Significance
Vitamin D deficiency is associated with HIV/AIDS progression and mortality. Seasonal decline in UVB radiation, darkly pigmented skin, low nutritional vitamin D intake, and genetic variation can increase risk of deficiency. Cape Town, South Africa, has a seasonal UVB regime and one of the world’s highest rates of HIV-1 infection, peaking in young adults. In two ethnically distinct groups of young adults in Cape Town we found high prevalence of seasonal vitamin D deficiency resulting from inadequate UVB exposure. This deficiency was associated with increased permissiveness of blood cells to HIV-1 infection which was reversed by vitamin D3 supplementation. Vitamin D may be a simple, cost-effective intervention, particularly in resource-poor settings, to reduce HIV-1 risk and disease progression.
Keywords: seasonal variation, infectious disease, polymorphism, pigmentation, nutrition
Abstract
Cape Town, South Africa, has a seasonal pattern of UVB radiation and a predominantly dark-skinned urban population who suffer high HIV-1 prevalence. This coexistent environmental and phenotypic scenario puts residents at risk for vitamin D deficiency, which may potentiate HIV-1 disease progression. We conducted a longitudinal study in two ethnically distinct groups of healthy young adults in Cape Town, supplemented with vitamin D3 in winter, to determine whether vitamin D status modifies the response to HIV-1 infection and to identify the major determinants of vitamin D status (UVB exposure, diet, pigmentation, and genetics). Vitamin D deficiency was observed in the majority of subjects in winter and in a proportion of individuals in summer, was highly correlated with UVB exposure, and was associated with greater HIV-1 replication in peripheral blood cells. High-dosage oral vitamin D3 supplementation attenuated HIV-1 replication, increased circulating leukocytes, and reversed winter-associated anemia. Vitamin D3 therefore presents as a low-cost supplementation to improve HIV-associated immunity.
Vitamin D is recognized as having diverse physiological and immunomodulatory functions, and deficiency is associated with a range of communicable and noncommunicable diseases, including HIV/AIDS progression and mortality (1, 2). Previtamin D3 is made in skin when UVB photons react with 7-dehydrocholesterol (7-DHC) in the cell membranes of keratinocytes (3). Constitutive and facultative skin pigmentation regulates the penetration of UV radiation into the skin, because eumelanin competes with 7-DHC for UVB photons, thus controlling the availability of UVB for previtamin D3 production (4). Therefore people with high eumelanin content (and thus darker skin) require greater UVB exposure to make previtamin D3 and are more prone to vitamin D deficiency. This requirement for greater exposure is exacerbated by seasonal UVB fluctuation; generally UVB levels exhibit a winter decline at latitudes >30° (5, 6).
Serum vitamin D [25-hydroxy-vitamin D, 25(OH)D] levels are determined by skin (dermal and epidermal) production, dietary intake, storage, and turnover. Each of these determinants is modified at a variety of levels: skin production by pigmentation, age, and the time, duration, and level of UVB exposure; dietary intake by vitamin D composition of foods; storage by body mass index (BMI) (7) and serum vitamin D-binding protein (DBP) (8); and turnover by polymorphic variation in genes encoding metabolizing enzymes [cytochrome P450 (CYP) 2R1, CYP27B1, CYP24A1, 7-DHC reductase (DHCR7)], the vitamin D receptor (VDR) and DBP (9–12), age (13), infection (14, 15), serum calcium and parathyroid hormone concentrations (16), and smoking habit (17). The two most significant determinants are UVB exposure and dietary intake, and although deconvolution of their relative impact is vital for understanding how to maintain vitamin D sufficiency for disease prevention, they are rarely investigated in the same study, nor are results adjusted for all other confounders. Furthermore, the majority of studies investigate vitamin D status in relation to chronic disease; there is a dearth of information regarding the determinants of vitamin D sufficiency in the healthy state and its relation to disease prevention.
Cape Town has a diverse population with respect to ethnicity, skin pigmentation, and socioeconomic status. The Xhosa, who migrated as part of the geographic expansion of north and northwest African agriculturalists, are a significant proportion of the population. Another major population group comprises people of self-identified Cape Mixed ancestry, who represent a complex admixture of Xhosa, Khoisan (the oldest inhabitants), European, South Asian, and Indonesian populations (18). Given their exposure to seasonal UVB variation and high infectious disease risk, the peoples of the Cape are of particular importance in studying the determinants and immunological consequence of vitamin D status. In the context of greatest HIV-1 risk, those of particular importance are females aged 15–24 y and males aged 20–29 y (19).
We thus undertook a longitudinal study of healthy young adults in Cape Town to assess the relative contribution of pigmentation, seasonal UVB exposure, dietary vitamin D intake, genetic variation, serum DBP, and smoking habit on vitamin D status and investigated whether seasonal vitamin D variation and vitamin D supplementation impact HIV-1 immunity in this high-risk population.
Results
Highly Prevalent Seasonal Vitamin D Deficiency and Its Reversal by Winter Supplementation.
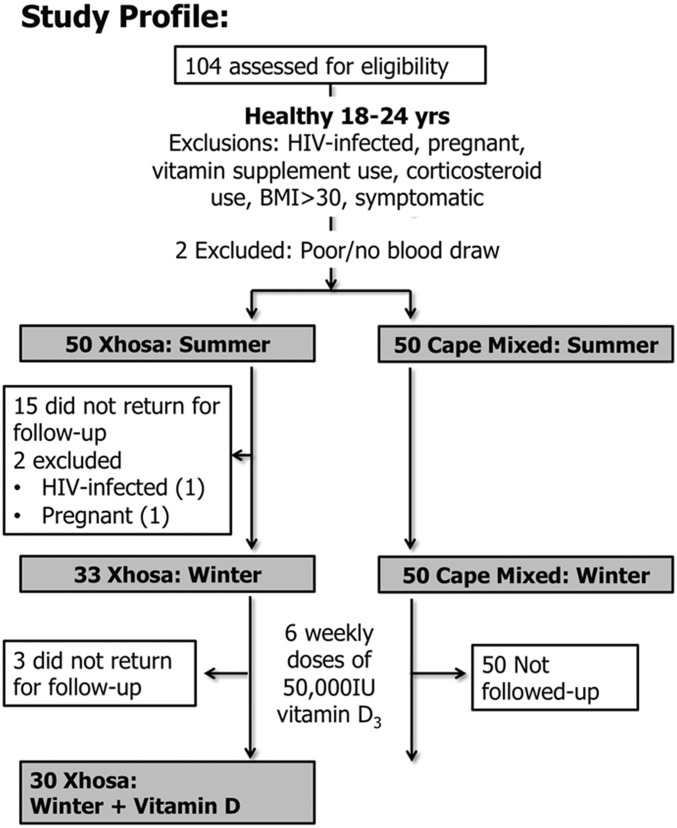
One hundred healthy (asymptomatic, BMI <30) young (18- to 24-y-old) adults (Xhosa, n = 50; Cape Mixed, n = 50) were recruited from two neighboring districts of Cape Town in the summer and were reassessed in the winter (for loss to follow-up, see Fig. S1). In winter, all participants received cholecalciferol (50,000 IU) weekly for 6 wk, and 30 Xhosa participants were followed up for 6 wk after their winter visit (Fig. 1A). All enrolled participants were asymptomatic with no evidence of infection (Fig. S2 A–C). The populations were well matched with similar female:male ratios and only a small difference in age (Xhosa 21 y vs. Cape Mixed 18.5 y; P < 0.0001) and smoking status (Xhosa 50% vs. 18%; P = 0.0014) (Table S1). Xhosa participants had darker skin pigmentation as measured by upper inner arm and forehead melanin index (MI) and erythema index (EI, a measure of tanning) (P < 0.0001; Table S2).
Fig. S1.
Study profile. We assessed 104 patients for eligibility to participate in the study between February 4 and February 28, 2013. Of these, two were ineligible: one did not attend for phlebotomy at visit 1, and one could not undergo successful phlebotomy. Of these subjects, 100 (50 Xhosa and 50 Cape Mixed) completed the summer visit. Winter participants were recruited between August 1 and August 30, 2013. All Cape Mixed and 35 Xhosa participants returned for follow-up in winter; two of the Xhosa participants were excluded because of pregnancy or HIV-infection, resulting in 33 eligible participants in winter. All participants received six oral capsules of 50,000 IU cholecalciferol at the winter visit. Administration of the first capsule was observed directly, and participants were told to take one capsule per week for the next 5 wk. Thirty Xhosa participants were followed up 6 wk (± 15 d; average +3 d) after receiving vitamin D supplementation (D3-50; Biotech Pharmaceutical).
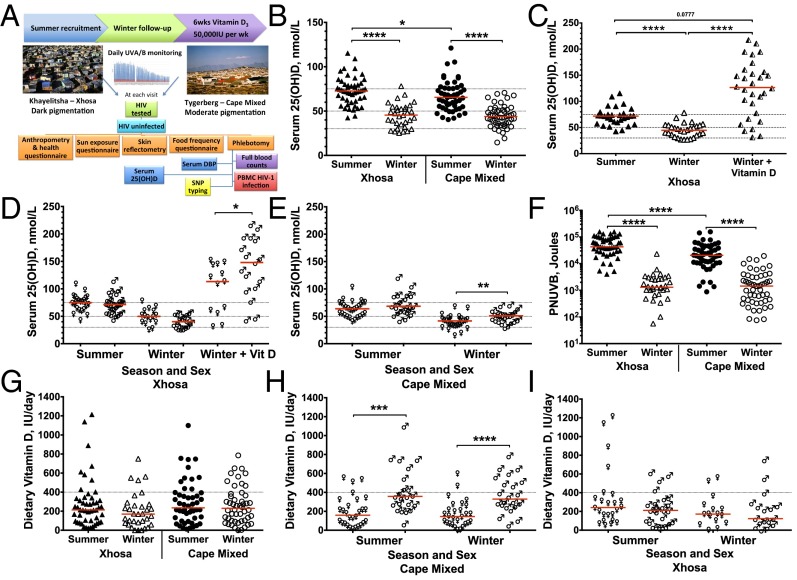
Fig. 1.
Vitamin D status, dietary vitamin D intake, and personal UVB exposure of Xhosa and Cape Mixed participants in Cape Town, South Africa, in summer, winter, and after receiving vitamin D3 in winter. (A) Study design. (B and C) Serum 25(OH)D concentration stratified by season (B) and after receiving vitamin D3 (C). Dotted lines indicate status thresholds: insufficiency, <75 nmol/L; deficiency, <50 nmol/L; severe deficiency, <30 nmol/L. (D and E) Serum 25(OH)D concentration stratified by sex (female, ♀; male, ♂) in Xhosa (D) and Cape Mixed (E) participants. (F) Dietary vitamin D intake measured by the food frequency questionnaire. Dotted lines indicate EAR. (G and H) Dietary vitamin D intake stratified by sex in Xhosa (G) and Cape Mixed (H) participants. (I) PNUVB. Xhosa: summer, n = 50; winter n = 33; winter + vitamin D, n = 30; Cape Mixed: summer and winter, n = 50. Medians are indicated by red lines. Significance was tested by the Wilcoxon rank test between seasons, by the Friedman test with Dunn’s multiple comparisons test for 25(OH)D postsupplementation (n = 30), and by the Mann–Whitney test between populations and sex; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
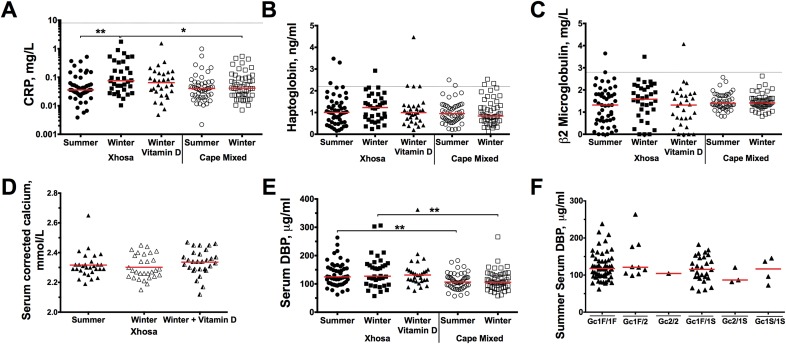
Fig. S2.
Acute-phase marker, DBP, and corrected calcium assessment. (A–C and E) In all participants the acute-phase markers serum CRP (A), haptoglobulin (B), and β2 microglobulin (C) and DBP (E) were measured at each time point. (D) Corrected calcium was measured in the 30 individuals who had 25(OH)D measured postsupplementation. (F) Summer serum DBP was stratified according to DBP Gc Haplotype. Red lines indicate the median (mean in D) and dotted lines indicate the upper level of the normal reference range for acute-phase markers. All participants fell within the normal range, except for one participant who had consistently high β2 microglobulin or haptoglobulin at more than one time point and three who had elevated levels of either β2 microglobulin or haptoglobulin at one time point. However, all participants’ CRP remained normal throughout the study, and therefore these participants were included in the study as healthy participants. There was a small but significant increase in CRP levels in Xhosa participants in winter as compared with summer, but this increase remained within the normal range. There was no effect of season or vitamin D supplementation on corrected calcium. Xhosa participants had significantly higher DBP levels in summer and in winter than Cape Mixed participants. There was no effect of season on DBP levels in either population, nor was there an effect of Gc haplotype on serum DBP levels. Significance was analyzed by a Kruskal–Wallis test with Dunn’s multiple comparisons (intra-Xhosa, except in D, which used Friedman’s test), Wilcoxon rank test (intra-Cape Mixed) or Mann–Whitney u test (intergroup); *P < 0.05; **P < 0.01.
Table S1.
Participant demographics
| Xhosa (n = 50) | Cape Mixed (n = 50) | P value* | |
| Age, median (IQR) | 21 (20–23) | 18.5 (18–20) | <0.0001 |
| BMI, Median (IQR) | 21.2 (19.8–24.8) | 20.5 (18.9–23.9) | 0.13 |
| Sex | |||
| Male, n (%) | 26 (52) | 22 (44) | 0.59 |
| Female, n (%) | 24 (48) | 28 (56) | |
| Smokers, n (%)† | 25 (50) | 9 (18) | 0.0014 |
| Follow-up | |||
| Winter, n (%) | 33 (66) | 50 (100) | |
| Supplementation, n (%)‡ | 30 (60) | — | |
P value determined by Mann–Whitney test or Fisher's exact test, bold type indicates statistically significant difference between Khosa and Cape Mixed groups.
Smokes at least 5 d a week, at at least one time point.
Cape Mixed cohort was not followed-up following supplementation.
Table S2.
Skin reflectance and sun exposure characteristics by season and population
| Measure | Summer | Winter | P value* |
| Arm MI, median (IQR) | |||
| Xhosa | 66.29 (61.92–71.38)† | 69.97 (65.25–73.90)† | 0.02 |
| Cape Mixed | 54.00 (46.03–58.28) | 50.53 (43.97–54.71) | <0.0001 |
| Forehead MI, median (IQR) | |||
| Xhosa | 70.41 (63.42–75.81)† | 73.69 (67.43–77.58)† | 0.0008 |
| Cape Mixed | 54.17 (48.314–59.91) | 50.59 (46.22–56.31) | <0.0001 |
| Arm EI, median (IQR) | |||
| Xhosa | 19.65 (18.69–20.24)† | 19.61 (18.61–20.31)† | 0.59 |
| Cape Mixed | 16.95 (14.09–19.29) | 15.16 (12.51–17.41) | <0.0001 |
| Forehead EI, median (IQR) | |||
| Xhosa | 19.28 (18.24–20.58)† | 19.84 (18.82–20.68)† | 0.37 |
| Cape Mixed | 22.18 (19.98–23.54) | 20.93 (18.55–22.37) | <0.0001 |
| Duration, median duration in minutes (IQR) | |||
| Xhosa | 1,335 (823–1,785) | 795 (465–1,410) | 0.0002 |
| Cape Mixed | 1,096 (795–1,545) | 540 (193–1,650) | 0.014 |
| Skin area exposed, %, mean (SD) | |||
| Xhosa | 30.00 (19.5–48.0)† | 12.50 (8.75–12.50) | <0.0001 |
| Cape Mixed | 22.63 (15.56–39.25) | 12.50 (6.25–12.50) | <0.0001 |
| Sunscreen used, n (%) | |||
| Xhosa | 6 (12) | 2 (6) | 0.47 |
| Cape Mixed | 7 (14) | 7 (14) | 1 |
| If used sunscreen, median SPF (range) | |||
| Xhosa | 15 (15–50) | 32.50 (15–50) | 0.82 |
| Cape Mixed | 15 (15–30) | 15 (15–30) | 1 |
EI, erythema index; MI, melanin index; bold type indicates statistically significant difference between summer and winter values.
Wilcoxon rank test or Fisher's exact test between seasons.
Mann–Whitney test significantly different between populations.
Although their higher melanin content reduced the rate of skin vitamin D production, Xhosa participants actually had higher serum 25(OH)D levels in summer than Cape Mixed participants (median 72.6 vs. 65.5 nmol/L; P = 0.038, Table 1). Cape Mixed participants also had a trend toward greater vitamin D deficiency (<50 nmol/L) in summer (16 vs. 4%; P = 0.077). Conversely, there was no difference in 25(OH)D levels between population groups in winter, when a significant drop in 25(OH)D levels was observed in both populations (P < 0.0001) and the majority of participants became vitamin D deficient (Fig. 1B). Severe deficiency (<30 nmol/L) occurred in 18% of Xhosa participants and 12% of Cape Mixed participants in the winter, and overall 64% and 70%, respectively, had deficient serum 25(OH)D levels (Table 1). After winter supplementation, 77% of the Xhosa group gained optimal levels (≥75 nmol/L) [median126.4 nmol/L, interquartile range (IQR) 74.631–57.1 nmol/L] (Fig. 1C), and there was no change in corrected serum calcium (winter mean ± SD 2.31 ± 0.08 mmol/L vs. postsupplementation 2.34 ± 0.09 mmol/L) (Fig. S2D). Cape Mixed females had lower 25(OH)D levels in winter than Cape Mixed males (median 41.46 vs. 50.80 nmol/L; P = 0.0054), and Xhosa females had lower 25(OH)D levels after supplementation than Xhosa males (median 113.3 vs. 147.9 nmol/L, P = 0.047), indicating that females in both groups are at risk for lower 25(OH)D (Fig. 1 D and E).
Table 1.
Serum 25(OH)D, dietary vitamin D intake, and UVB exposure by season and population
| Measure | Summer | Winter | P value* |
| Serum 25(OH)D, median nmol/L (range): Xhosa | 72.6 (62.1–80.43)† | 45.4 (35.7–51.2) | <0.0001 |
| Serum 25(OH)D, median nmol/L (range): Cape Mixed | 65.5 (54.6–76.1) | 43.8 (33.5–54.2) | <0.0001 |
| Vitamin D status, nmol/L (%) | <0.0001 | ||
| Xhosa: Severe deficiency, <30 nmol/L | 0 | 6 (18) | |
| Xhosa: Deficiency, <50 nmol/L | 2 (4) | 21 (64) | |
| Xhosa: Insufficiency, 50–75 nmol/L | 28 (56) | 11 (33) | |
| Xhosa: Sufficiency, >75 nmol/L | 20 (40) | 1 (3) | |
| Cape Mixed: Severe deficiency, <30 nmol/L | 0 | 6 (12) | |
| Cape Mixed: Deficiency, <50 nmol/L | 8 (16) | 35 (70) | |
| Cape Mixed: Insufficiency, 50–75 nmol/L | 27 (54) | 15 (30) | |
| Cape Mixed: Sufficiency, >75 nmol/L | 15 (30) | 0 | |
| Dietary vitamin D intake, median IU/d (IQR): Xhosa | 213 (94–335) | 170 (68–266) | 0.26 |
| Dietary vitamin D intake, median IU/d (IQR): Cape Mixed | 235 (120–395) | 230 (99–369) | 0.24 |
| Personal weekly UVB, median J (IQR): Xhosa | 42,982 (28,651–90,552)† | 1,304 (875.5–2,878) | <0.0001 |
| Personal weekly UVB, median J (IQR): Cape Mixed | 20,603 (10,887–42,897) | 1,450 (392.6–3,530) | <0.0001 |
Wilcoxon rank test or Fisher's exact test between seasons, bold type indicates statistically significant difference between summer and winter values.
Mann–Whitney test significant between populations, P < 0.04, bold type indicates statistically significant difference between summer and winter values.
Personal UVB Exposure, but Not Diet, Varies by Season.
Dietary intake of vitamin D was estimated using a 7-d quantitative food frequency questionnaire administered at each study visit. According to the estimated average requirement (EAR) cutoff-point method, 78–88% of participants in both populations had intakes below the EAR (400 IU) throughout the year, with median intakes ranging from 170 to 235 IU across groups and seasons (Fig. 1F and Table 1). There was no difference in intake between sexes in the Xhosa participants, but Cape Mixed females had lower intakes than Cape Mixed males in both seasons (P < 0.001), mirroring the sex patterns observed for 25(OH)D levels (Fig. 1 E, G, and H).
To understand the extent to which UVB exposure contributes to vitamin D deficiency, solar UVB was monitored daily for the duration of the study, and participants completed sun-exposure questionnaires. Both groups spent significantly longer in the sun in summer than in winter (P ≤ 0.014), with personal net UVB (PNUVB) exposure more than 10-fold higher in summer (P < 0.0001, Fig. 1I). Xhosa participants spent ∼4 h longer each week in the sun in both seasons than Cape Mixed participants (median: 1,335 vs. 1,096 minutes in summer and 795 vs. 540 minutes in winter) (Table S2), and they exposed larger areas of the body in the summer (median 30.0 vs. 22.6%; P = 0.0002). Both groups reduced their body exposure in winter to similar levels (12.5%), although the relative winter decrease was greater for Xhosa participants. There was limited sunscreen use in both groups (Table S2). Thus, Xhosa participants may partly compensate for their darker skin pigmentation by increasing their PNUVB in the summer (median 42,982 vs. 20,603 J; P < 0.0001), which is reflected in higher 25(OH)D levels in summer in Xhosa participants than in Cape Mixed participants (Table 1). However, Xhosa participants did not maintain higher UVB exposure in the winter (PNUVB 1,304 J vs. 1,450 J; P = 0.63), and they were at greater risk of deficiency, with 18% of participants exhibiting severe deficiency.
Genetic Variation Has a Larger Effect on the Response to Supplementation than on Seasonal Deficiency.
To investigate the effects of genetic variation on serum 25(OH)D concentrations, 10 SNPs in six genes associated with vitamin D deficiency were genotyped in all participants: DBP (rs7041 and rs4588), DHCR7 (7-DCH reductase and rs12785878), CYP2R1 (vitamin D 25-hydroxylase and rs10741657), CYP24A1 (vitamin D 24-hydroxylase and rs6013897), CYP27B1 (vitamin D 1α-hydroxylase and rs10877012), and VDR (rs2544037, rs10783219, rs10735810, and rs731236) (9–11, 20). All were in Hardy–Weinberg equilibrium, and five SNPs (CYP27B1 rs10877012; VDR rs10783219 and rs731236 TaqI; and DBP rs7041 and rs4588) had significantly higher minor allele frequency (MAF) in the Cape Mixed participants (P < 0.03, Table 2). DBP rs7041 and rs4588 are combined to form the Group component (Gc) haplotype of which there are three major alleles, Gc1F, Gc1S, and Gc2, with the Gc1F protein having higher binding affinity for serum 25(OH)D (8). Xhosa participants had significantly higher frequency of Gc1F/Gc1F carriers (76 vs. 34%), whereas the most common haplotype combination in the Cape Mixed participants was Gc1F/Gc1S (38 vs. 18%; P = 0.0002) (Table 2). Serum DBP levels also were measured in all participants; there was no effect of season, vitamin D supplementation or Gc haplotype on serum DBP levels in either population, but Cape Mixed participants had lower median levels in both seasons (summer/winter:106/107 vs. 125/127 µg/mL; P ≤ 0.0092) (Fig. S2 E and F).
Table 2.
SNP frequency in Xhosa and Cape Mixed participants
| Gene and SNP (also known as) | Location (function) | Allele* | Xh (Major) | Xh (Het) | Xh (Minor) | CM (Major) | CM (Het) | CM (Minor) | P-value† |
| CYP2R1 rs10741657 (rs2060793)‡ | Promoter | A/G | 0.72 | 0.22 | 0.06 | 0.54 | 0.38 | 0.08 | 0.17 |
| CYP27B1 rs10877012 (rs4646536)‡ | 5′ UTR | C/A | 0.86 | 0.12 | 0.02 | 0.54 | 0.42 | 0.04 | 0.002 |
| CYP24A1 rs6013897 | 3′ downstream | A/T | 0.52 | 0.42 | 0.06 | 0.66 | 0.26 | 0.08 | 0.24 |
| DBP | |||||||||
| rs7041 (Asp416Glu) | Exon 11 (nonsyn) | C/A§ | 0.8 | 0.18 | 0.02 | 0.5 | 0.44 | 0.06 | 0.007 |
| rs4588 (Thr420Lys) | Exon 11 (nonsyn) | T/G§ | 0.96 | 0.04 | 0 | 0.78 | 0.2 | 0.02 | 0.027 |
| VDR | |||||||||
| rs2544037 | Promoter | A/G | 0.52 | 0.38 | 0.1 | 0.58 | 0.3 | 0.12 | 0.7 |
| rs10783219 | Intron 0 | A/T | 0.9 | 0.1 | 0 | 0.72 | 0.22 | 0.06 | 0.044 |
| rs10735810 (FokI) | Exon 3 (nonsyn) | A/G | 0.64 | 0.3 | 0.06 | 0.56 | 0.38 | 0.06 | 0.69 |
| rs731236 (TaqI) | Intron 9 | A/G | 0.72 | 0.28 | 0 | 0.54 | 0.4 | 0.06 | 0.069 |
| DHCR7 rs12785878 (rs7944926; rs3794060)‡ | Intron 2 | G/T§ | 0.52 | 0.42 | 0.06 | 0.5 | 0.44 | 0.06 | 0.98 |
| DBP Gc Haplotype (alleles rs7041--rs4588)¶ | |||||||||
| Gc1F/Gc1F | Exon 11 (nonsyn) | CC-TT | 0.76 | 0.34 | |||||
| Gc1F/Gc2 | Exon 11 (nonsyn) | CC-TG | 0.04 | 0.14 | |||||
| Gc2/Gc2 | Exon 11 (nonsyn) | CC-GG | 0 | 0.02 | |||||
| Gc1F/Gc1S | Exon 11 (nonsyn) | CA-TT | 0.18 | 0.38 | |||||
| Gc2/Gc1S | Exon 11 (nonsyn) | CA-GT | 0 | 0.06 | |||||
| Gc1S/Gc1S | Exon 11 (nonsyn) | AA-TT | 0.02 | 0.06 |
CM, Cape Mixed; Het, heterozygous; Xh, Xhosa.
Major/minor alleles and frequencies.
P values for differences between sites tested by χ2 test for trend, bold type indicates statistically significant difference in SNP frequencies.
On reverse strand.
Predicted according to ref. 21.
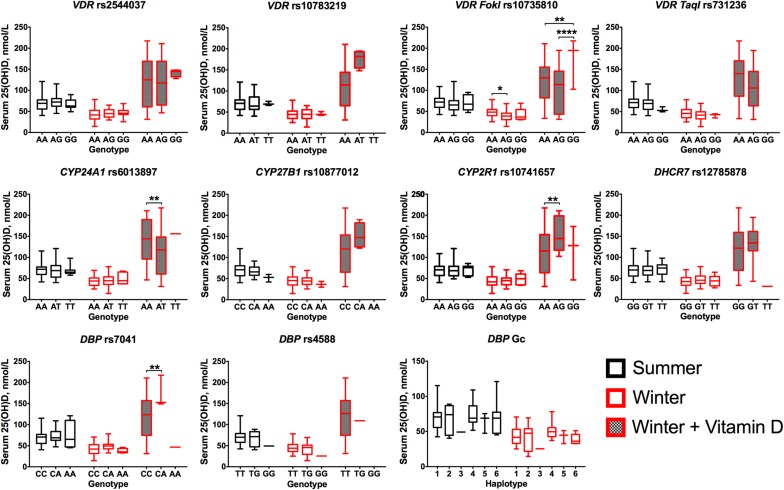
Although the powering was modest, genotypes were added to the stepwise regression and general linear models (GLM) for determinants of total 25(OH)D (Table 3 and Tables S3 and S4). In exploratory analyses of combined participants, a greater genotypic effect was observed after supplementation than with seasonal variation; those heterozygous for VDR FokI (rs10735810) had lower 25(OH)D in the winter and postsupplementation (P < 0.04); those heterozygous for CYP24A1 rs6013897 had lower 25(OH)D levels postsupplementation (P = 0.006); and those heterozygous for CYP2R1 rs10741657 and DBP rs7041 had higher 25(OH)D levels postsupplementation (P = 0.002) (Fig. S3).
Table 3.
Determinants of serum 25(OH)D concentration
| Variable | t-statistic | R-statistic | P value* |
| PNUVB | 9.502 | 0.579 | <0.0001 |
| Area of skin exposed | 5.855 | 0.401 | <0.0001 |
| Weekly duration of UVB | 4.401 | 0.312 | <0.0001 |
| Arm EI | 3.617 | 0.261 | 0.0004 |
| Gc1F/Gc1S | 3.026 | 0.221 | 0.0028 |
| VDR rs10735810 AA | 2.715 | 0.199 | 0.0073 |
| DBP rs7041 CA | 2.656 | 0.195 | 0.0086 |
| Forehead EI | 2.368 | 0.174 | 0.0190 |
| Cape Mixed ancestry | −2.271 | −0.167 | 0.0243 |
| VDR rs10735810 AG | −2.544 | −0.187 | 0.0118 |
P values derived using linear regression, with adjustment for covariates (smoking and age). The false-discovery rate was determined by the Benjamini–Hochberg; all <0.1.
Table S3.
Stepwise regression of serum 25(OH)D determinants, term added sequentially
| Df | Sum of Sq | Mean Sq | F value | P value | |
| PNUVB | 1.0000 | 3.6081 | 3.6081 | 123.2414 | <0.0001 |
| Gc1F/Gc1S | 1.0000 | 0.2139 | 0.2139 | 7.3067 | 0.0076 |
| VDR.rs731236 AA | 1.0000 | 0.1655 | 0.1655 | 5.6533 | 0.0186 |
| VDR.rs2544037 AA | 1.0000 | 0.1581 | 0.1581 | 5.4014 | 0.0213 |
| Weekly duration UVB | 1.0000 | 0.1847 | 0.1847 | 6.3082 | 0.0130 |
| % Skin area exposed | 1.0000 | 0.3125 | 0.3125 | 10.6747 | 0.0013 |
| Arm EI | 1.0000 | 0.1439 | 0.1439 | 4.9166 | 0.0280 |
| Arm MI | 1.0000 | 0.2166 | 0.2166 | 7.3985 | 0.0072 |
Df, degrees of freedom; EI, erythema index; MI, melanin index; PNUVB, personal net UVB exposure; Sq, square.
Table S4.
Determinants of severe vitamin D deficiency in winter and optimality in summer by generalized linear models
| Severe deficiency | Value | SE | t-value | Sufficiency | Value | SE | t-value |
| Gc2/Gc2 | 1.7086 | 0.4390 | 3.8919 | Weekly UVB duration | 0.7887 | 0.4043 | 1.9506 |
| VDR rs2544037 AA | 0.3698 | 0.1354 | 2.7306 | Arm EI | 1.9269 | 1.0812 | 1.7821 |
| Head MI | 1.8255 | 0.7504 | 2.4327 | Skin area exposed | 0.5448 | 0.3100 | 1.7577 |
| VDR rs10735810 AG | 0.3907 | 0.1862 | 2.0978 | Smoking | 0.2194 | 0.1519 | 1.4443 |
| CYP2R1 rs10741657 AA | 0.2244 | 0.1479 | 1.5172 | CYP24A1 rs6013897 AT | 0.2929 | 0.2622 | 1.1171 |
| Weekly UVB duration | 0.2710 | 0.1801 | 1.5052 | VDR rs731236 AA | 0.3883 | 0.3573 | 1.0866 |
| VDR rs2544037 AG | 0.2015 | 0.1500 | 1.3433 | CYP24A1 rs6013897 AA | 0.2555 | 0.2574 | 0.9926 |
| Gc1F/Gc2 | 0.3076 | 0.2395 | 1.2839 | VDR rs10735810 AA | 0.2279 | 0.2525 | 0.9026 |
| Skin area exposed | 0.2172 | 0.1705 | 1.2745 | VDR rs731236 AG | 0.3144 | 0.3532 | 0.8901 |
| VDR rs10735810 AA | 0.1988 | 0.1661 | 1.1969 | CYP27B1 rs10877012 CA | 0.4129 | 0.4655 | 0.8870 |
| VDR rs10783219 AT | 0.2847 | 0.2597 | 1.0960 | Gc1F/Gc2 | 0.3760 | 0.4469 | 0.8414 |
| CYP2R1 rs10741657 AG | 0.1266 | 0.1527 | 0.8292 | CYP27B1 rs10877012 CC | 0.3221 | 0.4510 | 0.7141 |
| Serum DBP | 0.1441 | 0.2172 | 0.6635 | Dietary Intake | 0.0602 | 0.1018 | 0.5911 |
| DHCR7 rs12785878 GG | 0.1283 | 0.2096 | 0.6121 | Gc2/Gc1S | 0.1970 | 0.3609 | 0.5459 |
| Gc1F/Gc1F | 0.1123 | 0.2012 | 0.5580 | VDR rs10783219 AA | 0.1816 | 0.3746 | 0.4848 |
| Gc1F/Gc1S | 0.1279 | 0.2322 | 0.5509 | VDR rs10735810 AG | 0.0684 | 0.2610 | 0.2620 |
| DHCR7 rs12785878 GT | 0.1068 | 0.2028 | 0.5264 | VDR rs10783219 AT | 0.0621 | 0.3876 | 0.1603 |
| VDR rs10783219 AA | 0.1286 | 0.2578 | 0.4989 | VDR rs2544037 AG | 0.0308 | 0.2196 | 0.1403 |
| VDR rs731236 AG | 0.1193 | 0.2481 | 0.4808 | BMI | 0.0735 | 0.7083 | 0.1038 |
| Gc2/Gc1S | 0.1413 | 0.3567 | 0.3961 | Gc1F/Gc1F | 0.0258 | 0.3447 | 0.0748 |
| VDR rs731236 AA | 0.0779 | 0.2450 | 0.3178 | Head EI | −0.1149 | 1.0197 | −0.1126 |
| CYP24A1 rs6013897 AA | 0.0537 | 0.1788 | 0.3004 | Head MI | −0.1974 | 1.0410 | −0.1896 |
| Smoking | 0.0319 | 0.1121 | 0.2844 | VDR rs2544037 AA | −0.0439 | 0.2103 | −0.2086 |
| CYP24A1 rs6013897 AT | 0.0337 | 0.1924 | 0.1752 | Gc1S/Gc1S | −0.2197 | 0.4920 | −0.4465 |
| Dietary intake | −0.0202 | 0.0760 | −0.2659 | CYP2R1 rs10741657 AG | −0.1421 | 0.2461 | −0.5772 |
| Arm EI | −0.2401 | 0.7573 | −0.3171 | CYP2R1 rs10741657 AA | −0.1532 | 0.2458 | −0.6233 |
| CYP27B1 rs10877012 CA | −0.2122 | 0.2939 | −0.7220 | Serum DBP | −0.2515 | 0.3219 | −0.7813 |
| PNUVB | −0.6304 | 0.8193 | −0.7695 | Gc1F/Gc1S | −0.6141 | 0.6661 | −0.9220 |
| CYP27B1 rs10877012 CC | −0.2297 | 0.2927 | −0.7849 | Arm MI | −1.2803 | 1.1883 | −1.0775 |
| BMI | −0.3770 | 0.4538 | −0.8307 | DHCR7 rs12785878 GT | −0.3737 | 0.2728 | −1.3699 |
| Head EI | −0.8837 | 0.8208 | −1.0766 | Sex | −0.1090 | 0.0755 | −1.4453 |
| Sex | −0.0718 | 0.0541 | −1.3278 | DHCR7 rs12785878 GG | −0.4582 | 0.2814 | −1.6283 |
| Arm MI | −1.4146 | 0.8913 | −1.5870 | PNUVB | −4.7016 | 2.6123 | −1.7998 |
Fig. S3.
Stratification of serum 25(OH)D concentration by genotype and season/supplementation. Data were combined for both populations. DBP Gc haplotypes: 1, Gc1F/Gc1F; 2, Gc1F/Gc2; 3, Gc2/Gc2; 4, Gc1F/Gc1S; 5, Gc2/Gc1S; 6, Gc1S/Gc1S. Data were analyzed by a Kruskal–Wallis with Dunn’s multiple comparisons test, within each season. The lines across the boxplots indicate the median (minimum–maximum); *P < 0.05; **P < 0.01; ****P < 0.0001. DBP rs7041, DHCR7 rs12785878, and CYP24A1 rs6013897 only had one individual homozygous for the minor allele at the supplementation visit, and these alleles were not analyzed for significance.
Personal UVB Exposure Is the Major Determinant of Vitamin D Status.
We next used regression models applied to all variables (SI Materials and Methods) to identify the determinants of serum 25(OH)D in both groups. Stepwise regression (Table S3) identified PNUVB as the dominant determinant (F = 123.2, P < 0.0001), followed by area of skin exposed (F = 10.7, P = 0.0013) and arm MI (F = 7.40, P = 0.0072). DBP haplotype Gc1F/Gc1S and two VDR SNPs, TaqI rs731236 and rs2544037, also contributed to the model, as did duration of UVB exposure and skin redness (arm EI), to a lesser extent. To determine the directionality of effect a GLM approach was applied adjusting for age and smoking status (Table 3). Again PNUVB was the dominant determinant, followed by area of skin exposed and weekly duration of exposure, all positively contributing to serum 25(OH)D, whereas VDR FokI rs10735810 AG and Cape Mixed ancestry where negatively correlated with 25(OH)D. The two measures of skin redness (arm and forehead EI), as well as Gc1F/Gc1S, VDR FokI rs10735810 AA, and DBP rs7041 CA, positively contributed to serum 25(OH)D.
The determinants of severe deficiency in winter and sufficiency in the summer also were examined using a GLM approach (Table S4). Sunlight exposure as a component of PNUVB did not prevent serious deficiency in the winter, but the area exposed and duration were important. The UVB content of winter sunlight is weak, but individuals who were in the sun for longer periods and who had the most surface area exposed produced more vitamin D. Darker skin (higher arm and forehead MI) also was associated with lower serum 25(OH)D status. Sex had an effect but correlated with area exposed and duration of exposure. Sufficiency in the summer was affected most strongly by duration of exposure and PNUVB and to a lesser extent by the degree of skin redness (arm EI). Possession of the DHCR7 rs12785878 GG genotype had a small effect on optimal serum 25(OH)D, but no other genes had a significant effect. Smoking status and sex also were influential in summer but only through correlation with patterns of sun exposure and skin pigmentation, respectively.
Winter Vitamin D3 Supplementation Increases Peripheral WBC Count and Counteracts Anemia.
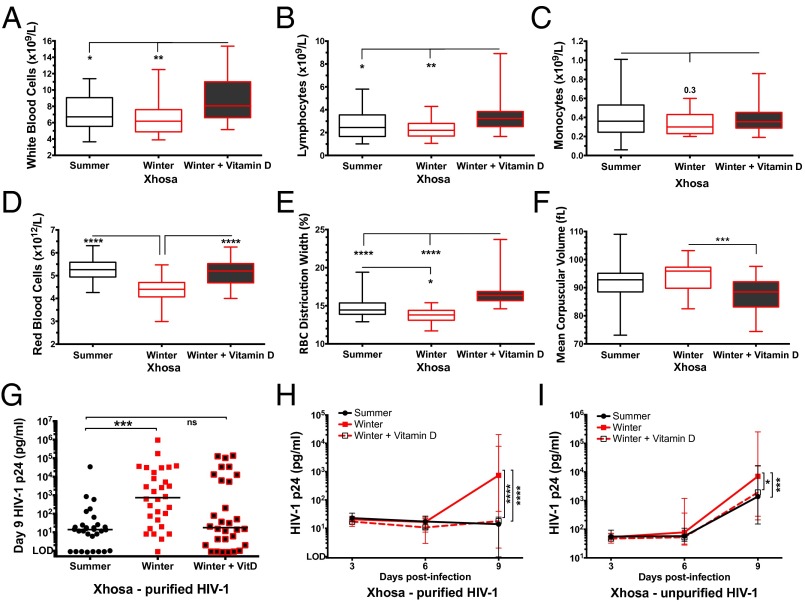
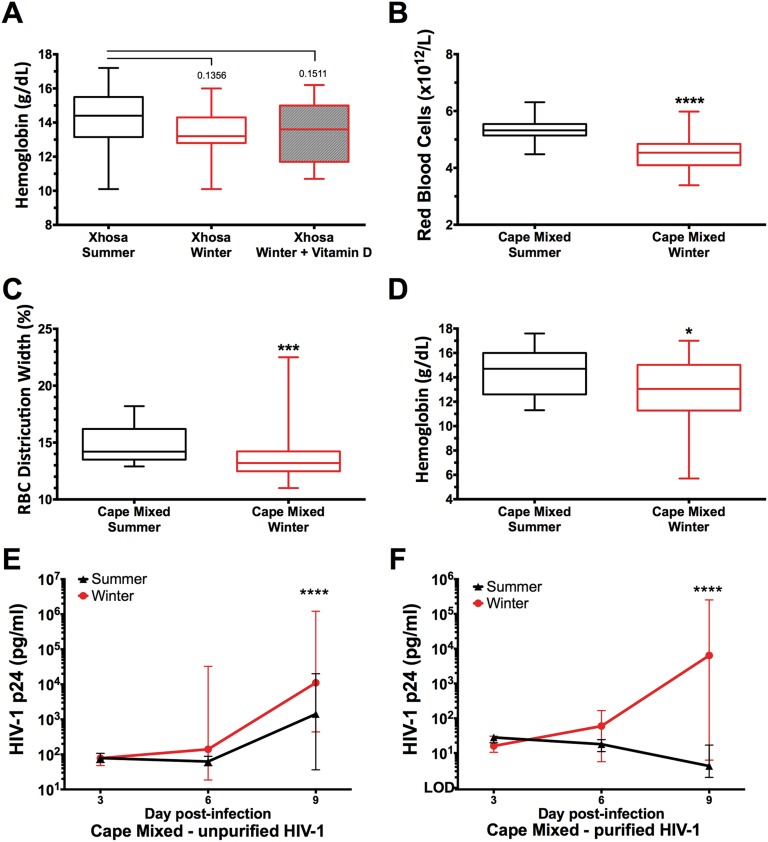
To investigate the functional consequence of seasonal serum 25(OH)D levels on the immune system, we next investigated seasonal differences in full blood count (FBC) and the effect of vitamin D3 supplementation on FBC in Xhosa participants. Vitamin D3 supplementation in the winter increased WBC count (P = 0.0016) and in particular lymphocyte count (P = 0.023), and there was a winter trend for decreased monocytes (Fig. 2 A–C). In the winter, participant’s RBC parameters tended toward macrocytic anemia [evidenced by decreased RBC and RBC distribution width (RDW), increased mean corpuscular volume, and a trend toward decreased Hb], and this tendency was reversed by supplementation (P ≤ 0.0007) (Fig. 2 D–F and Fig. S4A). The winter decline in RBC, RDW, and Hb also was seen in participants with Cape Mixed ancestry, although the effect of supplementation was not measured (Fig. S4 B–D).
Fig. 2.
FBC and HIV-1 replication in PBMCs from Xhosa participants in summer, winter, and after receiving winter vitamin D supplementation. (A–F) Box plots show WBC (A), lymphocyte (B), monocyte (C), and RBC (D) counts, RBC distribution width (E), and mean corpuscular volume (F) measured at each study visit (summer, n = 42; winter, n = 23; winter + vitamin D, n = 30). The lines across the box plots indicate the median (minimum–maximum); Kruskal–Wallis with Dunn’s multiple comparison test. (G) HIV-1 p24 concentration in culture supernatant 9 d postinfection of PBMCs (n = 30 longitudinally; the line indicates the median) with purified HIV-1 on the day of phlebotomy in 20% autologous serum; Friedman test with Dunn’s multiple comparison test. (H and I) HIV-1 p24 concentration in culture supernatant on day 3, 6, and 9 postinfection of PBMCs [n = 30 longitudinally, median (IQR)] with purified HIV-1 (H) or unpurified HIV-1 (I); repeated measures two-way ANOVA with Tukey’s multiple comparison test following log10 transformation. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. LOD, limit of detection (1 pg/mL); ns, not significant.
Fig. S4.
FBCs and HIV-1 replication in ex vivo PBMC infections. (A–D) Longitudinal Hb in Xhosa participants (A) and RBC counts (B), RDW (C), and Hb (D) in Cape Mixed participants. The lines across the boxplots indicate the median (minimum–maximum). Xhosa data were analyzed by the Kruskal–Wallis with Dunn’s multiple comparison test (summer, n = 41; winter, n = 23; winter + vitamin D, n = 25), and Cape Mixed data were analyzed by the Mann–Whitney test (summer, n = 31; winter, n = 50). (E and F) Analysis of HIV-1 p24 concentration in culture supernatant on day 3, 6, and 9 post ex vivo infection of PBMCs [summer, n = 49; winter, n = 6, median (IQR)] in 20% autologous serum with unpurified HIV-1 (E) or purified HIV-1 (F) by repeated-measures two-way ANOVA with Tukey’s multiple comparison test. *P < 0.05; ***P < 0.001; ****P < 0.0001.
Winter Vitamin D3 Supplementation Decreases HIV-1 Replication in Peripheral Blood Mononuclear Cells.
Because supplementation modified peripheral WBC and RBC counts, we next investigated the functional consequences on response to HIV-1 infection. The active vitamin D metabolite, 1α,25-dihydroxy-vitamin D, has been shown in vitro to inhibit HIV-1 replication in macrophages via induction of autophagy, mediated via cathelicidin induction (22), and 25(OH)D deficiency is associated with HIV-1 progression (1). Therefore we investigated the functional consequences of seasonal variation in serum 25(OH)D levels and winter vitamin D3 supplementation on the extent of HIV-1 replication in freshly isolated peripheral blood mononuclear cells (PBMCs), at each study visit. PBMCs were cultured in the presence of fresh 20% autologous serum, isolated at the same time as PBMCs, to maintain the in vivo cellular environment as best possible, with regard to seasonal serum 25(OH)D levels, autologous DBP, and other circulating chemokines/cytokines. Two preparations of HIV-1 BaL, purified and unpurified, were used for infection. Unpurified HIV-1 preparations contain exosomes, microvesicles, and conditioned medium from propagation and were tested for the likelihood that activating cells might result in greater productive infection of unstimulated cells.
Infection of PBMCs in winter, compared with summer, resulted in greater productive HIV-1 infection on day 9, as measured by culture supernatant p24 antigen levels. This result was seen in PBMCs isolated from both Xhosa (P = 0.0003, Fig. 2 G–I) and Cape Mixed (P < 0.0001, Fig. S4 E and F) participants. This winter increase in HIV-1 infection was seen with both preparations of HIV-1, with ∼1-log higher p24 measured from cells infected with unpurified virus (Xhosa median 7,038 pg/mL unpurified vs. 738 pg/mL purified) (Fig. 2 H and I).
After 6 wk of vitamin D3 supplementation in winter, the winter increase in HIV-1 p24 was attenuated, and Xhosa participants’ PBMCs showed a diminished capacity for productive HIV-1 infection: on day 9 p24 levels had dropped to the level as observed in summer (Fig. 2G). Again, this decrease occurred with infections using both purified and unpurified virus (Fig. 2 H and I), demonstrating the robustness of oral vitamin D3 supplementation in suppressing productive HIV-1 infection in peripheral blood cells ex vivo. Moreover there was a significant negative correlation between paired serum 25(OH)D and day 9 p24 concentrations from PBMCs infected with purified virus, across all time points (Spearman rs = −0.36; P < 0.0001), indicating a direct correlation between serum 25(OH)D levels and the ability of peripheral blood cells to limit productive HIV-1 infection.
Discussion
The multitude of, and complex interactions among, variables that modify vitamin D status and their impact on immunological function are poorly understood, particularly in the context of disease prevention in healthy individuals. In an urban African location with seasonal UVB variability and high infectious disease prevalence, we found that personal UVB exposure habit is the most important determinant of vitamin D status in healthy adults with moderate to dark skin pigmentation. Moreover, we found that the reversal of vitamin D deficiency in winter through oral supplementation can modulate the number and function of circulating WBCs, prevent anemia, and decrease productive HIV-1 infection. Furthermore, we found that UVB exposure habit can counterbalance the effects of pigmentation, genetic variation, and poor dietary intake.
The skin of the indigenous people of the Cape, the Khoisan, is considerably lighter than that of either study group (23) and may represent a long-established adaptation to seasonal UVB. The darker skin of both study populations denotes a degree of mismatch between skin pigmentation and environmental UVB resulting from their recent migration into the region; this effect is exacerbated by wearing concealing clothing and indoor living in the winter. The high prevalence of vitamin D deficiency in the winter in both groups indicates that people with moderate or dark pigmentation are at high risk of deficiency in the absence of significant dietary vitamin D intake when UVB radiation is limited by seasonal fluctuations.
We also noted significant polymorphic variation between the two populations for 5 of the 10 vitamin D-associated SNPs investigated, contributing further genetic insight into these understudied populations. Given the low MAF of the SNPs investigated, particularly in the Xhosa population, we identified only a minor effect of genetic variation on seasonal vitamin D status. This finding mirrors the recent genomewide association study in 16,125 individuals, from five cohorts, which showed the proportion of variation in 25(OH)D attributable to genetic variation was 1–4% (9). However, despite the small sample size, we identified four SNPs, in VDR, CYP24A1, CYP2R1, and DBP, which modify the response to high-dose supplementation. Although these results need to be confirmed in larger cohorts, such associations are supported by our previous finding that polymorphic variation in VDR modifies the effect of high-dose vitamin D supplementation on hastening time to sputum culture conversion in TB patients receiving intensive-phase treatment (24).
In South Africa, young females are at the greatest risk of HIV-1 infection (19). We found females in both populations also were at greater risk of deficiency, indicating this is an important target group for intervention. Dietary intake played a greater role in maintaining serum 25(OH)D levels when UVB levels were low. However, it is unlikely that a diet richer in vitamin D-containing foods alone can compensate for the seasonal changes in insolation and UVB at this or higher latitudes, especially when colder outdoor temperatures and the associated wearing of clothes and indoor living reduce the likelihood of exposure to weak UVB-containing sunlight. Recommendations for personal vitamin D supplementation or wide-scale food fortification may be considered, along with season-specific recommendations for short-duration sun exposure around noon in the winter and between ∼1.0 and 1.5 h sun exposure in the spring and autumn. Short periods of UVB exposure around solar noon are highly effective in raising serum 25(OH)D levels and pose a low risk to general and skin health (25).
Our findings of decreased winter WBC counts, particularly lymphocytes, and an increase in numbers following vitamin D supplementation corroborate a small longitudinal study in healthy Scandinavian adults with light pigmentation, which found a similar decrease in lymphocytes in winter, particularly CD4+ and CD8+ T cells, which was associated with reduced 25(OH)D (26). Higher serum 25(OH)D levels in children initiating antiretroviral therapy (ART) also were associated with higher CD4+ T-cell restoration (27). Further studies will investigate the detailed changes in innate and adaptive immune cell populations in our cohorts. Vitamin D deficiency also has been associated previously with anemia and low Hb in HIV-infected women (1). We found that vitamin D supplementation reversed winter-associated macrocytic anemia, suggesting that this adjunct therapy also may be effective in preventing anemia in HIV-infected individuals.
The demonstration that high-dosage oral vitamin D supplementation reversed serum 25(OH)D deficiency and attenuated the seasonal increase in ex vivo HIV-1 replication, similar to our previous finding that oral vitamin D reduces Mycobacterium tuberculosis replication in whole blood (28), provides strong evidence for the positive preventative effects of vitamin D supplementation for people with vitamin D deficiency and serious infectious diseases, conditions which apply to many cities in which the prevalence of vitamin D deficiency continues to rise. Furthermore, vitamin D may be a simple, cost-effective intervention, particularly in resource-poor settings, to prevent disease progression in persons infected with HIV-1 by suppressing viral replication, raising peripheral lymphocyte counts, and preventing anemia, potentially prolonging the time to ART initiation and enhancing the beneficial effects of ART once initiated.
SI Materials and Methods
Study Recruitment, Follow-Up, and Exclusion Criteria.
This prospective longitudinal study was designed to identify the environmental and physiological determinants of vitamin D status and the impact of vitamin D status on HIV-1 immunity in healthy young adults from two populations in neighboring suburbs of Cape Town, South Africa.
The summer visit was conducted in February 2013 (beginning 6 wk postsolstice), and the winter visit took place in August 2013 (beginning 6 wk postsolstice). Participants of Xhosa ancestry were recruited from the Site B Youth Centre and Community Health Centre in Khayelitsha, South Africa. Participants of Cape Mixed ancestry (self-identified as Cape Colored) were recruited from the Tygerberg district by a research social worker via word of mouth from communities situated within a 25-km radius of Tygerberg Hospital. Exclusion criteria were age <18 or >24 y; current use of vitamin supplements or corticosteroids; BMI ≥30; pregnancy known to participant; current symptoms of infection; and HIV-positive status known to the potential participant at first intake [following recent (≤7 d) testing for Xhosa] or determined by the HIV 1/2 Ag/Ab combo test (Alere) at study visit (for Cape Mixed). If a participant was positive for any exclusion criterion at the first visit, all that participant’s samples were excluded. If the participant was determined to be positive for any exclusion criterion at subsequent visits, only the samples from previous visits were included.
After the prescreening of individuals based on visual BMI prediction and known HIV status, 104 participants were assessed for eligibility, and 100 were enrolled (Xhosa, n = 50, Cape Mixed, n = 50). Participants underwent anthropometric measurements, the screening questionnaire, and, for Cape Mixed, HIV counseling and testing, to determine eligibility. Reasons that participants were not enrolled were HIV-infection (1 person), pregnancy (1 person), failure to return for first study visit (1 person), and unsuccessful phlebotomy (1 person). Seventeen Xhosa participants were lost to follow-up in winter because of pregnancy (1 person), HIV-infection (1 person), and inability to attend visit (15 persons) (Fig. S1).
Anthropometry and Screening Questionnaire.
At each study visit a screening questionnaire was conducted, and weight (in kilograms) and height (in centimeters) was measured according to standard anthropometric techniques. The questionnaire collected information on age, date of birth, sex, ethnicity, smoking status (nonsmoker or regularly smoke at least one cigarette 5 d/wk), current symptoms of illness (sore throat, runny nose, cough, fever, night sweats, sudden weight loss), supplement use, and medication use. Weight was measured using a digital weighing scale after removal of shoes and heavy outer layers of clothing. Height was measured using a portable stadiometer after removal of shoes, caps, and hats. Three independent measures were taken from each site, and the mean was calculated.
UVB Exposure.
Direct measurements of UVB were made at the University of Stellenbosch Solar Resource and Weather Station (33°55'41′′ S, 18°51'55′′ E, elevation 119 m), located at the northern limits of the Cape Flats and less than 30 km from the residential locations of the study populations. From March 2013 onward, direct UVB was measured using Model 2 dual-axis Sun Tracker (Kipp & Zonen) equipped with two CHP1 pyrheliometers for direct solar irradiance measurements and a UVS-AB-T sensor (Kipp & Zonen) for UVA (315–400 nm) and UVB (285–310 nm) measurements. These measurements are available online at weather.sun.ac.za/. Before March 2013, UVB was estimated from a model of UVA, and total solar insolation and the amount of scatter was estimated from the shadow ring. (Tests of this model showed little variation for the period when direct measures of UVB were available for comparison (correlation: R = 0.99945). UVB data were collected by minute, and averages were calculated for the sun exposure enumeration blocks.
Local direct UVB data collected for the study period and location were compared with remotely sensed UVB satellite data (from the NASA Omni Total Ozone Mapping Spectrograph) for purposes of broader temporal and spatial comparisons. Data used were total ozone column in Dobson units and UVB at 305 nm from both the clear sky and ground reflection. Information on solar output for the study period was recorded by the Solar Flux Solar Radio Monitoring Program of the National Research Council of Canada.
Sun exposure was assessed at each visit by a retrospective questionnaire that captured the duration and time of day of outdoor sun exposure and the amount of skin not covered by clothing or a hat at the time of exposure. Exposure intervals were divided more finely around noon and were longer near dawn and dusk when there was little UVB. Duration of exposure was assessed separately for weekdays and weekend. Personal net UVB exposure was calculated based on measured UVB for the period under consideration and was weighted by amount of skin exposed (calculated by applying the rule of nines used in determining the damaged body surface area in burn victims) and sunscreen use, based on a sun protection factor (SPF) <30 blocking half of the UVB for the body area and SPF >30 blocking all UVB.
Food Frequency Questionnaire.
The 7-d quantitative food frequency questionnaire was adapted from a food frequency questionnaire shown to be valid in providing a reasonable estimation of vitamin D intake in healthy young adults of diverse ancestry (30). Foods known to be good sources of vitamin D in South Africa and commonly consumed by South African adults were added, and foods not fortified with vitamin D were excluded. In South Africa, there is no mandatory fortification of foods with vitamin D; however, most margarines are vitamin D fortified at 180–880 IU/100 g, depending on the brand. Vitamin D intake from fish (eight oily fish and two whitefish), eggs, margarine, butter, and livers was estimated. The food type, frequency of intake, and portion usually consumed was estimated for each food item using tools to assist with brand identification and portion size estimation, and estimated portions were converted to grams (32). Estimated total daily vitamin D intakes for each participant at each visit were calculated using the South African Food Data System (Foodfinder III: Dietary analysis software, Medical Research Council), nutrition panels on product labels, and the US Department of Agriculture Nutrient Database and were taken to represent usual intakes. Because no reference intakes are available for the South African population, the Institute of Medicine’s Dietary Reference Intakes were used to assess adequacy of dietary vitamin D intake, using the EAR cutoff point of 400 IU for 19- to 30-y-old persons (16).
Biochemistry and FBCs.
Peripheral blood collected in serum tubes (Becton Dickinson) was centrifuged within 2–3 h of collection and was analyzed on the day of collection for 25(OH)D concentration by the chemiluminescent LIAISON 25 OH Vitamin D TOTAL Assay (DiaSorin) in the clinical diagnostic laboratories of PathCare, Western Cape. This method was chosen as the global standard diagnostic test for total 25(OH)D. The limit of detection was 4 ng/mL Serum also was stored at −80 °C and subsequently were batch analyzed for DBP and the acute-phase markers β2 microglobulin, haptoglobulin, and C-reactive protein (CRP) concentration by Luminex, using the Acute Phase 4-Plex Panel (LHC6006; all monoclonal antibodies; Life Technologies) on a Bio-Plex 200 (Bio-Rad) with Bio-Plex Manager 6.0 software. Sensitivity was <0.05 ng/mL for CRP, DBP, and α2 microglobulin and <1 ng/mL for haptoglobulin. All participants fell within the normal range for acute-phase markers, except one participant who had consistently high β2 microglobulin or haptoglobulin at more than one time point and three who had elevated levels of either β2 microglobulin or haptoglobulin at one time point. However, all participants’ CRP levels remained normal throughout the study; therefore, these participants were included in the study as healthy participants (Fig. S2 A–C). There was a small but significant increase in CRP levels in Xhosa participants in winter as compared with summer, but these levels remained within the normal range. Serum calcium and albumin (for corrected calcium) in longitudinal stored samples from the 30 individuals who received vitamin D supplementation were measured in the clinical diagnostic laboratories of National Health Laboratory Service, Groote Schuur Hospital. All participants fell within the normal range (minimum 2.12 mmol/L, maximum 2.65 mmol/L). FBCs were conducted on blood collected in sodium heparin tubes (BD) within 2–3 h after collection on a COULTER Ac·T diff analyzer(Beckman Coulter) and Hemavet 950 analyzer (Drew Scientific), with cross-referencing between systems. Not all samples had FBCs conducted in summer and in winter.
Genotyping.
PBMCs were prepared on a Ficoll–Paque density gradient, and 1 × 106 cells were pelleted and flash frozen in liquid nitrogen for storage at −80c until batch DNA extraction. Cell pellets were suspended in 200 μL PBS, and DNA was extracted using the QIAmp DNA Blood Mini Kit (Qiagen) following the manufacturer’s instructions and was stored at −80 °C. DNA was quantified on a NanoDrop 2000 (Thermo Scientific), and 10 ng was analyzed by the TaqMan genotyping assay (Life Technologies), in duplicate, with TaqMan Genotyping Master Mix and the probe sets for the following SNPs in genes: DBP (rs7041: C___3133594_30; rs4588: C___8278879_10); DHCR7 (7-dehydrocholesterol reductase; rs12785878: C__32063037_10); CYP2R1 (vitamin D 25-hydroxylase; rs10741657: C___2958430_10); CYP24A1 (vitamin D 24-hydroxylase; rs6013897: C__29958084_10); CYP27B1 (vitamin D 1α-hydroxylase; rs10877012: custom primer and probes: forward 5′-AACAGAGAGAGGGCCTGTCT-3′, reverse 5′-GGGAGTAAGGAGCAGAGAGGTAAA-3′; Vic probe 5′-CTGTGGGAGATTCTTTTAT-3′; Fam probe 5′-TGTGGGAGATTATTTTAT-3′) (33); and VDR (rs2544037: C__26075062_10; rs10783219: C___2880803_10; rs10735810: C__12060045_20; and rs731236: C___2404008_10) on a QuantStudio Flex-7 (Applied Biosystems) with data analysis in QuantStudio 7 software.
HIV-1 Stock Preparation.
The HIV-1 strain BaL from National Institute for Biological Standards and Controls was propagated in PBMCs in RPMI-1640 medium containing 50 mM glutamine with 20% (vol/vol) FCS, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 20 IU/mL IL-2 (Sigma) (34). Culture supernatants were passed through a 0.22-μM PVDF filter to remove cellular debris (Millipore) and were used directly for infection or were purified before use by ultracentrifugation through a 20% (wt/vol) sucrose buffer and wee resuspended in RPMI-1640 medium with 5% (vol/vol) human AB (HAB) serum (Invitrogen). HIV-1 virus preparations were titrated on the TZM-bl cells, a modified HeLa cell line stably transfected with CD4, CCR5, and CXCR4 and containing LTR-driven luciferase and LTR-driven α-galactosidase cassettes (35). The median tissue-culture infective dose (TCID50) of HIV-1 was determined 48 h postinfection following cell lysis in BrightGlo (Promega), and luminescence was read on a GloMax-96 (Promega).
HIV-1 PBMC Infection.
PBMCs were suspended in RPMI-1640 medium and 20% (vol/vol) autologous serum at 1 × 107/mL. Then 150-μL aliquots were mixed with 30-μL aliquots of purified HIV-1 Ba-L [multiplicity of infection (MOI) 0.004], unpurified HIV-1 Ba-L (MOI 0.002), or RPMI/5% (vol/vol) HAB serum (infection control), plated in triplicate in 96-well plates (60 μL per well), and incubated for 17 h at 37 °C/5% CO2. Cells were washed three times in warm RPMI and were resuspended in 200 μL RPMI/20% autologous serum for a further 9 d. After 3, 6, and 9 d, 100 μL of supernatant was removed and stored at −80 °C, and fresh autologous medium was added.
Supernatant p24 Quantification.
HIV-1 replication was measured by quantifying the HIV-1 p24 antigen concentration of lysed supernatant [incubated with 1% Triton X (Calbiochem) for 2 h at 37 °C] by Luminex. MagPlex carboxymethylated microspheres (region 42, 5.6 mm in diameter; Luminex) were coupled to a high-affinity anti-p24 monoclonal antibody (clone 4F6; ImmunoDiagnostics) using the antibody-coupling kit (Luminex). Then 1,750 beads per well were incubated with 50 μL of supernatant diluted 1:2 or 1:5 in assay buffer (PBA, 0.1% BSA, 0.1% mouse serum, 0.1% goat serum, 0.02% Tween 20, 1% Triton X) or 50 μL standard (HIV Gag-p24 #11695-V08E; Sino Biological; diluted 1:3 in assay buffer to generate an 11-point standard curve) in a range from 80,000–1 pg/mL for 1 h at room temperature with horizontal shaking, followed by incubation with 100 μL of the RD1-labeled anti-p24 KC57 antibody (1:1,000 dilution; Beckman Coulter) for 1 h at room temperature with horizontal shaking, followed by analysis on a BioPlex 200 (Bio-Rad) with BioPlex Manager 6.0 software. Data points <1pg/mL were designated 0 and indicated as the limit of detection.
Statistical Analysis.
All variables were tested for normality by the D’Agostino and Pearson omnibus test, and medians and interquartile ranges calculated. The Wilcoxon rank test or the Friedman or Kruskal–Wallis test with Dunn’s multiple comparison test was used for intragroup comparisons, and the Mann–Whitney test was used for intergroup comparisons. Smoking status, sex ratio, and SPF use were tested by Fisher’s exact test. Vitamin D status and genotype frequencies were tested by the χ2 test for trend. The Friedman test with Dunn’s multiple comparisons at a single time point and repeated-measures two-way ANOVA with Tukey’s multiple comparisons test over days of culture, between visits, was used to compare p24 concentration. Spearman’s rank-order correlation was used to test the 111 paired serum 25(OH)D concentrations with day 9 p24 concentrations from HIV infections of PBMCs collected at each time point.
Linear modeling to investigate the determinant of serum vitamin D concentration was conducted using Qlucore Omics Explorer 2.2 software. Continuous variable concentrations were log2 converted, and the variance was normalized to 1, whereas genotype and haplotype data were binomially transformed. Missing values were imputed by κ nearest neighbors (36). Variables that contributed to serum 25(OH)D concentration were identified using linear regression for GLM with adjustment for the covariates smoking and age. This analysis fits a multiple regression model to all covariates and subtracts the expression values predicted by this model from the observed values to remove covariate effects between patients (37). This analysis yielded t-statistics (calculated as the regression coefficient for each parameter divided by its SD); q values, which define the lowest false-discovery rate for which the hypothesis would be accepted under the Benjamini–Hochberg procedure for multiple testing correction, with a thresholds of 0.1 applied; and the R-statistic, which indicates the proportion of the total variation of that variable which is explained by the model tested (calculated as the square root of the R2-statistic, with the sign indicating the direction of the observed effect).
Stepwise regression and general linear models on vitamin D optimality and severe deficiency were conducted in S-Plus on ln-transformed continuous variables and binomially transformed genotype and haplotype data.
Variables Examined in This Study.
Identification and demographic variables examined were the center name and the participant’s ID, age in summer, sex, and ethnicity.
The anthropometric variables examined were weight, height, BMI, forehead EI, arm EI, forehead MI, and arm MI in summer and winter, age in winter, and smoking status.
The dietary vitamin D intake variables examined were summer and winter vitamin D food intake.
The duration and timing of UVB exposure were measured for every weekday and weekend day. This was corrected for the area of skin exposed, reduced by the SPF strength over the area of sunscreen application, if sunscreen was used, to determine UVB dosage (Joules UVB). The PNUVB was calculated as log10 (weekly total dosage weighted by exposure and SPF) (Joules UVB).
The following genotypes/haplotypes were considered:
VDR rs731236 AA
VDR rs731236 AG
DBP rs7041 CC
DBP rs7041 CA
DBP rs4588 TT
DBP rs4588 TG
DHCR7 rs12785878 GG
DHCR7 rs12785878 GT
CYP24A1 rs6013897 AA
CYP24A1 rs6013897 AT
CYP27B1 rs10877012 CC
CYP27B1 rs10877012 CA
CYP2R1 rs10741657 AA
CYP2R1 rs10741657 AG
VDR rs10735810 AA
VDR rs10735810 AG
Gc1F/Gc1F
Gc1F/Gc2
Gc2/Gc2
Gc1F/Gc1S
Gc2/Gc1S
Materials and Methods
Study Design.
The study was conducted in accordance with the Helsinki 1964 declaration, including subsequent revisions, and the South African Guidelines for Good Clinical Practice and the Medical Research Council Ethical Guidelines for Research. Ethical approval was received from the Human Research Ethics Review Boards of the Faculty of Health Sciences, University of Cape Town (ref. 003/2013) and the Faculty of Medicine and Health Sciences, Stellenbosch University (ref. N12/10/065) and from the Institutional Review Board of The Pennsylvania State University (ref. 41940). Written informed consent was obtained from all participants.
The 104 participants were assessed for eligibility, and 100 HIV-1–uninfected individuals (Xhosa, n = 50; Cape Mixed, n = 50) were enrolled. The summer and winter visits began 6 wk postsolstice. At the winter visit, all participants received six capsules of 50,000 IU cholecalciferol (D3-50; Biotech Pharmaceutical), to be taken weekly, and Xhosa participants were followed up 6 wk (± 15 d; mean +3 d) after their winter visit (Fig. S1). Previous studies have shown that serum 25(OH)D plateaus after ∼4–6 wk of supplementation (29); however, it is possible that the final equilibrated 25(OH)D level was underestimated at 6 wk. Ethics, recruitment, follow-up, and exclusion criteria are detailed in SI Materials and Methods. The procedures described below were conducted at each study visit.
UVB Exposure.
Sun exposure was assessed at each visit by a retrospective questionnaire that captured duration and time of day of outdoor sun exposure and the amount of skin not covered by clothing or a hat at time of exposure. PNUVB was calculated as described in SI Materials and Methods. Direct measurements of UVB were made at the University of Stellenbosch Solar Resource and Weather Station.
Skin Reflectometry.
Skin reflectance of all research subjects, expressed as EI and MI, was measured using a portable reflectometry device (DSM II ColorMeter; Cortex Technology). Constitutive pigmentation was measured on the upper inner arm site, and facultative pigmentation was measured on the forehead. Three independent measures were taken from each site, and the mean was calculated.
Food Questionnaire.
Intake of vitamin D (vitamin D2 and vitamin D3) was estimated using a 7-d quantitative food frequency questionnaire administered to every participant by a trained researcher at each study visit. The questionnaire (described in SI Materials and Methods) was adapted from a food frequency questionnaire shown to be valid in providing a reasonable estimation of dietary vitamin D intake in healthy young adults of diverse ancestry (30). Individuals taking vitamin D supplements were excluded.
Biochemistry and FBC.
Peripheral blood collected in serum tubes was analyzed on the day of collection for 25(OH)D concentration by the chemiluminescent LIAISON 25 OH Vitamin D TOTAL Assay (DiaSorin). Serum also was stored at −80 °C and subsequently batch analyzed for DBP, acute phase markers, calcium, and albumin. FBCs were conducted within 2–3 h of collection. Assays are described in SI Materials and Methods.
SNP Genotyping.
DNA was extracted from PBMCs using the QIAmp DNA Blood Mini Kit (QIAGEN), and 10 ng was analyzed by TaqMan Genotyping assay (Life Technologies), in duplicate, for the following SNPs in genes: DBP (rs7041 and rs4588), DHCR7 (rs12785878), CYP2R1 (rs10741657), CYP24A1 (rs6013897), CYP27B1 (rs10877012), and VDR (rs2544037, rs10783219, rs10735810, and rs731236), as described in SI Materials and Methods.
PBMC HIV-1 Infection and p24 Analysis.
After the isolation of PBMCs, cells were infected immediately with HIV-1, as described in SI Materials and Methods. HIV-1 replication was measured by quantifying the HIV-1 p24 antigen concentration of lysed supernatant by Luminex, as described in ref. 31, with slight modifications (SI Materials and Methods).
Statistical Analysis.
All univariate statistics were conducted in GraphPad Prism 6.0 software with an alpha of 0.05 and two-sided testing. GLM was conducted using Qlucore Omics Explorer 2.2 software and stepwise regression, and GLM on vitamin D optimality and deficiency was conducted in S-Plus. Full details are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank all participants for their voluntary participation; C. Diedrich, B. Alexander, and E. Geldenhuys for research assistance; and A. Martineau and D. Jolliffe for assistance with SNP selection. UVB data were collected at the Solar Technology and Energy Research Group solar observatory of Stellenbosch University, with assistance from P. Gauché and M. Mouzouris. This work was supported by a John Simon Guggenheim Fellowship; the Stellenbosch Institute for Advanced Study (N.G.J. and G.C.); a Sydney Brenner Postdoctoral Fellowship, Academy of Science of South Africa (A.K.C.); South African Sugar Association Research Grant 232 (to C.E.N.); UK Medical Research Council Grant U1175.02.002.00014; European Union 7th Framework Programme for Research and Technological Development Grant Health-F3-2012-305578; and Wellcome Trust Grants 084323 and 088316 (to R.J.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1500909112/-/DCSupplemental.
References
- 1.Mehta S, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS ONE. 2010;5(1):e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudfeld CR, et al. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS ONE. 2012;7(6):e40036. doi: 10.1371/journal.pone.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210(4466):203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: A D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 5.Webb AR. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol. 2006;92(1):17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Thieden E, Philipsen PA, Heydenreich J, Wulf HC. Vitamin D level in summer and winter related to measured UVR exposure and behavior. Photochem Photobiol. 2009;85(6):1480–1484. doi: 10.1111/j.1751-1097.2009.00612.x. [DOI] [PubMed] [Google Scholar]
- 7.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 8.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP) Hum Genet. 1993;92(2):183–188. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelman CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93(9):3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos-Lopez E, et al. Gestational diabetes mellitus and vitamin D deficiency: Genetic contribution of CYP27B1 and CYP2R1 polymorphisms. Diabetes Obes Metab. 2008;10(8):683–685. doi: 10.1111/j.1463-1326.2008.00879.x. [DOI] [PubMed] [Google Scholar]
- 12.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338(2):143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2(8671):1104–1105. doi: 10.1016/s0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 14.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 15.Pinzone MR, et al. LPS and HIV gp120 modulate monocyte/macrophage CYP27B1 and CYP24A1 expression leading to vitamin D consumption and hypovitaminosis D in HIV-infected individuals. Eur Rev Med Pharmacol Sci. 2013;17(14):1938–1950. [PubMed] [Google Scholar]
- 16.Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011;14(5):938–939. doi: 10.1017/S1368980011000565. [DOI] [PubMed] [Google Scholar]
- 17.Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999;53(12):920–926. doi: 10.1038/sj.ejcn.1600870. [DOI] [PubMed] [Google Scholar]
- 18.Patterson N, et al. Genetic structure of a unique admixed population: Implications for medical research. Hum Mol Genet. 2010;19(3):411–419. doi: 10.1093/hmg/ddp505. [DOI] [PubMed] [Google Scholar]
- 19.Abdool Karim Q, Abdool Karim SS, Singh B, Short R, Ngxongo S. Seroprevalence of HIV infection in rural South Africa. AIDS. 1992;6(12):1535–1539. doi: 10.1097/00002030-199212000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Uitterlinden AG, Fang Y, van Meurs JB, van Leeuwen H, Pols HA. Vitamin D receptor gene polymorphisms in relation to Vitamin D related disease states. J Steroid Biochem Mol Biol. 2004;89-90(1-5):187–193. doi: 10.1016/j.jsbmb.2004.03.083. [DOI] [PubMed] [Google Scholar]
- 21.Martineau AR, et al. Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J. 2010;35(5):1106–1112. doi: 10.1183/09031936.00087009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell GR, Spector SA. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem. 2011;286(21):18890–18902. doi: 10.1074/jbc.M110.206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiner JS, Harrison GA, Singer R, Harris R, Jopp W. Skin colour in southern Africa. Hum Biol. 1964;36(3):294–307. [PubMed] [Google Scholar]
- 24.Coussens AK, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci USA. 2012;109(38):15449–15454. doi: 10.1073/pnas.1200072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holick MF. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 26.Khoo A-L, et al. Seasonal variation in vitamin D3 levels is paralleled by changes in the peripheral blood human T cell compartment. PLoS ONE. 2012;7(1):e29250. doi: 10.1371/journal.pone.0029250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross AC, et al. Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antivir Ther. 2011;16(4):555–563. doi: 10.3851/IMP1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martineau AR, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176(2):208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 29.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69(5):842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 30.Wu H, et al. The development and evaluation of a food frequency questionnaire used in assessing vitamin D intake in a sample of healthy young Canadian adults of diverse ancestry. Nutr Res. 2009;29(4):255–261. doi: 10.1016/j.nutres.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Biancotto A, et al. A highly sensitive and dynamic immunofluorescent cytometric bead assay for the detection of HIV-1 p24. J Virol Methods. 2009;157(1):98–101. doi: 10.1016/j.jviromet.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langenhoven ML, Conradie PJ, Wolmarans P, Faber M. MRC Food Quantities Manual. South African Medical Research Council; Cape Town, South Africa: 1991. [Google Scholar]
- 33.Lange CM, et al. A genetic validation study reveals a role of vitamin D metabolism in the response to interferon-alfa-based therapy of chronic hepatitis C. PLoS ONE. 2012;7(7):e40159. doi: 10.1371/journal.pone.0040159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noursadeghi M, et al. Genome-wide innate immune responses in HIV-1-infected macrophages are preserved despite attenuation of the NF-kappa B activation pathway. J Immunol. 2009;182(1):319–328. doi: 10.4049/jimmunol.182.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei X, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46(6):1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Troyanskaya O, et al. Missing value estimation methods for DNA microarrays. Bioinformatics. 2001;17(6):520–525. doi: 10.1093/bioinformatics/17.6.520. [DOI] [PubMed] [Google Scholar]
- 37.Wichura MJ. The Coordinate-Free Approach to Linear Models. Cambridge Univ. Press; Cambridge, UK: 2006. [Google Scholar]