Significance
Epidermis, the outer layer of skin, is a protective barrier and a sensing interface. Although deviation of the ambient temperature is one of the most ubiquitous stimuli affecting the skin, the influence of mild cold on epidermal homeostasis is not well understood. Using a large range of techniques, we identified a novel mild-cold sensor protein in keratinocytes and demonstrate its location in the membrane of the endoplasmic reticulum, a major calcium store of the cell, which forms a Ca2+-permeable ion channel. Activation of this channel links the Ca2+ release to mitochondrial Ca2+ uptake and, thereby, modulates synthesis of ATP and superoxide involved in control of epidermal homeostasis. Molecular inactivation of this mild-cold sensor protein in mice impairs normal epidermal homeostasis.
Keywords: eTRPM8, cold, epidermal homeostasis, calcium, mitochondria bioenergetics
Abstract
Deviation of the ambient temperature is one of the most ubiquitous stimuli that continuously affect mammals’ skin. Although the role of the warmth receptors in epidermal homeostasis (EH) was elucidated in recent years, the mystery of the keratinocyte mild-cold sensor remains unsolved. Here we report the cloning and characterization of a new functional epidermal isoform of the transient receptor potential M8 (TRPM8) mild-cold receptor, dubbed epidermal TRPM8 (eTRPM8), which is localized in the keratinocyte endoplasmic reticulum membrane and controls mitochondrial Ca2+ concentration ([Ca2+]m). In turn, [Ca2+]m modulates ATP and superoxide () synthesis in a cold-dependent manner. We report that this fine tuning of ATP and levels by cooling controls the balance between keratinocyte proliferation and differentiation. Finally, to ascertain eTRPM8’s role in EH in vivo we developed a new functional knockout mouse strain by deleting the pore domain of TRPM8 and demonstrated that eTRPM8 knockout impairs adaptation of the epidermis to low temperatures.
The skin epidermis provides a protective barrier that guards the body against an uncongenial environment. Under the influence of a variety of ambient factors the skin epidermis undergoes continuous regeneration through so-called epidermal homeostasis (EH): the fine-tuning of the balance between proliferation, directional migration, differentiation, and death of keratinocytes. EH involves complex molecular and chemical pathways, regulating dynamic and continuous transition of keratinocytes from the proliferating state in the basal layer to the nonproliferating state in the suprabasal layer before the beginning of the differentiation in the stratum spinosum and stratum granulosum. The terminal differentiation step, characterized by keratinocyte death, transforms keratinocytes into corneocytes, which form the waterproof, mechanically resistant sheath of the stratum corneum (1).
Deviation of the ambient temperature is one of the most important stimuli that constantly affect mammals’ skin. At ambient temperatures from +10 °C to +30 °C, the unprotected human skin temperature settles at mean steady-state values within the range of +24 °C to +33 °C, respectively (2). Temperature is perceived by thermoreceptors, the ion channels that belong to the transient receptor potential (TRP) superfamily (for review see ref. 3). Of these, TRPV1 and TRPV2 are activated by heat (above 42 °C and above 52 °C, respectively) (4), whereas TRPM8 and likely TRPA1 are activated by mild (5, 6) and noxious (7–9) cold, respectively. Heat-stimulated keratinocytes have been shown to secrete ATP (10) and, taking into account that purinergic receptors are expressed in keratinocytes (11), TRPV3 is involved in a paracrine heat-induced regulator of EH. Surprisingly, Grifford et al. recently reported that local heating of human skin does not result in accumulation of interstitial ATP (12), which refutes the significance of TRPV thermoreceptors in normal temperature-dependent EH. TRPV3 also was suggested to be important for corneocyte formation through Ca2+-dependent activation of cross-linking enzymes such as transglutaminase (13–15). Apart from heat-activated TRP, no successful attempt to elucidate the role of cold-sensitive TRP channels in EH has been reported yet. The range of thermoactivation of TRPM8 channel fits well with human unprotected skin temperature, +24 °C to +33 °C (5, 6). Apart from the observation that topical application of TRPM8 chemical agonists can improve epidermal regeneration (16), no solid evidence for the expression of functional TRPM8 in epidermal keratinocytes has been presented yet and no alterations in epidermal homeostasis have been reported in trpm8−/− null mutant mice suppressing the full-length TRPM8 cold receptor (17, 18). However, suppression of the full-length TRPM8 expression does not necessarily affect expression of TRPM8 isoforms (19).
Here we report the cloning and characterization of a new four-transmembrane domain epidermal isoform of the TRPM8 cold receptor channel, dubbed epidermal TRPM8 (eTRPM8). We demonstrate that eTRPM8 is localized and functions in the keratinocyte endoplasmic reticulum (ER) membrane where its activation within ER–mitochondria contact sites sustains mitochondrial Ca2+ uptake, thus affecting mitochondrial Ca2+ concentration ([Ca2+]m). In turn, [Ca2+]m modulates ATP and superoxide () synthesis in a cold-dependent manner. We report that this fine-tuning of ATP and levels by cooling temperatures controls the balance between proliferation and differentiation of keratinocytes. Finally, to ascertain eTRPM8’s role in EH in vivo we developed a new functional knockout (KO) mouse line by deleting the active pore domain in all TRPM8 channel isoforms and demonstrated that eTRPM8 knockout impairs the epidermis adaptation to low temperatures and general skin homeostasis.
Results and Discussion
Novel TRPM8 Isoform Identified in Human Keratinocytes Is Composed of Four Transmembrane Domains.
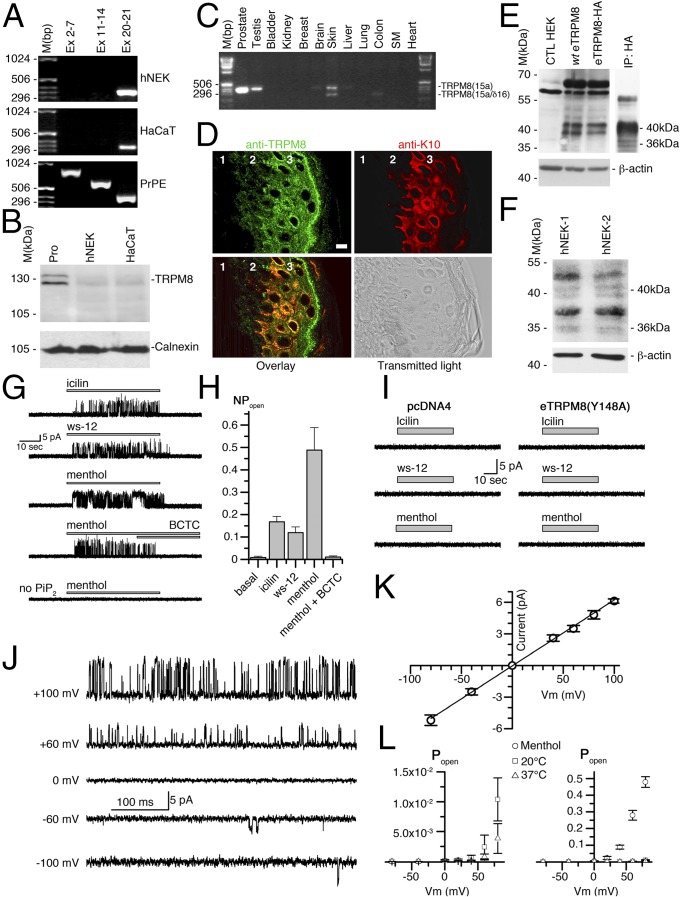
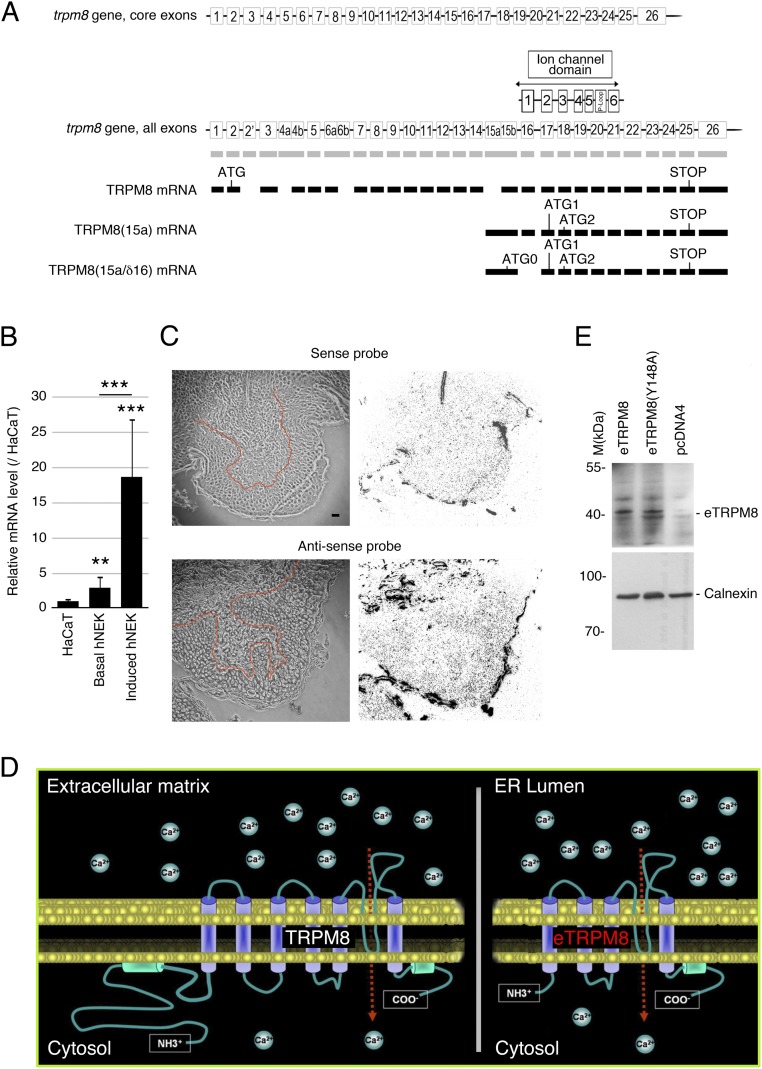
Using differential PCR screenings to characterize TRPM8 mRNA in human keratinocytes, we found that in both the HaCaT human keratinocyte cell line and human normal epidermal keratinocytes (hNEKs) TRPM8 mRNA is 5′ truncated. Indeed, in both cell types, PCR analysis reported the product of amplification of the pore-encoding mRNA region, but not exons 2–7 or exons 11–14 in the 5′ region of TRPM8 mRNA (Fig. 1A). Controls on primary prostate epithelial cells (PrPEs) were positive for all three regions tested, which validates the primers used for the detection of the classical TRPM8 mRNA. This demonstrates that the classical full-length TRPM8 is not expressed in keratinocytes. This conclusion was confirmed further by Western blot analysis that demonstrates the classical TRPM8 (the 130-kDa doublet) in total protein extract from human prostate, but not from hNEK and HaCaT cells (Fig. 1B). The 5′-RACE PCR, performed from pore-encoding exon 20, revealed a new alternate starting exon, labeled 15a. It was located upstream and was adjacent to core exon 15, which was renamed 15b, respectively (Fig. S1A). Internal quality control for the RACE PCR was performed with the cloning of the 5′ extremity of classical TRPM8 mRNA in human prostate. We succeeded in amplifying 2,900 bp (GenBank accession no. KC692993) and improved the definition of the first transcribed nucleotide (+1) of the TRPM8 mRNA, because we detected 10 additional bases upstream of the published +1 (NM_024080.4). This makes the new +1 nucleotide located 25 bases downstream of the TATAA box. Although the previously published +1 nucleotide was identified as located 35 bases downstream (20), this does not match the consensus distance between the TATAA box and +1 (from 15 bp to 25 bp). As presented in Fig. S1A, two sequences were cloned from hNEK mRNA: an alternate TRPM8 mRNA from exon 15a to exon 26, labeled TRPM8(15a) (GenBank accession no. KC692994), and a splice variant of this alternate mRNA with skipping of exon 16, labeled TRPM8(15a/δ16) (GenBank accession no. KC692995). TRPM8(15a) was found in human prostate, testis, skin, and brain, whereas TRPM8(15a/δ16) was detected in human skin and colon (Fig. 1C). Quantification of TRPM8(15a) mRNAs in keratinocytes demonstrated a similarly low level of the mRNAs in the HaCaT cell line and basal hNEKs, whereas induction of hNEKs (Materials and Methods) was associated with a significant increase in the level of TRPM8(15a) mRNAs (Fig. S1B). Furthermore, we verified eTRPM8 expression in human epidermis with in situ hybridization and immunohistofluorescence. In situ hybridization showed a positive detection of the pore-encoding region in epidermis, specifically with the antisense probe (Fig. S1C). Immunodetection of eTRPM8 with the antibody against the P loop of the TRPM8 channel confirmed epidermis-specific expression of eTRPM8 showing a marked increase of the channel expression in differentiated layers of epidermis (Fig. 1D).
Fig. 1.
Trpm8 gene encodes alternate TRPM8 mRNA variants and their associated proteins in human keratinocytes. (A) Representative PCR fingerprinting (n = 5) revealed expression of the pore-encoding region (exons 20 and 21) in the keratinocyte HaCaT cell line, in human normal epidermal keratinocytes (hNEK), and in primary culture of human prostate epithelial (PrPE) cells. Note that segments from exons 2–7 and from exons 11–14, encoding the cytosolic N terminus of the TRPM8 channel, were detected in PrPE cells but not in keratinocytes. (B) Representative immunoblotting (n = 3) of 100 µg total protein extracts from human prostate (Pro), human normal epidermal keratinocytes (hNEK), and HaCaT cell line. A classical full-length TRPM8 channel (126–128 kDa) was detected with rabbit anti-TRPM8 antibody (Alomone Laboratories; batch from 2009). Calnexin protein was used as a reporter of equal loading. (C) Full-length PCR illustrates human tissue profiling of alternate TRPM8(15a) transcripts (n = 3). (D) The gallery shows fluorescent confocal images of human female breast skin sections (n = 3) with immunostained eTRPM8 (Upper Left, green) and keratin 10 (Upper Right, red) and their overlay (Lower Left) and corresponding transmitted light image (Lower Right). Numbers on the images depict the following: 1, dermis; 2, basal layer of epidermis; and 3, spinal and granular layers of epidermis. (Scale bar: 5 µm.) (E) Wild-type eTRPM8 and HA-tagged eTRPM8 (WT eTRPM8 and eTRPM8-HA, respectively), detected with anti-TRPM8 antibody in total protein extract from HEK cells, show a strong doublet at 39–40 kDa and much weaker doublet at 35–36 kDa. β-actin was used as a control of the protein loading. Immunoprecipitation with anti-HA antibody followed by immunoblotting with anti-TRPM8 antibody (E, Right, IP: HA) confirms specificity of both doublets and invalidates nonspecific bands between 55 kDa and 70 kDa. The same results were obtained in three independent samples. (F) Native eTRPM8 protein detected with anti-TRPM8 immunoblotting in two independent samples (hNEK-1 and -2) of the induced hNEKs. β-actin was used as a control of the protein loading. (G and H) Sample activity of eTRPM8 in response to application of 10 µM icilin, 500 nM ws-12, and 200 µM menthol, as well as inhibition by 10 μM BCTC, with levels of eTRPM8 activity summarized in H. (I) Typical records upon application of icilin, ws-12, and menthol to liposomes prepared from cells expressing the nonfunctional pore mutant eTRPM8(Y148A) or transfected with empty vector (pcDNA44). (J) Sample activity of ws-12–activated eTRPM8 at different potentials, indicated at left. Note the characteristic dependence of open probability on the command potential. (K) Dependence of the amplitude on command voltage. Straight line presents a linear fit yielding the mean channel conductance of 63.1 ± 2.4 pS. (L) Comparison of Popen vs. voltage curves at 20 °C and 37 °C (Left) and basal and menthol stimulated at 20 °C (Right). Individual points show mean ± SEM values at the indicated voltages.
Fig. S1.
TRPM8 mRNA variants encode a four-transmembrane domain monomer. (A) Not-to-scale genomic structure of the trpm8 gene aligned with the exonic structure of classical TRPM8, TRPM8(15a), and TRPM8(15a/δ16) mRNAs. Transmembrane domains and the P-loop segment are positioned in accordance with their DNA-encoding sequences. The putative first ATG codon and STOP codon are presented. (B) Real-time PCR compares quantity of TRPM8(15a) mRNA in HaCaT cells and in basal or induced (Materials and Methods) hNEKs (n = 3). (C) Expression of eTRPM8 mRNA in human female breast skin sections (n = 3) detected with in situ hybridization using a either an antisense probe targeting the pore region of TRPM8 (C, Upper Right) or its sense counterpart (C, Lower Right). The boundary between epidermis and dermis is outlined (red) in the transmitted light images of the skin sections (C, Upper Left and Lower Left). (Scale bar: 10 µm.) (D) Schematic representation of the predicted tertiary structure of TRPM8 and eTRPM8 monomers and their cellular location. (E) Western blot showing the presence of eTRPM8 and nonfunctional mutant, eTRPM8(Y148A) in ER membrane-enriched extracts of transfected HEK cells. Control experiment was achieved by the transfection of an empty vector in HEK cells. eTRPM8 was detected at the expected size of 40 kDa. Internal control for protein amount was performed through the detection of calnexin.
Surprisingly, the putative ORFs of the two mRNAs were quite similar; however, TRPM8(15a/δ16) revealed an additional longer ORF (Fig. S1A). After quantification of TRPM8(15a) mRNA and TRPM8(15a/δ16) mRNA intensities with a gel imager, TRPM8(15a) in skin was about four times more expressed than its splice form, and we focused on TRPM8(15a) and defined its putative protein as epidermal TRPM8 (eTRPM8). Note that 5′ truncation removes the first 14 exons (Fig. S1A), which indicates that the two longest, putative ORFs encode four-transmembrane domain (4-TD) proteins, including TD3–6 and the P-loop–forming pore segment, with molecular masses of 40.94 kDa and 35.77 kDa, respectively (Fig. S1D). Immunoblotting of wild-type (WT) eTRPM8 or HA-tagged eTRPM8 expressed in HEK cells revealed a major 39- to 40-kDa doublet and a minor 35- to 36-kDa doublet (Fig. 1E), which are referred to as eTRPM8-40 and eTRPM8-36, respectively. Native eTRPM8 expression was confirmed in two independent hNEK samples (Fig. 1F) and it should be noted that a rather low level of protein expression correlates with a low level of eTRPM8 mRNA in keratinocytes.
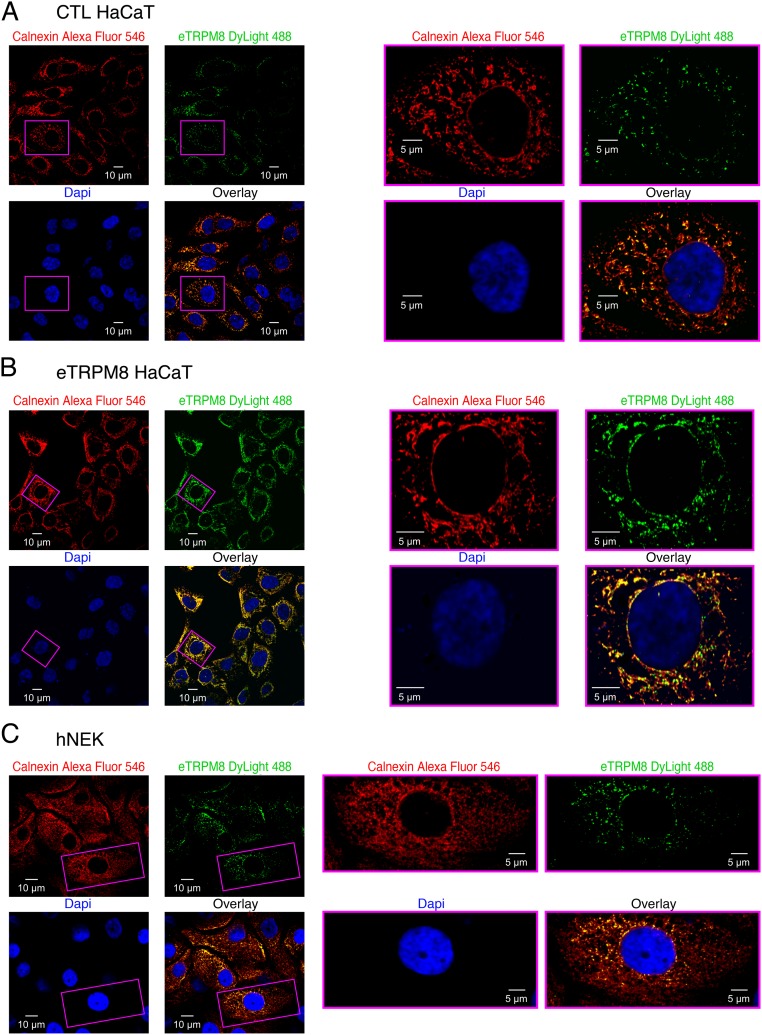
Cold (<32 °C) and cooling agents, such as menthol and icilin, activate the TRPM8 channel (5, 6). Although the precise position of the menthol and icilin binding sites in classical TRPM8 is not yet clearly identified, there is growing evidence that both agonists interact with transmembrane domains 3 and 4 of the channel (reviewed in ref. 21). This indirectly suggests that both agonists may bind to eTRPM8. In addition, because the cold sensor domain is located in the carboxy-terminus of classical TRPM8 (22), it is likely that eTRPM8 may function as a cold-sensitive protein. Nevertheless, no changes in transmembrane ion currents, recorded under whole-cell voltage clamp in eTRPM8-expressing HEK cells, were detected in response to cold, menthol, or icilin. This ruled out eTRPM8 as a plasma membrane functional channel. Based on our previous findings (19) we hypothesized that, similarly to TRPM8 in prostate cancer cells, eTRPM8 in keratinocytes may function as the ER Ca2+-release channel. To test this hypothesis, we first conducted double immunostaining of eTRPM8 and the ER protein calnexin. This revealed (Fig. S2) that eTRPM8 clusters coincide with the ER elements in CTL and eTRPM8-overexpressing HaCaT cells and in hNEKs. Because eTRPM8 is expressed in the ER of keratinocytes, to measure eTRPM8-mediated single-channel current we conducted patch-clamp recording on the giant unilamellar vesicles (GUVs) containing the proteins extracted from the ER membrane fractions of HEK293 cells expressing eTRPM8 (Fig. 1 G–L).
Fig. S2.
eTRPM8 is expressed in the keratinocyte endoplasmic reticulum (ER) but not in the cell plasma membrane. Detection of eTRPM8 and the ER marker, calnexin, was performed in (A) control HaCaT cells (CTL HaCaT), (B) HaCaT cells overexpressing epidermal TRPM8 (eTRPM8 HaCaT), and (C) human normal epidermal keratinocytes (hNEK), using indirect immunostaining. Primary antibody-specific binding to eTRPM8 and calnexin was visualized with DyLight 488- and Alexa Fluor 546-conjugated IgGs, respectively. Galleries (A–C, Left) show confocal images of Alexa Fluor 546 (the ER elements, red), DyLight 488 (eTRPM8, green), and DAPI (nuclei, blue) fluorescence and their overlay, as indicated. (A–C, Right) Enlarged images of the boxed regions.
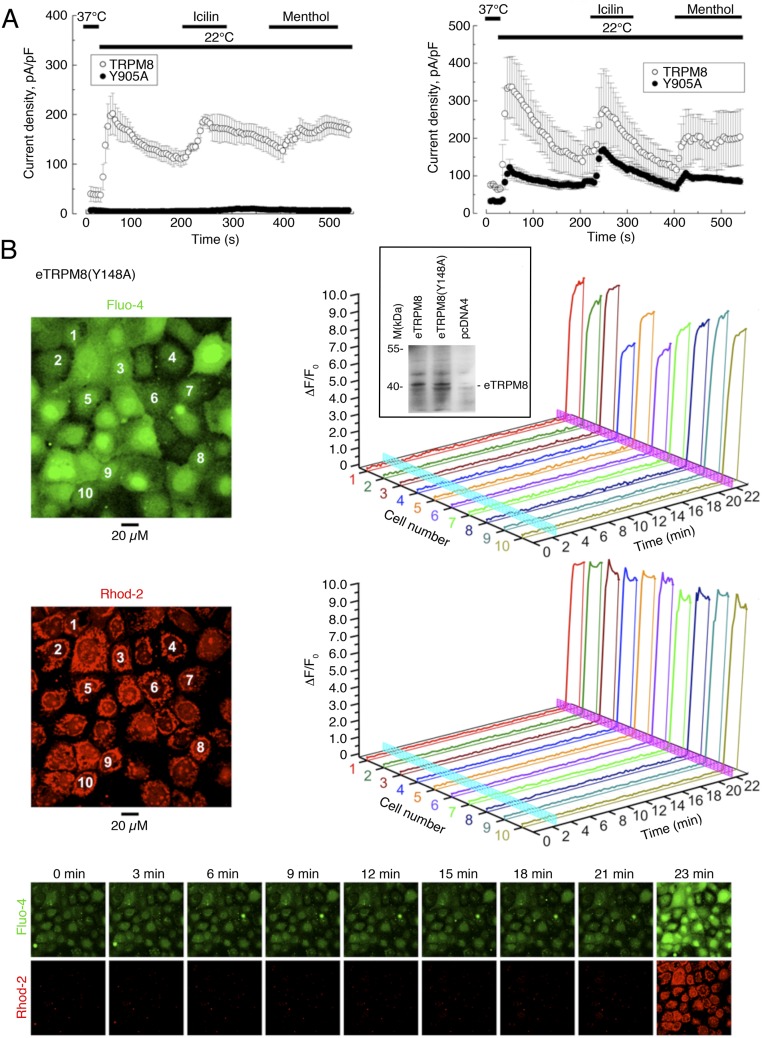
Biophysical properties of the single-channel activity measured under these conditions were characteristic of the TRPM8 channel: (i) single-channel conductance of 63.1 ± 2.4 pS and dependence of Popen on applied voltage at 20 °C, basal and in response to menthol, as well as at 37 °C (n = 33, 37, and 5); (ii) sensitivity to all currently known specific activators and inhibitors of TRPM8; and (iii) PiP2 dependence. The single-channel current was activated by 10 µM icilin (n = 16), 500 nM ws-12 (23) (n = 18), or 200 µM menthol (n = 31); required the presence of PiP2 in GUVs; and was blocked by 10 µM BCTC (n = 13). Stimulation with these activators of the patches made to the GUVs prepared from cells expressing the empty vector (n = 290) or nonfunctional mutant eTRPM8(Y148A) (n = 258) failed to induce the single-channel activity.
Thus, we have cloned a new kind of TRP channel, eTRPM8, which, in contrast to all previously known members of the TRP family, has the 4-TD structure. Furthermore, we demonstrated that keratinocytes express eTRPM8 instead of classical TRPM8, which raised the question about its role in epidermal homeostasis.
Functional TRPM8 Knockout Impairs Skin Differentiation in Mouse.
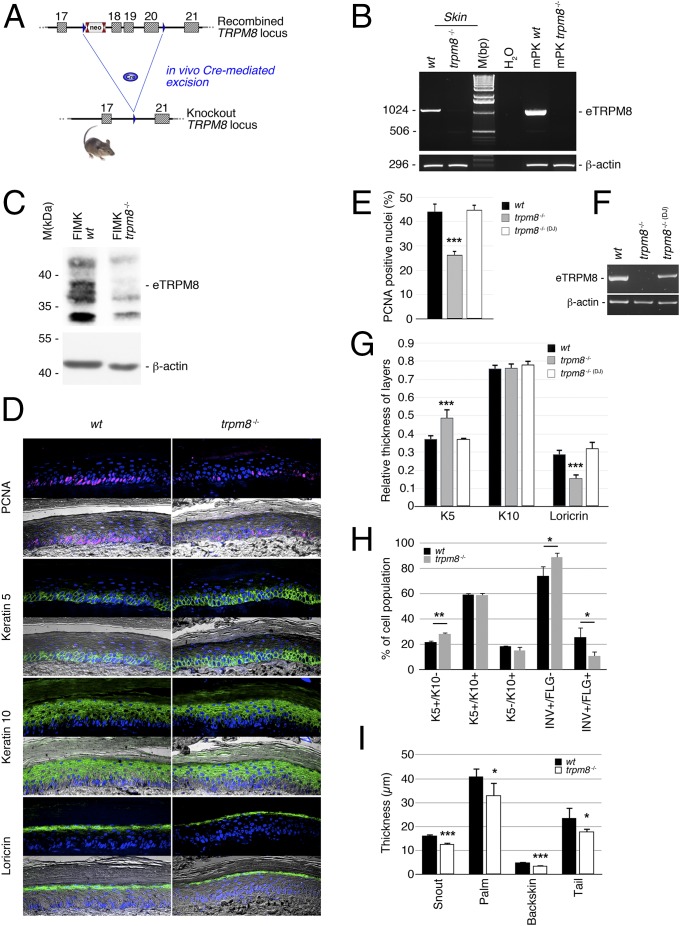
Several trpm8−/− mouse strains have been generated by deletion of either the first translated exon or exons in the first half of the TRPM8 sequence (17, 18). These mutations, however, did not target the pore region and, therefore, failed to suppress eTRPM8 expression and activity. In contrast, based on our previous detection of amino-terminal truncated TRPM8 isoforms (19), we have designed a functional KO mouse line by deleting exons 18, 19, and 20, which encode the active pore domain (Fig. 2A and Fig. S1A). Here we assessed the expression of mouse orthologous eTRPM8. PCR amplification of TRPM8(15a) was successfully achieved from exon 16 to exon 23 in total mRNA extracts (Fig. 2B), not only from skin sections of wild-type mice but also from primary culture of wild-type mouse keratinocytes (WT mPK) grown with 2% FCS + 1.8 mM Ca2+ (24). This implies that eTRPM8, detected in the skin sections, is hosted by keratinocytes. Moreover, the lack of amplification products for exons 14–23 is indicative of truncation of the first half of the 5′ exon characteristic of the eTRPM8 sequence. Specificity of TRPM8 detection was validated in the samples from TRPM8 KO mice that do not express TRPM8. Finally, we checked eTRPM8 protein expression in freshly isolated mouse keratinocytes (FIMKs) and detected a doublet of protein near 39 kDa from WT cells, which was not present in FIMK from trpm8−/− mice (Fig. 2C).
Fig. 2.
Epidermal TRPM8 isoform (eTRPM8) ablation in mouse epidermis partially impairs epidermal homeostasis. (A) Schematic representation of the strategy used to establish the trpm8 −/− mouse line. (B) PCR amplification from exon 16 to exon 22 demonstrates expression of mouse eTRPM8 mRNA in wild type (WT) skin as well as in mPKs derived from WT mouse skin and grown with 2% FCS and 1.8 mM Ca2+ (mPK WT). Note that no eTRPM8 expression was detected in keratinocytes derived from trpm8 −/− mouse skin (mPK trpm8 −/−) (n = 3). (C) Immunoblotting shows ∼38 kDa protein in the skin of WT but not trpm8 −/− mice (one of four readings for each mouse strain). β-actin was used as a control of the protein loading. (D) Representative immunohistofluorescence images of WT and trpm8 −/− mouse palm skin reveal a decreased number of cycling cells in trpm8 −/− epidermis (reported by PCNA) and a thicker granular layer (reported by loricrin, LN). (E) Bar diagram plot compares fractions of the cells with PCNA-positive nuclei in the keratin 5 (K5)-positive cell compartment counted in the images of skin sections of five WT mice, seven trpm8 −/− mice, and five trpm8 −/− (DJ) mice (lacking full-length TRPM8 channel only). Note that the trpm8 −/− mouse skin section (D) has a thicker basal layer (K5-positive cells) but a thinner granular layer (LN-positive cells), whereas the total thickness of SS + SG remains unaltered. (F) PCR amplification from exon 16 to exon 22 demonstrates expression of mouse eTRPM8 mRNA in skin sections of WT mice, trpm8 −/− mice, and trpm8 −/− (DJ) mice. (G) Bar diagram plot compares relative thickness (Materials and Methods) of K5-, K10-, and LR-positive compartments in five WT mice, seven trpm8 −/− mice, and five trpm8 −/− (DJ) mice. (H) Distribution of keratinocyte phenotypes in the suspension of cells, freshly isolated from the back skin samples of five WT and five trpm8 −/− mice, was measured with flow cytometry and compared. The phenotypes detected include basal cells (K5+/K10−), suprabasal and early spinal cells (K5+/K10+), late spinal cells (K10+/INV+/FLG−), and granular cells (INV+/FLG+). (I) Thickness of corneosum stratum (CS) was measured in trichrome-stained slides of paraffined skin samples obtained from the different skin regions (as indicated) and compared for five WT and five trpm8 −/− mice.
To address involvement of eTRPM8 in EH we tested whether eTRPM8 KO would impair EH. Immunohistofluorescence analysis revealed a significant decrease of the number of proliferating cell nuclear antigen (PCNA) positive cells in trpm8−/− basal/suprabasal compartments (B/SbS) labeled with keratin 5 (K5) (Fig. 2 D and E). This suggests that the proliferation level of the stratum basale (SB) is impaired in eTRPM8 KO epidermis. Because suppression of the channel pore region abolishes the activity of both eTRPM8 and classical TRPM8, to eliminate any possible involvement of the TRPM8 KO in the measurements (even though we obtained no evidence for the TRPM8 expression in keratinocytes) we conducted additional control experiments on the trpm8−/− (DJ) mouse strain, where only classical TRPM8, but not eTRPM8, is suppressed (Fig. 2F). No changes in the proportion of PCNA positive cells in comparison with that in WT mice were detected in these experiments (Fig. 2G). Furthermore, measurements of the thickness of the different epidermal layers (Fig. 2 D and G) revealed a significant increase of B/SbS, no change in stratum spinosum (SS) plus stratum granulosum (SG), and a significant decrease of the SG. These data indicate that the SS thickness is increased in proportion to the decrease of the SG thickness, so that total thickness of the two layers remains unchanged. The observed alterations were further confirmed by assessing the percentage of FIMKs positive for a specific epidermal layer marker (Fig. 2H). We have found that FIMKs from trpm8−/− mice were enriched in the number of basal keratin 5 (K5)-positive, keratin 10 (K10)-negative (K5+/K10−) proliferating cells, as well as in involucrin (INV)-positive, filaggrin (FLG)-negative (INV+/FLG−) late spinosum cells, but they were slightly depleted of filaggrin-positive (INV+/FLG+) granulosum cells, compared with the WT FIMKs. Finally, consistent with the decrease of proliferation and increase in late differentiation, we have detected the decrease in the thickness of the stratum corneum (SC) in the skin samples of different body regions from trpm8−/− mice: snout, palm, backskin, and tail (Fig. 2I).
Altogether, our data in the trpm8−/− mouse model demonstrated an impairment of EH, which was characterized by the decrease of proliferation rate and accumulation of keratinocytes in basal/suprabasal layers and early SS paralleled with a partial depletion of the cells in SG and SC. We therefore have concluded that eTRPM8 is an important modulator of EH. This prompted us to study molecular mechanisms of eTRPM8-dependent EH regulation.
eTRPM8 Is the ER Ca2+-Release Channel, Activity of Which Affects [Ca2+]m.
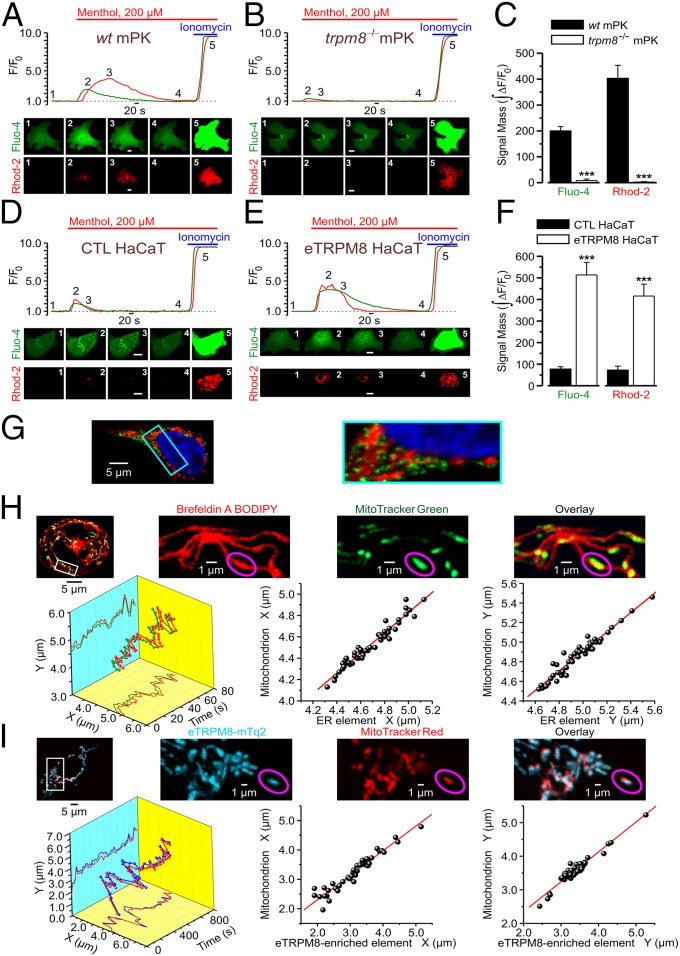
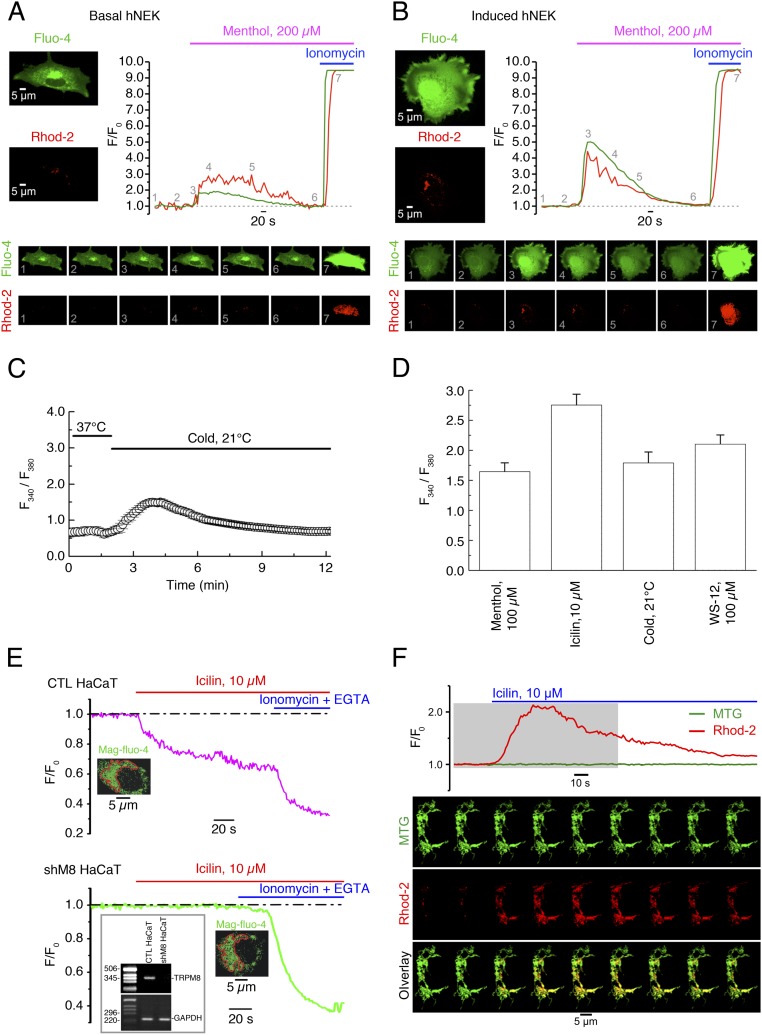
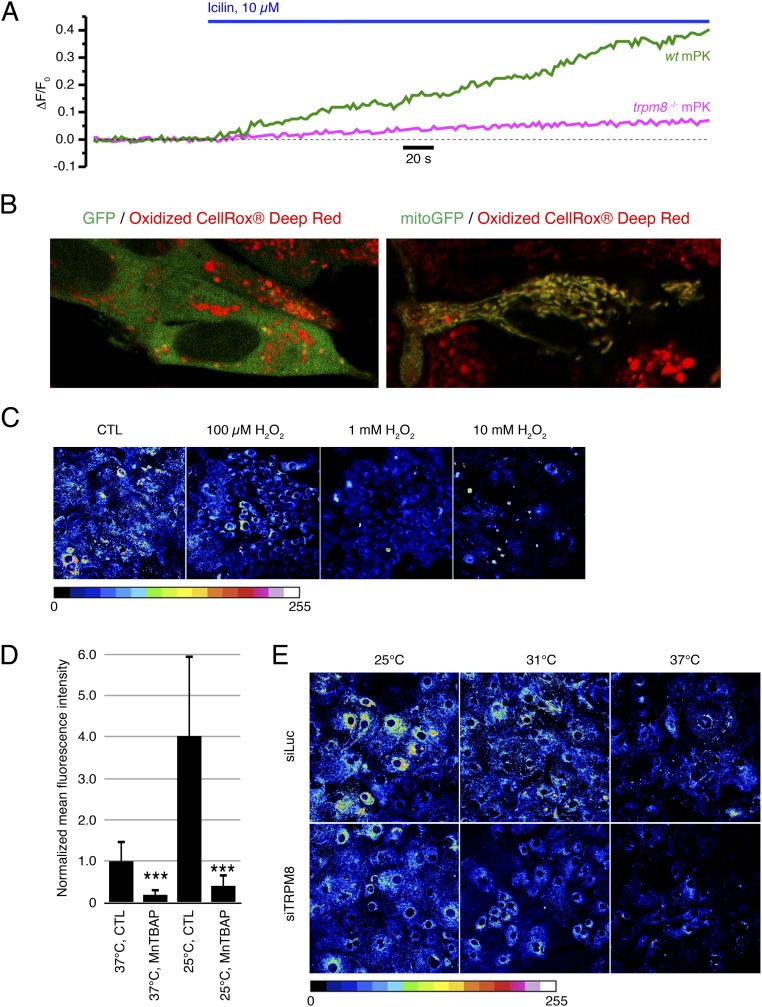
To assess immediate consequences of eTRPM8 activation in keratinocytes, we monitored menthol-induced changes of the ionized Ca2+ concentration in cytosol ([Ca2+]c) and mitochondria ([Ca2+]m), using fluorescence confocal imaging. In these experiments, to eliminate a possible contribution of capacitative Ca2+ entry and, thereby, to enable the detection of Ca2+ flow among intracellular compartments only, external solution, containing 70 µM Ca2+, was supplemented with 10 µM La3+ (25). Changes of [Ca2+]c and [Ca2+]m in response to external application of 200 µM menthol were simultaneously monitored at 37 °C, using fluo-4 and rhod-2, respectively. The [Ca2+]c and [Ca2+]m transients detected in mPKs from wild-type mice (Fig. 3A) were virtually abolished (Fig. 3C) in mPKs from trpm8−/− mice (Fig. 3B), whereas the transients observed in control HaCaT cells (Fig. 3D) were significantly augmented (Fig. 3F) following eTRPM8 overexpression (Fig. 3E). Induction (Materials and Methods) of hNEKs also increased the amplitude of both [Ca2+]c and [Ca2+]m transients (Fig. S3 A and B), which correlates with elevation of eTRPM8 protein expression (Fig. 1D). The sensitivity of eTRPM8 to menthol, icilin, cold, and WS-12 (synthetic TRPM8 agonist) was further confirmed by wide-field microscopy of the fura-2 responses (Fig. S3 C and D). Moreover, to verify the origin of Ca2+, giving rise to [Ca2+]c and [Ca2+]m transients, we monitored icilin-induced changes of the ER Ca2+ concentration ([Ca2+]ER) in permeabilized HaCaT cells. This revealed (Fig. S3E) that decrease of [Ca2+]ER in response to 10 µM icilin, observed in control HaCaT cells, was abolished following eTRPM8 knockdown (KD), achieved by transfection of HaCaT cells with shRNA targeting the eTRPM8-pore–encoding sequence (shM8). Mitochondrial origin of rhod-2 signal was confirmed by visualization of the mitochondria with MitoTracker Green FM (Fig. S2F).
Fig. 3.
eTRPM8 couples Ca2+ release from the ER to mitochondrial Ca2+ uptake. Changes of Ca2+ concentration in cytosol ([Ca2+]c) and mitochondria ([Ca2+]m) in response to external application of 200 µM menthol were monitored using x-y time series imaging of fluo-4 and rhod-2 fluorescence, respectively, in primary cultures of (A) wild-type mouse keratinocytes (WT mPK) and (B) trpm8 −/− mouse keratinocytes (trpm8 −/− mPK) and in (D) control HaCaT cells (CTL HaCaT) and (E) HaCaT cells overexpressing eTRPM8 (eTRPM8 HaCaT). The plots show the time course of normalized fluorescence (F/F0) of fluo-4 (green traces) and rhod-2 (red traces). The galleries below the plots demonstrate the images of fluo-4 and rhod-2 fluorescence (as indicated), captured at the moments depicted by the numbers on the plots, respectively. (Scale bar: 10 μm.) To eliminate capacitative Ca2+ entry, an external solution, containing 70 μM Ca2+, was supplemented with 10 μM La3+. To estimate the load of the Ca2+-sensitive indicators, the cells were exposed to 2.5 μM of ionomycin at the end of each experiment. Bar diagram plots compare masses, , of the fluo-4 and rhod-2 signals (as indicated) during the period between application of menthol and application of ionomycin in (C) trpm8 −/− mPKs (n = 35) vs. WT mPKs (n = 27) and in (F) eTRPM8 HaCaT cells (n = 15) vs. CTL HaCaT cells (n = 16). Immunodetection (G) of overexpressed eTRPM8 (green) in HaCaT cells expressing a DsRed targeted to mitochondria (red) illustrates that eTRPM8-expressing ER microdomains are in close proximity to mitochondria. (G, Right) Enlarged image of the boxed region. (H and I) Coordinated motility confirms tight coupling between eTRPM8-enriched ER elements and mitochondria in HaCaT cells. The ER elements were either stained with Brefeldin A BODIPY 558/568 (H) or identified by mTurquoise2 (mTq2) fluorescence, following eTRPM8-mTq2 expression (I). The mitochondria (H and I) were stained with either MitoTracker Green FM (MTG) or MitoTracker Red FM (MTR), respectively. Spatial distribution of the ER elements and mitochondria was analyzed using x-y time series confocal imaging. The galleries (H and I, Upper Right) show enlarged images of Brefeldin A BODIPY and MTG fluorescence (H) or mTq2 and MTR fluorescence (I) captured from the boxed region (H and I, Upper Left) and their overlays, as indicated. Motility analysis was conducted for the outlined (magenta ellipses) mitochondrion and the adjacent ER element. The x and y positions of the local maxima of the MTG and Brefeldin A BODIPY fluorescence (H) or mTq2 and MTR fluorescence (I) were computed and plotted over time. The 3D plots (H and I, Lower Left) show the trajectory of the motion of the mitochondrion (H, green; I, red) and adjacent (H, red) ER element or (I, blue) eTRPM8-enriched ER element. The x and y positions of the organelles in time are seen in the x-y and x-z projections on the 3D plot, respectively. The x vs. x and y vs. y positions for the mitochondrion and the ER element are plotted (H and I, Lower Center and Lower Right, respectively). Linear regression analysis revealed high correlation between the parameters in all four cases: R = 0.973 (H, Lower Center), R = 0.970 (H, Lower Right), R = 0.963 (I, Lower Center), and R = 0.967 (I, Lower Right).
Fig. S3.
eTRPM8-mediated Ca2+ release results in mitochondrial Ca2+ uptake in human keratinocytes. (A and B) Changes of Ca2+ concentration in cytosol ([Ca2+]c) and mitochondria ([Ca2+]m) elicited by external application of 200 µM menthol were monitored using x-y time series imaging of fluo-4 and rhod-2 fluorescence, respectively, in (A) primary culture of basal and (B) induced (Materials and Methods) human primary culture keratinocytes (hPK). To eliminate capacitative Ca2+ entry, external solution, containing 70 μM Ca2+, was supplemented with 10 μM La3+. To estimate the load of the Ca2+-sensitive indicators, the cells were exposed to 2.5 μM of ionomycin at the end of each experiment. The fluorescence intensity (F) was normalized to the averaged fluorescence intensity before menthol application (F0). The plots show the time course of normalized fluorescence (F/F0) of fluo-4 (green traces) and rhod-2 (red traces). The galleries below the plots demonstrate the images of fluo-4 and rhod-2 fluorescence (as indicated) captured at the moments, depicted by the numbers on the plots, respectively. (C) Application of cold solution (21 °C) induces [Ca2+]c transients in keratinocytes bathed in Ca2+-free medium. (D) Bar diagram plot shows mean amplitudes of the fura-2 responses (fluorescence intensity ratio at 340 nm and 380 nm) to 100 µM menthol (n = 30), 10 µM icilin (n = 32), 0.1 µM WS-12 (n = 25), and mild cold (21 °C) (n = 20) in the cells bathed in Ca2+-free solution. (E) Changes in the ER luminal Ca2+ concentration [Ca2+]ER were monitored at 37 °C in digitonin-permeabilized keratinocytes, using the low-affinity Ca2+ indicator mag-fluo-4. Application of 10 µM icilin induces a gradual decrease of the normalized mag-fluo-4 fluorescence (F/F0) in control HaCaT cells (CTL HaCaT; C, Upper) but not in eTRPM8 KD HaCaT cells (shM8 HaCaT; C, Lower). To verify whether mag-fluo-4 response reflects the decrease of [Ca2+]ER, the ER was depleted at the end of the experiment by exposure of the cells to the solution containing 1 µM ionomycin and 10 µM EGTA. The traces on the graphs show the time course of the normalized mag-fluo-4 fluorescence (F/F0) averaged within outlined (red) regions. (F) Visualization of mitochondria with MitoTracker Green FM (MTG) confirms mitochondrial origin of rhod-2 response to stimulation of eTRPM8 with icilin in HaCaT cells. The plot shows the time course of self-normalized (F/F0) MTG and rhod-2 fluorescence, as indicated. The fluorescence intensity (F) was normalized to the averaged fluorescence intensity before icilin application (F0). The galleries below the plot demonstrate the images of MTG fluorescence (Top), rhod-2 fluorescence (Middle), and their overlay (Bottom): Every 12th image captured from a single HaCaT cell during the period, highlighted on the plot by a gray background, is shown (from left to right). Note that elevation of mitochondrial Ca2+ concentration ([Ca2+]m) is reported in the overlay images by change in color of mitochondria from green (dominating MTG fluorescence) to yellow (the overlay of MTG and elevated rhod-2 fluorescence).
Another line of evidence, confirming that eTRPM8 functions as a Ca2+-permeable channel, was derived from the experiments on mutation of the channel pore region. We generated a pore-killer mutant of a classical TRPM8 channel by substituting tyrosine 905 with alanine that completely abolished the current through the homotetrameric channel and had a partial dominant-negative effect (Fig. S4A). By analogy, we created an identical pore-killer mutant for eTRPM8, eTRPM8(Y148A), and overexpressed it in HaCaT cells. In this experimental model no Ca2+ release from the ER and concurrent mitochondrial Ca2+ uptake was ever observed (Fig. S4B), whereas the protein expression was confirmed in Western-blot experiments. This implies that the pore-killer mutation abolishes the channel activity of eTRPM8, as it does in classical TRPM8 (Fig. S4A), and further confirms that the ER Ca2+ release leading to mitochondrial Ca2+ uptake is mediated via eTRPM8 (Fig. S4B).
Fig. S4.
Mutation in TRPM8 or eTRPM8 pore region abolishes the channel-mediated Ca2+ fluxes. (A) Whole-cell patch clamp recordings were conducted in WT TRPM8-, TRPM8(Y905A)-, and WT TRPM8 + TRPM8(Y905A)-transfected HEK cells. The cell membrane potential was repetitively altered by voltage ramps from −100 mV to +100 mV (applied at 0.2 Hz). The changes in the mean current density at +100 mV, elicited by the exposure to cold (22 °C), 500 μM menthol, and 10 µM icilin were compared in WT TRPM8-transfected vs. TRPM8(Y905A)-transfected cells (A, Left; n = 20 and n = 7, respectively), and in WT TRPM8-cotransfected vs. WT TRPM8 + TRPM8(Y905A)-cotransfected cells (A, Right; n = 15 and n = 8, respectively). Cotransfection of WT TRPM8 and TRPM8(Y905A) was performed at a 1:3 ratio. (B) No detectable changes of Ca2+ concentration in cytosol ([Ca2+]c) and mitochondria ([Ca2+]m) were observed in response to 200 µM menthol in HaCaT cells expressing eTRPM8(Y148A). Changes of [Ca2+]c and [Ca2+]m were monitored using x-y time series imaging of fluo-4 and rhod-2 fluorescence, respectively. To eliminate capacitative Ca2+ entry, external solution, containing 70 μM Ca2+, was supplemented with 10 μM La3+. The fluorescence intensity (F) was normalized to the averaged fluorescence intensity before menthol application (F0). Relative changes in the fluorescence intensity (ΔF/F0), averaged within each of 10 cells, denoted by the numbers on the images (B, Left), are plotted over time, respectively (B, Right). To estimate the load of the Ca2+-sensitive indicators, the cells were exposed to 2.5 μM of ionomycin at the end of the experiment. Menthol and ionomycin applications are depicted on the 3D plots by vertical cyan and magenta bars, respectively. The galleries below the plots demonstrate the images of fluo-4 and rhod-2 fluorescence (as indicated), captured at times indicated above the images. Inset (B, Top Right) shows the immunodetection of WT eTRPM8 and eTRPM8(Y148A) expressed in HEK cells. Negative control is achieved with protein extract from HEK transfected with the empty vector pcDNA4.
This suggests tight coupling between the ER and mitochondria, which facilitates mitochondrial Ca2+ uptake either by robust increase of Ca2+ concentration in the ER mitochondria “nanodomains” (26) or via direct “Ca2+ tunneling” from the ER to mitochondria (27). Immunofluorescence detection of native eTRPM8 in HaCaT cells, overexpressing DsRed targeted to mitochondria, revealed that eTRPM8 foci surround mitochondria (Fig. 3G). More evidence that eTRPM8-enriched ER elements are tightly coupled to mitochondria was obtained from the analysis of motility of the ER elements and mitochondria in living HaCaT cells (Movies S1 and S2). This revealed extremely high correlation in the step-by-step displacements of the ER element, visualized either with Brefeldin A BODIPY staining (Fig. 3H; n = 8) or following eTRPM8-mTurquoise2 expression (Fig. 3I; n = 10), and the adjacent mitochondrion. The coordinated motility of the organelles indicates that mitochondria are “anchored” to the adjacent eTRPM8-enriched ER elements.
Taken together, the above results imply that eTRPM8 is an ER functional channel, which, forming calcium nanodomains, couples the ER Ca2+ release to mitochondrial Ca2+ uptake in keratinocytes. We therefore analyzed the downstream molecular events engaged by eTRPM8 activation.
eTRPM8 Facilitates Mitochondrial ATP Synthesis at 37 °C and Adjusts ATP Production to Mild-Cold Adaptation Requirements.
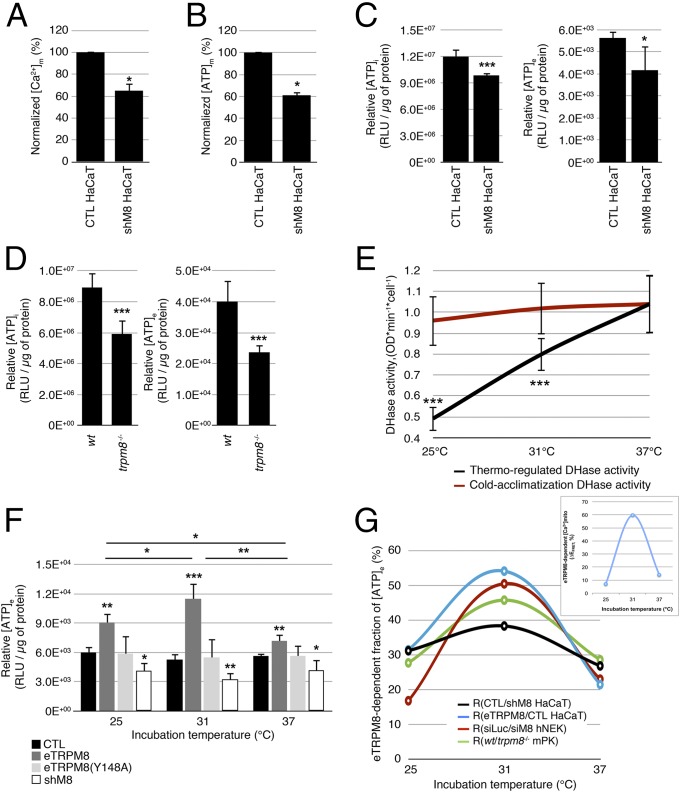
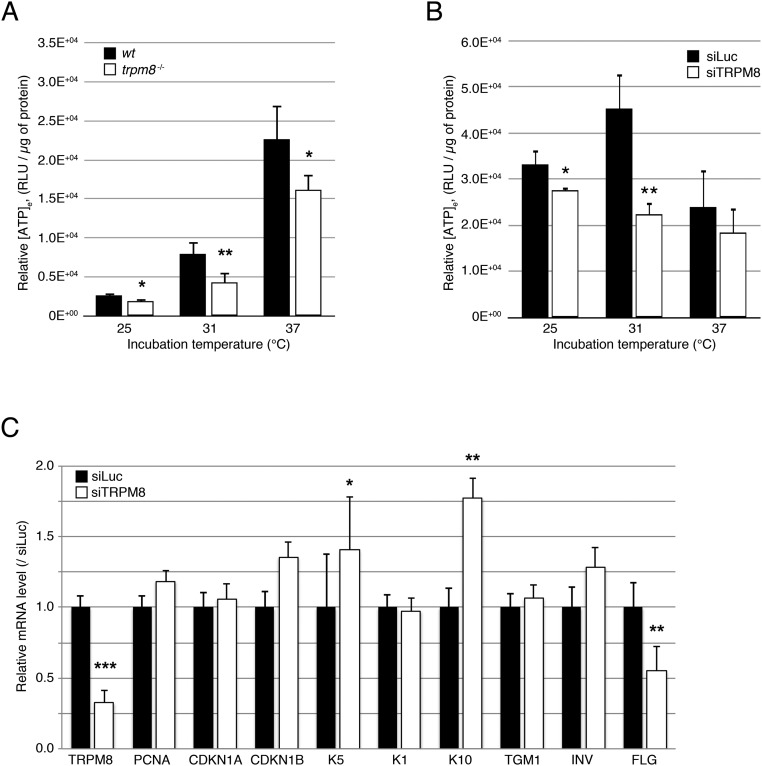
In pancreatic and hepatic cells, a correlated Ca2+ oscillation in both cytosol and mitochondria has been shown to support a cell-specific pacing of metabolism, which coordinates with cell function (28–30). Mitochondrial Ca2+ transients have been reported to increase mitochondrial dehydrogenase (DHase) activity that in turn enhances ATP synthesis. In addition, three key DHases of the tricarboxylic acid (TCA) cycle have been reported to be Ca2+ dependent, suggesting that mitochondrial Ca2+ regulates the NADH/NAD+ ratio, which in turn controls ATP synthesis (31–33). We therefore tested whether eTRPM8 expression and activity may influence ATP synthesis in keratinocytes. The eTRPM8 KD HaCaT cells showed almost 40% decrease of steady-state [Ca2+]m (Fig. 4A), which correlates with a 40% fall in steady-state [ATP]m detected with mitochondrial luciferase (Fig. 4B). Mitochondrial ATP is thought to represent between 40% and 60% of total cell ATP, depending on cell model and physiology. We therefore expected that the eTRPM8-dependent mitochondrial ATP synthesis would have a global impact on cell energy stores. Consistent with this, measurements of total intracellular ATP content ([ATP]i) in the eTRPM8 KD HaCaT cells and mPKs from trpm8−/− mice revealed a significant decrease compared with that in the respective controls (Fig. 4 C and D, Left).
Fig. 4.
eTRPM8 conveys cold-dependent enhancement of ATP synthesis. (A) A steady-state [Ca2+]m was assessed using mtAEQmut probe in control and eTRPM8 KD HaCaT cells (CTL HaCaT and shM8 HaCaT, respectively). Bar diagram plot compares mean ± SEM values in CTL HaCaT cells (n = 13) and ShM8 HaCaT cells (n = 11) in three independent experiments, after rescaling the mean control value to 100%. (B) Basal ATP concentration within mitochondria ([ATP]m) was measured with mitochondria targeted ATP-dependent luciferase in CTL HaCaT (n = 12) and shM8 HaCaT (n = 12) cells at 37 °C. Decrease of [ATP]m in shM8 HaCaT cells (presented as percentage of control) is summarized in the bar diagram plot. (C) Intracellular and extracellular ATP concentrations ([ATP]i and [ATP]e) were quantified using luciferase assay (Materials and Methods) in CTL HaCaT and shM8 HaCaT cells at 37 °C and compared (n = 5). (D) The same as C but for mPKs from WT and trpm8 −/− mice (n = 5). (E) Dehydrogenase (DHase) activity assay performed at 37 °C, 31 °C, or 25 °C demonstrates strong temperature dependence of DHase activity in hNEKs cultured at 37 °C (black curve, thermo-regulated DHase activity). No significant deviations in DHase activity associated with cold acclimatization of hNEKs cultured at 37 °C, 31 °C, or 25 °C were detected by DHase assay performed at 37 °C (red curve, cold acclimatization DHase activity). n = 3. (F) Quantification of [ATP]e HaCaT cells cultured at 37 °C, 31 °C, or 25 °C for 24 h following transfection with either vector (CTL), eTRPM8, pore-killer mutant eTRPM8(Y148A), or shRNA targeting eTRPM8 (shM8). n = 5. (G) Contribution of eTRPM8 to regulation of ATP synthesis was estimated from TRPM8-dependent fraction of [ATP]e: (i) the differences between WT/CTL/siLuc and KO/shM8/siTRPM8 [ATP]e values were divided by corresponding WT/CTL/siLuc [ATP]e values, for mPKs, HaCaT cells, and hNEKs, respectively and (ii) the difference between eTRPM8 HaCaT and CTL HaCaT [ATP]e values was divided by the eTRPM8 HaCaT [ATP]e value. (Inset) Using the same strategy the eTRPM8-dependent component of a steady-state [Ca2+]m was assessed by the measurements of 4mTM3cpv Cameleon FRET efficacy (EFRET) in HaCaT and eTRPM8 HaCaT cells. Smooth curves show parabolic interpolation of the mean values (n = 5). Note that in all cases, an impact of eTRPM8 activity on [ATP]e is maximal around 31 °C. Also note correlation between eTRPM8-dependent [Ca2+]m and [ATP]e.
Apart from being involved in all energy-dependent cellular processes, ATP is excreted as paracrine/autocrine messenger, which may contribute to EH. Indeed, purinergic receptors are expressed in the plasma membrane of keratinocytes and their activation modulates proliferation (34). Bearing this in mind, we measured extracellular ATP concentration ([ATP]e) and, as in the case of [ATP]i, detected [ATP]e decrease with both the KD HaCaT cells and mPKs from trpm8−/− mice (Fig. 4 C and D, Right). This revealed that [ATP]e represents a good correlate of [ATP]i. Altogether these data demonstrate that eTRPM8 is involved in modulation of mitochondrial ATP synthesis that defines both intra- and extracellular ATP concentration at 37 °C.
As eTRPM8 is a cold sensor, we tested whether mild cold could stimulate ATP production. The notion that ATP production could be enhanced by cold seems to be counterintuitive, because it is well appreciated that the optimal temperature for enzyme activity is 37 °C. To verify whether keratinocyte metabolism is sensitive to acute and chronic cold as expected, we assessed the temperature dependence of the total DHase activity in hNEKs and found that total DHase activity is gradually reduced during acute cooling for 1 h (Fig. 4E). Conversely, keratinocyte acclimatization to cold within 24 h prior to measuring total DHase activity at 37 °C revealed no difference in DHase activity in keratinocytes grown at 37 °C. This suggests that intrinsic temperature dependence of the enzyme activity dominates and masks a much weaker, if any, effect of the keratinocyte adaptation on DHase activity (i.e., increased expression, protein modifications increasing activity). We therefore assessed ATP content in the culture medium of control HaCaT cells grown at temperatures of 37 °C, 31 °C, and 25 °C for 24 h. This revealed no statistically significant alterations of [ATP]e (Fig. 4F), which is hardly consistent with the notion of decreased global metabolism at lowered temperatures. Overexpression of eTRPM8 increased [ATP]e relative to control HaCaT cells at all tested temperatures, whereas eTRPM8 knockdown (shM8) had an opposite effect. It is noteworthy that overexpression of the eTRPM8 pore-killer mutant [eTRPM8(Y148A)] reversed the effect observed with overexpression of functional eTRPM8, suggesting that eTRPM8 channel activity is required for full-scale ATP production, especially under mild-cold conditions. Nevertheless, the [ATP]e levels, observed in control HaCaT cells and following eTRPM8(Y148A) expression, were not statistically different, suggesting that the mutant is not negative dominant (Fig. 4F). Unexpectedly, [ATP]e in control mPKs gradually decreased with cooling and was reduced in mPKs from trpm8−/− mice with a maximal drop (about 50%) at 31 °C (Fig. S5A). In contrast, [ATP]e in hNEKs increased with cooling at 31 °C and was reduced by siTRPM8 expression (Fig. S5B). Thus, all cell models tested support the notion that eTRPM8 is involved in control of ATP synthesis. Despite the difference in temperature dependence of [ATP]e in different cell models, reflecting some contribution of eTRPM8-independent mechanisms, the impact of eTRPM8 on ATP synthesis was always maximal around 31 °C (Fig. 4G) and correlated with an eTRPM8-dependent fraction of [Ca2+]m (Fig. 4G, Inset). Although an increase in the eTRPM8-dependent fraction of [ATP]e at 31 °C can be attributed to elevation of the mitochondrial ATP synthesis boosted by the uptake of Ca2+ released from the ER via activated eTRPM8, subsequent decrease of this fraction at 25 °C cannot be explained by temperature dependence of TRPM8 and likely reflects an intrusion of a different mechanism, which decreases ATP synthesis despite elevated mitochondrial Ca2+ uptake. One of the possible candidates for such a mechanism is mitochondrial superoxide () production, which was reported to correlate well with metabolic rate (35, 36). It should be emphasized that keratinocytes are more prone to accumulation than other cell types due to reduced superoxide dismutase (SOD) activity (37).
Fig. S5.
(A) Extracellular ATP concentration ([ATP]e) assessed in the media with cultured keratinocytes, isolated from a wild-type mouse (WT mPK) and a trpm8 knockout mouse (trpm8 −/− mPK), and grown at 25 °C, 31 °C, or 37 °C for 3 d. Data are shown as mean ± SD for six WT mice and six trpm8 −/− mice. (B) Same as A but for induced hNEKs transfected with either control siRNA (siLuc) or siRNA targeting TRPM8 (siTRPM8) and cultured at 25 °C, 31 °C, or 37 °C for 3 d. (C) Real-time PCR experiment demonstrates the effect of eTRPM8 silencing (60% decrease) in hNEK cells (siTRPM8 vs. siLuc) on the expression of genes encoding PCNA, CDKN1A, CDKN1B, keratin 5 (K5), keratin 1 (K1), keratin 10 (K10), transglutaminase 1 (TGM1), involucrin (INV), and filaggrin (FLG). Experiment was performed three times, and values are presented as mean ± SD. The mean values on the plot are rescaled so that mean value in siLuc-treated cells for each gene tested is 1 ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
eTRPM8 Activation Facilitates Superoxide Production in Mitochondria and Triggers Its Accumulation Under Mild-Cold Conditions.
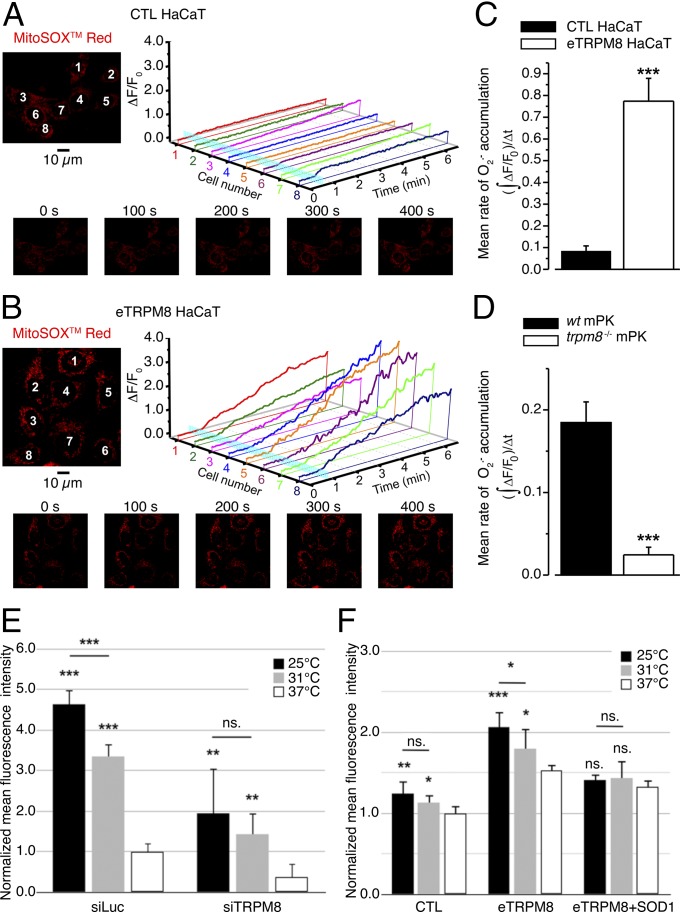
To test the above hypothesis we compared mitochondrial accumulation in response to eTRPM8 stimulation with 10 μM icilin in control (Fig. 5A) and eTRPM8-overexpressing (Fig. 5B) HaCaT cells and in WT mPKs and mPKs from trpm8−/− mice (Fig. S6A), using x-y time series confocal imaging of MitoSOX Red fluorescence in the living cells (Materials and Methods). This revealed that accumulation was augmented in eTRPM8-overexpressing HaCaT cells and was suppressed in mPKs from trpm8−/− mice. Indeed, the mean rate of accumulation, estimated as mass of MitoSOX fluorescent signal per second following icilin application, was found to be 9 times higher in eTRPM8-overexpressing HaCaT than control HaCaT cells (Fig. 5C) and 7.5 times lower in mPKs from trpm8−/− mice than in WT mPKs (Fig. 5D).
Fig. 5.
Icilin- and cold-induced O2•− accumulation depends on the level of eTRPM8 expression. (A and B) Confocal images of MitoSOX Red fluorescence revealed distinct mitochondrial staining in control HaCaT cells, CTL HaCaT (A), and HaCaT cells overexpressing epithelial TRPM8, eTRPM8 HaCaT (B). Changes in the MitoSOX fluorescence in response to stimulation with 10 μM icilin were monitored using the x-y time series imaging protocol. The MitoSOX fluorescence intensity (F) was normalized to the averaged fluorescence intensity before agonist application (F0). In A and B, relative changes in the fluorescence intensity (ΔF/F0), averaged within each of eight cells denoted by the numbers on the images (Upper Left), are plotted over time, respectively (Upper Right). Icilin application is depicted by a vertical cyan bar. The galleries (A and B, Lower) show the images of MitoSOX fluorescence captured at times indicated above the images. Mean rates of O2•− accumulation were estimated as masses of MitoSOX fluorescent signal per second following icilin application, , in CTL HaCaT (n = 28), in eTRPM8 HaCaT (n = 20), in primary cultures of either WT mouse keratinocytes (WT mPK) (n = 8) or trpm8 −/− mouse keratinocytes (trpm8 −/− mPK) (n = 10), and compared in C and D, respectively. (E) Steady-state superoxide concentration [O2•−] was assessed in living hNEKs after 72 h culturing at 37 °C, 31 °C, or 25 °C, using CellRox Deep Red Reagent. Cells were transfected either with control siRNA (siLuc) or with siRNA targeting the pore-encoding sequence of TRPM8 (siTRPM8). For each temperature and siRNA condition the measurements were performed in three Petri dishes, sequentially mounted on the microscope stage at the ambient temperature corresponding to the preincubation temperature. In each Petri dish confocal imaging of CellRox Deep Red fluorescence was performed from four fields of view, 230 × 230 µm (1,024 × 1,024 pixels) each. The O2•−- specific increases in the CellRox Deep Red signal mass (Materials and Methods) for each condition were averaged, normalized to the mean value detected at 37 °C in siLuc-transfected hNEKs, and compared. (F) The same as E, but for HaCaT cells transfected with empty vector (CTL), eTRPM8 plasmid, or eTRPM8 + SOD1 plasmids and cultured for 24 h at 37 °C, 31 °C, or 25 °C. The data were normalized to the mean value detected at 37 °C in HaCaT cells transfected with empty vector and compared. Note that the differences between corresponding values for different expression conditions (E and F) are statistically significant at all three temperatures tested with P < 0.05. Experiments in E and F were performed three times and include more than 200 cells per experiment.
Fig. S6.
Icilin-induced O2•− production is significantly reduced in primary culture of keratinocytes from trpm8−/− TRPM8 mice. (A) Relative changes in the MitoSOX Red fluorescence intensity (ΔF/F0), detected using confocal x-y time series imaging in primary culture of keratinocytes from wild-type mice, WT mPK (green trace), and keratinocytes from trpm8 −/− TRPM8 mice, trpm8 −/− mPK (magenta trace), reflect the time course of O2•− accumulation in response to application of 10 μM icilin. (B) Confocal images of oxidized CellRox deep red reagent in cells transfected with either GFP (B, Left) or mitoGFP (B, Right) revealed mitochondrial localization of CellRox deep red reagent. (C) Confocal images of CellRox deep red-loaded hNEKs incubated in solutions containing vehicle only (water; CTL) or increasing concentrations of hydrogen peroxide (H2O2), as indicated. The CellRox deep red fluorescence intensity was color coded as indicated by the bar. The same illumination intensity, photomultiplier gain, and offset were used in all of the experiments (n = 3). (D) Bar diagram plot compares mean intensity of the normalized CellRox deep red fluorescence in control hNEKs (CTL) with that in hNEKs pretreated with 100 µM MnTBAP for 30 min (MnTBAP) after 3-d cell culturing at either 37 °C or 25 °C, as indicated (n = 3). (E) Confocal images of CellRox deep red-loaded hNEKs transfected with either control siRNA (siLuc) or siRNA targeting TRPM8 (siTRPM8). Induced (Materials and Methods) cells were cultured at 25 °C, 31 °C, or 37 °C for 3 d. The CellRox deep red fluorescence intensity was color coded as indicated by the bar. The same illumination intensity, photomultiplier gain, and offset were used in all of the experiments (n = 3).
As natural stimulus-activating TRPM8 is cold, and because long-term incubation of keratinocytes in mild-cold conditions affects the ATP content (see above), we also tested the effect of long-term cooling on accumulation in different keratinocyte models, using CellRox Deep Red Reagent, a nonspecific reactive oxygen species (ROS) probe, previously reported to be confined to the cytosol. In contrast to that reported previously, we found that this probe accumulates in mitochondria, because it was colocalized with mitochondria-targeted GFP (Fig. S6B). Furthermore, we detected that CellRox Deep Red Reagent fluorescence decreased with increasing concentration of hydrogen peroxide, H2O2 (Fig. S6C). This indicates that an increase in the intensity of CellRox fluorescence is not a reporter of –H2O2 accumulation. On the other hand, a 30-min pretreatment of keratinocytes with MnTBAP, a superoxide () and peroxynitrite (ONOO−) scavenger, results in strong decrease of CellRox fluorescence. Because peroxynitrites are down-products of (38, 39), CellRox Deep Red Reagent seems to be a good reporter of the catalytic chain activity in mitochondria. Bearing this in mind, we assessed steady-state superoxide concentration [O2•−] in living hNEKs (Fig. 5E) or HaCaT cells (Fig. 5F) after 72-h or 24-h culturing at 37 °C, 31 °C, or 25 °C. The hNEKs were transfected either with control siRNA (siLuc) or with siRNA targeting the pore-encoding sequence of TRPM8 (siTRPM8), whereas HaCaT cells were transfected with empty vector (CTL), eTRPM8 plasmid, or eTRPM8 + SOD1 plasmids. Imaging of CellRox Deep Red fluorescence was performed using a confocal microscope equipped with a CO2 incubator. The -specific signals (Materials and Methods) obtained under different siRNA conditions were normalized to those in corresponding controls (37 °C; hNEKs, transfected with siLuc, or HaCaT cells, transfected with empty vector) and compared. This revealed that transfection of hNEKs with siRNA targeting the pore-encoding sequence of TRPM8 partially but significantly suppresses accumulation at all three temperatures tested (Fig. 5E and Fig. S6E). Bearing in mind that content depends on both its synthesis and dismutation by superoxide dismutases, we tested in HaCaT cells whether the effect of eTRPM8 overexpression can be reversed by overexpression of the Cu/Zn superoxide dismutase (SOD1). This revealed that significant increase of accumulation caused by eTRPM8 overexpression at all temperatures tested was partially reversed by concomitant overexpression of SOD1 (Fig. 5F). Furthermore, SOD1 overexpression suppresses cold dependence of accumulation, thus suggesting that the balance between dismutation and synthesis remains the same regardless of the temperature. However, simultaneous overexpression of eTRPM8 and SOD1 resulted in a small but significant increase of accumulation relative to cells transfected with empty vector (CTL) at all tested temperatures. This suggests that, with gradual cooling, elevation of eTRPM8 activity progressively overrides a native SOD activity, whereas when SOD1 is overexpressed, a cold-dependent increase of TRPM8 activity is masked.
Altogether, our data demonstrate that activation of eTRPM8 in the ER leads to the increase of [Ca2+]m that consequently enhances ATP and production in mitochondria. In line with this, 24–72 h incubation of keratinocytes under mild-cold conditions elevates both [ATP]i and []i. These findings are of great physiological importance, because ATP has been reported to be a key inducer of keratinocyte proliferation, whereas O2•− is involved in keratinocyte differentiation. Indeed, SOD1 was shown to be expressed in the basal layer, but to disappear in differentiated layers of epidermis (40). Based on this observation the authors speculated that in proliferating keratinocytes (which have elevated energy metabolism) SOD1 serves to maintain at a low level and, thereby, to protect the cells from oxidation, whereas in differentiated cells (which have low energy metabolism) enhanced accumulation of and perhaps peroxinitrites, resulting from down-regulation of SOD1, may be involved in regulation of keratinocyte differentiation. The latter was further supported by observation of correlation between ROS concentration and degree of keratinocyte differentiation (41, 42). We therefore hypothesized that eTRPM8-dependent production of both ATP and may control the proliferation/differentiation balance of keratinocytes.
eTRPM8 Regulates Proliferation in a Temperature-Dependent Manner.
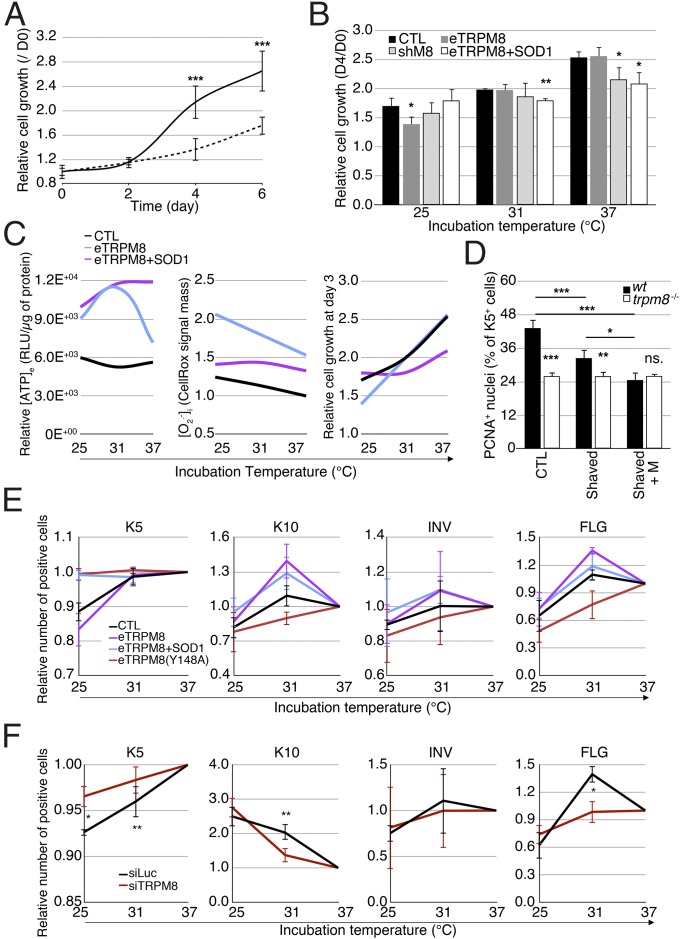
To test whether background, nonstimulated eTRPM8 activity at 37 °C is relevant to EH, hNEKs transfected with either control siRNA or anti-TRPM8 siRNA were subjected to a growth assay at 37 °C. The eTRPM8 KD hNEKs showed reduced growth rate in virtual absence of apoptosis (less than 1%), suggesting that lack of eTRPM8 suppresses proliferation (Fig. 6A). Assessment of thermosensitivity of keratinocyte proliferation revealed that control HEKs showed gradual decrease of growth rate with cooling, as expected, whereas eTRPM8 knockdown reduces the proliferation rate only at 37 °C (Fig. 6B), consistent with the notion that eTRPM8 activity at 37 °C encourages proliferation. eTRPM8 overexpression did not modify the growth at 37 °C, but significantly suppressed it at 25 °C. The latter was reversed by concomitant overexpression of SOD1, thus suggesting that the effect was mediated by (Fig. 6 B and C, Center and Right). Conversely, eTRPM8+SOD1 cotransfection reduced proliferation at both 37 °C and 31 °C, compared with control and eTRPM8-overexpressing cells. Paradoxically, even though eTRPM8+SOD1-overexpressing cells had higher ATP content than both control cells and eTRPM8-overexpressing cells at 37 °C (Fig. 6C, Left), their growth rate was significantly reduced (Fig. 6C, Right).
Fig. 6.
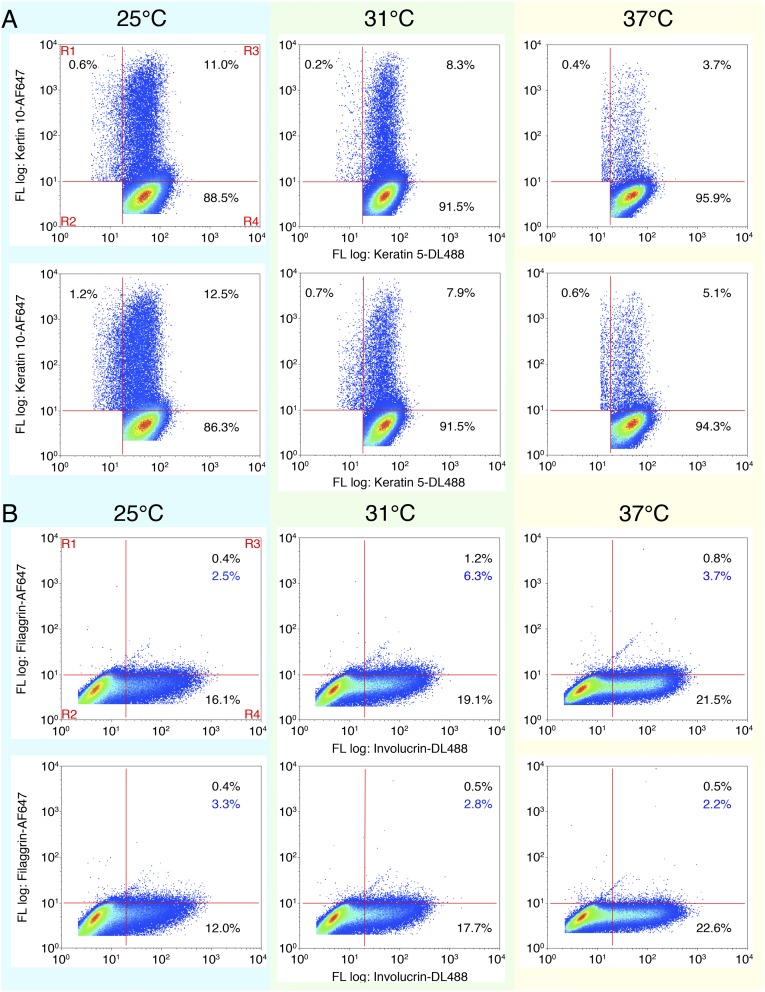
Mild cold suppresses proliferation and facilitates differentiation of keratinocytes in an eTRPM8-dependent manner. (A) The graph illustrates growth of induced (Materials and Methods) hNEKs transfected with either control siRNA (black solid line) or anti-TRPM8 siRNA (dashed line) during 6 d. Data are presented as fold increase of the cell number from day 0. n = 3. (B) Bar diagram plot compares growth of HaCaT cells transfected with empty vector (CTL), eTRPM8 vector (eTRPM8), or shRNA anti-TRPM8 vector (shM8) or cotransfected with eTRPM8 and SOD1 vectors (eTRPM8+SOD1) at 25 °C, 31 °C, and 37 °C. n = 3. (C) Charts illustrate dependence of [ATP]e (C, Left), [O2•−]i (C, Center), and cell growth (C, Right) on ambient temperature for HaCaT cells transfected with either empty vector (CTL) or eTRPM8 vector (eTRPM8) or cotransfected with eTRPM8 and SOD1 vectors (eTRPM8+SOD1). Smooth curves are the result of parabolic interpolation of the mean values (n = 3). (D) Count of PCNA-positive nuclei in basal epidermis compartment was performed on slices from three different areas of skin (Materials and Methods): furred (CTL), shaved (Shaved), and shaved with application of 1 mM Menthol twice a week (Shaved+M). Six WT and seven trpm8 −/− mice were treated for 3 wk before analysis was commenced. Bar diagram plot shows the ratio of the number of PCNA-positive cells divided by the number DAPI-positive nuclei in the basal compartment identified with anti-keratin 14 antibodies. (E) Distribution of keratinocyte phenotypes of HaCaT cells induced at 37 °C, 31 °C, or 25 °C for 24 h was measured with flow cytometry and compared for HaCaT cells transfected with empty vector (CTL), eTRPM8 vector (eTRPM8), or eTRPM8 mutant vector [eTRPM8(Y148A)] or cotransfected with eTRPM8 and SOD1 vectors (eTRPM8+SOD1). The distribution of keratinocyte phenotypes was estimated on the basis of the percentage of cells expressing basal differentiation marker, K5; early spinal differentiation marker, K10; late spinal differentiation marker, INV; and granular differentiation marker, FLG. Cold dependency of keratinocyte differentiation was calculated with normalization of values from cells grown at 25 °C and 31 °C by values from cells grown at 37 °C. Data are presented as mean ± SD (n = 3). Statistical significance was calculated for eTRPM8, eTRPM8+SOD1, and eTRPM8(Y148A) compared with control cells. (F) Same as E but for hNEKs transfected with either control siRNA (siLuc) or anti-TRPM8 siRNA (siTRPM8) for 4 d. Experiments in E and F were performed three times and include more than 100,000 cells per experiment. Data are presented as mean ± SD.
To assess the extent of eTRPM8 recruitment to keratinocyte proliferation in vivo, we examined whether cold/menthol stimulation of mouse epidermis would affect cell proliferation in SB. As most of mouse skin is protected from variations of ambient temperature by fur, which acts as a thermal insulator, we shaved the two sides of mice but kept the fur on the back, as a control. We applied 1 mM of menthol twice a day for 3 wk on the right side, whereas the left side remained untreated. Furred back skin was shaved after mice were euthanized, and three skin samples were compared: control (from the back), shaved (from the left side), and menthol-treated shaved (from the right side). Immunohistofluorescence detection of K14-positive cells, which form the basal transit amplifying compartment of epidermis cells (43), and PCNA-positive cells, which reflect the cell population undergoing the cell cycle, was performed with a confocal microscope. The proliferation rate was then estimated by dividing the number of PCNA+/K14+ cells by the number of PCNA−/K14+ cells. In control conditions (unshaved mice), this ratio was significantly reduced in trpm8−/− epidermis, which reveals a TRPM8-dependent, cold-independent regulatory component of keratinocyte proliferation. We also detected a significant decrease of this ratio in shaved trpm8+/+ epidermis and its further reduction in shaved trpm8+/+ epidermis subjected to menthol treatment (Fig. 6D), which demonstrates that cooling reduces proliferation in vivo. It should be emphasized that in all skin samples from trpm8−/− mice this ratio was reduced to the same extent, independently of shaving and menthol treatment. This result suggests that the decrease of the keratinocyte proliferation rate in vivo in response to cooling cannot be explained solely by customary temperature dependence of metabolic rate. Altogether, these results confirm that in vivo mild cold reduces the number of proliferating keratinocytes in an eTRPM8-dependent manner. Nevertheless, variations in the number of PCNA+ cells can be explained by either slowing down of the cell cycle or an increase of proliferation rate. The effect of eTRPM8 knockdown in HaCaT cells at 37 °C on cell growth (Fig. 6B) undoubtedly favors the second hypothesis and confirms that eTRPM8 expression is required for full-scale proliferation of basal keratinocytes at 37 °C and that mild cold reduces the proliferation rate of keratinocytes in a TRPM8-dependent manner. Regardless of the mechanism linking eTRPM8 expression to full-scale keratinocyte proliferation, reduction of keratinocyte proliferation rate may involve two different mechanisms: cell cycle arrest associated with quiescence phase or induction of differentiation.
Stimulation of eTRPM8 with Mild Cold Induces Keratinocyte Differentiation.
Upon quantification of the differentiation rate in 2D cultures of keratinocytes several issues have to be taken into account. First, it relies on the assumption that the initial proportion of different keratinocyte phenotypes (basal, spinous, granular, corneocytes) is well defined and constant in all Petri dishes. Second, induced changes in the proportion of the keratinocyte phenotypes, interpreted as differentiation, are generally assessed with a Western blot. This procedure, however, gives information on the total expression of the so-called differentiation markers in the cell population, but does not provide any information on either the number of cells in each phenotype or the mean expression level of differentiation markers in single cells. Third, on many occasions, quantification of the differentiation rate is based on detection of early differentiation markers only. Therefore, to quantify eTRPM8-linked cold dependency of the keratinocyte differentiation, we recently showed the efficiency of flow cytometry (24), as in the case of keratinocytes freshly isolated from mouse skin (Fig. 2G). Although the experiments were conducted with simultaneous detection of two of four markers (Fig. S7), to simplify visualization, the data are presented in charts showing the relative proportion of cells expressing one marker. Surprisingly, about 90% of cultured keratinocytes revealed the expression of basal markers K5 and K14, whereas less than 10% of the cells expressed differentiation markers K10 and INV. This could explain why we failed to detect any specific alterations of basal marker expression in HaCaT cells (Fig. 6E) and hNEKs (Fig. 6F) incubated at 31 °C, even though when cells were incubated at 25 °C, a significant decrease in the expression of these markers was observed. Intriguingly, in the case of eTRPM8 overexpression there were more K10-positive HaCaT cells at 37 °C than at 31 °C, whereas in the case of concomitant overexpression of SOD1 the situation was the opposite. This suggests a permissive role of O2•− in early differentiation. This hypothesis is supported by the observation that progressive cooling causes a gradual increase of K10 expression in hNEKs, which are known to express endogenous SOD1 at an early stage of differentiation. Expression of the late SS and SG INV marker was found to be weakly dependent on eTRPM8 expression and stimulation with mild cold. In contrast, the size fraction of cells with the granular marker, FLG, depended strongly on eTRPM8 expression/activity and showed a bell-shaped cold sensitivity with an optimum at the human physiological skin temperature of about 31 °C. Note that this bell-shaped cold sensitivity in the eTRPM8-overexpressing HaCaT cell population is identical to that of hNEKs. Furthermore, this cold sensitivity correlates with the cold sensitivity of ATP synthesis, suggesting that terminal differentiation of keratinocytes is a highly ATP-dependent process. These data together demonstrate that eTRPM8 is involved in the differentiation of keratinocytes not only in mouse (Fig. 2G) but also in human (Fig. 6 E and F) epidermis and conveys cold sensitivity of this process.
Fig. S7.
Mild cold effect on distribution of the induced hNEKs according to the expression of differentiation markers. (A) Flow cytometry codetection of keratin 5 and keratin 10 in hNEKs transfected with either control siRNA (siLuc) or siRNA targeting TRPM8 (siTRPM8) and grown at 25 °C, 31 °C, or 37 °C for 4 d. After compensation, regions of interest were chosen to highlight four cell populations (A and B, Upper Left): R1, cells expressing a single marker indicated on the y axis; R2, cells expressing nonspecific markers; R3, cells coexpressing two markers indicated on x and y axes; and R4, cells expressing a single marker indicated on the x axis. For each population (except R2) its fractional contribution is expressed as a percentage in the corresponding quadrant on the plots. Differentiation markers denoted on the x axis were immunodetected with Dyelight-488–conjugated IgG, whereas differentiation markers denoted on the y axis were immunodetected with AlexaFluor-647–conjugated IgG. (B) Same as A for codetection of involucrin and filaggrin labeled with Dyelight-488– and AlexaFluor-647–conjugated IgG, respectively.
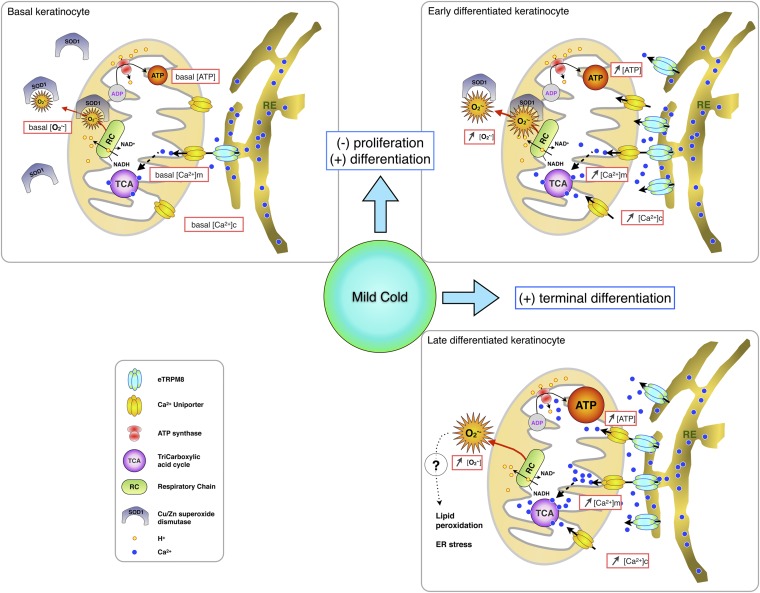
In conclusion, we have characterized a new archetype of the TRP channel encoded by a single gene. This channel is a TRPM8 isoform expressed in the endoplasmic reticulum of keratinocytes and serves as a central element of signaling pathways engaged in cold sensitivity of EH. Our results revealed a key role of eTRPM8 in keratinocyte bioenergetics: arbitration of the keratinocyte proliferation/differentiation balance (within the range of mild cold temperatures) via orchestrating the Ca2+/ATP/ triad (Fig. S8 and SI Results and Discussion). This eTRPM8-mediated temperature-dependent energy metabolism and signaling in keratinocytes might provide skin with the capacity to adapt to large variations of environmental temperature.
Fig. S8.
Schematic representation of the proposed mechanism by which eTRPM8 increases proliferation of basal keratinocytes and mediates cold-dependent potentiation of keratinocyte differentiation. In basal keratinocytes (Upper Left) the level of expression of eTRPM8 is lower whereas the level of expression of superoxide dismutase 1 (SOD1) is higher than in early differentiated keratinocytes (Upper Right). Activation of eTRPM8 in basal keratinocytes will, therefore, cause a moderate increase of mitochondrial Ca2+ concentration ([Ca2+]m), which, in turn, could potentiate the tricarboxylic acid cycle (TCA) and, as a result, increase the activity of the respiratory chain (RC) and ATP synthesis and secretion. However, concomitant accumulation will be limited by SOD1 activity. Moderate increase in ATP concentration will initiate cell cycle progression through paracrine activation of P2Y receptors. Induction of differentiation is associated with an increase of eTRPM8 expression and a decrease of SOD1 expression in differentiating keratinocytes (Upper Right and Lower Right). Therefore, activation of eTRPM8 in differentiated keratinocytes will boost ATP and synthesis. Augmented ATP secretion and accumulation in interstitial space will induce robust elevation of cytosolic Ca2+ concentration, [Ca2+]c (e.g., via activation of P2X-mediated Ca2+ entry). Enhanced synthesis will overcome the activity of down-regulated SOD1, thus resulting in accumulation of , which, in turn, will induce lipid peroxidation and ER stress. This, accompanied by a sustained [Ca2+]c elevation, will induce terminal differentiation of keratinocytes.
SI Results and Discussion
In this study, we have characterized a new archetype of TRP channel encoded by a single gene. This channel is a TRPM8 isoform expressed in the endoplasmic reticulum of keratinocytes and serves as a central element of signaling pathways engaged in cold sensitivity of epidermal homeostasis (EH). Our results revealed a key role of eTRPM8 in keratinocyte bioenergetics: arbitration of the keratinocyte proliferation/differentiation balance (within the range of mild cold temperatures) via orchestrating the Ca2+/ATP/O2•− triad.
eTRPM8: New Architecture of a Functional TRP Channel.
Our results show that a single gene from the TRP-channel family may in fact encode two fully functional channel isoforms with different transmembrane architecture, a classical TRPM8 with six-transmembrane domains (6-TD) and an unconventional eTRPM8 channel that consists of only 4-TD.
Recently it has been reported that topical application of TRPM8 agonists accelerated epidermal barrier recovery after tape stripping (16). Because the authors failed to demonstrate the expression and localization of the classical cold-menthol receptor in keratinocytes, they speculated that “TRPM8-like” protein is involved. In the present study we addressed this intriguing issue and report cloning of two TRPM8 variants referred to as TRPM8(15a) and TRPM8(15a/δ16). Our study was then focused on the TRPM8(15a) mRNA and its related protein, eTRPM8, which forms a functional Ca2+-release channel in the endoplasmic reticulum (ER). Patch clamp recordings on the GUVs formed from the ER membrane fractions of the HEK293 cells expressing this isoform have shown a characteristic activity, including its responsivity to conventional TRPM8 activators such as cold, menthol, icilin, and the specific agonist ws-12, as well as an inhibition by BCTC. In a view of these results, it seems likely that the N-terminal cytosolic segment and the first two TDs of the classical full-size TRPM8 channel do not play any principal role in its activation.
eTRPM8: A Novel Path for Ca2+ Channeling from the ER to Mitochondria.
The existence of a TRPM8 isoform that does not translocate to the plasma membrane, but is restricted to the ER membranes, where its activation triggers Ca2+ release, was demonstrated in our previous studies on human prostate cells (19, 44). Given the importance of Ca2+ homeostasis in keratinocyte differentiation, the role of the ER as a major intracellular Ca2+ store, and the lead of Ca2+ signaling impairment in pathology, the fact that a Ca2+-permeable eTRPM8 channel is specifically expressed in the ER grades this molecule as a potentially important therapeutic target. Indeed, it was recently demonstrated that in patients with Darier disease mutation of the atp2a2 gene encoding the ER Ca2+ pump, SERCA2b, impairs growth of keratinocytes and leads to the alteration of epidermis structure, whereas the same mutation in mice induces squamous-cell carcinoma (45, 46). Here we demonstrated that trpm8−/− mice are characterized by a reduced number of basal proliferating cells, a significant accumulation of spinal cells, and thinner granular and corneal compartments, which is indicative of the EH impairment.
Until now only inositol trisphosphate receptors (InsP3R) have been postulated to primarily regulate [Ca2+]m via tight ER–mitochondria connections (47, 48). We found that elevated steady-state [Ca2+]m correlates with the level of eTRPM8 expression and that eTRPM8 activation releases Ca2+ within ER–mitochondria functional nanodomains, which facilitates mitochondrial Ca2+ uptake leading to an abrupt elevation of [Ca2+]m. In turn, [Ca2+]m plays an important role in cell bioenergetics and ATP synthesis in particular. Ca2+-dependent regulation of ATP synthesis involves several mechanisms, including the tricarboxylic acid cycle’s Ca2+-dependent dehydrogenase regulation (for review see ref. 49) and Ca2+ binding by ATP synthase β-subunit (complex F1) itself (50).
eTRPM8 Signaling in Keratinocyte Proliferation.
ATP synthesized in keratinocytes is not only used in energy-dependent processes, but also secreted into interstitial compartment (51, 52) to support paracrine purinergic signaling. This signaling primarily targets metabotropic P2Y receptors, activation of which in basal keratinocytes stimulates their proliferation (53). Of P2Y-receptor subtypes, ADP/ATP-sensitive P2Y1 (EC50 ∼ 10–50 nM for ADP and ∼180–320 nM for ATP) and ATP/UTP-sensitive P2Y2 (EC50 ∼ 100–500 nM for ATP and UDP) were found in basal and suprabasal compartments (54). Induction of proliferation in stratum basale (SB) by extracellular ATP was demonstrated to be independent on extracellular Ca2+ and to involve Ca2+ release from the ER (55), which favors recruitment of P2Y vs. P2X receptors. In our ATP quantification assay, we estimated that [ATP]e in the media with basal nonstimulated keratinocytes grown at 37 °C ranges from 10 nM to 100 nM, which is close to the effective concentrations for P2Y receptor activation. This suggests that cold-induced eTRPM8 activation may promote purinergic signaling via enhanced synthesis and secretion of ATP. Indeed, our data demonstrate that both increased eTRPM8 expression and activity elevate ATP concentration in keratinocytes as well as in surrounding medium. Together with previous studies showing that optimal in vivo ATP secretion by keratinocytes is achieved at about 31 °C (12), our data indicate that ATP synthesis and/or secretion can be regarded as cold-dependent processes in which eTRPM8-mediated Ca2+ signaling plays an important role.
We have also demonstrated that eTRPM8 expression and activity correlate with accumulation of superoxide (O2•−) and likely a concomitant accumulation of peroxynitrite in mitochondria. Both matrix mitochondrial Mn superoxide dismutase (SOD2) and Cu/Zn cytosolic and interspace mitochondrial superoxide dismutase (SOD1) are expressed in basal/suprabasal keratinocytes and provide antioxidant defense of these cells by catalysis of dismutation (40). Our data suggest a minor role, if any, of in eTRPM8-dependent proliferation of basal keratinocytes. The proposed signaling pathways by which eTRPM8 stimulates proliferation of basal keratinocytes are summarized in Fig. S8 (Upper Left).
eTRPM8 Signaling in Keratinocyte Differentiation.
We have found that eTRPM8 expression is increased in differentiated layers of epidermis and that exposure to mild cold stimulates its activity. Furthermore, we have demonstrated that mild cold enhances the differentiation rate of keratinocytes in an eTRPM8-dependent manner. Although involvement of ATP in keratinocyte differentiation currently lacks direct evidence, the fact that a proportion of FLG-positive cells show the same cold dependence as ATP synthesis strongly supports this idea. In addition to P2Y receptors, keratinocytes also express Ca2+-permeable ionotropic P2X purinoceptors. Homomeric P2X5 receptor (EC50 ∼ 5–10 µM for ATP) was identified in the basal, suprabasal, and spinal compartments (54), whereas homomeric P2X7 receptor (EC50 ∼ 100 µM for ATP) was detected in the late spinal and granular layers, where it seems to be involved in late differentiation by mediating Ca2+-dependent activation of TGM1 and, thus, leading to keratinocyte death: transformation of keratinocytes into corneocytes.
We also demonstrated that mild cold stimulates eTRPM8-dependent accumulation of O2•−. Our data indicate that, at least partially, this O2•− originates from mitochondria, although it remains unclear whether long-term activation of eTRPM8 induces NADPH oxidase-dependent synthesis in cytosol. ROS and more specifically have been shown to be involved in keratinocyte differentiation (41). Furthermore, the ROS gradient from basal to upper layers is characteristic of the epidermis (56). However, strong accumulation of induces an increase of the number of suprabasal/spinal cells paralleled with the decrease of the quantity of granular cells. Thus, our results suggest that differentiation of keratinocyte is a fine-tuned process involving O2•− accumulation and elevation of ATP concentration in eTRPM8-dependent manner. The optimal progression of this process is achieved at a physiological skin temperature of about 31 °C. Based on these findings we summarized in Fig. S8, Upper Right and Lower Right the signaling pathways involved in the cold-stimulated eTRPM8-dependent differentiation of keratinocytes.
eTRPM8 vs. TRPV3 in Epidermal Homeostasis.
Identified in keratinocytes, warmth-activated TRPV3 has been proposed to participate in activation of transglutaminase (13, 14) and, as consequence, to be involved in terminal differentiation of keratinocytes. However, for a healthy human the mean physiological skin temperature is about 32 °C (2), which is slightly below the threshold for TRPV3 activation. Interestingly, TRPV3 and eTRPM8 complement each other in thermosensitivity (i.e., activation by warmth vs. cooling) and the Ca2+ signaling mechanisms they engage (i.e., mediation of Ca2+ entry vs. Ca2+ release). Thus, relative expression and activity of eTRPM8 and TRPV3 may provide skin with an adaptive capacity to variations in environmental temperatures.
In conclusion, we report the identification of a new TRPM8 isoform, eTRPM8, which enables cold-dependent regulation of epidermal homeostasis. eTRPM8 is a key controller of temperature-dependent energy metabolism and signaling in keratinocytes that provides skin with the capacity to adapt to large variations of environmental temperature.
Materials and Methods
Cell Culture.
Establishment of trpm8−/− Mice.
To suppress ion channel activity of every channel-like TRPM8 isoform, we inserted LoxP sites with homologous recombination in introns 17 and 20. This ultimately led to a 2,500-bp deletion including exons 18–20, which encode transmembrane domains 3–5 in addition to the first part of the P loop of the TRPM8 channel. Please refer to SI Materials and Methods for extended description of the methods. This study was carried out in strict accordance with the French Law (Articles R214-117 to 121 of the rural code) and recommendations in the Guide for the Care and Use of Laboratory Animals (www.recherche-animale.org).
Molecular Biology and Biochemistry.
See SI Materials and Methods and Table S1.
Table S1.
Oligonucleotides used in the present study
| Gene | 5′ forward 3′ | 5′ reverse 3′ |
| Cloning | ||
| TRPM8(15a) | TAAGAATGGACTCACGCACAGG | TCTCAAGGTCTCAGCACACTA |
| PCR | ||
| hTRPM8(ex 2–7) | GAAGGAATGACACTCTGGAC | GCCATTGTCCACGAGCAGC |
| hTRPM8 (11-14) | GATTTTCACCAATGACCGCCG | CCCCAGCAGCATTGATGTCG |
| hTRPM8 (20-21) | TGCTGCAGAGGATGCTGATCG | GGTGCCCACCGTGTAGCCAA |
| hTRPM8 (15a-17) | GTGCTGATGTCGCTGTAGAGC | GAGGAAGGCGATGTAGAAGACC |
| mTRPM8(ex16-18) | CGAGACACGAAGAACTGGAAG | CTGCCTCACTTCATCACAGAAG |
| mGAPDH | CTGCGACTTCAACAGCAACTC | TCCACCACCCTGTTGCTGTA |
| Real-time PCR | ||
| GAPDH | ACCCACTCCTCCACCTTTG | CTCTTGTGCTCTTGCTGGG |
| TRPM8 | CGGTCATCTACGAGCCCTAC | CACACACAGTGGCTTGGACT |
| PCNA | CGACACCTACCGCTGCGACC | TAGCGCCAAGGTATCCGCGT |
| CDKN1A | TCAGGGTCGAAAACGGCGGC | TTTGAGGCCCTCGCGCTTCC |
| CDKN1B | AGCGGAGCAATGCGCAGGAA | GGCGTCTGCTCCACAGAACCG |
| Keratin 1 | ATTTCTGAGCTGAATCGTGTGATC | CTTGGCATCCTTGAGGGCATT |
| Keratin 5 | CTGCTGGAGGGCGAGGAATGC | CCACCGAGGCCACCGCCATA |
| Keratin 10 | TGATGTGAATGTGGAAATGAATGC | GTAGTCAGTTCCTTGCTCTTTTCA |
| Involucrin | CTGCCTCAGCCTTACTGTGA | GGAGGAGGAACAGTCTTGAGG |
| Filaggrin | CTGGACACTCAGGTTCCCAT | TTTCGTGTTTGTCTGCTTGC |
| Transglutaminase 1 | TCACTGTTTCATTGTCTCCA | CCCTCACCAATGTCGTCTTC |
Electrophysiology.
Visualization of the ER and Mitochondria.
Visualization of Agonist-Induced Changes of Ca2+ Concentration in Cytosol ([Ca2+]c) and Mitochondria ([Ca2+]m).
Changes of [Ca2+]c and [Ca2+]m were imaged using the high-affinity fluorescent Ca2+ indicator fluo-4 and Ca2+ indicator rhod-2. Mitochondrial Ca2+ signal was also evaluated 48 h after transfection of M8-CTL and M8-KD cells with an adenoviral mutated mitochondria-targeted aequorin probe (mtAEQmut). For extended procedures, refer to SI Materials and Methods.
Superoxide Assessment.
Steady-state superoxide concentration [] was assessed in living hNEKs or HaCaT cells after 72 h or 24 h culturing at 37 °C, 31 °C, or 25 °C, using CellRox Deep Red Reagent (Molecular Probes), loaded by 30-min incubation of the cells with 2.5 µM of the dye at 37 °C. Please refer to SI Materials and Methods for extended description of the methods.
Time domain–fluorescence lifetime imaging microscopy (TM-FLIM) was achieved to estimate the difference in steady-state [Ca2+]m with a Cameleon bioindicator. For details, see SI Materials and Methods.
Data Analysis and Statistical Procedures.
Each experiment was repeated at least three times independently. Data are expressed as mean ± SD when not indicated in legends. The data were analyzed and graphs plotted using Origin 5.0 software (Microcal). InStat3 (GraphPad Software) was used for statistical analysis and the mean values were compared using either an unpaired t test with Welch’s corrected test (two groups) or a one-way ANOVA with a Dunnett multiple-comparison posttest (three or more groups). Statistical significances were as follows: *P < 0.05, **P < 0.01, and ***P < 0.001. Smooth curves on the graphs were obtained by interpolation of data points, using a parametric Lagrange second-degree polynomial: parabola for interpolation of three data points.
SI Materials and Methods
Cell Culture.
The HaCaT cell line was grown in Dulbecco’s minimal essential media (DMEM) (Gibco) supplemented with 2% (vol/vol) FCS and kanamycin (100 µg/mL), and [Ca2+] was adjusted to 1.8 mM to induce differentiation. hNEK cells were obtained from Lifescience and plated in 10-cm round Petri dishes in basal Keratinocyte-SFM (serum free medium) media at a density of 500,000 cells per dish. Basal Keratinocyte-SFM media were supplemented with bovine pituitary extract, EGF, glutamine, and kanamycin (Invitrogen). Induction of differentiation, “induced cells,” was achieved by adding 2% FCS and 1.8 mM Ca2+ to basal Keratinocyte-SFM, as described previously (24).
Establishment of trpm8−/− Mice.
To suppress ion channel activity of every channel-like TRPM8 isoform, we inserted LoxP sites with homologous recombination in introns 17 and 20. This ultimately led to a 2,500-bp deletion including exons 18–20, which encode transmembrane domains 3–5 in addition to the first part of the P loop of the TRPM8 channel. After homologous recombination in 129SV/PAS ES cells and G418 selection, insertion of vectors was controlled with Southern blot and PCR. Selected ES cells were used for blastocyte injection and led to the generation of male chimeras. These were bred with CMV-Cre C57BL/6J females to generate a heterozygous TRPM8 knockout. Agouti-colored pups were selected for transmission of mutant chromosome in the germ line prior to PCR validation of the deletion for the TRPM8 knockout line. F1 animals were interbred to obtain F2 homozygote trpm8−/− mice as well as control homozygote trpm8+/+ mice, as confirmed by PCR after blood collection. Scale-up of trpm8−/− colonies was achieved by backcrossing the trpm8−/− line with a control trpm8+/+ line. According to the Jackson Laboratory recommendations for breeding strategies, interbreeding was performed for eight generations to prevent substrain apparition and genetic divergence. This study was carried out in strict accordance with the law (Articles R214-117 to 121 of the rural code), and the recommendations in the Guide for the Care and Use of Laboratory Animals of the French National Institute for Medical Research (www.recherche-animale.org/). The protocol has been approved by the Committee on the Ethics of Animal Experiments of the University of Lille 2. All efforts were made to minimize animal suffering.
Primary Culture of Mouse Keratinocytes.
After euthanasia, back skin of C57BL/6J mice was shaved before skin removal. Pieces of skin were either fixed in formol prior to inclusion in paraffin or used for keratinocyte (mPK) isolation. To isolate the cells, s.c. tissues were removed and pieces of the skin, dermis side down, were put into the solution supplemented with 0.25% trypsin. Freely floating pieces of the skin (with unsubmerged epidermis) were incubated overnight at 4 °C. Then, dermis was removed and remaining epidermal tissue was cut into 250-mm2 pieces, which were transferred to Defined-SFM media (Gibco) supplemented with 10% FCS to inhibit trypsin. The pieces of epidermis were then triturated with a wide-bore pipette and residual hair, connective tissue, and debris were removed from the suspension by sieving with a 70-µm nylon mesh. The cell suspension was then centrifuged for 10 min at 250 × g and keratinocytes were resuspended in serum-free Defined-SFM media (to suppress fibroblast growth) and were grown in Petri dishes. After 5 d in culture the cells were transferred to the serum-free Ca2+-free Keratinocyte-SFM media to stimulate the cell growth without differentiation. When required, induction of differentiation was achieved by adding 2% FCS and 1.8 mM Ca2+ to Keratinocyte-SFM media.
Transient Expression Studies.
Plasmid transfection was carried out with X-tremeGENE HP DNA Transfection Reagent (Roche) at the concentration of 4 µg/1 million cells. SiRNA transfection was performed with 50 nM of HiPerfect transfection reagent (Qiagen). hNEKs transfection was conducted twice with a 2-d interval to improve the transfection rate.
RACE-PCR and Cloning of eTRPM8.
RACE-PCR was performed as reported previously (57). Briefly, 5′ extremities were cloned from total mRNA after the first RACE amplification followed by a Nested-RACE PCR, using materials derived from the SMART RACE-PCR kit (Clontech). Full-length variants were cloned from the same set of total mRNA, using Prime Script reverse transcriptase. Long-distance PCR was conducted using Phusion polymerase (Finnzymes), GC rich buffer, and specific primers designed on extremities of RACE-amplified DNA (for primers, see Table S1). The DNA amplification procedure included 30 s denaturation at 98 °C, followed by 15 cycles of 5 s at 98 °C, 15 s at 68 °C, and 30 s/kbp at 72 °C; 20 cycles of 5 s at 98 °C, 15 s at 64 °C, and 30 s/kbp at 72 °C; and finally 5 min at 72 °C. After agarose gel purification and DNA recovery, amplicons were subjected to terminal adenylation with TaqGold polymerase (Applera) and cloned overnight in pGem-T easy vector with T4 ligase at 14 °C. Finally, wild-type inserts were transferred into pcDNA4-TO-A vector with appropriate restrictases. Alternatively, inserts were modified with a consensus KOZAK sequence and fused in frame with HA tag at their C terminus.
siRNA and shRNA plasmids were built in pENTER vector (Invitrogen) following the manufacturer’s recommendations. Control shRNA targeted TRPM8 exon 7 and shRNA anti-eTRPM8 targeted exon 20. shRNA sense sequences are CACCAtctctgagcgcactattcaGAGAGtgaatagtgcgctcagaga and CACCAtattccgttcggtcatctaGAGAGtagatgaccgaacggaata (uppercase letters refer to the structure of the short hairpin RNA and lowercase letters refer to the siRNA sequence). Control of silencing efficiency was achieved by immunoblotting of total proteins extracted from Hek cells, cotransfected with either shCTL + eTRPM8pcDNA4 or shM8 + eTRPM8pcDNA4.
Control siRNA targeted Luciferase (siLuc) and siRNA anti-eTRPM8 (siTRPM8) targeted exon 20. The sense sequences are CUUACGCUGAGUACUUCGA(dTdT) and GUAUUCUGGACGAGUCAUU(dTdT), respectively.
Analysis of TRPM8 mRNA Expression.
Total RNA was isolated from different cell lines, using Tri Reagent mix (Sigma-Aldrich). After a DNase I (Invitrogen) treatment and phenol/chloroform purification, 2 µg of total RNA was reverse transcribed into cDNA at 42 °C, using random hexamer primers (Applera) and MuLV reverse transcriptase (Applera).
Qualitative PCR was performed with TaqGold polymerase (Applera) and controlled on an agarose gel.
Real-time quantitative PCR was performed on a Cfx C1000 system (Bio-Rad). For each reaction, 12.5 ng of cDNA was placed in 15 μL of final reaction medium containing 7.5 µL of 2× SsoFast EvaGreen Supermix (Bio-Rad) and 200 nM primer pairs (Table S1). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control to normalize variations in RNA extracts, the degree of RNA degradation, and variability in RT efficiency. To quantify the results, we used the comparative Ct method. PCR protocol consisted of 30 s denaturation at 95 °C followed by 40 cycles of (4 s at 95 °C and 30 s at 60 °C) and a final dissociation curve to control specificity of the amplification.
In Situ Hybridization.
Human skin sections were obtained from resection specimens after breast reduction surgery according to the Declaration of Helsinki Principles and the guidelines of the ethical committee of the Medical Center, CHRU Lille prepared in agreement with French law. The skin sections (10 µm thick) were mounted onto gelatin-coated slides and air-dried. After PCR amplification, the sense and antisense probe sequences were cloned into pcR2.1 TA cloning (Invitrogen) and checked for sequencing. The vector was linearized, and the probes were transcribed with the SP6/T7 transcription kit (Roche Diagnostics) and [35S]-dUTP (1,300 Ci/mmol; Amersham Biosciences). In situ hybridization (ISH) was performed as previously described (58), and finally radioactivity was revealed using X-ray film (Biomax-MR; Kodak) before digitalization. The sense sequence of the probe (318 bp) was cloned with forward primer CGTTCGGTCATCTACGAGC and backward primer GCAGTACTCCTGCACCAGG and correspond to the sequence of the pore domain. Antisense probe was generated using the complementary primer sequences.
Immunoblotting.
Tissues were collected and stored in liquid nitrogen until protein extraction. Pieces of skin were transferred in beads containing tubes and 500 µL of an ice-cold buffer (pH 7.2) containing 10 mM PO4Na2/K buffer, 150 mM NaCl, 1 g/100 mL sodium deoxycholate, 1% Triton X-100, 1% Nonidet P-40, and a mixture of protease inhibitors (Sigma-Aldrich), and a phosphatase inhibitor (sodium orthovanadate; Sigma-Aldrich) was added. Protein extraction was achieved with two cycles of 30 s at 6,500 × g in a high-throughput PRECELLYS24 tissue homogenizer cooled down with CRYOLIS at 4 °C.
Cultured cells were collected in a PBS solution and then pelleted prior to extraction in the ice-cold buffer described above. After 30 min incubation on ice, the protein extract was further subjected to sonication and debris were pelleted at 15,000 × g at 4 °C for 10 min. Protein concentration was measured by the mean of a BCA protein assay (Pierce). A total of 25 µg of total protein was loaded onto a 10% polyacrylamide gel before SDS-PAGE was performed. After electrophoresis, proteins were transferred to a nitrocellulose membrane, using a semidry electroblotter (Bio-Rad). The membrane was blocked in TNT + 5% (wt/vol) milk (15 mM Tris buffer, pH 8, 140 mM NaCl, 0.05% Tween 20, and 5% nonfat dried milk) for 30 min at room temperature and then soaked in primary antibody diluted in TNT + 1% milk for either 2 h at room temperature or overnight at +4 °C. After three washes in TNT, the membrane was soaked in secondary antibody diluted in TNT + 1% milk for 1 h at room temperature. The membrane was processed for chemiluminescence detection using Luminata Forte Western HRP Substrate (Millipore) according to the manufacturer’s instructions. After a 10-min bath in Reblot PLus Mild SOlution (Millipore), the membrane was blotted again. The primary antibodies were rabbit anti-TRPM8 channel [Anti-TRPM8 (extracellular), cat. no. ACC-049, lot AN-03; Alomone, 2009 batch] or anti-TRPM8 channel (Anti-TRPM8 ab109308, lot GR47573-2; Abcam, 2011–2012 batches) at 1 μg/mL, rabbit anti-HA tag (Sc-805; Santa Cruz, 1/200), mouse anti-actin (Lab Vision; 1/1,000), and mouse anti-Calnexin (MAB3126; Millipore, 1/1,000).
Immunohistofluorescence.
Experiments were performed on 6-µm-thick sagittal slices of the palm skin from WT, five trpm8−/− mice, or five trpm8−/− (DJ) mice, obtained with Microm HM355S (Thermo Scientific). After paraffin removal, antigen retrieval was achieved in a citrate buffer boiled four times for 5 min in a microwave oven. After three washes in PBS, the tissue sections were blocked with PBS supplemented with 1.2% gelatin (PBS/gelatin) for 30 min at 37 °C and then coincubated with primary antibodies diluted in PBS/gelatin for 2 h at 37 °C. After thorough rinsing in PBS/gelatin, the slides/dishes were treated with the corresponding secondary antibody: either Dye light 488-labeled anti-rabbit IgG (Jackson ImmunoResearch; dilution 1/2,000) or Alexa fluor 546-labeled anti-mouse IgG (Molecular Probes; dilution 1/4,000) diluted in PBS/gelatin for 1 h at room temperature (RT). After rinsing twice in PBS/gelatin and once in PBS with 1/200 DAPI for 10 min at RT, the slides were mounted with Mowiol and examined under a confocal microscope. Primary antibodies used were anti-Keratin 5 (K5) (Covance) at 1/2,000 dilution, anti-Keratin 10 (K10) (Covance) at 1/10 dilution, anti-Involucrin (INV) (Sigma) at 1/1,000 dilution, anti-Loricrin (LR) (Abcam) at 1/1,000 dilution, anti-PCNA (Santa Cruz) at 1/100 dilution, and anti-TRPM8 channel [Anti-TRPM8 (extracellular), cat. no. ACC-049, lot AN-03; Alomone, 2009 batch] or anti-TRPM8 channel (Anti-TRPM8 ab109308, lot GR47573-2; Abcam, 2011–2012 batches) at 1 μg/mL. The two anti-TRPM8 antibodies recognize close regions of TRPM8 pore encoded by exons 21–22 and 21, respectively, and can thus detect classical full-length TRPM8 and TRPM8 isoforms as well. Analysis of the epidermis structure was performed using an LSM 780 confocal workstation (Zeiss). In each skin slice, imaging was performed on four randomly selected 500-μm-wide regions of interest (ROI). In each ROI, the thickness of K5-, K10-, and LN-positive compartments was measured at 3 x positions. The measurements were performed on the slices from five WT and five trpm8−/− mice, giving in total 60 measurements per condition. To compensate for the effect of deviations in the cut angle on the apparent thickness of the epidermis crosscuts, the thickness of K5-, K10-, or LR-positive compartments was normalized to the total thickness of stratum basale (SB) + stratum spinosum (SS) + stratum granulosum (SG), measured from the overlay of the transmitted light image and confocal image of DAPI fluorescence. Mean ± SED values were compared for WT and trpm8−/− mice.
Measurement of the Stratum Corneum Thickness.
Deparaffinized slides of mouse skin from snout, palm, back skin, and tail were subjected to Hemalum-erythrosin-safran trichrome staining. The slides were analyzed on an upright Axio Imager.A1 microscope (Zeiss). Images were acquired with an AxioCam MRc5 digital camera (Zeiss) and Axionvision software was used for analysis. Measurements were performed on intact samples of stratum corneum of eight WT mice and eight trpm8−/− mice.
Flow cytometry was conducted with a CyAn ADP Analyzer. Cells were harvested, split into 1 million samples in 15-mL tubes, and fixed overnight with 1 mL of 70% ethanol at −20 °C. Cells were then washed twice with PBS, supplemented with 4% BSA and 0.1% Triton X100, and incubated at RT for 30 min. After that, the cells were transferred for 1 h to PBS-BT supplemented with the primary antibodies at the same dilutions as described above. After two cycles of washout in PBS the cells were incubated for 30 min at RT with secondary antibodies: anti-rabbit IgG coupled to DL-488 and anti-mouse IgG coupled to AF-647, diluted at 1/2,000 and 1/4,000, respectively. Following three washout cycles at RT flow cytometry was commenced. The flow cytometer was calibrated with rainbow beads before each experiment. Laser lines of 488 nm and 642 nm were used. Data were analyzed with FlowJo software (v 8.7).
Dehydrogenase Quantization Assay.
The cells were trypsinized, counted, and transferred to 96-well plates: 20,000 cells per well in 100 µL of medium. After 2–4 h of recovery at the desired incubation temperature (37 °C, 31 °C, or 25 °C), the cells were subjected to a CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay (Promega). Although this assay kit is commonly used for quantization of growth, it is based on the assessment of dehydrogenase (DHase) activity by means of measuring optical density (OD) reflecting absorption of light by formazan at 490 nm. We modified this procedure to measure DHase activity by fixing the cell number at a constant level. Briefly, 100 µL of MTS solution (Promega) was added to each well and OD was measured 30 min, 60 min, 90 min, and 120 min later. Apparent enzymatic activity (OD/min) was estimated from the fit of linear function f(OD) = a•T(min) + b to the data, after correction for the background OD, measured in a cell-free well containing 100 µL of medium and 100 µL of MTS solution.
Electrophysiology.
Membrane currents in TRPM8-expressing HEK cells were recorded in the whole-cell configuration, using the patch-clamp technique and a computer-controlled EPC-9 amplifier (HEKA Electronic). Patch pipettes were made using a P-97 puller (Sutter) from borosilicate glass capillaries (WPI). Patch pipettes (resistance 3–5 MΩ) were filled with the following solution: 120 mM Cs-Met, 10 mM CsCl, 6 mM MgCl2, 10 mM BAPTA, 2.5 mM CaCl2 (calculated free Ca2+ concentration: 150 nM), 10 mM Hepes, pH adjusted to 7.3 with CsOH (osmolality: 305 mosmol/L). The extracellular solution used to record TRPM8 current contained 140 mM NaCl, 5 mM KCl, 10 mM CaCl2, 2 mM MgCl2, 0.3 mM Na2HPO4, 0.4 mM KH2PO4, 4 mM NaHCO3, 5 mM glucose, 10 mM Hepes, pH adjusted to 7.3 with NaOH (osmolality, 330 mosmol/L).
For giant unilamellar vesicles (GUV) patch-clamp experiments, we used Axopatch 200B amplifier and pClamp 10.0 software (Molecular Devices) for data acquisition and analysis. Patch pipettes were fabricated from borosilicate glass capillaries (World Precision Instruments) on a horizontal puller (Sutter Instruments) and had a resistance in the range of 7–10 MΩ. Prepared vesicles were immersed in a bath solution containing 150 mM NaCl, 5 mM glucose, 10 mM Hepes, pH 7.2. To prevent activity of IP3R and RyR channels, commonly present in the ER, this solution was supplemented with 1 mM MgCl2, 1 mM EGTA, and 1 μM dantrolene (59). Patch pipettes were filled with the same solution, supplemented by 2 μM PiP2 and 1 mM MgATP to enhance TRPM8 activity (60).
Experimental Solutions.
In confocal microscopy experiments the cells were bathed in physiological salt solution (PSS) containing 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, 10 mM Hepes, pH adjusted to 7.4 with NaOH. PSS was supplemented with 70 μM or 1.7 mM CaCl2, depending on experimental protocol, as described in the text.
Preparation of GUVs.
GUVs were prepared from the 1:5 mixtures of the ER-containing fraction with 10:1 diphytanoylphosphatidylcholine (DPhPC)/cholesterol lipid combination (5 mM). This mixture was supplemented by 0.2 mM phosphatidylinositol 4,5-bisphosphate (PiP2) to sustain TRPM8 activity (60).
Wide-Field Ca2+ Imaging.
Calcium imaging experiments were performed as described previously (44). Briefly, [Ca2+]c was measured using the ratiometric dye fura-2 and quantified according to the Grynkiewicz equation (61). The bath solution contained 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 0.3 mM Na2HPO4, 0.4 mM KH2PO4, 4 mM NaHCO3, 5 mM glucose, 10 mM Hepes, pH adjusted to 7.3 with NaOH (osmolality 330 mosmol/L).
Visualization of the ER and Mitochondria.
The ER was visualized using Brefeldin A BODIPY 558/568 (62, 63), which was loaded by a 20-min incubation of the cells with 2 µM of the dye followed by a 1.5-h wash. The mitochondria were visualized using MitoTracker Green FM, which was loaded by a 30-min incubation of the cells with 2 µM of the dye followed by a 30-min wash.
Visualization of Agonist-Induced Changes of Ca2+ Concentration in Cytosol ([Ca2+]c) and Mitochondria ([Ca2+]m).
Changes of [Ca2+]c were imaged using the high-affinity fluorescent Ca2+ indicator fluo-4, as described previously (44, 63). Fluo-4 was loaded by a 1-h incubation of the cells with 5 µM fluo-4 acetoxymethyl ester (fluo-4 AM; diluted from a stock containing 2 mM fluo-4 AM and 0.025% (wt/vol) pluronic F-127 in dimethyl sulfoxide). Changes of [Ca2+]m were imaged using the fluorescent Ca2+ indicator rhod-2: A total of 50 μg of rhod-2 AM was dissolved in 10 μL of DMSO [containing 0.025% (wt/vol) pluronic F-127], which was then mixed with 4 mL of PSS and superfused to the experimental chamber for 20 min. The incubation of the cells with the dyes was followed by a 1-h wash in PSS containing 1.7 mM CaCl2 to allow time for deesterification. The dye loading was performed at RT. The cells were then kept for 30 min at 37 °C. Before imaging was commenced the cells were superfused with PSS containing 70 μM CaCl2 and supplemented with 10 μM LaCl3 to eliminate capacitative Ca2+ entry, unless stated otherwise. The cells were stimulated with either 200 µM menthol or 10 μM icilin. The intensity of fluo-4 or rhod-2 fluorescence (F) was normalized to the average fluorescence intensity in the images acquired before agonist application (F0). The temporal profiles of the agonist-induced [Ca2+]c and [Ca2+]m transients are illustrated by the plots showing the time course of the normalized fluo-4 or rhod-2 fluorescence intensity (F/F0) averaged within a confocal optical slice of the cell.
Superoxide Assessment.
The hNEKs were transfected either with control siRNA (siLuc) or with siRNA targeting the pore-encoding sequence of TRPM8 (siTRPM8), whereas HaCaT cells were transfected with empty vector (CTL), eTRPM8 plasmid, or eTRPM8 + SOD1 plasmids. Cells were incubated in the medium supplemented with 2.5 µM CellRox Deep Red reagent for 30 min at 37 °C. After two washes, cells were incubated for 30 min at 37 °C before confocal imaging. For each temperature and siRNA condition, the measurements were performed in three Petri dishes, sequentially mounted on the microscope stage at the ambient temperature corresponding to the preincubation temperature. In each Petri dish, confocal imaging of CellRox Deep Red fluorescence was performed from four regions of interest, 230 × 230 µm (1,024 × 1,023 pixels) each. The same illumination intensity, photomultiplier gain, and offset were used in all of the experiments. Data were processed with ImageJ64 freeware. After setting offset (pixel fluorescence intensity below 20 a.u.) and the mean cell background (pixel fluorescence intensity 100 a.u.) in the control condition (cells grown at 37 °C) specific to , increase in the CellRox Deep Red fluorescence was computed according to the formula , where PixI(20–255) and PixI(100–255) are the pixels with intensities lying within the range of 20–255 a.u. and 100–255 a.u., respectively. The O2•−-specific increases in the CellRox Deep Red signal mass for each condition were averaged, normalized to the mean value, detected at 37 °C either in hNEKs transfected with siLuc or HaCaT cells transfected with empty vector, and compared. Data are presented as mean ± SD.
Specificity of CellRox Deep Red oxidation by superoxide or peroxynitrite was assessed with Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP). Cells were incubated for 30 min at 37 °C in the medium supplemented with 100 µM MnTBAP. After three washes in MnTBAP-free medium, the cells were then incubated with CellRox Deep Red as described above.
Icilin-induced accumulation in mitochondria was performed using MitoSOX Red. Briefly, 50 μg of MitoSOX Red was dissolved in 20 μL of DMSO [containing 0.025% (wt/vol) pluronic F-127] and aliquoted at 2 μL. One aliquot was then dissolved in 2 mL of external solution and superfused to the experimental chamber. The cells were stained for 10 min followed by a 30-min wash, all at 37 °C. Mean rates of accumulation were estimated as mass of MitoSOX fluorescent signal per second following icilin application, . Experiments were conducted at 37 °C.
ATP Quantization Assay.
Intra- and extracellular ATP concentrations were quantified with the ATP bioluminescence assay kit HS II (Roche). For extracellular ATP, 500 µL of 24-h conditioned media was picked up and filtered with 0.2-µm filters to remove floating cells. A total of 100 µL of media was mixed with luciferase-containing solution before luminescence quantization in a LUMAT LB 9507 system (Berthold LUMAT LB 9507). Intracellular ATP was measured as follows. After trypsinization 1 million cells were gently spun down at 180 × g for 8 min. The pellet was then resuspended in lysis buffer for 10 min on ice, and the cells were spun down again at 12,000 × g for 10 min. Supernatant (100 µL) was then diluted 10× and 100× with dilution buffer and finally 100 µL of diluted solution was mixed with 100 µL of luciferase-containing solution prior to luminescence quantization. For both intra- and extracellular ATP quantization, the cells were counted and total cellular protein was extracted. The protein extracts were quantified with a BCA protein assay and luminescence intensity (RLU) was normalized to the weight of the extracted protein (RLU/µg).
Confocal Microscopy.
Experimental chambers with the cells were placed on the stage of an Axiovert 200M inverted microscope attached to an LSM 510 META laser-scanning unit (Zeiss). The SCSi interface of the confocal microscopes was hosted by a Pentium PC (32-bit Windows NT 4.0 operating system) running LSM 510 software (Zeiss). During the time series protocol, the x-y confocal images of the fluo-4, rhod-2, MitoSOX Red, Brefeldin A BODIPY 558/568, or MitoTracker Green fluorescence were acquired at 0.1–0.5 Hz, using a Zeiss plan-Apochromat 40× 1.3 N.A. or 63× 1.4 N.A. oil-immersion objective. Fluo-4 and MitoTracker Green fluorescence was excited by the 488-nm line of a 500-mW argon ion laser (Laser-Fertigung) and was captured at wavelengths 505–530 nm. Rhod-2, Brefeldin A BODIPY 558/568, and MitoTracker Red fluorescence was excited by the 543-nm line of 15-mW helium/neon ion laser and was detected at wavelengths 560–615 nm or above 560 nm. The mTurquoise2 fluorescence was excited by a 405-nm line of a blue diode laser and was captured at wavelengths 475–525 nm. The MitoSOX fluorescence was excited by a 514-nm line of a 500-mW argon ion laser and was captured at wavelengths above 560 nm. The illumination intensity was attenuated to 0.5–6% (depending on the laser line) with an acousto-optical tunable filter (Zeiss). To optimize signal quality the pinhole was set to provide a confocal optical section of 0.5–1.8 µm, depending on experimental protocol. To avoid any bleed-through of the fluorescence signal in multistaining experiments, fluorochromes with well-separated excitation and emission spectra were used and imaging was performed using the frame-by-frame multitrack mode of the confocal scanner: sequential acquisition via well-separated optical channels of the x-y images produced by fluorescence of different fluorochromes. The photomultiplier gain and offset in each optical channel were set individually to achieve similar signal intensity at each channel and remove subsignal noise from the images. The adequacy of the imaging protocol applied to the multilabeled cells was confirmed by control experiments on the monolabeled cells.
Time Domain–Fluorescence Lifetime Imaging Microscopy.
For live-cell imaging, cells were placed on 35-mm glass-bottom dishes (MatTek Corporation), filled with L-15 medium without phenol red (Life Technologies), and kept at 37 °C using a stage incubator (Life Imaging Services). Fluorescence lifetime imaging microscopy (FLIM) was performed with a Leica TCS SP5 X confocal head (Leica Microsystems) with the SMD upgrade, mounted on an inverted microscope (DMI6000; Leica Microsystems). A pulsed diode laser, PDL 800-B (PicoQuant GMBH), delivered 40-MHz repetitive rate pulses at 405 nm. The confocal pinhole was set to 1 Airy, for a 0.921-µm optical slice. Single photon events that originated from the illuminated voxel were collected through a 63×/1.2 N.A. water-immersion objective and recorded by a TCSPC detector (HydraHarp 400; PicoQuant GMBH). Fluorescence was detected through a 483/32 single-bandpass filter (Semrock) on single photon avalanche photodiodes (SPAD) (MPD), set up at 256 × 256 pixels. Arrival time of single photons was measured with SymPhoTime software (PicoQuant GMBH) and images were taken with LAS AF software (Leica Microsystems). To obtain the best resolution of organelles, a fivefold zoom factor was applied, giving a pixel size of 0.193 µm and an image size of 49.21 × 49.21 µm. Because the statistical determination of the distribution of the single photon arrival time requires a minimum of 100 photons per pixel, 120 frames were acquired at 200 Hz and summed in the final image.
Aequorin Measurements.
Mitochondrial Ca2+ signal was evaluated 48 h after transfection of M8-CTL and M8-KD cells with adenoviral mutated mitochondria-targeted aequorin probe (mtAEQmut). mtAEQ was reconstituted with 5 μM coelenterazine for 2 h at 37 °C in Krebs–Ringer modified buffer composed of 125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM Na3PO4, 5.5 mM glucose, 20 mM Hepes, pH adjusted to 7.4 with NaOH. Aequorin measurements were carried out in a purpose-built luminometer. The experiments were terminated by the cell lysis with 100 μM digitonin in a hypotonic Ca2+-rich solution (10 mM CaCl2 in H2O), which discharges the remaining aequorin pool. The light signal was collected and calibrated into [Ca2+] values, as previously described (64).
Data Analysis and Statistical Procedures.
Each experiment was repeated at least three times independently. Data are expressed as mean ± SD when not indicated in legends. The data were analyzed and graphs plotted using Origin 5.0 software (Microcal). InStat3 (GraphPad Software) was used for statistical analysis and the mean values were compared using either an unpaired t test with Welch’s corrected test (two groups) or a one-way ANOVA with Dunnett’s multiple-comparison posttest (three or more groups). Statistical significances were *P < 0.05, **P < 0.01, and ***P < 0.001. Smooth curves on the graphs were obtained by interpolation of data points, using a parametric Lagrange second-degree polynomial: parabola for interpolation of three data points.
Supplementary Material
Acknowledgments
The authors thank Delphine Taillieu and Mélanie Besegher for their technical assistance for animals housing at “Animalerie High-Tech de l’Institut de Médecine Prédictive et de Recherche Thérapeutique de Lille” and for their help in the menthol experiment on shaved mice. The trpm8−/− mouse line was designed by G.B. and Genoway. The Trpm8−/− mouse line was established by Genoway and housed at Charles River Laboratories. The authors thank Marie Norbert and all of the Genoway team for their support and their competence. The trpm8−/− (DJ) mouse line is a generous gift from Prof. David Julius. The authors thank Dr. Jérôme Busserolles for providing them with these animals. D1ER cameleon and mt Cameleon are generous gifts from Prof. Roger Tsien. The authors thank Prof. Jean-Pierre Julien (Centre de Recherche, Centre Hospitalo-Universitaire de Laval) for his generous gift of pcDNA3-FlagN-SOD1 plasmid. This work has been supported by grants from Institut National de la Recherche Médicale, Ministère de l’Education Nationale, Ligue Nationale Contre le Cancer, Region Nord-Pas-de-Calais, and Agence Nationale de Recherche Grant REPROD. G.B. is supported by the Cosmetics Department of Johnson & Johnson Laboratories. A.-s.B. is supported by the Fondation pour la Recherche Médicale. D.G. is supported by the State Foundation for Fundamental Research (F 46.2/001). B.B. was supported by the French association “Fondation pour la Recherche Médicale.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database, www.ncbi.nlm.nih.gov/nuccore/ (accession nos. KC692993, KC692994, and KC692995).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423357112/-/DCSupplemental.
References
- 1.Fuchs E. Epidermal differentiation: The bare essentials. J Cell Biol. 1990;111(6 Pt 2):2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith AD, Crabtree DR, Bilzon JL, Walsh NP. The validity of wireless iButtons and thermistors for human skin temperature measurement. Physiol Meas. 2010;31(1):95–114. doi: 10.1088/0967-3334/31/1/007. [DOI] [PubMed] [Google Scholar]
- 3.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445(7130):858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 4.Caterina MJ, Julius D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 5.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 6.Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 7.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 8.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50(2):277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Obata K, et al. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115(9):2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandadi S, et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458(6):1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koizumi S, et al. Ca2+ waves in keratinocytes are transmitted to sensory neurons: The involvement of extracellular ATP and P2Y2 receptor activation. Biochem J. 2004;380(Pt 2):329–338. doi: 10.1042/BJ20031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gifford JR, Heal C, Bridges J, Goldthorpe S, Mack GW. Changes in dermal interstitial ATP levels during local heating of human skin. J Physiol. 2012;590(Pt 24):6403–6411. doi: 10.1113/jphysiol.2012.240523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moqrich A, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307(5714):1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 14.Peier AM, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296(5575):2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 15.Cheng X, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010;141(2):331–343. doi: 10.1016/j.cell.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denda M, Tsutsumi M, Denda S. Topical application of TRPM8 agonists accelerates skin permeability barrier recovery and reduces epidermal proliferation induced by barrier insult: Role of cold-sensitive TRP receptors in epidermal permeability barrier homoeostasis. Exp Dermatol. 2010;19(9):791–795. doi: 10.1111/j.1600-0625.2010.01154.x. [DOI] [PubMed] [Google Scholar]
- 17.Bautista DM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448(7150):204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 18.Dhaka A, et al. TRPM8 is required for cold sensation in mice. Neuron. 2007;54(3):371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Bidaux G, et al. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J Clin Invest. 2007;117(6):1647–1657. doi: 10.1172/JCI30168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bidaux G, et al. Evidence for specific TRPM8 expression in human prostate secretory epithelial cells: Functional androgen receptor requirement. Endocr Relat Cancer. 2005;12(2):367–382. doi: 10.1677/erc.1.00969. [DOI] [PubMed] [Google Scholar]
- 21.Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G. ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium. 2007;42(4–5):427–438. doi: 10.1016/j.ceca.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci. 2006;26(18):4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck B, et al. Prospects for prostate cancer imaging and therapy using high-affinity TRPM8 activators. Cell Calcium. 2007;41(3):285–294. doi: 10.1016/j.ceca.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Borowiec AS, Delcourt P, Dewailly E, Bidaux G. Optimal differentiation of in vitro keratinocytes requires multifactorial external control. PLoS ONE. 2013;8(10):e77507. doi: 10.1371/journal.pone.0077507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanden Abeele F, Roudbaraki M, Shuba Y, Skryma R, Prevarskaya N. Store-operated Ca2+ current in prostate cancer epithelial cells. Role of endogenous Ca2+ transporter type 1. J Biol Chem. 2003;278(17):15381–15389. doi: 10.1074/jbc.M212106200. [DOI] [PubMed] [Google Scholar]
- 26.Poburko D, Fameli N, Kuo KH, van Breemen C. Ca2+ signaling in smooth muscle: TRPC6, NCX and LNats in nanodomains. Channels. 2008;2(1):10–12. doi: 10.4161/chan.2.1.6053. [DOI] [PubMed] [Google Scholar]
- 27.Shkryl VM, Shirokova N. Transfer and tunneling of Ca2+ from sarcoplasmic reticulum to mitochondria in skeletal muscle. J Biol Chem. 2006;281(3):1547–1554. doi: 10.1074/jbc.M505024200. [DOI] [PubMed] [Google Scholar]
- 28.Pralong WF, Spät A, Wollheim CB. Dynamic pacing of cell metabolism by intracellular Ca2+ transients. J Biol Chem. 1994;269(44):27310–27314. [PubMed] [Google Scholar]
- 29.Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82(3):415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 30.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96(24):13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormack JG, Denton RM. Role of calcium ions in the regulation of intramitochondrial metabolism. Properties of the Ca2+-sensitive dehydrogenases within intact uncoupled mitochondria from the white and brown adipose tissue of the rat. Biochem J. 1980;190(1):95–105. doi: 10.1042/bj1900095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denton RM, McCormack JG, Edgell NJ. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansford RG, Zorov D. Role of mitochondrial calcium transport in the control of substrate oxidation. Mol Cell Biochem. 1998;184(1–2):359–369. [PubMed] [Google Scholar]
- 34.Pillai S, Bikle DD. Adenosine triphosphate stimulates phosphoinositide metabolism, mobilizes intracellular calcium, and inhibits terminal differentiation of human epidermal keratinocytes. J Clin Invest. 1992;90(1):42–51. doi: 10.1172/JCI115854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Campo R, López-Torres M, Cadenas S, Rojas C, Barja G. The rate of free radical production as a determinant of the rate of aging: Evidence from the comparative approach. J Comp Physiol B. 1998;168(3):149–158. doi: 10.1007/s003600050131. [DOI] [PubMed] [Google Scholar]
- 36.Sohal RS, Allen RG. Relationship between metabolic rate, free radicals, differentiation and aging: A unified theory. Basic Life Sci. 1985;35:75–104. doi: 10.1007/978-1-4899-2218-2_4. [DOI] [PubMed] [Google Scholar]
- 37.Hornig-Do HT, et al. Human epidermal keratinocytes accumulate superoxide due to low activity of Mn-SOD, leading to mitochondrial functional impairment. J Invest Dermatol. 2007;127(5):1084–1093. doi: 10.1038/sj.jid.5700666. [DOI] [PubMed] [Google Scholar]
- 38.Bringold U, Ghafourifar P, Richter C. Peroxynitrite formed by mitochondrial NO synthase promotes mitochondrial Ca2+ release. Free Radic Biol Med. 2000;29(3–4):343–348. doi: 10.1016/s0891-5849(00)00318-x. [DOI] [PubMed] [Google Scholar]
- 39.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33(11):1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 40.Carraro C, Pathak MA. Characterization of superoxide dismutase from mammalian skin epidermis. J Invest Dermatol. 1988;90(1):31–36. doi: 10.1111/1523-1747.ep12462534. [DOI] [PubMed] [Google Scholar]
- 41.Tamiji S, et al. Induction of apoptosis-like mitochondrial impairment triggers antioxidant and Bcl-2-dependent keratinocyte differentiation. J Invest Dermatol. 2005;125(4):647–658. doi: 10.1111/j.0022-202X.2005.23885.x. [DOI] [PubMed] [Google Scholar]
- 42.Muramatsu S, et al. Differentiation-specific localization of catalase and hydrogen peroxide, and their alterations in rat skin exposed to ultraviolet B rays. J Dermatol Sci. 2005;37(3):151–158. doi: 10.1016/j.jdermsci.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Rangarajan A, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20(13):3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thebault S, et al. Novel role of cold/menthol-sensitive transient receptor potential melastatine family member 8 (TRPM8) in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. J Biol Chem. 2005;280(47):39423–39435. doi: 10.1074/jbc.M503544200. [DOI] [PubMed] [Google Scholar]
- 45.Sakuntabhai A, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21(3):271–277. doi: 10.1038/6784. [DOI] [PubMed] [Google Scholar]
- 46.Prasad V, et al. Haploinsufficiency of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump, predisposes mice to squamous cell tumors via a novel mode of cancer susceptibility. Cancer Res. 2005;65(19):8655–8661. doi: 10.1158/0008-5472.CAN-05-0026. [DOI] [PubMed] [Google Scholar]
- 47.Robb-Gaspers LD, et al. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998;17(17):4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robb-Gaspers LD, et al. Coupling between cytosolic and mitochondrial calcium oscillations: Role in the regulation of hepatic metabolism. Biochim Biophys Acta. 1998;1366(1–2):17–32. doi: 10.1016/s0005-2728(98)00118-2. [DOI] [PubMed] [Google Scholar]
- 49.Harris DA, Das AM. Control of mitochondrial ATP synthesis in the heart. Biochem J. 1991;280(Pt 3):561–573. doi: 10.1042/bj2800561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hubbard MJ, McHugh NJ. Mitochondrial ATP synthase F1-beta-subunit is a calcium-binding protein. FEBS Lett. 1996;391(3):323–329. doi: 10.1016/0014-5793(96)00767-3. [DOI] [PubMed] [Google Scholar]
- 51.Dixon CJ, et al. Regulation of epidermal homeostasis through P2Y2 receptors. Br J Pharmacol. 1999;127(7):1680–1686. doi: 10.1038/sj.bjp.0702653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burrell HE, et al. Human keratinocytes release ATP and utilize three mechanisms for nucleotide interconversion at the cell surface. J Biol Chem. 2005;280(33):29667–29676. doi: 10.1074/jbc.M505381200. [DOI] [PubMed] [Google Scholar]
- 53.Lee WK, et al. Purinoceptor-mediated calcium mobilization and proliferation in HaCaT keratinocytes. J Dermatol Sci. 2001;25(2):97–105. doi: 10.1016/s0923-1811(00)00117-1. [DOI] [PubMed] [Google Scholar]
- 54.Greig AV, Linge C, Cambrey A, Burnstock G. Purinergic receptors are part of a signaling system for keratinocyte proliferation, differentiation, and apoptosis in human fetal epidermis. J Invest Dermatol. 2003;121(5):1145–1149. doi: 10.1046/j.1523-1747.2003.12567.x. [DOI] [PubMed] [Google Scholar]
- 55.Inoue K, et al. Characterization of multiple P2X receptors in cultured normal human epidermal keratinocytes. J Invest Dermatol. 2005;124(4):756–763. doi: 10.1111/j.0022-202X.2005.23683.x. [DOI] [PubMed] [Google Scholar]
- 56.Thiele JJ, Hsieh SN, Briviba K, Sies H. Protein oxidation in human stratum corneum: Susceptibility of keratins to oxidation in vitro and presence of a keratin oxidation gradient in vivo. J Invest Dermatol. 1999;113(3):335–339. doi: 10.1046/j.1523-1747.1999.00693.x. [DOI] [PubMed] [Google Scholar]
- 57.Bidaux G, et al. Regulation of transient receptor potential melastatin 8 (TRPM8) channel activity by its short isoforms. J Biol Chem. 2011;287(5):2948–2962. doi: 10.1074/jbc.M111.270256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lesage J, Blondeau B, Grino M, Bréant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142(5):1692–1702. doi: 10.1210/endo.142.5.8139. [DOI] [PubMed] [Google Scholar]
- 59.Nelson TE, Lin M, Zapata-Sudo G, Sudo RT. Dantrolene sodium can increase or attenuate activity of skeletal muscle ryanodine receptor calcium release channel. Clinical implications. Anesthesiology. 1996;84(6):1368–1379. doi: 10.1097/00000542-199606000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Rohács T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8(5):626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 61.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440–3450. [PubMed] [Google Scholar]
- 62.Vanden Abeele F, et al. Functional implications of calcium permeability of the channel formed by pannexin 1. J Cell Biol. 2006;174(4):535–546. doi: 10.1083/jcb.200601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Povstyan OV, Harhun MI, Gordienko DV. Ca2+ entry following P2X receptor activation induces IP3 receptor-mediated Ca2+ release in myocytes from small renal arteries. Br J Pharmacol. 2011;162(7):1618–1638. doi: 10.1111/j.1476-5381.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chami M, Ferrari D, Nicotera P, Paterlini-Bréchot P, Rizzuto R. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J Biol Chem. 2003;278(34):31745–31755. doi: 10.1074/jbc.M304202200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.