Significance
Cancer-related inflammation promotes progression and therapy resistance in tumors of adulthood. Knowledge concerning the significance of inflammation in childhood malignancies has been limited. Neuroblastoma is an embryonal tumor of early childhood with poor prognosis despite intensified therapy, and biological understanding is necessary to develop novel therapies. We found high-risk neuroblastoma, in particular the therapy-resistant subset with chromosome 11q-deletion, to be inflammatory driven and characterized by high expression of the COX/microsomal prostaglandin E synthase-1 (mPGES-1)/prostaglandin E2 (PGE2) pathway that correlates with metastatic stage and poor clinical outcome. We further detected infiltrating cancer-associated fibroblasts expressing mPGES-1, the essential enzyme for synthesis of PGE2, promoting tumor growth, angiogenesis, and metastatic spread. Treatment targeting this inflammatory pathway provides a therapeutic option for neuroblastoma and other cancers.
Keywords: mPGES-1, PGE2, neuroblastoma, tumor microenvironment, cancer-associated fibroblasts
Abstract
The majority of solid tumors are presented with an inflammatory microenvironment. Proinflammatory lipid mediators including prostaglandin E2 (PGE2) contribute to the establishment of inflammation and have been linked to tumor growth and aggressiveness. Here we show that high-risk neuroblastoma with deletion of chromosome 11q represents an inflammatory subset of neuroblastomas. Analysis of enzymes involved in the production of proinflammatory lipid mediators showed that 11q-deleted neuroblastoma tumors express high levels of microsomal prostaglandin E synthase-1 (mPGES-1) and elevated levels of PGE2. High mPGES-1 expression also corresponded to poor survival of neuroblastoma patients. Investigation of the tumor microenvironment showed high infiltration of tumor-promoting macrophages with high expression of the M2-polarization markers CD163 and CD206. mPGES-1–expressing cells in tumors from different subtypes of neuroblastoma showed differential expression of one or several cancer-associated fibroblast markers such as vimentin, fibroblast activation protein α, α smooth muscle actin, and PDGF receptor β. Importantly, inhibition of PGE2 production with diclofenac, a nonselective COX inhibitor, resulted in reduced tumor growth in an in vivo model of 11q-deleted neuroblastoma. Collectively, these results suggest that PGE2 is involved in the tumor microenvironment of specific neuroblastoma subgroups and indicate that therapeutic strategies using existing anti-inflammatory drugs in combination with current treatment should be considered for certain neuroblastomas.
Neuroblastoma is the most common and deadliest tumor of childhood. Although the survival of children with neuroblastoma has improved during the last decade, patients with high-risk disease still have a poor prognosis, despite advanced and intensive treatments, with overall survival rates less than 40% (1). The International Neuroblastoma Risk Group (INRG) classification system defines neuroblastoma risk groups as low, intermediate, and high, based on age at diagnosis, histology, and genetic aberrations (2). Among high-risk neuroblastomas, amplification of the neuroblastoma MYC (MYCN) oncogene is the most frequent genetic aberration, seen in 30–40% of the patients. Another common genetic change in the high-risk group is deletion of the long arm of chromosome 11 (11q-deletion). Deletion of 11q occurs in tumors with multiple genetic aberrations and chromosome instability but commonly without MYCN-amplification and therefore is a useful prognostic marker in adverse-stage tumors lacking MYCN-amplification (3). These patients are often older at disease onset and have slow disease progression but often develop therapy resistance and have poor clinical outcome. Among low- and intermediate-risk neuroblastomas are the so-called “special neuroblastomas” (4S) that show a metastatic phenotype but are associated with spontaneous regression and a survival rate of 90% (1, 4, 5).
Prostaglandins are bioactive lipids involved in many biological processes both in physiological processes e.g., blood pressure, smooth muscles contraction, and protection of the intestinal mucosa, and in pathological conditions such as autoimmune diseases and cancer (6). Prostaglandins are formed by the conversion of arachidonic acid to prostaglandin H2 (PGH2) by the cyclooxygenases COX-1 and COX-2, followed by further processing by terminal enzymes, the prostaglandin synthases. Prostaglandin E2 (PGE2) is a proinflammatory and immunomodulatory lipid mediator formed from PGH2 by microsomal prostaglandin E synthase 1 (mPGES-1). Elevated levels of mPGES-1 and its enzymatic product PGE2 have been found in several different cancers, including colon cancer (7–9), nonsmall cell lung cancer (10), and prostate cancer (11, 12). PGE2 signaling contributes to increased proliferation (13, 14) and invasiveness (15) of cancer cells, stimulates tumor angiogenesis (16, 17), inhibit apoptosis (18), induces chemoresistance (19), and mediates suppression of anti-tumor immunity (20, 21). Because tumor-promoting inflammation has been included as one of the hallmarks of cancer (22), chronic inflammation and its impact on tumorigenesis in adult tumors have been immensely investigated.
Less is known about the inflammatory component in childhood tumors; however, in a recent study, Asgharzadeh et al. (23) showed that infiltration of immunosuppressive M2-polarized macrophages in metastatic MYCN-nonamplified neuroblastoma tumors contributed to the metastatic phenotype and worsened the outcome of these patients. Cancer-associated fibroblasts (CAFs) also have been found to contribute to tumor development and metastasis (24), and in a study by Zeine et al. (25) a high number of CAFs was found to correlate with more aggressive Schwannian stroma-poor neuroblastoma tumors.
We previously reported the effect of anti-inflammatory drugs on tumor growth in preclinical in vivo models of neuroblastoma (26–28). We also have found high expression of the PGE2 receptors EP1–EP4 in neuroblastoma tumor tissue as well as effects of PGE2 on neuroblastoma cell growth in vitro (29). These results suggest that inflammatory processes are important for neuroblastoma growth. Therefore we investigated the importance of proinflammatory prostaglandins and their enzymes in neuroblastoma subgroups.
Results
mPGES-1 mRNA Expression in Primary Neuroblastoma.
To study the expression levels of mPGES-1, COX-1, and COX-2 in primary neuroblastoma, quantitative real-time PCR analysis of tumor tissue was performed. When the relative expression was analyzed, significantly higher expression of mPGES-1 mRNA was seen in the 11q-deleted tumors than in low-risk tumors alone (P = 0.04), and there was a strong tendency toward a difference in mPGES-1 mRNA expression between 11q-deleted tumors vs. MYCN-amplified and low-risk tumors considered together (P = 0.06, Mann–Whitney; P = 0.04, t test). The expression of COX-1 was significantly different in 11q-deleted tumors and low-risk tumors (P = 0.03) and in 11q-deleted tumors compared with MYCN-amplified and low-risk tumors considered together (P = 0.02). No significant differences were found for COX-2 (Fig. 1A).
Fig. 1.
mPGES-1 expression and PGE2 levels are associated with 11q-deleted neuroblastoma. (A) Expression of mPGES-1, COX-1, and COX-2 mRNA in neuroblastoma tissue samples was measured by the TaqMan Gene Expression Assay. Expression of mPGES-1 and COX-1 was significantly higher in 11q-deleted tumors than in low-risk tumors (P = 0.04 and P = 0.03, respectively). No difference in COX-2 levels was found. (B) Analysis of an expression cohort shows high expression of mPGES-1 associated with worse overall survival in metastatic neuroblastoma (INSS stage 4). (C) PGE2 levels in neuroblastoma samples as measured by LC-MS/MS were significantly higher in 11q-deleted tumors than in MYCN-amplified (NMA) (P = 0.02) and low-risk tumors (P = 3.0e-04). Bars in A and C represent median values.
Using publicly available expression cohorts, we analyzed mPGES-1 gene expression levels and its correlation to overall survival in patients with International Neuroblastoma Staging System (INSS) stage 4 tumors. Patients with tumors expressing high levels of mPGES-1 had an overall survival of only 10%, compared with 62% in patients with tumors expressing low levels of mPGES-1 (P = 9.6e-04) (Fig. 1B).
Prostaglandin Profiling of Neuroblastoma Tumors.
Prostaglandin levels were measured by LC-MS/MS analysis of liquid–liquid extracted tissue homogenates from 29 primary neuroblastoma samples (four 11q-deleted, eight MYCN-amplified, and 17 low-risk tumors; Table S1). There were significantly higher levels of PGE2 in the 11q-deleted tumors than in the MYCN-amplified tumors (P = 0.02) or in the low-risk tumors (P = 3.0e-04) (Fig. 1C). No significant differences were found in the levels of other prostanoids analyzed (Fig. S1 and Table S2).
Table S1.
Clinical characteristics of neuroblastoma patients involved in the study
| Sample | Age at diagnosis, months | Sex | CGH class | INRG-HR | INRG stage | Biology | Pretreated | Outcome | Survival, months |
| NB2 | 41 | M | 11q- | + | M | Unfavorable | Yes | AWD | 19+ |
| NB11 | 29 | F | 11q- | − | L | Unfavorable | Yes | NED | 210+ |
| NB12 | 31 | M | 11q- | + | M | Unfavorable | Yes | DOC | 6 |
| NB19 | 33 | F | 11q- | + | M | Unfavorable | Yes | NED | 32+ |
| NB43 | 35 | F | 11q- | + | M | Unfavorable | Yes | NED | 31+ |
| NB44 | 27 | M | 11q- | + | M | Unfavorable | Yes | AWD | 32+ |
| NB45 | 55 | F | 11q- | + | M | Unfavorable | Yes | NED | 20+ |
| NB1 | 41 | M | NMA | + | L | Unfavorable | No | DOD | 17 |
| NB6 | 79 | M | NMA | + | L | Unfavorable | Yes | NED | 70+ |
| NB13 | 19 | F | NMA | + | M | Unfavorable | Yes | DOD | 4 |
| NB14 | 14 | F | NMA | + | M | Unfavorable | Yes | DOD | 13 |
| NB39 | 15 | F | NMA | + | M | Unfavorable | Yes | NED | 13+ |
| NB41 | 136 | M | NMA | + | M | Unfavorable | Yes | DOD | 10 |
| NB34 | 30 | M | NMA | + | M | Unfavorable | Yes | DOD | 5 |
| NB37 | 31 | F | NMA | + | M | Unfavorable | Yes | NED | 59+ |
| NB4 | 10 | M | NO | − | MS | Favorable | No | NED | 49+ |
| NB10 | 0 | M | NO | − | MS | Favorable | No | NED | 126+ |
| NB25 | 11 | F | n.a. | − | MS | Favorable | No | NED | 76+ |
| NB8 | 31 | M | NO | − | L | Favorable | No | NED | 140+ |
| NB9 | 0 | M | NO | − | L | Favorable | No | DOC | 0 |
| NB18 | 43 | F | NO | − | L | Favorable | No | NED | 174+ |
| NB20 | 6 | M | 17q+ | − | L | Unfavorable | No | NED | 44+ |
| NB22 | 6 | M | NO | − | L | Favorable | No | NED | 148+ |
| NB26 | 33 | F | OS | − | L | Favorable | No | NED | 153+ |
| NB28 | 25 | F | NO | − | L | Favorable | No | NED | 40+ |
| NB29 | 0 | F | NO | − | L | Favorable | No | NED | 72+ |
| NB30 | 4 | F | NO | − | L | Favorable | No | NED | 71+ |
| NB31 | 13 | F | NO | − | L | Favorable | No | NED | 84+ |
| NB33 | 110 | M | OS | − | L | Favorable | No | NED | 32+ |
| NB36 | 12 | F | OS | − | L | Favorable | No | NED | 131+ |
| NB38 | 13 | M | OS | − | L | Favorable | No | NED | 76+ |
| NB42 | 12 | F | NO | − | L | Favorable | No | NED | 270+ |
Clinical characteristics of neuroblastoma patients involved in the study. Comparative genomic hybridization (CGH) class (3): NMA, MYCN-amplification; n.a., not annotated; NO, numerical only; OS, other structural; 11q, 11q−; 17q, 17q+ without NMA/11q−. INRG–high-risk (HR) (2): HR +, NMA or INRG stage M, age at diagnosis >18 mo. INRG stage (2): L, localized (INSS stage 1, 2, and 3); M, metastatic (INSS stage 4); MS, metastatic special (INSS stage 4S). Outcome: AWD, alive with disease; DOC, dead of complications; DOD, dead of disease; NED, no evidence of disease. Survival months: survival in completed months from diagnosis; +, still alive.
Fig. S1.
Levels of PGD2, PGF2α, thromboxane B2 (TxB2), and 6-keto-PGF1α in neuroblastoma samples measured by LC-MS/MS. No significant differences were observed. Bars represent median values.
Table S2.
Prostanoid profiling of neuroblastoma tumor tissue
| Biology | PGE2 | PGD2 | 6-keto-PGF1α | Thromboxane B2 | PGF2α |
| 11q− | 4.56 ± 4.39*,† | 0.03 ± 0.01 | 0.14 ± 0.09 | 0.07 ± 0.05 | 0.40 ± 0.30 |
| NMA | 0.05 ± 0.02 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.05 ± 0.01 |
| Low-risk | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.01 ± 0.01 | 0.03 ± 0.02 | 0.03 ± 0.01 |
Prostanoid levels in tumor tissue samples from neuroblastoma patients resected during surgery [11q-, n = 4; MYCN-amplified (NMA), n = 8; low risk, n = 17]. Mean values are reported as pmol/mg extracted tumor ± SEM.
P = 3.0e-04 (11q- vs. low-risk).
P = 0.02 (11q- vs. NMA).
Immunohistochemical Analysis of Prostaglandin Synthases.
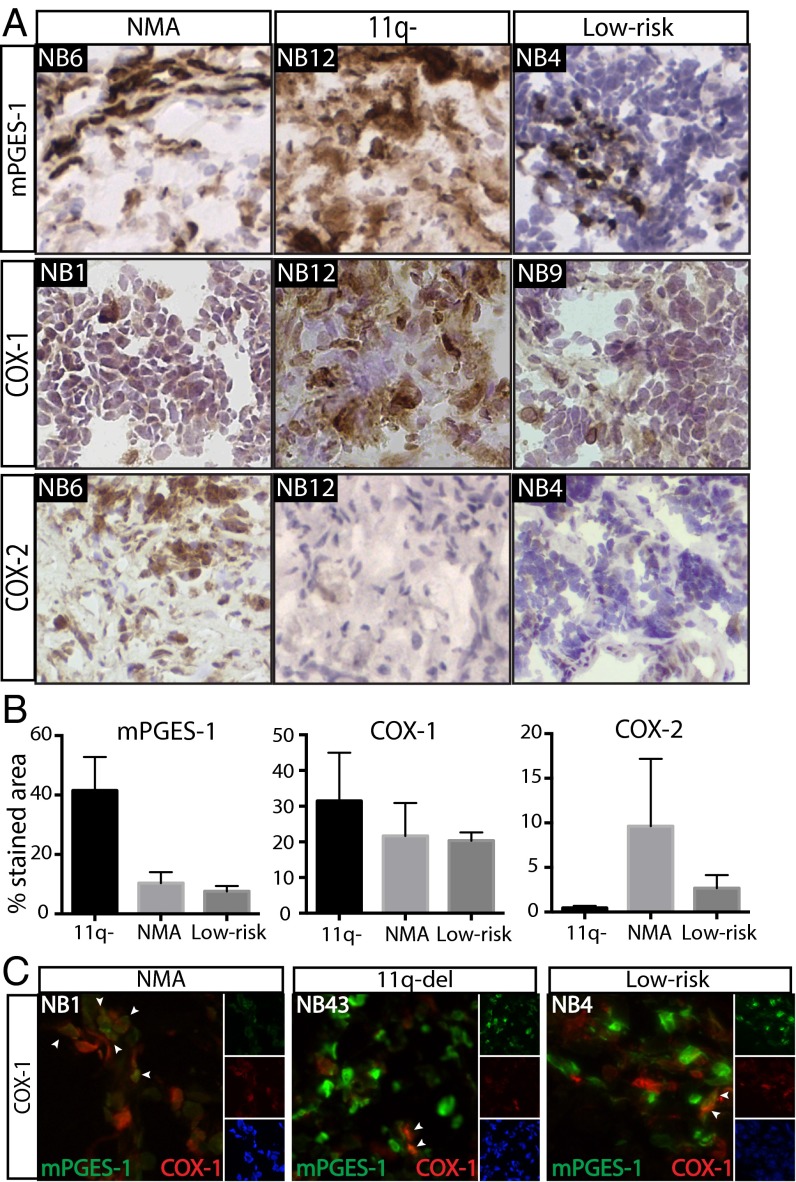
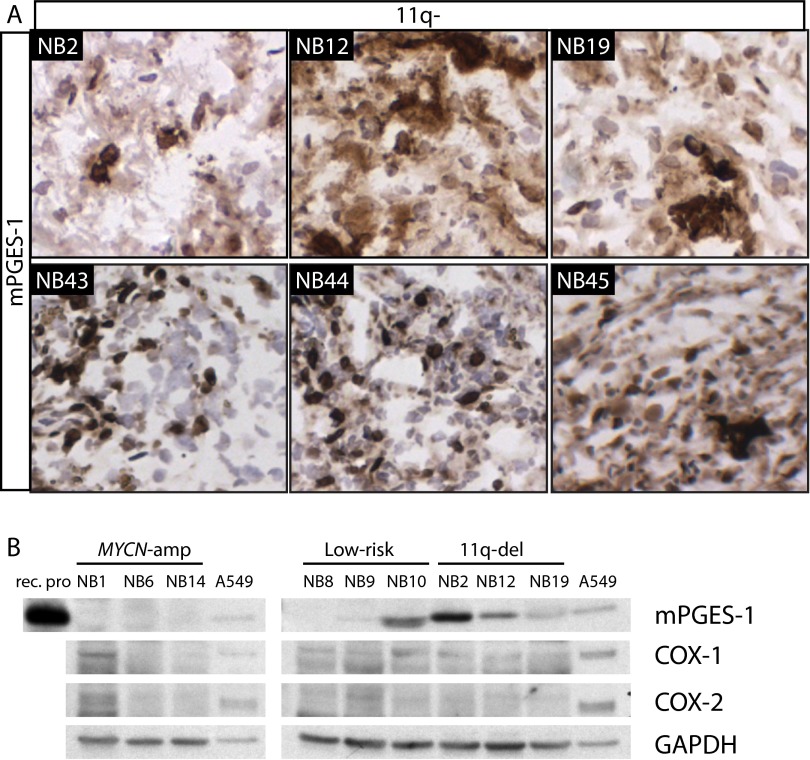
The expression of enzymes involved in synthesis of prostaglandins in neuroblastoma was analyzed using immunohistochemistry (IHC). COX-1, COX-2, mPGES-1, lipocalin-type prostaglandin D synthase (L-PGDS), hematopoietic-type prostaglandin D synthase (H-PGDS), and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) were analyzed in tumors from 11 neuroblastoma patients (four 11q-deleted, three MYCN-amplified, and four low-risk tumors; Fig. 2 and Fig. S2). Expression of mPGES-1 was found in all tumors tested, with significantly higher levels in the 11q-deleted tumors than in the low-risk tumors alone (P = 0.02) or in the MYCN-amplified and low-risk tumors considered together (P = 0.004). mPGES-1 expression was confirmed with IHC in additional 11q-deleted tumors (Fig. S3) and with Western blot. Western blot analysis clearly showed higher levels of mPGES-1 in the 11q-deleted tumors than in the MYCN-amplified and low-risk tumors. COX-1 was detected in one of three MYCN-amplified tumors, and a weak band was seen in all low-risk and 11q-deleted tumors. Of all tumors analyzed, COX-2 was detected in only one of the MYCN-amplified tumors (Fig. S3). Consistent with Western blot analysis, IHC analysis showed that there was more expression of COX-1 than COX-2 in the neuroblastoma tumors, and the difference between COX-1 and COX-2 expression was more evident in the 11q-deleted tumors, but no significant difference between the tumor subgroups was found for either COX-1 or COX-2 (Fig. 2B). Both prostaglandin D2 (PGD2) synthases were present in significantly higher levels in low-risk tumors than in 11q-deleted tumors (L-PGDS, P = 6.0e-04; H-PGDS, P = 0.03). Elevated levels of H-PGDS also were detected in MYCN-amplified tumors as compared with 11q-deleted tumors (P = 0.02) (Fig. S2). The 11q-deleted tumors expressed significantly lower levels of 15-PGDH, an enzyme responsible for PGE2 and PGD2 degradation, than did either low-risk tumors alone (P = 0.005) or MYCN-amplified and low-risk tumors taken together (P = 0.008).
Fig. 2.
COX/mPGES-1 expression in human neuroblastoma tumors. (A) IHC analysis of mPGES-1, COX-1, and COX-2 in primary human neuroblastoma tumors with different biology: MYCN-amplification (NMA), 11q-deletion, and low-risk. Sections were counterstained with hematoxylin. One representative image was selected from tumors from at least three patients in each subgroup. (B) Quantification was performed on tumors from 11 patients in three clinical subgroups (four with 11q-deleted tumors, three with NMA tumors, and four with low-risk tumors). Data are presented as mean ± SEM. mPGES-1 expression was higher in 11q-deleted tumors than in low-risk tumors (P = 0.02), and there was a strong tendency toward higher mPGES-1 levels in 11q-deleted tumors compared with NMA tumors (P = 0.07). No differences in COX-1 or COX-2 levels were seen. (C) Dual labeling of mPGES-1 (green) and COX-1 (red) counterstained with a nuclear dye, Hoechst (blue). In the NB1 tumor, most cells expressing mPGES-1 also expressed COX-1. There also were cells expressing only COX-1. In the NB43 and NB4 tumors, cells expressed either COX-1 or mPGES-1 with only a few points of colocalization. Arrowheads indicate cells coexpressing both enzymes.
Fig. S2.
Expression of PGD synthases and 15-PGDH in human neuroblastoma (NB) tumors. (A) IHC analysis of H-PGDS, L-PGDS, and 15-PGDH in primary human neuroblastoma tumors with different biology: MYCN-amplification (NB1), 11q-deletion (NB2 and NB12), and low-risk (NB4). Sections were counterstained with Mayer’s hematoxylin. One representative image selected from the staining of at least three tumors from each neuroblastoma subtype is shown. (B) Sections from three tumors in each subgroup were quantified. Significantly higher levels of L-PGDS were found in low-risk tumors than in 11q-deleted tumors (P = 6.0e-04). H-PGDS was expressed at higher levels in MYCN-amplified (P = 0.02) and low-risk tumors (P = 0.03) than in 11q-deleted tumors. Also, 15-PGDH was expressed at higher levels in low-risk tumors than in 11q-deleted tumors (P = 0.004). Data are represented as mean ± SEM.
Fig. S3.
mPGES-1 expression in 11q-deleted tumors. (A) IHC analysis of mPGES-1 expression in additional 11q-deleted tumors showed strong mPGES-1 expression in all tested tumors. Sections were counterstained with Mayer’s hematoxylin. (B) Western blot analysis of mPGES-1, COX-1, and COX-2 in neuroblastoma tumor tissue. The expression of mPGES-1, COX-1, and COX-2 was analyzed in tumors from three patients in each neuroblastoma subgroup [11q-deleted, MYCN-amplified (NMA), and low-risk]. mPGES-1 was detected in all 11q-deleted tumors.
Because of the relatively high levels of COX-1 compared with COX-2 in the neuroblastoma tumors, double staining of mPGES-1 and COX-1 was performed. In one of the three tumors tested a considerable overlap between COX-1 and mPGES-1 was observed. In two of the three tumors only single cells expressed both enzymes, but cells expressing the respective enzymes were in proximity to each other (Fig. 2C).
IHC Analysis of the Tumor Microenvironment in Relation to mPGES-1 Expression.
IHC analysis of mPGES-1 in neuroblastoma samples showed positive staining of mPGES-1 in nontumorigenic cells, suggesting that stromal or tumor-infiltrating cells express mPGES-1 (Fig. 2A). We therefore performed IHC analysis using an mPGES-1 antibody in combination with antibodies detecting different immune cells, stromal and endothelial cells, and fibroblasts known to be part of the tumor microenvironment (Table 1). The double staining was performed on a tumor from one patient in each of the subgroups; we designated these tumors “NB1” (untreated, MYCN-amplified, INSS stage 2, unfavorable prognosis), “NB4” (untreated, low-risk, INSS 4S, favorable prognosis), and tumor “NB43” (pretreated, 11q-deleted, INSS stage 4, unfavorable prognosis).
Table 1.
Localization of different components of the tumor microenvironment in relation to mPGES-1
| Cell type | Marker | NB1, NMA | NB43, 11q-deleted | NB4, low-risk |
| Neuroblastoma tumor | GD2 | No | No | No |
| Myeloid cells | CD68 | No | No | No |
| CD163 | No | No | No | |
| CD11b | No | No | No | |
| CD11c | No | No | No | |
| T cell | CD3 | No | No | No |
| CD4 | No | No | No | |
| B cell | CD20 | No | No | No |
| Epithelial | CD31 | No | No | Yes |
| Mesenchymal | Vimentin | Yes | No | Yes |
| CAF | αSMA | Yes | No | No |
| FAP | Yes | No | No | |
| PDGFRβ | Yes | No | Yes | |
| PDGFRα | No | No | Yes | |
| FSP-1 | No | Yes | Yes |
Colocalization was scored as negative (no) or positive (yes) in tumors from three patients, one from each subgroup [11q-deleted (tumor NB43), MYCN-amplified (tumor NB1), and low-risk (tumor NB4)].
Disialoganglioside (GD2) is expressed almost exclusively on tumors of neuroectodermal origin and is used as therapeutic target in neuroblastoma (30). Double staining of GD2 with mPGES-1 showed a considerably different staining pattern. No coexpression of GD2 and mPGES-1 was found in any of the three tumors analyzed (NB1, NB4, or NB43). The expression was mutually exclusive, with a clear compartmentalization of cells expressing the respective proteins (Fig. 3A).
Fig. 3.
Investigation of the tumor microenvironment in relation to mPGES-1. (A) In total three human neuroblastoma tumors from one patient in each subgroup, NB1 (MYCN-amplified), NB43 (11q-deleted), and NB4 (low-risk), were stained with mPGES-1 (green) and a neuroblastoma tumor cell marker, GD2 (red). Dual labeling showed no colocalization of mPGES-1 with the neuroblastoma tumor marker GD2. (B) Double staining of mPGES-1 (green) and a pan-macrophage marker (CD68) (red). No colocalization of mPGES-1 and CD68 was found. (C) Dual labeling of vimentin (red), an intermediate filament found in cells of mesenchymal origin, and mPGES-1 (green) showed colocalization in both the NB1 and NB4 tumors but not in the NB43 tumor.
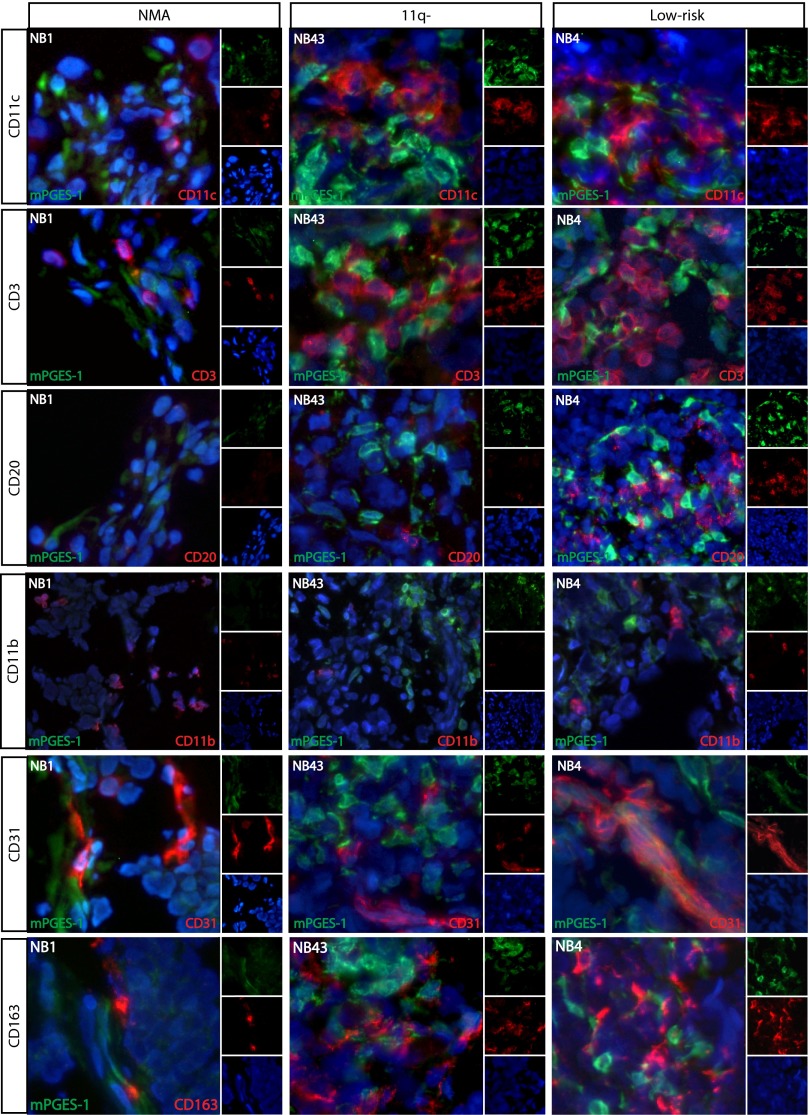
Because macrophages are a sign of inflammation, are a typical source of mPGES-1 expression (31), and are present in tumor microenvironments as tumor-associated macrophages, double staining of mPGES-1 in combination with a pan-macrophage marker (CD68) and a marker for M2-polarized macrophages (CD163) was used. All analyzed tumors showed widespread expression of CD68 throughout the sections, thus showing the presence of macrophages (Fig. 3B). Interestingly, no colocalization of mPGES-1 and CD68 was seen. CD163 also was expressed abundantly throughout the tumor sections, but no colocalization between mPGES-1 and CD163 was found in any of the neuroblastoma samples analyzed (Fig. S4). To consider the extent of macrophage infiltration and macrophage polarization, tumors from three patients in each subgroup were stained with CD163, CD206 (M2-polarization), and MHC class II (MHCII; higher expression in M1). There were significantly more M2-polarized macrophages in 11q-deleted and MYCN-amplified tumors than in low-risk tumors (see Fig. S5), suggesting a more immunosuppressive microenvironment in high-risk neuroblastoma.
Fig. S4.
Immune cell infiltration in neuroblastoma tumors in relation to mPGES-1 expression. Tumors from three neuroblastoma patients, one from each subgroup, NB1 (MYCN-amplified), NB43 (11q-deleted), and NB4 (low-risk), were double stained with mPGES-1 (green) and CD11c (dendritic cells), CD3 (T cells), CD20 (B cells), CD11b (myeloid cells), CD31 (endothelial cells), and CD163 (M2-macrophages) (red), respectively. Sections were counterstained with Hoechst (blue). The NB1 tumor displayed few dendritic cells and T cells and no B cells. Both the NB43 tumor and the NB4 tumor had profuse dendritic cells and T cells, which in the NB4 tumor were localized to the same areas as mPGES-1–expressing cells. Only the NB4 tumor had B cells, which also were in proximity to mPGES-1–expressing cells. The endothelial marker CD31 showed colocalization with mPGES-1 in some areas of the NB4 tumor. No colocalization of mPGES-1 with the myeloid cell marker (CD11b) or M2-polarized macrophage marker (CD163) was detected.
Fig. S5.
Macrophage infiltration in neuroblastoma tumors. IHC analysis of surface antigens expressed on M1 (MHCII)- and M2 (CD163 and CD206)-polarized macrophages was performed on consecutive tumor sections from three patients in each subgroup [11q-deleted (NB43), MYCN-amplified (NB6), and low-risk (NB10)]. Similar staining patterns in the same area of the tumor were seen for CD163 and CD206 and to a lesser extent for MHCII. Quantification revealed a shift in polarization toward M2 in 11q-deleted (CD206, P = 0.03) and MYCN-amplified (CD163, P = 0.01) tumors compared with low-risk tumors. No difference in the expression of MHCII was found between the tumor subgroups (11q- vs. low-risk, P = 0.62; NMA vs. low-risk, P = 0.85).
Apart from macrophages, other myeloid cells, including myeloid-derived suppressor cells (MDSCs), are known to infiltrate tumor tissue. To cover all myeloid cells, double staining of mPGES-1 with CD11b was performed, but no colocalization was seen (Fig. S4).
To determine if dendritic cells contribute to mPGES-1 expression in neuroblastoma, double staining of mPGES-1 and CD11c was performed, but no colocalization was found. However, in the NB4 and NB43 tumors the CD11c+ cells were found only in areas with mPGES-1+ cells (Fig. S4). In the MYCN-amplified tumor, NB1, there was an overall pattern of weak CD11c staining, and no positive CD11c staining was detected in the proximity of mPGES-1+ cells.
Next we studied lymphocyte infiltration in the proximity of mPGES-1+ cells (Fig. S4). CD3+ T cells were present in all tumors to a varying extent. The NB1 and NB4 tumors had no T cells in mPGES-1− areas. Tumor NB1 showed few CD3+ cells in mPGES-1+ areas, whereas CD3+ T cells were abundant in presence in the mPGES-1–expressing areas of the NB4 tumor. These T cells also were CD4+. CD3+ T cells were abundant throughout the NB43 tumor and were slightly enriched in mPGES-1+ areas. Interestingly, B cells were abundant only in the NB4 tumor and only in proximity to mPGES-1–expressing cells (Fig. S4).
Subsequently we studied the contribution of mPGES-1 expression from endothelial cells. Double staining of mPGES-1 and the endothelial cell marker CD31 showed colocalization with mPGES-1–expressing cells in some areas of the NB4 tumor, but no colocalization was detected in the NB1 or NB43 tumors (Fig. S4).
To determine if the mPGES-1–expressing cells could be of mesenchymal origin, we performed double staining of mPGES-1 and vimentin (Fig. 3C). A majority of the mPGES-1+ cells also showed positive staining for vimentin in the NB4 tumor. In the NB1 tumor the majority of cells were positive for vimentin, including cells expressing mPGES-1 (Fig. 3C). The NB43 tumor showed weak vimentin staining, and only a few of these cells coexpressed mPGES-1.
Because the majority of mPGES-1+ cells in the NB1 and NB4 tumors also expressed vimentin, we performed IHC analysis with markers for CAFs, i.e., fibroblast activation protein α (FAP), PDGF receptor β (PDGFRβ), fibroblast-specific protein 1 (FSP-1), α smooth muscle actin (αSMA), and PDGF receptor α (PDGFRα), (Fig. 4). In the NB1 tumor there was a clear colocalization of cells positive for mPGES-1 and CAF markers. Cells expressing mPGES-1 exhibited strong positive staining for FAP and αSMA and weak positive staining for PDGFRβ, whereas none of the cells were positive for PDGFRα or FSP-1 (Fig. 4). In the NB4 tumor there was colocalization of mPGES-1 with PDGFRα, PDGFRβ, and FSP-1 but no colocalization with FAP and αSMA (Fig. 4). Only weak positive staining for CAF markers was detected in the NB43 tumor, and only FSP-1 showed colocalization with mPGES-1.
Fig. 4.
Cellular localization of mPGES-1 expression in neuroblastoma tumors. Three human neuroblastoma tumors, NB1 (MYCN-amplified), NB43 (11q-deleted), and NB4 (low-risk 4S), were stained for mPGES-1 (green) and FAP (A), PDGFRβ (B), FSP-1 (C), αSMA (D), or PDGFRα (E), markers for cancer-associated fibroblast (CAFs), in red. All sections were counterstained with the nuclear dye Hoechst (blue). Colocalization was seen for FAP, αSMA, and PDGFRβ in the NB1 tumor. Colocalization of PDGFRα, PDGFRβ, and FSP-1 also was seen in the NB4 tumor. In the NB43 tumor mPGES-1 colocalized with FSP-1. Taken together, these results suggest that CAFs are a source for mPGES-1 expression and PGE2 production.
COX Inhibition of an in Vivo Xenograft Mouse Model of 11q-Deleted Neuroblastoma.
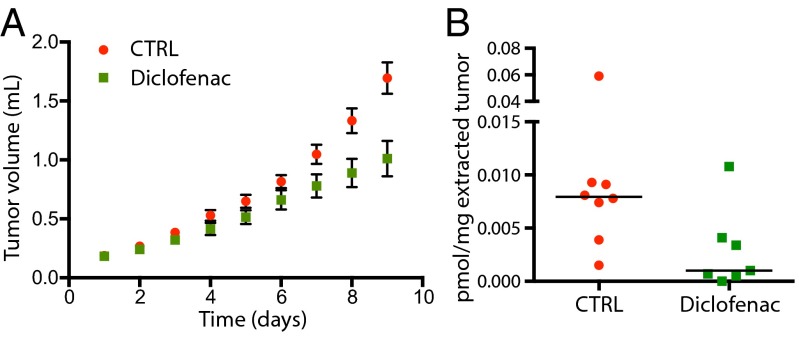
The effect of COX inhibition on neuroblastoma tumors in vivo was assessed in an 11q-deleted neuroblastoma cell line (32) (SK-N-AS) xenograft mouse model treated with diclofenac (Fig. 5). Tumor volume was measured and compared with untreated animals. A significant reduction in tumor growth was found for diclofenac-treated animals compared with control animals on day 8 (P = 0.01) and day 9 (P = 0.008) (Fig. 5A). Endogenous levels of PGE2 were measured in the tumors using LC-MS/MS of solid-phase extracted tumor tissue. There was a significant decrease of PGE2 in tumors from diclofenac-treated animals compared with controls (P = 0.04) (Fig. 5B).
Fig. 5.
COX inhibition reduces PGE2 levels and reduces tumor growth in vivo. Nude mice were inoculated with SK-N-AS neuroblastoma cells harboring an 11q-deletion and were treated with diclofenac (250 mg/L drinking water) for nine consecutive days. (A) Tumor volumes were measured each day, and a significant difference between diclofenac-treated and control mice was seen on day 8 (P = 0.01) and day 9 (P = 0.008). (B) PGE2 was measured in the tumors using solid-phase extraction and LC-MS/MS analysis. There was a significant decrease in PGE2 in tumors from diclofenac-treated mice compared with control mice (P = 0.04). Bars represent median values.
Discussion
The involvement of chronic inflammation in the initiation and progression of adult tumors of epithelial origin has been well established (6). Much less is known about the contribution of inflammation and proinflammatory mediators in solid childhood cancers, and we therefore investigated the involvement of prostaglandins in different subsets of neuroblastoma.
We found that mPGES-1 is expressed in human primary neuroblastoma samples. Expression of mPGES-1 was present in all tumors tested and was significantly higher in the 11q-deleted subset of high-risk tumors than in low-risk tumors on both the mRNA (Fig. 1A) and the protein level (Fig. 2). Our lipid analysis showed significantly higher levels of PGE2, the enzymatic product of mPGES-1, in the 11q-deleted subset than in either MYCN-amplified or low-risk tumors (Fig. 1C), confirming the observations in the IHC analysis. In addition, significantly lower levels of 15-PGDH, the enzyme responsible for metabolizing PGE2, were detected in the 11q-deleted tumors, possibly contributing to the high PGE2 levels found in these tumors (Fig. S2). All patients with high-risk neuroblastomas are subjected to the same induction treatment before surgical excision of the tumor (Table S3) (33), and all patients had at least 2 wk off chemotherapy before surgery. Any effect of the chemotherapeutic drugs on prostaglandin levels would have affected the patients in both of the high-risk subgroups (i.e., patients with 11q-deleted tumors and those with MYCN-amplified tumors) similarly.
Table S3.
Presurgical treatment of high-risk neuroblastoma patients
Induction treatment of high-risk neuroblastoma patients. In total, carboplatin 1,500 mg/m2, cisplatin 320 mg/m2, etoposide 700 mg/m2, cyclophosphamide 2,100 mg/m2, and vincristine 12 mg/m2 were administered according to the COJEC induction regimen (48) as part of the HR-NBL1/SIOPEN protocol (33).
Block A: carboplatin, 750 mg/m2; etoposide, 175 mg/m2; vincristine, 1.5 mg/m2.
Block B: cisplatin, 80 mg/m2; vincristine, 1.5 mg/m2.
Block C: cyclophosphamide, 1,050 mg/m2; etoposide, 175 mg/m2; vincristine, 1.5 mg/m2.
The mPGES-1 staining pattern in the IHC analysis suggested that cells located in the tumor microenvironment, and not tumor cells, are responsible for the production of PGE2. To confirm this suggestion, double staining with mPGES-1 and GD2, a neuroblastoma tumor cell marker, was performed. In all three tumors analyzed a clear discrepancy in staining pattern was seen. The areas with mPGES-1–expressing cells and GD2-expressing cells were mutually exclusive, indicating that PGE2 is produced by cells in proximity to tumor cells but not by the tumor cells themselves. To our surprise, low levels of COX-2 were detected in the majority of neuroblastoma samples analyzed (Fig. 2), but the pattern of COX-1 staining was similar to that of mPGES-1. Double staining with mPGES-1 and COX-1 showed coexpression of both enzymes in some cells. We also detected cells with only COX-1 or mPGES-1 expression, perhaps because the enzyme was present at levels below the limit of detection for IHC analysis. However, COX-1 also has been shown to exert paracrine distribution of PGH2 to mPGES-1 (34). PGH2, which is needed for conversion to PGE2, also could be supplied from circulating cells expressing COX-1 or COX-2.
As discussed by Pistoia et al. (35), there is an immunosuppressive tumor microenvironment in neuroblastoma that potentially enables tumor cells to evade host immune responses. This immunosuppressive state has been shown in human tumor samples by the infiltration of M2-polarized macrophages (23) and in neuroblastoma mouse models by the infiltration of MDSCs (26, 36). Our data show predominance toward M2-polarization of the tumor-infiltrating macrophages (i.e., tumor-associated macrophages) in both 11q-deleted tumors and MYCN-amplified tumors, with elevated levels of CD206 and CD163, respectively (Fig. S5), supporting the findings by Asgharzadeh et al. (23).
Because CD68+ and CD163+ macrophages did not stain positive for mPGES-1 in the tumor samples, double staining with the dendritic cell marker CD11c was performed. No colocalization was found; however, CD11c-expressing cells were in cell-to-cell contact with the mPGES-1–expressing cells in the NB4 tumor and to some extent in the NB43 tumor (Fig. S4). This finding led us to explore further different populations of immune cells present in the tumor samples in relation to mPGES-1+ cells. Strikingly, in the 4S tumor NB4 there were CD3+ CD4+ T cells and CD20+ B cells only in clusters together with mPGES-1–expressing cells and CD11c+ cells. Outside these immunological hotspots, no B cells or T cells were present. In the NB43 tumor, CD3+ CD4+ T cells were widespread in the tumor sections, although they were slightly more concentrated in the mPGES-1–expressing cell clusters. Nonetheless, essentially no CD20+ B cells could be found in either the NB1 or the NB43 tumor. Spontaneous regression is an intriguing feature of 4S tumors, and the finding that only the 4S neuroblastoma sample had B cells is interesting and should be investigated further.
Endothelial cells have been shown to express mPGES-1 and to secrete PGE2 (31). Colocalization between mPGES-1 and CD31 was detected in the 4S neuroblastoma sample NB4 (Fig. S4). In the NB4 tumor and in the MYCN-amplified NB1 tumor, we also identified colocalization of mPGES-1 with vimentin. Vimentin is an intermediate filament found in nerve cell progenitors and also in cells of mesenchymal origin and is used as a marker of epithelial-to-mesenchymal transition (EMT), a process promoting tumor metastasis (37). In a recent publication, epithelial tumor cells induced with EGF led to overexpression of mPGES-1 concomitant with increased vimentin expression and down-regulation of E-cadherin, initiating the phenotypic change of EMT (38). Both the NB1 and the NB4 tumors had relatively high vimentin expression, whereas only low, sporadic vimentin expression was found in the 11q-deleted tumor NB43, possibly because of chemotherapeutic treatment of this tumor before resection (Tables S1 and S3). Because vimentin also is expressed in fibroblasts, we looked at CAFs. In a recent study Alcolea et al. (39) showed that head and neck squamous cell carcinoma cells stimulated both dermal and tumor-derived fibroblasts to express mPGES-1, and they also observed PGE2 release in tumor-adjacent mucosa (39). Erez et al. (40) showed that CAFs educated by tumor cells mediated tumor-enhancing inflammation in an NF-κB–dependent manner. In line with these results, we found that mPGES-1+ cells in all three tumors analyzed (NB1, NB4, and NB43) expressed one or more markers for CAFs. The MYCN-amplified tumor NB1 coexpressed mPGES-1 and CAF markers such as vimentin, FAP, αSMA, and PDGFRβ but not FSP-1 and PDGFRα (41). Although mPGES-1+ cells in NB4 expressed vimentin, PDGFRα, PDGFRβ, and FSP-1, no colocalization with FAP or αSMA was detected. In the 11q-deleted tumor NB43, FSP-1 was the only CAF marker present in mPGES-1+ cells, and the expression of CAF markers was generally low (Fig. 4). Even though referred to as one population of cells, CAFs are heterogeneous in their function and origin as well as in their expression of markers (41, 42), possibly reflecting the differential expression of CAF markers seen in the neuroblastoma tumors. Recently, CAFs also have been found to contribute to an immunosuppressive tumor microenvironment (43).
Next we wanted to investigate the effect of PGE2 inhibition on tumor growth in an in vivo model of 11q-deleted neuroblastoma. Treatment with diclofenac, a nonselective COX inhibitor, resulted in reduced tumor growth. A reduction in PGE2 in the tumors was confirmed also. Based on our data, we propose that mPGES-1 is expressed in subpopulations of CAFs; therefor only a fraction of cells in the whole tumor produce PGE2, explaining the relatively low levels of PGE2 found. Still, the decrease in PGE2 production resulting from diclofenac treatment is enough to have a significant effect on tumor progression in the in vivo 11q-deleted model, suggesting that targeting PGE2 could be of benefit for children with aggressive, chemoresistant 11q-deleted neuroblastomas, as was indicated recently for bladder cancer (19).
In conclusion, we have identified an activated COX/mPGES-1/PGE2 pathway in 11q-deleted neuroblastoma with high expression of mPGES-1, low expression of 15-PGDH, and elevated levels of PGE2 as compared with MYCN-amplified and low-risk tumors. Analysis of expression cohorts revealed a worse outcome for high-risk patients (INSS stage 4) with high mPGES-1 expression. In addition, COX inhibition in an in vivo model of 11q-deleted neuroblastoma resulted in reduced tumor growth. These findings collectively suggest that PGE2 signaling in 11q-deleted high-risk tumors could be targeted using existing nonsteroidal anti-inflammatory drugs or by developing new compounds directly targeting mPGES-1. In addition there are convincing observations that mPGES-1/PGE2 may play a role in the complex network of the tumor microenvironment in general and specifically in CAFs (39, 44), further strengthening the suggestion that mPGES-1 be targeted in neuroblastoma and in other tumors with activated COX/mPGES-1/PGE2 signaling.
Materials and Methods
The animal experiment was approved by the Stockholm North Animal Research Committee (approval N231/14) in accordance with the Animal Protection Law (SJVFS 2012:26), the Animal Protection Regulation (SFS 1988:539), and the Regulation for the Swedish National Board for Laboratory Animals (SFS 1988: 541).
Detailed materials and methods are provided in SI Materials and Methods.
Patient Material.
Neuroblastoma tumor tissue was obtained from the Karolinska University Hospital. Ethical approval was obtained by the Stockholm Regional Ethical Review Board and the Karolinska University Hospital Research Ethics Committee (approval nos. 2009/1369-31/1 and 03/736). Tumor and patient characteristics are summarized in Table S1. Thirty-two neuroblastoma samples representing all clinical subsets were used.
Prostaglandin Profiling.
Approximately 40 mg of human primary tumor tissue from 29 individuals was used in the analysis. Liquid–liquid extraction and analysis by LC-MS/MS was performed essentially as described previously (26).
Quantitative Real-Time PCR Analysis.
RNA was prepared from ∼30 mg of primary tumor tissue, and 100 ng of RNA was used to synthesize cDNA. The TaqMan Gene Expression Assay (Applied Biosystems) was used to evaluate the relative expression levels of mPGES-1, COX-1, and COX-2 mRNA.
The Gene-Expression Database R2.
Gene-expression analysis of the impact of mPGES-1 on overall survival in neuroblastoma patients was performed using a publicly available database [R2: microarray analysis and visualization platform (r2.amc.nl)]. In the public Versteeg-88 dataset, mRNA from 88 human neuroblastoma samples are included, 40 of whom are high-risk patients (INSS stage 4).
IHC Analysis of Neuroblastoma Tumor Tissue.
Frozen tumor tissue, sectioned at 7 μm and fixed in 2% formaldehyde, was used. Staining was preformed as described earlier (31). All antibodies used in the study are listed in Table S4.
Table S4.
Antibodies used for IHC and immunofluorescence (IF) analysis
| Antigen | Antibody | Source | Dilution | |
| IHC | IF | |||
| COX-1 | sc-1752 | Santa Cruz Biotechnology | 1:200 | |
| COX-1 | 160108 | Cayman | 1:3,200 | |
| COX-2 | 160112 | Cayman | 1:50 | |
| mPGES-1 | (31) | In house | 1:5,000 | 1:4,000 |
| L-PGDS | 160003 | Cayman | 1:800 | |
| H-PGDS | 160013 | Cayman | 1:800 | |
| 15-PGDH | 10007950 | Cayman | 1:3,200 | |
| GD2 | MAB2052 | Millipore | 1:15000 | |
| CD31 | M0823 | DAKO | 1:80 | |
| CD68 | M0718 | DAKO | 1:16000 | |
| CD163 | M0794 | DAKO | 1:100 | 1:80 |
| CD206 | MCA2155 | AbD Serotec | 1:200 | |
| HLA-DR (MHCII) | M0746 | DAKO | 1:3,000 | |
| CD11b | 301302 | BioLegend | 1:200 | |
| CD11c | 301615 | BioLegend | 1:5,000 | |
| CD3 | 347340 | BD Biosciences | 1:640 | |
| CD20 | M0755 | DAKO | 1:200 | |
| Vimentin | M0725 | DAKO | 1:10000 | |
| αSMA | M0851 | DAKO | 1:200 | |
| FAP | BMS168 | eBioscience | 1:400 | |
| FSP-1 | AMAb90599 | Atlas Antibodies | 1:2,500 | |
| PDGFRα | sc-21789 | Santa Cruz Biotechnology | 1:250 | |
| PDGFRβ | 3175 | Cell Signaling | 1:100 | |
COX Inhibition of in Vivo 11q-Deleted Xenograft Tumor Growth.
A xenograft mouse model harboring 11q-deletion tissue was treated with diclofenac for 9 d essentially as described previously (45).
Statistical Analysis.
For statistical comparisons a two-tailed t test and/or a Mann–Whitney test were used when applicable. Tumors included in the present study were collected in an ongoing national study and are part of a consecutive unbiased series of neuroblastoma patients. Samples available for analysis were included in the study.
SI Materials and Methods
Quantitative Real-Time PCR Analysis.
RNA was prepared from ∼30 mg of primary tumor tissue using RNeasy (Qiagen), and 100 ng of RNA was used to synthesize cDNA using the High Capacity RNA-to-cDNA Kit (Applied Biosystems). TaqMan Gene Expression Assays (Applied Biosystems) were used to evaluate the relative expression levels of mPGES-1, COX-1, and COX-2 mRNA. Each reaction contained 2×TaqMan Universal PCR Master Mix and 20×TaqMan Gene Expression Assay [mPGES-1 (PTGES), Hs01115610-m1; COX-1 (PTGS1), Hs00377726-m1; COX-2 (PTGS2), Hs01573471-m1; HPRT1, Hs02800695-m1] and 2 µL cDNA for detection of the reference gene Hypoxanthine guanine phosphoribosyl transferase 1 (HPRT) or 10 µL cDNA for detection of mPGES-1, COX-1, and COX-2, respectively. The final volume was adjusted to 25 µL with RNase-free water. The PCR was performed in MicroAmp optical 96-well plates covered with MicroAmp optical caps (Applied Biosystems). The expression levels were detected on an ABI PRISM 7500 sequence detection system (Applied Biosystems). Expression was normalized to an endogenous control (HPRT), and differences in fold change were calculated using a calibrator sample. NB9, which had the median mPGES-1 level in the low-risk group, was selected as the calibrator sample.
Prostaglandin Profiling.
Approximately 40 mg of human primary tumor tissue from 29 individuals was used in the analysis. Liquid–liquid extraction and analysis by LC-MS/MS was performed. As internal standards, 75 µL of deuterated PGE2, PGD2, prostaglandin F2α (PGF2α), 6-keto-PGF1α, and thromboxane B2 were added to the tumor samples. Before homogenization, 20 µL methanol and 125 µL 0.1% formic acid were added. After mixing for 5 min, 600 µL ethyl acetate was added to extract all lipids. This step was repeated twice. For mice tumors ∼30 mg of tumor tissue was homogenized by mixing after the addition of 75-µL internal standards (deuterated PGE2, PGD2, PGF2α, 6-keto-PGF1α, and thromboxane B2), 20 µL methanol, and 125 µL 0.1% formic acid. After mixing for 5 min, 600 µL methanol was added, and samples were mixed for 5 min and centrifuged to pellet cell debris. This step was repeated twice. The methanol from the supernatant was evaporated under vacuum before solid-phase extraction using Oasis HLB extraction columns (Waters Corporation) as described previously (46).
Prostanoid screening was performed by LC-MS/MS on a Waters 2795 HPLC coupled to an Acquity TQ Detector triple quadrupole mass spectrometer (Waters Corporation). Samples were eluted from a Synergy Hydro-RP column (100 mm, 2 mm i.d., 2.5 mm particle size, and 100-Å pore size) using a 45-min stepwise gradient ranging from 10–90% solvent B (acetonitrile acidified with 0.05% formic acid). The mobile phase was composed of MilliQ water as solvent A and acetonitrile acidified with 0.05% formic acid as solvent B. Prostanoids were detected in multiple reaction monitoring mode at mass transitions of m/z 351.1 > 315.1 for PGE2 and PGD2, eluting at 23.3 min and 24.3 min, respectively; PGF2α was detected at m/z 353.2 > 309.1, eluting at 22.7 min, TXB2 at m/z 369.1 > 169.1, eluting at 21.7 min, and 6-keto-PGF1α at m/z 369.1 > 245.2, eluting 17.0 min. An internal standard calibration curve was used for calibration.
Western Blot Analysis of mPGES-1 in Neuroblastoma Tumor Tissue.
Tumor tissue was lysed using T-PER (Thermo Fisher Scientific) containing complete protease inhibitor mixture (Roche) and was incubated on ice for 30 min. Tumor lysates were sonicated for 10 min and centrifuged briefly. Concentrations of the samples were determined using bicinchoninic acid (BCA) assay (Pierce). Approximately 85 μg of tumor lysate was separated by SDS/PAGE and transferred to PVDF membranes (GE Healthcare). Membranes were probed with α-mPGES-1 antibody (1:250; Cayman 160140), α-COX-1 antibody (1:1,000; Cayman 160108), and α-COX-2 antibody (1:200; Cayman 160106). Secondary α-rabbit was diluted 1:50,000 (GE Healthcare), and α-mouse was diluted 1:10,000 (GE Healthcare). Recombinant mPGES-1 was loaded as positive control for mPGES-1 and IL-1β–stimulated A549 cells were used as positive control for COX-1 and COX-2.
IHC Analysis of Neuroblastoma Tumor Tissue.
Frozen tumor tissue was sectioned using a cryostat in 7-μm thin sections and fixed in 2% (vol/vol) formaldehyde for 20 min. Dilutions and washes were performed using PBS containing 0.1% saponin, pH 7.4. Endogenous peroxidase activity was blocked using 1% H2O2, and biotin was blocked using an avidin/biotin blocking kit (Vector Laboratories). Tumor sections were incubated overnight at room temperature with primary antibody containing 3% (vol/vol) human serum. After incubation for 15 min with 1% goat serum (or horse serum, depending on the secondary antibody), sections were incubated for 30 min at room temperature with biotin-conjugated secondary antibodies (goat α-rabbit IgG, 1:1,600; horse α-mouse IgG, 1:320; Vector Laboratories) containing 1% goat or horse serum and 3% human serum. After incubation with ABC complex (Elite ABC kit, Vector Laboratories), the sections were developed for 6 min using diaminobenzidine (DAB Peroxidase Substrate Kit; Vector Laboratories) as the chromogen. Sections were counterstained with Mayer’s hematoxylin (Histolab). Tumors from at least three patients from each subgroup (11q-deleted, MYCN-amplified, and low-risk) were quantified for expression of mPGES-1, COX-1, COX-2, L-PGDS, H-PGDS, 15-PGDH, and macrophages (MHCII, CD206, and CD163) using Leica Qwin IM500 software as described previously (47). An unpaired two-tailed t test was used for statistical analysis. For antibody concentrations, see Table S4.
Immunofluorescent Staining of Neuroblastoma Tumor Tissue.
Tumor sections were washed for 10 min and blocked in 20% normal human serum for 45 min at room temperature. Sections then were incubated overnight at room temperature with primary antibodies containing 3% human serum. Slides were washed and incubated with secondary antibodies conjugated with fluorescent dye at room temperature for 30 min. After additional washes the sections were counterstained with Hoechst diluted 1:1,000 for 30 s and were washed in PBS. Sections were mounted in glycerol with PBS (1:10). Dilutions and washes were performed using PBS containing 0.1% saponin, pH 7.4, if not stated otherwise. The double-staining procedure was performed using an in-house–made mPGES-1 antibody (1:4,000) produced in rabbit in combination with different cell markers produced in mice. The secondary reagent used was a mixture of Alexa Fluor 488 goat α-rabbit and Alexa Fluor 594 goat α-mouse (1:1,000) antibody. An additional section was used with a mixture of matched isotype controls. For information about antibodies used, see Table S4.
COX Inhibition of in Vivo 11q-Deleted Xenograft Tumor Growth.
Female NMRI nu/nu mice (4–8 wk old) obtained from Taconic Laboratories were maintained under pathogen-free conditions and were given sterile water and food ad libitum. Each mouse was inoculated on the right flank with 107 SK-N-AS cells harboring the 11q-deletion. When tumors reached a volume of 0.15 mL, the animals were randomized to receive 250 mg/L diclofenac (Cayman Chemicals) in the drinking water (n = 7) or no treatment (n = 9) for nine consecutive days. Tumors were measured every day, and the volume was calculated as (width)2 × length × 0.44. When mice were killed, tumor tissue was snap frozen for ex vivo studies.
Supplementary Material
Acknowledgments
This work was supported by grants from the Swedish Research Council, The Swedish Cancer Society, the Swedish Childhood Cancer Foundation, The Swedish Rheumatism Association, King Gustaf V 80 Years Foundation, Swedish Foundation for Strategic Research, and The Stockholm County Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424355112/-/DCSupplemental.
References
- 1.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362(23):2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn SL, et al. INRG Task Force The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J Clin Oncol. 2009;27(2):289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carén H, et al. High-risk neuroblastoma tumors with 11q-deletion display a poor prognostic, chromosome instability phenotype with later onset. Proc Natl Acad Sci USA. 2010;107(9):4323–4328. doi: 10.1073/pnas.0910684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodeur GM, Bagatell R. Mechanisms of neuroblastoma regression. Nat Rev Clin Oncol. 2014;11(12):704–713. doi: 10.1038/nrclinonc.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Angio GJ, Evans AE, Koop CE. Special pattern of widespread neuroblastoma with a favourable prognosis. Lancet. 1971;1(7708):1046–1049. doi: 10.1016/s0140-6736(71)91606-0. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimatsu K, et al. Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin Cancer Res. 2001;7(12):3971–3976. [PubMed] [Google Scholar]
- 8.van Rees BP, et al. Expression of microsomal prostaglandin E synthase-1 in intestinal type gastric adenocarcinoma and in gastric cancer cell lines. Int J Cancer. 2003;107(4):551–556. doi: 10.1002/ijc.11422. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki Y, et al. Microsomal prostaglandin E synthase-1 is involved in multiple steps of colon carcinogenesis. Oncogene. 2012;31(24):2943–2952. doi: 10.1038/onc.2011.472. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimatsu K, et al. Inducible prostaglandin E synthase is overexpressed in non-small cell lung cancer. Clin Cancer Res. 2001;7(9):2669–2674. [PubMed] [Google Scholar]
- 11.Jain S, Chakraborty G, Raja R, Kale S, Kundu GC. Prostaglandin E2 regulates tumor angiogenesis in prostate cancer. Cancer Res. 2008;68(19):7750–7759. doi: 10.1158/0008-5472.CAN-07-6689. [DOI] [PubMed] [Google Scholar]
- 12.Hanaka H, et al. Microsomal prostaglandin E synthase 1 determines tumor growth in vivo of prostate and lung cancer cells. Proc Natl Acad Sci USA. 2009;106(44):18757–18762. doi: 10.1073/pnas.0910218106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pai R, et al. Prostaglandin E2 transactivates EGF receptor: A novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8(3):289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 14.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276(21):18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 15.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278(37):35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 16.Pai R, et al. PGE(2) stimulates VEGF expression in endothelial cells via ERK2/JNK1 signaling pathways. Biochem Biophys Res Commun. 2001;286(5):923–928. doi: 10.1006/bbrc.2001.5494. [DOI] [PubMed] [Google Scholar]
- 17.Trompezinski S, Pernet I, Schmitt D, Viac J. UV radiation and prostaglandin E2 up-regulate vascular endothelial growth factor (VEGF) in cultured human fibroblasts. Inflamm Res. 2001;50(8):422–427. doi: 10.1007/PL00000265. [DOI] [PubMed] [Google Scholar]
- 18.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58(2):362–366. [PubMed] [Google Scholar]
- 19.Kurtova AV, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517(7533):209–213. doi: 10.1038/nature14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obermajer N, et al. PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Invest. 2012;41(6-7):635–657. doi: 10.3109/08820139.2012.695417. [DOI] [PubMed] [Google Scholar]
- 21.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Asgharzadeh S, et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol. 2012;30(28):3525–3532. doi: 10.1200/JCO.2011.40.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kharaishvili G, et al. The role of cancer-associated fibroblasts, solid stress and other microenvironmental factors in tumor progression and therapy resistance. Cancer Cell Int. 2014;14:41–48. doi: 10.1186/1475-2867-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeine R, et al. Presence of cancer-associated fibroblasts inversely correlates with Schwannian stroma in neuroblastoma tumors. Mod Pathol. 2009;22(7):950–958. doi: 10.1038/modpathol.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlson LM, et al. Low-dose aspirin delays an inflammatory tumor progression in vivo in a transgenic mouse model of neuroblastoma. Carcinogenesis. 2013;34(5):1081–1088. doi: 10.1093/carcin/bgt009. [DOI] [PubMed] [Google Scholar]
- 27.Johnsen JI, et al. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res. 2004;64(20):7210–7215. doi: 10.1158/0008-5472.CAN-04-1795. [DOI] [PubMed] [Google Scholar]
- 28.Ponthan F, et al. Celecoxib prevents neuroblastoma tumor development and potentiates the effect of chemotherapeutic drugs in vitro and in vivo. Clin Cancer Res. 2007;13(3):1036–1044. doi: 10.1158/1078-0432.CCR-06-1908. [DOI] [PubMed] [Google Scholar]
- 29.Rasmuson A, et al. Autocrine prostaglandin E2 signaling promotes tumor cell survival and proliferation in childhood neuroblastoma. PLoS ONE. 2012;7(1):e29331. doi: 10.1371/journal.pone.0029331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu AL, et al. Children’s Oncology Group Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westman M, et al. Expression of microsomal prostaglandin E synthase 1 in rheumatoid arthritis synovium. Arthritis Rheum. 2004;50(6):1774–1780. doi: 10.1002/art.20286. [DOI] [PubMed] [Google Scholar]
- 32.Kryh H, et al. Comprehensive SNP array study of frequently used neuroblastoma cell lines; copy neutral loss of heterozygosity is common in the cell lines but uncommon in primary tumors. BMC Genomics. 2011;12:443–453. doi: 10.1186/1471-2164-12-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ladenstein R, et al. Randomized Trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: The European HR-NBL1/SIOPEN study. J Clin Oncol. 2010;28(21):3516–3524. doi: 10.1200/JCO.2009.27.3524. [DOI] [PubMed] [Google Scholar]
- 34.Salvado MD, Alfranca A, Escolano A, Haeggström JZ, Redondo JM. COX-2 limits prostanoid production in activated HUVECs and is a source of PGH2 for transcellular metabolism to PGE2 by tumor cells. Arterioscler Thromb Vasc Biol. 2009;29(7):1131–1137. doi: 10.1161/ATVBAHA.109.188540. [DOI] [PubMed] [Google Scholar]
- 35.Pistoia V, et al. Immunosuppressive microenvironment in neuroblastoma. Front Oncol. 2013;3:167–174. doi: 10.3389/fonc.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santilli G, et al. Polyphenon [corrected] E enhances the antitumor immune response in neuroblastoma by inactivating myeloid suppressor cells. Clin Cancer Res. 2013;19(5):1116–1125. doi: 10.1158/1078-0432.CCR-12-2528. [DOI] [PubMed] [Google Scholar]
- 37.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Donnini S, et al. EGFR signaling upregulates expression of microsomal prostaglandin E synthase-1 in cancer cells leading to enhanced tumorigenicity. Oncogene. 2012;31(29):3457–3466. doi: 10.1038/onc.2011.503. [DOI] [PubMed] [Google Scholar]
- 39.Alcolea S, et al. Interaction between head and neck squamous cell carcinoma cells and fibroblasts in the biosynthesis of PGE2. J Lipid Res. 2012;53(4):630–642. doi: 10.1194/jlr.M019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17(2):135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 41.Cortez E, Roswall P, Pietras K. Functional subsets of mesenchymal cell types in the tumor microenvironment. Semin Cancer Biol. 2014;25:3–9. doi: 10.1016/j.semcancer.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Paulsson J, Micke P. Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin Cancer Biol. 2014;25:61–68. doi: 10.1016/j.semcancer.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Kakarla S, Song XT, Gottschalk S. Cancer-associated fibroblasts as targets for immunotherapy. Immunotherapy. 2012;4(11):1129–1138. doi: 10.2217/imt.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li HJ, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov. 2012;2(9):840–855. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baryawno N, et al. Tumor-growth-promoting cyclooxygenase-2 prostaglandin E2 pathway provides medulloblastoma therapeutic targets. Neuro-oncol. 2008;10(5):661–674. doi: 10.1215/15228517-2008-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Idborg H, et al. Effects of mPGES-1 deletion on eicosanoid and fatty acid profiles in mice. Prostaglandins Other Lipid Mediat. 2013;107:18–25. doi: 10.1016/j.prostaglandins.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Melin E, Lindroos E, Lundberg IE, Borg K, Korotkova M. Elevated expression of prostaglandin E2 synthetic pathway in skeletal muscle of prior polio patients. J Rehabil Med. 2014;46(1):67–72. doi: 10.2340/16501977-1230. [DOI] [PubMed] [Google Scholar]
- 48.Pearson AD, et al. European Neuroblastoma Study Group Children’s Cancer and Leukaemia Group (CCLG formerly United Kingdom Children’s Cancer Study Group) High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: A randomised trial. Lancet Oncol. 2008;9(3):247–256. doi: 10.1016/S1470-2045(08)70069-X. [DOI] [PubMed] [Google Scholar]