Abstract
Nonalcoholic fatty liver (NAFL) is an emerging global epidemic which progresses to nonalcoholic steatohepatitis (NASH) and cirrhosis in a subset of subjects. Various reviews have focused on the etiology, epidemiology, pathogenesis and treatment of NAFLD. This review highlights specifically the triggers implicated in disease progression from NAFL to NASH. The integrating role of genes, dietary factors, innate immunity, cytokines and gut microbiome have been discussed.
Keywords: adiponectin, cytokines, gut microbiota, lipotoxicity, PNPLA3
Abbreviation: AGE, Advanced glycation end products; ALT, Alanine aminotransferase; AMPK, AMP-activated protein Kinase; APPL1 and 2, Adaptor protein 1 and 2; ATP, Adenosine tri-phosphatase; BMI, Basal Metabolic Index; CD, Cluster of differentiation; COL13A1, Collagen, type XIII, alpha 1; DAMP, Damage assocauted molecular pattern molecules; EFCAB4B, EF-hand calcium binding domain 4B; FA, Fatty acid; FDFT1, Farnesyl-diphosphate farnesyltransferase 1; FFA, Free fatty acid; GCKR, Glucokinase regulatory protein; GLUT 5, Glucose transporter type 5; GWAS, Genome wide association studies; HDL, High density lipoprotein; Hh, Hedgehog; HMGB1, High-mobility group protein B1; HOMA-IR, Homoestatic model assessment-insulin resistance; HSC, Hepatic Stellate Cells; IL6, Interleukin 6; IR, Insulin Resistance; KC, Kupffer Cells; LPS, Lipopolysacharrides; LYPLAL1, Lypophospholipase like 1; MCP, Monocyte chemotactic protein; NAD, Nicotinamide adenine dinucleotide; NAFL, Nonalcoholic fatty liver; NAFLD, Nonalcoholic fatty liver disease; NASH, Nonalcoholic steatohepatitis; NCAN, Neurocan gene; NF-KB, Nuclear Factor Kappa B; NK, Natural Killer; NKL, Natural Killer T cells; NLR, NOD like receptor; NNMT, Nicotinamide N-methyltransferase gene; OXLAM, Oxidized linolenic acid metabolite; PAMP, Pathogen-associated Molecular pattern; PARVB, Beta Parvin Gene; PDGF, Platelet-derived growth factor; PNPLA3, Patatin-like phospholipase domain-containing protein 3; PPAR-α, Peroxisome proliferator activated receptor alpha; PPP1R3B, Protein phosphatase 1 R3B; PUFA, Poly unsaturated fatty acid; PZP, Pregnancy-zone protein; ROS, Reactive oxygen species; SAMM, Sorting and assembly machinery component; SCAP, SREBP cleavage-activating protein; SFA, Saturated fatty acid; SNP, Single nucleotide polymorphism; SOCS3, Suppressor of cytokine signaling 3; SOD2, Superoxide dismutase 2 gene; SREBP-1C, Sterol regulatory Element—Binding Protein 1-C gene; TLR, Toll like receptor; TNF α, Tumor necrosis factor Alpha; UCP3, Uncoupling protein 3 gene
Non alcoholic fatty liver disease (NAFLD) is an emerging medical problem worldwide which affects a significant proportion of the western population and there is gradual spread of this epidemic to south-east Asian countries. NAFLD encompasses two entities: Non-alcoholic fatty liver (NAFL) and Non-alcoholic steatohepatitis (NASH). NAFL is defined as the evidence of hepatic steatosis without inflammation either by imaging or by histology in individuals without significant alcohol consumption in whom secondary causes of steatosis are absent.1 NASH on the other hand, is characterized by the presence of both steatosis and inflammation with evidence of hepatocyte injury in the form of ballooning with or without fibrosis.1
The prevalence of NAFLD has been gradually increasing and one third adult Americans have NAFLD.2 The prevalence in obese population may be as high 57.55–74%.3,4 The global spread of this epidemic is evidenced by the presence NAFL in 36.8% of Mediterranean, 21.5% of Iranians and 27% of urban Chinese adults.5–7 However, the prevalence varies between countries and continents. The prevalence in Europe is 20–30% while that in Japan varies between 9 and 30%8,9 and in China between 5 and 24%.10 In India, the prevalence of NAFLD in urban population is 16%–32% while that in rural areas is approximately 9%.11–13 Among the Asian countries the lowest prevalence is observed in Singapore at 5%.10 Globally NAFLD has been related to obesity and sedentary lifestyle. Interestingly both NAFL and NASH have been observed in non-obese subjects in Asians, which is referred to as the Asian paradox.11,14,15 Mere presence of fat in the hepatocytes is not considered as a disease. As most of the subjects with NAFL do not progress to NASH, differentiation between these two conditions is paramount. Though liver histology is the gold standard in diagnosis of NAFL, the commonest method used is transabdominal ultrasonography which has a sensitivity of 100% and specificity of 90% when fat on liver biopsy exceeds 20%.16 Other non-invasive modalities used to diagnose NAFL includes transient elastography (Fibroscan) and Acoustic Resonance Magnetic Imaging (ARFI) which measures liver stiffness and corresponds to presence of fibrosis. Magnetic resonance spectroscopy is a quantitative method of measuring liver fat but is limited in clinical use due to lack of widespread availability and cost.17 However, none of these available noninvasive tests can distinguish simple steatosis from NASH. Although raised aspartate aminotransferase and alanine aminotransferase levels are considered by some as a marker of NASH, yet these enzymes may be normal in many subjects with biopsy proven NASH.18 A recent study using non-invasive tools have found 81% probability of differentiating NAFL from NASH using Bayesian approach combining clinical, laboratory, and imaging data.19 Based on the modality used to diagnose NAFL, the detection of disease varies. Ultrasound based study from India has shown the prevalence of NAFLD to be 16.6%12 while in a study based on liver biopsy the presence of NASH was 53%.20 In another study from costal eastern India, one fourth of patients had evidence of NASH on liver biopsy on presentation.21 In another Asian study involving 52 patients, biopsy proven NASH at presentation was found in 32.6% patients while 23% patients with baseline NAFL progressed to NASH.22 In contrast, a recent study from the West with mean 6.6 years follow up, baseline NASH was found in 75% patients with disease progression from NAFL to NASH in 44% patients. Among those patients with simple steatosis fibrosis progression was observed in follow up liver biopsy which was statistically more in diabetic steatosis thereby suggesting that simple steatosis can progress to clinically significant fibrosis.23 Similar data from west suggests that among those with NASH only 21–26% progress to cirrhosis over 8.2 years.24 Meta-analysis have demonstrated that NAFLD increases the risk of all-cause mortality.25 Therefore, aggressive management in the form of dietary and lifestyle modification is required in patients who has NASH when compared to those who have simple steatosis. The riddle of progression from NAFL to NASH and subsequently to cirrhosis is poorly understood. In this review, we present new insights into progression of NAFL to NASH, integrating the role of genes, diet, immunological profile, cytokines, liver cell types, and gut microbiota.
Journey from nonalcoholic fatty liver disease to nonalcoholic steatohepatitis
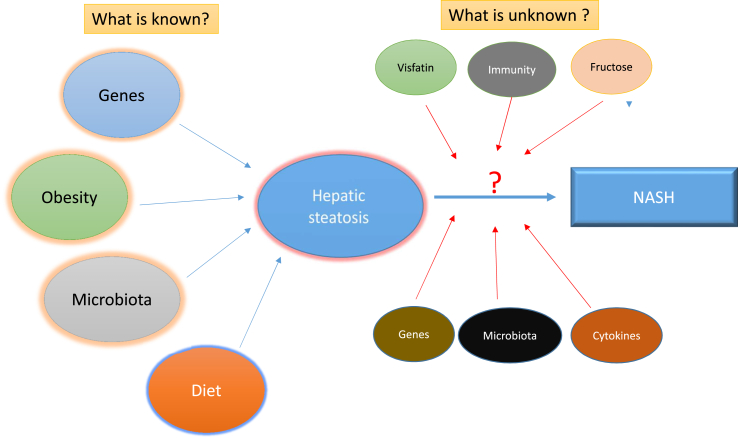
The spectrum of NAFLD ranges from simple steatosis to NASH. The accumulation of fat above the physiological level (<5%) in the hepatocytes (steatosis) is the pre-requisite for development of NASH. Although NAFL is almost universally present in obese individuals yet not all of them progress to NASH. Similarly there are many individuals who may be non-obese physically but may be metabolically obese and have NASH. This indicates that multiple factors other than obesity and insulin resistance are involved in the disease progression. The original “two-hit hypothesis” considered steatosis to be the “first hit”, which increased the sensitivity of the liver to the “second hits” that leads to hepatocyte injury and ultimately inflammation (NASH) and fibrosis.26 However, emerging evidence suggests that the pathogenesis involves “multiple hits”27 which progress parallel to each other and progression to NASH depends on the close interaction and cross talk between host genes, environmental influences, gut microbiota and host immune system.28 The progression from NAFL to NASH is poorly understood. The following sections will elaborate the various factors implicated in disease progression (Figure 1).
Figure 1.
Graphical illustration showing the unknown factors in the riddle of progression of NAFL to NASH.
Genes in nonalcoholic fatty liver disease
Considerable variation in severity of the disease and rate of progression suggests a genetic predisposition for the disease. It is a polygenic disease with involvement of multiple loci, environmental and nutrient interactions. Furthermore, in a substantial percentage (25–35%) of individuals, the disease is thought to be contributed by a genetic component.29 Common genetic variants at various loci have consistently been associated with fatty infiltration. Studies have shown that Asian Indians have a genetic predisposition to have up to twofold increased hepatic fat accumulation despite having a low BMI due to their tendency to have more visceral adiposity.15 The major locus among these is 22q13.31 which harbors the PNPLA3 gene. A particular single nucleotide polymorphism (SNP) namely rs738409 showed strongest association with NAFLD across various Genome wide association studies (GWAS) in various populations30–32 and this is probably one of the very few SNPs that were identified by GWAS which was significant across various ethnicities. The SNP is located in the 3rd exon of the gene and is mainly expressed by liver and adipose tissue. The gene is involved in triglyceride hydrolysis activity and the SNP introduces an amino acid substitution from isoleucine to methionine (I148M) that abolishes the function.33 PNPLA-I148M polymorphism has been shown to be associated with increased necroinflammation, severe steatohepatitis and advanced fibrosis.34 In India, higher frequency of C/G and G/G genotypes of the rs738409 polymorphism was noted in NAFLD subjects and this was associated with significantly higher fasting insulin, HOMA-IR, alanine transaminase and aspartate transaminase levels.35 In addition to predisposing patients to severe disease, patients with PNPLA3 rs738409 GG homozygous genotype has also fivefold increased risk of developing hepatocellular carcinoma though the findings needs to be validated in larger studies.36 On the other side of the spectrum PNPLA3 S453 polymorphism is associated with lower hepatic fat content in African Americans, making them the group at lowest risk of NAFLD. This protective effect is independent of the presence of PNPLA3—I148M polymorphism.37
Other GWA studies identified common genetic variants in or near LYPLAL1, PPP1R3B, NCAN and GCKR that are associated with steatosis apart from NASH, fibrosis and metabolic traits in patients with European ancestry.30 Importantly, the rs780094 SNP was associated with a higher risk of type2 diabetes, higher levels of triglycerides, reduced fasting plasma glucose levels and lower Homoestatic model assessment–insulin resistance (HOMA-IR) in Japanese population. Furthermore, it was hypothesized that the effect of the SNP on diabetes is probably mediated through impaired beta cell function rather than through obesity.38 Likewise another study in Japanese identified SAMM50, PARVB and PNPLA3 as risk loci associated with NAFLD. A GWA study in Caucasians identified FDFT1, COL13A1, EFCAB4B, PZP as causative loci for steatosis, NAFLD activity score, degree of fibrosis, lobular inflammation and serum levels of alanine amino transferase (ALT). A pooled genetic study that identified SNPs from the above GWA studies and genotyped the same in Indian NAFLD patients identified PNPLA3, SAMM50, PARVB and PZP as risk loci for NAFLD apart from association of variants in NCAN and PZP being associated with higher levels of ALT.39 Individual hypothesis or candidate gene approach based studies have identified various loci that are either protective, or confer susceptibility to NAFLD, steatosis or faster progression. It was shown that the SCAP rs2101247 “A” allele might decrease the risk of NAFLD especially in females with metabolic syndrome, however the reasons and mechanisms are unclear.40 SNPs namely rs2276736, rs3772630 and rs3772627 appear to be protective especially in the Indians against NAFLD.41 A Val277Ala substitution in the PPAR-α (peroxisome proliferator activated receptor alpha) was associated with NAFLD. However it was associated with decreased waist circumference and waist-to-hip ratio suggesting a protective role in obesity.42 In contrast, variants in uncoupling protein 3 gene (UCP3),43 Sterol Regulatory Element-Binding Protein 1C gene (SREBP-1C),44 Adaptor protein (APPL1 and 2),45 Nicotinamide N-methyltransferase gene (NNMT)46 and mitochondrial superoxide dismutase 2 gene (SOD2)47 conferred susceptibility.
Another gene which has been linked to NAFLD is apolipoprotein 3 (APOC3) gene. Carriers of APOC3 variant alleles (c-482T, T-455C or both) in non-Asian population had increased NAFLD and insulin resistance.48 Further studies in Asian Indians has shown varied results. Polymorphism T-455C in APOC3 gene has been associated with NAFLD and higher serum triglyceride levels in Southern Indian population.49 Another study in the same region of India, on the other hand, found that PNPLA3 rs738409 polymorphism to be associated with NAFLD but failed to show any independent association of APOC C3 gene rs2854116 and rs2854117 polymorphism with NAFLD.50
Environmental influences on the genotype and the effect they have on the phenotype have been studied to a certain extent in the context of NAFLD. Since identifying disease susceptibility alone would not necessarily aid in disease outcomes, understanding the environmental interactions with the genotype will help in managing the disease in a better way. Recent studies have identified the effect of n3 and n6 poly unsaturated fatty acids (PUFAs) on the genotype. It was seen that there was significantly higher fatty infiltration in individuals who consumed more of n6 versus n3 PUFAs and with GG genotype in the PNPLA3 gene.51 Similarly another study which included subjects with both GG and CC genotype, showed that. Individuals in both groups lost 3.1 kg weight on an average. However, individuals in the GG genotype group lost 45% as against only 18% of liver fat in the CC genotype group, suggesting that individuals with the GG genotype may have a better advantage with weight loss and fatty infiltration.52 In another significant study it was seen that the G allele of the rs738409 in the PNPLA3 gene was associated with both alcoholic and non-alcoholic fatty liver disease, so it is prudent for an individual with the GG genotype to avoid alcohol for an overall better health of the liver.53
The role of hepatic iron in progression of NAFL to NASH is unclear. Dysregulation of iron regulatory molecules or genetic factors may be responsible for iron overload in NAFLD patients. The C282Y mutation (HFE gene) which is common in Europeans are not found in Indian population. However, H63D heterozygosity was found in 16.9% of Asian Indians with NASH when compared to only 12% in controls, suggesting that primary iron overload in Indians is non HFE type.54 Although genetic susceptibility has been identified for NAFLD, further research should focus on gene–gene and gene–nutrient interactions employing advanced genomic techniques.
Obesity, insulin resistance and dietary factors in nonalcoholic fatty liver disease
Lipids and Lipotoxicity
The composition of food intake plays an important role in NAFL. Not all lipids are equally harmful. Polyunsaturated fatty acids (PUFAs) are of two type (i) n-6 PUFAs which includes linoleic acid and arachadonic acid and (ii) n-3 PUFAs which includes alpha-linoleic acid, eicosapentanoic acid and docosahxanoic acid. The n-6 PUFAs have the capability of producing proinflammatory eicosanoids55 and are harmful while the n-3 PUFAs have anti-inflammatory properties leading to decreased lipogenesis and lower hepatic steatosis.56,57 Studies have shown that a high ratio of n6:n3 PUFA in hepatocytes and circulation is associated with severity of NAFLD.58 Based on this, a meta-analysis showed n-3 supplementation may decrease hepatic fat, though herterogenicity of the study population warrants the need of well-designed randomized control trials.59 Reducing n-6 intake is an interesting alternative as linoleic acid is associated with formation of oxidized linoleic acid metabolite (OXLAMs) which are found increased in NASH and correlate with severity of NASH.60
The “lipotoxicity” represents the major mechanism underlying hepatocyte dysfunction leading to disease progression in NASH. Lipotoxic injury appears to occur in the setting of excess free fatty acid (FFA) traffic, especially saturated fatty acids (SFAs), rather than due to simple triglyceride accumulation. This view is supported by studies that demonstrate that increased hepatocyte triglyceride formation61 or reduced export of lipid62 does not, of itself, increase inflammation. Furthermore, evidence suggests that triglyceride accumulation may actually be a protective mechanism to counter lipotoxicity.63 A number of other studies have shown lipotoxic injury is reduced if alternative pathways of fatty acid (FA) disposal are available.64 This suggests that triglycerides themselves are unlikely to be the cause of hepatocyte injury in NASH and probably occurs in parallel with the generation of toxic metabolites, with these lipotoxic metabolites being primarily responsible for disease progression.65 The current theory of lipotoxicity centres on an increase in the flux of FFAs within hepatocytes. This is a direct consequence of increased influx (through increased dietary intake of SFAs as well as de novo lipogenesis and adipose lipolysis in the setting of insulin resistance and impairment of compensatory oxidative processes.66 The net result is the generation of toxic lipid metabolites, such as ceramides, diacylglycerols, lysophosphatidyl choline, and oxidized cholesterol metabolites, which act as reactive oxygen species (ROS),67 although the absolute and relative levels of each of these substances in NAFLD and its progression remain unconfirmed.
Insulin Resistance
Obesity and metabolic syndrome are risk factors for NAFL and NASH in the western world. However, in Asians, it has been observed that subjects with no components of the metabolic syndrome can still have insulin resistance and also significant NASH.68 Abdominal obesity has been found in only 7.1% subjects among lean Asian Indians with NAFL.69 Visceral adipose tissue content was initially tough to be the most important factor in pathogenesis of NASH among Asians. However, in an Indian study involving biopsy proven NAFL patients, both subcutaneous adipose tissue and total adipose tissue contents has been found to be positively correlated with the disease severity.70 Indian studies have shown that presence of metabolic syndrome among Indian NAFL to be between 21% and 41%.71,72 However, in contrast, a recent study from India has shown that 50% of Indian subjects with NAFLD did not have insulin resistance and one third of these patients had significant fibrosis.73 The lack of insulin resistance may be associated with less severe disease in lean Indian fatty liver subjects.69 In contrast, other studies from India found the prevalence of insulin resistance in around 97.5% subjects with NAFLD.74 In support of the previous study, another study from the subcontinent has found that 98% of Indian NAFL subjects have reduced insulin sensitivity and 50% of them were associated with features of metabolic syndrome.20 The Indian cohort of NAFLD appears to be a heterogeneous subset of patient with variable insulin resistance with more resistance in subjects with features of metabolic syndrome and lesser in lean NAFLD.
Fructose
Fructose is an isomer of glucose with a hydroxyl group on carbon-4 reversed in position.75 Over the years, the consumption of fructose has enormously increased as it has been increasingly used as artificial sweetener in preference over sucrose due to its lower cost and ability to induce less satiety.76 With the help of fructose specific Glucose transporter type 5 (GLUT 5), fructose is absorbed from the small intestine, and reaches the liver via the portal vein. In the liver, it undergoes phosphorylation to produce 3 carbon intermediates.77 Fructose may cause hepatotoxicity indirectly by worsening metabolic syndrome and has been linked in humans to increase triglyceride levels and decrease HDL cholesterol. Peroxisome proliferator activated receptor gamma coactivator 1 beta also plays a crucial role in fructose induced insulin resistance.78,79
Fructose metabolism involving hexokinase causes depletion of ATP which induces metabolic stress.70 High fructose intake has also been linked to intestinal dysbiosis, which activates the gut–liver axis and induces a low grade endotoxemia which in turn predisposes to NASH.80 Copper depletion and iron overload which are indirectly associated with NASH has been found with increased fructose consumption.81,82 Whether visceral fat accumulation is directly related to progression of NAFL remains an area of research. However, some studies have linked increased fructose to significant increase in waist circumference and the proportion of visceral adipose tissue with no change in total body fat.83,84 The direct effects of fructose toxicity may be due to the accumulation of fructose 1–phosphate in the hepatocytes. Fructose produces advanced glycation end products (AGE) 17 times faster than glucose. High AGE levels, common in western diet, exacerbate liver injury, inflammation and fibrosis via oxidative stress, cytokine synthesis (TNF-α and IL-6), and through hepatic stellate cell activation.85 Fructose over consumption, thereby contributes to development of obesity, dyslipidemia, and impaired glucose tolerance, producing AGEs responsible for producing dysfunctional proteins.86 Therefore, AGE and its receptor pathway could be considered a new target for nutritional or pharmacological strategy to slow NAFLD progression.
Innate immunity and cytokines
Immune Cells
Lymphoid cells of liver encompass resident lymphocytes, which are functionally and phenotypically distinct from their counterparts in the peripheral circulation and in other organs as well as hordes conventional (i.e. B cells, CD4+ and CD8C+ T cells, natural killer cells) and non-conventional lymphoid cells(i.e., γδ TCR+ T cells, natural killer T cells). A key role in specific immune function is also exerted by mucosal associated invariant T cells that are a highly specialized T cell population in the vascular network of the liver.87 Regulatory T cell populations seem to have an important role in maintaining a beneficial balance in the liver between immuno-tolerance and activation.88 In addition to classic parenchymal hepatocytes and cholangiocytes, the liver contains other cell types responsible for the homeostasis of the innate and adaptive immune system. Among the non-lymphoid cells, Kupffer cells (KC) and dendritic cells (DC) from the myeloid lineage have a major role in the immune response. While dendritic cells are the primary antigen-presenting cells of the liver, the cholangiocytes can also act as antigen-presenting cells89 thus playing an additional role in the hepatic immune function. Dendritic cells in patients with NAFLD exhibits immature yet functionally activated phenotype in response to lipo-polysaccharide stimulation which secrete inflammatory cytokines and contribute to worsening of disease.90 The innate immune response is an important effector of parenchymal inflammation in liver diseases, such as NASH, and is mediated by innate immune cells, including neutrophils, macrophages (Kupffer cells), natural killer (NK) cells, and natural killer T (NKT) cells. The following section elaborates the role of each cell types in NAFL.
Resident or monocyte-derived Kupffer cells are the largest population of mononuclear phagocytes in the body. They are present throughout the liver, but there is variation in the population density, cytological characteristics, and physiologic functions of KC in different zones of the hepatic acinus/lobule. During liver injury and diseases, monocytes rapidly differentiate into mature cells that are indistinguishable from genuine KC, independently from the circulating monocytes.91 They are strategically located in liver sinusoids, therefore they are the first cells to come in contact with exogenous immunoreactive material or endogenous signals phagocytosing, processing and presenting antigen, and secreting various pro-inflammatory mediators such as cytokines, prostanoids, nitric oxide, and reactive oxygen intermediates. Expression of the Fc receptor results in non-specific phagocytosis of immune complexes as well as antibody-coated particles such as microorganisms and allows KC to have a significant role in control of inflammatory and immunologic processes. It has been shown that high-fat diet increase Kupffer cells number and induce their pro-inflammatory status i.e. attainment of M1 phenotype from M2 status. Pro-inflammatory activated Kupffer cells by lipid promote hepatic NKT cell over-activation and cell death, which lead to further hepatic NKT cell deficiency in the development of NASH.92 Generally, KCs are exposed to low levels of gut-derived lipopolysachharides (LPS). This stimulus allows KC to trigger an escape mechanism that involves IL-10, which in turn contributes to the down-regulation of pro-inflammatory cytokines.93 On the other hand, following massive Toll like receptor 4 (TLR4) stimulation, KC produce several chemokines and cytokines, such as TNF-α, IL-1β, IL-6, IL-12, and IL-18.94 Conversely, adiponectin was recently shown to shift KC polarization to the M2/anti-inflammatory phenotype, preventing progression of NASH in mice. Adiponectin decrease, as well as adiponectin gene deletion, induces hepatic steatosis progression, fibrosis, and tumor development.95 Moreover, KCs have also metabolic function, regulating fatty acids oxidation, increasing hepatic lipid storage and insulin resistance (IR), as mechanisms of adaptation to increased caloric intake. This event which is triggered by secretion of inflammatory cytokines, suggests a beneficial role for alternatively M2-activated KCs in metabolic derangements.96,97
Natural killer cells are abundant in the liver and may have a role in the progression of NAFLD as they are capable of inducing apoptosis of both HSCs and hepatocytes, via IFNγ.98 Although circulating NK cell numbers and cytotoxic activity appear to be reduced in obesity,99 it is likely that the hepatic NK cell population is significantly increased in NASH.100 NKT cells regulate host responses to tissue damage by inducing an adaptive immune response (both Type 1 and Type 2) and are seen in association with necroinflammation.101 Although animal models and studies in other chronic liver diseases have shown conflicting roles in hepatic fibrogenesis,102 NKT cells are activated by lipid antigens and therefore may contribute to the progression of fibrosis in NAFLD. Studies in humans and animals suggest that NKT cells may play a protective role in limiting steatosis but a deleterious role in the progression of inflammation and fibrosis.103
Hepatocytes apart from their metabolic and detoxifying functions, express TLR4, although they require high doses of LPS to induce significant effects.104 Under inflammatory conditions, the expression of TLR2, rather than TLR4, is up-regulated, indicating a major responsiveness to TLR2 activation and involvement of downstream MyD88 signaling pathway following an insult.105 In comparison to KCs, hepatocytes are more able to clear LPS from systemic circulation, through its uptake and release into the bile, where TLR4, CD14, and myeloid differentiation (MD)-2 have an obligatory role for LPS uptake by hepatocytes.106 Furthermore, TLR4/MyD88 signaling in hepatocytes has been shown to play a pivotal role during the early progression of high fructose diet induced NAFLD, in which free High-mobility group protein B1 (HMGB1) served as a Damage-associated molecular pattern molecules (DAMP) mediating TLR4 activation.107
Hepatic stellate cells (HSCs) trans-differentiate from quiescent to active state under the influence of activators like platelet-derived growth factor (PDGF) and transforming growth factor β1 (TGF-β1) secreted by activated KC, infiltrating monocytes, platelets, and damaged hepatocytes.108 The resting HSCs may acquire adipogenic or myogenic phenotype during the trans-differentiation,109 determined by adipogenic and myogenic gene expression. Indubitably, adipogenic genes are down-regulated under ischemia and inflammation and up-regulated by peroxisome proliferator-activated receptor-γ (PPAR-γ). Other factors implicated in HSCs activation include the Hedgehog (Hh) pathway,110 cytokine stimulation (particularly TNF-α, IL-1β, and IL-6),111 and leptin.112 Conversely, adiponectin released from adipose tissue reduces HSC migration and proliferation.113 Finally, TLR4 contributes to the activation of HSCs through the MyD88–NF-kB-dependent pathway.114
Murine biliary cells express CD14, MD-2, and TLR2, 3, 4, and 5 and after LPS stimulation, murine biliary cells activates the NF-kB pathway and synthesized TNFα.115 Human biliary epithelial cells express TLR 1 to TLR10. Subsequent to TLR2 and TLR4 activation, Homeobox protein CDX-2 and mucus core protein-2 expression increases.115,116 The progression of NAFLD in humans has been related to the increase in bile ductules, and their senescence markers. Moreover, such senescent bile ductules express chemotactic protein, such as Monocyte chemotactic protein 1 (MCP-1), which are likely responsible for HSC activation.117
Hepatic dendritic cells are the antigen-presenting cells in the liver. In inflammatory conditions, they are recruited in to liver sinusoids, and migrate to periportal and pericentral areas. They express TLR4/MD-2 complex, produce inflammatory cytokines (i.e. IL-12 and TNF-α), and express co-stimulatory molecules (CD40, CD80, and CD86) following several stimuli, such as LPS, peptidoglycan, poly-I: C, and cytidine-phosphate guanosine (CpG)-DNA.114
Cytokines
The synthesis of cytokines, such as TNF-α and IL-6, both involved in inflammatory and metabolic alterations, characterizes the earliest phases of liver injury, leading to the synthesis of other cytokines that, jointly, induce cell migration and initiate healing processes, including fibrosis.118 A correlation has been found between TNF-α levels and fibrosis degree in NASH patients,119 indeed gene expression of either TNF-α or its receptor is significantly elevated in their hepatic and adipose tissues.120 Similar correlation has been found in NAFLD patients, whose circulating TNF-α are significantly elevated concomitantly with the increase in the Nonalcoholic steatosis (NAS) score which is a histologic scoring system recognized as standard reference in the evaluation and gradation of hepatic inflammation and damage.121 In addition, progression of NAFLD correlates with polymorphisms in the TNF-α promoter region and serum level of soluble Tumor Necrosis Factor (TNF)receptor-2.122
It has been also shown that adipose tissue-derived IL-6 regulates hepatic IR via up-regulation of Suppressor of cytokine signaling 3 (SOCS3).123 Indeed, over expression of SOCS-3 in the mouse liver causes IR and an increase in sterol regulatory element-binding protein (SREBP-1c) that regulates fatty acid synthesis. Conversely, inhibition of SOCS3 in obese diabetic mice improves insulin sensitivity, normalizing the increased SREBP- 1c expression.124 In humans, a significant association of high IL 6, IR and increased oxidative stress have been found in NAFLD subjects and correlated with advanced disease on histology.125 When oxidative stress and cytokine levels of NAFLD subjects were compared with those of chronic viral hepatitis and healthy volunteers, NAFLD patients showed significantly higher levels of oxidative stress as well as cytokine levels.126 Similarly, higher oxidative stress has also been documented in both diabetic and non-diabetic Indian NAFLD patients.127
Recent studies have established a link between NASH and increased IL-1β production,128,129 potentially via an increase in inflammasome activation.129 Activation of the inflammasome and release of IL-1β requires 2 signals: an endogenous danger signal and a TLR ligand.130 A recent study has demonstrated that saturated fatty acids (SFAs) can elicit inflammasome activation.131 SFAs are known to increase sensitivity of primary hepatocytes to LPS in vitro, and administration of exogenous LPS in vivo can enhance IL-1β levels and inflammasome activation in the setting of steatohepatitis. Evidence that SFAs induce hepatocyte apoptosis132,133 and DAMPs from dying hepatocytes can induce inflammasome activation and IL-1β further suggests that SFAs “prime” the fatty liver for LPS-induced inflammasome activation.133
Adiponectin/Visfatin/Leptin
Adiponectin, a hormone synthesized and secreted by adipocytes is considered as an anti-inflammatory adipokine134 which trim down inflammation by stimulating secretion of anti-inflammatory cytokines (e.g., IL-10), slabing NF-KB activation, and stalling release of TNF-α, IL-6 and chemokines. It has been shown that adiponectin levels were reduced in NASH in comparison to control and simple steatosis patients.135 Nevertheless, adiponectin levels was inversely associated only with intrahepatic fat but not with inflammation and fibrosis.136 It has been suggested that adiponectin may represent a link between hepatic fat and insulin resistance.137
Visfatin, an adipocytokine predominantly expressed and secreted by visceral adipose tissue is of central importance in Nicotinamide adenine dinucleotide (NAD) biosynthesis, innate immunity and inflammation.138 Our study showed that there was a significant decline in visceral adipose tissue visfatin level which was associated with degree of steatosis in NAFLD patients.139 Moreover, our own unpublished data documented reduction of body weight as well as liver weight in high fat diet induced obese rats upon exogenous administration of visfatin in comparison to the untreated group of obese rats. It is to be noted that visfatin being important in NAD biosynthesis may be playing a role in energy balance which needs further investigations.
Leptin an adipocyte-secreted, negative feedback hormone whose levels are elevated in obesity is considered to have a lipostatic function, and is released into the bloodstream upon increase in the amount of fat stored in the adipocytes. Acting indirectly though the central neural pathway, and directly through the activation of AMPK (AMP-activated protein kinase), it prevents the occurrence of NAFLD where leptin levels are directly correlated to the severity of the disease.140 However, the correlation of circulating levels of leptin in NAFLD progression is still obscure. Increased level of leptin in nonalcoholic steatohepatitis has been reported with higher in patients with advanced disease which was independently of BMI.141 An increase in serum leptin levels associated with a decrease in soluble leptin receptor, has been observed in subjects with moderate to severe steatosis. This strong negative correlation between serum leptin and soluble leptin receptor found in NAFLD implicates the role of peripheral leptin resistance in these patients.142
Role of gut microbiota
Gut microbiota is now considered as a major metabolic internal organ, composed of >104 microorganisms and containing a second genome which is up to 100–400 times that of humans.143 The intestinal microbiome is composed of bacteria, viruses, yeast and archae.144,145 Recently, there has been a growing body of evidence that implicate gut microbiota in NAFLD and its progression to NASH.146 The advances of “culture independent techniques” focused on 16s ribosomal RNA gene sequencing, having led to linking of gut microbiota to obesity related NAFLD in humans as well as in animal models through the concept of gut–liver axis gut derived products like lipopolysaccharides, bacterial DNA and peptidoglycans reach the liver through the portal vein which activates cell surface receptors and triggers a cascade of signal transductions leading to hepatic inflammation and fibrosis.147 The microbiome composition of obese humans are distinct from normal weight individuals with a lower proportion of bacteroidetes and a higher proportion of firmicutes-Bacteroidetes ratio in NASH148 though a recent study only found a relative lower abundance of bacteroidetes in NASH which was independent of BMI and energy intake from diet. The same was not found in simple steatosis and healthy controls.127 An intact intestinal mucosal barrier acts as a filter to release of products of gut microbiome into circulation. A study by Miele et al has shown that both intestinal permeability and the prevalence of small intestinal bacterial overgrowth are increased in patients with NAFLD and correlate with the severity of steatosis. They have also suggested that the disruption of tight junction integrity in NAFLD leads to cross talk between the gut and the liver. The microbiota causes increased release of endogenous ethanol,146 metabolizes dietary choline causing deficiency and releases LPS.149 TLRs on hepatocytes, recognize these pathogen associated molecular patterns (PAMPs) associated with LPS, unmethylated DNA and lipopeptides and trigger pro inflammatory gene expression and nod-like receptors (NLR) family which upon activation forms molecular machines (“inflammasomes”) that regulate immune responses by processing cytokine precursors into active forms. In a study by Zhu et al, the composition of gut bacterial communities of NASH, obese and healthy children were determined by 16s ribosomal RNA pyrosequencing revealed that each group of subjects had a unique gut microbial composition. Proteobacteria, Enterobacteriaceae and Escherichia were the only phylum, family and genus types, exhibiting significant difference between obese and NASH microbiomes. Alcohol producing bacteria were also significantly higher in NASH microbiome possibly leading to increased oxidative stress and hepatic inflammation.150
Whether the gut microbiome in non-obese NASH is similar to obese NASH is still an area of active and interesting research. The key is to identify a metagenomic sequence which will co relate to NAFL or NASH and translate it into a non invasive diagnostic tool for risk stratification of progression to NASH. Microbiota modulation to prevent or improve NAFL/NASH is an area on developing new therapeutic targets.
Conclusion
In the setting of NAFL, increased influx of FFAs within hepatocytes along with enhanced denovo lipogenesis with associated derangement of compensatory oxidative and endoplasmic retinaculum (ER) stress processes can generate Damp molecules to induce TLRs in various liver cells. These in turn induce downstream production of proinflammatory cytokines to augment inflammatory processes towards progression to NASH. Alternation in gut microbiota or a leaky gut can further aggravate the TLR sensitivity to LPS resulting in a shift from tolerance to immune-active condition in the liver contributing inflammatory processes towards NASH. In addition, emerging evidence suggest that there is a genetic susceptibility for hepatic steatosis in subjects who may or may not have the conventional risks. SNPs in PNPLA3 have been reported to be strongly associated with NAFL and progression to NASH. Whether all the above cross talks between genes, diet, metabolic abnormalities, immunological changes and gut microbiome translate phenotypically to disease progression from NAFL to NASH is still an area of future research.
Conflicts of interest
All authors have none to declare.
References
- 1.Chalasani N., Younossi Z., Lavine J.E. The diagnosis and management of non-alcoholic fatty liver disease: practice guidelines by the American Association for the study of Liver diseases, American College of Gastroenterology, and the American Gastroenterological Association. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Browning J.D., Szczepaniak L.S., Dobbins R. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Bellentani S., Saccoccio G., Masutt F. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 4.Luyckx F.H., Desaive C., Thiry A. Liver abnormalities in severely obese subjects. Effect of drastic weight loss after gastroplasty. Int J Obes. 1998;22:222–226. doi: 10.1038/sj.ijo.0800571. [DOI] [PubMed] [Google Scholar]
- 5.Chiloiro M., Caruso M.G., Cisternino A.M. Ultrasound evaluation and correlates of fatty liver disease: a population study in a Mediterranean area. Metab Syndr Relat Disord. 2013;11:349–358. doi: 10.1089/met.2012.0169. [DOI] [PubMed] [Google Scholar]
- 6.Fan J.G. Epidemiology of alcoholic and non-alcoholic fatty liver disease in china. J Gastroenterol Hepatol. 2013;28(suppl 1):11–17. doi: 10.1111/jgh.12036. [DOI] [PubMed] [Google Scholar]
- 7.Lankarani K.B., Ghaffarpasand F., Mahmoodi M. Non alcoholic fatty liver disease in Southern Iran: a population based study. Hepat Mon. 2013;13:e9248. doi: 10.5812/hepatmon.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eguchi Y., Hyogo H., Ono M. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a muticentre large retrospective study. J Gastroenterol. 2012;47:586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 9.Loomba R., Abraham M., Unalp A. Association between diabetes, family history of diabetes and risk factors of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrell G.C., Wong V.W., Chitturi S. NAFLD in Asia – as common and important as in West. Nat Rev Gastroenterol Hepatol. 2013;10:307–318. doi: 10.1038/nrgastro.2013.34. [DOI] [PubMed] [Google Scholar]
- 11.Das K., Das K., Mukherjee P.S. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 12.Amarapurkar D., Kamani P., Patel N. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161–163. [PubMed] [Google Scholar]
- 13.Singh S.P., Nayak S., Swain M. Prevalence of nonalcoholic fatty liver disease in costal eastern India: a preliminary ultrasonographic survey. Trop Gastroenterol. 2004;25:76–79. [PubMed] [Google Scholar]
- 14.Fan J.G., Li F., Cai X.B., Peng Y.D., Ao Q.H., Gao Y. Effects of nonalcoholic fatty liver disease on development of metabolic disorders. J Gastroenterol Hepatol. 2007;22:1086–1091. doi: 10.1111/j.1440-1746.2006.04781.x. [DOI] [PubMed] [Google Scholar]
- 15.Petersen K.F., Dufour S., Feng J. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian men. Proc Natl Acad Sci U S A. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasarathy S., Dasarathy J., Khiyami A., Joseph R., Lopez R., Mc Cullough A.J. Validity of realtime ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061–1067. doi: 10.1016/j.jhep.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sczepaniak L.S., Nurenberg P., Leonard D.S. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 18.Mofrad P., Contos M.J., Haque M. Clinical and histological spectrum of non-alcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz Y., Eren F. A Bayesian approach to an integrated multimodal noninvasive diagnosis of definitive nonalcoholic steatohepatitis in the spectrum of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2014;26:1292–1295. doi: 10.1097/MEG.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 20.Duseja A., Das A., Das R. The clinicopathological profile of Indian patients with nonalcoholic fatty liver disease (NAFLD) is different from that in the West. Dig Dis Sci. 2007;52:2368–2374. doi: 10.1007/s10620-006-9136-y. [DOI] [PubMed] [Google Scholar]
- 21.Singh S.P., Kar S.K., Panigrahi M.K. Profile of patients with incidentally detected nonalcoholic fatty liver disease (IDNAFLD) in costal eastern India. Trop Gastroenterol. 2013;34:144–152. doi: 10.7869/tg.118. [DOI] [PubMed] [Google Scholar]
- 22.Wong V.W., Wong G.L., Choi P.C. Disease progression of nonalcoholic fatty liver disease: a prospective study with paired liver biopsy at 3 years. Gut. 2010;59:969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 23.Mc Pherson S., Hardy T., Henderson E., Burt A.D., Day C.P., Anstee Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implication for prognosis and clinical management. J Hepatol. 2014;29 doi: 10.1016/j.jhep.2014.11.034. S0168–8278(14)00883-6. [DOI] [PubMed] [Google Scholar]
- 24.Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 25.Musso G., Gambino R., Cassader M., Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 26.Day C.P., James O.F. Steatohepatitis: a tale of two“hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 27.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hit hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 28.Wree A., Broderick Lori, Canbay All, Hoffmann Hal M., Feldstein Ariel E. From NAFLD to NASH to cirrhosis – new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627–636. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 29.Schwimmer J.B., Celedon M.A., Lavine J.E. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speliotes E.K., Yerges-Armstrong L.M., Wu J. Genome- wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawaguchi T., Sumida Y., Umemura A. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PLoS One. 2012;7:e38322. doi: 10.1371/journal.pone.0038322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalasani N., Guo X., Loomba R. Genome-wide association study identifies variants associated with histologic features of nonalcoholic fatty liver disease. Gastroenterology. 2010;139:1567–1576. doi: 10.1053/j.gastro.2010.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He S., McPhaul C., Li J.Z. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rotman Y., Koh C., Zmuda J.M., Kleiner D.E., Liang T.J. The association of genetic variability in palatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;50:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatt S.P., Nigam P., Misra A., Guleria R., Pandey R.M., Pasha M.A. Genetic variation in the patatin-like phospholipase domain-containing protein-3 (PNPLA-3) gene in Asian Indians with nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2013;11:329–335. doi: 10.1089/met.2012.0064. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y.L., Patman G.L., Leathart J.B.S. Carriage of PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated with hepatocellular carcinoma. J Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 37.Stefano R., Julia K., Chao X. Genetic variation in PNPLA3 confers susceptibility to non-alcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onuma H., Tabara Y., Kawamoto R. The GCKR rs780094 polymorphism is associated with susceptibility of type 2 diabetes, reduced fasting plasma glucose levels, increased triglycerides levels and lower HOMA-IR in Japanese population. J Hum Genet. 2010;55:600–604. doi: 10.1038/jhg.2010.75. [DOI] [PubMed] [Google Scholar]
- 39.Kanth V.V., Sasikala M., Rao P.N. Pooled genetic analysis in ultrasound measured non-alcoholic fatty liver disease in Indian subjects: a pilot study. World J Hepatol. 2014;6:435–442. doi: 10.4254/wjh.v6.i6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun S., Wang M., Song H. SCAP gene polymorphisms decrease the risk of nonalcoholic fatty liver disease in females with metabolic syndrome. J Genet. 2013;92:565–570. doi: 10.1007/s12041-013-0280-9. [DOI] [PubMed] [Google Scholar]
- 41.Zain S.M., Mohamed Z., Mahadeva S. Susceptibility and gene interaction study of the angiotensin II type 1 receptor (AGTR1) gene polymorphisms with non-alcoholic fatty liver disease in a multi-ethnic population. PLoS One. 2013;8:e58538. doi: 10.1371/journal.pone.0058538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S., Li Y., Li S., Yu C.A. Val227Ala substitution in the peroxisome proliferator activated receptor alpha (PPAR alpha) gene associated with non-alcoholic fatty liver disease and decreased waist circumference and waist-to-hip ratio. J Gastroenterol Hepatol. 2008;23:1415–1418. doi: 10.1111/j.1440-1746.2008.05523.x. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y.P., Liang L., Wang C.L. Association between UCP3 gene polymorphisms and nonalcoholic fatty liver disease in Chinese children. World J Gastroenterol. 2013;19:5897–5903. doi: 10.3748/wjg.v19.i35.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musso G., Bo S., Cassader M., DeMichieli F., Gambino R. Impact of sterol regulatory element-binding factor-1c polymorphism on incidence of nonalcoholic fatty liver disease and on the severity of liver disease and of glucose and lipid dysmetabolism. Am J Clin Nutr. 2013;98:895–906. doi: 10.3945/ajcn.113.063792. [DOI] [PubMed] [Google Scholar]
- 45.Barbieri M., Esposito A., Angellotti E., Rizzo M.R., Marfella R., Paolisso G. Association of genetic variation in adaptor protein APPL1/APPL2 loci with non-alcoholic fatty liver disease. PLoS One. 2013;8:e71391. doi: 10.1371/journal.pone.0071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sazci A., Ozel M.D., Ergul E., Aygun C. Association of nicotinamide-N-methyltransferase gene rs694539 variant with patients with nonalcoholic steatohepatitis. Genet Test Mol Biomarkers. 2013;17:849–853. doi: 10.1089/gtmb.2013.0309. [DOI] [PubMed] [Google Scholar]
- 47.Namikawa C., Shu-Ping Z., Vyselaar J.R. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non-alcoholic steatohepatitis. J Hepatol. 2004;40:781–786. doi: 10.1016/j.jhep.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 48.Peterson K.F., Dufour S., Hariri A. Apolipoprotein C3 gene variants in non-alcoholic fatty liver disease. N Eng J Med. 2010;362:1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puppala J, Bhrugumalla S, Kumar A, et al Apolipoprotein C3 gene polymorphism in Southern Indian patients with nonalcoholic fatty liver disease. Indian J Gastroenterol [epub] 2014.Oct 17 [DOI] [PubMed]
- 50.Ravikanth V.V., Sasikala M., Urmila S.A. PNPLA3 gene polymorphism but not APOC3 enhances risk for nonalcoholic fatty liver disease in Indian subjects. J Clin Exp Hepatol. 2013;3:S24. [Google Scholar]
- 51.Santoro N., Savoye M., Kim G. Hepatic fat accumulation is modulated by the interaction between the rs738409 variant in the PNPLA3 gene and the dietary omega6/omega3 PUFA intake. PLoS One. 2012;7:e37827. doi: 10.1371/journal.pone.0037827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sevastianova K., Kotronen A., Gastaldelli A. Genetic variation in PNPLA3 (adiponutrin) confers sensitivity to weight loss-induced decrease in liver fat in humans. Am J Clin Nutr. 2011;94:104–111. doi: 10.3945/ajcn.111.012369. [DOI] [PubMed] [Google Scholar]
- 53.Stickel F., Buch S., Lau K. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in Caucasians. Hepatology. 2011;53:86–95. doi: 10.1002/hep.24017. [DOI] [PubMed] [Google Scholar]
- 54.Dhillon B.K., Das R., Garewal G. Frequency of primary iron overload and HFE gene mutations (C282Y, H63D and S65C0 in chronic liver disease patients in North India. World J Gastroenterol. 2007;13:2956–2959. doi: 10.3748/wjg.v13.i21.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Das U.N. Biological significance of essential fatty acids. J Assoc Physicians India. 2006 Apr;54:309–319. [PubMed] [Google Scholar]
- 56.Seki H., Tani Y., Arita M. Omega-3 PUFA derived anti-inflammatory lipid mediator resolving E1. Prostaglandins Other Lipid Mediat. 2009;89:126–130. doi: 10.1016/j.prostaglandins.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Dentin R., Benhamed F., Pégorier J.P. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005;15:2843–2854. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Araya J., Rodrigo R., Videla L.A. Increase in long chain polyunsaturated fatty acid n6/n3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2004;106:635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 59.Parker H.M., Johnson N.A., Burdon C.A., Cohn J.S., O'Connor H.T., George J. Omega 3 supplementation and non alcoholic fatty liver disease: a systemic review and meta-analysis. J Hepatol. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 60.Feldstein A.E., Lopez R., Tamimi T.A. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monetti M., Levin M.C., Watt M.J. Dissociation of hepatic steatosis and insulin resistance in mice over expressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Liao W., Hui T.Y., Young S.G., Davis R.A. Blocking microsomal triglyceride transfer protein interferes with apoB secretion without causing retention or stress in the ER. J Lipid Res. 2003;44:978–985. doi: 10.1194/jlr.M300020-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Li Z.Z., Berk M., McIntyre T.M., Feldstein A.E. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009 Feb 27;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ntambi J.M., Miyazaki M., Stoehr J.P. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 66.Scorletti E., Bhatia L., McCormick K.G. Effects of purified eicosapentaenoic and docosahexaenoic acids in non-alcoholic fatty liver disease: results from the *WELCOME study. Hepatology. 2014;60:1211–1221. doi: 10.1002/hep.27289. [DOI] [PubMed] [Google Scholar]
- 67.Han M.S., Park S.Y., Shinzawa K. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 68.Sinn D.H., Gwak G.Y., Park H.N. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic middle-aged Asian adults. Am J Gastroenterol. 2012;107:561–567. doi: 10.1038/ajg.2011.400. [DOI] [PubMed] [Google Scholar]
- 69.Kumar R., Rastogi A., Sharma M.K. Clinicopathological characteristics and metabolic profiles of non-alcoholic fatty liver disease in Indian patients with normal body mass index: do they differ from obese or overweight non-alcoholic fatty liver disease? Indian J Endocrinol Metab. 2013;17:665–671. doi: 10.4103/2230-8210.113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choudhary N.S., Duseja A., Kalra N., Das A., Dhiman R.K., Chawla Y.K. Correlation of adipose tissue with liver histology in Asian Indian patients with nonalcoholic fatty liver disease (NAFLD) Ann Hepatol. 2012;11:478–486. [PubMed] [Google Scholar]
- 71.Sanal M.G., Sarin S.K. Association of nonalcoholic fatty liver disease with metabolic syndrome in Indian population. Diabetes Metab Syndr. 2011;5:76–80. doi: 10.1016/j.dsx.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 72.Bajaj S., Nigam P., Luthra A. A case control study on insulin resistance, metabolic co-variates and prediction score in non-alcoholic fatty liver disease. Indian J Med Res. 2009;129:285–292. [PubMed] [Google Scholar]
- 73.Singh S.P., Misra B., Kar S.K. Nonalcoholic fatty liver disease (NAFLD) without insulin resistance: Is it different? Clin Res Hepatol Gastroenterol. 2014 Dec 17 doi: 10.1016/j.clinre.2014.08.014. pii: S2210-7401(14)00285-X. http://dx.doi.org/10.1016/j.clinre.2014.08.014. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 74.Bhat G., Baba C.S., Pandey A., Kumari N., Choudhuri G. Insulin resistance and metabolic syndrome in nonobese Indian patients with nonalcoholic fatty liver disease. Trop Gastroenterol. 2013;34:18–24. doi: 10.7869/tg.2012.86. [DOI] [PubMed] [Google Scholar]
- 75.Stanhope K.L., Havel P.J. Fructose consumption: recent results and their potential implications. Ann N Y Acad Sci. 2010;1190:15–24. doi: 10.1111/j.1749-6632.2009.05266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lustig R.H. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc. 2010;110:1307–1321. doi: 10.1016/j.jada.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 77.Lim J.S., Mietus-Snyder M., Valente A., Schwarz J.M., Lustig R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 78.Lê K.A., Tappy L. Metabolic effects of fructose. Curr Opin Clin Nutr Metab Care. 2006;9:469–475. doi: 10.1097/01.mco.0000232910.61612.4d. [DOI] [PubMed] [Google Scholar]
- 79.D'Angelo G., Elmarakby A.A., Pollock D.M., Stepp D.W. Fructose feeding increases insulin resistance but not blood pressure in Sprague–Dawley rats. Hypertension. 2005 Oct;46:806–811. doi: 10.1161/01.HYP.0000182697.39687.34. [DOI] [PubMed] [Google Scholar]
- 80.Nolan J.P. The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology. 2010;52:1829–1835. doi: 10.1002/hep.23917. [DOI] [PubMed] [Google Scholar]
- 81.Aigner E., Strasser M., Haufe H. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am J Gastroenterol. 2010;105:1978–1985. doi: 10.1038/ajg.2010.170. [DOI] [PubMed] [Google Scholar]
- 82.Nelson J.E., Klintworth H., Kowdley K.V. Iron metabolism in nonalcoholic fatty liver disease. Curr Gastroenterol Rep. 2012;14:8–16. doi: 10.1007/s11894-011-0234-4. [DOI] [PubMed] [Google Scholar]
- 83.Ibrahim M.M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 84.Rodríguez A., Catalán V., Gómez- Ambrosi J., Frühbeck G. Visceral and subcutaneous adiposity: are both potential therapeutic targets for tackling the metabolic syndrome? Curr Pharm Des. 2007;13:2169–2175. doi: 10.2174/138161207781039599. [DOI] [PubMed] [Google Scholar]
- 85.Leung C., Herath C.B., Jia Z. Dietary glycotoxins exacerbate progression of experimental fatty liver disease. J Hepatol. 2014;60:832–838. doi: 10.1016/j.jhep.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 86.Mastrocola R., Collino M., Rogazzo M. Advanced glycation end products promote hepatosteatosis by interfering with SCAP-SREBP pathway in fructose-drinking mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G398–G407. doi: 10.1152/ajpgi.00450.2012. [DOI] [PubMed] [Google Scholar]
- 87.Tang X.Z., Jo J., Tan A.T. IL-7 licenses activation of human liver intra sinusoidal mucosal-associated invariant T cells. J Immunol. 2013;190:3142–3152. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- 88.Longhi M.S., Ma Y., Mieli-Vergani G., Vergani D. Regulatory T cells in autoimmune hepatitis. J Hepatol. 2012;57:932–933. doi: 10.1016/j.jhep.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 89.Lleo A., Invernizzi P. Apotopes and innate immune system: novel players in the primary biliary cirrhosis scenario. Dig Liver Dis. 2013;45:630–636. doi: 10.1016/j.dld.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 90.Rana D., Duseja A., Dhiman R.K., Chawla Y., Arora S.K. Maturation defective myeloid dendritic cells in nonalcoholic fatty liver disease patients release inflammatory cytokines in response to endotoxin. Hepatol Int. 2013;7:562–569. doi: 10.1007/s12072-012-9371-6. [DOI] [PubMed] [Google Scholar]
- 91.Schulz C., Gomez P.E., Chorro L. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 92.Tang T., Sui Y., Lian M., Li Z., Hua J. Pro-inflammatory activated kupffer cells by lipids induce hepatic NKT cells deficiency through activation-induced cell death. PLoS One. 2013;8:e81949. doi: 10.1371/journal.pone.0081949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knolle P., Schlaak J., Uhrig A., Kempf P., Meyerzum Buschenfelde K.H., Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide(LPS) challenge. J Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 94.Seki E., Tsutsui H., Nakano H. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor88 that is critically involved in induction of production of IL-12and IL-1b. J Immunol. 2001;166:2651–2657. doi: 10.4049/jimmunol.166.4.2651. [DOI] [PubMed] [Google Scholar]
- 95.Gatselis N.K., Ntaios G., Makaritsis K., Dalekos G.N. Adiponectin: a key play-maker adipocytokine in non-alcoholic fatty liver disease. Clin Exp Med. 2014;14:121–131. doi: 10.1007/s10238-012-0227-0. [DOI] [PubMed] [Google Scholar]
- 96.Han M.S., Jung D.Y., More I.C. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;11;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y., Chen K., Wang C. Cell surface receptor FPR2 promotes antitumor host defense by limiting M2 polarization of macrophages. Cancer Res. 2013;73:550–560. doi: 10.1158/0008-5472.CAN-12-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhan Y.T., An W. Roles of liver innate immune cells in nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:4652–4660. doi: 10.3748/wjg.v16.i37.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O'Shea D., Cawood T.J., O'Farrelly C., Lynch L. Natural killer cells in obesity: impaired function and increased susceptibility to the effects of cigarette smoke. PLoS One. 2010;5:e8660. doi: 10.1371/journal.pone.0008660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kahraman A., Schlattjan M., Kocabayoglu P. Major histocompatibility complex class I-related chains A and B (MIC A/B): a novel role in nonalcoholic steatohepatitis. Hepatology. 2010;51:92–102. doi: 10.1002/hep.23253. [DOI] [PubMed] [Google Scholar]
- 101.Bendelac A., Savage P.B., Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 102.Park O., Jeong W.I., Wang L. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Syn W.K., Oo Y.H., Pereira T.A. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu S., Gallo D.J., Green A.M. Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun. 2002;70:3433–3442. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsumura T., Degawa T., Takii T. TRAF6-NF-kappaB pathway is essential for interleukin-1-induced TLR2 expression and its functional response to TLR2 ligand in murine hepatocytes. Immunology. 2003;109:127–136. doi: 10.1046/j.1365-2567.2003.01627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mimura Y., Sakisaka S., Harada M., Sata M., Tanikawa K. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology. 1995;109:1969–1976. doi: 10.1016/0016-5085(95)90765-3. [DOI] [PubMed] [Google Scholar]
- 107.Li L., Chen L., Hu L. Nuclear factor high-mobility group box1 mediating the activation of toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology. 2011;54:1620–1630. doi: 10.1002/hep.24552. [DOI] [PubMed] [Google Scholar]
- 108.Fujii H., Kawada N. Inflammation and fibrogenesis in steatohepatitis. J Gastroenterol. 2012;47:215–225. doi: 10.1007/s00535-012-0527-x. [DOI] [PubMed] [Google Scholar]
- 109.Tsukamoto H., She H., Hazra S., Cheng J., Miyahara T. Anti-adipogenic regulation underlies hepatic stellate cell transdifferentiation. J Gastroenterol Hepatol. 2006;(suppl 3):S102–S105. doi: 10.1111/j.1440-1746.2006.04573.x. [abstract] [DOI] [PubMed] [Google Scholar]
- 110.Choi S.S., Witek R.P., Yang L. Activation of Rac1 promotes hedgehog-mediated acquisition of the myofibroblastic phenotype in rat and human hepatic stellate cells. Hepatology. 2010;52:278–290. doi: 10.1002/hep.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Minicis S., Svegliati-Baroni G. Fibrogenesis in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5:179–187. doi: 10.1586/egh.11.28. [DOI] [PubMed] [Google Scholar]
- 112.Choi S.S., Syn W.K., Karaca G.F. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem. 2010; 19;285:36551–36560. doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ikejima K., Takei Y., Honda H. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122:1399–1410. doi: 10.1053/gast.2002.32995. [DOI] [PubMed] [Google Scholar]
- 114.Seki E., Brenner D.A. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 115.Ikeda H., Sasaki M., Ishikawa A. Interaction of Toll-like receptors with bacterial components induces expression of CDX2 and MUC2 in rat biliary epithelium in vivo and in culture. Lab Invest. 2007;87:559–571. doi: 10.1038/labinvest.3700556. [DOI] [PubMed] [Google Scholar]
- 116.Harada K., Isse K., Nakanuma Y. Interferon gamma accelerates NF-kappaB activation of biliary epithelial cells induced by Toll-like receptor and ligand interaction. J Clin Pathol. 2006;59:184–190. doi: 10.1136/jcp.2004.023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chiba M., Sasaki M., Kitamura S., Ikeda H., Sato Y., Nakanuma Y. Participation of bile ductular cells in the pathological progression of non-alcoholic fatty liver disease. J Clin Pathol. 2011;64:564–570. doi: 10.1136/jcp.2011.090175. [DOI] [PubMed] [Google Scholar]
- 118.Tilg H., Diehl A.M. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000; 16;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 119.Jarrar M.H., Baranova A., Collantes R. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412–421. doi: 10.1111/j.1365-2036.2007.03586.x. [DOI] [PubMed] [Google Scholar]
- 120.Crespo J., Cayon A., Fernandez-Gil P. Gene expression of tumor necrosis factor alpha and TNF-receptors,p55 and p75,in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 121.Manco M., Marcellini M., Giannone G., Nobili V. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;127:954–960. doi: 10.1309/6VJ4DWGYDU0XYJ8Q. [DOI] [PubMed] [Google Scholar]
- 122.Tokushige K., Takakura M., Tsuchiya-Matsushita N., Taniai M., Hashimoto E., Shiratori K. Influence of TNF gene polymorphisms in Japanese patients with NASH and simple steatosis. J Hepatol. 2007;46:1104–1110. doi: 10.1016/j.jhep.2007.01.028. PMID:17395331. [DOI] [PubMed] [Google Scholar]
- 123.Sabio G., Das M., Mora A. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 125.Kumar R., Prakash S., Chhabra S. Association of pro-inflammatory cytokines, adipokines and oxidative stress with insulin resistance and non-alcoholic fatty liver disease. Indian J Med Res. 2012;136:229–236. [PMC free article] [PubMed] [Google Scholar]
- 126.Kumar A., Sharma A., Duseja A. Patients with nonalcoholic fatty liver disease (NAFLD) have higher oxidative stress in comparison to chronic viral hepatitis. J Clin Exp Hepatol. 2013;3:12–18. doi: 10.1016/j.jceh.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Narasimhan S., Gokulakrishnana K., Sampathkumar R. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clin Biochem. 2011;40:815–821. doi: 10.1016/j.clinbiochem.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 128.Miura K., Kodama Y., Inokuchi S. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–334. doi: 10.1053/j.gastro.2010.03.052. e7. Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011 Jul;54(1):133-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Csak T., Ganz M., Pepsia J., Kodys K., Dolganiuc A., Szabo G. Fatty liver and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–144. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Netea M.G., Nold-Petry C.A., Nold M.F. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;5;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Csak T., Dolganiuc A., Kodys K. Mitochondrial antiviral signaling protein defect links impaired antiviral response and liver injury in steatohepatitis in mice. Hepatology. 2011;53:1917–1931. doi: 10.1002/hep.24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ricchi M., Odoardi M.R., Carulli L. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24:830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 133.Ji J., Zhang L., Wang P. Saturated free fatty acid, palmitic acid, induces apoptosis in fetal hepatocytes in culture. Exp Toxicol Pathol. 2005;56:369–376. doi: 10.1016/j.etp.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 134.Tsochatzis E.A., Papatheodoridis G.V., Archimandritis A.J. Adipokines in nonalcoholic steatohepatitis: from pathogenesis to implications in diagnosis and therapy. Mediators Inflamm. 2009;2009:831670. doi: 10.1155/2009/831670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tilg H., Moschen A.R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;10:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 136.Hui J.M., Hodge A., Farrell G.C., Kench J.G., Kriketos A., George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 137.Hijona E., Hijona L., Arenas J.I., Bujanda L. Inflammatory mediators of hepatic steatosis. Mediat Inflamm. 2010;2010:837419. doi: 10.1155/2010/837419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bajaj M., Suraamornkul S., Piper P. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:200–206. doi: 10.1210/jc.2003-031315. [DOI] [PubMed] [Google Scholar]
- 139.Gaddipati R., Sasikala M., Padaki N. Visceral adipose tissue visfatin in nonalcoholic fatty liver disease. Ann Hepatol. 2010;9:266–270. [PubMed] [Google Scholar]
- 140.Myers M.G., Cowley M.A., Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 141.Uygun A., Kadayifci A., Yesilova Z. Serum leptin levels in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2000;95:3584–3589. doi: 10.1111/j.1572-0241.2000.03297.x. [DOI] [PubMed] [Google Scholar]
- 142.Xiao D.H., Yan F., Hen Z. Serum leptin and soluble leptin receptor in non-alcoholic fatty liver disease. World J Gastroenterol. 2008;14:2888–2893. doi: 10.3748/wjg.14.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qin J., Li R., Raes J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Whitman W.B., Coleman D.C., Wiebe W.J. Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mouzaki M., Comelli E.M., Arendt B.M. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 146.Miele L., Valenza V., La Torre G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 147.Compare D., Coccoli P., Rocco A. Gut–liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:471–476. doi: 10.1016/j.numecd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 148.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006; 21;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 149.Jacob A.I., Goldberg P.K., Bloom N., Degenshein G.A., Kozinn P.J. Endotoxin and bacteria in portal blood. Gastroenterology. 1977;72:1268–1270. [PubMed] [Google Scholar]
- 150.Zhu L., Baker S.S., Gill C. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]