Significance
Human populations are rapidly expanding along Earth's coastlines, increasing stress to coastal ecosystems and the services they provide. One of these stressors comes from an increase in nutrient inputs from coastal land development that can enter estuaries and coastal seas leading to algal blooms that deplete oxygen from the water, a condition known as hypoxia. In this study, we investigated how coastal hypoxia influences fish diversity, the nursery habitat for juvenile fish, and fisheries production in adjacent offshore waters. We found that severity in hypoxia corresponded with declines to fish diversity, nursery quality, and offshore fisheries. Furthermore, intensification of El Niño mediated hypoxia by improving dissolved oxygen conditions, demonstrating that climate can influence anthropogenic stressors affecting coastal ecosystem services.
Keywords: ecosystem services, El Niño, fisheries, hypoxia, resilience
Abstract
Coastal ecosystems provide numerous important ecological services, including maintenance of biodiversity and nursery grounds for many fish species of ecological and economic importance. However, human population growth has led to increased pollution, ocean warming, hypoxia, and habitat alteration that threaten ecosystem services. In this study, we used long-term datasets of fish abundance, water quality, and climatic factors to assess the threat of hypoxia and the regulating effects of climate on fish diversity and nursery conditions in Elkhorn Slough, a highly eutrophic estuary in central California (United States), which also serves as a biodiversity hot spot and critical nursery grounds for offshore fisheries in a broader region. We found that hypoxic conditions had strong negative effects on extent of suitable fish habitat, fish species richness, and abundance of the two most common flatfish species, English sole (Parophrys vetulus) and speckled sanddab (Citharichthys stigmaeus). The estuary serves as an important nursery ground for English sole, making this species vulnerable to anthropogenic threats. We determined that estuarine hypoxia was associated with significant declines in English sole nursery habitat, with cascading effects on recruitment to the offshore adult population and fishery, indicating that human land use activities can indirectly affect offshore fisheries. Estuarine hypoxic conditions varied spatially and temporally and were alleviated by strengthening of El Niño conditions through indirect pathways, a consistent result in most estuaries across the northeast Pacific. These results demonstrate that changes to coastal land use and climate can fundamentally alter the diversity and functioning of coastal nurseries and their adjacent ocean ecosystems.
Over a third of Earth’s human population is concentrated along coastal margins (1), and much of the planet is dependent on the many functions and services provided by coastal ecosystems. Coastal ecosystems face multiple threats that include habitat loss and modification through urban development, intensification of agriculture and subsequent eutrophication, climate change, and overfishing, all of which decrease ecosystem functioning and diminish the ecological and economic value of continental shelves around the world (2–6). The effect of multiple stressors, such as climate change and hypoxia, over spatial and temporal scales relevant to the diversity and function of coastal systems is poorly understood. Furthermore, there are very few predictions on how climate change will interact with other anthropogenic threats to influence ecosystem functioning and services.
Certain critical functions and services of coastal ecosystems, such as estuaries, are potentially affected by anthropogenic threats. These services include supporting biodiversity (7) and the provision of nursery habitat for species, where estuaries can contribute disproportionately to offshore fisheries productivity (8, 9). The nursery function, in particular, could be affected by a suite of anthropogenic stressors, manifesting in declines to offshore fisheries production. Along the California Current, factors potentially influencing the coastal nursery function include climatic effects, such as El Niño and upwelling (10–12), as well as anthropogenic factors operating on multiple scales, such as ocean warming on ocean basin scales (12–14), or anthropogenic nutrient loading on local to regional scales. The latter can drive the depletion of oxygen from the water column, hypoxia, with negative consequences to aquatic life (2, 14–17).
Using a highly altered, albeit regionally important estuarine ecosystem, we examined how anthropogenically induced hypoxia influences vital ecosystem services, such as the maintenance of biodiversity and nursery function, and investigated whether climate indirectly drives these ecosystem services through the modulation of hypoxia. By determining the climatic drivers of hypoxia and its association with fish diversity and nursery function, we are able to show the linkages between human stressors, climate, and ecosystem services.
Study System: Anthropogenic Stress Threatening a Valuable Nursery and Biodiversity Hot Spot
Elkhorn Slough is an estuary on the central California coast that is representative of temperate estuaries worldwide facing multiple anthropogenic threats. Although it has a relatively small area, Elkhorn Slough provides important juvenile habitat for several species of cultural, ecological, and commercial importance, which include marine mammals and birds (18), sharks (19), and commercially important flatfish (20). Additionally, it is the only major estuary along 350 km of coastline, making it an important system for supporting regional biodiversity. Finally, Elkhorn Slough has a rare combination of long-term fish, water quality, and regional climatic and oceanographic datasets that span 40 y in the estuary and >20 y in the adjacent offshore region, making it possible to test the relationship between anthropogenic stressors, climate, and ecosystem services, specifically fish diversity and the estuarine nursery function. Systems like Elkhorn Slough, where it is possible to examine climate and anthropogenic influences on ecosystem services over meaningful spatial and temporal scales are essential for making predictions on the effects of anthropogenic stressors in a changing climate.
Despite its importance as a nursery and biodiversity hot spot, the estuary is threatened by anthropogenic stressors, most notably enhanced nutrient loading. Elkhorn Slough has some of the highest recorded nutrient loading in temperate estuaries worldwide (21, 22), a consequence of a highly agricultural landscape surrounding the estuary. This nutrient loading has created eutrophic and hypoxic conditions in the estuary (22–24), which are among the most severe in the United States (Fig. S1). Consequently, spatially and temporally variable hypoxic conditions develop in the estuary (24, 25), a condition that is known to cause declines in fish populations through reduced survival, growth, and reproduction (3, 15, 16, 26).
As an indicator of biodiversity, we quantified fish species richness. As an indicator of nursery function of the estuary, we focused on the two most abundant flatfish species in the estuary, English sole (Parophrys vetulus) and speckled sanddab (Citharichthys stigmaeus) (present in 29.3% and 45.8%, respectively, in surveys from 1970 to 2010; n = 371). The juvenile life history stage for both English sole (19–250 mm) (27) (µElkhorn Slough = 55.9 ± 29.7 SD) and speckled sanddab (20–90 mm) (27) (µElkhorn Slough = 73.4 ± 22.1 SD) was the most common life history stage caught in surveys (95.3% and 69.1%, respectively), emphasizing the nursery role of the estuary. Both species are known to use estuaries as nurseries (20, 28); for English sole, the majority leave estuaries as age-0 (<1 y; ∼120 mm) juveniles (29) and nearly all leave by age 1 (<2 y; ∼180 mm) (28). English sole is a commercially important fish, and it has been estimated that ∼50% of adults caught in the offshore Monterey Bay region used Elkhorn Slough as juveniles (20). Elkhorn Slough is also at the southern end of the range for English sole (30), making it potentially more susceptible to both hypoxia and temperature stress.
Results and Discussion
Impaired Water Quality Drives the Loss of Nursery Function and Fish Diversity.
Hypoxia had negative consequences for the maintenance of fish diversity and nursery function for flatfish. The negative effects of hypoxia on fish have been demonstrated, yet studies on hypoxic effects are usually on short timescales (hours to <10 y) or are not spatially and temporally explicit (16, 17). Here, we present results from 40 y of monitoring that suggests that the effects of anthropogenic nutrient loading and hypoxia on fish are variable through time and space. We define hypoxia as any dissolved oxygen (DO) value that drops below mean DO conditions (µElkhorn Slough = 8.23 mg⋅L−1), a similar value reported in the literature (16), where it has been reported that even minor declines in DO have been found to alter species responses, especially for many fish that will avoid areas of depleted DO. Furthermore, we used the mean DO condition in the estuary to define hypoxic conditions as fish may respond to changes in local conditions more than thresholds defined from other systems.
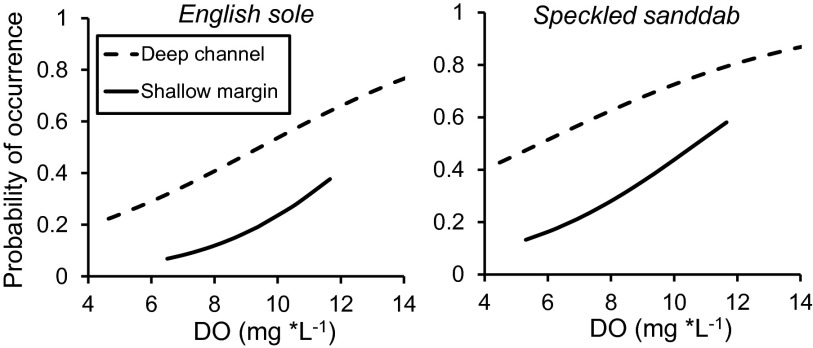
Presence of our two target flatfish species was positively correlated with greater DO conditions in Elkhorn Slough, indicating that hypoxia can negatively affect habitat quality in the flatfish nursery. Sequential logistic regression revealed that of all potential predictor variables [temperature, upwelling, El Niño Southern Oscillation (ENSO), Pacific Decadal Oscillation (PDO), salinity, and sampling effort], DO was the only factor that consistently correlated with flatfish presence (Table S1). The two target flatfish species demonstrated significant declines as a function of decreasing DO based on logistic regression (Fig. 1 and Table S1), suggesting a general negative effect of low DO on fish presence in two habitat types: deep channels (∼2–10 m in depth) and shallow margins (<2 m in depth). Temperature was also consistently included in the best-fit models using Akaike information criterion (AIC) (Table S1); however, the direction of the correlation varied from positive to negative and was often marginally significant (P = 0.05–0.10) (Table S1). Climate and oceanographic drivers (upwelling, ENSO, and PDO) were also significant predictors in the logistic regression models; however, there were few consistent patterns among species in both deep-channel and shallow-margin habitats, suggesting that local habitat conditions, such as DO, are more important factors influencing flatfish presence in the estuary. Deep-channel surveys indicated that speckled sanddab presence was also positively correlated with El Niño conditions (Table S1). On the other hand, presence of English sole was positively correlated with increased upwelling (Table S1), and this pattern was consistent for both deep-channel and shallow-margin habitats, indicating upwelling could be driving the recruitment of English sole to the estuary.
Fig. 1.
Logistic regression analysis of the predicted probability of occurrence for two species of flatfish as function of DO concentration (in milligrams per liter) for deep-channel (dotted line) (n = 169) and shallow-margin (solid line) (n = 78) habitats for English sole and speckled sanddab. See Table S1 for logistic regression results.
We determined the spatial extent of flatfish habitat quality in the estuary by modeling flatfish presence as a function of DO. We targeted DO because it was the strongest and most consistent predictor of flatfish presence based on the logistic regressions described above. The lower section of the main channel of Elkhorn Slough provided the highest quality habitat for flatfish (Fig. S2) based on higher levels of DO and the strong positive relationship between DO and fish presence. Flatfish habitat quality, as defined by DO levels, generally worsened outside of the lower main channel of the estuary. Peripheral areas of the estuary where tides were restricted by water control structures were the poorest habitat for flatfish in the estuary, due to more frequent and intense hypoxic conditions (24). Also, difficulty in fish passage through water control structures could compound the effects of severe hypoxia. Spatial patterns of the predicted probability of flatfish presence identified by habitat modeling (Fig. S2) corresponded to areas with low-to-moderate eutrophication from a previous study (24). These results indicated that hypoxia can have negative effects on fish habitat extent and potentially the nursery function for flatfish in the estuary.
The logistic regression model and the spatial modeling of predicted probabilities were validated using a 2005 spatially explicit survey of 16 water quality (Fig. S2A) and fish (Fig. S3) (31) sampling stations around Elkhorn Slough (Table S2). The logistic regression analysis of flatfish presence indicated that the probability of flatfish occurrence decreases with decreased DO (P = 0.040). Sites that were more hypoxic and behind water control structures were devoid of flatfish during the 2005 surveys. The threshold for absence was ∼4 mg⋅L−1 DO (Fig. S2) in daytime DO samples; DO levels are typically much lower at nighttime, and anoxic conditions occur commonly in tidally restricted sites in Elkhorn Slough (24). This value was consistent with the lower threshold from the logistic regressions at long-term fish sampling locations (Fig. 1 and Fig. S2A) and is also consistent with previous studies documenting flatfish density declines occurring below 3 mg⋅L−1 DO (32, 33). These results also suggest that negative hypoxic effects to flatfish are compounded by additional stressors, in this case habitat alterations through water control structures.
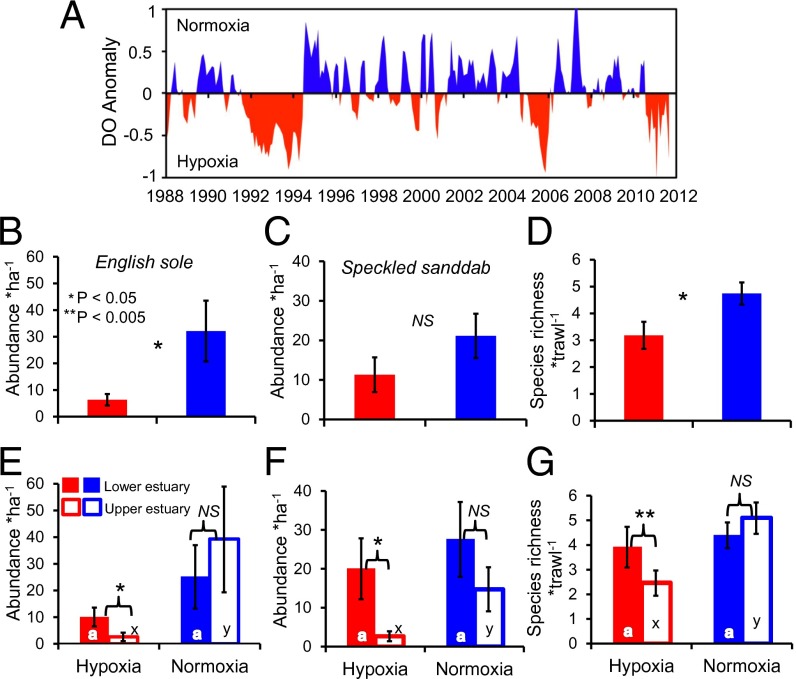
The flatfish nursery and suitable fish habitat in Elkhorn Slough is dynamic in time and space as revealed by a dissolved oxygen anomaly (DOA) (SI Methods), which identified periods of hypoxia and normoxia in nonartificially restricted areas of the estuary (Fig. 2A, Fig. S2, and Table S3). During periods of normoxia, English sole abundance increased by 367% (P = 0.034; Fig. 2B) and fish species richness increased by 49% (P = 0.029; Fig. 2D). Although there was no significant difference in normoxic and hypoxic periods for speckled sanddab (P = 0.175), there was a trend of greater abundance during normoxic periods (Fig. 2C).
Fig. 2.
(A) Time series of the 3-mo moving average of the DOA (in milligrams per liter ± 0.38 SD). (B–D) Results from an independent-samples t tests comparing English sole and speckled sanddab abundance and fish species richness between hypoxic and normoxic periods using data from deep-channel survey data pooled from both the upper and lower estuary (see Table S3 for statistical results and sample sizes). (E–G) Results from both paired-samples t tests comparing differences in the lower and upper estuary on fish parameters during hypoxia and normoxia, respectively (see Table S3 for statistical results and sample sizes); and independent-samples t tests comparing the hypoxic and normoxic periods on fish parameters for both the lower and upper estuary, respectively (see Table S3 for statistical results and sample sizes). Differences in letters indicate significant differences (P < 0.10). All error bars represent ±1 SE.
During periods of normoxia, fully tidal areas in the upper half of the estuary become suitable flatfish nursery habitat and supported greater fish species richness compared with hypoxic periods (Table S3). Therefore, fish diversity and flatfish nursery habitat are only provided for in one-half of the estuary during hypoxic periods, but nearly the entire estuary is available during normoxia. During periods of normoxia, there were no significant differences between the upper and lower estuary for species richness (P = 0.153) and both English sole (P = 0.561) and speckled sanddab (P = 0.305) abundances, indicating the upper estuary is suitable habitat for fish when conditions are favorable (Fig. 2 E–G and Table S3). However, significant differences between the upper and lower estuary emerged during hypoxic conditions, as significantly fewer species (P = 0.005), English sole (P = 0.018), and speckled sanddab (P = 0.042) used the upper estuary (Table S3). Additionally, there were no significant differences between hypoxic and normoxic conditions in the lower estuary for species richness and English sole and speckled sanddab abundances (all P > 0.10; Fig. 2 E–G and Table S3). However, there were significantly lower species richness (P = 0.002) (51.8% reduction) and abundance of English sole (P = 0.068) (93.3% reduction) and speckled sanddab (P = 0.054) (82.0% reduction) in the upper estuary during hypoxic conditions compared with normoxic conditions (Table S3). Based on our spatial models of available flatfish habitat in the estuary (Fig. S2), we estimated that the estuary-wide decline of fish associated with hypoxia could result in an average annual loss of ∼7,000 speckled sanddabs and ∼18,000 English sole, the latter of which could translate to a substantial loss of recruitment to the offshore adult population with consequences to the overall population and fishery, thus decreasing the nursery function for flatfish in the estuary.
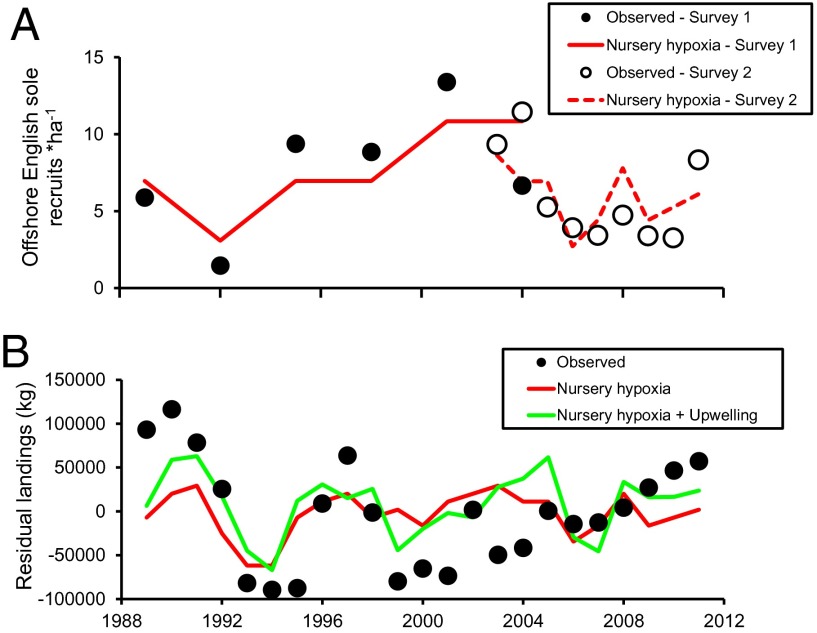
Indeed, periods of hypoxia corresponded to declines in the offshore English sole population (Table S4). The majority of juvenile English sole recruit to the offshore adult population after their first or second year in the nursery (34), so we predicted that the 80% decline in juvenile English sole abundance observed in Elkhorn Slough during hypoxic years (Fig. 2B) would translate to a significantly reduced abundance in offshore catch in the following year. As predicted, cross-correlation analysis detected a 1-y lag effect on the offshore recruitment of English sole based on National Marine Fisheries Service (NMFS) trawl surveys in the Monterey Bay region (Fig. S4). Increases in the nursery hypoxia (measured as the number of hypoxic months according to the DOA), were correlated with lower English sole recruitment (recruits per hectare, 2003–2011) in this fishery-independent dataset. Using the 1-y lag correlation, we modeled the relationship between nursery hypoxia (measured as the number of hypoxic months according to the DOA) and DO in the offshore region using multiple regression with two independent and overlapping NMFS surveys, one from 1989 to 2004 and the other from 2003 to 2011. We were interested in offshore DO because it too went hypoxic in English sole habitats during the 1989–2011 study period (µMonterey Bay = 2.66 ± 0.88 SD). Nursery hypoxia was the sole predictor of offshore adult English sole recruitment in Monterey Bay in both independent trawl surveys (Fig. 3A): 1989–2004 (P = 0.099, R2 = 0.534) and 2003–2011 (P = 0.075, R2 = 0.385), and these relationships were negative (Table S4). There are two potential mechanisms driving the poor recruitment to the offshore population following hypoxic periods in the nursery: either hypoxia in the estuary caused increased mortality or it spurred movement to areas of poorer nursery quality (e.g., nearshore or offshore), resulting in decreased growth and survival rates. Using a fishery-dependent dataset (1989–2011), we modeled the relationship between the English sole fishery (using residual landings to correct for effort) and nursery hypoxia along with several other factors known to drive fish populations: offshore DO, ENSO, PDO, North Pacific Gyre Oscillation (NPGO), and upwelling. Nursery hypoxia was a significant predictor of declines in the English sole fishery (P = 0.006; Fig. 3B), but increased upwelling (P = 0.023) was also a significant predictor that correlated with decreases in English sole landings (full model: P = 0.011, R2 = 0.299; Table S4). Taken together, these results suggest that negative effects of hypoxia on the coastal nursery function are strongest on new recruits in the offshore region, and the combination of nursery hypoxia and unfavorable offshore conditions, such as low DO, that occur with strong upwelling can lead to declines in the English sole fishery.
Fig. 3.
Regression models showing the significant contributors to (A) English sole adult recruitment to the offshore Monterey Bay population using two independent surveys (n = 6 and 9), and (B) English sole landings with effort (year) removed (n = 23). Observed data (circles) are included to compare with model projections. Nursery hypoxia was negatively correlated with English sole recruits and fishery landings (A and B), and upwelling was only negatively correlated with fishery landings (B); see Table S4 for model results.
Moderating Influences of Climate on Coastal Eutrophication, Hypoxia, and Nursery Function.
By causing variation in precipitation and runoff, climate can be a powerful moderator of coastal hypoxia stress from anthropogenic nutrient loading (3, 35, 36). There was high variation in hypoxic and normoxic conditions in Elkhorn Slough from 1988 to 2012 (Fig. 2A). This result indicated additional drivers are also modulating hypoxia in addition to the exponential increase in nutrient loading documented in Elkhorn Slough over the last four decades (22).
Structural equation modeling (SEM) (SI Methods) identified mean annual salinity as the key correlate of the annual lower limit of DO in the upper estuary (µ = 4.85 mg⋅L−1; ±0.51 SD; Fig. S2A and Fig. 4A). Path analysis revealed that increases in El Niño conditions, including increased precipitation and decreased salinity, were associated with reduced hypoxic conditions in the upper estuary. These relationships were most likely due to increased flushing rates in the estuary (24). In the lower estuary (Fig. S2A), several factors including increased upwelling intensity, occurring primarily as springtime events (37), along with salinity, temperature, and solar radiation, all correlated negatively with DO (Fig. 4B). However, the annual lower limit in the lower estuary (µ = 5.81; ±0.51 SD) rarely approached the 4 mg⋅L−1 threshold for flatfish, indicating that upwelling and factors associated with dry conditions (high salinity, temperature, and solar radiation) lowered DO, but probably not enough to cause severe deleterious effects to fish.
Fig. 4.
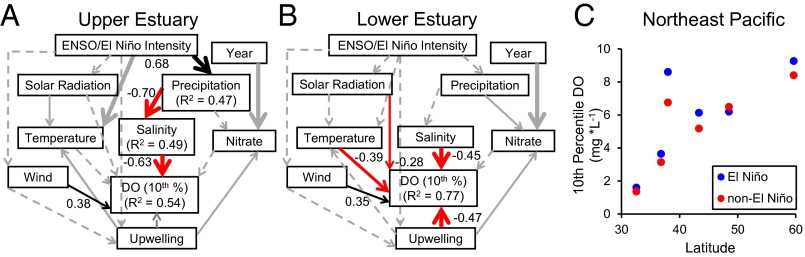
Drivers of hypoxia in (A and B) Elkhorn Slough and (C) northeast Pacific estuaries. (A and B) Path analysis from results of SEM (SI Methods) showing the direct and indirect drivers of lower DO (i.e., degree of hypoxia measured in annual 10th percentile) in upper (A) (n = 15, χ2 = 5.76, df = 6, P = 0.45) and lower (B) (n = 10, χ2 = 6.90, df = 10, P = 0.74) Elkhorn Slough, respectively. Solid lines indicate significant correlations (P < 0.10); dashed lines indicate insignificant correlations (P > 0.10); line widths are proportional to regression coefficients, and only paths with significant effects on DO and their corresponding coefficients and R2 are shown. Black lines indicate positive correlations and red lines indicate negative correlations. Gray lines were significant correlations that were not used in the final model because their paths did not lead to DO. (C) Hypoxia in northeast Pacific estuaries of the United States as a function of latitude and El Niño (n = 3) and non-El Niño (n = 3) years (SI Methods). Each point represents the mean among stations at each estuary.
The results of the SEM suggest that low salinity as well as lower upwelling indices, factors both driven by El Niño, had a positive effect on the oxygen condition of the estuary. Increases in El Niño intensity result in increased local precipitation along with warmer ocean waters; the latter can relax upwelling intensity (38, 39). An increase in the frequency and intensity of El Niño events could mitigate Elkhorn Slough’s hypoxic condition and thus support flatfish in two ways: (i) increased precipitation (as indicated by decreases in salinity) increases the flushing of the estuary, which has been shown to decrease eutrophication in hypoxic estuaries (40); and (ii) relaxation of local upwelling that brings hypoxic water from the deep sea over the continental shelf during intense upwelling years (37, 41), although this effect was not detected in our analysis. It should be noted that, despite hypoxia occurring in both the lower and upper estuary, the greatest declines to fish occurred in the upper estuary (Fig. 2 E–G) and could be due to a combination of factors, which include more frequent and severe hypoxia (Fig. S2), higher temperatures (24), and fewer refugia due to shallower depths in the upper estuary.
Finally, we hypothesized that if severity in estuarine hypoxia is mediated by large-scale climatic factors, such as El Niño, then patterns in hypoxic conditions should be consistent in estuaries across the entire northeast Pacific. Using paired-samples t tests comparing hypoxia in El Niño (ENSO index, >0.5) and neutral non-El Niño (ENSO index, −0.5 to 0.5) years (SI Methods), we found significant negative associations with El Niño and severity of hypoxic conditions in estuaries across the northeast Pacific (P = 0.018; t(18) = 2.609; Fig. 4C), indicating that cyclical climatic patterns are indeed strong predictors of hypoxia severity. El Niño conditions measured from 1997 to 2009 were associated with improved oxygen conditions in five of the six estuaries we investigated. In our analysis, the only estuary, Padilla Bay, Washington, that did not indicate positive effects from El Niño conditions is located inland ∼200 km from the exposed outer coast, and is likely not as exposed to upwelling-driven hypoxia as the other five estuaries with mouths opening directly or closer to the outer coast. Furthermore, Padilla Bay is situated in an area of Puget Sound that does not receive high precipitation (and increased flushing) during El Niño years (42). Conversely, the estuary that experienced the greatest DO differences due to El Niño was San Francisco Bay, where DO sensors were situated along a deep channel near the coastal mouth of the estuary. Future El Niño conditions may be especially important for estuaries in the southern range as they experience more severe hypoxia (Fig. S1) likely due to higher temperatures, a condition that has been predicted to increase due to climate change (14).
Conclusion
Our results demonstrate that climate variation drives ecosystem resilience to anthropogenic threats. Here, we provide the first evidence (to our knowledge) linking hypoxia in estuaries with a reduction in flatfish nursery habitat, fish species richness, and offshore fisheries production, and demonstrate how ENSO cycles drive hypoxic conditions in estuaries to mediate anthropogenic nutrient loading. We have demonstrated that anthropogenic threats to available fish habitat in one small, albeit important, estuary can have consequences for a larger region and adjacent ecosystems, and that climate interacts with these threats to influence ecosystem services. In this case, El Niño alleviated stress to the system by improving oxygen conditions.
Determining how climate change will affect threatened coastal ecosystems is a difficult challenge facing researchers and resource managers (43, 44) but one that is enhanced by the availability of long-term monitoring datasets (10, 11, 41). Our results offer insight as to how climate change may impact the effects of anthropogenic nutrient loading, which is a threat that is likely to persist given Earth’s human population growth in coastal environments (1). Here, the effect of climate change is dependent on the direction of change, the context of other stressors, and the life history and physiological tolerances of the species of concern. For example, if El Niño conditions are predicted to intensify with climate change, then this could enhance certain ecosystem services through the suppression of upwelling, increased flushing, improved oxygen conditions, and enhanced diversity and nursery function of northeast Pacific estuaries that suffer from hypoxia resulting from anthropogenic nutrient loading. However, if climate change results in moderate El Niño conditions and upwelling in the northeast Pacific intensifies, as predicted (45, 46), then it could result in poor oxygen conditions in coastal zones, especially when combined with increased anthropogenic nutrient loading, resulting in an overall decline in estuarine diversity and nursery function. As it currently stands, future ENSO conditions are highly unpredictable and there is little understanding of its long-term variation (47, 48). These results demonstrate the need to incorporate local-scale anthropogenic threats along with climate variation into models to predict and mitigate the impact of climate change on estuarine ecosystem services.
Methods
See SI Methods for descriptions of spatial modeling, DOA, DO drivers, and data sources.
Correlating Habitat Condition with Presence of Select Flatfish Species.
To test for the potential effects of hypoxia on flatfish species, we examined differences in presence/absence data for English sole and speckled sanddab. To determine the key correlates with individual flatfish species, we used sequential logistic regression with the GLM package in R, version 3.0.2. The sequential predictor variables were selected based on our a priori hypothesis that DO was the most important driver of fish in Elkhorn Slough, and on F values from preliminary multiple linear regressions in the following order: DO (in milligrams per liter), temperature (in degrees Celsius), Monterey Bay upwelling, ENSO index, PDO index, salinity (in parts per thousand), and daily sampling effort (number of trawls or seines). We omitted nitrate and the NPGO index from the sequential logistic regression because they were not significant predictors (P < 0.10) in the preliminary multiple regressions. We did not include sampling location or season as factors in the analysis because flatfish were generally caught at all times of the year throughout the primary sampling stations (Fig. S2A) in Elkhorn Slough. To confirm the relationship between environmental patterns and presence/absence for each flatfish species, we replicated the logistic regression analysis using both deep-channel and shallow-margin habitat data, respectively.
We determined the key correlates of flatfish presence using AIC. We applied a reduced logistic regression by correlating the presence/absence data to the significant individual environmental predictors to determine the direction of the correlation. We used model selection based on AIC weighting (49) to confirm that the final sequential logistic regression was the most appropriate model. Last, we applied DO as the only predictor variable to determine general patterns in the relationship between DO and flatfish presence. All α values were set at 0.10 to reduce type II errors that fail to reject the null hypothesis given the challenges of large-scale field sampling (SI Methods, Model Validation of Flatfish Logistic Regressions).
Spatiotemporal Associations of the DO Anomaly and the Estuarine Fish Assemblage.
We next investigated how variation in DO conditions in the estuary explained patterns in fish species richness and abundance of English sole and speckled sanddab (number of individuals per hectare). Only data from standardized trawl surveys (1991–2003) that had surveyed deep-channel habitats from both the lower and upper estuary within a month of each other were used in the analysis. We used the 3-mo running average of DOA to characterize each sampling date as either hypoxic or normoxic. We tested for the effects of hypoxia (hypoxic or normoxic) and region (lower vs. upper) on fish diversity (fish species richness) and abundance of flatfish. The estuary was divided into lower and upper based on known hydrological, oceanographic (50, 51), and biological (24) breaks, including differences in the severity of hypoxia and eutrophication (Fig. S2A). We used a series of t tests (SPSS, version 20; IBM) to determine differences in hypoxia and region. We first pooled all data among hypoxic (n = 16) and normoxic (n = 34) regimes and compared them using an independent-samples t test. Next, we compared the paired samples (upper and lower estuary) during hypoxic and normoxic conditions, respectively, to determine specific habitat use during the two regimes using a paired-samples t test. Last, we compared fish variables in the lower and upper estuary, respectively, during hypoxic and normoxic conditions using an independent-samples t test. For independent-samples t tests, we tested for equal variances using a Levene’s test; if the test was significant (P < 0.10), then we used a Welch’s t test of unequal variances.
Offshore English Sole Population and Fishery Relationships with the DO Anomaly.
To determine whether variable nursery conditions can have consequences to the offshore English sole population, we correlated nursery hypoxia with (i) English sole recruitment using two fisheries-independent annual to triennial bottom-trawl surveys from NMFS (52, 53), and (ii) a fisheries (landings)-dependent dataset from the California Department of Fish and Wildlife. We used the standardized abundance (recruits per hectare) from depths where greater than 90% of English sole recruit to (36–200 m) for each year sampled in the two NMFS survey types (survey 1: 1989–2004, n = 6; survey 2: 2003–2011, n = 9). We only used surveys around Monterey Bay (36.5°N and 37°N, and east of −122.5°W) where adult and subadult English sole are known to recruit from the Elkhorn Slough nursery (20).
Using cross-correlation analysis in R, version 3.0.2, we determined the time lag (in years) that had the greatest correlation between the number of hypoxic months in the nursery (predictor variable) calculated from monthly means of the DOA across sites and offshore English sole recruitment (dependent variable). We then used backward stepwise multiple regression and AIC model selection to determine the environmental drivers of adult English sole recruitment using two key predictors: (i) nursery hypoxia and (ii) mean DO sampled near the sea floor in English sole habitat (200 m) in the offshore Monterey Bay region. For English sole landings (in kilograms; n = 23) in Monterey Bay, we included the additional factors NPGO, PDO, ENSO, and upwelling into the multiple regression model because it had higher replication than the recruitment dataset, allowing us to include more factors. To remove the confounding effects in annual fishing effort on the total English sole landings, we calculated the residuals from the correlation of fish landings and year, and used the residuals as the dependent variable in the final analysis (54).
For English sole subadult and young adult recruitment to the offshore population, we chose age-1 (<2 y) and age-2 (<3 y) individuals (<250-mm female, <230-mm male), most likely to have used Elkhorn Slough as a nursery the year before recruitment. We assumed that all new subadult and adult recruits would be targeted by bottom trawls along with older size classes because the minimum required net mesh size for the commercial fishery was 115 mm (55), and most English sole reach a minimum size of 120 mm by the time they reach age 1 according to Von Bertalanffy parameters from Elkhorn Slough (56), which is around the time when English sole begin to emigrate from the estuary. We considered our analysis to detect nursery hypoxia effects on the English sole offshore fishery robust, because 53.1% of fish are older juveniles or young adults <3 y of age (<25 cm) according to the NMFS trawl surveys, which accounts for the majority of fish in the population.
Supplementary Material
Acknowledgments
We thank S. Newkirk and The Nature Conservancy for supporting this work. Additionally, we thank the following group of people for support for this project: K. Wasson, P. Raimondi, M. Carr, R. Kudela, S. Munch, S. Williams, G. Cailliet, S. Norris, and L. Pendleton. This manuscript was greatly improved by comments from the editor and two anonymous reviewers. We thank J. Hastie, B. Horness, and NMFS for use of offshore trawl data, and the National Estuarine Research Reserve (NERR) program for water quality data funded through an award from the Estuarine Reserves Division, Office of Ocean and Coastal Resource Management, National Ocean Service, National Oceanic and Atmospheric Administration. Additional financial support was provided to B.B.H. through the NERR Graduate Research Fellowship and the Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, and to F.P.C. through the David and Lucile Packard Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7892.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505815112/-/DCSupplemental.
References
- 1.Barbier EB, et al. Coastal ecosystem-based management with nonlinear ecological functions and values. Science. 2008;319(5861):321–323. doi: 10.1126/science.1150349. [DOI] [PubMed] [Google Scholar]
- 2.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293(5530):629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 3.Rabalais NN, Turner RE, Wiseman WJ. Gulf of Mexico hypoxia, aka “The dead zone.”. Annu Rev Ecol Syst. 2002;33:235–263. [Google Scholar]
- 4.Lotze HK, et al. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science. 2006;312(5781):1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- 5.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319(5865):948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 6.Selman M, Greehhalgh S, Diaz R, Sugg Z. 2008. Eutrophication and Hypoxia in Coastal Areas: A Global Assessment of the State of Knowledge. World Resources Institute Policy Note (World Resources Institute, Washington, DC)
- 7.Millennium Ecosystem Assessment . Ecosystems and Human Well-Being: Biodiversity Synthesis. World Resources Institute; Washington, DC: 2005. [Google Scholar]
- 8.Beck MW, et al. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience. 2001;51:633–641. [Google Scholar]
- 9.Sheaves M, Baker R, Nagelkerken I, Connolly RM. 2014. True value of estuarine and coastal nurseries for fish: Incorporating complexity and dynamics. Estuaries Coasts 38:401–414.
- 10.Cloern JE, Jassby AD, Thompson JK, Hieb KA. A cold phase of the East Pacific triggers new phytoplankton blooms in San Francisco Bay. Proc Natl Acad Sci USA. 2007;104(47):18561–18565. doi: 10.1073/pnas.0706151104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cloern JE, et al. Biological communities in San Francisco Bay track large-scale climate forcing over the North Pacific. Geophys Res Lett. 2010;37:1–6. [Google Scholar]
- 12.Todd CD, et al. Detrimental effects of recent ocean surface warming on growth condition of Atlantic salmon. Glob Change Biol. 2008;14:958–970. [Google Scholar]
- 13.Sherman K, Belkin IM, Friedland KD, O’Reilly J, Hyde K. 2009. Accelerated warming and emergent trends in fisheries biomass yields of the world’s large marine ecosystems. Ambio 38(4):215–224. [DOI] [PubMed]
- 14.Altieri AH, Gedan KB. 2015. Climate change and dead zones. Glob Chang Biol 21(4):1395–1406. [DOI] [PubMed]
- 15.Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321(5891):926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- 16.Vaquer-Sunyer R, Duarte CM. Thresholds of hypoxia for marine biodiversity. Proc Natl Acad Sci USA. 2008;105(40):15452–15457. doi: 10.1073/pnas.0803833105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breitburg DL, Hondorp DW, Davias LA, Diaz RJ. Hypoxia, nitrogen, and fisheries: Integrating effects across local and global landscapes. Annu Rev Mar Sci. 2009;1:329–349. doi: 10.1146/annurev.marine.010908.163754. [DOI] [PubMed] [Google Scholar]
- 18.Harvey JT, Connors S. Birds and mammals. In: Caffrey JM, Brown M, Tyler WB, Silberstein M, editors. Changes in a California Estuary: A Profile of Elkhorn Slough. Elkhorn Slough Foundation; Moss Landing, CA: 2002. pp. 187–214. [Google Scholar]
- 19.Carlisle AB, Starr RM. Habitat use, residency, and seasonal distribution of female leopard sharks Triakis semifasciata in Elkhorn Slough, California. Mar Ecol Prog Ser. 2009;380:213–228. [Google Scholar]
- 20.Brown JA. Using the chemical composition of otoliths to evaluate the nursery role of estuaries for English sole Pleuronectes vetulus populations. Mar Ecol Prog Ser. 2006;306:269–281. [Google Scholar]
- 21.Caffrey J. Biogeochemical cycling. In: Caffrey JM, Brown M, Tyler WB, Silberstein M, editors. Changes in a California Estuary: A Profile of Elkhorn Slough. Elkhorn Slough Foundation; Moss Landing, CA: 2002. pp. 215–236. [Google Scholar]
- 22.Hughes BB, et al. Recovery of a top predator mediates negative eutrophic effects on seagrass. Proc Natl Acad Sci USA. 2013;110(38):15313–15318. doi: 10.1073/pnas.1302805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caffrey J, Hollibaugh J, Bano N, Haskins J. Effects of upwelling on short-term variability in microbial and biogeochemical processes in estuarine sediments from Elkhorn Slough, California, USA. Aquat Microb Ecol. 2010;58:261–271. [Google Scholar]
- 24.Hughes BB, Haskins JC, Wasson K, Watson E. Identifying factors that influence the expression of eutrophication in a central California estuary. Mar Ecol Prog Ser. 2011;439:31–43. [Google Scholar]
- 25.Beck NG, Bruland KW. Diel biogeochemical cycling in a hyperventilating shallow estuarine environment. Estuaries. 2000;23:177–187. [Google Scholar]
- 26.Diaz RJ. Overview of hypoxia around the world. J Environ Qual. 2001;30(2):275–281. doi: 10.2134/jeq2001.302275x. [DOI] [PubMed] [Google Scholar]
- 27.Love MS. Certainly More Than You Want to Know About the Fishes of the Pacific Coast. Really Big Press; Santa Barbara, CA: 2011. [Google Scholar]
- 28.Baxter R, Hieb K, DeLeon S, Fleming K, Orsi J. 1999. Report on the 1980–1995 Fish, Shrimp, and Crab Sampling in the San Francisco Bay Estuary, California (Interagency Ecological Program for the San Francisco Bay-Delta Estuary, Sacramento, CA), Technical Report 63.
- 29.Krygier EE, Pearcy WG. The role of estuarine and offshore nursery areas for young English sole, Parophrys vetulus Girard, of Oregon. Fish Bull. 1986;84:119–132. [Google Scholar]
- 30.Emmett RL, Stone S, Hinton SA, Monaco M. 1991. Distribution and Abundance of Fishes and Invertebrates in West Coast Estuaries. Species Life History Summaries. (NOAA, Rockville, MD), Vol II.
- 31.Ritter AF, et al. Ecological signatures of anthropogenically altered tidal exchange in estuarine ecosystems. Estuaries Coasts. 2008;31:554–571. [Google Scholar]
- 32.Levings CD. 1980. Demersal and benthic communities in Howe Sound basin and their responses to dissolved oxygen deficiency (Government of Canada, Fisheries and Oceans, West Vancouver, BC, Canada), Canadian Technical Report of Fisheries and Aquatic Sciences No. 951.
- 33.Eby LA, Crowder LB. Hypoxia-based habitat compression in the Neuse River Estuary: Context dependent shifts in behavioral avoidance thresholds. Can J Fish Aquat Sci. 2002;59:952–965. [Google Scholar]
- 34.Gunderson DR, Armstrong DA, Shi Y-B, McConnaughey RA. Patterns of estuarine use by juvenile English sole (Parophrys vetulus) and Dungeness crab (Cancer magister) Estuaries. 1990;13:59–71. [Google Scholar]
- 35.Rabalais NN, et al. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences. 2010;7:585–619. [Google Scholar]
- 36.Scully ME. Wind modulation of dissolved oxygen in Chesapeake Bay. Estuaries Coasts. 2010;33:1164–1175. [Google Scholar]
- 37.Booth JAT, et al. Natural intrusions of hypoxic, low pH water into nearshore marine environments on the California coast. Cont Shelf Res. 2012;45:108–115. [Google Scholar]
- 38.Chavez FP. Forcing and biological impact of onset of the 1992 El Niño in central California. Geophys Res Lett. 1996;33:265–268. [Google Scholar]
- 39.Friederich GE, Walz PM, Burczynski MG, Chavez FP. Inorganic carbon in the central California upwelling system during the 1997-1999 El Niño-La Niña event. Prog Oceanogr. 2002;54:185–203. [Google Scholar]
- 40.Paerl HW, et al. Ecosystem responses to internal and watershed organic matter loading: Consequences for hypoxia in the eutrophying Neuse River Estuary, North Carolina, USA. Mar Ecol Prog Ser. 1998;166:17–25. [Google Scholar]
- 41.Chan F, et al. Emergence of anoxia in the California Current Large Marine Ecosystem. Science. 2008;319(5865):920. doi: 10.1126/science.1149016. [DOI] [PubMed] [Google Scholar]
- 42.Fleming SW, Whitfield PH, Moore RD, Quilty EJ. Regime-dependent streamflow sensitivities to Pacific climate modes cross the Georgia–Puget transboundary ecoregion. Hydrol Processes. 2007;21:3264–3287. [Google Scholar]
- 43.Côté IM, Darling ES. Rethinking ecosystem resilience in the face of climate change. PLoS Biol. 2010;8(7):e1000438. doi: 10.1371/journal.pbio.1000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Micheli F, et al. Evidence that marine reserves enhance resilience to climatic impacts. PLoS One. 2012;7(7):e40832. doi: 10.1371/journal.pone.0040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auad G, Miller A, Di Lorenzo E. Long-term forecast of oceanic conditions off California and their biological implications. J Geophys Res. 2006;111:1–14. [Google Scholar]
- 46.Sydeman WJ, et al. Climate change. Climate change and wind intensification in coastal upwelling ecosystems. Science. 2014;345(6192):77–80. doi: 10.1126/science.1251635. [DOI] [PubMed] [Google Scholar]
- 47.Collins M, et al. The impact of global warming on the tropical Pacific Ocean and El Niño. Nat Geosci. 2010;3:391–397. [Google Scholar]
- 48.Giese BS, Ray S. El Niño variability in simple ocean data assimilation (SODA), 1871–2008. J Geophys Res. 2011;116:C02024. [Google Scholar]
- 49.Johnson JB, Omland KS. Model selection in ecology and evolution. Trends Ecol Evol. 2004;19(2):101–108. doi: 10.1016/j.tree.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Largier JL, Hollibaugh JT, Smith SV. Seasonally hypersaline estuaries in Mediterranean-climate regions. Estuar Coast Shelf Sci. 1997;45:789–797. [Google Scholar]
- 51.Nidzieko NJ, Monosmith SG. Contrasting seasonal and fortnightly variations in the circulation of a seasonally inverse estuary, Elkhorn Slough, California. Estuaries Coasts. 2013;36:1–17. [Google Scholar]
- 52. NMFS, Data sources are from the NOAA Alaska Fisheries Science Center's West Coast Groundfish Triennial Shelf Bottom Trawl Survey database (Racebase) and the NOAA Northwest Fisheries Science Center's West Coast Groundfish Bottom Trawl Survey. Available upon request from www.nwfsc.noaa.gov/research/divisions/fram/popeco/index.cfm.
- 53.Zimmermann M. Benthic fish and invertebrate assemblages within the National Marine Fisheries Service US West Coast Triennial Bottom Trawl Survey. Cont Shelf Res. 2006;26:1005–1027. [Google Scholar]
- 54.Graham MH. Confronting multicollinearity in ecological multiple regression. Ecology. 2003;84:2809–2815. [Google Scholar]
- 55.Pacific Fishery Management Council 2014. Pacific Coast Groundfish Fishery Management Plan, for the California, Oregon, and Washington Groundfish Fishery (Pacific Fishery Management Council, Portland, OR)
- 56.Smith JG, Nitsos RJ. Age and growth studies of English sole, Parophrys vetulus, in Monterey Bay, California. Pac Mar Fish Comm Bull. 1969;7:73–79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.