Abstract
The compounds 4-vinylcyclohexene 1,2-monoepoxide (VCM) and 4-Vinylcyclohexene diepoxide (VCD) are the two downstream metabolites of 4-vinylcyclohexene (VCH), an ovotoxic agent in mammals. In addition, VCM and VCD may be found as by-products of VCH oxidation in the environment. Recently, we reported the involvement of oxidative stress in the toxicity of VCH in Drosophila melanogaster. However, it was not possible to determine the individual contributions of VCM and VCD in VCH toxicity. Hence, we investigated the toxicity of VCM and VCD (10–1000 µM) in flies after 5 days of exposure via the diet. Our results indicated impairments in climbing behaviour and disruptions in antioxidant balance and redox status evidenced by an increase in DCFH oxidation, decreases in total thiol content and glutathione-S-transferase (GST) activity in the flies exposed to VCM and VCD (p<0.05). These effects were accompanied by disruptions in the transcription of the genes encoding the proteins superoxide dismutase (SOD1), kelch-like erythroid-derived cap-n-collar (CNC) homology (ECH)-associated protein 1 (Keap-1), mitogen activated protein kinase 2 (MAPK-2), catalase, Cyp18a1, JAFRAC 1 (thioredoxin peroxidase 1) and thioredoxin reductase 1 (TrxR-1) (p<0.05). VCM and VCD inhibited acetylcholinesterase (AChE) and delta aminolevulinic acid dehydratase (δ-ALA D) activities in the flies (p<0.05). Indeed, here, we demonstrated that different target enzymes and genes were modified by the electrophiles VCM and VCD in the flies. Thus, D. melanogaster has provided further lessons on the toxicity of VCM and VCD which suggest that the reported toxicity of VCH may be mediated by its transformation to VCM and VCD.
Keywords: 4-Vinylcyclohexene 1,2-monoepoxide; 4-Vinylcyclohexene diepoxide; Reactive oxygen and nitrogen species; Changes in mRNA levels; Disruption of antioxidant homoeostasis
Abbreviations: VCM, 4-vinylcyclohexene 1,2-monoepoxide; VCH, 4-vinylcyclohexene; VCD, 4-vinylcyclohexene diepoxide; RT PCR, reverse transcription polymerase chain reaction; mRNA, messenger RNA; DCFH-DA, 2′,7′-dichlorofluorescein diacetate; RONS, reactive oxygen and nitrogen species; TrxR-1, thioredoxin reductase 1; Keap-1, kelch-like erythroid-derived cap-n-collar (CNC) homology (ECH)-associated protein 1; MAPK-2, mitogen-activated protein kinase 2; SOD1, superoxide dismutase1; δ-ALA D, delta aminolevulinic acid dehydratase; mEH, microsomal epoxide hydrolase; AChE, acetylcholinesterase; GST, glutathione-S-transferase.
Graphical abstract
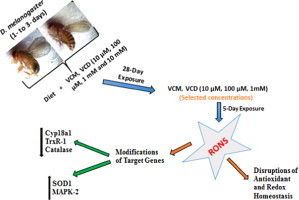
Introduction
Environmental exposure to chemicals such as polycyclic aromatic hydrocarbons, 4-vinylcyclohexene (VCH) and 7,12-dimethylbenz[a]anthracene have long been of toxicological concerns because they are potential inducers of early ovarian failure (menopause) in women [1–3]. Recently, attention has been focused by the European Chemicals Agency on VCH, which is a dimer of 1,3-butadiene, produced during the process of tire curing. It is also formed as a by-product during the manufacture of plastics, pesticides, rubber and flame-retardants [4,5]. 4-Vinylcyclohexene 1,2-monoepoxide (VCM), is the first metabolite formed by the epoxidation of VCH in the liver by the enzyme cytochrome P450 isoform 2A (CYP 2A). Thereafter, VCM is further metabolized by CYP2B to form the diepoxide form, VCD. The metabolism of VCD involves bioactivation to form inactive tetrol metabolite [4-(1,2-dihydroxy)ethyl-1,2-dihydroxycyclohexane] with the aid of microsomal epoxide hydrolase (mEH) that is expressed in multiple tissues [6]. Additionally, GST can conjugate VCD with GSH to yield VCD–SG conjugates [7] (Scheme 1).
Scheme 1.
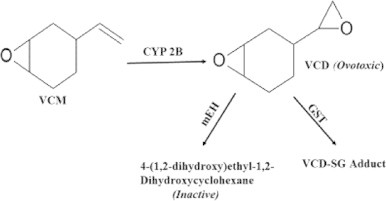
Metabolism of 4-vinyvlcyclohexene 1,2-monoepoxide (VCM) and 4-vinyvlcyclohexene diepoxide (VCD) in tissues (e.g. liver). VCD: 4-vinylcyclohexene diepoxide; mEH: microsomal epoxide hydrolase; GST: glutathione-S-transferase.
Toxicological studies on VCH and its metabolites, VCM and VCD, revealed that they can cause pre-antral follicle loss in rats and mice, by accelerating the natural apoptotic process of atresia [8]. For instance, VCM has been implicated in germ cell destruction in female mice and rats [9]. In the industry, VCD is manufactured through a process of epoxidation of 4-vinylcyclohexene with peroxyacetic acid in an inert solvent [10]. The demand for VCD is high, because it provides commercial outlet for VCH [11], and it is used as a reactive diluent for other diepoxides and epoxy resins derived from epichlorohydrin and bisphenol A [12]. Exposure to VCD may occur when it is being manufactured. People may also get exposed to VCD during the production and use of VCH, epoxy-based polyglycols and resins. In addition, laboratory workers may be exposed during embedding of biological tissues using epoxy polymer for electron microscopy [13,14]. Indeed, between 1981 and 1983, about 6200 employees were exposed to a product contaminated with VCD [15]. VCD is presently being used as a model of ovotoxicity due to its capacity to selectively destroy primordial and small primary follicles in ovaries of mice and rats [16,17].
Substantial evidence indicates that environmental toxicants can induce oxidative stress by altering cellular redox balance, and the activities of antioxidant enzymes such as catalase and GST, leading to excessive production of free radicals [18]. In turn, free radicals can damage lipids, proteins, carbohydrates and nucleic acids, thereby, inhibiting their normal functions [19]. Consequently, a myriad of enzymatic and nonenzymatic antioxidant systems regulate the redox state of the cells. For instance, glutathione (GSH) is a cysteine-containing tripeptide responsible for scavenging free radicals through either direct chemical reactions or reduction of peroxides as a cofactor for glutathione peroxidases [20]. In addition, the activity of glutathione reductase, which requires NADPH as an energy source, ensures that most GSH is present in cells in the reduced form. Furthermore, glutathione-S-transferases (GSTs), a large family of enzymes that covalently link reactive chemicals with GSH, aid in the detoxification and excretion of toxic substances. Also, the thioredoxin/thioredoxin reductase system also serves a similar function by providing reducing potential for different biochemical reactions [21,22]. Moreover, several other antioxidant enzymes directly detoxify ROS in order to prevent cellular damage. For example, superoxide dismutases (SODs) react with superoxide anion radicals to form oxygen and H2O2 [23], while catalase together with various peroxidases such as glutathione peroxidases (GPXs) convert peroxides to water [24,25]. Additionally, oxidative stress is primarily responsible for the regulation of the expression of the genes encoding antioxidant enzymes [26].
Recently, our research team reported the involvement of oxidative stress in VCH-induced toxicity in Drosophila melanogaster [3]. However, in the previous study [3], it was not possible to determine the individual contributions of VCM and VCD in VCH toxicity. Since the metabolism of VCH results in the formation of reactive electrophilic epoxides VCM and VCD, we hypothesize that VCM and VCD are potentially more reactive and toxic than the parent compound-VCH. Of note, the mechanisms of toxicity of VCM and VCD are still unravelled, though VCD has been reported to change apoptotic signalling [27–29], MAPK/AP-1 signalling [30], and KIT/KIT ligand signalling [31–33]. Oxidative stress has been identified as a possible mechanism underlying VCD-induced toxicity; however, this was not fully elucidated and depletion of GSH levels was reported in the liver and ovary of rats exposed to VCD [34,35]. Thus, here we have sought to elucidate the mechanisms of VCM and VCD toxicity using D. melanogaster as a model for the first time. Specifically, we determined the impact of exposure of flies to VCM as well as VCD using different endpoints of toxicity, including quantification of mortality, and the activity or expression of electrophile sensitive enzymes and genes. The study was extended to examine the neurobehavioral effect of VCM and VCD on the flies.
Materials and methods
Chemicals
All chemicals used were of analytical grade. The 4-vinylcyclohexene 1,2-monoepoxide (VCM), 4-vinylcyclohexene diepoxide (VCD), 2′,7′-dichlorofluorescein diacetate (DCFH-DA), 1-chloro-2,4-dinitrobenzene, 5,5′-dithiobis(2-nitro-benzoic acid) (DTNB) and acetylthiocholine iodide were purchased from Sigma Aldrich (St Louis, MO).
Fly strains
D. melanogaster Harwich strain was obtained from the National Species Stock Center, Bowling Green, OH, USA, and maintained at constant temperature and humidity (23±1 °C; 60% relative humidity, respectively) under 12 h dark/light cycle. We used standard Drosophila medium composed of cornmeal medium (1% w/v), brewer's yeast (2% w/v), sucrose (1% w/v), powdered milk (1% w/v), agar and nipagin (0.08% w/v). The maximum average lifespan of flies used here (D. melanogaster, Harwich strain) is about 50–58 days, and about 50% of the flies generally die within 41–45 days. However, this can vary depending on a variety of factors such as diet composition and temperature [36].
Survival curve after VCM exposure
In three independent experiments, 30 flies of both genders (1–3 days old) were exposed to various respective concentrations of VCM and VCD (10, 100 µM, 1 and 10 mM; prepared in 98% ethanol) in three replicates for 28 days as previously described [3]. The number of live and dead flies were scored daily till the end of the experiment and the survival rate was expressed as percentage of live flies. Based on the survival curves of flies exposed to VCM and VCD (Fig. 1), we decided to expose the flies to 10, 100 µM and 1 mM of VCM or VCD for 5 days in order to detect early molecular and behavioural signs of toxicity associated with VCM or VCD exposure (Scheme 2). The 5 days survival rate was also determined.
Fig. 1.
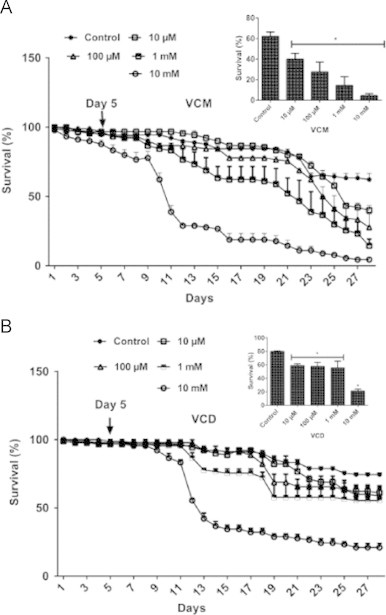
VCM and VCD reduced survival rates of Drosophila melanogaster. (A) 28 days survival of flies treated with VCM and (B) 28 days survival of flies treated with VCD. Data are presented as mean±SEM of three independent biological replicates carried out in duplicates. Each assay was carried out in three independent experiments. *p<0.05 vs control.
Scheme 2.
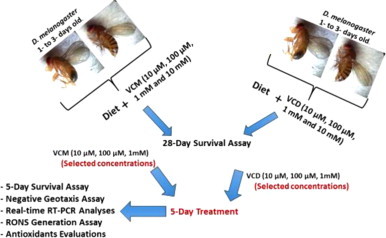
Summary of Experimental Design.
Negative geotaxis assay
The locomotor (Climbing) performance of VCM- and VCD-exposed and control flies were investigated using the negative geotaxis assay [37]. Briefly, ten (10) VCM- or VCD-exposed and control flies were immobilized under mild ice anaesthesia and placed separately in labelled vertical glass columns (length, 15 cm; diameter, 1.5 cm). After the recovery period (about 20 min), the flies were gently tapped to the bottom of the column. Following 6 s, the number of flies that climbed up to the 6 cm mark of the column, as well as those that remained below this mark were recorded. Data were expressed as the percentage of flies that escaped beyond the 6 cm mark in 6 s. The score of each group was an average of three trials for each group of treated and control flies.
The isolation of RNA and analysis of mRNA levels by quantitative real-time RT-PCR
Total RNA (about 2 µg) from 25 flies of VCM- or VCD-treated and control groups were extracted using Trizol® Reagent (Invitrogen®) following the manufacturer's protocol as previously described [3]. The primers sequences used for this study (SOD1, catalase, Keap-1, MAPK-2, TrxR-1, JAFRAC 1 and Cyp18a1, Table 1) were based on published sequences in GenBank Overview (http://www.ncbi.nlm.nih.gov/genbank/). They were designed using Primer3 program version 0.4.0 (http://frodo.wi.mit.edu/primer3/) and custom made by Invitrogen®. After treatment of the total RNA with DNase I (Invitrogen®), cDNA was synthesized with M-MLV reverse transcriptase enzyme and random primers by following the manufacturer's protocol (Invitrogen®), and stored at −20 °C till further use. Thereafter, qPCR was performed in 20 µL reaction volumes consisting of 1 µL RT product (cDNAs) as template, 1× PCR Buffer, 25 µM dNTPs, 0.2 µM of each primer (Table 1), 1.5–2.5 mM MgCl2, 0.1× SYBR Green I (molecular probes®) and 1 U Taq DNA polymerase (Invitrogen®). Next, thermal cycle was performed using StepOne Plus real time PCR systems (Applied Biosystems, NY) which involves, activation of the Taq DNA polymerase at 95 °C for 5 min, 40 cycles of 15 s at 95 °C, 15 s at 60 °C, and 25 s at 72 °C. After this, we determined the threshold and baselines using the StepOne Software v2.0 (Applied Biosystems, NY). This was followed by the analysis of SYBR fluorescence using StepOne software version 2.0 (Applied Biosystems, NY). Then, we calculated and recorded the CT (cycle threshold) value for each sample using 2-ΔΔCT [38]. Lastly, we analysed and obtained ΔCT value by subtracting the endogenous reference genes Tubulin and GPDH CT values from the CT value of each of the genes of interest.
Table 1.
Sequences of qPCR primers.
| Primer sequence | |
|---|---|
| SOD1 LEFT | 5′-GGAGTCGGTGATGTTGACCT-3′ |
| SOD1 RIGHT | 5′-GTTCGGTGACAACACCAATG-3′ |
| CATALASE LEFT | 5′-ACCAGGGCATCAAGAATCTG-3′ |
| CATALASE RIGHT | 5′-AACTTCTTGGCCTGCTCGTA-3′ |
| TrxR-1 LEFT | 5′-CGTTCTATTGTGCTGCGTGG-3′ |
| TrxR-1 RIGHT | 5′-AGCTTGCCATCATCCTGCTT-3′ |
| Cyp18a1 LEFT | 5′-TCTTGTTCTTGGCCGTCGAG-3′ |
| Cyp18a1 RIGHT | 5′-AGCAATGTGATCTGCAGCCT-3′ |
| JAFRAC 1 LEFT | 5′-TGGATCAACACGCCAAGGAA-3′ |
| JAFRAC 1 RIGHT | 5′-GGATGCCAGTCTCCTCATCG-3′ |
| MAPK-2 LEFT | 5′-GGCCACATAGCCTGTCATCT-3′ |
| MAPK-2 RIGHT | 5′-ACCAGATACTCCGTGGCTTG-3′ |
| KEAP-1 LEFT | 5′-CCAACTTCCTCAAGGAGCAG-3′ |
| KEAP-1 RIGHT | 5′-CGGCGACAAATATCATCCTT-3′ |
| GPDH LEFT | 5′-ATGGAGATGATTCGCTTCGT-3′ |
| GPDH RIGHT | 5′-GCTCCTCAATGGTTTTTCCA-3′ |
| TUBULIN LEFT | 5′-ACCAATGCAAGAAAGCCTTG-3′ |
| TUBULIN RIGHT | 5′-ATCCCCAACAACGTGAAGAC-3′ |
Sample preparation for biochemical assays
At the end of the exposure period (5 days), the flies (50) from each group of control and VCM- or VCD-treated flies were anaesthetized in ice, weighed, and homogenized in 0.1 M phosphate buffer, pH 7.0 (1 mg: 10 µL), and centrifuged for 10 min at 4000g (temperature, 4 °C). The supernatants obtained were used to determine the activities of catalase (CAT), Glutathione-S-transferase (GST) and acetylcholinesterase (AChE), and total thiol content. The assays were performed in duplicates for each of the three replicates of VCM or VCD concentrations and control in three independent experiments.
Measurement of DCFH oxidation
2′,7′-Dichlorofluorescein (DCFH) oxidation was measured as an index of oxidative stress according to the method of Perez-Severiano et al. [39]. Five microlitres (5 µL, 1:10 dilution) of each supernatant from VCM- and VCD-treated and control flies, 150 µL of 0.1 M potassium phosphate buffer (pH 7.4) and 40 µL of distilled water were transferred into a 96-well plate. After adding 5 µL of 200 µM DCFH-DA (final concentration of 5 µM) to the samples, the fluorescence product of DCFH oxidation (i.e. DCF), was measured for 10 min (30 s intervals), using SpectraMax plate reader (Molecular Devices, USA) with excitation 488 and 525 nm emission. All experiments were monitored in duplicate for each of the three replicates of VCM- or VCD-treated and control flies. The rate of DCF formation was expressed in percentage of the control group.
Total thiol determination
Total thiol content was determined using the method of Ellman [40]. The reaction mixture contained 170 µL potassium phosphate buffer (0.1 M, PH 7.4), 20 µL of sample as well as 10 µL of DTNB (10 mM). After incubation for 30 min at room temperature, the absorbance was measured at 412 nm and used to calculate the sample total thiol levels (in µmol/mg protein) using GSH as standard.
Glutathione-S-transferase (GST) activity
The activity of glutathione-S-transferase (GST; EC 2.5.1.18) was determined by the method of Habig and Jacoby [41] using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate. The assay reaction mixture contained 270 µL of solution A (20 mL of 0.25 M potassium phosphate buffer, pH 7.0 with 2.5 mM EDTA, 10.5 mL of distilled water and 500 µL of 0.1 M GSH at 25 °C), 20 µL of sample (1:5 dilution) and 10 µL of 25 mM CDNB. An increase in absorbance was measured at 340 nm for 5 min at 10 s interval using SpectraMax plate reader (Molecular Devices, USA). The data were expressed in mmol/min/mg of protein using the molar extinction coefficient (ε) of 9.6 mM−1 cm−1 for the coloured GS–DNB conjugate formed by GST.
Catalase (CAT) activity
The measurement of catalase (CAT; EC 1.11.1.6) activity was followed by a procedure described by Aebi [42]. The reaction mixture containing 1.8 mL of potassium phosphate buffer, pH 7.0, 180 µL of 300 mM H2O2, and 20 µL of sample (1:50 dilution) was carried out by monitoring the clearance of H2O2 at 240 nm at 25 °C. The decrease in H2O2 was monitored for 2 min (10 s intervals), at 240 nm using a UV–visible spectrophotometer (Shimadzu) and expressed as µmol of H2O2 consumed/min/mg of protein.
Acetylcholinesterase (AChE) activity
AChE activity was determined by the method described by Ellman et al. [43]. To the reaction mixture containing 135 µL of distilled water, 20 µL of 100 mM potassium phosphate buffer (pH 7.4), 10 mM DTNB, and 5 µL of sample, 20 µL of 8 mM acetylthiocholine was added. The change in absorbance was monitored at 412 nm for 5 min at 15 s intervals, using a SpectraMax plate reader (Molecular Devices, USA). The data were calculated against blank, and sample blank and the results were corrected by the protein content. The enzyme activity was expressed as µmole/min/mg of protein.
The determination of delta aminolevulinic acid dehydratase (δ-ALA-D) activity
The δ-ALA-D activity was determined by the modified method of Sassa [44], by measuring the rate of formation of porphobilinogen. Eighty (80) flies/vial were treated with VCM or VCD (10, 100 µM and 1 mM) for 5 days as described above. Consequently, the flies were anaesthetized using ice, weighed and homogenized in Tris–HCl buffer (5 mM, pH 8.5, 1 g of flies: 3 mL of buffer). The homogenates were centrifuged at 4000 rpm for 10 min at 4 °C to obtain supernatant fractions used to determine the activity of δ-ALA-D. The reaction mixture containing 10 µL of Tris–Glycine (0.5 M, pH 8.5), distilled water (7 µL), sample (25 µL) and 8 µL of 5-aminolevulinic acid hydrochloride (31.25 mM) was incubated for 3 h at 37 °C. Thereafter, 100 µL of TCA (10%) containing CuSO4 (20 mM) was added to each of the reaction mixtures, and centrifuged at 5000 rpm for 10 min. Subsequently, 100 µL of each of the supernatants obtained was added to 100 µL of Erlich reagent and incubated for 30 min at room temperature. Then, the absorbance was measured at 555 nm in a SpectraMax plate reader (Molecular Devices, USA) and the data were expressed as percentage activity of the control. Each assay was carried out in duplicates of the three replicate of the respective concentrations of VCM or VCD in three independent experiments.
Determination of protein
Protein concentrations in the whole fly homogenates were determined as described by Lowry et al. [45] using bovine serum albumin as the standard.
Statistical analysis
The data are expressed as mean±SEM (standard error of mean), and the statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Turkey's post-hoc test. The results were considered statistically significant at p<0.05.
Results
VCM and VCD reduced survival rates and impaired climbing behaviour of D. melanogaster
VCM and VCD reduced survival rates of the flies (Fig. 1A and B) along the 28 days of survival assay. However, the concentrations of 10, 100 and 1000 µM of VCM or VCD did not decrease survival rates after the 5 days of treatment (Fig. 2A and B). To investigate the neurobehavioral effects of VCM or VCD in the exposed flies, we determined the climbing activity of D. melanogaster after 5 days of exposure. As shown in Fig. 2C and D exposure to VCM or VCD (10 µM–1 mM) significantly decreased climbing/locomotor behaviour when compared with the control flies (p<0.05).
Fig. 2.
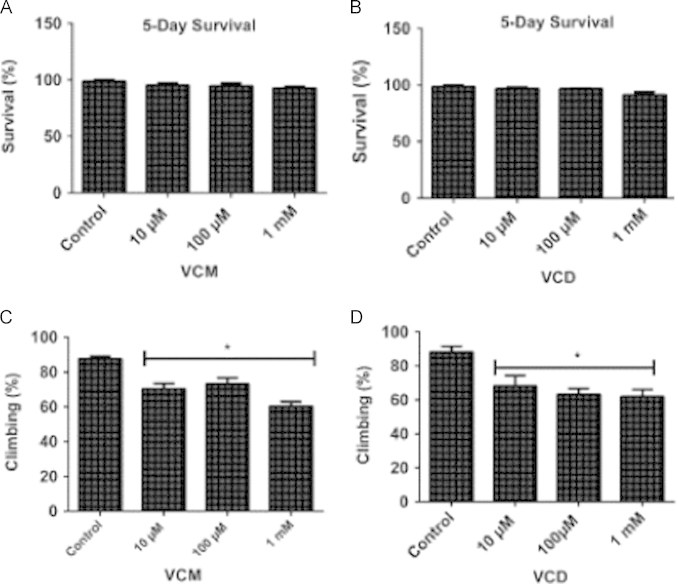
VCM and VCD did not alter survival of D. melanogaster, but impaired climbing behaviour after 5 days of exposure. (A) Five days survival rate and (C) negative geotaxis (climbing rate) of flies treated with VCM; (B) 5 days survival rate and (D) negative geotaxis (climbing rate) of flies treated with VCD. Data are presented as mean±SEM of three independent biological replicates carried out in duplicates. Each assay was carried out in three independent experiments. *p<0.05 vs control.
VCM and VCD enhanced reactive oxygen and nitrogen species (RONS) generation after 5 days of exposure in D. melanogaster
To ascertain if VCM and VCD could induce RONS production, DCFH oxidation was measured as an index of oxidative stress in the homogenates of flies following VCM or VCD exposure. VCM or VCD (10 µM–1 mM) caused significant increase in DCFH oxidation when compared with the control flies (p<0.05, Fig. 3A and B).
Fig. 3.
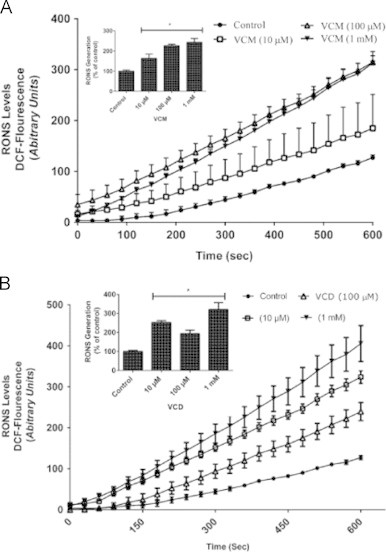
VCM and VCD enhanced RONS generations after 5 days of exposure in Drosophila melanogaster. (A) RONS levels after treatment of flies with VCM for 5 days; (B) RONS levels after treatment of flies with VCD for 5 days. Data are presented as mean±SEM of three independent biological replicates carried out in duplicates. Each assay was carried out in three independent experiments. *p<0.05 vs control.
Changes in antioxidant enzymes and total thiol content of D. melanogaster after 5 days of exposure to VCM and VCD
To examine whether the increased RONS generation measured by the DCFH oxidation is associated with decreased activities of antioxidant enzymes and total thiol content, we investigated the activities of catalase (CAT) and glutathione-S-transferase (GST), as well as total thiols content. VCM exposure significantly increased CAT activity at all the concentrations tested (p<0.05; Fig. 4A), while VCD increased CAT activity at 10 and 100 µM concentrations when compared to control (p<0.05; Fig. 4B). A statistically significant depletion in total thiol content was observed in the flies exposed to 10 µM and 1 mM VCM for 5 days (p<0.05; Fig. 4C), while VCD decreased total thiol content at all the tested concentrations compared with the control (p<0.05; Fig. 4D). Exposure of flies to VCM (10 and 100 µM) resulted in significant decrease in GST activity (p<0.05; Fig. 4E) while VCD exposure decreased GST activity in all the tested concentrations, when compared with the control group (p<0.05; Fig. 4F).
Fig. 4.
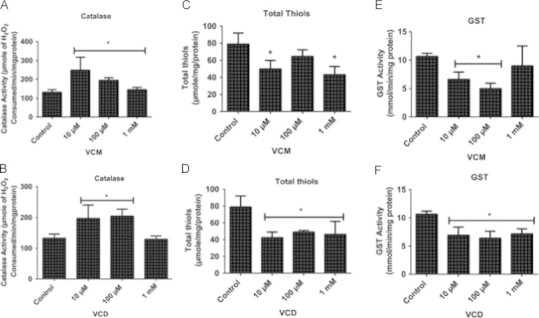
Changes in antioxidant enzymes activities and total thiol content of D. melanogaster after 5 days of exposure to VCM and VCD. (A) Catalase activity, (C) Total thiols content and (E) GST activity of flies treated with VCM for 5 days; (B) catalase activity, (D) Total thiols content and (F) GST activity of flies treated with VCD for 5 days. Data are presented as mean±SEM of three independent biological replicates carried out in duplicate. *p<0.05 vs control. Each assay was carried out in three independent experiments.
Effects of VCM or VCD exposure on superoxide dismutase (SOD1) and catalase (CAT) mRNA expressions in D. melanogaster
The qRT-PCR assays on SOD1 indicated that flies exposed to VCM (10 µM) and VCD (10 and 100 µM) exhibited significant increase in SOD1 mRNA level after 5 days of exposure as compared with the control (p<0.05; Fig. 5A and B). In contrast, VCM (10, 100 and 1000 µM) and VCD (10 µM) significantly decreased CAT mRNA levels in the treated flies in comparison with the control (p<0.05; Fig. 5C and D).
Fig. 5.
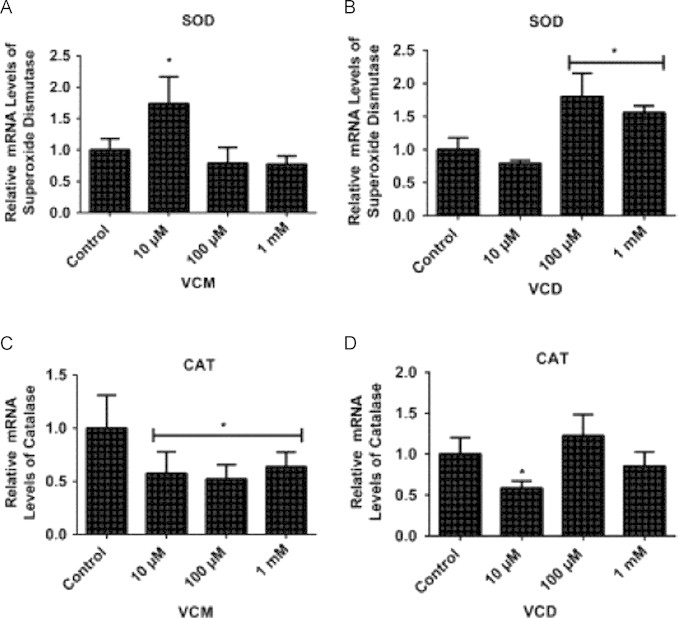
Quantitative real time RT-PCR (qRT-PCR) analyses of SOD1 and CAT gene expressions in D. melanogaster exposed to VCM and VCD for 5 days. (A) SOD1 and (C) cat mRNA levels of flies treated with VCM for 5 days; (B) SOD1 and (D) CAT mRNA levels of flies treated with VCD for 5 days. Data are presented as mean±SEM of three independent biological replicates carried out in quadruplicates. Each assay was carried out in three independent experiments. *p<0.05 vs control.
VCM and VCD induced changes in Keap-1 and MAPK-2 mRNA gene expressions in D. melanogaster after 5 days of exposure
Exposure of flies for 5 days to 10 and 100 µM of VCM decreased Keap-1 mRNA levels (p<0.05; Fig. 6A). In contrast, exposure to 100 and 1000 µM of VCD enhanced the expression of Keap-1 mRNA compared with the control (p<0.05; Fig. 6B). Additionally, while only the highest concentration of VCM (1 mM) increased MAPK-2 mRNA levels, all the concentrations of VCD (10, 100 and 1000 µM) increased MAPK-2 mRNA levels (Fig. 6C and D), when compared with the control group (p<0.05).
Fig. 6.
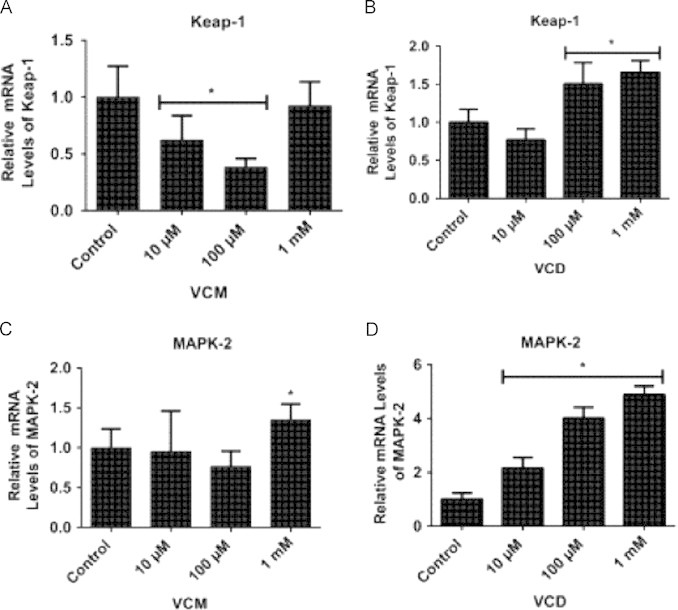
Quantitative real time RT-PCR (qRT-PCR) analyses of Keap-1 and MAPK-2 mRNA gene expressions of D. melanogaster after 5 days of exposure to VCM and VCD. (A) Keap-1 and (C) MAPK-2 mRNA levels of flies exposed to VCM for 5 days; (B) Keap-1 and (D) MAPK-2 mRNA levels of flies exposed to VCD for 5 days. Data are presented as mean±SEM of three independent biological replicates carried out in quadruplicates. Each assay was carried out in three independent experiments. *p<0.05 vs control.
VCM and VCD altered Cyp18a1, JAFRAC 1 and TrxR-1 mRNA gene expressions in D. melanogaster after 5 days of exposure
After the 5 days of treatment, VCM significantly decreased the expressions of the mRNA levels of Cyp18a1 (Fig. 7A) and JAFRAC 1 (Fig. 7C) in all the tested concentrations, while TrxR-1 mRNA levels (Fig. 7E) were significantly reduced in the flies exposed to the 10 µM VCM concentration (p<0.05). We noted that VCD (10 and 1000 µM) significantly decreased the expressions of the mRNA levels of Cyp18a1 (Fig. 7B) and TrxR-1 (Fig. 7F). In contrast, exposure to 100 and 1000 µM of VCD increased the expressions of JAFRAC 1 mRNA levels in comparison with the control group (p<0.05; Fig. 7D).
Fig. 7.
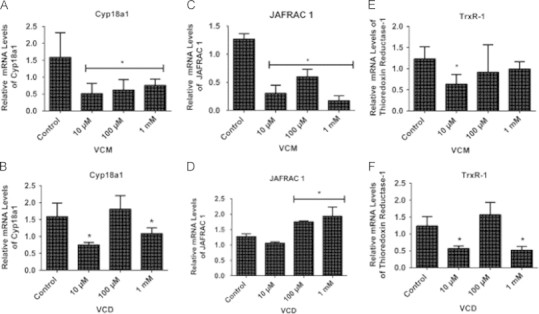
Quantitative real time RT-PCR (qRT-PCR) analyses of Cyp18a1, JAFRAC 1 and TrxR-1 mRNA gene expressions of D. melanogaster after 5 days of exposure to VCM and VCD. (A) Cyp18a1, (C) JAFRAC 1 and (E) TrxR-1 mRNA levels of flies treated with VCM for 5 days; (B) Cyp18a1, (D) JAFRAC 1 and (F) TrxR-1 mRNA levels of flies treated with VCD for 5 days. Data are presented as mean±SEM of three independent biological replicates carried out in quadruplicates. Each assay was carried out in three independent experiments. *p<0.05 vs control.
VCM and VCD inhibited AChE and δ-ALA-D activities after 5 days of exposure in D. melanogaster
We observed that VCM (10 and 1000 µM) and VCD (10, 100 and 1000 µM) significantly inhibited AChE activity following 5 days of exposure, (Fig. 8A and B, p<0.05). In addition, VCM and VCD caused significant inhibition of δ-ALA-D activity in all the tested concentrations (p<0.05; Fig. 8C and D).
Fig. 8.
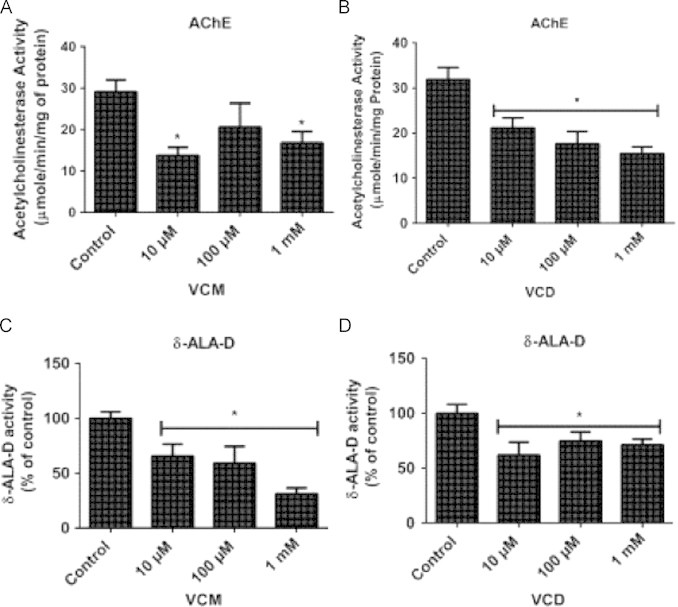
VCM and VCD inhibited AChE and δ-ALA-D activities after 5 days of exposure to Drosophila melanogaster. (A) AChE and (C) δ-ALA-D activities of flies exposed to VCM for 5 days; (B) AChE and (D) δ-ALA-D activities of flies exposed to VCD for 5 days. Data are presented as mean±SEM of three independent biological replicates carried out in duplicates. Each assay was carried out in three independent experiments. *p<0.05 vs control.
Discussion
Exposure to environmental toxicants can generate reactive oxygen and nitrogen species (RONS), and cause oxidative damage to cellular components [46]. For instance, in the human reproductive tissues, environmental toxicants may deplete follicles at all stages of development in females [47], or alter gene expression that is pertinent to spermatogenesis in males [48]. In the present investigation, the mechanisms of toxicity of the downstream metabolites of 4-vinylcyclohexene (VCH), i.e. 4-vinylcyclohexene 1,2-monoepoxide (VCM) and 4-vinylcyclohexene diepoxide were investigated in D. melanogaster. The results indicated that exposure to VCM as well as VCD induced RONS-mediated disruption of antioxidant balance in the flies.
Although, ROS are important in several biochemical processes, their overproduction can induce oxidative damage to cellular biomolecules such as DNA, carbohydrates, lipids and proteins [49]. Similar to our previous study with VCH [3], 5 days of VCM or VCD exposure induced overproduction of RONS. Accordingly, we observed increased CAT activity and SOD1 mRNA gene expression in VCM- and VCD-exposed flies. The increased CAT activity may be an adaptive response to degrade hydrogen peroxide overproduced in the fly’s tissues (here evidenced by an increase in DCFH-oxidation). In contrast to CAT activity, the mRNA level of catalase decreased after exposure to VCM as well as VCD. The discrepancies between activity and mRNA can be related to changes in posttranscriptional processing of catalase gene products. In our previous study involving VCH, we found an increase in CAT activity without any change in CAT mRNA level [3]. This suggests that the response of the flies to VCH and its metabolites, VCM and VCD is not exactly the same.
In addition, we sought to understand the response of the flies to VCM or VCD exposure with respect to the transcription of other genes such as kelch-like erythroid-derived cap-n-collar (CNC) homology (ECH)-associated protein 1 (Keap-1), mitogen-activated protein kinase 2 (MAPK-2), Cyp18a1, JAFRAC 1 and TrxR-1. We noted that VCM and VCD increased MAPK-2 and decreased Cyp18a1 and TrxR-1 transcription. In addition, VCM and VCD had different effects on the transcription of Keap-1 and JAFRAC 1 genes. While VCM reduced the levels of mRNA of Keap-1 and JAFRAC 1 genes, VCD enhanced their mRNA levels.
Keap-1, a cytosolic repressor of nuclear factor erythroid 2-related factor 2 (Nrf2), is a sensor of redox regulation. Importantly, the Drosophila cap′n’collar C (CncC), the counterpart of Nrf2 [50], and Keap-1 proteins, regulate antioxidant response elements (ARE)-mediated transcription, and they are crucial for cellular defence against oxidative stress. Of note, when Keap-1 senses disturbance in the redox homoeostasis, it switches Nrf2 on or off. As a result of this, Keap-1 protein undergoes a conformational change which leads to Nrf2 liberation and migration to the nucleus, where it promotes the transcription of antioxidant genes [51]. In a study by Jung and Kwak [52], Keap1-knockdown HT29 cells exhibited enhanced levels of Nrf2 and its target gene expressions, which conferred greater resistance to hydrogen peroxide and 4-hydroxynonenal insults than the control cells. Thus, flies treated with VCM may be more resistant to oxidative stress compared with VCD-treated flies, due to the reduced expression of Keap-1 transcription in VCM-exposed flies. Possibly, the net impact of VCM and VCD on Keap-1 was what we noted in our previous study in which VCH, the parent compound, enhanced the transcription of Keap-1 [3,53].
MAPKs are proapoptotic signalling molecules activated via phosphorylation by MAPK kinases (MAPKKs). Thus, the up-regulation of MAPK-2 gene, as reported here, may be connected with VCM – as well as VCD-induced toxicity, since MAPK-2 has been shown to be induced by ROS accumulation [54].
In order to understand the possible roles of VCM and VCD on flies development, we investigated the expression of Cyp18a1 gene that encodes a cytochrome P450 enzyme (CYP18A1) with 26-hydroxylase activity, a prominent step in Drosophila ecdysteroid catabolism. The ecdysteroids are steroids hormones that play fundamental roles in the metamorphosis of D. melanogaster [55,56]. Thus, the reduced expression of Cyp18a1 transcription, due to VCM or VCD exposure, may impair the fly’s developmental stages. For instance, Guittard et al. [57] have demonstrated that knockdown of Cyp18a1 gene expression led to the inactivation of CYP18A1 in Drosophila, which resulted in pupal lethality. The observation that VCM and VCD changed Cyp18a1 mRNA expression indicates that VCM and VCD may represent potential disruptors of the normal development of D. melanogaster.
In view of the lethality caused to the flies after 28 days of exposure to VCM or VCD, as well as the accumulation of RONS after 5 days of treatment, we sought to understand the impact of VCM and VCD on JAFRAC 1 (thioredoxin peroxidase 1), which is believed to extend longevity and promote resistance to oxidative stress in flies. Indeed, JAFRAC 1 is the fly homologue of human peroxiredoxin II (Prx II), regulated by JNK/FOXO signalling [58]. Thus, the VCM-induced reduction of the mRNA gene expression of JAFRAC 1, indicates another potential molecular mechanism involved in VCM-induced toxicity, which is different from VCD-induced induction of JAFRAC 1 gene, which in this case, may represent an adaptive response of the flies to VCD insult alone. This further indicates that VCM and VCD impacts on the flies are not exactly similar.
Further, because the thioredoxin system is responsible for the reduction of glutathione disulphide in D. melanogaster due to the absence of glutathione reductase [59], we sought to understand the impact of VCM and VCD on the transcription of thioredoxin reductase 1 (TrxR-1) gene in the flies. The TrxRs possess a rather broad substrate spectrum due to the presence of additional redox centre on the flexible –COOH terminal extension of the protein [60]. TrxR-1 is vital in maintaining the antioxidant capacity of the system via the transfer of reducing equivalents from NADPH to thioredoxin (Trx), which in turn reduces other proteins such as Trx peroxidase [61]. Here, VCM and VCD decreased the transcription of TrxR-1 gene, which might have contributed to the disruption in the antioxidant capacity of the flies. At least in part, this can help in explaining the overproduction of RONS as noted here. Since reduced transcription does not always imply reduced activity of the corresponding enzyme, one limitation of the present study is the fact that we did not investigate the activity of TrxR-1. However, the enhanced oxidation of DCFH supports the notion that VCM and VCD induced overproduction of RONS, and overwhelmed the antioxidant buffering capacity of the flies. Another limitation of the present investigation is that we did not perform the immunodetection of the proteins due to limitation in the available techniques with respect to D. melanogaster.
We also evaluated the effects of exposure to VCM and VCD on total GST activity and total thiols content in flies. GST is an enzyme involved in conjugation reactions during phase II xenobiotic metabolism. It catalyses the reactions between reduced glutathione (GSH) and a variety of potentially toxic electrophilic compounds [62,63]. This helps to protect the cell against free radicals [64]. Similarly, total thiols (protein and non-protein thiols), are important targets of RONS and useful biochemical indicators of oxidative stress [65]. Membranes are also susceptible to free radicals resulting in lipid peroxidation and decreased concentration of –SH groups in membrane proteins [66]. In the present study, the activity of GST and the levels of total –SH groups were significantly reduced following 5 days of exposure to VCM and VCD. These observations could be due to increased free radicals/RONS and reactive products of lipid peroxidation produced under our experimental conditions. Of note, in our previous study, VCH failed to alter the level of total thiols after 5 days of exposure in flies [3], indicating that exposure to the more reactive epoxides (VCM and VCD) were more damaging to thiols than the parent compound (VCH).
Further, we sought to know if VCM and VCD could affect the behaviour of the flies by assessing the climbing activity and the acetylcholinesterase (AChE) activity in the flies. We noticed significant reductions in the climbing rates of VCM- and VCD-exposed flies, which was associated with the inhibition of AChE activity. The work of Sharma et al. [67] indicated a positive correlation between inhibition of AChE activity and climbing/locomotor activity in flies. Our results further suggest that AChE activity determination is a sensitive marker of neurotoxicity, since inhibition of its activity can be an indicator of poor locomotor activity [68] and general toxicity [69].
Since some studies have reported that δ-aminolevulinic acid dehydratase (δ-ALA-D) can be sensitive to pro-oxidants, and that its activity can be decreased in situations associated with oxidative stress [70], we investigated the effects of VCM and VCD on its activity. The δ-ALA-D is an enzyme essential for all aerobic organisms because it catalyses the asymmetric condensation of two molecules of 5-aminolevulinate to produce porphobilinogen, a precursor of porphyrins [71]. Similar to our observation with VCH, VCM and VCD significantly inhibited δ-ALA-D activity after 5 days of exposure. The inhibition of δ-ALA-D can exacerbate the over-generation of free radicals (which may account for VCM and VCD toxicity) via an accumulation of aminolevulinic acid (ALA), a pro-oxidant [72], and therefore further contribute to oxidative stress induced by VCM and VCD. Since D. melanogaster δ-ALA-D contains essential sulfhydryl (–SH) groups in its structure, another explanation for δ-ALA-D inhibition can be the oxidation of these residues, leading to enzyme inactivation [73].
In summary, the results presented here evidenced some of the mechanisms associated with VCM – as well as VCD-induced toxicity in D. melanogaster. The contributions of the induction of RONS/Free radicals, as well as disruptions in the antioxidant and redox balance, followed by the inhibition of the transcription of important genes such as TrxR-1 and Cyp18a1, are part of these mechanisms (Scheme 3). Also, VCM- and VCD-induced neurotoxicity in the flies was indicated by AChE inhibition. Taken together, these results elucidate important features of the electrophilic metabolites of VCH, i.e. VCM and VCD, thus contributing to a better understanding of the mechanisms involved in the toxicity of VCH, using D. melanogaster as a model. Hence, in view of some similarities in the mechanisms of toxicity of VCH and its metabolites, VCM and VCD, we can confirm that the toxicity of VCH may be mediated by its transformation to VCM and VCD.
Scheme 3.
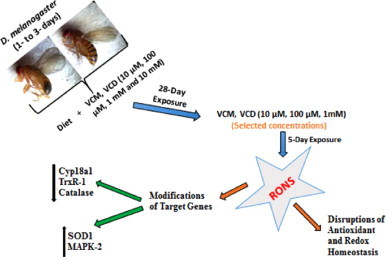
Summary of the mechanisms of 4-vinylcyclohexene 1,2-monoepoxide (VCM)- and 4-vinyvlcyclohexene diepoxide (VCD)-induced toxicity in Drosophila melanogaster.
Conflicts of interest
The authors declare that there is no conflict of interest associated with this study.
Acknowledgements
A.A.O. is grateful to TWAS-CNPq for the financial support. A.A.O. is a beneficiary of the TWAS-CNPq 2012 Postdoctoral Fellowship. In addition, this study was supported by CNPq, CAPES, FAPERGS, FAPERGS-PRONEX, FAPERGS-PRONEX-CNPq, VITAE Fundation, Rede Brasileira de Neurociências (IBNET-FINEP), FINEP-CTINFRA, INCT-CNPq for excitotoxicity and Neuroprotection and CNPQ-Institute for Excitotoxicity and Neuroprotection.
Contributor Information
Amos O. Abolaji, Email: amos_abolaji@yahoo.com, ao.abolaji@ui.edu.ng.
João B.T. Rocha, Email: jbtrocha@yahoo.com.br.
References
- 1.Hoyer P.B., Davis J.R., Bedrnicek J.B., Marion S.L., Christian P.J., Barton J.K., Brewer M.A. Ovarian neoplasm development by 7,12-dimethylbenz[a]anthracene (DMBA) in a chemically-induced rat model of ovarian failure. Gynecol. Oncol. 2009;112(3):610–615. doi: 10.1016/j.ygyno.2008.12.013. 19150572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganesan S., Bhattacharya P., Keating A.F. 7,12-Dimethylbenz[a]anthracene exposure induces the DNA repair response in neonatal rat ovaries. Toxicol. Appl. Pharmacol. 2013;272(3):690–696. doi: 10.1016/j.taap.2013.08.013. 23969067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abolaji A.O., Kamdem J.P., Lugokenski T.H., Nascimento T.K., Waczuk E.P., Farombi E.O., Loreto É.L., Rocha J.B. Involvement of oxidative stress in 4-vinylcyclohexene-induced toxicity in Drosophila melanogaster. Free Radic. Biol. Med. 2014;71:99–108. doi: 10.1016/j.freeradbiomed.2014.03.014. 24681254 [DOI] [PubMed] [Google Scholar]
- 4.Rappaport S.M., Fraser D.A. Gas chromatographic–mass spectrometric identification of volatiles released from a rubber stock during simulated vulcanization. Anal. Chem. 1976;48(3):476–481. [Google Scholar]
- 5.International Agency for Research on Cancer (IARC), 4-Vinylcyclohexene. IARC Monographs on the Evaluation of Carcinogenic risks to Humans: Some Industrial Chemicals, vol. 60, 1994, IARC, Lyon, France, p. 347.
- 6.Bhattacharya P., Sen N., Hoyer P.B., Keating A.F. Ovarian expressed microsomal epoxide hydrolase: role in detoxification of 4-vinylcyclohexene diepoxide and regulation by phosphatidylinositol-3 kinase signaling. Toxicol. Appl. Pharmacol. 2012;258(1):118–123. doi: 10.1016/j.taap.2011.10.014. 22061827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajapaksa K.S., Cannady E.A., Sipes I.G., Hoyer P.B. Involvement of CYP 2E1 enzyme in ovotoxicity caused by 4-vinylcyclohexene and its metabolites. Toxicol. Appl. Pharmacol. 2007;221(2):215–221. doi: 10.1016/j.taap.2007.03.009. 17462685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kappeler C.J., Hoyer P.B. 4-Vinylcyclohexene diepoxide: a model chemical for ovotoxicity. Syst. Biol. Reprod. Med. 2012;58(1):57–62. doi: 10.3109/19396368.2011.648820. 22239082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith B.J., Mattison D.R., Sipes I.G. The role of epoxidation in 4-vinylcyclohexene-induced ovarian toxicity. Toxicol. Appl. Pharmacol. 1990;105(3):372–381. doi: 10.1016/0041-008x(90)90141-g. 2237912 [DOI] [PubMed] [Google Scholar]
- 10.Wallace J.G. Encyclopedia of Chemical Technology. second edition. Vol. 8. John Wiley & Sons; New York: 1964. pp. 249–265. [Google Scholar]
- 11.Chemical Manufacturers Association . Hygiene Sampling for 4-Vinylcyclohexene in the Work Place. Chemical Manufacturers Association; Washington DC: 1991. [Google Scholar]
- 12.Union Carbide Crop. Bakelite Epoxy Resin ERL-4206, Technical Bulletin, 4-1456, New York,1964 .
- 13.Fluka Chemika-BioChemika . SAF Bulk Chemicals. Fluka Chemika-BioChemika; Buchs: 1993. p. 1366. [Google Scholar]
- 14.Ringo D.L., Brennan E.F., Cota-Robles E.H. Epoxy resins are mutagenic: implications for electron microscopists. J. Ultrastruct. Res. 1982;80(3):280–287. doi: 10.1016/s0022-5320(82)80041-5. 6752439 [DOI] [PubMed] [Google Scholar]
- 15.US National Institute for Occupational Safety and Health (1981–1983) US National Institute for Occupational Safety and Health; Cincinnati, OH.: 1993. [Google Scholar]
- 16.Smith B.J., Mattison D.R., Sipes I.G. The role of epoxidation in 4-vinylcyclohexene-induced ovarian toxicity. Toxicol. Appl. Pharmacol. 1990;105(3):372–381. doi: 10.1016/0041-008x(90)90141-g. 2237912 [DOI] [PubMed] [Google Scholar]
- 17.Doerr J.K., Hooser S.B., Smith B.J., Sipes I.G. Ovarian toxicity of 4-vinylcyclohexene and related olefins in B6C3F1 mice: role of diepoxides. Chem. Res. Toxicol. 1995;8(7):963–969. doi: 10.1021/tx00049a010. 8555412 [DOI] [PubMed] [Google Scholar]
- 18.Franco R., Sánchez-Olea R., Reyes-Reyes E.M., Panayiotidis M.I. Environmental toxicity, oxidative stress and apoptosis: Ménage á trois. Mutat. Res. 2009;674(1–2):3–22. doi: 10.1016/j.mrgentox.2008.11.012. 19114126 [DOI] [PubMed] [Google Scholar]
- 19.Davies K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50(4–5):279–289. doi: 10.1080/713803728. 11327322 [DOI] [PubMed] [Google Scholar]
- 20.Anderson M.E., Luo J.L. Glutathione therapy: from prodrugs to genes. Semin. Liver Dis. 1998;18(4):415–424. doi: 10.1055/s-2007-1007174. 9875558 [DOI] [PubMed] [Google Scholar]
- 21.Kemp M., Go Y.M., Jones D.P. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic. Biol. Med. 2008;44(6):921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. 18155672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Go Y.M., Jones D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta. 2008;1780(11):1273–1290. doi: 10.1016/j.bbagen.2008.01.011. 18267127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho Y.-S., Gargano M., Cao J., Bronson R.T., Heimler I., Hutz R.J. Reduced fertility in female mice lacking copper–zinc superoxide dismutase. J. Biol. Chem. 1998;273(13):7765–7769. doi: 10.1074/jbc.273.13.7765. 9516486 [DOI] [PubMed] [Google Scholar]
- 24.Jones D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006;8(9–10):1865–1879. doi: 10.1089/ars.2006.8.1865. 16987039 [DOI] [PubMed] [Google Scholar]
- 25.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295(4):C849–C868. doi: 10.1152/ajpcell.00283.2008. 18684987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joo M.S., Lee C.G., Koo J.H., Kim S.G. miR-125b transcriptionally increased by Nrf2 inhibits AhR repressor, which protects kidney from cisplatin-induced injury. Cell Death Dis. 2013;4:e899. doi: 10.1038/cddis.2013.427. 24176857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Springer L.N., McAsey M.E., Flaws J.A., Tilly J.L., Sipes I.G., Hoyer P.B. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol. Appl. Pharmacol. 1996;139(2):394–401. doi: 10.1006/taap.1996.0180. 8806857 [DOI] [PubMed] [Google Scholar]
- 28.Springer L.N., Tilly J.L., Sipes I.G., Hoyer P.B. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicol. Appl. Pharmacol. 1996;139(2):402–410. doi: 10.1006/taap.1996.0181. 8806858 [DOI] [PubMed] [Google Scholar]
- 29.Hu X., Christian P., Sipes I.G., Hoyer P.B. Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol. Reprod. 2001;65(5):1489–1495. doi: 10.1095/biolreprod65.5.1489. 11673266 [DOI] [PubMed] [Google Scholar]
- 30.Hu X., Flaws J.A., Sipes I.G., Hoyer P.B. Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-Vinylcyclohexene Diepoxide in rats. Biol. Reprod. 2002;67(3):718–724. doi: 10.1095/biolreprod.102.004259. 12193377 [DOI] [PubMed] [Google Scholar]
- 31.Fernandez S.M., Keating A.F., Christian P.J., Sen N., Hoying J.B., Brooks H.L., Hoyer P.B. Involvement of the KIT/KITL signaling pathway in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in rats. Biol. Reprod. 2008;79(2):318–327. doi: 10.1095/biolreprod.108.067744. 18448842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keating A.F., J Mark C., Sen N., Sipes I.G., Hoyer P.B. Effect of phosphatidylinositol-3 kinase inhibition on ovotoxicity caused by 4-Vinylcyclohexene Diepoxide and 7,12- Dimethylbenz[a]anthracene in neonatal rat ovaries. Toxicol. Appl. Pharmacol. 2009;241(2):127–134. doi: 10.1016/j.taap.2009.08.012. 19695275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mark-Kappeler C.J., Sen N., Keating A.F., Sipes I.G., Hoyer P.B. Distribution and responsiveness of rat anti-mullerian hormone during ovarian development and VCD-Induced ovotoxicity. Toxicol. Appl. Pharmacol. 2010;249(1):1–7. doi: 10.1016/j.taap.2010.08.024. 20816688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devine P.J., Sipes I.G., Hoyer P.B. Effect of 4-vinylcyclohexene diepoxide dosing in rats on GSH levels in liver and ovaries. Toxicol. Sci. 2001;62(2):315–320. doi: 10.1093/toxsci/62.2.315. 11452144 [DOI] [PubMed] [Google Scholar]
- 35.Devine P.J., Sipes I.G., Hoyer P.B. Initiation of delayed ovotoxicity by in vitro and in vivo exposures of rat ovaries to 4-vinylcyclohexene diepoxide. Reprod. Toxicol. 2004;19(1):71–77. doi: 10.1016/j.reprotox.2004.06.002. 15336714 [DOI] [PubMed] [Google Scholar]
- 36.Golombieski R.M., Graichen D.ÂS., Rocha J.B.T.d, Valente V.L.dS., da Silva L.E. Over-activation of the Drosophila melanogaster hsp83 gene by selenium intoxication. Genet. Mol. Biol. 2008;31(1):128–135. [Google Scholar]
- 37.Feany M.B., Bender W.W. A drosophila model of Parkinson’s disease. Nature. 2000;404(6776):394–398. doi: 10.1038/35006074. 10746727 [DOI] [PubMed] [Google Scholar]
- 38.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. 11846609 [DOI] [PubMed] [Google Scholar]
- 39.Pérez-Severiano F., Santamaría A., Pedraza-Chaverri J., Medina-Campos O.N., Ríos C., Segovia J. Increased formation of reactive oxygen species, but no changes in glutathione peroxidase activity, in striata of mice transgenic for the Huntington’s disease mutation. Neurochem. Res. 2004;29(4):729–733. doi: 10.1023/b:nere.0000018843.83770.4b. 15098934 [DOI] [PubMed] [Google Scholar]
- 40.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. 13650640 [DOI] [PubMed] [Google Scholar]
- 41.Habig W.H., Jakoby W.B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. 7329316 [DOI] [PubMed] [Google Scholar]
- 42.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. 6727660 [DOI] [PubMed] [Google Scholar]
- 43.Ellman G.L., Courtney K.D., Andres V. Jr, Feather-stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. 13726518 [DOI] [PubMed] [Google Scholar]
- 44.Sassa S. Delta-aminolevulinic acid dehydratase assay. Enzyme. 1982;28(2–3):133–145. doi: 10.1159/000459097. 7140716 [DOI] [PubMed] [Google Scholar]
- 45.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. 14907713 [PubMed] [Google Scholar]
- 46.Igawa Y., Keating A.F., Rajapaksa K.S., Sipes I.G., Hoyer P.B. Evaluation of ovotoxicity induced by 7, 12-dimethylbenz[a]anthracene and its 3,4-diol metabolite utilizing a rat in vitro ovarian culture system. Toxicol. Appl. Pharmacol. 2009;234(3):361–369. doi: 10.1016/j.taap.2008.10.009. 19027032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathur P.P., D’Cruz S.C. The effect of environmental contaminants on testicular function. Asian J. Androl. 2011;13(4):585–591. doi: 10.1038/aja.2011.40. 21706039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0. 25646037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennett M.R. Reactive oxygen species and death: oxidative DNA damage in atherosclerosis. Circ. Res. 2001;88(7):648–650. doi: 10.1161/hh0701.089955. 11304484 [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi M., Itoh K., Suzuki T., Osanai H., Nishikawa K., Katoh Y., Takagi Y., Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7(8):807–820. doi: 10.1046/j.1365-2443.2002.00561.x. 12167159 [DOI] [PubMed] [Google Scholar]
- 51.Tian H., Zhang B., Di J., Jiang G., Chen F., Li H., Li L., Pei D., Zheng J. Keap1: one stone kills three birds Nrf2, IKKβ and Bcl-2/Bcl-xL. Cancer Lett. 2012;325(1):26–34. doi: 10.1016/j.canlet.2012.06.007. 22743616 [DOI] [PubMed] [Google Scholar]
- 52.Jung K.A., Kwak M.K. Enhanced 4-hydroxynonenal resistance in KEAP1 silenced human Colón cancer cells. Oxid. Med. Cell. Longev. 2013;2013:423965. doi: 10.1155/2013/423965. 23766854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abolaji A.O., Kamdem J.P., Lugokenski T.H., Nascimento T.K., Waczuk E.P., Farombi E.O., Loreto E.L.S., Rocha J.B.T. Corrigendum to “Involvement of oxidative stress in 4-vinylcyclohexene-induced toxicity in Drosophila melanogaster” [Free Radic. Biol. Med. 71 (2014) 99–108] Free Radic. Biol. Med. 2015;82:204–205. doi: 10.1016/j.freeradbiomed.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 54.Iida C., Fujii K., Kishioka T., Nagae R., Onishi Y., Ichi I., Kojo S. Activation of mitogen activated protein kinase (MAPK) during carbon tetrachloride intoxication in the rat liver. Arch. Toxicol. 2007;81(7):489–493. doi: 10.1007/s00204-007-0181-x. 17285312 [DOI] [PubMed] [Google Scholar]
- 55.Williams D.R., Fisher M.J., Rees H.H. Characterization of ecdysteroid 26-hydroxylase: an enzyme involved in molting hormone inactivation. Arch. Biochem. Biophys. 2000;376(2):389–398. doi: 10.1006/abbi.2000.1731. 10775427 [DOI] [PubMed] [Google Scholar]
- 56.You L. Steroid hormone biotransformation and xenobiotic induction of hepatic steroid metabolizing enzymes. Chem. Biol. Interact. 2004;147(3):233–246. doi: 10.1016/j.cbi.2004.01.006. 15135080 [DOI] [PubMed] [Google Scholar]
- 57.Guittard E., Blais C., Maria A., Parvy J.P., Pasricha S., Lumb C., Lafont R., Daborn P.J., Dauphin-Villemant C. CYP18A1, a key enzyme of drosophila steroid hormone inactivation, is essential for metamorphosis. Dev. Biol. 2011;349(1):35–45. doi: 10.1016/j.ydbio.2010.09.023. 20932968 [DOI] [PubMed] [Google Scholar]
- 58.Lee K.S., Iijima-Ando K., Iijima K., Lee W.J., Lee J.H., Yu K., Lee D.S. JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends life span. J. Biol. Chem. 2009;284(43):29454–29461. doi: 10.1074/jbc.M109.028027. 19720829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanzok S.M., Fechner A., Bauer H., Ulschmid J.K., Müller H.M., Botella-Munoz J., Schneuwly S., Schirmer R., Becker K. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science. 2001;291(5504):643–646. doi: 10.1126/science.291.5504.643. 11158675 [DOI] [PubMed] [Google Scholar]
- 60.Bauer H., Kanzok S.M., Schirmer R.H. Thioredoxin-2 but not thioredoxin-1 is a substrate of thioredoxin peroxidase-1 from Drosophila melanogaster: isolation and characterization of a second thioredoxin in D. melanogaster and evidence for distinct biological functions of Trx-1 and Trx-2. J. Biol. Chem. 2002;277(20):17457–17463. doi: 10.1074/jbc.M200636200. 11877442 [DOI] [PubMed] [Google Scholar]
- 61.Mustacich D., Powis G. Thioredoxin reductase. Biochem. J. 2000;346(1):1–8. 10657232 [PMC free article] [PubMed] [Google Scholar]
- 62.Hayes J.D., Flanagan J.U., Jowsey I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. 15822171 [DOI] [PubMed] [Google Scholar]
- 63.Lushchak V.I. Glutathione homeostasis and functions: potential targets for medical interventions. J. Amino Acids. 2012;2012:736837. doi: 10.1155/2012/736837. 22500213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rinaldi R., Eliasson E., Swedmark S., Morgenstern R. Reactive intermediates and the dynamics of glutathione transferases. Drug Metab. Dispos. 2002;30(10):1053–1058. doi: 10.1124/dmd.30.10.1053. 12228179 [DOI] [PubMed] [Google Scholar]
- 65.LoPachin R.M., Barber D.S. Synaptic cysteine sulfhydryl groups as targets of electrophilic neurotoxicants. Toxicol. Sci. 2006;94(2):240–255. doi: 10.1093/toxsci/kfl066. 16880199 [DOI] [PubMed] [Google Scholar]
- 66.Staroń A., Mąkosa G., Koter-Michalak M. Oxidative stress in erythrocytes from patients with rheumatoid arthritis. Rheumatol. Int. 2012;32(2):331–334. doi: 10.1007/s00296-010-1611-2. 21082319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma A., Mishra M., Shukla A.K., Kumar R., Abdin M.Z., Chowdhuri D.K. Organochlorine pesticide, endosulfan induced cellular and organismal response in Drosophila melanogaster. J. Hazard. Mater. 2012;221–222:275–287. doi: 10.1016/j.jhazmat.2012.04.045. 22579458 [DOI] [PubMed] [Google Scholar]
- 68.Kavitha P., Venkateswara Rao J. Oxidative stress and locomotor behaviour response as biomarkers for assessing recovery status of mosquito fish, Gambusia affinis after lethal effect of an organophosphate pesticide, monocrotophos. Pestic. Biochem. Physiol. 2007;87(2):182–188. [Google Scholar]
- 69.Sanchez-Hernandez J.C., Sanchez B.M. Lizard cholinesterases as biomarkers of pesticide exposure: enzymological characterization. Environ. Toxicol. Chem. 2002;21(11):2319–2325. 12389909 [PubMed] [Google Scholar]
- 70.Rocha J.B.T., Saraiva R.A., Garcia S.C., Gravina F.S., Nogueira C.W. Aminolevulinate dehydratase (δ-ALA-D) as marker protein of intoxication with metals and other pro-oxidant situations. Toxicol. Res. 2012;1(2):85–102. [Google Scholar]
- 71.Sassa S., Fujita H., Kappas A. Genetic and chemical influences on heme biosynthesis. In: Kotyk A., Skoda J., Paces V., Kostka V., editors. Vol. 1. VSP; Utrecht: 1989. pp. 329–338. (Highlights Mod. Biochem.). [Google Scholar]
- 72.Jain S.K., Palmer M. The effect of oxygen radicals metabolites and vitamin E on glycosylation of proteins. Free Radic. Biol. Med. 1997;22(4):593–596. doi: 10.1016/s0891-5849(96)00377-2. 9013122 [DOI] [PubMed] [Google Scholar]
- 73.Golombieski R.M., Graichen D.A.S., Pivetta L.A., Nogueira C.W., Loreto E.L.S., Rocha J.B.T. Diphenyl diselenide [(PhSe)2] inhibits Drosophila melanogaster δ-aminolevulinate dehydratase (δ-ALA-D) gene transcription and enzyme activity. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2008;147(2):198–204. doi: 10.1016/j.cbpc.2007.09.007. [DOI] [PubMed] [Google Scholar]


