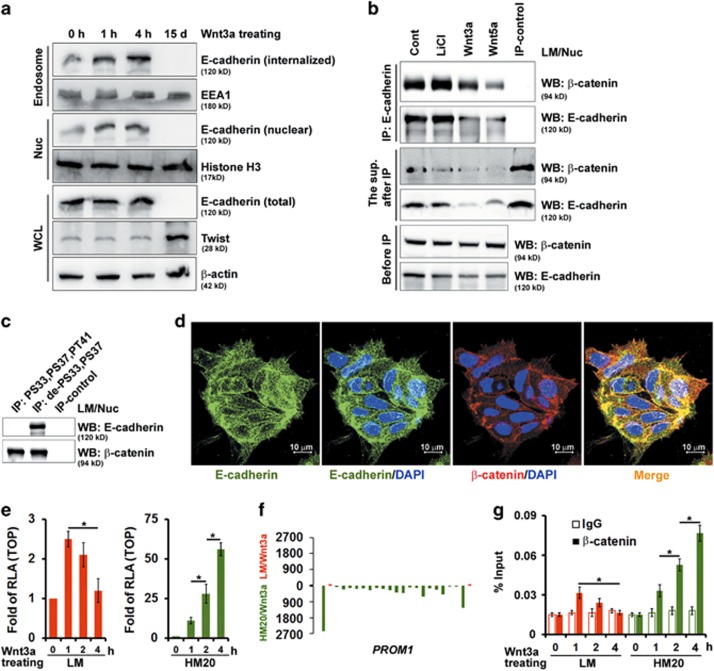
Figure 6.
The internalized E-cadherin by endosomal sorting is translocated into the nucleus and negatively regulates Wnt/β-catenin-elicited transcriptional activity. A549 cells were cultivated in suspension for 24 days and then migrated back onto the plate to reform a monolayer (LM cells). LM cells were functionally fractionated by invasive assays, 5 times (HM5 cells), 10 times (HM10 cells) and 20 times (HM20 cells). (a) LM cells were treated with Wnt3a-containing medium for 1 h, 4 h and 15 days as indicated. Total cell lysates, endosomes (purified by sucrose gradient centrifugation) and nuclear (Nuc) fractions were subjected to western blotting (WB). (b) WB analysis of Nuc extracts of LM cells treated with control (Cont), LiCl, Wnt5a, or Wnt3a before immunoprecipitation (IP; bottom panel), in the supernatant recovered after IP with anti-E-cadherin (middle panel), and in the immunoprecipitates (top panel). (c) Nuc extracts were prepared from LM cells and then immunoprecipitated using antibodies against phosphorylated-S33,S37,PT41 or dephosphorylated-S33,S37 β-catenin followed by WB. (d) LM cells were immunostained for E-cadherin (green) and counterstained with DAPI (4',6-diamidino-2-phenylindole). Representative images taken by confocal laser microscopy are shown. Scale bars=10 μm. (e) TOPflash luciferase reporter assays were performed in LM (left panel) and HM (right panel) cells after treatment with control or Wnt3a-containing medium for 1, 2 and 4 h as indicated. RLA, relative luciferase activity. (f) ChIP sequencing (ChIP-seq) profiles for binding of β-catenin complexes in LM (red; top panel) and HM20 (green; bottom panel) cells after stimulation with Wnt3a. The illustrative region indicates PROM1 (15,964,699–16,086,001). (g) ChIP–quantitative PCR analysis to validate the results of the ChIP-seq experiment described in f, showing the fold change of β-catenin antibody compared with the IgG control. Data in e and g were derived from three independent experiments and are presented as the mean±s.d. *P<0.05 (t-test).