning liver cirrhosis can be seen as strong. This interest could potentially suggest that this may be the most likely etiology for the advancement of stem cell therapy.
There is evidence of a variety of methods utilizing different stem cell types across the trials. However, the representation of the evidence is unevenly distributed in both quality and quantity. This makes comparisons difficult between and consequently a valid conclusion cannot be drawn regarding the approach that would be deemed most efficacious.
There is also a low quality of evidence present as the majority of the trial fall under safety trials. Only study 1030 can be defined as a randomized control trial, the primary objective of which was to determine efficacy. The problem lies in the very narrow inclusion criteria employed by this trial, choosing only to include patients that present with the Hepatitis C virus as the causative etiology for cirrhosis. Whilst positive results regarding the efficacy of stem cell therapy were noted, there is no data to guarantee that the same effect will be present in those patients presenting without infection.
The primary aim of the bulk of the trails was to determine the safety of this therapy. All the trials concluded that infusion of stem cells through either the hepatic artery or portal vein was safe except Study 626 which reported severe side effects in one of the patients undergoing therapy. However, due to the isolated nature of this adverse effect, it is hard to fully attribute it to the re-infusion of stem cell therapy.
The requirement for a framework on which to base future stem cell therapy trials is evident. However, further evidence may be needed before this structure can be implemented. Until the most efficacious and safe stem cell type and administration technique has been determined, through in vitro and in vivo models, this step cannot be taken. Another stumbling block that faces the advancement of stem cell therapy in the treatment of liver disease is the lack of definitive mechanism by which the action appears to occur. Two primary models have been suggested: the theory of transdifferentiation15,33 and the theory of cell fusion.34,35 Neither model has yet been able to disprove the other and consequently both are still possibilities. Once these hurdles have been cleared, it will be easier to make larger advances in expanding the evidence base in this novel topic area. If the evidence appears positive, larger clinical trials will be granted approval and the results from those may allow the application of this technology is standard clinical practice.
While there is great excitement at the potential that stem cell therapy may have in the treatment of liver cirrhosis, it is plain that the therapy is still within the clinical experimentation phase. While there is reasonable evidence present in this topic area, it is very sporadic and variable and cannot be easily compared to give us valid conclusions. However, the majority of the evidence base supports further advancement and research into stem cell therapy in cirrhotic liver disease so that it may become commonplace in clinical practice in the decades to follow.
Conclusion
The treatment of cirrhotic liver disease using stem cell therapy has the most evidence in what is currently a very novel treatment option. However, due to a lack of structure for these trials, the nature of the results is very sporadic. Consequently, valid conclusions are hard to draw from the data. The results support the suggestion that further research needs to be carried out to fully understand stem cell therapy and to further expand the evidence base of its use in the treatment of liver disease.
Conflicts of interest
All authors have none to declare.
References
- 1.Hoyert D.L., Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2013; October 10;61(6) [PubMed] [Google Scholar]
- 2.American Cancer Society . American Cancer Society; Atlanta: 2013. Cancer Facts and Figures 2013. [Google Scholar]
- 3.Zhang Z., Lin H., Shi M. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012; Mar;27(suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 4.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005; Feb;115(2):209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisseleva T., Gigante E., Brenner D.A. Recent advances in liver stem cell therapy. Curr Opin Gastroenterol. 2010; Jul;26(4):395–402. doi: 10.1097/MOG.0b013e32833a6bec. [DOI] [PubMed] [Google Scholar]
- 6.Khan A.A., Parveen N., Mahaboob V.S. Safety and efficacy of autologous bone marrow stem cell transplantation through hepatic artery for the treatment of chronic liver failure: a preliminary study. Transpl Proc. 2008; May;40(4):1140–1144. doi: 10.1016/j.transproceed.2008.03.111. [DOI] [PubMed] [Google Scholar]
- 7.Kim W.R., Kremers W.K. Benefits of “the benefit model” in liver transplantation. Hepatology. 2008; July;48:697–698. doi: 10.1002/hep.22497. [DOI] [PubMed] [Google Scholar]
- 8.Houlihan D.D., Newsome P.N. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology. 2008; Aug;135(2):438–450. doi: 10.1053/j.gastro.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 9.Almeida-Porada G., Zanjani E.D., Porada C.D. Bone marrow stem cells and liver regeneration. Exp Hematol. 2010; Jul;38(7):574–580. doi: 10.1016/j.exphem.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissman I.L. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000; Jan 7;100(1):157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 11.Lakshmipathy U., Verfaillie C. Stem cell plasticity. Blood Rev. 2005; Jan;19(1):29–38. doi: 10.1016/j.blre.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Blau H.M., Brazelton T.R., Weimann J.M. The evolving concept of a stem cell: entity or function? Cell. 2001;105(7):829–841. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 13.Bryder D., Rossi D.J., Weissman I.L. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006; Aug;169(2):338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagasse E., Connors H., Al-Dhalimy M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000; Nov;6(11):1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 15.Jang Y.Y., Collector M.I., Baylin S.B., Diehl A.M., Sharkis S.J. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004; Jun;6(6):532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 16.Mallet V.O., Mitchell C., Mezey E. Bone marrow transplantation in mice leads to a minor population of hepatocytes that can be selectively amplified in vivo. Hepatology. 2002; Apr;35(4):799–804. doi: 10.1053/jhep.2002.32530. [DOI] [PubMed] [Google Scholar]
- 17.Ding D.C., Shyu W.C., Lin S.Z. Mesenchymal stem cells. Cell Transpl. 2011;20(1):5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 18.Russo F.P., Parola M. Stem cells in liver failure. Best Pract Res Clin Gastroenterol. 2012; Feb;26(1):35–45. doi: 10.1016/j.bpg.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Stock P., Staege M.S., Müller L.P. Hepatocytes derived from adult stem cells. Transpl Proc. 2008; March;40(2):620–623. doi: 10.1016/j.transproceed.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 20.Oh S., Hatch H.M., Petersen B.E. Hepatic oval ‘stem’ cell in liver regeneration. Seminars Cell Dev Biology. 2002; December;13(6):405–409. doi: 10.1016/s1084952102001271. [DOI] [PubMed] [Google Scholar]
- 21.Lowes K.N., Brennan B.A., Yeoh G.C., Olynyk J.K. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999; Feb;154(2):537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakinuma S., Tanaka Y., Chinzei R. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21(2):217–227. doi: 10.1634/stemcells.21-2-217. [DOI] [PubMed] [Google Scholar]
- 23.Gaia S., Smedile A., Omede P. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006; Jul;45(1):13–19. doi: 10.1016/j.jhep.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Terai S., Ishikawa T., Omori K. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006; Oct;24(10):2292–2298. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- 25.Mohamadnejad M., Alimoghaddam K., Mohyeddin-Bonab M. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007; Oct;10(4):459–466. [PubMed] [Google Scholar]
- 26.Mohamadnejad M., Namiri M., Bagheri M. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007; Jun 28;13(24):3359–3363. doi: 10.3748/wjg.v13.i24.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai M., Zacharoulis D., Milicevic M.N. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008; Aug;103(8):1952–1958. doi: 10.1111/j.1572-0241.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 28.Kharaziha P., Hellstrom P.M., Noorinayer B. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009; Oct;21(10):1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 29.Khan A.A., Shaik M.V., Parveen N. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transpl. 2010;19(4):409–418. doi: 10.3727/096368910X498241. [DOI] [PubMed] [Google Scholar]
- 30.Salama H., Zekri A.R., Bahnassy A.A. Autologous CD34+ and CD133+ stem cells transplantation in patients with end stage liver disease. World J Gastroenterol. 2010; Nov 14;16(42):5297–5305. doi: 10.3748/wjg.v16.i42.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikeghbalian S., Pournasr B., Aghdami N. Autologous transplantation of bone marrow-derived mononuclear and CD133(+) cells in patients with decompensated cirrhosis. Arch Iran Med. 2011; Jan;14(1):12–17. [PubMed] [Google Scholar]
- 32.Lin H., Zhang Z., Shi M. Prospective controlled trial of safety of human umbilical cord derived-mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2012; Jul;20(7):487–491. doi: 10.3760/cma.j.issn.1007-3418.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Terai S., Sakaida I., Yamamoto N. An in vivo model for monitoring trans-differentiation of bone marrow cells into functional hepatocytes. J Biochem. 2003; Oct;134(4):551–558. doi: 10.1093/jb/mvg173. [DOI] [PubMed] [Google Scholar]
- 34.Vassilopoulos G., Russell D.W. Cell fusion: an alternative to stem cell plasticity and its therapeutic implications. Curr Opin Genet Dev. 2003; Oct;13(5):480–485. doi: 10.1016/s0959-437x(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Willenbring H., Akkari Y. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003; Apr 24;422(6934):897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]