Abstract
Neuroblastoma is the second most common paediatric cancer. It develops from undifferentiated simpatico-adrenal lineage cells and is mostly sporadic; however, the aetiology behind the development of neuroblastoma is still not fully understood. Intracellular calcium ([Ca2+]i) is a secondary messenger which regulates numerous cellular processes and, therefore, its concentration is tightly regulated. This review focuses on the role of [Ca2+]i in differentiation, apoptosis and proliferation in neuroblastoma. It describes the mechanisms by which [Ca2+]i is regulated and how it modulates intracellular pathways. Furthermore, the importance of [Ca2+]i for the function of anti-cancer drugs is illuminated in this review as [Ca2+]i could be a target to improve the outcome of anti-cancer treatment in neuroblastoma. Overall, modulations of [Ca2+]i could be a key target to induce apoptosis in cancer cells leading to a more efficient and effective treatment of neuroblastoma.
Keywords: neuroblastoma, intracellular calcium([Ca2+]i), PI3K/AKT, ALK, FAK, NGF signalling, differentiation, apoptosis, proliferation, chemotherapeutic treatment, chemotherapy, drug resistance
1. Introduction
Paediatric cancers—like neuroblastoma—transpire during the neonatal epoch of an infant [1]. Neuroblastoma are solid malignant tumours which are widely diagnosed during infancy [2,3,4] and are the second most common paediatric tumours representing about 10% of all paediatric cancers [5]. According to the childhood and adolescent cancer statistics published by the American Cancer Society (2014), neuroblastoma (7%) is the third most frequent cancer in childhood only preceded by acute lymphocytic leukaemia (26%) and cancers of the brain and CNS (21%) [6]. In the United Kingdom, neuroblastoma is slightly more frequent and counts for nearly 8% of the childhood malignancies [7]. The aetiology of neuroblastoma is not fully understood [7]. Its incidence rate is slightly higher in boys than girls with a ratio of 1.2 to 1 [6,7]. Its prevalence and severity varies in different ethnicities around the world with more cases being reported among whites than other ethnicities [6].
Neuroblastoma arises from undifferentiated simpatico-adrenal lineage cells which derive from neural crest cells [8]. The reason for the failure of these sympatico-adrenal lineages is still unknown. Neuroblast masses develop mostly at the abdomen (~65%) but are also found at chest (~20%), neck (~5%) or pelvis (~5%) [3]. Neuroblastoma is a highly heterogeneous tumour, which varies from relentless progression of the disease to spontaneous regression [5]. Like ganglio-neuroblastoma and ganglio-neuroma, it is categorized as a peripheral neuroblastic tumour [3,9]. Neuroblastoma occurs mostly sporadic, with familial neuroblastoma being rare (only 1%–2% of all neuroblastoma cases) [10].
The International Neuroblastoma Risk Group Staging System (INRGSS) has introduced a standardized classification of the disease in order to guide clinical trials at different regions across the world [11,12]. INRGSS divides the disease in four major stages L1, L2, M and MS (Table 1) [11,13], also referred as “very low”, “low”, “intermediate” and “risk” patients. Other classifications of neuroblastoma are based on the age, tumour differentiation histologic appearance and genetic factors including oncogene MYCN, aberrations in 11q and tumour cell ploidy [11,13].
Table 1.
Four major stages in “the international neuroblastoma risk group staging system”.
| Stage | Description |
|---|---|
| L1 | Localized tumour without any detectable image-defined risk factors |
| L2 | Localized tumour with one or more image defined risk factors |
| M | Metastatic disease |
| MS | Metastatic disease with metastases confined to skin, liver, and/or bone marrow (confined to children ≤ 18 month) |
In physiology terms, cancers could be described as the end-result of either of eight vital cellular modifications which include the (1) escape of cell from apoptosis, (2) development of an autocrine system (for synthesizing growth factors resulting in a higher rate of proliferation), (3) development of an insensitivity to anti-growth signals, (4) uncontrolled replication potential, (5) incessant angiogenesis, (6) ability for tissue invasion and metastasis, (7) reprogramming of cellular energetics and (8) escape from immune cells[14,15]. Thus, the altered balance between apoptosis and proliferation could result in switching a normal cell to a malignant cell.
The secondary messenger calcium plays key role in regulating cellular processes and hence cellular calcium homeostasis is crucial. In cancer cells, the intracellular calcium concentration ([Ca2+]i) is in prominence as it modulates the apoptotic or proliferative pathways of the cell. Several studies have conferred the impact of anti-cancer drugs on [Ca2+]i levels. In this review, the following chapters will highlight the essential role of [Ca2+]i in the development and treatment of neuroblastoma by interacting and exerting its effect on cell survival signalling, differentiation, proliferation and apoptosis [16,17].
2. Role of [Ca2+]i in Neuroblastoma
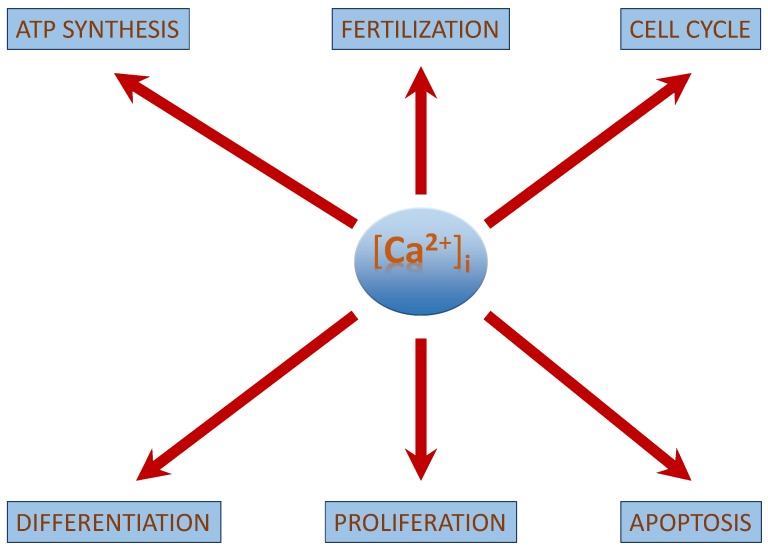
[Ca2+]i regulates multiple cellular processes such as fertilization, cell differentiation, proliferation, transcription factor activation, ATP-synthesis and apoptosis [18]. The concentration of [Ca2+]i is vital to elicit a specific physiological function in normal functioning cells (Figure 1). This review discusses the mechanism by which [Ca2+]i regulates particularly differentiation, proliferation and apoptosis in neuroblastoma and how its manipulation can be used to improve the efficiency of an anti-cancer therapy. It also focuses on the tumour suppressor role of cancer-sensing receptors (CaSR) in neuroblastoma. An overview on the role of [Ca2+]i is discussed in Table 2. Increase in [Ca2+]i is not restricted to neuroblastoma alone, as its found to have an apoptotic effect on breast cancer cellline MCF7 cells [19,20], making it a key molecule in regulation of cancers.
Figure 1.
Role of [Ca2+]i:[Ca2+]i regulates a range of cellular processes of which differentiation, proliferation and apoptosis is discussed for neuroblastoma. As the [Ca2+]i signal defines the fate of the cell, it is critical to regulate its concentration.
Table 2.
An overview on the role of [Ca2+]i in neuroblastoma.
| [Ca2+]i in Neuroblastoma |
|---|
| [Ca2+]i interacts with the growth factor signaling cascade in neuroblastoma. |
| Three main kinases involved in cell survival signaling in neuroblastoma include PI3K/AKT, ALK and FAK. |
| [Ca2+]i activated CAM kinases activates ERK1/2 exerts its role in neuroblastoma differentiation. |
| [Ca2+]i regulated apoptosis in neuroblastoma involves the intrinsic pathway and the activation of CaSR. |
| Chemotherapeutic drug treatment shows an increase in [Ca2+]i concentrations. |
3. [Ca2+]i—Regulation and Signalling
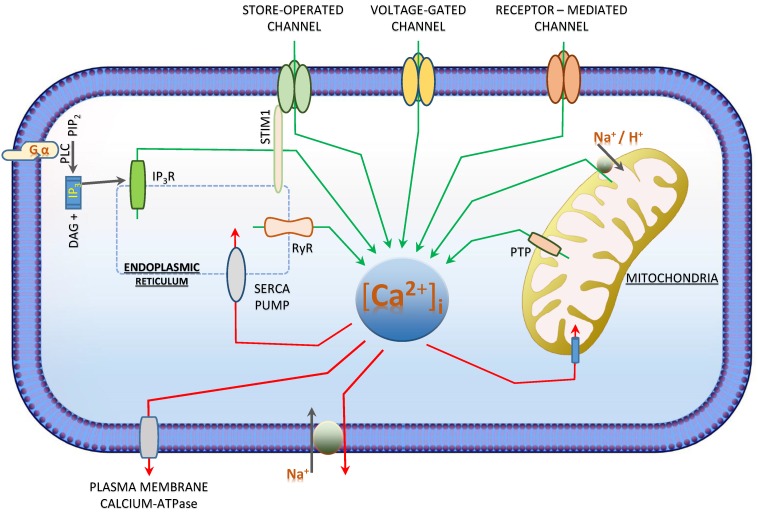
Cells fate chiefly depends on the spatial, magnitude and temporal characteristic of [Ca2+]i [17,21]. Resting cells have an [Ca2+]i concentration between 10 and 100nM which could rise up to 100 times upon Ca2+-entry from the extracellular space or its release from the internal Ca2+-stores [18,22]. To maintain a low [Ca2+]i concentration and to secure clearly defined [Ca2+]i signals, calcium entry and extrusion mechanisms are tightly regulated [23]. As Ca2+ is high in the extracellular compartment as well as in the intracellular calcium stores, Ca2+-entry (from extracellular) or release (from the stores) will elevate the cytosolic [Ca2+]i-concentration [17,18]. [Ca2+]i is reduced by active transport mechanisms which move the cytosolic calcium either back to the stores (Endoplasmic Reticulum (ER) and mitochondria) or the extracellular space (Figure 2). In cancer, [Ca2+]i regulation mechanisms such as Na+/Ca2+-exchangers, calcium pumps and calcium channels are altered [17,21].
Figure 2.
[Ca2+]i-Homeostasis by calcium influx and efflux: Intracellular calcium increases (green arrows) via the (1) voltage-gated channels, (2) receptor-mediated channels, (3) Na2+/Ca2+and Na2+/H+-Exchangers (4) store-operated channels which includes the IP3R and RYR (5) GPCR activated IP3R activation. [Ca2+]i decreases (red arrows) via the (1) SERCA pump, (2) plasma membrane calcium ATPase pump (PMCA), (3) permeability transition pore (PTP) and (4) Na2+/Ca2+ Exchangers. In neuroblastoma, receptor activated for calcium influx are GPCRs (1) [24,25,26,27,28,29] and Sigma Receptors (2) [30]. Calcium release is more often from the stores (3) [24,25,27,28,29,31,32,33] or store-operated channel (4) [26].
The influx mechanisms are regulated by voltage-, receptor- (binding of transmitters such as ATP, glutamate or acetylcholine to their corresponding receptors) and store-operated channels. As mentioned earlier, the internal stores of calcium includes ER or a similar organelle named sacroplasmic reticulum (SR) in muscle cells, of which the calcium release is mainly controlled by the inositol-1,4,5 triphosphate receptor (InsP3R) and the ryanodine receptor (RYR). Calcium is released from the ER by the binding of InsP3 and cyclic ADP ribose (cADPR) to the InsP3R and RYR, respectively. InsP3 is released in response to binding of hormones, growth factors and the neurotransmitteracetylcholine to their cell surface receptors by the cleavage of phosphatidyl inositol 4,5-bisphosphate, a phospholipid present in the cell membrane. In addition, Ca2+ is released from the ER by the nicotinic acid dinucleotide phosphate (NAADP) and sphingosine-1 phosphate (S1P) to the NAADP receptor and sphingolipid calcium release-mediating protein.
4. Signalling Pathways in Neuroblastoma and Their Dependence on [Ca2+]i
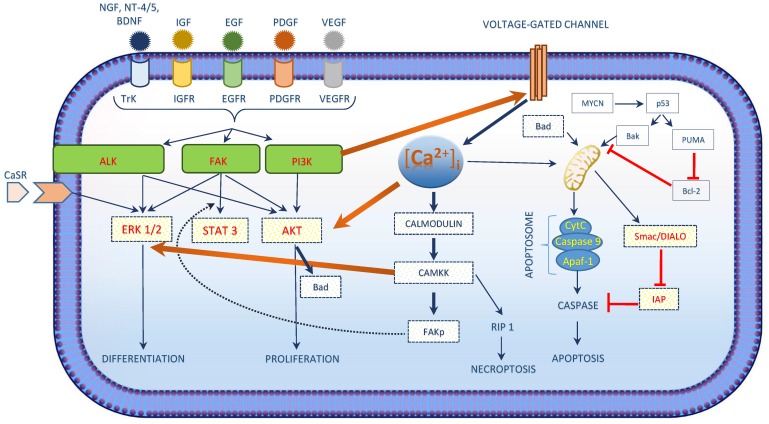
Development of neuroblastoma tumourgenesis and malignancy arises due to unbalanced cell survival and apoptotic pathways. Nerve Growth Factor (NGF), Insulin-like Growth Factor (IGF), Epidermal Growth Factor (EGF), Platelet-Derived Growth Factor (PDGF) and Vascular Endothelial Growth Factor (VEGF) are major growth factor signalling pathways in Neuroblastoma (Figure 3). Cell survival signalling pathways are activated by these growth factors leading to the activation of downstream proteins by the intermediate kinases (PI3K/AKT, ALK and FAK) including the activation of transcription factors (MYCN, NF-KB and p53) (Figure 3) [34]. Of this, PI3K/AKT is of great importance as it is a major pathway involved in neuroblastoma development [35,36] and was described in primary neuroblastoma cells [37] and other cell lines (including SH-SY5Y, SK-N-SH, SK-N-BE, SK-N-EP and IMR32) [34,35]. PI3K/AKT promotes cell survival by activating survival associated proteins and by inhibiting the apoptotic pathway [38,39,40]. Several studies focused on the up-regulation of apoptosis in neuroblastoma cells by down regulation of the PI3K/AKT pathway and found that cells with MYCN amplification show a greater inhibition of the PI3K/AKT pathway which is considered to be a major factor for the prognosis of neuroblastoma [34,41,42]. Currently, AKT inhibitors including temsirolimus (a rapamycin analogue), perifosine (synthetic oral alkylphospholipid) are on clinical trials and its safety in children is being studied.
Figure 3.
Intracellular calcium and cell survival: Intracellular calcium activates the intermediate proteins in the signalling pathways in neuroblastoma. Figure illustrates calcium regulating three kinases (AKT, ERK and FAK) that are involved in the cell survival signalling in neuroblastoma. The intracellular calcium and PI3K/AKT pathway influences one another forming a loop, while its impact on other two kinases is mainly via the activation of calmodulins and the CaM dependent protein kinase kinase.
Anaplastic Lymphoma Kinase (ALK) is also associated with cell survival signalling of neuroblastoma. It belongs to the family of insulin receptors with the trans-membrane receptor tyrosine kinase [43] and exerts its role in cell growth and development, predominantly through the central nervous system [44]. In nearly 90% of neuroblastoma tumour samples, expression of ALK protein was observed [45] and was associated with ALK gene mutations [46]. ALK gene mutations are associated with both familial and sporadic neuroblastoma. Downstream signalling cascade of ALK signalling includes AKT, ERK1/2 and STAT3 [47].Calcium phosphorylates three kinases (AKT, ERK and FAK) that are involved in the cell survival signalling in neuroblastoma (Figure 3) [48,49].
4.1. [Ca2+]i as a Key Factor which Determines the Fate of Neuroblastoma Cells
[Ca2+]i also exert its effect on intermediate kinases that are involved in cell survival signalling. NGF signalling is a predominant signalling pathway in neuroblastoma. Trktyrosine kinase receptor family is associated with the NGF signalling and includes TrkA for NGF, TrkB for brain-derived neurotrophic factor (BDNF) and neurotrophin-4/5 (NT-4/5) and TrkC for neurotrophin-3 (NT-3) (Figure 3) [50]. Another receptor for NGF is p75NTFwhich exerts a lower affinity to NGF compared to TrkA. Expression of Trk in neuroblastoma varies with the type of neuroblastoma. High levels of TrkA and TrkC is expressed in biologically favourable neuroblastoma while high levels of TrkB and its ligand BDNF is expressed in biologically unfavourable aggressive neuroblastoma with MYCN amplification [51,52]. In PC12 cells, NGF ligand binding leads to the activation of ERK-MAP kinases for exerting its cellular functions. In addition, the NGF signal transduction is associated with an increase in [Ca2+]i with a corresponding decrease in both internal stores and the extracellular spaces via the Trk receptors [53,54,55]. A study in monocytes and neurons revealed that PI3K/AKT pathway could phosphorylate CaVβ2a which would end up being associated with the trafficking proteins and opening of voltage-gated calcium channels [56]. Thus, this increase in [Ca2+]i could be due to the opening of the voltage-gated calcium channels across the plasma membrane by P13K [56] which is mainly activated in neuroblastoma.
ERK signalling cascade regulates cellular processes like development, differentiation and proliferation [57,58,59]. Both ERK signalling and elevated [Ca2+]i are considered as key regulators for intracellular signalling initiated by extracellular ligands like growth factors and hormones as they determine the fate of the signalling pathway (Figure 3) [60]. In addition, extracellular ligand could lead to an increase of [Ca2+]i [61,62] having an effect on the sub-cellular localization of ERK by interfering with its protein–protein interaction properties [60]. An elevated [Ca2+]i-concentration could either lead to activation or inhibition of ERK cascade signalling. Chuderland and co-workers showed that in rat cells, the translocation of phosphorylated ERKs from the cytosol to the nucleus is delayed by elevated [Ca2+]i, thereby affecting the localization of ERK [63]. However, molecular mechanisms to activate the ERK cascade by elevated [Ca2+]i are more prevalent [64,65]. This is achieved by the activation of calmodulin dependent kinases 1 and 2 [66], PYK2 kinase [67], Ras-GRF [68] or by the inhibition of Ras-GAPs [69,70]. In support to the effect of [Ca2+]ion ERK signalling cascade, studies on PC12 cells also confirmed that activation of ERK by NGF signalling is regulated by both [Ca2+]i and CaM[71]. Thus, the activation of ERK cascade activation by growth factor signalling is regulated by [Ca2+]i and hence could have a role in neuroblastoma development as well. Studies using NG108 cells shows that an increase in [Ca2+]i could help in cell survival by activating Protein Kinase B or AKT. Increased [Ca2+]i activates Ca2+/CaM dependent protein kinase kinase (CaM-KK) which activates AKT thereby phosphorylating the serine residue 136 of BAD and hence promote cell survival [72].
FAK plays a key role in growth, survival, proliferation and migration of cells and is closely associated with neuroblastoma [34,73]. [Ca2+]i phosphorylates FAK at its serine residues, as shown for Swiss 3T3 cells where [Ca2+]i activates CaM-KII which leads to the activation of FAK by phosphorylating FAK at it Ser-843 residue (Figure 3).
4.2. [Ca2+]i Induces Differentiation and Proliferation in Neuroblastoma
Neuroblastoma is associated with a block in cell differentiation, since the spontaneous regression of neuroblastoma is achieved partly through the neuronal differentiation of the cells. Treatment by inducing differentiation is considered as one of the most effective therapeutic strategies. Retinoic acid induces differentiation in neuroblastoma cell lines [2,74,75]. Currently, high-risk neuroblastoma patients are treated with retinoic acid and cells exhibit the inhibition of proliferation and the induction of differentiation [76]. The induction of differentiation in neuroblastoma cell lines is associated with an increase of [Ca2+]i.
Both neuroblastoma tumours and neuroblastoma cell lines consists of multipotent precursor cells which differentiate into discrete cell lineages of the neural crest [26,77]. Three main cellular phenotypes observed in neuroblastoma cell lines include neuroblastic N-type cells, substrate adherent S-type cells and intermediate I-type cells [78,79]. N-type cells consist of immature nerve cells and are precursors to the sympatho-adrenal cell lineage [26,79,80]. S-type cells are the non-neuronal lineage of the neural crest and are precursor cells of the Schwann, glial, and melanocytic cells of the neural crest [78,81]. With respect to the morphological characteristics and the biological markers, the I-type cells are considered to be intermediate to both N- and S-type cells [78,81]. I-type cells represent either a stem cell stage or an intermediate stage in between the trans-differentiation of N- and S-type cells [81,82]. N-type cells are more malignant in nature, while S-type cells are of a non-malignant nature [26,83,84].
An increase in [Ca2+]i was observed in 9cRA-induced differentiation of SH-SY5Y and the differentiated N- and S-type cells from SH-SY5Y cells.SH-SY5Y cells shows a [Ca2+]i concentration of 98 ± 4 nM and after treatment with 10 µM retinoic acid showed an increase in [Ca2+]i [25]. These cells endogenously express muscarinic M3 and bardykinin B2 receptors and upon differentiation with 10 µM of retinoic acid followed by the stimulation of the receptors with methacholine and bradykinin resulted in greater elevation of [Ca2+]i (Table 3) [85,86]. The [Ca2+]i elevation measured 727 ± 30 nM and 535 ± 41 nM with the activation of 1mM methacholine and 10µM bradykinin. The authors concluded that the differentiation of these SH-SY5Y cells was associated with enhanced phosphoinositide signalling and that it was not receptor specific. Thus, this study confirmed that the increase of [Ca2+]i was mediated by the release of Ca2+ from intracellular stores via Ins(1,4,5)P3 [25] (Figure 2).
Table 3.
Increase of [Ca2+]i in human neuroblastoma cell lines: Color code in the table illustrates different receptors that initiates an increase of [Ca2+]i in neuroblastoma cell lines of human origin. Two main categories of receptors mentioned include the G-protein coupled receptor and Sigma receptors (subtypes were not differentiated).
| Sl.No | Cell Lines | Orgin | Treatment | Receptor | Concentration | Ca2+ Release | Basal [Ca2+]i | Increased [Ca2+]i | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | IMR-32 | H | Orexin-A (GPCR) | Orexin Type 1 Receptor (GPCR) | 3 nM | Store Release (IP3R) | 50 nM | 4 fold | [24] |
| 2 | SH-SY5Y | H | Retinoic Acid | Retinoid X receptor (Nuclear Receptors) | 10 µM | Store Release | 98 nM | No increase | [25] |
| 3 | SH-SY5Y | H | Retinoic Acid | Retinoid X receptor (Nuclear Receptors) | 1 µM | Store Operated calcium Channel | 10 nM | 4 fold | [26] |
| 4 | SH-SY5Y | H | Oxotremorine-M | Muscarinic Receptor (GPCR) | 10 µM | Store Release (IP3R) | 50 nM | 2 fold | [27] |
| 5 | SH-SY5Y | H | Methacholine | Muscarinic Receptor (GPCR) | 1 mM | Store Release (IP3R) | 98 nM | 2 fold | [25] |
| 6 | SH-SY5Y | H | Carbachol | Muscarinic Receptor (GPCR) | 1 mM | Store Release | - | 3.5 fold | [28] |
| 7 | SK-N-SH | H | Carbachol | Muscarinic Receptor (GPCR) | 100 µM | Store Release | 59 nM | 2 fold | [29] |
| 8 | SH-SY5Y | H | Bradykinin | Bradykinin Receptor (GPCR) | 10 µM | Store Release (IP3R) | 98 nM | 1 fold | [25] |
| 9 | SH-SY5Y | H | Bradykinin | Bradykinin Receptor (GPCR) | 10 µM | Store Release | - | 2 fold | [28] |
| 10 | SH-SY5Y | H | Arsenic Trioxide | - | 1 µM | Store Release (IP3R and RyR) | 75 nM | 2 fold | [31] |
| 11 | SH-SY5Y | H | Arsenic Trioxide | - | 1 µM | Store Release (IP3R and RyR) | 70 nM | 3 fold | [32] |
| 12 | SH-SY5Y | H | Trimethyltin Chloride | - | 0.1 µM | Store Release | - | 2 fold | [33] |
| 13 | SH-SY5Y | H | Cisplatin | - | 1 µM | Extracellular Space | 75 nM | 2 fold | [31] |
| 14 | SK-N-SH | H | CB-64D | Sigma 2 receptor | 100 µM | Thapsigargin insensitive calcium store | - | 4 fold | [30] |
| 15 | SK-N-SH | H | JL-II-147 | Sigma 2 receptor | 100 µM | Thapsigargin insensitive calcium store | - | 2 fold | [30] |
| 16 | SK-N-SH | H | BD737 | Sigma 2 receptor | 100 µM | Thapsigargin insensitive calcium store | - | 1 fold | [30] |
| 17 | SK-N-SH | H | LR172 | Sigma 2 receptor | 100 µM | Thapsigargin insensitive calcium store | - | 1 fold | [30] |
| 18 | SK-N-SH | H | BD1008 | Sigma 2 receptor | 100 µM | Thapsigargin insensitive calcium store | - | 1 fold | [30] |
| 19 | SK-N-SH | H | Haloperidol | Sigma 2 receptor | 100 µM | Thapsigargin insensitive calcium store | - | 1 fold | [30] |
| 20 | SK-N-SH | H | Ibogaine | Sigma 2 receptor | 100 µM | Thapsigargin insensitive calcium store | - | 1 fold | [30] |
Increase of [Ca2+]i either from the extracellular space or from the internal stores upon treatment of neuroblastoma cell lines with several GPCR ligands like retinoic acid as in current neuroblastoma treatment, Sigma 2 ligands and other compounds like cisplatin, arsenic trioxide or tri-methyl-tin chloride (TMT) results either in differentiation or induction of apoptosis (Figure 2). This confers the role of [Ca2+]i in inducing these pathways in the neuroblastoma cells and hence an insight into the possible role of [Ca2+]i in the development and an efficient mechanism of action for the treatment of neuroblastoma.
4.3. [Ca2+]i Induces Apoptosis in Neuroblastoma
Apoptosis is defined as programmed cell death (PCD) by which cells are eliminated in a biological system and is crucial for physiological processes like cell maintenance, development and tumour development and regression [87]. Apoptosis is induced either by extrinsic (death receptor) or intrinsic (mitochondrial) pathways, both leading to the activation of caspase 3, 6 and 7 which in turn cleave several other proteins and activate DNAses leading to cell death [50,88,89,90]. Diminution of cell apoptosis could be mediated by an altered balance of the pro- and anti-apoptotic proteins, decreased caspase activation and impaired extrinsic pathways. Studies revealed that under pathophysiological conditions, a greater quantity of mitochondrial Ca2+-uptake from the cytoplasm occurs thereby initiating cell apoptosis [91] (Figure 4). Mitochondrial uptake of Ca2+ from the ER also activates apoptosis with the opening of permeability transition pore (PTP) across the mitochondrial membrane by creating a negative mitochondrial membrane potential [92,93].
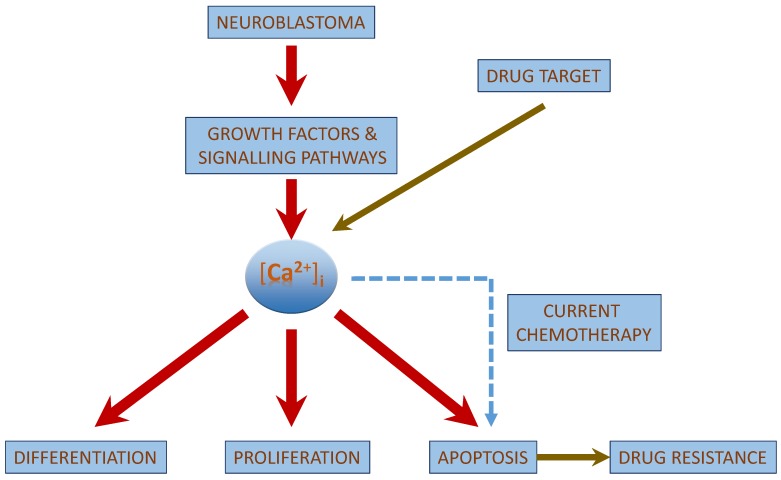
Figure 4.
[Ca2+]i as a possible drug target: [Ca2+]i is involved in the signalling pathway of neuroblastoma pathophysiology and could be a possible drug target for the treatment of neuroblastoma.
Mitochondrial Ca2+-uptake is channelled by mitochondrial inner membrane and the Ca2+-release is initiated by the mitochondrial outer membrane. Mitochondria Ca2+-Uniporters (MCU) are located in the mitochondrial inner membrane supporting the mitochondrial Ca2+-uptake across the membrane while creating a negative mitochondrial membrane potential. A mitochondrial membrane potential (Δψm) of −180 mV developed across the membrane which facilitates MCU move Ca2+ across the membrane without interfering with the mitochondrial ATP hydrolysis or the transport of other ions [92,94]. Na+/Ca2+ and H+/Ca2+ exchangers support the transport of Ca2+ across the mitochondrial outer membrane. However, when Ca2+ is accumulated in the mitochondrial matrix it interacts with the prolyl isomerase cyclophilin D within the mitochondrial matrix resulting in the formation of PTP across the mitochondrial membrane [95]. This traverses the channel across the mitochondrial inner and outer membrane and allows Ca2+ (together with other ions and small molecules) to pass [92,96,97]. In addition, PTP opening is initiated by reactive oxygen species (ROS) and free fatty acids which are released with higher Ca2+-concentration in the matrix [98,99]. However, if the cytoplasm is filled with very high Ca2+-concentrations, PTPs remains open and permits the entry of solutes into the mitochondrial matrix which results in the expansion of the mitochondrial matrix. This results in the Mitochondrial Outer Membrane Permeabilization (MOMP) with the release of inter membrane space proteins like cytochrome c, Smac, DIABLO and caspase activation ensuing apoptotic cell death [100,101]. MOMP is sequestered with the activation of a cascade of both pro-apoptotic andanti-apoptotic members of the Bcl-2 family proteins [101].
Favourable neuroblastoma is often associated with a deregulated apoptotic pathway [50]. The sympathetic lineage neuroblast entails NGF, a neurotrophic factor for its cell survival and differentiation. Studies confirmed that the deletion or deficiency of these NGFs results in the activation of apoptotic cell death via intrinsic pathway in both normal cells and in tumour cells. Furthermore, when NGF is limited, apoptosis is triggered in favourable NB cells expressing TrkA and p75NTF [50,102,103]. TrkB and their ligands are often related to MYCN amplification which is mainly associated with aggressive NB [50,104]. The genomic amplification of the transcription factor MYCN is a strong predictable clinical marker for the poor survival rate of the patients [105,106]. MYCN induces its role in differentiation, proliferation, cell growth, apoptosis, metabolism and protein synthesis [107]. Several studies reported that with the down regulation of MYCN expression in neuroblastoma cells induced cell apoptosis, morphological differentiation and cell growth arrest at the G1phase [108,109,110]. In the absence of NT-3, TrkC mediates apoptosis via the activation of caspase 9 [50,111], and since human aggressive NB is associated with higher expression of NT-3, caspase activated apoptosis is narrowed in these NBs [112]. These neurotrophins exert their survival role by Ras/MAPK and PI3K/AKT pathway [111,113].
Tumourgenesis is associated with the inactivation of p53, a classical tumour suppressor protein. In favourable NB, studies revealed the fact that apoptosis in NB is associated with p53.Nearly 25% of NB is associated with MYCN amplification. In MYCN amplified NB cell lines, cell apoptosis is mediated by p53 and is the direct transcriptional target of MYCN [114]. MYCN could also induce apoptosis in NB cells via activating the pro-apoptotic targets PUMA and Bax which are transactivated by p53 [114,115,116]. In addition, NB cells apoptosis is associated with MYCN amplification occurring via the activation of extrinsic apoptotic pathways [117,118].
In cultured cortical neurons, both BDNF and NT-3 lead to an elevation of [Ca2+]i [119,120]. In addition, NGF induces an increase of Ca2+-uptake in both 3T3-Trk and 3T3-p75 cells in which the 3T3 cells are transfected with the high affinity NGF receptor p140trkand low affinity NGFR receptor p75NGF, respectively. 3T3-Trk also showed an increase of [Ca2+]i irrespective of 3T3-p75 cells. Additionally, NGF helps in [Ca2+]i signalling via voltage-gated channels in mouse nociceptive neurons. A suggestion by Bonnington and McNaughton is that out of the three pathways activated by NGF induced TrKA receptor activation, PI3K pathway leads to the sensitization of TRPV1. This sensitization increased Ca2+-influx via voltage-gated channels. Two other pathways activated by TrKA receptors are the PLC gamma and ShC induced Ras pathway [121]. Studies on basal forebrain neurons confirmed that upon culturing cells with NGF and BDNF for four to six days, a considerable increase in L- and N-type calcium channel currents were observed in NGF treated cells. Whereas, in BDNF treated cells, no change in calcium channel currents was observed [120]. Baldelli and co-workers describes that treatment of developing hippocampal neurons with NGF, NT-3 and BDNF for six to 20 days, voltage gated Ca2+-currents increased in a time and concentration dependent manner. BDNF exerted its Ca2+-influx via N, P/Q and R channels while NGF and NT-3 exerted its calcium influx via L-channels [122]. Others confirmed that calcium induces apoptosis via mitochondria [123,124].
Despite of the knowledge on MYCN amplification associated with neuroblastoma cell apoptosis, studies still confers the role of [Ca2+]i in inducing intrinsic apoptotic pathway. In addition, studies have confirmed the role of [Ca2+]i in inducing necroptosis in human neuroblastoma cells (Figure 3) via the activation of receptor interacting protein kinase 1 (RIP1) by the CAMKK [125].
4.4. Tumour Suppressor Functions of the Calcium-Sensing Receptor (CaSR) in Neuroblastoma
Calcium-sensing receptor (CaSR), a G-protein coupled receptor is a key mediator protein in maintaining the cellular responses and cell fate in response to external Ca2+-concentrations between 0.05–5 mM [126,127]. Change of extracellular Ca2+-concentration within this range transforms the proliferative cellular response to a stage of differentiation or quiescence [126]. In addition, the activation of CaSR is associated with an increased expression and secretion of parathyroid hormone-related peptide (PTHrP) which has a role in the development of hypercalcemia in cancer cells [126]. Loss or up-regulation of CaSR could result in the development of a neoplastic disease like cancer. However, CaSR activation exerts a different role in different types of cancers [9,128], as in prostate cancers, CaSR activation alleviates tumour cell progression and bone metastasis while in colon cancer; expression of CaSR is associated with differentiation in colon epithelium thereby acting as a tumour suppressor protein [129,130].
Recent studies with neuroblastoma brought more insights into calcium and calcium sensing receptors. The expression of CaSR and smaller levels of parathyroid hormone-related protein were observed in differentiated, favourable neuroblastic tumours [23,128]. CaSR exerts a tumour suppressor role in neuroblastoma [23]. Studies on human patient samples with relatively superior prognostic variables like low clinical stage and clinical risk and age of diagnosis below one year had confirmed CaSR expression on nearly 50% of both fully and partially differentiating neuroblastoma, along with the expression of PTHrP. CaSR expression was restricted mainly to the early phases of differentiation and only when the neuroblasts differentiation reaches a certain level, the PTHrP expression was observed [128].
In addition, Masdival and co-workers reported CaSR expression in developmental malignancy, confirming that both CaSR and PTHrP are expressed in differentiated favourable neuroblastoma and are unregulated upon inducing differentiation [23]. In undifferentiated unfavourable neuroblastoma, CaSR expression is silenced by several genetic and epigenetic mechanisms [23,128]. Also, in vitro experiments confirmed that when cells were exposed to Ca2+, cell vacuolization was observed along with an increase in number of dead cells. Thus, it was clear that CaSR was over expressed in neuroblastoma cells and when exposed to Ca2+ cells underwent apoptosis via ERK1/2signalling cascade [9].
Genome-wide association studies in relation to neuroblastoma were on focus and the susceptibility loci and alleles allied with the low and high risk neuroblastoma were revealed. The genetic variations on disease susceptibility included FLJ22536 (6p22) [23,131,132], BARD1(2q35) [23,132,133], LMO1(11p15) [23,132,134], HSD17B12 (11p11.2) [23,135], DUSP12(1q23.3) [23,135], LINC00340(6p22) [23,135], HACE1 and LIN28B(6q16) [23,136]. These studies confer that the genetic variations area plausible rationale for the development of benign and malignant neuroblastoma [133,135]. Genetic variants at the carboxy terminal of the CaSR were studied and three single nucleotide polymorphisms at rs1042636, rs1801725 and rs1801726 were observed and this tri-locus haplotype could be considered as an outcome predictor in neuroblastoma patients. The work also showed that the malignant nature of neuroblastoma is associated with the inactivation of CaSR gene in patients [23].
5. [Ca2+]i Modulations with Chemotherapeutic Treatment of Neuroblastoma
Traditional treatment for neuroblastoma includes surgery, radiation therapy, chemotherapy, radiation therapy with stem cell rescue and the recent treatment therapies include targeted therapy and vaccines. Treatment protocols vary with the stage of disease (Table 4). Common chemotherapeutic drugs used for the neuroblastoma treatment includes Carboplatin, Cisplatin, Cyclophosphamide, Doxorubicin, Etoposide, Ifosfamide, Melphalan, Vincristine, Teniposide and Topotecan [137].
Table 4.
Different stages in the development of neuroblastoma.
| Stage of Neuroblastoma | Tumour Characteristics | Treatment Protocol |
|---|---|---|
| Stage 1 (Low) | Single site specific | Surgery |
| Stage 2A (Low) | Single site specific and could not be removed completely by surgery. | Surgery and Chemotherapy |
| Stage 2B (Low) | Single site specific and could be removed completely by surgery. Cancer development could be present at lymph nodes around the tumour. | Surgery |
| Stage 3 (Intermediate Risk) | Cancer could be present in one or both sides of the body and lymph nodes. | Chemotherapy |
| Stage 4 (High Risk) | Cancer spread to distant body parts (bone, liver, skin, bone marrow and other organs) and distant lymph nodes. | Surgery, Chemotherapy, Radiotherapy, Immunotherapy and Retinoid Therapy |
| Stage 4S (High Risk) | Child is younger than 12 months with cancer spread on one side of the body. Lymph nodes on the same side of the body also affected. | Surgery, Chemotherapy and Radiotherapy |
| Relapsed/Recurrent | - | Chemotherapy, Immunotherapy, Retinoid Therapy, Tyrosine kinase and Aurora kinase inhibitors and targeted delivery of radionuclides. |
Chemotherapeutic drugs used for the treatment of cancer modulate [Ca2+]i with the induction of necrosis or apoptosis. Studies report that arsenic trioxide (As2O3) and cisplatin (CDDP) [31,32] and trimethyltin chloride (TMT) [33] induce apoptosis in neuroblastoma cells by interfering with [Ca2+]i-homeostasis. Cisplatin is considered as the most effective chemotherapeutic drug for the treatment of neuroblastoma. Cisplatin exerts its anti-cancer therapeutic role by cytotoxicity (interacting with the purine bases in DNA at the nucleophilic N7-sites forming a interstrand and intrastrand of DNA-DNA and DNA-protein crosslinks) [138] and apoptosis (increase in activity of caspase-8 and caspase 9) [139]. Ciplatin also interacts with calcium signaling, p53 and reactive oxygen species [138,139] and apoptosis by an increase in activity of caspase-8 and caspase 9 and up regulation ofp53 expression [22]. Followed by the application, [Ca2+]i was elevated by Ca2+ entry from the extracellular space [22,139]. The studies concluded that the rise of [Ca2+]i was CDDP-concentration dependent, leading to apoptosis by the activation of calpain mediated by ionsitol tri-phosphate (IP3) [22,139]. It was suggested that by increasing [Ca2+]i through a different activation of IP3, the efficacy of cisplatin could be increased resulting in a higher rate of apoptosis. Cisplatin increased [Ca2+]i by Ca2+-entry and As2O3 by Ca2+-release from the internal stores [32,140]. Cisplatin induced increase in [Ca2+]i is allied with a cellular apoptosis with the activation of calpain and not caspase-8, confirming that cisplatin induced calcium influx via the IP3 receptors lead to cellular apoptosis with the activation of calpain [22].
6. Drug Resistance in Neuroblastoma
Despite of the above mentioned advanced treatment in neuroblastoma, treatment protocols are often ineffective in patients with aggressive neuroblastoma due to the development of drug resistance to chemotherapeutic drugs [141,142] (Figure 4). Drug resistance leads to a greater rate of disease relapse than normally expected, and in neuroblastoma, resistance is often related to the expression of a multi-drug resistant-associated protein (MRP1) [141,143] or due to a mutations or loss of function of p53 [144]. However, apart from the drug resistance, chemotherapeutic treatment could also lead to an increase in malignancy and metastasis of the disease, thereby affecting its biological properties [142].
Studies on resistant neuroblastoma cell lines UKF-NB-2rVCR20 and UKF-NB-2rDOX100 showed a greater resistance to vincristine (VCR) and doxorubicin (DOX), respectively, whereas these cell lines still responded to cisplatin. In addition, with respect to the normal cell lines, these cell lines showed a variation in the expression levels of P-glycoprotein, GD2 and neural cell adhesion molecule (NCAM), an adhesion receptor resulting in a greater rate of tumourgenesis in in vivo studies [145]. Drug resistance also leads to an increase in adhesion of the resistant neuroblastoma cell lines to the endothelium [145,146]. As2O3 is more effective in normoxia and hypoxia than the common drug etoposide in multi-drug resistance cell lines. Thus, As2O3 could be considered as a more effectual drug in the treatment of patients with aggressive neuroblastoma where a greater level of hypoxia is usually observed. Therefore, it is vital to identify the molecular mechanism behind the development of drug resistance as it will help to design new chemotherapeutic drug(s) with better efficacy and also will help to predict the possible outcomes of a new drug when introduced [147].
7. Future Studies with [Ca2+]i in Neuroblastoma
The role of [Ca2+]i in neuroblastoma development, proliferation and apoptosis can be better understood when the complexity behind the Ca2+-concentration regulating the cellular process is considered. In neuroblastoma, [Ca2+]i is a key mediator and might not be restricted to growth factor signalling and the kinase activation. Apart from these, studies relating to [Ca2+]i on MYCN expression is questionable as neuroblastoma is often associated with MYCN over expression. Since [Ca2+]i rises with chemotherapeutic treatment, elevating [Ca2+]i agonist could improve the current treatment protocol. However, the studies should be extended to other biological and environmental factors like Vitamin D deficiency and calcium absorption as the disease prevalence is higher in regions with less sunlight. In addition, further insights into calcium deficiency in pregnancy and the scope of [Ca2+]i in treatment of other types of cancers are to be discussed.
8. Conclusions
[Ca2+]i regulates different cellular functions and has a key role in the development and prevalence of neuroblastoma. In neuroblastoma, these [Ca2+]i form a network with the intermediate kinase of major signalling pathways, either directly or indirectly, and its concentration determines the fate of the cells. Hence, studies could be more focused on [Ca2+]i, which could lead to the development of new Ca2+ based drugs or could also help in improving the efficacy of chemotherapeutic drugs that are currently used in treatment of neuroblastoma.
Acknowledgements
This work was supported by NPRP 6-089-3-021, Qatar National Research Fund (QNRF).The authors thank Faizal Sherif for drawing the figures.
Abbreviations
- INRGSS
International Neuroblastoma Risk Group Staging System
- Ca2+
Calcium Ions
- [Ca2+]i
Intracellular Calcium
- CaSR
Cancer-Sensing Receptors
- ATP
Adenosine Triphosphate
- ER
Endoplasmic Reticulum
- IP3
Ionsitol Triphosphate
- InsP3R
Inositol-1,4,5 Triphosphate Receptor
- RyR
Ryanodine Receptor
- NAADP
Nicotinic Acid Dinucleotide Phosphate
- S1P
Sphingosine-1 Phosphate
- cADPR
Cyclic ADP Ribose
- NGF
Nerve Growth Factor
- IGF
Insulin-like Growth Factor
- EGF
Epidermal Growth Factor
- PDGF
Platelet-derived Growth Factor
- VEGF
Vascular Endothelial Growth Factor
- ALK
Anaplastic Lymphoma Kinase
- BDNF
Brain-Derived Neurotrophic Factor
- NT-4/5
Neurotrophin-4/5
- MCU
Mitochondria Calcium Uniporter
- Δψm
Mitochondrial Membrane Potential
- MOMP
Mitochondrial Outer Membrane Permeabilization
- As2O3
Arsenic Trioxide
- TMT
Trimethyltin Chloride
- MRP1
Multi-Drug Resistant-Associated Protein
- VCR
Vincristine
- DOX
Doxorubicin
- NCAM
Neural Cell Adhesion Molecule
Author Contributions
Noothan Jyothi Satheesh: drafting of the manuscript; Dietrich Büsselberg: concept and revisions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Orbach D., Sarnacki S., Brisse H.J., Gauthier-Villars M., Jarreau P.H., Tsatsaris V., Baruchel A., Zerah M., Seigneur E., Peuchmaur M., et al. Neonatal cancer. Lancet Oncol. 2013;14:609–620. doi: 10.1016/S1470-2045(13)70236-5. [DOI] [PubMed] [Google Scholar]
- 2.Maris J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colon N.C., Chung D.H. Neuroblastoma. Adv. Pediatr. 2011;58:297–311. doi: 10.1016/j.yapd.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domingo-Fernandez R., Watters K., Piskareva O., Stallings R.L., Bray I. The role of genetic and epigenetic alterations in neuroblastoma disease pathogenesis. Pediatr. Surg. Int. 2013;29:101–119. doi: 10.1007/s00383-012-3239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamijo T., Nakagawara A. Molecular and genetic bases of neuroblastoma. Int. J. Clin. Oncol. 2012;17:190–195. doi: 10.1007/s10147-012-0415-7. [DOI] [PubMed] [Google Scholar]
- 6.Ward E., DeSantis C., Robbins A., Kohler B., Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 7.Fisher J.P., Tweddle D.A. Neonatal neuroblastoma. Semin Fetal Neonatal Med. 2012;17:207–215. doi: 10.1016/j.siny.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Jiang M., Stanke J., Lahti J.M. The connections between neural crest development and neuroblastoma. Curr. Top Dev. Biol. 2011;94:77–127. doi: 10.1016/B978-0-12-380916-2.00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casala C., Gil-Guinon E., Ordonez J.L., Miguel-Queralt S., Rodriguez E., Galvan P., Lavarino C., Munell F., de Alava E., Mora J., et al. The calcium-sensing receptor is silenced by genetic and epigenetic mechanisms in unfavorable neuroblastomas and its reactivation induces ERK1/2-dependent apoptosis. Carcinogenesis. 2013;34:268–276. doi: 10.1093/carcin/bgs338. [DOI] [PubMed] [Google Scholar]
- 10.Maris J.M., Weiss M.J., Mosse Y., Hii G., Guo C., White P.S., Hogarty M.D., Mirensky T., Brodeur G.M., Rebbeck T.R., et al. Evidence for a hereditary neuroblastoma predisposition locus at chromosome 16p12–13. Cancer Res. 2002;62:6651–6658. [PubMed] [Google Scholar]
- 11.Monclair T., Brodeur G.M., Ambros P.F., Brisse H.J., Cecchetto G., Holmes K., Kaneko M., London W.B., Matthay K.K., Nuchtern J.G., et al. The international neuroblastoma risk group (INRG) staging system: An inrg task force report. J. Clin. Oncol. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brisse H.J., McCarville M.B., Granata C., Krug K.B., Wootton-Gorges S.L., Kanegawa K., Giammarile F., Schmidt M., Shulkin B.L., Matthay K.K., et al. Guidelines for imaging and staging of neuroblastic tumors: Consensus report from the international neuroblastoma risk group project. Radiology. 2011;261:243–257. doi: 10.1148/radiol.11101352. [DOI] [PubMed] [Google Scholar]
- 13.Cohn S.L., Pearson A.D., London W.B., Monclair T., Ambros P.F., Brodeur G.M., Faldum A., Hero B., Iehara T., Machin D., et al. The international neuroblastoma risk group (INRG) classification system: An INRG task force report. J. Clin. Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber R. Ca2+ signaling, intracellular pH and cell volume in cell proliferation. J. Membr. Biol. 2005;205:129–137. doi: 10.1007/s00232-005-0778-z. [DOI] [PubMed] [Google Scholar]
- 17.Capiod T., Shuba Y., Skryma R., Prevarskaya N. Calcium signalling and cancer cell growth. Subcell. Biochem. 2007;45:405–427. doi: 10.1007/978-1-4020-6191-2_15. [DOI] [PubMed] [Google Scholar]
- 18.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell. Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 19.Al-Taweel N., Varghese E., Florea A.M., Busselberg D. Cisplatin (CDDP) triggers cell death of MCF-7 cells following disruption of intracellular calcium ([Ca2+]i) homeostasis. J. Toxicol. Sci. 2014;39:765–774. doi: 10.2131/jts.39.765. [DOI] [PubMed] [Google Scholar]
- 20.Varghese E., Busselberg D. Auranofin, an anti-rheumatic gold compound, modulates apoptosis by elevating the intracellular calcium concentration ([Ca2+]i) in MCF-7 breast cancer cells. Cancers (Basel) 2014;6:2243–2258. doi: 10.3390/cancers6042243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prevarskaya N., Skryma R., Shuba Y. Targeting Ca2+ transport in cancer: Close reality or long perspective? Expert Opin. Ther. Targets. 2013;17:225–241. doi: 10.1517/14728222.2013.741594. [DOI] [PubMed] [Google Scholar]
- 22.Florea A.M., Busselberg D. Anti-cancer drugs interfere with intracellular calcium signaling. Neurotoxicology. 2009;30:803–810. doi: 10.1016/j.neuro.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Masvidal L., Iniesta R., Casala C., Galvan P., Rodriguez E., Lavarino C., Mora J., de Torres C. Polymorphisms in the calcium-sensing receptor gene are associated with clinical outcome of neuroblastoma. PLoS ONE. 2013;8:e59762. doi: 10.1371/journal.pone.0059762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasman J., Bart G., Larsson K., Louhivuori L., Peltonen H., Akerman K.E. The orexin OX1 receptor regulates Ca2+ entry via diacylglycerol-activated channels in differentiated neuroblastoma cells. J. Neurosci. 2006;26:10658–10666. doi: 10.1523/JNEUROSCI.2609-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin A.K., Nahorski S.R., Willars G.B. Complex relationship between Ins(1,4,5)P3 accumulation and Ca2+-signalling in a human neuroblastoma revealed by cellular differentiation. Br. J. Pharmacol. 1999;126:1559–1566. doi: 10.1038/sj.bjp.0702464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell N., Hann V., Redfern C.P., Cheek T.R. Store-operated Ca2+ entry in proliferating and retinoic acid-differentiated N- and S-type neuroblastoma cells. Biochim. Biophys. Acta. 2013;1833:643–651. doi: 10.1016/j.bbamcr.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grudt T.J., Usowicz M.M., Henderson G. Ca2+ entry following store depletion in SH-SY5Y neuroblastoma cells. Brain Res. Mol. Brain Res. 1996;36:93–100. doi: 10.1016/0169-328X(95)00248-Q. [DOI] [PubMed] [Google Scholar]
- 28.Marini P., Moriello A.S., Cristino L., Palmery M., De Petrocellis L., di Marzo V. Cannabinoid CB1 receptor elevation of intracellular calcium in neuroblastoma SH-SY5Y cells: Interactions with muscarinic and delta-opioid receptors. Biochim. Biophys. Acta. 2009;1793:1289–1303. doi: 10.1016/j.bbamcr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 29.McGinnis K.M., Shariat-Madar Z., Gnegy M.E. Cytosolic calmodulin is increased in SK-N-SH human neuroblastoma cells due to release of calcium from intracellular stores. J. Neurochem. 1998;70:139–146. doi: 10.1046/j.1471-4159.1998.70010139.x. [DOI] [PubMed] [Google Scholar]
- 30.Vilner B.J., Bowen W.D. Modulation of cellular calcium by sigma-2 receptors: Release from intracellular stores in human SK-N-SH neuroblastoma cells. J. Pharmacol. Exp. Ther. 2000;292:900–911. [PubMed] [Google Scholar]
- 31.Gunes D.A., Florea A.M., Splettstoesser F., Busselberg D. Co-application of arsenic trioxide (As2O3) and cisplatin (CDDP) on human SY-5Y neuroblastoma cells has differential effects on the intracellular calcium concentration ([Ca2+]i) and cytotoxicity. Neurotoxicology. 2009;30:194–202. doi: 10.1016/j.neuro.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Florea A.M., Splettstoesser F., Busselberg D. Arsenic trioxide (As2O3) induced calcium signals and cytotoxicity in two human cell lines: SY-5Y neuroblastoma and 293 embryonic kidney (HEK) Toxicol. Appl. Pharmacol. 2007;220:292–301. doi: 10.1016/j.taap.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Florea A.M., Splettstoesser F., Dopp E., Rettenmeier A.W., Busselberg D. Modulation of intracellular calcium homeostasis by trimethyltin chloride in human tumour cells: Neuroblastoma SY5Y and cervix adenocarcinoma HeLa S3. Toxicology. 2005;216:1–8. doi: 10.1016/j.tox.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Megison M.L., Gillory L.A., Beierle E.A. Cell survival signaling in neuroblastoma. Anticancer Agents Med. Chem. 2013;13:563–575. doi: 10.2174/1871520611313040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnsen J.I., Segerstrom L., Orrego A., Elfman L., Henriksson M., Kagedal B., Eksborg S., Sveinbjornsson B., Kogner P. Inhibitors of mammalian target of rapamycin downregulate MYCN protein expression and inhibit neuroblastoma growth in vitro and in vivo. Oncogene. 2008;27:2910–2922. doi: 10.1038/sj.onc.1210938. [DOI] [PubMed] [Google Scholar]
- 36.Abid M.R., Guo S., Minami T., Spokes K.C., Ueki K., Skurk C., Walsh K., Aird W.C. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:294–300. doi: 10.1161/01.ATV.0000110502.10593.06. [DOI] [PubMed] [Google Scholar]
- 37.Opel D., Poremba C., Simon T., Debatin K.M., Fulda S. Activation of Akt predicts poor outcome in neuroblastoma. Cancer Res. 2007;67:735–745. doi: 10.1158/0008-5472.CAN-06-2201. [DOI] [PubMed] [Google Scholar]
- 38.Kwon S.H., Hong S.I., Kim J.A., Jung Y.H., Kim S.Y., Kim H.C., Lee S.Y., Jang C.G. The neuroprotective effects of Lonicera japonica Thunb. Against hydrogen peroxide-induced apoptosis via phosphorylation of MAPKs and PI3K/Akt in SH-SY5Y cells. Food Chem. Toxicol. 2011;49:1011–1019. doi: 10.1016/j.fct.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Wang L., Yang H.J., Xia Y.Y., Feng Z.W. Insulin-like growth factor 1 protects human neuroblastoma cells SH-EP1 against MPP+-induced apoptosis by AKT/GSK-3beta/JNK signaling. Apoptosis. 2010;15:1470–1479. doi: 10.1007/s10495-010-0547-z. [DOI] [PubMed] [Google Scholar]
- 40.Ho R., Minturn J.E., Hishiki T., Zhao H., Wang Q., Cnaan A., Maris J., Evans A.E., Brodeur G.M. Proliferation of human neuroblastomas mediated by the epidermal growth factor receptor. Cancer Res. 2005;65:9868–9875. doi: 10.1158/0008-5472.CAN-04-2426. [DOI] [PubMed] [Google Scholar]
- 41.Brodeur G.M., Seeger R.C., Schwab M., Varmus H.E., Bishop J.M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 42.Brodeur G.M. Neuroblastoma: Biological insights into a clinical enigma. Nat. Rev. Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 43.Morris S.W., Kirstein M.N., Valentine M.B., Dittmer K.G., Shapiro D.N., Saltman D.L., Look A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 44.Palmer R.H., Vernersson E., Grabbe C., Hallberg B. Anaplastic lymphoma kinase: Signalling in development and disease. Biochem J. 2009;420:345–361. doi: 10.1042/BJ20090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamant L., Pulford K., Bischof D., Morris S.W., Mason D.Y., Delsol G., Mariame B. Expression of the ALK tyrosine kinase gene in neuroblastoma. Am. J. Pathol. 2000;156:1711–1721. doi: 10.1016/S0002-9440(10)65042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Passoni L., Longo L., Collini P., Coluccia A.M., Bozzi F., Podda M., Gregorio A., Gambini C., Garaventa A., Pistoia V., et al. Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Res. 2009;69:7338–7346. doi: 10.1158/0008-5472.CAN-08-4419. [DOI] [PubMed] [Google Scholar]
- 47.Del Grosso F., De Mariano M., Passoni L., Luksch R., Tonini G.P., Longo L. Inhibition of N-linked glycosylation impairs ALK phosphorylation and disrupts pro-survival signaling in neuroblastoma cell lines. BMC Cancer. 2011;11 doi: 10.1186/1471-2407-11-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng F., Soellner D., Nunez J., Wang H. The basal level of intracellular calcium gates the activation of phosphoinositide 3-kinase-Akt signaling by brain-derived neurotrophic factor in cortical neurons. J. Neurochem. 2008;106:1259–1274. doi: 10.1111/j.1471-4159.2008.05478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chun-Jen Lin C., Summerville J.B., Howlett E., Stern M. The metabotropic glutamate receptor activates the lipid kinase PI3K in drosophila motor neurons through the calcium/calmodulin-dependent protein kinase II and the nonreceptor tyrosine protein kinase DFaK. Genetics. 2011;188:601–613. doi: 10.1534/genetics.111.128561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y., Nakagawara A. Apoptotic cell death in neuroblastoma. Cells. 2013;2:432–459. doi: 10.3390/cells2020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eggert A., Ikegaki N., Liu X.G., Brodeur G.M. Prognostic and biological role of neurotrophin-receptor TrkA and TrkB in neuroblastoma. Klin. Padiatr. 2000;212:200–205. doi: 10.1055/s-2000-9677. [DOI] [PubMed] [Google Scholar]
- 52.Brodeur G.M., Minturn J.E., Ho R., Simpson A.M., Iyer R., Varela C.R., Light J.E., Kolla V., Evans A.E. Trk receptor expression and inhibition in neuroblastomas. Clin. Cancer Res. 2009;15:3244–3250. doi: 10.1158/1078-0432.CCR-08-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Bernardi M.A., Rabins S.J., Colangelo A.M., Brooker G., Mocchetti I. TrkA mediates the nerve growth factor-induced intracellular calcium accumulation. J. Biol. Chem. 1996;271:6092–6098. doi: 10.1074/jbc.271.11.6092. [DOI] [PubMed] [Google Scholar]
- 54.Jiang H., Ulme D.S., Dickens G., Chabuk A., Lavarreda M., Lazarovici P., Guroff G. Both p140(trk) and p75(NGFR) nerve growth factor receptors mediate nerve growth factor-stimulated calcium uptake. J. Biol. Chem. 1997;272:6835–6837. doi: 10.1074/jbc.272.11.6835. [DOI] [PubMed] [Google Scholar]
- 55.Nikodijevic B., Guroff G. Nerve growth factor-induced increase in calcium uptake by PC12 cells. J. Neurosci. Res. 1991;28:192–199. doi: 10.1002/jnr.490280206. [DOI] [PubMed] [Google Scholar]
- 56.Viard P., Butcher A.J., Halet G., Davies A., Nurnberg B., Heblich F., Dolphin A.C. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat. Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- 57.Yoon S., Seger R. The extracellular signal-regulated kinase: Multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 58.Raman M., Chen W., Cobb M.H. Differential regulation and properties of mapks. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 59.Avruch J. Map kinase pathways: The first twenty years. Biochim. Biophys. Acta. 2007;1773:1150–1160. doi: 10.1016/j.bbamcr.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chuderland D., Seger R. Calcium regulates ERK signaling by modulating its protein-protein interactions. Commun. Integr. Biol. 2008;1:4–5. doi: 10.4161/cib.1.1.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rottingen J., Iversen J.G. Ruled by waves? Intracellular and intercellular calcium signalling. Acta Physiol. Scand. 2000;169:203–219. doi: 10.1046/j.1365-201x.2000.00732.x. [DOI] [PubMed] [Google Scholar]
- 62.Munaron L. Calcium signalling and control of cell proliferation by tyrosine kinase receptors (review) Int. J. Mol. Med. 2002;10:671–676. [PubMed] [Google Scholar]
- 63.Chuderland D., Marmor G., Shainskaya A., Seger R. Calcium-mediated interactions regulate the subcellular localization of extracellular signal-regulated kinases. J. Biol. Chem. 2008;283:11176–11188. doi: 10.1074/jbc.M709030200. [DOI] [PubMed] [Google Scholar]
- 64.Agell N., Bachs O., Rocamora N., Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell. Signal. 2002;14:649–654. doi: 10.1016/S0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 65.Cullen P.J., Lockyer P.J. Integration of calcium and Ras signalling. Nat. Rev. Mol. Cell. Biol. 2002;3:339–348. doi: 10.1038/nrm808. [DOI] [PubMed] [Google Scholar]
- 66.Tebar F., Villalonga P., Sorkina T., Agell N., Sorkin A., Enrich C. Calmodulin regulates intracellular trafficking of epidermal growth factor receptor and the MAPK signaling pathway. Mol. Biol. Cell. 2002;13:2057–2068. doi: 10.1091/mbc.01-12-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dikic I., Tokiwa G., Lev S., Courtneidge S.A., Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 68.Farnsworth C.L., Freshney N.W., Rosen L.B., Ghosh A., Greenberg M.E., Feig L.A. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 69.Chen H.J., Rojas-Soto M., Oguni A., Kennedy M.B. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by cam kinase II. Neuron. 1998;20:895–904. doi: 10.1016/S0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 70.Liu Q., Walker S.A., Gao D., Taylor J.A., Dai Y.F., Arkell R.S., Bootman M.D., Roderick H.L., Cullen P.J., Lockyer P.J. CAPRI and RASAL impose different modes of information processing on Ras due to contrasting temporal filtering of Ca2+ J. Cell. Biol. 2005;170:183–190. doi: 10.1083/jcb.200504167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egea J., Espinet C., Soler R.M., Peiro S., Rocamora N., Comella J.X. Nerve growth factor activation of the extracellular signal-regulated kinase pathway is modulated by Ca(2+) and calmodulin. Mol. Cell. Biol. 2000;20:1931–1946. doi: 10.1128/MCB.20.6.1931-1946.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yano S., Tokumitsu H., Soderling T.R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 73.Golubovskaya V.M., Kweh F.A., Cance W.G. Focal adhesion kinase and cancer. Histol. Histopathol. 2009;24:503–510. doi: 10.14670/HH-24.503. [DOI] [PubMed] [Google Scholar]
- 74.Thiele C.J., Reynolds C.P., Israel M.A. Decreased expression of N-MYC precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nature. 1985;313:404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- 75.Sidell N., Altman A., Haussler M.R., Seeger R.C. Effects of retinoic acid (RA) on the growth and phenotypic expression of several human neuroblastoma cell lines. Exp. Cell. Res. 1983;148:21–30. doi: 10.1016/0014-4827(83)90184-2. [DOI] [PubMed] [Google Scholar]
- 76.Matthay K.K., Reynolds C.P., Seeger R.C., Shimada H., Adkins E.S., Haas-Kogan D., Gerbing R.B., London W.B., Villablanca J.G. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children’s oncology group study. J. Clin. Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walton J.D., Kattan D.R., Thomas S.K., Spengler B.A., Guo H.F., Biedler J.L., Cheung N.K., Ross R.A. Characteristics of stem cells from human neuroblastoma cell lines and in tumors. Neoplasia. 2004;6:838–845. doi: 10.1593/neo.04310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ross R.A., Spengler B.A., Domenech C., Porubcin M., Rettig W.J., Biedler J.L. Human neuroblastoma I-type cells are malignant neural crest stem cells. Cell. Growth Differ. 1995;6:449–456. [PubMed] [Google Scholar]
- 79.Ross R.A., Biedler J.L., Spengler B.A. A role for distinct cell types in determining malignancy in human neuroblastoma cell lines and tumors. Cancer Lett. 2003;197:35–39. doi: 10.1016/S0304-3835(03)00079-X. [DOI] [PubMed] [Google Scholar]
- 80.Ross R.A., Spengler B.A. Human neuroblastoma stem cells. Semin. Cancer. Biol. 2007;17:241–247. doi: 10.1016/j.semcancer.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 81.Ciccarone V., Spengler B.A., Meyers M.B., Biedler J.L., Ross R.A. Phenotypic diversification in human neuroblastoma cells: Expression of distinct neural crest lineages. Cancer Res. 1989;49:219–225. [PubMed] [Google Scholar]
- 82.Ross R.A., Spengler B.A., Biedler J.L. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J. Natl. Cancer Inst. 1983;71:741–747. [PubMed] [Google Scholar]
- 83.Spengler B.A., Lazarova D.L., Ross R.A., Biedler J.L. Cell lineage and differentiation state are primary determinants of MYCN gene expression and malignant potential in human neuroblastoma cells. Oncol. Res. 1997;9:467–476. [PubMed] [Google Scholar]
- 84.Piacentini M., Piredda L., Starace D.T., Annicchiarico-Petruzzelli M., Mattei M., Oliverio S., Farrace M.G., Melino G. Differential growth of N- and S-type human neuroblastoma cells xenografted into scid mice. Correlation with apoptosis. J. Pathol. 1996;180:415–422. doi: 10.1002/(SICI)1096-9896(199612)180:4<415::AID-PATH684>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 85.McDonald R.L., Kaye D.F., Reeve H.L., Ball S.G., Peers C., Vaughan P.F. Bradykinin-evoked release of [3h]noradrenaline from the human neuroblastoma SH-SY5Y. Biochem. Pharmacol. 1994;48:23–30. doi: 10.1016/0006-2952(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 86.Willars G.B., Nahorski S.R. Quantitative comparisons of muscarinic and bradykinin receptor-mediated ins (1,4,5)p3 accumulation and Ca2+ signalling in human neuroblastoma cells. Br. J. Pharmacol. 1995;114:1133–1142. doi: 10.1111/j.1476-5381.1995.tb13325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wong R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30 doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Assuncao Guimaraes C., Linden R. Programmed cell deaths. Apoptosis and alternative deathstyles. Eur. J. Biochem. 2004;271:1638–1650. doi: 10.1111/j.1432-1033.2004.04084.x. [DOI] [PubMed] [Google Scholar]
- 89.Henriquez M., Armisen R., Stutzin A., Quest A.F. Cell death by necrosis, a regulated way to go. Curr. Mol. Med. 2008;8:187–206. doi: 10.2174/156652408784221289. [DOI] [PubMed] [Google Scholar]
- 90.Hotchkiss R.S., Strasser A., McDunn J.E., Swanson P.E. Cell death. N. Engl. J. Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krieger C., Duchen M.R. Mitochondria, Ca2+ and neurodegenerative disease. Eur. J. Pharmacol. 2002;447:177–188. doi: 10.1016/S0014-2999(02)01842-3. [DOI] [PubMed] [Google Scholar]
- 92.Hajnoczky G., Csordas G., Das S., Garcia-Perez C., Saotome M., Sinha Roy S., Yi M. Mitochondrial calcium signalling and cell death: Approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell. Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rong Y., Distelhorst C.W. Bcl-2 protein family members: Versatile regulators of calcium signaling in cell survival and apoptosis. Annu. Rev. Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 94.Kirichok Y., Krapivinsky G., Clapham D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 95.Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., et al. Loss of cyclophilin d reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 96.Basso E., Fante L., Fowlkes J., Petronilli V., Forte M.A., Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J. Biol. Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 97.Giorgio V., Soriano M.E., Basso E., Bisetto E., Lippe G., Forte M.A., Bernardi P. Cyclophilin D in mitochondrial pathophysiology. Biochim. Biophys. Acta. 2010;1797:1113–1118. doi: 10.1016/j.bbabio.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scorrano L., Penzo D., Petronilli V., Pagano F., Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha aopototic signaling. J. Biol. Chem. 2001;276:12035–12040. doi: 10.1074/jbc.M010603200. [DOI] [PubMed] [Google Scholar]
- 99.Starkov A.A., Chinopoulos C., Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell. Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 100.Green D.R., Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 101.Parsons M.J., Green D.R. Mitochondria in cell death. Essays Biochem. 2010;47:99–114. doi: 10.1042/bse0470099. [DOI] [PubMed] [Google Scholar]
- 102.Nakagawara A., Arima-Nakagawara M., Scavarda N.J., Azar C.G., Cantor A.B., Brodeur G.M. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N. Engl. J. Med. 1993;328:847–854. doi: 10.1056/NEJM199303253281205. [DOI] [PubMed] [Google Scholar]
- 103.Wright K.M., Vaughn A.E., Deshmukh M. Apoptosome dependent caspase-3 activation pathway is non-redundant and necessary for apoptosis in sympathetic neurons. Cell. Death Differ. 2007;14:625–633. doi: 10.1038/sj.cdd.4402024. [DOI] [PubMed] [Google Scholar]
- 104.Nakagawara A., Azar C.G., Scavarda N.J., Brodeur G.M. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol. Cell. Biol. 1994;14:759–767. doi: 10.1128/mcb.14.1.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Caren H., Kryh H., Nethander M., Sjoberg R.M., Trager C., Nilsson S., Abrahamsson J., Kogner P., Martinsson T. High-risk neuroblastoma tumors with 11q-deletion display a poor prognostic, chromosome instability phenotype with later onset. Proc. Natl. Acad. Sci. USA. 2010;107:4323–4328. doi: 10.1073/pnas.0910684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Westermark U.K., Wilhelm M., Frenzel A., Henriksson M.A. The MYCN oncogene and differentiation in neuroblastoma. Semin. Cancer Biol. 2011;21:256–266. doi: 10.1016/j.semcancer.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Eilers M., Eisenman R.N. Myc’s broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bell E., Premkumar R., Carr J., Lu X., Lovat P.E., Kees U.R., Lunec J., Tweddle D.A. The role of MYCN in the failure of MYCN amplified neuroblastoma cell lines to G1 arrest after DNA damage. Cell Cycle. 2006;5:2639–2647. doi: 10.4161/cc.5.22.3443. [DOI] [PubMed] [Google Scholar]
- 109.Kang J.H., Rychahou P.G., Ishola T.A., Qiao J., Evers B.M., Chung D.H. MYCN silencing induces differentiation and apoptosis in human neuroblastoma cells. Biochem. Biophys. Res. Commun. 2006;351:192–197. doi: 10.1016/j.bbrc.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nara K., Kusafuka T., Yoneda A., Oue T., Sangkhathat S., Fukuzawa M. Silencing of MYCN by RNA interference induces growth inhibition, apoptotic activity and cell differentiation in a neuroblastoma cell line with MYCN amplification. Int. J. Oncol. 2007;30:1189–1196. [PubMed] [Google Scholar]
- 111.Tauszig-Delamasure S., Yu L.Y., Cabrera J.R., Bouzas-Rodriguez J., Mermet-Bouvier C., Guix C., Bordeaux M.C., Arumae U., Mehlen P. The TrkC receptor induces apoptosis when the dependence receptor notion meets the neurotrophin paradigm. Proc. Natl. Acad. Sci. USA. 2007;104:13361–13366. doi: 10.1073/pnas.0701243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bouzas-Rodriguez J., Cabrera J.R., Delloye-Bourgeois C., Ichim G., Delcros J.G., Raquin M.A., Rousseau R., Combaret V., Benard J., Tauszig-Delamasure S., et al. Neurotrophin-3 production promotes human neuroblastoma cell survival by inhibiting trkc-induced apoptosis. J. Clin. Investig. 2010;120:850–858. doi: 10.1172/JCI41013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaplan D.R., Miller F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10:381–391. doi: 10.1016/S0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 114.Chen L., Iraci N., Gherardi S., Gamble L.D., Wood K.M., Perini G., Lunec J., Tweddle D.A. P53 is a direct transcriptional target of mycn in neuroblastoma. Cancer Res. 2010;70:1377–1388. doi: 10.1158/0008-5472.CAN-09-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakano K., Vousden K.H. Puma, a novel proapoptotic gene, is induced by p53. Mol. Cell. 2001;7:683–694. doi: 10.1016/S1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 116.Seoane J., Le H.V., Massague J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- 117.Fulda S., Lutz W., Schwab M., Debatin K.M. MYCN sensitizes neuroblastoma cells for drug-induced apoptosis. Oncogene. 1999;18:1479–1486. doi: 10.1038/sj.onc.1202435. [DOI] [PubMed] [Google Scholar]
- 118.Cui H., Li T., Ding H.F. Linking of N-MYC to death receptor machinery in neuroblastoma cells. J. Biol. Chem. 2005;280:9474–9481. doi: 10.1074/jbc.M410450200. [DOI] [PubMed] [Google Scholar]
- 119.Berninger B., Garcia D.E., Inagaki N., Hahnel C., Lindholm D. BDNF and NT-3 induce intracellular Ca2+ elevation in hippocampal neurones. Neuroreport. 1993;4:1303–1306. doi: 10.1097/00001756-199309150-00004. [DOI] [PubMed] [Google Scholar]
- 120.Levine E.S., Dreyfus C.F., Black I.B., Plummer M.R. Differential effects of NGF and BDNF on voltage-gated calcium currents in embryonic basal forebrain neurons. J. Neurosci. 1995;15:3084–3091. doi: 10.1523/JNEUROSCI.15-04-03084.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bonnington J.K., McNaughton P.A. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J. Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baldelli P., Forni P.E., Carbone E. BDNF, NT-3 and NGF induce distinct new Ca2+ channel synthesis in developing hippocampal neurons. Eur. J. Neurosci. 2000;12:4017–4032. doi: 10.1046/j.1460-9568.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- 123.Giorgi C., Romagnoli A., Pinton P., Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr. Mol. Med. 2008;8:119–130. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]
- 124.Verkhratsky A. Calcium and cell death. Subcell Biochem. 2007;45:465–480. doi: 10.1007/978-1-4020-6191-2_17. [DOI] [PubMed] [Google Scholar]
- 125.Nomura M., Ueno A., Saga K., Fukuzawa M., Kaneda Y. Accumulation of cytosolic calcium induces necroptotic cell death in human neuroblastoma. Cancer Res. 2014;74:1056–1066. doi: 10.1158/0008-5472.CAN-13-1283. [DOI] [PubMed] [Google Scholar]
- 126.Rodland K.D. The role of the calcium-sensing receptor in cancer. Cell. Calcium. 2004;35:291–295. doi: 10.1016/j.ceca.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 127.Saidak Z., Mentaverri R., Brown E.M. The role of the calcium-sensing receptor in the development and progression of cancer. Endocr. Rev. 2009;30:178–195. doi: 10.1210/er.2008-0041. [DOI] [PubMed] [Google Scholar]
- 128.de Torres C., Beleta H., Diaz R., Toran N., Rodriguez E., Lavarino C., Garcia I., Acosta S., Sunol M., Mora J. The calcium-sensing receptor and parathyroid hormone-related protein are expressed in differentiated, favorable neuroblastic tumors. Cancer. 2009;115:2792–2803. doi: 10.1002/cncr.24304. [DOI] [PubMed] [Google Scholar]
- 129.Chakrabarty S., Wang H., Canaff L., Hendy G.N., Appelman H., Varani J. Calcium sensing receptor in human colon carcinoma: Interaction with Ca(2+) and 1,25-dihydroxyvitamin d(3) Cancer Res. 2005;65:493–498. [PubMed] [Google Scholar]
- 130.Singh N., Promkan M., Liu G., Varani J., Chakrabarty S. Role of calcium sensing receptor (CaSR) in tumorigenesis. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:455–463. doi: 10.1016/j.beem.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 131.Maris J.M., Mosse Y.P., Bradfield J.P., Hou C., Monni S., Scott R.H., Asgharzadeh S., Attiyeh E.F., Diskin S.J., Laudenslager M., et al. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N. Engl. J. Med. 2008;358:2585–2593. doi: 10.1056/NEJMoa0708698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nguyen le B., Diskin S.J., Capasso M., Wang K., Diamond M.A., Glessner J., Kim C., Attiyeh E.F., Mosse Y.P., Cole K., et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility loci. PLoS Genet. 2011;7:e1002026. doi: 10.1371/journal.pgen.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Capasso M., Devoto M., Hou C., Asgharzadeh S., Glessner J.T., Attiyeh E.F., Mosse Y.P., Kim C., Diskin S.J., Cole K.A., et al. Common variations in bard1 influence susceptibility to high-risk neuroblastoma. Nat. Genet. 2009;41:718–723. doi: 10.1038/ng.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang K., Diskin S.J., Zhang H., Attiyeh E.F., Winter C., Hou C., Schnepp R.W., Diamond M., Bosse K., Mayes P.A., et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature. 2011;469:216–220. doi: 10.1038/nature09609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Capasso M., Diskin S.J., Totaro F., Longo L., De Mariano M., Russo R., Cimmino F., Hakonarson H., Tonini G.P., Devoto M., et al. Replication of GWAS-identified neuroblastoma risk loci strengthens the role of BARD1 and affirms the cumulative effect of genetic variations on disease susceptibility. Carcinogenesis. 2013;34:605–611. doi: 10.1093/carcin/bgs380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Diskin S.J., Capasso M., Schnepp R.W., Cole K.A., Attiyeh E.F., Hou C., Diamond M., Carpenter E.L., Winter C., Lee H., et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat. Genet. 2012;44:1126–1130. doi: 10.1038/ng.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bhatnagar S.N., Sarin Y.K. Neuroblastoma: A review of management and outcome. Indian J. Pediatr. 2012;79:787–792. doi: 10.1007/s12098-012-0748-2. [DOI] [PubMed] [Google Scholar]
- 138.Siddik Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 139.Florea A.M., Busselberg D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Splettstoesser F., Florea A.M., Busselberg D. IP(3) receptor antagonist, 2-APB, attenuates cisplatin induced Ca2+-influx in HeLa-S3 cells and prevents activation of calpain and induction of apoptosis. Br. J. Pharmacol. 2007;151:1176–1186. doi: 10.1038/sj.bjp.0707335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Florea A.M., Yamoah E.N., Dopp E. Intracellular calcium disturbances induced by arsenic and its methylated derivatives in relation to genomic damage and apoptosis induction. Environ. Health Perspect. 2005;113:659–664. doi: 10.1289/ehp.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Keshelava N., Seeger R.C., Groshen S., Reynolds C.P. Drug resistance patterns of human neuroblastoma cell lines derived from patients at different phases of therapy. Cancer Res. 1998;58:5396–5405. [PubMed] [Google Scholar]
- 143.Peaston A.E., Gardaneh M., Franco A.V., Hocker J.E., Murphy K.M., Farnsworth M.L., Catchpoole D.R., Haber M., Norris M.D., Lock R.B., et al. MRP1 gene expression level regulates the death and differentiation response of neuroblastoma cells. Br. J. Cancer. 2001;85:1564–1571. doi: 10.1054/bjoc.2001.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Keshelava N., Zuo J.J., Chen P., Waidyaratne S.N., Luna M.C., Gomer C.J., Triche T.J., Reynolds C.P. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res. 2001;61:6185–6193. [PubMed] [Google Scholar]
- 145.Kotchetkov R., Cinatl J., Blaheta R., Vogel J.U., Karaskova J., Squire J., Hernaiz Driever P., Klingebiel T., Cinatl J., Jr. Development of resistance to vincristine and doxorubicin in neuroblastoma alters malignant properties and induces additional karyotype changes: A preclinical model. Int. J. Cancer. 2003;104:36–43. doi: 10.1002/ijc.10917. [DOI] [PubMed] [Google Scholar]
- 146.Blaheta R.A., Michaelis M., Natsheh I., Hasenberg C., Weich E., Relja B., Jonas D., Doerr H.W., Cinatl J., Jr. Valproic acid inhibits adhesion of vincristine- and cisplatin-resistant neuroblastoma tumour cells to endothelium. Br. J. Cancer. 2007;96:1699–1706. doi: 10.1038/sj.bjc.6603777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Karlsson J., Edsjo A., Pahlman S., Pettersson H.M. Multidrug-resistant neuroblastoma cells are responsive to arsenic trioxide at both normoxia and hypoxia. Mol. Cancer Ther. 2005;4:1128–1135. doi: 10.1158/1535-7163.MCT-05-0047. [DOI] [PubMed] [Google Scholar]