Abstract
Background
Currently, undifferentiated cells are found in all tissue and term as local stem cells which are quiescent in nature and less in number under normal healthy conditions but activate upon injury and repair the tissue or organs via automated activating mechanism. Due to very scanty presence of local resident somatic local stem cells in healthy organs, isolation and expansion of these adult stems is an immense challenge for medical research and cell based therapy. Particularly organ like liver, there is an ongoing controversy about existence of liver stem cells.
Methods
Herein, Hepatic stem cells population was identified during culture of primary hepatocyte cells upon immediate isolation of primary hepatocyte cells. These liver stem cells has been expanded extensively and differentiated into primary hepatocytes under defined culture conditions in a nanostructured self assembling peptides modular bioreactor that mimic the state of art of liver microenvironment and compared with Matrigel as a positive control. Nanostructured self assembling peptides were used a defined extracellular matrix and Matrigel was used for undefined extracellular matrix. Proliferation of hepatic stem cells was investigated by two strategies. First strategy is to provide high concentration of hepatocyte growth factor (HGF) and second strategy is to evaluate the role of recombinant human erythropoietin (rHuEPO) in presence of trauma/ischemia cytokines (IL-6, TNF-α). Expansion to hepatic differentiation is observed by morphological analysis and was evaluated for the expression of hepatocyte-specific genes using RT-PCR and biochemical methods.
Results
Hepatocyte-specific genes are well expressed at final stage (day 21) of differentiation period. The differentiated hepatocytes exhibited functional hepatic characteristics such as albumin secretion, urea secretion and cytochrome P450 expression. Additionally, immunofluorescence analysis revealed that hepatic stem cells derived hepatocytes exhibited mature hepatocyte markers (albumin, CK-19, CPY3A1, alpha 1-antitrypsin). Expansion and hepatic differentiation was efficiently in nanostructured self assembling peptides without such batch to batch variation while there was much variation in Matrigel coated bioreactor. In conclusion, the results of the study suggest that the nanostructured self assembling peptides coated bioreactor supports expansion as well as hepatic differentiation of liver stem cells which is superior than Matrigel.
Conclusion
This defined microenvironment conditions in bioreactor module can be useful for research involving bioartificial liver system, stem cell research and engineered liver tissue which could contribute to regenerative cell therapies or drug discovery and development.
Keywords: bioreactor, defined culture conditions, hepatic stem cells, Matrigel, nanostructured self assembling peptides
Abbreviations: HGF, Hepatocyte growth factor; rHuEPO, Recombinant human erythropoietin; IL-6, Interleukin 6; TNF-α, Tumor necrosis factor alpha; CK-19, Cytokeratin 19; CPY3A1, Cytochrome P450 3A 1; A1AT, Alpha 1-antitrypsin; GaIN, D-galactosamine; CK 7, Cytokeratin 7; AFP, α-fetoprotein; Thy1, Thy-1 cell surface antigen; EROD, Ethoxyresorufin O-deethylase; MROD, Methoxyresorufin O-demethylase; PROD, Pentoxyresorufin O-depentylase
Adult stem cells renew our damaged and aged cells throughout life. Currently adult stem cell based therapies is central focus for scientist and clinicians. Generally adult stem cells are small in numbers and quite silent or inactive state and activated during disease or tissue damage conditions for regeneration. Local adult stem cells do two major roles such: manipulate themselves to increase the numbers, differentiated required tissue or cells to repair diseased tissue or aged tissue under specific microenvironments. Identifying, isolating, expanding and hepatic differentiation in a defined microenvironment of hepatic stem cells is a really a challenge for next generation of adult stem cell based therapies. There is an ongoing controversy about existence of hepatic stem cells. Recently, Watanabe et al., reported a novel monoclonal antibody to detect hepatic stem-like cells in rats and recommended that liver epithelial cells, oval cells, and fetal liver cells share a common cell marker of liver stem cells.1 Theoretically, Oval cells or small hepatocytes, hepatic stem cells are all considered as facultative hepatic stem cells which have potential to differentiate into hepatocytes and cholangiocytes and activated when hepatocytes are blocked and unable to replicated or repaired. Hepatic stem-like cells lines have been established from normal adult porcine liver2, normal adult rat liver3 and human postnatal livers and fetal livers.4 Hirata et al., reported that their hepatic stem cell like populations differ from small hepatocytes.3 There is a confusing terminology about oval cells and small hepatocytes.5 Liminre et al., concluded that duct cells of terminal bile ductular system can generate both oval cells and small hepatocytes in response to D-galactosamine (GaIN).6 Avril et al., reported that mature hepatocytes are the source of small hepatocytes in a retrorsine model of liver injury.7 Oval cells or small hepatocytes, hepatic stem cells share many characters but still under debate due lack of unique specific marker for hepatic stem cells. Oval cells appear in rat liver when treated with hepatoxin. The main markers of oval cells are CK 7 and CK 19, α-fetoprotein (AFP), CD34, c-kit, and Thy1.8-11 Recently it was report that hepatic differentiation of oval cells is occurred sequentially from Thy1+CD44-, to Thy1+CD44+, and then Thy1-CD44+ cells and finally these CD 44 + cells became to mature hepatocytes.12,13 It has been widely accepted that CD 44 is well known cancer stem cell surface markers.14 Kon et al., reported that CD 44 is a specific marker for small hepatocytes.15 Small hepatocyte termed as a sub population of hepatocyte which can be isolated from normal healthy rat liver16, 17 and human liver18 without giving hepatoxin. It was reported that bone marrow cells are also sources of oval cells.19, 20 Numerous reports demonstrated that hepatic bile ductular system is main source of oval cells.21, 22 Stem-like populations of cells are also involved to regenerate the lost liver mass.23, 24 Further it is a controversial question whether the major hepatic stem cells are epithelial cells which exist in the liver or are in part from the circulating pool of hematopoietic stem cells and become mature hepatocyte via hepatic stem cell or oval cells or small hepatocytes. It is also hypothesized that hepatic stem cells may be derived from hematopoietic stem cells and share hematopoietic markers, such as CD34, thy1, c-kit and CD133. However, another group also reported that these markers are not representative of hepatic stem cells.25, 26 Taken all together, there is growing controversy of hepatic stem cells, however, all still remain unclear.
Isolation and expansion of adult hepatic stem cells from a healthy rat liver and their efficient hepatic differentiation of under well-defined vivo hepatic like microenvironment in a nanostructured self assembling peptides coated multiwell bioreactor was conducted. There two types of well-defined vivo hepatic like microenvironment were conducted separately for proliferation of hepatic stem cells and efficient hepatic differentiation under special combination of growth factors, cytokines. Further, two different strategies were used for proliferation of hepatic stem cells. First strategy to provide of high concentration of HGF and second strategy is to evaluate the role of rHuEPO in presence of trauma/ischemia cytokines (IL-6, TNF-α). Because rHuEPO has been used for many organ regeneration including liver regeneration in clinical setting. Schmeding et al., investigated that rHuEPO reduces ischemia-reperfusion injury transplanted rat livers and significantly improved survival conditions.27 Further, TNF-α and IL-6 plasma levels remains significantly elevated in infected cirrhotic patients compared than noninfected cirrhotic patients.28 Liver regeneration in rat is also associated with TNF-alpha/IL-6 signals.29 IL-6 has significant role for protection of liver from ischemia and stimulates hepatocyte to proliferate after reperfusion.30 IL-6 has important role for survivability of mice after partial hepatectomy.31 Numerous evidences reported that TNF-α and IL-6 are required for liver regeneration.32-34 Under some stress conditions, TNF-α and IL-6 released in hepatic microenvironments which stimulate hepatocytes to reenter the proliferation state.35-38 We hypothesize that whether TNF-alpha and IL-6 on hepatic stem cells under influence of rHuEPO. So we attempted to establish an in vitro trauma model for proliferation of hepatic liver stem cells.
Small number of hepatic stem cells population was noticed during culture of rat primary hepatocyte cells upon immediate isolation of primary hepatocytes cell. Nanostructured self assembling peptides coated multiwell bioreactor system was used from expansion of these cells under influence of hepatocyte growth factors initially up to 1 week and compared with Matrigel coated bioreactor. Herein it was designed to integrate self-assembling peptides (Puramtarix, we named it here as Nanomatrix), is completely defined, and composed of short, repeating units of amino acids self-organize spontaneously to build nanostructures of interwoven nanofibers with diameters of 10–20 nm. We compare the potential of nanostructured self-assembling peptides with Matrigel. Matrigel is composed of laminin, collagen IV, and enactin and several growth factors which originally from Englebreth-Holm-Swarm tumors of mice. Although it is considered an undefined extracellular matrix but it has been used as optimal matrix in wide range in primary cells, cancer cell including stem cell culture. Matrigel has contributed significantly in stem cell culture for self-renewal and pluripotency but has major limitations due to undefined contribution. In this study, the potential of hepatic stem cells to differentiate into functional mature hepatocytes within designed self assembling peptides has been investigated along with comparison with Matrigel. Expansion potential hepatic stem cells and biochemical and molecular features of hepatocyte-like cells differentiated from hepatic stem cells on the nanostructured self assembling peptides coated multiwall bioreactor were used to show the role of self assembling peptides to enhance efficient differentiation and spontaneous generation under defined microenvironment.
1. Experimental section
1.1. Isolation of Hepatocytes
Hepatocytes were isolated from male Sprague-Dawley rats (weighing 200–250 g) by the two-step collagenase perfusion method, as previously described.39 The isolated cells were purified by Percoll iso-density centrifugation and their viability was examined by the trypan blue exclusion test (more than 85 to 95% in this experiment). Cell viability was assessed by trypan blue exclusion and hepatocytes with a viability of greater than 85–90% were used. Hepatocytes were cultured in Williams' E medium supplemented with L-glutamine 2 mM, penicillin 100 U/ml, streptomycin 100 μg/ml, dexamethasone 1 μM, insulin 0.2 U/ml, glucagon 4 ng/ml without serum supplement. Hepatocytes were plated on mini bioreactor coated with nanostructured self assembling peptides and incubated in at 37 °C and 5% CO2. After 5 hours after plating, the medium was replaced for removal of non-adherent cells.
1.2. Detection and Expansion of Hepatic Stem Cell in Primary Rat Hepatocyte Culture
The exact location of hepatic stem cell is remains unclear, however numerous researchers hypothesized that canal of Hering is probably origin of hepatic stem cell. Hypothesized diagrammatic representation of location of hepatic stem cell in live lobule is given in Figure 11A. After about 2 d, few hepatic stem cell colonies were appeared in culture of culture of primary adult rat hepatocytes nanostructured self assembling peptides bioreactor. High concentration of HGF (100 ng/ml) was given to quick proliferation of such hepatic stem population. After 7 d, there hepatic stem cells were confluent over hepatocytes. Then the cells were splitted using accutase and replated at nanostructured self assembling peptides bioreactor under influence of HGF. The another strategy is to evaluate the role of rHuEPO (10 ng/ml) for proliferation of hepatic stem cells in the presence and absence of trauma cytokines (IL-6 (10 ng/ml) and TNF alpha (10 ng/ml) in nanostructured self assembling peptides bioreactor under defined condition and Matrigel coated bioreactor.
Figure 11.
(A): Hypothethesized diagrammatic representation of location of hepatic stem cell in liver. (a) The rat liver, (b) Hepatic lobular units is the structural functional units or lobules of the liver (c) Single lobular unit, (d) Overall details of single structural functional unit or lobule of the liver. Hepatic stem cells niches have been identified within the liver in the canals of Hering. Generally, hepatocyte has potential to regenerate itself upon injury or aging. When hepatocyte unable to proliferate, hepatic stem cell activate and convert to hepatocyte. It may classified as three types of hepatocytes in structural functional lobule of the liver, (1) fully mature hepatocytes or about to terminate, present towards central vein (2) immature hepatocyte regarded as small hepatocytes, present towards bile duct (3) There are some hepatocytes are present in between the fully mature hepatocytes and small hepatocytes, is termed as maturing hepatocytes. (B). Preparation of nanostructured self assembling peptides coated bioreactor.
1.3. Proliferation Assay
To determine the rate of hepatic stem cell proliferation (fold increase) of liver stem cells in nanostructured self assembling peptides bioreactor, hepatic stem cells were seeded with HGF and without HGF at an initial density of 2 x 104 cells per cm2. Hepatic stem cells were incubated for 7 days at 37 °C in a humidified 5% CO2 incubator. Cell growth at different time points (day 1, day 3 and day 7) was measured by quick proliferation assay kit/(abcam) and BrdU cell proliferation assay kit. Similar proliferation assay was conducted to evaluate the role of rHuEPO for proliferation of hepatic stem cells.
1.4. Preparation of Nanostructured Self Assembling Peptides Coated Bioreactor and Matrigel Coated Bioreactor
Nanostructured self assembling peptides coated multiwall bioreactor are prepared based on previous method.40, 41 Briefly, the overall procedure is represented diagrammatically is Figure 11B. Expansion (WE) media were changed daily for proliferation and specific combination of growth factors and cytokines were explored for hepatic differentiation. Matrigel (BD Biosciences, Germany) was also used as a positive control for hepatic differentiation of liver stem cells. Thin Matrigel layer coated bioreactor according to manufacture guidelines like previously described (Hong and Glauert, 2000). Matrigel (470 μg/cm2 Matrigel) was uniformly spread over the bottom of an ice-cold bioreactor. The plate was transferred to a 37 °C incubator for 1- 4 h to allow gelation in multiwall bioreactor for starting hepatic differentiation experiment. Although our clinically relevant bioreactor which has been tested the in a preclinical porcine model,42 is differ in size but the fundamental design of our flat membrane clinically relevant bioreactor43 and mini bioreactor is similar.44 The present experiment utilized mini multiwall bioreactor for expansion and differennation in a defined culture conditions.
1.5. Hepatic Differentiation Protocols
For efficient hepatic differentiation, hepatic stem cells having low passage number were cultivated at 30% confluence on the self-assembling peptides coated bioreactor. Here, three major step protocols in both experimental setups were used (sequentially to mimic in vivo liver development). Cells sequentially exposed to hepatogenic factors were cultured in the presence of liver-specific cytokines and growth factors, added either sequentially (days 0–3: William's E Medium + Activin A (100 ng/ml) + WNT 3a (50 ng/ml); days 3–6: William's E Medium + BMP-4 (50 ng/ml) + FGF-2 (10 ng/ml); days 6-8: William's E Medium + FGF-10 (20 ng/ml); days 8-12; William's E Medium + RA 1 μM + FGF-10 (20 ng/ml); days 12-20: William's E Medium + HGF (50 ng/m) + FGF -4 (20 ng/ml) + EGF (20 ng/ml) + Oncostatin M (10 ng/ml) + Dex (0.1 μM). Differentiation media were changed every 2 days. Bile duct-like differentiation protocol is the addition high concentration of HGF in addition to sequential protocol. To evaluate the differentiation potential of hepatic stem cells into cholangiocytes, hepatic stem cells were cultured with high concentration of HGF (200 ng/ml) for 21 day until the special bile duct-like structures appeared.
1.6. Albumin Secretion Assay
Albumin production by hepatic stem cells derived hepatocytes cells in culture was measured in supernatant medium collected every other day using our previous described ELISA method.39,40 Albumin secretion was determined immunochemically with an ELISA technique, using supernatants collected on a daily basis for 2 weeks. Cell culture supernatants were collected every 24 h up to 21 days in culture and stored at 4 °C for further analysis. Chromatographically purified rat albumin and the monoclonal antibody for rat albumin were purchased from Cappel (Durham, NC). Ninety-six-well plates (Nunc, Wiesbaden, Germany) were coated with albumin and left overnight at 4 °C. The coating buffer contained 1 mg/ml albumin. After washing the plate four times, 50 ml of cell culture supernatant was added to the wells and incubated with 50 ml of anti-rat albumin antibody conjugated to horseradish peroxidase. After 24 h at 4 °C, substrate buffer containing O-phenylenediamine dihydrochloride and H2O2 was added for 6 min. The reaction was stopped with 100 ml of 8 N H2SO4. Absorbance was measured at 490 nm using a Dynatech M5000 spectrophotometer. The standard curve ranged between 1 and 100 mg albumin/ml.
1.7. Urea Assay
Urea concentration was assayed by the enzymatic urease method (Sigma).39,40
1.8. Ammonia Removal Efficiency
The ammonia detoxification functions of cells in the bioreactor were estimated by assessing the ability of cells to eliminate ammonia in the presence of 1 mM of ammonia in the culture medium using previous described method. 45,46.
1.9. RNA Isolation, cDNA Synthesis and RT- PCR
Total RNA was isolated from cultured cells using a RNeasy MinElute Cleanup Kit and then 8 μg of the RNA was reverse transcribed into cDNA from cultured rat primary liver cells prepared using the Fastlane Cell cDNA kit (Qiagen, Hilden, Germany) according to manufacturer's instructions. Quantitative real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems). Primers for rat albumin and CYPA31 and GAPDH genes were selected (Rat CYP3A1-specific primer set: forward, 5′ GCCATCACGGACACAGAAATA 3′; reverse, 5′ GAACGTGGGTGACAGTAAGGCT 3′; rat albumin forward, 5′ ATACACCCAGAAAGCACCTC 3′; reverse, 5′ CACGAATTGTGCGAATGTCAC 3′; Rat housekeeping gene forward 5′ CAG TTC CAC CCA CCT CAG AT 3′ and reverse 5′ TTT TGG GCT CCT TCA GAG TG 3′, CK19: forward, 5′-GACTTCCTATAGCTATCGCC-3′; reverse, 5′-TCTGGT-ACCAGTCGCGAATC-3′; 359 bp. α-Fetoprotein (AFP): forward, 5′-TGAAA-TTTGCCACGAGACGG -3′; reverse, 5′-TGTCATACTGA-GCGGCTAAG-3′; 272 bp.. GAPDH: forward, 5′-ACCACAGTCCATGCCATCAC-3′; reverse, 5′-TCCACCACCCTGTTGCTGTA-3′; 452 bp; alpha 1-antitrypsin, forward 5′ AACAATGGGGCTGACCTC 3′;reverse, 5′ CCACAAAGATGGGGCTCT – 3′). Primer (Sigma Genosys) concentrations were optimized before use. SYBR Green Master Mix (1×) was used with 1 μM of forward and reverse primers in a total volume of 12 μl that also included 1 μl cDNA. All PCR reactions were performed in duplicate. PCR amplification was performed as follows: 95 °C for 10 min, 40 cycles of 95 °C for 15 sec, 60 °C for 10 sec and 68 °C for 1 min on a Mastercycler Realplex (Eppendorf). The PCR products were then analyzed using the electrophoresis of 2% agarose gels stained with ethidium bromide for visualization. The gel images were captured using a Molecular Imager FX (Bio-Rad Laboratories, Hercules, CA). The housekeeping gene (GAPDH) was used as an internal standard to determine relative levels of albumin, albumin and CYP3A1 gene expression. Adult hepatocyte was used as a positive control. Following PCR, samples were run on a 2% agarose gel, stained with ethidium bromide, and imaged.
1.10. ECOD, EPOD and EROD Assay
To determine the metabolic activity of the liver stem cells derived hepatocyt, 7-Ethoxyresorufin O-deethylation (EROD) and 7-ethoxycoumarin O-deethylation (ECOD) were measured as previously described.44
1.11. Immunofluorescence
Iimmunofluorescence of mature hepatocyte and bile duct markers were carrying out based on methods described elsewhere.47 Briefly, The expression of each mature hepatocyte and bile duct markers were examined on cells from 3 independent experiments. Briefly, after blocking with 1% BSA in PBS, cells were incubated at 4 °C with primary antibodies (Albumin, CK 19, CYP3A1, alpha 1-antitrypsin) overnight. The primary antibodies (abcam) against CK 19 (1:200), albumin (1:200), CYP3A1 (1:500), alpha 1-antitrypsin (1:400), Rat E-Cadherin Monoclonal Antibody(1:400) (Life technologies), DLK1 Antibody (1:500) (Cell signaling), EpCAM Antibody (1:600) (Novus Biological), CD90/Thy1 antibody (1: 500) (abcam) were used. Following washing, they were incubated with the secondary species-specific fluorescence conjugated antibodies (Alexa Fluor 555) 1:400, 1 h at RT, including 1 μl/ml DAPI and observed fluorescence microscopy analysis was performed using a Zeiss Zeta microscope.
1.12. Statistical Analysis
Results from three independent experiments are presented as the means ± standard deviations. Experiments were conducted in triplicate and data were expressed as mean-standard error. Statistical analyses were performed using paired-end t-tests for means with significance set at P<0.05, P< 0.01 and P<0.001 using Microsoft Excel 8.0.
2. Results and discussion
2.1. Appearance of Very Less Number of Hepatic Stem Cells in Nanostructured Self Assembling Peptides Coated Bioreactor and Matrigel Coated Bioreactor
Very few small hepatic stem cells like colonies were detected in on day 3 of culture of primary hepatocyte (Figure 1). We observed that certain hepatic stem cells are present in form one spherical form during hepatocytes culture. Repeated experiments had done to detect these very small number cells in form single colony and found the same few number of hepatic stem cell colonies on day 3 in all five repeated experiments. Morphological evaluation for proliferation was performed under inverted microscopy at day 3, day 5 and day 7 in nanostructured self assembling peptides coated bioreactor. Hepatocyte culture was under defined condition without any fetal calf serum (FCS). To expansion of these small number of hepatocytes cells; we supplied high concentration of hepatocyte growth factors (100 ng/ml) each day up to 7 days. After 3 days, the cells divided very rapidly. Primary hepatocytes disappear gradually (Figure 1) during rapid proliferation of hepatic stem cells by influence of high concentration of HGF. After confluences of hepatic stem cells, accutase used to splitting these cells and again seeded in bioreactor for more expansion of these hepatic stem cells to increase the numbers for future experiments and cryopreserved. Light microscopic evaluation showed that there are uniform size with an average cell diameter (10 um) which has large nucleus and very scanty cytoplasm. The high nucleus/cytoplasm ratio persists in long term culture up to 35 passages. The appearance is quite similar like human hepatic stem cells.48 They mentioned that colonies human hepatic stem cells have morphology similar to that of embryonic stem cell colonies. Previously, establishment of such human hepatic stem cells require either embryonic mesenchymal feeders, such as embryonic mouse stromal cell line (STO) feeders, or specific matrix components, such as hyaluronans, for survival in ex vivo conditions.3-50 However, all existing expansion culture conditions were undefined. In contrast to previous existing culture conditions, herein we established defined expansion culture conditions using nanostructured self assembling peptides as extracellular matrix for proliferation of hepatic stem cells in a multiwell bioreactor.
Figure 1.
(A). Phase-contrast photomicrographs of hepatic stem cells (day 3) from an adult rat liver in culture on nanostructured self assembling peptides coated bioreactor during hepatocyte culture and their proliferation at day 5, day 7. (B) Quick proliferation test for proliferation of hepatic stem cells nanostructured self assembling peptides coated bioreactor and Matrigel coated bioreactor under influence of HGF. Cell numbers was counted as fold change of 2 × 104 cells/cm2. Results are presented as the mean ± SD from three independent experiments. Statistically significant difference compared with controls (P < 0.05). (C) BrdU-incorporation assay proliferation of hepatic stem cells nanostructured self assembling peptides coated bioreactor and Matrigel coated bioreactor under influence of HGF. Cell numbers were counted in BrdU-incorporation assay by fold change of 2 × 104 cells. Results are presented as the mean ± SD from three independent experiments. Statistically significant difference compared with controls (P < 0.05).
To measure the growth of hepatic stem cells in two different extracellular matrix (nanostructured self assembling peptides and Matrigel) under influence of HGF, hepatic stem cells was seeded at a density of 2 × 104 cm2cells into nanostructured self assembling peptides and Matrigel coated bioreactor with HGF and without HGF. Expansion media (WE) was supplement with high concentration HGF (100 ng/ml). At day 3, 5, and 7 hepatic stem cells were digested with accutase and evaluated by quick proliferation test and BrdU incorpartion assay. To analyze the proliferation of hepatic stem cells, cells were plated at 2 × 104 cells/cm2 on nanostructured self assembling peptides and Matrigel coated bioreactor. Proliferation and quantification of hepatic stem cells was assessed using quick proliferation test and BrdU incorpartion assay on days 3, 5 and 7 for both conditions. It was found that high concentration of HGF supports manipulation of hepatic stem cells in both Matrigel and nanostructured self assembling peptides coated multiwall bioreactor. Matrigel coated bioreactor showed fluctuate proliferation but there is no such fluctuate in nanostructured self assembling peptides coated bioreactor (Figure 1B). Cell proliferation rate was fluctuate and batch to batch variation of three independent experiments in Matrigel-coated bioreactors compared to nanostructured peptide-coated bioreactors. (Figure 1B) because of many growth factors and cytokines are spontnous secreted by Matrigel during cell proliferation. The significant point of these two conditions is peptide is purely defined but Matrigel is not defined. Matrigel is a gelatinous protein mixture of laminin, growth factors, entactin, type IV collage which all are secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma. It is believed that this mixture resembles the complex extracellular environment found in many tissues and has been often used by researcher for cell culture as a substrate. High concentration of HGF-treated hepatic stem cells exhibited higher and stable proliferation rates in nanostructured self assembling peptides coated bioreactor than Matrigel coated bioreactor.
2.2. Role of rHuEPO in Proliferation of Hepatic Stem Cells in Presence of Trauma Cytokines (IL-6 and TNF-α) Nanostructured Self Assembling Peptides Coated Bioreactor and Matrigel Coated Bioreactor
As shown in Figure 2, low concentrations of rHuEPO interacted with trauma cytokines (IL-6 and TNF-α) boost the proliferation of hepatic stem cells in comparison to other combinations such as rHuEPO, rHuEPO + IL- 6+ TNF-α, or IL- 6+ TNF-α. The proliferation assay was tested by quick proliferation test which is more sensitive than trypan blue method. DNA synthesis was measured using BrdU cell proliferation assay also used to evaluate the DNA content of proliferated hepatic cells. DNA synthesis was significantly higher in rHuEPO (10 ng/mL) + TNF-α (10 ng/mL) + IL- 6 (10 ng/mL) than IL- 6 (10 ng/mL) + TNF-α (10 ng/mL) or alone rHuEPO. It was confirmed by both tests that rHuEPO synergized with IL- 6, TNF-α for boosting the proliferation rate of hepatic stem cells. In our previous report, it was showed that low doses of rHuEPO increase the hepatic regenerative capacity after partial hepatectomy in rats by enhancing hepatocyte proliferation.51 It was reported that rHuEPO has potential for in vitro proliferation of mesenchymal stem cells under the acute kidney injury microenvironment.52
Figure 2.
(A) Effects of low concentration of rHuEPO on hepatic stem cells proliferation. Cellular proliferation was assessed by quick proliferation test kit. Cell numbers was counted as fold change of 2 × 104 cells/cm2. Data represent mean + SD (n = 3) in conditions such as rHuEPO + IL- 6 + TNF-α, IL- 6 + TNF-α, and rHuEPO. (B) Cellular proliferation was assessed by BrdU cell proliferation assay at days 3, 5 and 7. Data represent mean + SD (n = 3) in conditions such as rHuEPO + IL- 6 + TNF-α, IL- 6 + TNF-α and rHuEPO. Cell numbers were counted in BrdU-incorporation assay by fold change of 2 × 104 cells. Results are presented as the mean ± SD from three independent experiments. Statistically significant difference compared with controls (P < 0.05).
2.3. Morphological Characterization during Differentiation Process in Nanostructured Self Assembling Peptides coated Bioreactor and Matrigel Coated Bioreactor
Morphological characterization of hepatic stem cells was examined during differentiation process of initial stage day 1) and final stage (day 21). On Day 1, hepatic stem cells showed a round morphology with a big nucleus and notched nucleus and a scanty cytoplasm. Alterations in the morphology of cells were quantified using images of cultured cells captured by an invert microscope and a digital camera before and after hepatic differentiation. There is no significant difference of hepatocytes cells at day 21 between nanostructured self assembling peptides and Matrigel coated bioreactor. Nanostructured self assembling peptides supported the creation of a high number of functional hepatocytes compared to Matrigel coated bioreactor. Cell morphology was observed by phase construct microscopy at final stage (day 21) of hepatic differentiation. We observed that cells treated with Matrigel had more heterogeneous shapes. At day 21, we found more polygonal shapes and binucleated hepatocytes which resemble the typical morphology of rat mature hepatocytes. An overview of distinguishable stages (initial and final stage) of efficient hepatic differentiation is provided in Figure 3. Hepatic stem cells were cultured and explored with special growth factors and cytokines in specific time (sequential protocol). At the beginning of differentiation culture, Hepatic stem cells were small and high ratio of nucleus and small cytoplasm (Figure 3). As early as day 21, we observed more enlarged and more flattened differentiated cells which look like typical of hepatocytes in form of polygonal shape in nanostructured self assembling peptides coated bioreactor than Matrigel coated bioreactor.
Figure 3.
Morphological changes of hepatic stem cells were documented by phase construct microscopy of hepatic differentiation at day 1 and day 21. Cells sequentially exposed to hepatogenic factors were cultured in the presence of liver-specific cytokines and growth factors, added either sequentially (days 0–3: William's E Medium + Activin A (100 ng/ml) + WNT 3a (50 ng/ml); days 3–6: William's E Medium + BMP-4 (50 ng/ml) + FGF-2 (10 ng/ml); days 6–8: William's E Medium + FGF-10 (20 ng/ml); days 8-12; William's E Medium + RA 1 μM + FGF-10 (20 ng/ml); days 12-20: William's E Medium + HGF (50 ng/m) + FGF -4 (20 ng/ml) + EGF (20 ng/ml) + Oncostatin M (10 ng/ml)+ Dex (0.1 μM). Differentiation media were changed every 2 days.
2.4. Hepatic Stem cells Grown on Nanostructured Self Assembling Peptides coated Bioreactor could Differentiate into Efficiently Functional Mature Hepatocytes than Matrigel Coated Bioreactor
2.4.1. Hepatic Gene Expression (Albumin, AFP, CYP3A1, AAT, CK-19) of Differentiated Cells at the mRNA Level
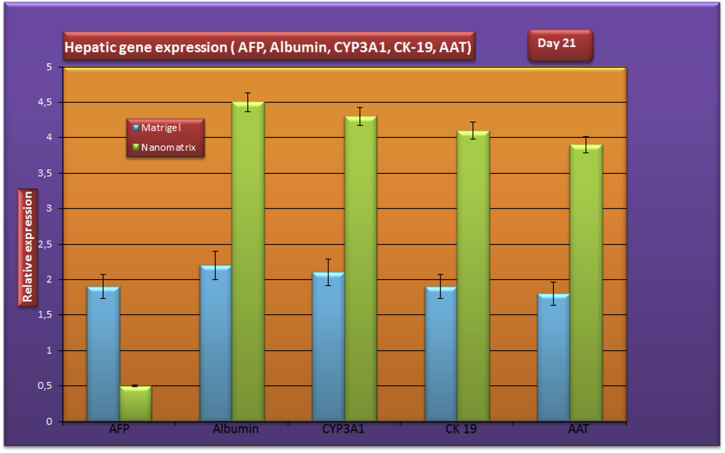
RT-PCR analysis indicated that albumin, AFP, CK-19, CYP3A1, Alpha-1-antitrypsin (AAT) genes were not expressed in hepatic stem cells before differentiation in both conditions (nanostructured self assembling peptides coated and Matrigel coated) whereas their mature hepatic genes expressions were detected in after differentiated process at day 21. The differentiated hepatocytes expressed those genes (CK-19, CYP3A4 and AAT) normally found on mature hepatocytes. After sequential protocol treatment, expression of the mature hepatic genes was detected in the induced cells on day 21 of differentiation. RT-PCR analysis revealed that immature hepatic markers (AFP) had significantly higher gene expression in Matrigel coated bioreactor than nanostructured self assembling peptides coated bioreactor. It was confirmed the mature liver markers of gene expression of the albumin, CK-19, CYP3A4, alpha 1-antitrypsin genes observed that their mature gene expression levels were one-fold higher in cells nanostructured self assembling peptides coated bioreactor than Matrigel coated at day 21 (Figure 4). The mRNA expression level of immature marker (AFP) was also significantly different between Matrigel and nanostructured self assembling peptides coated. The gene expression of albumin, CK-19, CYP3A1 and AAT genes were detected at day 21 of culture in nanostructured self assembling peptides coated, which was also significantly higher than in Matrigel coated bioreactor (Figure 4). As shown in Figure, gene expression of the hepatic genes increased on day 21 of differentiation. The expression of β-actin, used as an internal control, was the same in both the undifferentiated at day 1 and differentiated cells at day 21.
Figure 4.
Hepatic gene expression (albumin, AFP, CYP3A1, CK-19 and AAT) of differentiated cells at end stage of hepatic differentiation on nanostructured self assembling peptides coated bioreactor and in Matrigel coated bioreactor. Results are presented as the mean ± SD from three independent experiments. Statistically significant difference compared with controls (P < 0.05).
2.4.2. Evaluation of Major Liver-specific Biochemical Functions (Albumin Secretion is Superior in Nanostructured Self Assembling Peptides Coated Bioreactor than Matrigel Coated)
The negligible albumin synthesis was 0.04 ± 0.01 pg/h/cell in the nanostructured self assembling peptides coated bioreactor and 0.03 ± 0.09 pg/h/cell in the Matrigel coated bioreactor on the first day (Figure 5). The difference between peptide coated bioreactor and the Matrigel coated bioreactor at day 1 was not statistically significant (P = 0.401). The albumin synthesis rate was 2.56 ± 0.68 pg/h/cell in the Matrigel coated bioreactor on day 21. The value of albumin synthesis (3.76 ± 0.21 pg/h/cell) by the nanostructured self assembling peptides coated bioreactor occurred on day 21. The albumin production of the nanostructured self assembling peptides coated bioreactor coated bioreactor was 1 to 1.19 pg/h/cell higher than that of the Matrigel coated bioreactor, and this difference was significant (P < 0.001).
Figure 5.
Albumin synthesis rate hepatic stem cells derived hepatocytes of end stage of differentiation period (day 21) on nanostructured self assembling peptides coated bioreactor and in Matrigel coated bioreactor. Results are presented as the mean ± SD from three independent experiments. Statistically significant difference compared with controls (P < 0.05).
2.4.3. Urea Synthesis is Significantly Higher in Nanostructured Self Assembling Peptides Coated Bioreactor than in Matrigel Coated Bioreactor
The Urea synthesis was 0.11 ± 0.13 pg/h/cell in the nanostructured self assembling peptides coated bioreactor and 0.67 ± 0.08 pg/h/cell in the Matrigel coated bioreactor on the first day of culture. We observed a significant increase (P < 0.01) in both groups (Figure 6). The Urea synthesis was 1.01 ± 0.21 pg/h/cell in the in nanostructured self assembling peptides coated bioreactor on the seventh day and 2.26 ± 0.18 pg/h/cell in the Matrigel coated bioreactor group on day 21. The difference between nanostructured self assembling peptides coated bioreactor and the Matrigel coated bioreactor at day 21 was statistically significant (P < 0.05).
Figure 6.
Urea synthesis rate hepatic stem cells derived hepatocytes of end stage of differentiation period (day 21) on nanostructured self assembling peptides coated bioreactor and in Matrigel coated bioreactor. Results are presented as the mean ± SD from three independent experiments. Statistically significant difference compared with controls (P < 0.05).
2.4.4. Ammonia Removal Efficiency is Enhanced in Nanostructured Self Assembling Peptides Coated Bioreactor than in Matrigel Coated Bioreactor
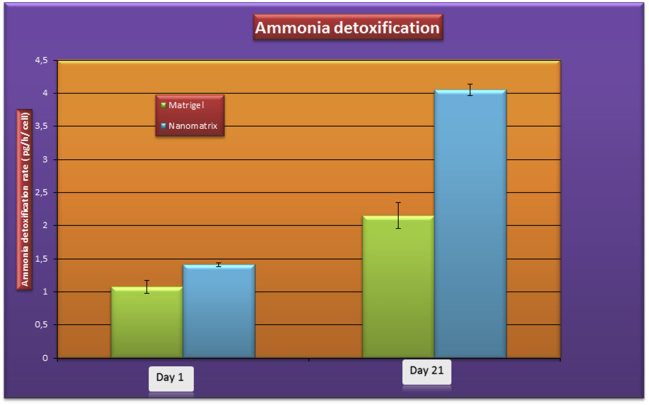
The ammonia detoxification rate was 1.41 ± 0.02 pg/h/cell in the in nanostructured self assembling peptides coated bioreactor and 1.07 ± 0.98 pg/h/cell in the Matrigel coated bioreactor group at day 1 (Figure 7). The elimination of ammonia in the nanostructured self assembling peptides coated bioreactor was 4.05 ± 0.83 pg/h/cell in nanostructured self assembling peptides coated bioreactor and 2.15 ± 0.96 pg/h/cell in Matrigel coated bioreactor on the day 21. The difference between the peptide coated bioreactor and the Matrigel coated bioreactor group at day 21 was statistically significant (P < 0.05).
Figure 7.
Ammonia detoxification rate hepatic stem cells derived hepatocytes of end stage of differentiation period (day 21) on nanostructured self assembling peptides coated bioreactor and in Matrigel coated bioreactor. Results are presented as the mean ± SD from three independent experiments. Statistically significant difference compared with controls (P < 0.05).
2.4.5. Cytochrome P450 Enzymes Expression in Nanostructured Self Assembling Peptides Coated Bioreactor and in Matrigel Coated Bioreactor
EROD (specific for CYP1A1/2 forms), ECOD (CYP1A/2B) and PROD (CYP2B) were assayed in differentiated hepatocytes of cultured on nanostructured self assembling peptides coated bioreactor and in Matrigel coated bioreactor at the final day (day 21) of the differentiation period. Cytochrome P450 (CYP450) expression was evaluated by the ethoxyresorufin o-deethylase (EROD) assay, methoxy- (MROD), pentoxy- (PROD), or benzyloxy- (BROD) resorufin. Assay which are chiefly catalyzed by the isoenzyme CYP450IA1 respectively. At day 21 differentiated hepatocytes of nanostructured self assembling peptides coated bioreactor exhibited EROD activity of 48 ± 2 pmol/min per mg and Matrigel coated bioreactor showed EROD activity of 25 ± 5 pmol/min per mg after induction with 3-methylcholanthrene(Figure 8A). PROD activity was also induced by phenobarbital (PB) (0.75 mM; 72 h incubation). PROD activities were 21 ± 3 and 12 ± 6 pmol/min per mg in nanostructured self assembling peptides coated bioreactor and in Matrigel coated bioreactor respectively at day 21, which is significantly different (Figure 8B). The ECOD activity was 11 pmol/min/mg protein and 4 pmol/min/mg protein in on nanostructured self assembling peptides coated bioreactor and in Matrigel coated bioreactor respectively (Figure 8C), which is significant difference (P< 0.005).
Figure 8.
Hepatic metabolic function was assessed by determining the activities of Phase I enzymes EROD (A) and ECOD (B), and (C) PROD in nanostructured self assembling peptides coated bioreactor and in Matrigel coated bioreactor. EROD, or ECOD, PROD activity was induced by 3-MC (5 mM) or PB (1 mM), respectively at end stage of differentiation period (day 21).
2.4.6. Evaluation of Immature Hepatic Markers (AFP) and Mature Hepatocyte Markers (albumin, CK 18, alpha 1-antitrypsin, bile duct marker markers (CK 19) at Final Hepatic Differentiation (day 21) in Nanostructured Self Assembling Peptides Coated Bioreactor and Matrigel Coated Bioreactor
At day 21, more positive mature hepatic markers observed in the nanostructured self assembling peptides coated bioreactor, and in Matrigel coated bioreactor (Figure 9A and B), suggesting that both extracellular matrix has good potential for hepatic differentiation. However, defined point of view, nanostructured self assembling peptides coated bioreactor were much more effective for hepatic differentiation. Upon sequential differentiation protocol, there was a higher expression of mature liver markers (albumin, CK-19, CPY3A1, AAT) in hepatic stem cell derived hepatocytes of nanostructured self assembling peptides coated bioreactor (Figure 9A). Hepatic stem cell derived hepatocytes in Matrigel coated bioreactor also exhibited mature hepatocyte markers (Figure 9 B). We conclude that nanostructured self assembling peptides coated bioreactor can be alternative for Matrigel.
Figure 9.
Immunofluorescence analysis of in vitro hepatic differentiations of hepatic stem cells at end stage of differentiation in nanostructured self assembling peptides coated bioreactor and in Matrigel coated bioreactor. Immunofluorescence staining showed that differentiated hepatic stem cells stained positively for albumin, CK-19, CPY3A1, AAT. DAPI (middle panels) was used for nuclear staining. A: Matrigel (a1: albumin; a2: CPY3A1; a3: CK19; a4: AAT), B: Nanomatrix (b1: albumin; b2: CPY3A1; b3: CK19; b4: AAT).
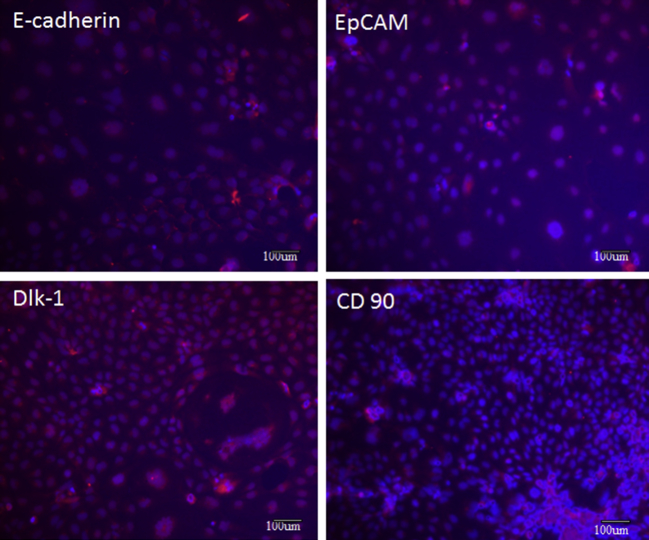
We detected major makers of hepatic stem cells such as Delta-like 1 protein (Dlk-1) EpCAM+, CD 90 and E-cadherin (Figure 10). Dlk-1, also known as preadipocyte factor 1 (Pref-1), a member of the delta-like family of cell surface transmembrane proteins which is highly expressed in human and rodent fetal liver but absent from mature hepatocytes in neonatal and adult rodent liver.53–55 Numerous evidences accumulated that Dlk-1 may play an important role in tumourigenesis as well as organogenesis. The CD90 marker is considered as a marker for various kinds of stem cells including liver cancer stem cells.56 Okabe et al., reported that hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver.57 Ueberham et al., reported that E-cadherin is a reliable cell surface marker for the identification of liver specific stem cells.58
Figure 10.
Immunofluorescence analysis of hepatic stem cells markers (Delta-like 1 protein (Dlk-1) EpCAM+, CD 90 and E-cadherin). Immunofluorescence staining showed that heatic stem cells population exhibited positively for Delta-like 1 protein (Dlk-1) EpCAM+, CD 90 and E-cadherin DAPI (middle panels) was used for nuclear staining.
3. Conclusions
Establishments of hepatic stem cell like population from healthy liver is still a challenging task since very few researcher inaugurated for isolation of hepatic stem cells population in healthy human liver56, porcine liver57, rat liver58, mouse liver59 where isolation and expansion methodology is differ each other and associated with undefined culture conditions. The main of existing protocols for expansion and differentiation methodology is undefined culture conditions, associated with FCS or other substances which are barrier for either preclinical or clinical setting of cell base therapy. Herein, we established a very simple protocol for isolation and expansion of hepatic stem cells from healthy rat liver. Hepatic differentiation of hepatic stem cells was conducted in nanostructured self assembling peptides multiwell bioreactor module and compare with Matrigel coated bioreactor. It was showed that HGF alone is sufficient to stimulate for proliferation of hepatic stem cells. The role of rHuEPO in presence of trauma/ischemia cytokines (IL-6, TNFα) was evaluated and found that significant proliferation of hepatic stem cells in presence of trauma/ischemia cytokines (IL-6, TNFα). The sequential differentiation protocol is preferred to use for hepatic differentiation which is based on the secretion pattern of the developing human embryonic liver to establish in vitro conditions. This sequential differentiation protocol has been widely accepted by researcher recently which yields several benefits and creates specific microenvironments in a manner consistent with in vivo human liver development. Further, we established a chemically defined, efficient, scalable, expansion and differentiation of hepatic stem cells using nanostructured self assembling peptides in multiwell bioreactor module. Nanostructured self assembling peptides is composed of natural amino acids (arginine, alanine and aspartic acid), which can be used by cells upon transplantation during cellular therapy. Nanostructured self assembling peptides coated multiwell bioreactor module might be alternative extracellular matrix of existing Matrigel culture system for expansion of wide variety of stem cells in either preclinical therapeutic purposes or academic research.
Conflicts of interest
All authors have none to declare
Acknowledgments
The authors wish to thank Angela Henning her excellent technical support and Frank Struck for supply of primary rat hepatocytes. Funding of this project was provided by Medicine faculty of University of Leipzig.
References
- 1.Watanabe G., Nanjo H., Nagai H. A el monoclonal antibody identified hepatic stem-like cells in rats. Med Mol Morphol. 2011;44:103–110. doi: 10.1007/s00795-010-0516-1. [DOI] [PubMed] [Google Scholar]
- 2.Kano J., Ishiyama T., Nakamura N., Iijima T., Morishita Y., Noguchi M. Establishment of hepatic stem-like cell lines from normal adult porcine liver in a poly-d-lysine-coated dish with NAIR-1 medium. In Vitro Cell Dev Biol Anim. 2003;39:440–448. doi: 10.1290/1543-706X(2003)039<0440:EOHSCL>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Schmelzer E., Zhang L., Bruce A. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirata M., Amano K., Miyashita A., Yasunaga M., Nakanishi T., Sato K. Establishment and characterization of hepatic stem-like cell lines from normal adult rat liver. J Biochem. 2009;145:51–58. doi: 10.1093/jb/mvn146. [DOI] [PubMed] [Google Scholar]
- 5.Strain A.J., Crosby H.A. Hepatic stem cells. Gut. 2000;46:743–745. doi: 10.1136/gut.46.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemire J.M., Shiojiri N., Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by d-galactosamine. Am J Pathol. 1991;139:535–552. [PMC free article] [PubMed] [Google Scholar]
- 7.Avril A., Pichard V., Bralet M.P., Ferry N. Mature hepatocytes are the source of small hepatocyte-like progenitor cells in the retrorsine model of liver injury. J Hepatol. 2004;41:737–743. doi: 10.1016/j.jhep.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 8.Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology. 2001;33:738–750. doi: 10.1053/jhep.2001.21900. [DOI] [PubMed] [Google Scholar]
- 9.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 10.Santoni-Rugiu E., Jelnes P., Thorgeirsson S.S., Bisgaard H.C. Progenitor cells in liver regeneration: molecular responses controlling their activation and expansion. APMIS. 2005;113:876–902. doi: 10.1111/j.1600-0463.2005.apm_386.x. [DOI] [PubMed] [Google Scholar]
- 11.Bird T.G., Lorenzini S., Forbes S.J. Activation of stem cells in hepatic diseases. Cell Tissue Res. 2008;331:283–300. doi: 10.1007/s00441-007-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kon J., Ichinohe N., Ooe H., Chen Q., Sasaki K., Mitaka T. Thy1-positive cells have bipotential ability to differentiate into hepatocytes and biliary epithelial cells in galactosamine-induced rat liver regeneration. Am J Pathol. 2009;175:2362–2371. doi: 10.2353/ajpath.2009.080338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichinohe N., Tanimizu N., Ooe H. Differentiation capacity of hepatic stem/progenitor cells isolated from d-galactosamine-treated rat livers. Hepatology. 2013;57:1192–1202. doi: 10.1002/hep.26084. [DOI] [PubMed] [Google Scholar]
- 14.Du L., Wang H., He L. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- 15.Kon J., Ooe H., Oshima H., Kikkawa Y., Mitaka T. Expression of CD44 in rat hepatic progenitor cells. J Hepatol. 2006;45:90–98. doi: 10.1016/j.jhep.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Mitaka T., Kojima T., Mizuguchi T., Mochizuki Y. Growth and maturation of small hepatocytes isolated from adult rat liver. Biochem Biophys Res Commun. 1995;214:310–317. doi: 10.1006/bbrc.1995.2289. [DOI] [PubMed] [Google Scholar]
- 17.Mitaka T., Sato F., Mizuguchi T., Yokono T., Mochizuki Y. Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology. 1999;29:111–125. doi: 10.1002/hep.510290103. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki K., Kon J., Mizuguchi T. Proliferation of hepatocyte progenitor cells isolated from adult human livers in serum-free medium. Cell Transplant. 2008;17:1221–1230. doi: 10.3727/096368908787236666. [DOI] [PubMed] [Google Scholar]
- 19.Petersen B.E., Bowen W.C., Patrene K.D. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 20.Crosby H.A., Hubscher S., Fabris L. Immunolocalization of putative human liver progenitor cells in livers from patients with end-stage primary biliary cirrhosis and sclerosing cholangitis using the monoclonal antibody OV-6. Am J Pathol. 1998;152:771–779. [PMC free article] [PubMed] [Google Scholar]
- 21.Theise N.D., Saxena R., Portmann B.C. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 22.Roskams T.A., Theise N.D., Balabaud C. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 23.Oh S.H., Hatch H.M., Petersen B.E. Hepatic oval 'stem' cell in liver regeneration. Semin Cell Dev Biol. 2002;13:405–409. doi: 10.1016/s1084952102001271. [DOI] [PubMed] [Google Scholar]
- 24.Thorgeirsson S.S. Hepatic stem cells in liver regeneration. FASEB J. 1996;10:1249–1256. [PubMed] [Google Scholar]
- 25.Hsia C.C., Evarts R.P., Nakatsukasa H., Marsden E.R., Thorgeirsson S.S. Occurrence of oval-type cells in hepatitis B virus-associated human hepatocarcinogenesis. Hepatology. 1992;16:1327–1333. doi: 10.1002/hep.1840160604. [DOI] [PubMed] [Google Scholar]
- 26.Crosby H.A., Hubscher S.G., Joplin R.E., Kelly D.A., Strain A.J. Immunolocalization of OV-6, a putative progenitor cell marker in human fetal and diseased pediatric liver. Hepatology. 1998;28:980–985. doi: 10.1002/hep.510280412. [DOI] [PubMed] [Google Scholar]
- 27.Schmeding M., Rademacher S., Boas-Knoop S. rHuEPo Reduces ischemia-reperfusion injury and improves survival after transplantation of fatty livers in rats. Transplantation. 2010;89:161–168. doi: 10.1097/TP.0b013e3181c425fd. [DOI] [PubMed] [Google Scholar]
- 28.Byl B., Roucloux I., Crusiaux A., Dupont E., Deviere J. Tumor necrosis factor alpha and interleukin 6 plasma levels in infected cirrhotic patients. Gastroenterology. 1993;104:1492–1497. doi: 10.1016/0016-5085(93)90361-f. [DOI] [PubMed] [Google Scholar]
- 29.Jin L.M., Liu Y.X., Zhou L. Ischemic preconditioning attenuates morphological and biochemical changes in hepatic ischemia/reperfusion in rats. Pathobiology. 2010;77:136–146. doi: 10.1159/000292647. [DOI] [PubMed] [Google Scholar]
- 30.Camargo C.A., Jr., Madden J.F., Gao W., Selvan R.S., Clavien P.A. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513–1520. doi: 10.1002/hep.510260619. [DOI] [PubMed] [Google Scholar]
- 31.Blindenbacher A., Wang X., Langer I., Savino R., Terracciano L., Heim M.H. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology. 2003;38:674–682. doi: 10.1053/jhep.2003.50378. [DOI] [PubMed] [Google Scholar]
- 32.Teoh N., Leclercq I., Pena A.D., Farrell G. Low-dose TNF-alpha protects against hepatic ischemia-reperfusion injury in mice: implications for preconditioning. Hepatology. 2003;37:118–128. doi: 10.1053/jhep.2003.50009. [DOI] [PubMed] [Google Scholar]
- 33.Teoh N., Field J., Farrell G. Interleukin-6 is a key mediator of the hepatoprotective and pro-proliferative effects of ischaemic preconditioning in mice. J Hepatol. 2006;45:20–27. doi: 10.1016/j.jhep.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 34.Sudo K., Yamada Y., Saito K. TNF-alpha and IL-6 signals from the bone marrow derived cells are necessary for normal murine liver regeneration. Biochim Biophys Acta. 2008;1782:671–679. doi: 10.1016/j.bbadis.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Selzner M., Camargo C.A., Clavien P.A. Ischemia impairs liver regeneration after major tissue loss in rodents: protective effects of interleukin-6. Hepatology. 1999;30:469–475. doi: 10.1002/hep.510300215. [DOI] [PubMed] [Google Scholar]
- 36.Streetz K.L., Luedde T., Manns M.P., Trautwein C. Interleukin 6 and liver regeneration. Gut. 2000;47:309–312. doi: 10.1136/gut.47.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ledda-Columbano G.M., Curto M., Piga R. In vivo hepatocyte proliferation is inducible through a TNF and IL-6-independent pathway. Oncogene. 1998;17:1039–1044. doi: 10.1038/sj.onc.1202018. [DOI] [PubMed] [Google Scholar]
- 38.Jin L.M., Jin S.F., Liu Y.X. Ischemic preconditioning enhances hepatocyte proliferation in the early phase after ischemia under hemi-hepatectomy in rats. Hepatobiliary Pancreat Dis Int. 2012;11:521–526. doi: 10.1016/s1499-3872(12)60217-3. [DOI] [PubMed] [Google Scholar]
- 39.Bader A., Fruhauf N., Tiedge M. Enhanced oxygen delivery reverses anaerobic metabolic states in prolonged sandwich rat hepatocyte culture. Exp Cell Res. 1999;246:221–232. doi: 10.1006/excr.1998.4295. [DOI] [PubMed] [Google Scholar]
- 40.Giri S., Nieber K., Acikgoz A., Pavlica S., Keller M., Bader A. Telomerase activity and hepatic functions of rat embryonic liver progenitor cell in nanoscaffold-coated model bioreactor. Mol Cell Biochem. 2010;336:137–149. doi: 10.1007/s11010-009-0266-3. [DOI] [PubMed] [Google Scholar]
- 41.Giri S., Acikgoz A., Pathak P. Three dimensional cultures of rat liver cells using a natural self-assembling nanoscaffold in a clinically relevant bioreactor for bioartificial liver construction. J Cell Physiol. 2012;227:313–327. doi: 10.1002/jcp.22738. [DOI] [PubMed] [Google Scholar]
- 42.Fruhauf N.R., Oldhafer K.J., Holtje M. A bioartificial liver support system using primary hepatocytes: a preclinical study in a new porcine hepatectomy model. Surgery. 2004;136:47–56. doi: 10.1016/j.surg.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Maringka M., Giri S., Bader A. Preclinical characterization of primary porcine hepatocytes in a clinically relevant flat membrane bioreactor. Biomaterials. 2010;31:156–172. doi: 10.1016/j.biomaterials.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Schmitmeier S., Langsch A., Jasmund I., Bader A. Development and characterization of a small-scale bioreactor based on a bioartificial hepatic culture model for predictive pharmacological in vitro screenings. Biotechnol Bioeng. 2006;95:1198–1206. doi: 10.1002/bit.21089. [DOI] [PubMed] [Google Scholar]
- 45.van Anken H.C., Schiphorst M.E. A kinetic determination of ammonia in plasma. Clin Chim Acta. 1974;56:151–157. doi: 10.1016/0009-8981(74)90223-x. [DOI] [PubMed] [Google Scholar]
- 46.Calligaris S.D., Almada L.L., Guibert E.E., Tiribelli C., Rodriguez J.V. Ammonium detoxifying activity is maintained after 72 hours of cold preservation of rat hepatocytes in University of Wisconsin (UW) solution. Cryo Letters. 2002;23:245–254. [PubMed] [Google Scholar]
- 47.Cho C.H., Berthiaume F., Tilles A.W., Yarmush M.L. A new technique for primary hepatocyte expansion in vitro. Biotechnol Bioeng. 2008;101:345–356. doi: 10.1002/bit.21911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McClelland R., Wauthier E., Zhang L. Ex vivo conditions for self-replication of human hepatic stem cells. Tissue Eng Part C Methods. 2008;14:341–351. doi: 10.1089/ten.tec.2008.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner W.S., Schmelzer E., McClelland R., Wauthier E., Chen W., Reid L.M. Human hepatoblast phenotype maintained by hyaluronan hydrogels. J Biomed Mater Res B Appl Biomater. 2007;82:156–168. doi: 10.1002/jbm.b.30717. [DOI] [PubMed] [Google Scholar]
- 50.Turner W.S., Seagle C., Galanko J.A. Nuclear magnetic resonance metabolomic footprinting of human hepatic stem cells and hepatoblasts cultured in hyaluronan-matrix hydrogels. Stem Cells. 2008;26:1547–1555. doi: 10.1634/stemcells.2007-0863. [DOI] [PubMed] [Google Scholar]
- 51.Bader A., Pavlica S., Deiwick A. Proteomic analysis to display the effect of low doses of erythropoietin on rat liver regeneration. Life Sci. 2011;89:827–833. doi: 10.1016/j.lfs.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Liu N., Tian J., Wang W., Cheng J., Hu D., Zhang J. Effect and mechanism of erythropoietin on mesenchymal stem cell proliferation in vitro under the acute kidney injury microenvironment. Exp Biol Med (Maywood) 2011;236:1093–1099. doi: 10.1258/ebm.2011.011001. [DOI] [PubMed] [Google Scholar]
- 53.Yanai H., Nakamura K., Hijioka S. Dlk-1, a cell surface antigen on foetal hepatic stem/progenitor cells, is expressed in hepatocellular, colon, pancreas and breast carcinomas at a high frequency. J Biochem. 2010;148:85–92. doi: 10.1093/jb/mvq034. [DOI] [PubMed] [Google Scholar]
- 54.Nishina H. hDlk-1: a cell surface marker common to normal hepatic stem/progenitor cells and carcinomas. J Biochem. 2012;152:121–123. doi: 10.1093/jb/mvs069. [DOI] [PubMed] [Google Scholar]
- 55.Oertel M., Menthena A., Chen Y.Q., Teisner B., Jensen C.H., Shafritz D.A. Purification of fetal liver stem/progenitor cells containing all the repopulation potential for normal adult rat liver. Gastroenterology. 2008;134:823–832. doi: 10.1053/j.gastro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Herrera M.B., Bruno S., Buttiglieri S. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24:2840–2850. doi: 10.1634/stemcells.2006-0114. [DOI] [PubMed] [Google Scholar]
- 57.Okabe M., Tsukahara Y., Tanaka M. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–1960. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- 58.Ueberham E., Aigner T., Ueberham U., Gebhardt R. E-cadherin as a reliable cell surface marker for the identification of liver specific stem cells. J Mol Histol. 2007;38:359–368. doi: 10.1007/s10735-007-9098-1. [DOI] [PubMed] [Google Scholar]
- 59.Li W.L., Su J., Yao Y.C. Isolation and characterization of bipotent liver progenitor cells from adult mouse. Stem Cells. 2006;24:322–332. doi: 10.1634/stemcells.2005-0108. [DOI] [PubMed] [Google Scholar]