Abstract
Stereotactic body radiotherapy (SBRT) has become a standard treatment option for early stage, node negative non-small cell lung cancer (NSCLC) in patients who are either medically inoperable or refuse surgical resection. SBRT has high local control rates and a favorable toxicity profile relative to other surgical and non-surgical approaches. Given the excellent tumor control rates and increasing utilization of SBRT, recent efforts have focused on limiting toxicity while expanding treatment to increasingly complex patients. We review toxicities from SBRT for lung cancer, including central airway, esophageal, vascular (e.g., aorta), lung parenchyma (e.g., radiation pneumonitis), and chest wall toxicities, as well as radiation-induced neuropathies (e.g., brachial plexus, vagus nerve and recurrent laryngeal nerve). We summarize patient-related, tumor-related, dosimetric characteristics of these toxicities, review published dose constraints, and propose strategies to reduce such complications.
Keywords: stereotactic body radiation therapy (SBRT), stereotactic ablative radiotherapy (SABR), non-small cell lung cancer (NSCLC), toxicity, complications
1. Introduction
Few recent developments in the treatment of non-small cell lung cancer (NSCLC) have matched the impact that stereotactic body radiotherapy (SBRT), also referred to as stereotactic ablative radiotherapy (SABR), has had on outcomes in early stage, medically inoperable NSCLC [1]. Prior to the advent of SBRT, these NSCLC patients had dismal outcomes, with poor local control rates, and 5-year survival rates of 20%–30% with conventionally fractionated external beam radiation (EBRT) [2,3,4]. Although escalation of doses above 80 Gy improved local control and survival, dose limiting toxicities were reached at doses of 84–90 Gy [5,6,7]. Intensification of treatment through the addition of concurrent chemotherapy and hypofractionation were also attempted with limited success; however, improvements in localization and radiation delivery allowed for hypofractionated, dose escalated regimens to be delivered safely [8], with 2-year local control rates above 90% [9,10].
Currently, SBRT is the treatment of choice for early stage, node negative NSCLC in patients who are either medically inoperable or refuse surgical resection. These stereotactic techniques reduce target uncertainty and planning target volume (PTV) margins while improving dose gradients across tissue, allowing delivery of high cumulative doses and improving local control while reducing dose related toxicity by minimizing exposure to organs at risk (OAR) [11]. While retrospective comparisons of patients treated by surgery and SBRT are limited by an imbalance of poor prognostic factors in SBRT cohorts [12], after statistical adjustments for these differences, similar outcomes between SBRT and both limited sublobar resection and lobectomy have been reported [12,13].
While treatment of early stage NSCLC with SBRT has proven to be an effective treatment modality with acceptable toxicity, careful patient evaluation, simulation, treatment planning, and eventual radiation delivery is required to minimize complications. With this in mind, we review the literature on treatment considerations for delivering lung SBRT.
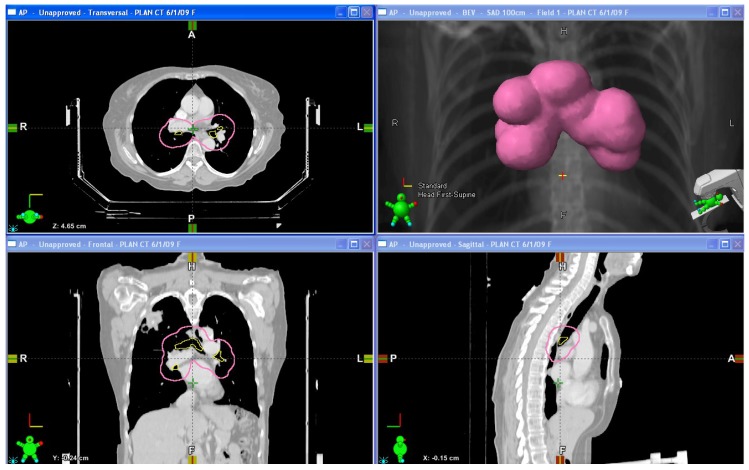
2. Centrally Located Tumors
For early stage NSCLC, centrally located lesions are defined as any lesion existing within a 2 cm zone surrounding the proximal bronchial tree, from the carina to the lobar and lingular bronchi [14] (Figure 1). Previously, central lesions treated with traditional SBRT doses have been shown to cause excessive toxicity, as was shown in a Phase II study from Indiana University where patients with centrally located (hilar/pericentral) tumors treated to 60 to 66 Gy in three fractions had an 11-fold higher risk of developing grade 3–5 toxicities when compared to similarly treated peripherally located tumors [10]. Updated results from this study [15] at a median follow up of 50.2 months confirmed a high incidence of grade 3 to 5 toxicity (27.3%), almost three times the rate for peripheral lesions (10.4%). Other studies have had similar observations. For instance, a phase I single fraction dose escalation trial for patients with primary or metastatic lung lesions by Le et al. reported late treatment-related toxicities in 25% of patients, of which the majority of overall toxicity (62.5%) and all three grade 5 toxicities (pneumonitis/pleural effusion, tracheoesophageal fistula, and pulmonary embolism/recall pneumonitis) were seen in centrally located tumors [16,17]. Thus, centrally located tumors represent a high-risk tumor location, predisposing such patients to a unique toxicity profile.
Figure 1.
Diagrams of the proximal bronchial tree and the surrounding 2 cm avoidance zone. Upper left: Axial view; Lower left: Coronal view; Upper right: 3-dimensional representation of the avoidance zone; Lower right: Sagittal view.
3. Central Airway Toxicities
Given the proximity of centrally located lesions to major airways, patients with these tumors are at higher risk for a dose-related major airway toxicity and consequent atelectasis, stenosis/stricture, airway necrosis, and/or fistula formation. Although its pathogenesis is poorly understood, airway toxicity has been hypothesized to be caused by a dose-dependent radiation-induced damage of the bronchial wall, leading to fibrosis and consequent stenosis and stricture. Data from more traditional conventionally fractionated radiation have showed narrowing caliber of mainstem bronchi, as measured by post-treatment CT as early as three months after high dose EBRT to doses of ≥73.6 Gy [18]. The data for central airway toxicity for SBRT is more limited; however, a retrospective study by Song et al., showed complete or partial bronchial stricture in 8/9 (89%) patients with central tumors treated with SBRT (at doses of 40–60 Gy in 3–4 fractions) at a median follow-up time of 26.5 months [16]. Perhaps data from high dose rate (HDR) brachytherapy can provide the greatest information about central airway toxicity from ablative radiation doses. A study by Speiser et al. examined radiation reactions for a total of 342 patients treated with endobronchial brachytherapy at doses of 750–1000 cGy at 5–10 mm depth for three fractions [19]. For the entire cohort, there was a 12% bronchial stenosis rate with higher rates seen in patients with large cell histology, treated with curative intent, prior laser photoresection, and concurrent EBRT [19].
More severe central airway late toxicity could lead to complete bronchial stenosis with resultant atelectasis, bronchial necrosis, or fatal hemoptysis. A retrospective study by Karlsson et al. demonstrated findings of radiation-induced atelectasis in 24.3% of patients with prescribed doses of 20–50 Gy in 2–5 fractions for tumors close to the bronchial tree [20]. On subsequent survival hazard function analysis, the median dose to 0.1 cc of bronchial tree in patients who developed atelectasis was 210 Gy3 vs. 105 Gy3 in 2 Gy equivalent doses for patients who did not develop atelectasis (p = 0.031). Another study by Rowe et al. described one bronchial necrosis-related hemorrhage and death occurring 10.5 months after SBRT to a 5.7 cm metastasis abutting the left mainstem bronchus, with the area of necrosis having received a maximum dose above the dose prescribed [21]. Corradetti et al. presented a similar case of central-airway necrosis and subsequent fatal hemoptysis in a NSCLC patient receiving a fractionation scheme of 50 Gy in five fractions [22].
4. Esophageal Toxicity
Esophageal toxicity is another well-known complication of radiotherapy for centrally located tumors treated with SBRT [23], ranging from mild esophagitis to stricture, perforation, and/or tracheoesophageal fistula [9,24]. Previous data from a number of detailed analyses of conventional EBRT suggest that the volume of esophagus exposed to higher doses of radiation (Vd) is the most meaningful metric. For instance, Palma et al. found that on multivariable analysis, V60 formed the best predictive model for radiation esophagitis following 3D-CRT or IMRT (for ≥grade 2, OR 1.34 per 10% increase, p < 0.001; for ≥grade 3, OR 1.33 per 10% increase, p < 0.001) [25]. Interestingly, even when staying at or below previously reported safe thresholds (limiting the esophagus in a single fraction to D5cc of 14.5 Gy, D2cc of 15 Gy, and to Dmax of 19 Gy) [26,27], SBRT fractionation regimens could still have single-fraction biologically effective doses (SFBEDs) to the gross tumor volume (GTV) exceeding these thresholds (calculated via linear quadratic (LQ) modeling), resulting in esophageal adverse events [28]. For instance, the two occurrences of high-grade esophageal toxicity events (tracheoesophageal fistula and esophageal perforation) in the study by Abelson et al. occurred at dose-volume points within or near the safe thresholds, at SFBED to D5cc of 16.5 and 11.4 Gy, to D2cc of 18.2 and 14.1 Gy, and to Dmax of 21.0 and 18.5 Gy, respectively, by LQ modeling [28].
Additionally, post-SBRT administration of chemotherapy can be an important modifier of radiation sensitivity. One patient in a study by Abelson et al. who experienced grade 5 toxicity received adjuvant chemotherapy three months after SBRT (25 Gy in one fraction), consisting initially of carboplatin and paclitaxel and then switched to gemcitabine two cycles later, at which time the patient developed (within the high-dose radiation volume) a tracheoesophageal fistula and ultimately fatal hemoptysis [28]. It is possible that the post-SBRT chemotherapy contributed to the patient’s adverse events, especially given that chemotherapy in the setting of conventionally fractionated radiotherapy has been found to increase the risk of esophageal toxicity [29]. Although the mechanism of fistula formation is poorly understood, it is likely related to the impaired angiogenesis leading to delayed and dysfunctional wound healing [30], as well as predisposition to fistula formation by radiation and chronic inflammation [31]. There is a new clinical trial (NCT02319889) looking at Abraxane (nab-paclitaxel) after SBRT which should better elucidate the potential role of SBRT and combination chemotherapy in improving treatment response as well as toxicity [32].
5. Vascular Injury
SBRT to central tumors also increases the dose to the central vascular structures, particularly the aorta. Although typically thought of as relatively radio-resistant, elevated doses seen in SBRT and the re-irradiation setting may lead to severe toxicity. In a landmark study by Evans et al., patients were evaluated for dosimetric correlates for aortic toxicities, defined as hemoptysis secondary to aortic damage, exsanguination secondary to aortic rupture, aortic aneurysm in the irradiated region, or aortic dissection [33] (Table 1). At a median follow up of 42 months, 2/35 (5.7%) patients had developed grade 5 aortic toxicity, ranging up to 39 months after the last radiation treatment [33]. In their dosimetric analysis, there was a 25% rate of grade 5 aortic toxicity for patients receiving maximum composite doses to 1 cm3 of the aorta ≥120 Gy versus 0% rate for those receiving <120 Gy (p = 0.047) [33].
Table 1.
Dosimetric considerations.
| Endpoint | Organ | Dosimetric Constraint (Comment) | Study |
|---|---|---|---|
| Vocal Cord Paralysis | Recurrent Laryngeal Nerve (RLN), Vagus Nerve (VN) | Of 12 patients with significant dose to either the RLN or VN, 2 patients developed vocal cord paresis, at a cumulative single fraction equivalent dose (SFED3; α/β = 3 Gy) to VN of 64.5 Gy and 16 Gy and SFED3 to the RLN 15.3 Gy and 19.5 Gy. | Shultz et al., 2014 [34] |
| Aortic Toxicity | Aorta | Recommended dose threshold of 120.0 Gy as a raw dose, 90.0 Gy when dose is corrected for long-term recovery during retreatment interval | Evans et al., 2013 [33] |
| Brachial Plexopathy | Brachial Plexus | Doses >26 Gy in 3–4 fractions resulted in an increased 2 year risk of brachial plexopathy, with similar cutoffs noted for BED >100 Gy3 and SFED-4 >15 Gy | Forquer et al., 2009 [35] |
Other potentially life threatening vascular complications from SBRT include hemoptysis and pulmonary hemorrhage. Potential risk factors for its occurrence include central location of tumor (OR = 3.003, p = 0.113) [36] (OR = 6.976, p = 0.017) [37], squamous histology (OR = 5.491, p = 0.040) [36], baseline major tumor cavitation (OR = 17.878, p = 0.001) [36], and suspicion of endobronchial involvement (OR = 12.8, p = 0.024) [38].
Of these risk factors, central location of tumor is a notable cause of fatal hemoptysis. Death following treatment of centrally located tumors, even at lower dose per fraction, has been reported in many studies and is related to inclusion of the esophagus and/or bronchus in the high dose volume, which leads to bronchial stricture or tissue ulceration and necrosis [39]. For example, Milano et al. cite a case of hemoptysis following SBRT for a central NSCLC that exposed the bronchus to a cumulative dose of 98 Gy [40]. Moreover, retreatment of central lung lesions with SBRT also increases the risk for hemoptysis. Oshiro et al. reported a patient who previously received two courses of thoracic radiotherapy (brachytherapy and SBRT) with grade 5 (fatal) hemoptysis 18 months post-SBRT with BED10 of 87.5 Gy [41].
6. Spontaneous Pneumothorax and Other Pulmonary Toxicities
Another rare complication of SBRT for NSCLC is spontaneous pneumothorax, which may occur as a result of radiation-induced pulmonary changes, apical pleural injury, and/or parenchymal injury [42]. Although previously seen predominantly in patients treated for Hodgkin’s lymphoma for whom large volumes of pleura were within the radiotherapy (RT) field, Onishi et al. described a case of asymptomatic spontaneous pneumothorax detected during a routine follow-up by chest X-ray and chest computed tomography (CT) two months after receiving SBRT for NSCLC to separate right upper and right lower lobe lesions [42]. Postulated contributing factors include tumor related air trapping and consequent alveolar space rupture, and RT-induced dense pulmonary/pleural fibrosis leading to rupture of subpleural blebs [43]. However, given the rarity of such a complication, specific data on its incidence and/or risk factors for its development are lacking.
Although concern exists for changes in post treatment pulmonary function (PF) after SBRT, data suggests these changes may only be only of minimal clinical relevance. Investigators from the Indiana studied the effects of SBRT on PF, and found no significant long-term change in FEV1, FEV1%, or DLCO after treatment [44,45]. Similar studies [9,46,47] also suggest minimal PF changes following SBRT, comparable to those expected from physiologic aging [9,44,46,47,48]. Furthermore, there has been no determination of a minimum PF necessary for the safe practice of SBRT [46]. Thus, baseline PF alone may not be an absolute contraindication to treatment with SBRT [49], and dose reduction aimed at preserving long term PF may be unjustified [44].
7. Radiation Pneumonitis
Radiation pneumonitis (RP) is one of the major toxicities which limits the maximal radiation dose that can be safely delivered to thoracic tumors [50], with severity ranging from asymptomatic cases detected only radiographically to clinically evident cases involving cough, shortness of breath, and fever. In severe cases, there may be dense fibrotic lung changes and respiratory compromise requiring supplemental oxygen or assisted ventilation [51]. Reported rates of post-SBRT RP requiring clinical intervention range from 0% to 29% [52,53,54,55], with the occurrence of severe (≥grade 3) RP usually uncommon, even among patients with compromised lung function [56,57]. This is likely due to the small target sizes and limited planning margins allowed by SBRT, thus minimizing the volume of normal lung tissue exposed to escalated doses [56,57]. Nevertheless, life-threatening toxicities have been reported in up to 12% of cases in various studies [10,53]. Therefore, understanding the risk factors for its occurrence, including dosimetric, diagnostic (e.g., biological, radiological), and patient-related (e.g., pulmonary-related comorbidities) predictors, are warranted.
7.1. Dosimetric Predictors of RP
The best supported dosimetric correlates of RP is mean lung dose (MLD), followed by V5 and V20 (percentage of the lung volume receiving >5 Gy and >20 Gy, respectively) [8,56,58,59]. Barriger et al. noted unadjusted total lung mean doses above 4 Gy and V20 greater than 4% being associated with increased RP on univariate analysis [8]. Interestingly, Ong et al. showed the best predictor of RP to be the contralateral lung V5, with all patients in their series developing RP with a contralateral lung V5 >26% [59].
Additional predictors of RP relate to increasing radiation target volume, as larger tumor sizes result in larger volumes of lung treated to higher doses [59]. Similarly, the risk of RP is also related to the conformity index (CI), defined as a ratio of the volume encompassed by the prescription isodose line (IDL) and volume of the PTV [51,58]. Although only indirectly related to the treatment volume, applying constraints to this additional parameter ensures the high dose region is restricted to the area immediately adjacent to the target. Clinically, Yamashita et al. found that none of the traditional dosimetric variables, including V5, V10, V20, and MLD, correlated with the CI, and that high CI values correlated significantly with RP occurrence (p = 0.0394) [53].
The anatomical location of the treatment region within the lung may also correlate with the development of RP. Kayas et al. reported that the portion of the lung below its geometric mid-line appears to be more radiosensitive than the upper portion and consequently more susceptible to RP [60]. This finding is consistent with those of other studies, where inferior tumor location [50,61] and ipsilateral lower lobe mean effective dose [62] correlated with RP. This phenomenon is likely explained by the greater target cell density [63] and greater functional importance of the lower lung [64], resulting in more severe symptoms upon damage. One must also take into account the greater respiratory motion seen for lower lobe tumors, which may expose larger volumes to radiation dose during treatment delivery, particularly in the absence of robust motion management [62].
7.2. Diagnostic Predictors of RP
Along with dose-volume factors, several biomarkers and radiologic imaging findings can help predict the risk for severe RP after SBRT [65]. Higher levels of the biomarkers serum Krebs von den Lungen-6 (KL-6), a circulating mucin-like glycoprotein produced and secreted from type II pneumocytes, and surfactant protein-D (SP-D) were associated with increased risk of severe RP after receiving SBRT [66,67]. Mechanistically, serum levels of KL-6 are dependent on the number of regenerating pneumocytes and integrity of the alveolar-capillary membrane [68], which may be compromised before treatment and/or in the setting of RP. In addition to elevated pre-treatment KL-6 levels (>500.0 U/mL), significant independent factors of high grade RP noted in the Yamashita experience included elevated levels of SP-D (>110.0 ng/mL) and presence of an interstitial pneumonitis (IP) shadow on pre-treatment CT.
These markers have also shown correlations with RP when measured after initiation of RT. Patients with elevated post treatment KL-6 levels in the months after RT relative to baseline (>1.5 to 1.7 times the pretreatment value) have shown correlations with symptomatic, grade 2 to 3 RP, values of which decrease in response to steroid use [67,69]. In a separate cohort, radiographic changes were seen in all patients who developed grade 3 RP after SBRT, the incidence of which was 75%, 40%, and 1.2% in patients with imaging findings at one, two, and greater than three months after SBRT, respectively [70]. Although these markers await prospective validation and widespread clinical adoption, they demonstrate the potential for individualized patient-specific markers to predict clinical toxicities in thoracic SBRT.
7.3. Patient-Related Predictors
Many patient-related factors have been investigated for their relationship with the incidence of RP, including co-morbid pulmonary disease, smoking history, and female gender. Underlying subclinical interstitial lung disease (ILD), defined by characteristic chest CT findings, may predispose patients to uncharacteristically extensive and fatal RP extending beyond the irradiated field [71]. In contrast, a retrospective series of patients with GOLD stage III-IV Chronic Obstructive Pulmonary Disease (COPD) treated with SBRT demonstrated low rates (1.7%) of grade 3 RP with risk adapted fractionation schemes [72]. The presence of pulmonary emphysema also was not found to predict for RP after SBRT, with at least one group demonstrating lower rates compared to those with normal function [71,73]. Although these data support the safety and efficacy of SBRT in this patient population, the underlying comorbid disease appears to dictate overall survival, as patients with GOLD IV COPD had worse median survival than those with GOLD III COPD (17 vs. 36 months, log-rank p = 0.01) [72].
Smoking also appears to be inversely correlated with the development of RP, particularly with regards to smoking status at the time of RT [74] and pack-years smoked [75]. Possible explanations for the protective effect of smoking on the incidence and degree of RP include possible reduction in radiation-induced inflammation [76] and increases in smoking-associated pulmonary and/or plasma glutathione, and consequent prevention of oxidant lung injury [77].
At least one study did show that female gender was a significant factor for predicting grade 2 RP in multivariate analysis [78], with a potential confounding of reduced total lung volume among females, which may result in relatively higher doses delivered to the remaining normal lung [78].
8. Chest Wall and Skin Toxicities
Chest wall toxicities are usually associated with SBRT for peripherally located lung tumors [79,80], and include skin toxicity, rib fracture, and chronic chest wall pain [57]. Given the dosimetric nature of chest wall toxicities, patients with tumors more than 1–2 cm from the chest wall and 5 cm from the posterior skin are at very low risk of toxicity [81].
8.1. Chest Wall Pain and Rib Fracture
Chest wall pain (CWP) after SBRT, especially for treatment to lesions in close proximity to the chest wall, has been reported in 5%–25% of patients [80,81,82,83] and can present with or without demonstrable rib fracture. Although the mechanism of chest wall pain following SBRT is poorly understood, possible target tissues responsible for this toxicity include the underlying large peripheral intercostal nerves [84] and bone [85]. Patient-related factors may also increase the risk of CWP, including younger patient age [86] and continued smoking [81]. Additional reports demonstrate obesity as a significant risk factor for CWP [82], with one study showing a nearly double increased in risk of chronic CWP for patients with ≥29 BMI compared to patients with <29 BMI (27% vs. 13%, respectively; p = 0.01) [82]. Among the group with elevated BMI, diabetes mellitus (DM) was also highly correlated with pain [82].
It appears that CWP may be at least partially independent of direct rib injury, as Andolino et al. reported only 19% of CWP episodes coincided with a documented rib fracture [80], and most patients in a series by Creach et al. who developed rib fracture did not report CWP prior to the rib fracture [87]. Additionally, the characteristic timing of onset after RT appears to differ between the two toxicities (Table 2). In terms of rib fracture alone, there is a strong association between individual rib dose-volume histogram parameters and fracture risk in the small-volume/high dose region, such as V60 [88,89]. Other reported parameters for chest wall toxicity and radiation-induced rib fracture include chest wall dose of 30 Gy being received by >30 to 70 cm3 [80,81,82,84,89], dose to 2 cm3 of the chest wall [84,88], and maximum dose to the rib or chest wall >50 Gy [80]. Limiting chest wall toxicities may also be possible through more protracted hyperfractionated regimens, as some investigators have found comparable local control with reduced CWP incidence (<6%) [90].
Table 2.
Chest wall and related toxicity characteristics.
| Toxicity | Incidence | Timing | Dosimetric Correlates | Study | ||
|---|---|---|---|---|---|---|
| Volume | Dose | Fraction | ||||
| Chest Wall Pain | 30% risk (Range: 10% to 44%) | 12.6 months post-SBRT (Range: 4.3–35.9 months) | 30 cc of chest wall | 30 Gy | 3 (Range:3–5) | Dunlap et al., 2010 [89] Mutter et al., 2012 [84] Creach et al., 2012 [87] |
| Rib Fracture | 5% risk | 19.2 months post-SBRT | 2 cc of rib | 27 Gy | 3 | Pettersson et al., 2009 [88] |
| 50% risk | 2 cc of rib | 50 Gy | 3 | Creach et al., 2012 [87] | ||
| Skin Toxicity | 1.2%–14% | 3–6 weeks post-SBRT | <10 cc volume, volume maximum of 30 Gy | 6 Gy/fraction | 5 | RTOG 0813 [91] Hoppe et al., 2008 [92] |
| maximum point dose of 32 Gy, maximum posterior skin dose ≥50% of actual prescribed dose | 6.4 Gy/fraction | 5 | ||||
8.2. Skin Toxicity
Although observed in a limited number of patients, selective subsets remain at elevated risk for skin toxicity, including those with a large body habitus [57], and posterior tumors location with limited distance between the lesion and overlying skin [82,92]. Additional dosimetric risk factors include treatment delivery through a limited number of coplanar beams, maximum skin doses 50% or greater than the prescribed dose [92], and among lesions within 2.5 cm of the chest wall, volume of the chest wall receiving 30 Gy (V30) (<50 mL, 22% vs. ≥51 mL, 44%, p = 0.02) [82].
9. Brachial Plexopathy
Tumors located in the apex of the lung pose unique local treatment challenges due to their close proximity to the brachial plexus and resulting radiation-induced brachial plexopathy (RIBP), with symptoms of upper extremity paresthesias, motor weakness, and neuropathic pain [93]. Mechanistically, RIBP is thought to be due to demyelination leading to axon loss [94], consistent with the observation that multiple traumas, including that from tumor invasion and/or previous surgery, may reduce the threshold for development of symptoms [94,95,96].
Limited dosimetric studies for RIBP indicate a threshold effect. Forquer et al. found that SBRT using absolute brachial plexus doses >26 Gy in three to four fractions resulted in an increased 2-year risk of brachial plexopathy compared to doses ≤26 Gy (46% vs. 8%, p = 0.04), with similar cutoffs noted for BED >100 Gy3 (p = 0.04) and SFED-4 (single fraction equivalent dose, where Dq = 4 Gy) >15 Gy (p = 0.06) [35]. Chang et al. similarly found that centrally located lesions treated to doses of 50 Gy in four fractions had a risk of RIBP limited to those where brachial plexus Dmax >35 Gy and V30 >0.2 cm3 (p = 0.001) [97]. Given the limited experience with RIBP, practitioners should exercise caution when treating apical tumors.
10. Vagus Nerve Injury
Although rare, thoracic SBRT delivered to central structures may result in vagus nerve (VN) injury due to its location within mediastinum [34]. Anatomically, the left vagus nerve is at risk when left upper lobe lesions are treated given its close association with the lung parenchyma, while in the right hemithorax, the VN branches into the right recurrent laryngeal nerve (RLN), where it is at risk for injury as it drapes over the apical pleura and courses back toward the tracheoesophageal groove [34]. While data from Shultz et al. suggest that generally both VN and RecLN are relatively resistant to SBRT, 2 of 12 (17%) patients who received significant doses to either of these structures ultimately presented with observable nerve injury in the form of vocal cord paralysis from ipsilateral VN and RecLN injury (Table 1). Although no clear dose response was found, possible predisposing factors to toxicity include high cumulative doses due to re-irradiation in one patient, and pre-existing connective tissue disease (rheumatoid arthritis) in increased radiation sensitivity in another patient [34].
11. Ways to Avoid Complications
11.1. Patient Selection
The initial patient evaluation should include an evaluation of the patient’s functional status and any comorbid conditions that may impact the safety and efficacy of SBRT. Specifically, patients should be evaluated both functionally and radiographically for baseline pulmonary abnormalities, including IP shadows on pretreatment CT scans [66]. Although not used in routine practice, consideration may be given to evaluation of serum KL-6 and SP-D levels both before and after SBRT to identify those at higher risk for complications [67]. Although these markers await prospective validation, Yamashita et al. was able to show a reduction in pneumonitis risk (18.8% to 3.5% grade 4–5 RP) when patients with elevated levels of KL-6 and SP-D and obvious interstitial pneumonitis (IP) shadows on CT were excluded from receiving SBRT [66]. Additionally, practitioners should be aware of the timing of SBRT relative to any chemotherapy, where added caution may be necessary in the SBRT planning phase to avoid toxicity [28].
11.2. Simulation/Motion Management
In general, a very robust immobilization system [14] and an accurate pretreatment verification of PTV positioning and delivery of SBRT [57,97] are crucial in the prevention of inadvertent overdosing of OARs, though unnecessary immobilization may lead to added skin toxicity and thus should be avoided [92,98]. Respiration management can reduce the internal target volume (ITV) and consequently the normal tissue irradiated [65], while minimization of target uncertainty with techniques such as active breath control (ABC), cone-beam CT [99], and/or respiratory gating [100] can decrease PTV margins and thus toxicities associated with chest wall, mediastinal, and other soft tissue structures.
11.3. Treatment Planning
11.3.1. Dose/Fractionation
Local control of lung tumors generally requires a biologically effective dose (BED) of at least 100 Gy [21,101,102,103,104]. Therefore, centrally located tumors require modulation of the dose size and/or fractionation to allow for safe delivery without increased toxicity [97]. Less intensive fractionation schemes for SBRT or risk-adapted approaches have been advocated regardless of tumor location within the chest [44,56,103,105,106,107,108]. Currently, 50 Gy delivered over five fractions has been widely adopted for the treatment of centrally located tumors [22,109], although more protracted and presumably safer regimens have been explored by several institutions [90,97,110]. As efforts to reduce toxicity may lead to unexpected changes in local control [22,24,101,103,111], cooperative group trials, such as Radiation Therapy Oncology Group (RTOG) 0813 [91], 0915 [112], and 0236 [113], have looked to build off of previous experiences by clearly defining the efficacy of SBRT regimens, while prospectively evaluating dose constraints and consequent toxicity with various fractionation schemes (Table 3).
Table 3.
Published stereotactic body radiotherapy (SBRT) dose constraints.
| Organ | Endpoint (≥Grade 3) | Dosimetric Constraints | Fractions | Prescription Dose (Gy) | Reference | |
|---|---|---|---|---|---|---|
| Volume | Constraint | |||||
| Esophagus | Stenosis/Fistula | <5 cc | 11.9 Gy | 1 | 34 | RTOG 0915 [112] |
| Max Point Dose | 15.4 Gy | |||||
| Max Point Dose | 27 Gy | 3 | 60 | RTOG 0236 [113] | ||
| <5 cc | 18.8 Gy | 4 | 48 | RTOG 0915 [112] | ||
| Max Point Dose | 30 Gy | |||||
| <5 cc | 27.5 Gy | 5 | 40–60 | RTOG 0813 [91] | ||
| Max Point Dose | 105% of PTV Prescription | |||||
| Brachial Plexus | Neuropathy | <3 cc | 14 Gy | 1 | 34 | RTOG 0915 [112] |
| Max Point Dose | 17.5 Gy | |||||
| Max Point Dose | 24 Gy | 3 | 60 | RTOG 0236 [113] | ||
| <3 cc | 23.6 Gy | 4 | 48 | RTOG 0915 [112] | ||
| Max Point Dose | 27.2 Gy | |||||
| <3 cc | 30 Gy | 5 | 40–60 | RTOG 0813 [91] | ||
| Max Point Dose | 32 Gy | |||||
| Great Vessels | Aneurysm | <10 cc | 31 Gy | 1 | 34 | RTOG 0915 [112] |
| Max Point Dose | 37 Gy | |||||
| <10 cc | 43 Gy | 4 | 48 | RTOG 0915 [112] | ||
| Max Point Dose | 49 Gy | |||||
| <10 cc | 47 Gy | 5 | 40–60 | RTOG 0813 [91] | ||
| Max Point Dose | 105% of PTV Prescription | |||||
| Trachea and Large Bronchus | Stenosis/Fistula | <4 cc | 10.5 Gy | 1 | 34 | RTOG 0915 [112] |
| Max Point Dose | 20.2 Gy | |||||
| Max Point Dose | 30 Gy | 3 | 60 | RTOG 0236 [113] | ||
| <4 cc | 15.6 Gy | 4 | 48 | RTOG 0915 [112] | ||
| Max Point Dose | 34.8 Gy | |||||
| <4 cc | 18 Gy | 5 | 40–60 | RTOG 0813 [91] | ||
| Max Point Dose | 105% of PTV Prescription | |||||
| Rib | Pain or Fracture | <1 cc | 22 Gy | 1 | 34 | RTOG 0915 [112] |
| Max Point Dose | 30 Gy | |||||
| <1 cc | 32 Gy | 4 | 48 | RTOG 0915 [112] | ||
| Max Point Dose | 40 Gy | |||||
| Skin | Ulceration | <10 cc | 23 Gy | 1 | 34 | RTOG 0915 [112] |
| Max Point Dose | 26 Gy | |||||
| <10 cc | 33.2 Gy | 4 | 48 | RTOG 0915 [112] | ||
| Max Point Dose | 40 Gy | |||||
| <10 cc | 30 Gy | 5 | 40–60 | RTOG 0813 [91] | ||
| Max Point Dose | 32 Gy | |||||
| Lung | Basic Lung Function | 1500 cc | 7 Gy | 1 | 34 | RTOG 0915 [112] |
| 1500 cc | 11.6 Gy | 4 | 48 | RTOG 0915 [112] | ||
| 1500 cc | 12.5 Gy | 5 | 40–60 | RTOG 0813 [91] | ||
| Pneumonitis | 1000 cc | 7.4 Gy | 1 | 34 | RTOG 0915 [112] | |
| 1000 cc | 12.4 Gy | 4 | 48 | RTOG 0915 [112] | ||
| 1000 cc | 13.5 Gy | 5 | 40–60 | RTOG 0813 [91] | ||
Abbreviations: PTV, planning target volume; RTOG, Radiation Therapy Oncology Group.
11.3.2. Target Delineation
All at-risk structures/organs should be contoured individually, with planning organ at risk volume (PRV) margins allowed for intrafraction movement, to avoid preventable complications [35,57,59,92,98]. When possible, care should be taken to avoid including immediately adjacent OARs in the target volume [114]. In general, parallel OARs require a determination of the critical organ volume and threshold dose required to avoid end-organ damage [57], whereas for serial OARs maximum point doses are of more clinical concern, and more protracted regimens may be required to avoid catastrophic complications [57].
11.3.3. Plan Optimization/Beam Arrangement/Weighting
Dose distributions can be altered at the time of plan optimization to reduce doses to at-risk structures. Use of SBRT beam angle/weighting optimization with tight aperture margins is crucial for creating a sharp dose gradient that provides adequate target coverage while avoiding overdosing critical structures [97]. Planning techniques, such as using RapidArc treatment delivery, increasing the number of non-coplanar beam angles, and/or using alternative beam arrangements and beam weighting, have been shown to reduce dose to the chest wall toxicity [83,89,98,115]. Use of alternative dose constraints should also be considered, including the CI and R50% (defined as a ratio of the 50% prescription IDL to PTV volume), which have been recently adopted by the RTOG in their most recent protocols to facilitate plan optimization [116].
Caution must be exercised when using published dose constraints, as uncertainty exists regarding the effect of dose and fractionation on toxicity such that tolerances may not be readily extrapolated from the conventionally fractionated to the hypofractionated setting [23]. In addition, practitioners must be aware of the curative potential of early stage lesions treated with SBRT, as the risk of low grade toxicity may not warrant reduction in target SBRT doses [23]. With increasing application of SBRT in the central lung region, improved delineation of risk will be necessary to prevent avoidable complications [23].
Moreover, advances in plan optimization may also improve outcomes while minimizing toxicity. With a technique developed by Dong et al., the highly non-coplanar 4π planning algorithm utilizes simultaneous optimization of PTVs and OARs, including specific dose constraints for lung V20, V10, and V5 to arrive at a final plan. Using this technique, improvements were seen compared to standard IMRT and VMAT plans, with improved target dose coverage and reduced doses to at OARs, allowing for potential dose escalation [117].
11.3.4. Treatment Delivery
Lastly, new technologies in treatment delivery also hold the potential for reducing radiation toxicities. Real-time tumor tracking via 4D SBRT may allow better dose delivery to the tumor as it moves throughout respiration, thus reducing ITV and PTV margins, and consequently adjacent normal tissue dose [118]. Helical tomotherapy (HT) may improve OAR sparing for those in very close proximity to the PTV, as is the case with central lung tumors [119]. Additionally, flattening filter-free (FFF) linear accelerators, capable of delivering dose rates up to four times that of conventional linear accelerators, may allow for shorter treatment delivery times and thus reduce the opportunity for intrafraction motion [120] and exposure of normal tissue to scattered dose outside the field [121]. Volume-adapted dosing strategies hold promise for decreased radiotherapy intensity for smaller tumors with similar tumor control, and consequently, reduced dose to OARs [122].
12. Conclusions
SBRT has become a standard treatment option for early stage, node negative NSCLC, largely due to high local control rates and favorable toxicity profile compared with both surgical and non-surgical approaches. With excellent tumor control rates, the recent focus of lung SBRT strategies has been to limit toxicity while treating increasingly complex patients with a diverse array of presentations. Improvements in our understanding of patient-specific risk factors and prognosticators of outcomes will play a larger role in evaluating patients for SBRT candidacy, as well as stratifying those amenable to escalation and de-escalation of treatment. Traditional and novel dosimetric constraints will continue to be redefined, particularly in the re-irradiation setting, as improvements are made in our understanding of critical organ tolerances and dose-volume thresholds required to deliver safe treatments. Developments in radiation planning and delivery are also expected, which will complement developments in our understanding of the radiobiology of hypofractionated ablative radiotherapy on both tumor and normal tissue. Although we expect larger, prospective multi-institutional experiences to validate previous work, broaden our understanding, and present new challenges, at present it remains imperative to recognize current toxicity limitations of SBRT and treat patients accordingly.
Author Contributions
Kylie H. Kang has contributed to the collection of literature and writing of the manuscript. Christian C. Okoye and Ravi B. Patel have contributed to the collection of literature, writing, and review of the manuscript. Shankar Siva, Tithi Biswas, Rodney J. Ellis, Min Yao, and Mitchell Machtay have contributed to the writing and review of the manuscript. Simon S. Lo has contributed to the design, writing, and review of the manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Senan S. Stereotactic body radiotherapy: Do central lung tumors still represent a “no-fly zone”? Onkologie. 2012;35:406–407. doi: 10.1159/000341091. [DOI] [PubMed] [Google Scholar]
- 2.Haffty B.G., Goldberg N.B., Gerstley J., Fischer D.B., Peschel R.E. Results of radical radiation therapy in clinical stage I, technically operable non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 1988;15:69–73. doi: 10.1016/0360-3016(88)90348-3. [DOI] [PubMed] [Google Scholar]
- 3.Gauden S., Ramsay J., Tripcony L. The curative treatment by radiotherapy alone of stage I non-small cell carcinoma of the lung. Chest. 1995;108:1278–1282. doi: 10.1378/chest.108.5.1278. [DOI] [PubMed] [Google Scholar]
- 4.Fang L.C., Komaki R., Allen P., Guerrero T., Mohan R., Cox J.D. Comparison of outcomes for patients with medically inoperable stage I non-small-cell lung cancer treated with two-dimensional vs. Three-dimensional radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006;66:108–116. doi: 10.1016/j.ijrobp.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Rosenzweig K.E., Fox J.L., Yorke E., Amols H., Jackson A., Rusch V., Kris M.G., Ling C.C., Leibel S.A. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer. 2005;103:2118–2127. doi: 10.1002/cncr.21007. [DOI] [PubMed] [Google Scholar]
- 6.Bradley J., Graham M.V., Winter K., Purdy J.A., Komaki R., Roa W.H., Ryu J.K., Bosch W., Emami B. Toxicity and outcome results of rtog 9311: A phase I–II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2005;61:318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 7.Narayan S., Henning G.T., Ten Haken R.K., Sullivan M.A., Martel M.K., Hayman J.A. Results following treatment to doses of 92.4 or 102.9 gy on a phase I dose escalation study for non-small cell lung cancer. Lung Cancer. 2004;44:79–88. doi: 10.1016/j.lungcan.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Barriger R.B., Forquer J.A., Brabham J.G., Andolino D.L., Shapiro R.H., Henderson M.A., Johnstone P.A., Fakiris A.J. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:457–462. doi: 10.1016/j.ijrobp.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman R., Papiez L., McGarry R., Likes L., DesRosiers C., Frost S., Williams M. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–1955. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 10.Timmerman R., McGarry R., Yiannoutsos C., Papiez L., Tudor K., DeLuca J., Ewing M., Abdulrahman R., DesRosiers C., Williams M., et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J. Clin. Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 11.Amini A., Yeh N., Gaspar L.E., Kavanagh B., Karam S.D. Stereotactic body radiation therapy (sbrt) for lung cancer patients previously treated with conventional radiotherapy: A review. Radiat. Oncol. 2014;9 doi: 10.1186/1748-717X-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng X., Schipper M., Kidwell K., Lin J., Reddy R., Ren Y., Chang A., Lv F., Orringer M., Spring Kong F.M. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: A meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2014;90:603–611. doi: 10.1016/j.ijrobp.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree T.D., Denlinger C.E., Meyers B.F., El Naqa I., Zoole J., Krupnick A.S., Kreisel D., Patterson G.A., Bradley J.D. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2010;140:377–386. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 14.Lo S.S., Fakiris A.J., Chang E.L., Mayr N.A., Wang J.Z., Papiez L., Teh B.S., McGarry R.C., Cardenes H.R., Timmerman R.D. Stereotactic body radiation therapy: A novel treatment modality. Nat. Rev. Clin. Oncol. 2010;7:44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 15.Fakiris A.J., McGarry R.C., Yiannoutsos C.T., Papiez L., Williams M., Henderson M.A., Timmerman R. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase ii study. Int. J. Radiat. Oncol. Biol. Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 16.Song S.Y., Choi W., Shin S.S., Lee S.W., Ahn S.D., Kim J.H., Je H.U., Park C.I., Lee J.S., Choi E.K. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer. 2009;66:89–93. doi: 10.1016/j.lungcan.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Le Q.-T., Loo B.W., Ho A., Cotrutz C., Koong A.C., Wakelee H., Kee S.T., Constantinescu D., Whyte R.I., Donington J. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J. Thorac. Oncol. 2006;1:802–809. doi: 10.1097/01243894-200610000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Kelsey C.R., Kahn D., Hollis D.R., Miller K.L., Zhou S.M., Clough R.W., Marks L.B. Radiation-induced narrowing of the tracheobronchial tree: An in-depth analysis. Lung Cancer. 2006;52:111–116. doi: 10.1016/j.lungcan.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Speiser B.L., Spratling L. Radiation bronchitis and stenosis secondary to high dose rate endobronchial irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1993;25:589–597. doi: 10.1016/0360-3016(93)90003-E. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson K., Nyman J., Baumann P., Wersall P., Drugge N., Gagliardi G., Johansson K.A., Persson J.O., Rutkowska E., Tullgren O., et al. Retrospective cohort study of bronchial doses and radiation-induced atelectasis after stereotactic body radiation therapy of lung tumors located close to the bronchial tree. Int. J. Radiat. Oncol. Biol. Phys. 2013;87:590–595. doi: 10.1016/j.ijrobp.2013.06.2055. [DOI] [PubMed] [Google Scholar]
- 21.Rowe B.P., Boffa D.J., Wilson L.D., Kim A.W., Detterbeck F.C., Decker R.H. Stereotactic body radiotherapy for central lung tumors. J. Thorac. Oncol. 2012;7:1394–1399. doi: 10.1097/JTO.0b013e3182614bf3. [DOI] [PubMed] [Google Scholar]
- 22.Corradetti M.N., Haas A.R., Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N. Engl. J. Med. 2012;366:2327–2329. doi: 10.1056/NEJMc1203770. [DOI] [PubMed] [Google Scholar]
- 23.Wu A.J., Williams E., Modh A., Foster A., Yorke E., Rimner A., Jackson A. Dosimetric predictors of esophageal toxicity after stereotactic body radiotherapy for central lung tumors. Radiother. Oncol. 2014;112:267–271. doi: 10.1016/j.radonc.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onimaru R., Shirato H., Shimizu S., Kitamura K., Xu B., Fukumoto S., Chang T.C., Fujita K., Oita M., Miyasaka K., et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int. J. Radiat. Oncol. Biol. Phys. 2003;56:126–135. doi: 10.1016/S0360-3016(03)00095-6. [DOI] [PubMed] [Google Scholar]
- 25.Palma D.A., Senan S., Oberije C., Belderbos J., de Dios N.R., Bradley J.D., Barriger R.B., Moreno-Jimenez M., Kim T.H., Ramella S., et al. Predicting esophagitis after chemoradiation therapy for non-small cell lung cancer: An individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013;87:690–696. doi: 10.1016/j.ijrobp.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Gomez D.R., Hunt M.A., Jackson A., O’Meara W.P., Bukanova E.N., Zelefsky M.J., Yamada Y., Rosenzweig K.E. Low rate of thoracic toxicity in palliative paraspinal single-fraction stereotactic body radiation therapy. Radiother. Oncol. 2009;93:414–418. doi: 10.1016/j.radonc.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmerman R.D. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin. Radiat. Oncol. 2008;18:215–222. doi: 10.1016/j.semradonc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Abelson J.A., Murphy J.D., Loo B.W., Jr., Chang D.T., Daly M.E., Wiegner E.A., Hancock S., Chang S.D., Le Q.T., Soltys S.G., et al. Esophageal tolerance to high-dose stereotactic ablative radiotherapy. Dis. Esophagus. 2012;25:623–629. doi: 10.1111/j.1442-2050.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 29.Werner-Wasik M., Pequignot E., Leeper D., Hauck W., Curran W. Predictors of severe esophagitis include use of concurrent chemotherapy, but not the length of irradiated esophagus: A multivariate analysis of patients with lung cancer treated with nonoperative therapy. Int. J. Radiat. Oncol. Biol. Phys. 2000;48:689–696. doi: 10.1016/S0360-3016(00)00699-4. [DOI] [PubMed] [Google Scholar]
- 30.Scappaticci F.A., Fehrenbacher L., Cartwright T., Hainsworth J.D., Heim W., Berlin J., Kabbinavar F., Novotny W., Sarkar S., Hurwitz H. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J. Surg Oncol. 2005;91:173–180. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 31.Goodgame B., Veeramachaneni N., Patterson A., Govindan R. Tracheo-esophageal fistula with bevacizumab after mediastinal radiation. J. Thorac. Oncol. 2008;3:1080–1081. doi: 10.1097/JTO.0b013e3181858eba. [DOI] [PubMed] [Google Scholar]
- 32. NCT02319889: Pilot Study of SBRT Plus Chemotherapy for Non-Small Cell Lung Carcinoma. [(accessed on 3 January 2015)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02319889.
- 33.Evans J., Gomez D., Amini A., Rebueno N., Allen P., Martel M., Rineer J., Ang K., McAvoy S., Cox J., et al. Aortic dose constraints when reirradiating thoracic tumors. Radiother. Oncol. 2013;106:327–332. doi: 10.1016/j.radonc.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shultz D.B., Trakul N., Maxim P.G., Diehn M., Loo B.W., Jr. Vagal and recurrent laryngeal neuropathy following stereotactic ablative radiation therapy in the chest. Pract. Radiat.Oncol. 2014;4:272–278. doi: 10.1016/j.prro.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Forquer J.A., Fakiris A.J., Timmerman R.D., Lo S.S., Perkins S.M., McGarry R.C., Johnstone P.A. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: Dose-limiting toxicity in apical tumor sites. Radiother. Oncol. 2009;93:408–413. doi: 10.1016/j.radonc.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Ito M., Niho S., Nihei K., Yoh K., Ohmatsu H., Ohe Y. Risk factors associated with fatal pulmonary hemorrhage in locally advanced non-small cell lung cancer treated with chemoradiotherapy. BMC Cancer. 2012;12 doi: 10.1186/1471-2407-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim Y.-H., Kim E.-Y., Ban H.-J., Oh I.-J., Kim K.-S., Kim Y.-C., Ahn S.-J. Risk factors for fatal hemoptysis after concurrent chemoradiation therapy in patients with non-small cell lung carcinoma. Chonnam Med. J. 2010;46:19–24. doi: 10.4068/cmj.2010.46.1.19. [DOI] [Google Scholar]
- 38.Reck M., Barlesi F., Crino L., Henschke C.I., Isla D., Stiebeler S., Spigel D.R. Predicting and managing the risk of pulmonary haemorrhage in patients with nsclc treated with bevacizumab: A consensus report from a panel of experts. Ann. Oncol. 2012;23:1111–1120. doi: 10.1093/annonc/mdr463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chi A., Nguyen N.P., Komaki R. The potential role of respiratory motion management and image guidance in the reduction of severe toxicities following stereotactic ablative radiation therapy for patients with centrally located early stage non-small cell lung cancer or lung metastases. Front. Oncol. 2014;4 doi: 10.3389/fonc.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milano M.T., Chen Y., Katz A.W., Philip A., Schell M.C., Okunieff P. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiother. Oncol. 2009;91:301–306. doi: 10.1016/j.radonc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Oshiro Y., Aruga T., Tsuboi K., Marino K., Hara R., Sanayama Y., Itami J. Stereotactic body radiotherapy for lung tumors at the pulmonary hilum. Strahlenther. Onkol. 2010;186:274–279. doi: 10.1007/s00066-010-2072-y. [DOI] [PubMed] [Google Scholar]
- 42.Ohnishi K., Shioyama Y., Nomoto S., Sasaki T., Ohga S., Yoshitake T., Toba T., Atsumi K., Shiinoki T., Terashima H., et al. Spontaneous pneumothorax after stereotactic radiotherapy for non-small-cell lung cancer. Jpn. J. Radiol. 2009;27:269–274. doi: 10.1007/s11604-009-0333-4. [DOI] [PubMed] [Google Scholar]
- 43.Pezner R.D., Horak D.A., Sayegh H.O., Lipsett J.A. Spontaneous pneumothorax in patients irradiated for hodgkin’s disease and other malignant lymphomas. Int. J. Radiat. Oncol. Biol. Phys. 1990;18:193–198. doi: 10.1016/0360-3016(90)90284-Q. [DOI] [PubMed] [Google Scholar]
- 44.Stephans K.L., Djemil T., Reddy C.A., Gajdos S.M., Kolar M., Machuzak M., Mazzone P., Videtic G.M.M. Comprehensive analysis of pulmonary function test (pft) changes after stereotactic body radiotherapy (sbrt) for stage I lung cancer in medically inoperable patients. J. Thorac. Oncol. 2009;4:838–844. doi: 10.1097/JTO.0b013e3181a99ff6. [DOI] [PubMed] [Google Scholar]
- 45.Henderson M., McGarry R., Yiannoutsos C., Fakiris A., Hoopes D., Williams M., Timmerman R. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage i non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;72:404–409. doi: 10.1016/j.ijrobp.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 46.Guckenberger M., Kestin L.L., Hope A.J., Belderbos J., Werner-Wasik M., Yan D., Sonke J.-J., Bissonnette J.P., Wilbert J., Xiao Y., et al. Is there a lower limit of pretreatment pulmonary function for safe and effective stereotactic body radiotherapy for early-stage non-small cell lung cancer? J. Thorac. Oncol. 2012;7:542–551. doi: 10.1097/JTO.0b013e31824165d7. [DOI] [PubMed] [Google Scholar]
- 47.Ohashi T., Takeda A., Shigematsu N., Kunieda E., Ishizaka A., Fukada J., Deloar H.M., Kawaguchi O., Takeda T., Takemasa K., et al. Differences in pulmonary function before vs. 1 year after hypofractionated stereotactic radiotherapy for small peripheral lung tumors. Int. J. Radiat. Oncol. Biol. Phys. 2005;62:1003–1008. doi: 10.1016/j.ijrobp.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 48.Takeda A., Enomoto T., Sanuki N., Handa H., Aoki Y., Oku Y., Kunieda E. Reassessment of declines in pulmonary function ≥1 year after stereotactic body radiotherapy. Chest. 2013;143:130–137. doi: 10.1378/chest.12-0207. [DOI] [PubMed] [Google Scholar]
- 49.Stanic S., Paulus R., Timmerman R.D., Michalski J., Barriger R.B., Bezjak A., Videtic G.M., Bradley J. No clinically significant changes in pulmonary function following stereotactic body radiation therapy (sbrt) among medically inoperable patients with early stage peripheral non-small cell lung cancer: An analysis of RTOG 0236. Int. J. Radiat. Oncol. Biol. Phys. 2012;84 doi: 10.1016/j.ijrobp.2012.07.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seppenwoolde Y., De Jaeger K., Boersma L.J., Belderbos J.S.A., Lebesque J.V. Regional differences in lung radiosensitivity after radiotherapy for non–small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004;60:748–758. doi: 10.1016/j.ijrobp.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 51.Tucker S.L., Jin H., Wei X., Wang S., Martel M.K., Komaki R., Liu H.H., Mohan R., Chen Y., Cox J.D., et al. Impact of toxicity grade and scoring system on the relationship between mean lung dose and risk of radiation pneumonitis in a large cohort of patients with non–small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010;77:691–698. doi: 10.1016/j.ijrobp.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 52.Nagata Y., Takayama K., Matsuo Y., Norihisa Y., Mizowaki T., Sakamoto T., Sakamoto M., Mitsumori M., Shibuya K., Araki N., et al. Clinical outcomes of a phase I/II study of 48 gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int. J. Radiat. Oncol. Biol. Phys. 2005;63:1427–1431. doi: 10.1016/j.ijrobp.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 53.Yamashita H., Nakagawa K., Nakamura N., Koyanagi H., Tago M., Igaki H., Shiraishi K., Sasano N., Ohtomo K. Exceptionally high incidence of symptomatic grade 2–5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat. Oncol. 2007;2 doi: 10.1186/1748-717X-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guckenberger M., Heilman K., Wulf J., Mueller G., Beckmann G., Flentje M. Pulmonary injury and tumor response after stereotactic body radiotherapy (sbrt): Results of a serial follow-up ct study. Radiother. Oncol. 2007;85:435–442. doi: 10.1016/j.radonc.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 55.Zimmermann F.B., Geinitz H., Schill S., Thamm R., Nieder C., Schratzenstaller U., Molls M. Stereotactic hypofractionated radiotherapy in stage I (t1-2 n0 m0) non-small-cell lung cancer (nsclc) Acta Oncol. 2006;45:796–801. doi: 10.1080/02841860600913210. [DOI] [PubMed] [Google Scholar]
- 56.Guckenberger M., Baier K., Polat B., Richter A., Krieger T., Wilbert J., Mueller G., Flentje M. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother. Oncol. 2010;97:65–70. doi: 10.1016/j.radonc.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 57.Lo S.S., Sahgal A., Chang E.L., Mayr N.A., Teh B.S., Huang Z., Schefter T.E., Yao M., Machtay M., Slotman B.J., et al. Serious complications associated with stereotactic ablative radiotherapy and strategies to mitigate the risk. Clin. Oncol. (R. Coll. Radiol.) 2013;25:378–387. doi: 10.1016/j.clon.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Borst G.R., Ishikawa M., Nijkamp J., Hauptmann M., Shirato H., Onimaru R., van den Heuvel M.M., Belderbos J., Lebesque J.V., Sonke J.J. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiother. Oncol. 2009;91:307–313. doi: 10.1016/j.radonc.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Ong C.L., Palma D., Verbakel W.F., Slotman B.J., Senan S. Treatment of large stage I–II lung tumors using stereotactic body radiotherapy (SBRT): Planning considerations and early toxicity. Radiother. Oncol. 2010;97:431–436. doi: 10.1016/j.radonc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Kyas I., Hof H., Debus J., Schlegel W., Karger C.P. Prediction of radiation-induced changes in the lung after stereotactic body radiation therapy of non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007;67:768–774. doi: 10.1016/j.ijrobp.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 61.Hope A.J., Lindsay P.E., El Naqa I., Alaly J.R., Vicic M., Bradley J.D., Deasy J.O. Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int. J. Radiat. Oncol. Biol. Phys. 2006;65:112–124. doi: 10.1016/j.ijrobp.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 62.Yorke E.D., Jackson A., Rosenzweig K.E., Merrick S.A., Gabrys D., Venkatraman E.S., Burman C.M., Leibel S.A., Ling C.C. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002;54:329–339. doi: 10.1016/S0360-3016(02)02929-2. [DOI] [PubMed] [Google Scholar]
- 63.Tucker S.L., Liao Z.X., Travis E.L. Estimation of the spatial distribution of target cells for radiation pneumonitis in mouse lung. Int. J. Radiat. Oncol. Biol. Phys. 1997;38:1055–1066. doi: 10.1016/S0360-3016(97)00131-4. [DOI] [PubMed] [Google Scholar]
- 64.West J.B. Respiratory Physiology: The Essentials. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2012. [Google Scholar]
- 65.Matsuo Y., Shibuya K., Nakamura M., Narabayashi M., Sakanaka K., Ueki N., Miyagi K., Norihisa Y., Mizowaki T., Nagata Y., et al. Dose-volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:e545–e549. doi: 10.1016/j.ijrobp.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 66.Yamashita H., Kobayashi-Shibata S., Terahara A., Okuma K., Haga A., Wakui R., Ohtomo K., Nakagawa K. Prescreening based on the presence of ct-scan abnormalities and biomarkers (kl-6 and sp-d) may reduce severe radiation pneumonitis after stereotactic radiotherapy. Radiat. Oncol. 2010;5 doi: 10.1186/1748-717X-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iwata H., Shibamoto Y., Baba F., Sugie C., Ogino H., Murata R., Yanagi T., Otsuka S., Kosaki K., Murai T., et al. Correlation between the serum kl-6 level and the grade of radiation pneumonitis after stereotactic body radiotherapy for stage i lung cancer or small lung metastasis. Radiother. Oncol. 2011;101:267–270. doi: 10.1016/j.radonc.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 68.Kong F.M., Ao X., Wang L., Lawrence T.S. The use of blood biomarkers to predict radiation lung toxicity: A potential strategy to individualize thoracic radiation therapy. Cancer Control. 2008;15:140–150. doi: 10.1177/107327480801500206. [DOI] [PubMed] [Google Scholar]
- 69.Hara R., Itami J., Komiyama T., Katoh D., Kondo T. Serum levels of kl-6 for predicting the occurrence of radiation pneumonitis after stereotactic radiotherapy for lung tumors. Chest. 2004;125:340–344. doi: 10.1378/chest.125.1.340. [DOI] [PubMed] [Google Scholar]
- 70.Takeda A., Ohashi T., Kunieda E., Enomoto T., Sanuki N., Takeda T., Shigematsu N. Early graphical appearance of radiation pneumonitis correlates with the severity of radiation pneumonitis after stereotactic body radiotherapy (sbrt) in patients with lung tumors. Int. J. Radiat. Oncol. Biol. Phys. 2010;77:685–690. doi: 10.1016/j.ijrobp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 71.Yamaguchi S., Ohguri T., Ide S., Aoki T., Imada H., Yahara K., Narisada H., Korogi Y. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: The potential risk of extensive radiation pneumonitis. Lung Cancer. 2013;82:260–265. doi: 10.1016/j.lungcan.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 72.Palma D., Lagerwaard F., Rodrigues G., Haasbeek C., Senan S. Curative treatment of stage I non-small-cell lung cancer in patients with severe copd: Stereotactic radiotherapy outcomes and systematic review. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:1149–1156. doi: 10.1016/j.ijrobp.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 73.Kimura T., Matsuura K., Murakami Y., Hashimoto Y., Kenjo M., Kaneyasu Y., Wadasaki K., Hirokawa Y., Ito K., Okawa M. Ct appearance of radiation injury of the lung and clinical symptoms after stereotactic body radiation therapy (SBRT) for lung cancers: Are patients with pulmonary emphysema also candidates for sbrt for lung cancers? Int. J. Radiat. Oncol. Biol. Phys. 2006;66:483–491. doi: 10.1016/j.ijrobp.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 74.Jin H., Tucker S.L., Liu H.H., Wei X., Yom S.S., Wang S., Komaki R., Chen Y., Martel M.K., Mohan R., et al. Dose-volume thresholds and smoking status for the risk of treatment-related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy. Radiother. Oncol. 2009;91:427–432. doi: 10.1016/j.radonc.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takeda A., Kunieda E., Ohashi T., Aoki Y., Oku Y., Enomoto T., Nomura K., Sugiura M. Severe COPD is correlated with mild radiation pneumonitis following stereotactic body radiotherapy. Chest. 2012;141:858–866. doi: 10.1378/chest.11-1193. [DOI] [PubMed] [Google Scholar]
- 76.Bjermer L., Cai Y., Nilsson K., Hellstrom S., Henriksson R. Tobacco smoke exposure suppresses radiation-induced inflammation in the lung: A study of bronchoalveolar lavage and ultrastructural morphology in the rat. Eur. Respir. J. 1993;6:1173–1180. [PubMed] [Google Scholar]
- 77.Bhattathiri V.N. Possible role of plasma GSH in modulating smoking related radiation pneumonitis. Radiother. Oncol. 1999;51:291–292. doi: 10.1016/s0167-8140(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 78.Takeda A., Ohashi T., Kunieda E., Sanuki N., Enomoto T., Takeda T., Oku Y., Shigematsu N. Comparison of clinical, tumour-related and dosimetric factors in grade 0–1, grade 2 and grade 3 radiation pneumonitis after stereotactic body radiotherapy for lung tumours. Br. J. Radiol. 2012;85:636–642. doi: 10.1259/bjr/71635286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stephans K.L., Djemil T., Reddy C.A., Gajdos S.M., Kolar M., Mason D., Murthy S., Rice T.W., Mazzone P., Machuzak M., et al. A comparison of two stereotactic body radiation fractionation schedules for medically inoperable stage I non-small cell lung cancer: The cleveland clinic experience. J. Thorac. Oncol. 2009;4:976–982. doi: 10.1097/JTO.0b013e3181adf509. [DOI] [PubMed] [Google Scholar]
- 80.Andolino D.L., Forquer J.A., Henderson M.A., Barriger R.B., Shapiro R.H., Brabham J.G., Johnstone P.A., Cardenes H.R., Fakiris A.J. Chest wall toxicity after stereotactic body radiotherapy for malignant lesions of the lung and liver. Int. J. Radiat. Oncol. Biol. Phys. 2011;80:692–697. doi: 10.1016/j.ijrobp.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 81.Stephans K.L., Djemil T., Tendulkar R.D., Robinson C.G., Reddy C.A., Videtic G.M. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT) Int. J. Radiat. Oncol. Biol. Phys. 2012;82:974–980. doi: 10.1016/j.ijrobp.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 82.Welsh J., Thomas J., Shah D., Allen P.K., Wei X., Mitchell K., Gao S., Balter P., Komaki R., Chang J.Y. Obesity increases the risk of chest wall pain from thoracic stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:91–96. doi: 10.1016/j.ijrobp.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voroney J.P., Hope A., Dahele M.R., Purdie T.G., Franks K.N., Pearson S., Cho J.B., Sun A., Payne D.G., Bissonnette J.P., et al. Chest wall pain and rib fracture after stereotactic radiotherapy for peripheral non-small cell lung cancer. J. Thorac. Oncol. 2009;4:1035–1037. doi: 10.1097/JTO.0b013e3181ae2962. [DOI] [PubMed] [Google Scholar]
- 84.Mutter R.W., Liu F., Abreu A., Yorke E., Jackson A., Rosenzweig K.E. Dose-volume parameters predict for the development of chest wall pain after stereotactic body radiation for lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:1783–1790. doi: 10.1016/j.ijrobp.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fajardo L.F., Berthrong M., Anderson R.E. Radiation Pathology. Oxford University Press; New York, NY, USA: 2001. Musculoskeletal system; pp. 365–377. [Google Scholar]
- 86.Bongers E.M., Haasbeek C.J., Lagerwaard F.J., Slotman B.J., Senan S. Incidence and risk factors for chest wall toxicity after risk-adapted stereotactic radiotherapy for early-stage lung cancer. J. Thorac. Oncol. 2011;6:2052–2057. doi: 10.1097/JTO.0b013e3182307e74. [DOI] [PubMed] [Google Scholar]
- 87.Creach K.M., El Naqa I., Bradley J.D., Olsen J.R., Parikh P.J., Drzymala R.E., Bloch C., Robinson C.G. Dosimetric predictors of chest wall pain after lung stereotactic body radiotherapy. Radiother. Oncol. 2012;104:23–27. doi: 10.1016/j.radonc.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 88.Pettersson N., Nyman J., Johansson K.A. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy of non-small cell lung cancer: A dose- and volume-response analysis. Radiother. Oncol. 2009;91:360–368. doi: 10.1016/j.radonc.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 89.Dunlap N.E., Cai J., Biedermann G.B., Yang W., Benedict S.H., Sheng K., Schefter T.E., Kavanagh B.D., Larner J.M. Chest wall volume receiving >30 gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010;76:796–801. doi: 10.1016/j.ijrobp.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 90.Li Q., Swanick C.W., Allen P.K., Gomez D.R., Welsh J.W., Liao Z., Balter P.A., Chang J.Y. Stereotactic ablative radiotherapy (SABR) using 70 gy in 10 fractions for non-small cell lung cancer: Exploration of clinical indications. Radiother. Oncol. 2014;112:256–261. doi: 10.1016/j.radonc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 91.Radiation Therapy Oncology Group RTOG 0813: Seamless Phase I/II Study of Stereotactic Lung Radiotherapy (SBRT) for Early Stage, Centrally Located, Non-Small Cell Lung Cancer (NSCLC) in Medically Inoperable Patients. [(accessed on 3 January 2015)]. Available online: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0813.
- 92.Hoppe B.S., Laser B., Kowalski A.V., Fontenla S.C., Pena-Greenberg E., Yorke E.D., Lovelock D.M., Hunt M.A., Rosenzweig K.E. Acute skin toxicity following stereotactic body radiation therapy for stage i non-small-cell lung cancer: Who’s at risk? Int. J. Radiat. Oncol. Biol. Phys. 2008;72:1283–1286. doi: 10.1016/j.ijrobp.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 93.Schierle C., Winograd J.M. Radiation-induced brachial plexopathy: Review. Complication without a cure. J. Reconstr. Microsurg. 2004;20:149–152. doi: 10.1055/s-2004-820771. [DOI] [PubMed] [Google Scholar]
- 94.Ferrante M.A. Brachial plexopathies: Classification, causes, and consequences. Muscle Nerve. 2004;30:547–568. doi: 10.1002/mus.20131. [DOI] [PubMed] [Google Scholar]
- 95.Amini A., Yang J., Williamson R., McBurney M.L., Erasmus J., Jr., Allen P.K., Karhade M., Komaki R., Liao Z., Gomez D., et al. Dose constraints to prevent radiation-induced brachial plexopathy in patients treated for lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:e391–e398. doi: 10.1016/j.ijrobp.2011.06.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kori S.H., Foley K.M., Posner J.B. Brachial plexus lesions in patients with cancer: 100 cases. Neurology. 1981;31:45–50. doi: 10.1212/WNL.31.1.45. [DOI] [PubMed] [Google Scholar]
- 97.Chang J.Y., Li Q.Q., Xu Q.Y., Allen P.K., Rebueno N., Gomez D.R., Balter P., Komaki R., Mehran R., Swisher S.G., et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: How to fly in a “no fly zone”. Int. J. Radiat. Oncol. Biol. Phys. 2014;88:1120–1128. doi: 10.1016/j.ijrobp.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 98.Lo S.S., Fakiris A.J., Wang J.Z., Mayr N.A. In regard to Hoppe et al. (Int. J Radiat. Oncol. Biol. Phys. 2008;72:1283–1286) Int. J. Radiat. Oncol. Biol. Phys. 2009;74:977. doi: 10.1016/j.ijrobp.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 99.Wang X., Zhong R., Bai S., Xu Q., Zhao Y., Wang J., Jiang X., Shen Y., Xu F., Wei Y. Lung tumor reproducibility with active breath control (ABC) in image-guided radiotherapy based on cone-beam computed tomography with two registration methods. Radiother. Oncol. 2011;99:148–154. doi: 10.1016/j.radonc.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 100.Guckenberger M., Kavanagh A., Webb S., Brada M. A novel respiratory motion compensation strategy combining gated beam delivery and mean target position concept—A compromise between small safety margins and long duty cycles. Radiother. Oncol. 2011;98:317–322. doi: 10.1016/j.radonc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 101.Taremi M., Hope A., Dahele M., Pearson S., Fung S., Purdie T., Brade A., Cho J., Sun A., Bissonnette J.P., et al. Stereotactic body radiotherapy for medically inoperable lung cancer: Prospective, single-center study of 108 consecutive patients. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:967–973. doi: 10.1016/j.ijrobp.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 102.Onishi H., Araki T., Shirato H., Nagata Y., Hiraoka M., Gomi K., Yamashita T., Niibe Y., Karasawa K., Hayakawa K., et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a japanese multiinstitutional study. Cancer. 2004;101:1623–1631. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 103.Chang J.Y., Balter P.A., Dong L., Yang Q., Liao Z., Jeter M., Bucci M.K., McAleer M.F., Mehran R.J., Roth J.A., et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;72:967–971. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nuyttens J.J., van der Voort van Zyp N.C., Praag J., Aluwini S., van Klaveren R.J., Verhoef C., Pattynama P.M., Hoogeman M.S. Outcome of four-dimensional stereotactic radiotherapy for centrally located lung tumors. Radiother. Oncol. 2012;102:383–387. doi: 10.1016/j.radonc.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 105.Lagerwaard F.J., Haasbeek C.J., Smit E.F., Slotman B.J., Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:685–692. doi: 10.1016/j.ijrobp.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 106.Onishi H., Shirato H., Nagata Y., Hiraoka M., Fujino M., Gomi K., Niibe Y., Karasawa K., Hayakawa K., Takai Y., et al. Hypofractionated stereotactic radiotherapy (hypofxsrt) for stage I non-small cell lung cancer: Updated results of 257 patients in a japanese multi-institutional study. J. Thorac. Oncol. 2007;2:S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 107.Uematsu M., Shioda A., Suda A., Fukui T., Ozeki Y., Hama Y., Wong J.R., Kusano S. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: A 5-year experience. Int. J. Radiat. Oncol. Biol. Phys. 2001;51:666–670. doi: 10.1016/S0360-3016(01)01703-5. [DOI] [PubMed] [Google Scholar]
- 108.Senan S., Haasbeek N.J., Smit E.F., Lagerwaard F.J. Stereotactic radiotherapy for centrally located early-stage lung tumors. J. Clin. Oncol. 2007;25:464. doi: 10.1200/JCO.2006.09.8178. [DOI] [PubMed] [Google Scholar]
- 109.Olsen J.R., Robinson C.G., El Naqa I., Creach K.M., Drzymala R.E., Bloch C., Parikh P.J., Bradley J.D. Dose-response for stereotactic body radiotherapy in early-stage non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:e299–e303. doi: 10.1016/j.ijrobp.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 110.Haasbeek C.J., Lagerwaard F.J., Slotman B.J., Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J. Thorac. Oncol. 2011;6:2036–2043. doi: 10.1097/JTO.0b013e31822e71d8. [DOI] [PubMed] [Google Scholar]
- 111.Guckenberger M., Wulf J., Mueller G., Krieger T., Baier K., Gabor M., Richter A., Wilbert J., Flentje M. Dose-response relationship for image-guided stereotactic body radiotherapy of pulmonary tumors: Relevance of 4d dose calculation. Int. J. Radiat. Oncol. Biol. Phys. 2009;74:47–54. doi: 10.1016/j.ijrobp.2008.06.1939. [DOI] [PubMed] [Google Scholar]
- 112.Radiation Therapy Oncology Group RTOG 0915: A Randomized Phase ii Study Comparing 2 Stereotactic Body Radiation Therapy (SBRT) Schedules for Medically Inoperable Patients with Stage I Peripheral Non-Small Cell Lung Cancer. [(accessed on 3 January 2015)]. Available online: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0915. [DOI] [PMC free article] [PubMed]
- 113.Radiation Therapy Oncology Group RTOG 0236: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I/II Non-Small Cell Lung Cancer. [(accessed on 3 January 2015)]. Available online: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0236.
- 114.Nagata Y., Wulf J., Lax I., Timmerman R., Zimmermann F., Stojkovski I., Jeremic B. Stereotactic radiotherapy of primary lung cancer and other targets: Results of consultant meeting of the international atomic energy agency. Int. J. Radiat. Oncol. Biol. Phys. 2011;79:660–669. doi: 10.1016/j.ijrobp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 115.Verbakel W.F., Senan S., Cuijpers J.P., Slotman B.J., Lagerwaard F.J. Rapid delivery of stereotactic radiotherapy for peripheral lung tumors using volumetric intensity-modulated arcs. Radiother. Oncol. 2009;93:122–124. doi: 10.1016/j.radonc.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 116.Yang J., Fowler J.F., Lamond J.P., Lanciano R., Feng J., Brady L.W. Red shell: Defining a high-risk zone of normal tissue damage in stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2010;77:903–909. doi: 10.1016/j.ijrobp.2009.12.069. [DOI] [PubMed] [Google Scholar]
- 117.Dong P., Lee P., Ruan D., Long T., Romeijn E., Low D.A., Kupelian P., Abraham J., Yang Y., Sheng K. 4pi noncoplanar stereotactic body radiation therapy for centrally located or larger lung tumors. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:407–413. doi: 10.1016/j.ijrobp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 118.Prevost J.B., Voet P., Hoogeman M., Praag J., Levendag P., Nuyttens J.J. Four-dimensional stereotactic radiotherapy for early stage non-small cell lung cancer: A comparative planning study. Technol. Cancer Res. Treat. 2008;7:27–33. doi: 10.1177/153303460800700103. [DOI] [PubMed] [Google Scholar]
- 119.Chi A., Ma P., Fu G., Hobbs G., Welsh J.S., Nguyen N.P., Jang S.Y., Dai J., Jin J., Komaki R. Critical structure sparing in stereotactic ablative radiotherapy for central lung lesions: Helical tomotherapy vs. Volumetric modulated arc therapy. PLoS ONE. 2013;8:e59729. doi: 10.1371/journal.pone.0059729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Prendergast B.M., Dobelbower M.C., Bonner J.A., Popple R.A., Baden C.J., Minnich D.J., Cerfolio R.J., Spencer S.A., Fiveash J.B. Stereotactic body radiation therapy (SBRT) for lung malignancies: Preliminary toxicity results using a flattening filter-free linear accelerator operating at 2400 monitor units per minute. Radiat. Oncol. 2013;8 doi: 10.1186/1748-717X-8-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kragl G., Baier F., Lutz S., Albrich D., Dalaryd M., Kroupa B., Wiezorek T., Knoos T., Georg D. Flattening filter free beams in sbrt and imrt: Dosimetric assessment of peripheral doses. Z. Med. Phys. 2011;21:91–101. doi: 10.1016/j.zemedi.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 122.Trakul N., Chang C.N., Harris J., Chapman C., Rao A., Shen J., Quinlan-Davidson S., Filion E.J., Wakelee H.A., Colevas A.D., et al. Tumor volume-adapted dosing in stereotactic ablative radiotherapy of lung tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012;84:231–237. doi: 10.1016/j.ijrobp.2011.10.071. [DOI] [PubMed] [Google Scholar]