Significance
Understanding the host response to HIV-1 infection may provide important clues to design new strategies to prevent further infection and viral spread. In this report, we show that the cellular protein Target Of Egr1 (TOE1) can specifically bind to a HIV-1 regulatory sequence, called the transactivator response element, and inhibit its activity. This inhibition is shown to be sufficient to impair viral transcription and replication in HIV-1–infected T cells. We show that TOE1 is secreted from activated primary T cells, mimicking antigen presentation. Secreted forms of TOE1 cross the plasma membrane of neighboring cells and retain HIV-1 inhibitory activity. These results provide new information on a cellular mediator of HIV-1 inhibition and may guide further therapeutic anti–HIV-1 approaches.
Keywords: TOE1, HIV-1, replication, cell penetrating peptide, granzyme B
Abstract
Target of Egr1 (TOE1) is a nuclear protein localized primarily in nucleoli and Cajal bodies that was identified as a downstream target of the immediate early gene Egr1. TOE1 displays a functional deadenylation domain and has been shown to participate in spliceosome assembly. We report here that TOE1 can function as an inhibitor of HIV-1 replication and show evidence that supports a direct interaction of TOE1 with the viral specific transactivator response element as part of the inhibitory mechanism. In addition, we show that TOE1 can be secreted by activated CD8+ T lymphocytes and can be cleaved by the serine protease granzyme B, one of the main components of cytotoxic granules. Both full-length and cleaved TOE1 can spontaneously cross the plasma membrane and penetrate cells in culture, retaining HIV-1 inhibitory activity. Antiviral potency of TOE1 and its cell-penetrating capability have been identified to lie within a 35-amino-acid region containing the nuclear localization sequence.
We previously reported the cloning of a gene encoding the Target Of Egr1 (TOE1) protein as a target for the immediate early transcription factor Egr1 using ChIP-based methodology and its partial characterization as an inhibitor of cell growth (1). Mechanistically, TOE1 overexpression correlates with up-regulation of the cyclin-dependent kinase inhibitor p21 and produces an accumulation of cells in the G2/M phase of the cell cycle. Subsequently, we found that TOE1 is able to bind to the C-terminal tetramerization domain of the p53 tumor suppressor protein and enhances its transcriptional activity, further underscoring the potential importance of a role for TOE1 in the cellular growth inhibition through cell cycle modulation (2).
A previous report from another group studying RNA turnover and investigating human homologs for the yeast Ccr4p and Caf1p/Pop2p found that TOE1 possesses RNA deadenylase catalytic activity in vitro (3). Interestingly, only transfection of TOE1 with a deletion in its nuclear localization signal, restricting cytoplasmic localization of TOE1, displayed deadenylase activity in cultured cells.
Although heterokaryon experiments using HeLa and NIH 3T3 cells detected nucleocytoplasmic shuttling, it remains uncertain whether retention of TOE1 in the cytoplasm occurs to permit effective deadenylation of RNA substrates. An additional report from another group studying Cajal body structure and function identified a role for TOE1 in Cajal body formation, as well as in RNA splicing, although its precise role in RNA splicing is yet to be determined (4). Together, the combination of the published work points to the involvement of TOE1 in the regulation of multiple cellular functions including those involving growth regulation and RNA metabolism.
With regard to human disease pathology, clues for a biological role for TOE1 were revealed in a study by investigators in search for the identity of an endogenous noncytotoxic proteinaceous factor secreted from CD8+ T lymphocytes able to control the replication of HIV-1 in infected CD4+ T cells. This study used a subtractive hybridization and PCR strategy to examine genes differentially regulated in discordant HIV-1–positive identical twins and confirmed in additional HIV-1–positive individuals displaying a CD8+ T-cell noncytotoxic antiviral response (CNAR). RNA transcripts for three genes were found to have significantly elevated levels in CD8+ T cells from the twin displaying anti–HIV-1 activity, with one of these transcripts being TOE1 identified as flj13949 therein (5). The sought factor, termed CAF (CD8+ cell antiviral factor), has been proposed to endow HIV-1–positive individuals with protection from progression to AIDS by inhibiting viral replication and thereby maintaining a low viral load without the need for antiviral therapy (6). Consistent with this proposal is the finding that HIV-1–infected individuals termed long-term nonprogressors who maintain stable CD4+ T-cell counts and a subset of these patients defined as “elite controllers” whose viral RNA levels are maintained below the level of detection, do not require treatment to prevent disease progression and show enhanced CAF levels in fluids from cultured cells (7). Subsequent studies using microarray screening for genes with enhanced expression in cDNA samples from CNAR-positive donors failed to confirm TOE1 as a CNAR overexpressed gene, and the identity of CAF remains to be determined (8, 9). Nonetheless, a host of biochemical features that this factor possesses has been determined and appears to function by a transcriptional mechanism acting on the viral long terminal repeat (LTR) domain (6, 10, 11). It should be noted that HIV-1 suppression in the absence of antiviral therapy has also been linked to a genetic component in a population of long-term nonprogressors who are enriched in HLA B57 alleles preferentially targeting the HIV-1 Gag protein, suggesting that specific amino acids that present viral peptides play a role in host control of HIV-1 as determined by a genome-wide association analysis (12). However, genetics cannot entirely account for viremic control because it was found that 67% of long-term nonprogressors possess enriched HLA B57 and other protective HLA alleles, whereas the same alleles were also found in 37% of HIV-1 progressors (13, 14). The purpose of the present study is to further explore a possible relationship between the expression of TOE1 and HIV-1 replication and to define whether TOE1 may function in an innate immune, CAF-like manner.
Results
TOE1 Expression Interferes with Tat Transactivation of the HIV-1 LTR.
To determine whether TOE1 might impact HIV-1 replication at the transcriptional level, we first sought to examine the effect of exogenous expression of TOE1 in an in vitro assay measuring viral LTR transcriptional activation driven by Tat. For this, 293T cells were transfected with an HIV-1 LTR-driven luciferase reporter construct and expression vectors to produce Tat and TOE1. We found that, although Tat strongly activated the HIV-1 LTR domain as expected, expression of TOE1 alone did not appear to affect HIV-1 LTR-dependent transcriptional activity. However, coexpression of TOE1 together with Tat was effective in inhibiting Tat-driven luciferase expression in a dose-dependent manner (Fig. 1A). Although TOE1 is known to have an effect on cell proliferation, this activity is noted following an extended time frame of several days. The experiments presented above, however, were developed 16–18 h following transfections where no difference in cell numbers is observed. To assess the specificity of this response, we tested whether TOE1 would impact the transcriptional activation of an alternate c-fos minimal promoter containing three tandem Egr1 binding sites. As shown in Fig. 1B, TOE1 did not appear to affect activation of an Egr1 responsive promoter. Transfected TOE1 expression levels were confirmed by Western blotting (Figs. 1 A and B, Lower). This effect was not the result of cytotoxicity as shown in Fig. 1C. The effect of TOE1 on the HIV-1 LTR may function by interfering with Tat directly or by binding to TAR RNA. To distinguish between these, we replaced the TAR sequence with the Rev responsive element (RRE) within the HIV-1 LTR-Luc plasmid and examined reporter activation in the presence of a Tat/Rev fusion protein. We found that in the context of the intact HIV-1 LTR, Tat/Rev activation was inhibited by TOE1. In contrast, replacement of the TAR with RRE showed activation by Tat/Rev, but this activation was not TOE1 inhibitable (Fig. 1D). These results indicated that TOE1 could specifically inhibit the activity of Tat at the level of transcriptional activation and that this inhibition appeared to be due to a mechanism involving TAR binding. We further examined the specificity of TOE1 inhibition by testing the effect of TOE1 expression on the activation of an alternate retroviral LTR promoter. Using the Moloney Murine Leukemia Virus (Mo-MLV) LTR, we observed at the highest concentrations of TOE1, an approximate 30–35% inhibition of activity (Fig. 1E). This result contrasts with the approximate 80% inhibition seen on the HIV-1 LTR using 10-fold less TOE1. Interestingly, CAF has also been shown to inhibit HIV-1 replication by a transcriptional mechanism of action (10, 11, 15). As mentioned above, CAF is a factor secreted from CD8+ T cells isolated from HIV-1–infected individuals able to control viremia, but TOE1 is localized to the nucleus/nucleolus and has not been reported to be secreted extracellularly. Therefore, we next addressed whether TOE1 is expressed in primary human CD8+ T cells and, if so, whether it could be secreted upon cellular activation.
Fig. 1.
TOE1 inhibits Tat-driven transactivation of HIV-1 LTR. Luciferase assay of 293T cells transfected with various constructs. (A) HIV-1 LTR reporter construct alone or in the combination with TOE1, and HIV-1 LTR (10 ng/well) plus Tat (5 ng/well) and the indicated amounts of TOE1. (B) pEBS3-fos alone or in combination with its activator Egr1 plus or minus TOE1 (30 ng each). (Lower) TOE1 expression levels by Western blotting from immunoprecipitation with α-FLAG and probed with α-TOE1 Ab-86. (C) LDH assay for cytotoxicity. (D) HIV-1 LTR alone or in combination with Tat/Rev or Tat/Rev + TOE1 (10, 5, and 20 ng, respectively) or with reporter construct RRE-LTR alone or in combination with Tat/Rev or Tat/Rev + TOE1 (15, 15, and 60 ng, respectively). (E) Mo-MLV LTR (20 ng) alone or in combination with TOE1 at the amounts indicated. The error bars represent the SD of one or two separate experiments performed in triplicate and are representative of multiple experiments. One asterisk indicates a significant and two or more asterisks a highly significant difference as assessed by one-way ANOVA followed by Bonferroni post hoc test. In A, P < 0.002 for 0 vs. 1 ng, P < 0.0095 for 0 vs. 2 ng, P < 0.004 for 0 vs. 3 ng, P < 0.002 for 0 vs. 5 ng (F = 62.40, P = 0.0001). In D, P < 0.004 for F = 15.95, P < 0.0001. In E, P < 0.004 for 20 ng and P < 0.004 for 50 ng (F = 15.95, P < 0.0001).
TOE1 Is Secreted Following Immune Response Activation.
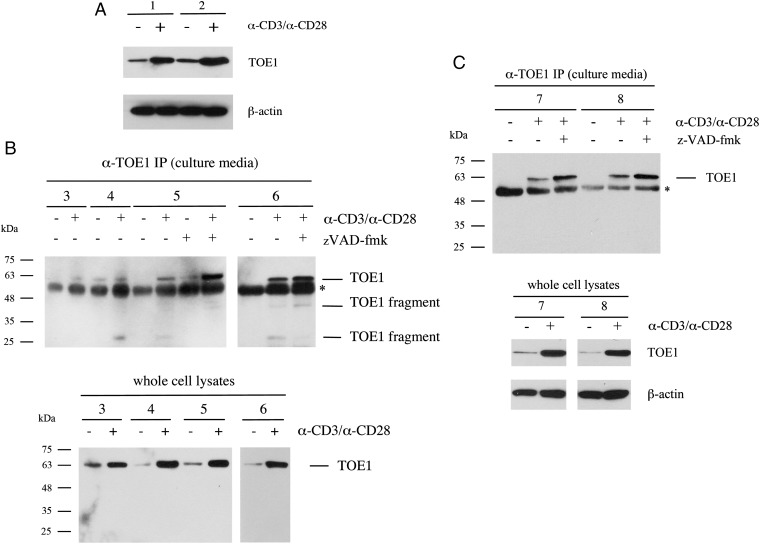
To first examine TOE1 expression in primary human CD8+ T cells, blood samples were obtained from healthy donors, and CD8+ T cells were isolated, placed in culture, and activated by the addition of anti-CD3/anti-CD28 antibodies (an experimental condition mimicking antigen presentation). Following activation, cell lysates were tested for TOE1 expression by Western blotting, and the results are shown in Fig. 2A. We found that TOE1 was expressed in CD8+ T cells and furthermore that expression was significantly increased following T-cell receptor activation in two independent donors. Further probing of this blot using an antibody against β-actin served to confirm equal protein loading (Fig. 2A, Lower). We then proceeded to test whether TOE1 could be secreted from its nuclear localization by collecting the conditioned media from untreated and activated cells. Results from CD8+ T cells collected from four different donors are shown in Fig. 2B. In all four donor samples tested (donors 3–6), TOE1 could be found in immunoprecipitates from the conditioned media suggesting that TOE1 can be secreted following activation of these cells. Interestingly, in three of the four donors, smaller-molecular-weight species were detected in the immunoprecipitates from the conditioned media together with full-length protein, suggesting that TOE1 may be subject to proteolytic cleavage either during or after secretion. To distinguish secretion from cell death following activation, we treated cells with the apoptosis inhibitor zVAD-fmk. As seen for donor samples 5 and 6 in Fig. 2B, the presence of TOE1 in the culture media was maintained and, in fact, appeared to be enhanced. When cell lysates from donor samples 3–6 were tested for TOE1 expression, we found increased TOE1 in activated cells as before (Fig. 2B, Lower). Interestingly, CD8+ T cells from donor 4 appear to secrete full length TOE1 before activation, which may indicate a possible underlying immune response involving TOE1 in this donor. Together, these experiments provide evidence that TOE1 can be both up-regulated and secreted from activated CD8+ T cells and that this appears to be accompanied by at least partial TOE1 fragmentation. We also tested for the expression and secretion of TOE1 from primary CD4+ T cells. As shown in Fig. 2C (donors 7 and 8), we also observed TOE1 expression in CD4+ T cells that was induced upon activation of the cells with anti-CD3/CD28 antibodies. In addition, although TOE1 secretion could be detected in the media of activated CD4+ T cells, we did not detect any fragments of the full-length TOE1 protein.
Fig. 2.
TOE1 is up-regulated and secreted from T cells following stimulation of the T-cell receptor. (A) Western blot analysis of whole lysates from primary human CD8+ T cells from two distinct donors (donor 1 and donor 2) before and after stimulation with α-CD3/α-CD28 loaded beads. The upper blot is probed with a mouse monoclonal α-TOE1 antibody. The blot was subsequently probed with anti−β-actin to confirm equal loading (Lower). (B) Western blot analysis of immunoprecipitates from culture media. CD8+ T cells were obtained from four additional donors (donors 3–6) and stimulated with α-CD3/α-CD28 loaded beads. At 24 to 72 h following stimulation, immunoprecipitation was performed on cleared culture media using the rabbit α-TOE1 Ab-86 + α-TOE (GeneTex); zVAD-fmk was added at 50 μM where indicated. Western blot was performed with mouse α-TOE1 (Abnova). Asterisk shows the presence of cross-reacting IgG. (Lower) α-TOE1 Western blot analysis of cell lysates for donors 3–6. (C) Western blot analysis performed as in B from CD4+ T cells from donors 7 and 8. (Lower) α-TOE1 Western blot analysis of cell lysates for donors 7 and 8 and corresponding β-actin probing for loading control.
TOE1 Can Be Proteolytically Cleaved by Granzyme B.
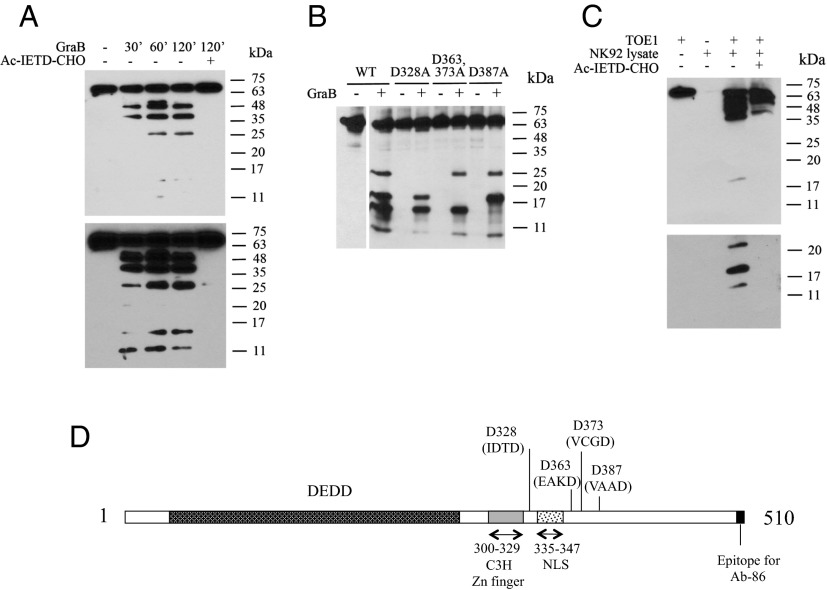
Granzyme B (GraB) and other members of the granzyme family are potent secreted proteases found in granules of cytotoxic lymphocytes that function in collaboration with perforins to eliminate target cells, especially virally infected and transformed cells (16, 17). Recent research has found that, in addition to its cytotoxic role, GraB may also possess a noncytotoxic activity (18, 19). To determine whether GraB could be the CD8+ T-cell–derived protease responsible for TOE1 cleavage, we performed in vitro digestions using purified GraB together with purified, recombinant TOE1 proteins followed by Western blotting. Fig. 3A shows a time course digestion of TOE1 with GraB over a 2-h period and analyzed using a polyclonal antibody raised against the central region of TOE1. Using 100 nM enzyme concentration, we observed the appearance of several cleavage products while the use of the tetrapeptide GraB inhibitor Ac-IETD-CHO completely abrogated the processing of TOE1, thus confirming the GraB specificity of the observed cleavage products. Fig. 3A, Lower is a longer exposure of the blot in the upper panel showing the presence of additional lower-molecular-weight fragments. To help identify the specific cleavage sites, we used an alternate rabbit polyclonal anti-TOE1 antibody that was raised against a single epitope at the extreme C-terminal end of TOE1 (Ab-86). GraB is a serine protease that displays a strong preference for cleavage after aspartate residues in the P1 position of a tetrapeptide recognition site. Therefore, using site-directed mutagenesis, we proceeded to mutate a number of aspartate residues corresponding to potential GraB cleavage sites. Ab-86 recognized fragments requiring the presence of the C-terminal epitope and allowed us to define in vitro GraB cleavage sites at residues 328, 363, 373, and 387 of full-length TOE1 (Fig. 3B). We also performed similar experiments using GraB from an endogenous source. For these experiments, we prepared a freeze/thaw extract from the natural killer cell line NK92 that expresses high levels of GraB. The cleared lysate was then incubated with recombinant TOE1. As shown in Fig. 3C, we obtained a similar proteolytic pattern to that seen in the experiments using recombinant GraB, and, consistent with our experiments using a recombinant enzyme, the cleavage was inhibited by the addition of Ac-IETD-CHO. The 26- and 48-kDa fragments were observed from activated primary human CD8+ T cells thus suggesting that the protease responsible for TOE1 processing in cytotoxic cells lysates is GraB. The lower panel is a longer exposure of the lower-molecular-weight region of the blot shown in the upper panel. These results represent the first report, to our knowledge, defining TOE1 as a substrate for GraB. Fig. 3D represents a summary of the identified GraB cleavage sites in TOE1, as well as showing the positions of the deadenylation domain (DEDD), C3H zinc finger, and lysine/arginine rich nuclear localization sequence (NLS).
Fig. 3.
TOE1 is a substrate for GraB. (A) Time course in vitro digestion of recombinant TOE1 with 100 nM recombinant active GraB. Cleavage products of TOE1 are revealed by Western blot analysis using a rabbit polyclonal antibody (GeneTex) targeting a central region of TOE1. Incubation with the GraB inhibitor tetrapeptide IETD-CHO (100 μM) prevents the processing. (Lower) Longer exposure of the Upper panel. (B) Identification of D328, D363/373, and D387 cleavage sites in TOE1 by GraB. Constructs expressing either WT FLAG-tagged TOE1 or recombinant forms carrying point mutations in the indicated aspartate residues were transfected into 293T cells; α-FLAG immunoprecipitates from cell lysates were incubated with 100 nM GraB for 2 h. Rabbit α-TOE1 (Ab-86) antibody was used for Western blotting. (C) Western blot analysis of recombinant TOE1 incubated for 2 h with 50 μL of cleared freeze/thaw extract from NK92 cells shows processing of TOE1 that is prevented by the GraB inhibitor Ac-IETD-CHO (100 μM). (Lower) Longer exposure of the Upper panel. (D) Summary of the location of GraB cleavage sites and the positions of the deadenylation (DEDD), C3H zinc finger, and NLS. The C-terminal epitope position for the Ab-86 antibody is also indicated. The GraB tetrapeptide recognition sites for each cleavage site are indicated in brackets.
TOE1 Contains Endogenous Cell-Penetrating Ability.
From the results presented above, we found that TOE1 inhibited Tat activity on the HIV-1 LTR, could be secreted by activated CD8+ T cells, and could be cleaved by GraB in the extracellular space. If secreted TOE1, or a fragment thereof, were to function as an inhibitor of HIV-1 LTR activation, it would require subsequent transport into a target cell. Interestingly, and similarly to Tat, TOE1 harbors a long lysine/arginine-rich NLS (amino acids 335–347) composed of a largely uninterrupted sequence of 12 basic residues (Table 1). For Tat, this basic region (amino acids 49–57) is indispensable for the recognition and binding to the TAR sequence in the viral LTR, as well as for efficient elongation and capping of the viral transcript, and it also serves as the protein's cell penetrating peptide (20–23). Therefore, we tested the hypothesis that the TOE1 NLS might also function as a cell-penetrating peptide. We generated recombinant TOE1 fusion proteins comprising EGFP and engineered both full-length TOE1 and a fragment representing the GraB-generated fragment spanning amino acids 329–363. This fragment was chosen for these experiments because it is the smallest fragment that maintains the lysine/arginine-rich NLS. Recombinant TOE1-EGFP proteins were then administered to the culture media of HeLa cells, and cells were imaged using live cell confocal imaging. We chose to perform these experiments on live cells because previous reports examining protein transduction on fixed cells found that fixation protocols can generate artifactual positive results for proteins that merely bind nonspecifically to the cell membrane (24).
Table 1.
Basic region sequence comparison
| Protein | Sequence |
| HIV-1 Tat | 49-RKKRRQRRR-57 |
| TOE1 | 335-KRRRRRRREKRKR-347 |
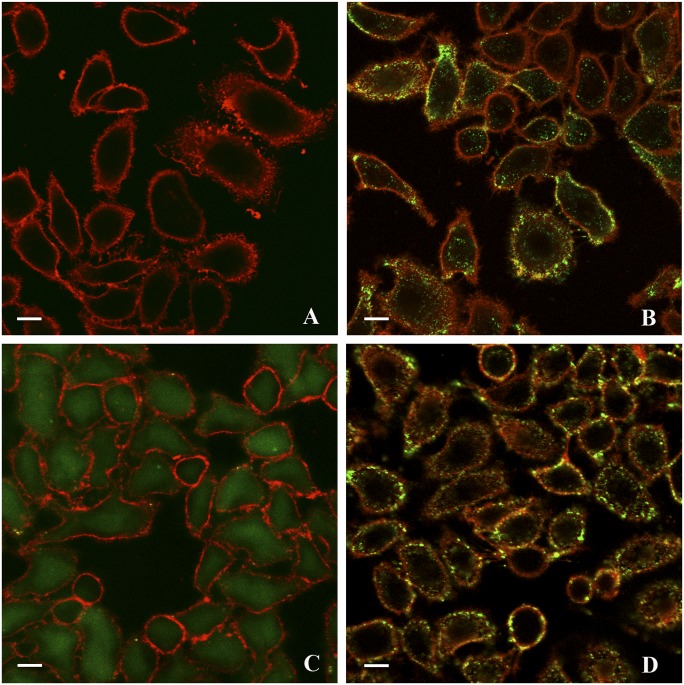
Following administration of full-length TOE1-EGFP, we observed a rapid intracellular uptake of the fusion protein but not of the control EGFP alone (Fig. 4 A and B). TOE1-EGFP promptly gathered around the cell surface and within 1 h of incubation, it could be seen internalized in endosomal-like foci. Similarly, EGFP-tagged Tat appeared to be distributed in similar structures in the cytoplasm and to a smaller extent within the nucleus (Fig. 4D). In contrast, when we added the small TOE1 fragment consisting of residues 329–363 fused to EGFP, we observed a diffuse intracellular import of this protein. Thus, it appeared that this 35-amino-acid fragment containing the lysine/arginine rich domain was sufficient to allow transduction into cells (Fig. 4C). Moreover, this fragment did not appear to be localized into foci.
Fig. 4.
TOE1 and the 35-amino-acid GraB cleavage product 329–363 can penetrate live cells. Live cell confocal images of HeLa cells 1 h after administration of recombinant EGFP-TOE1 fusion protein, EGFP fusions with the GraB processing product 329–363, or Tat. EGFP alone was used as control. All fusion proteins included a 6×His tag and were purified on a nickel column and administered to cultures of HeLa cells at a 1 μM concentration. CellMask Deep-Red plasma membrane stain was added immediately before live imaging at 0.25 μg/mL. (A) Control, EGFP only; (B) TOE1-EGFP; (C) 329–363-EGFP; (D) Tat-EGFP. (Scale bar, 10 μm.)
These results represent the first demonstration, to our knowledge, that TOE1 harbors a 35-amino-acid region comprising the lysine/arginine-rich domain capable of penetrating a target cell and delivering an EGFP cargo to the cell interior.
Extracellular Administration of TOE1 Can Inhibit HIV-1 LTR Transactivation by Tat.
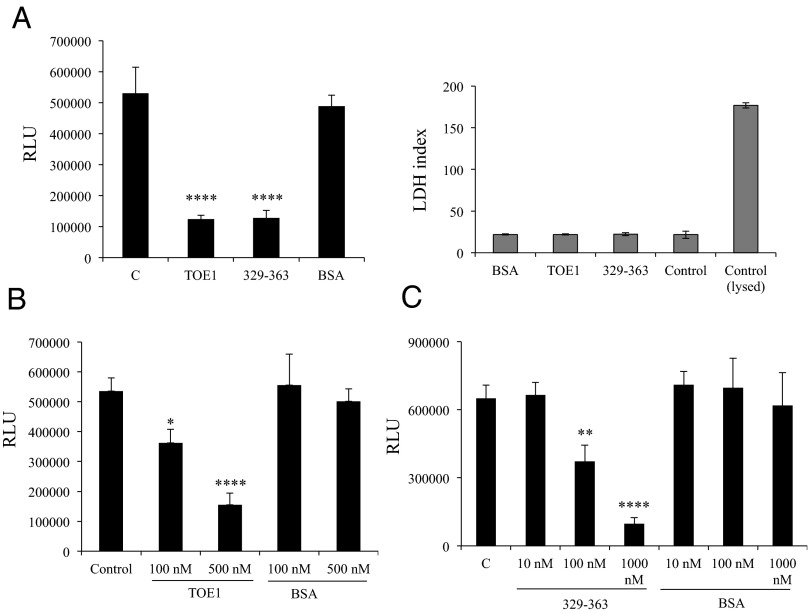
We next wanted to test whether extracellularly delivered TOE1 could achieve the same transcriptional modulation of HIV-1 LTR obtained in cotransfection experiments. This activity would require that on cellular uptake, TOE1 can be delivered to the nucleus in a similar manner as has been demonstrated for Tat (20, 25, 26). We therefore transfected 293T cells with a combination of Tat and HIV-1 LTR-driven luciferase constructs. After 5 h, the medium was removed and replaced with fresh medium to which recombinant TOE1, 329–363 peptide, or BSA for control was added. The left panel of Fig. 5A shows that TOE1 added to the medium was able to inhibit Tat-driven HIV-1 LTR luciferase activity, demonstrating that exogenous TOE1 could reproduce the transcriptional inhibition seen using a transfected TOE1 expression vector. Moreover, the 329–363 cell-penetrating fragment of TOE1 was also able to reproduce this HIV-1 LTR-driven inhibitory activity, whereas adding BSA had no effect on Tat transactivation of HIV-1 LTR. This decrease in luciferase expression was not the result of cytotoxicity as verified by LDH assay (Fig. 5A, Right). From these results, we concluded that extracellular TOE1 could not only cross the plasma membrane, but also be active in inhibiting Tat-driven HIV-1 LTR transcriptional activity. Fig. 5 B and C show a dose–response effect of TOE1 and the 329–363 fragment on Tat transactivation of HIV-1 LTR, with a 70% and 85% reduction of expression, respectively, at the highest concentrations used. Taken together, these results demonstrate that following internalization, a functional version of TOE1 retaining Tat inhibitory activity is effectively delivered to the nucleus.
Fig. 5.
TOE1 administered to cells is a functionally active inhibitor of HIV-1 LTR expression. (A) Luciferase assay (Left) of 293T cells transfected with HIV-1 LTR reporter construct plus pCEP4-Tat and then treated with 500 nM recombinant TOE1, 329–363, or BSA; in the control sample, only PBS was added. (Right) Result of LDH assay performed on the culture media measuring cytotoxicity. (B and C) Luciferase assays on 293T cells treated with increasing concentrations of recombinant TOE1 and 329–363, respectively. Error bars represent SD of triplicate values from a representative of multiple experiments. One asterisk indicates a significant and two or more asterisks a highly significant difference as assessed by one-way ANOVA, followed by Bonferroni post hoc test. In A, P < 0.0001 for BSA vs. TOE1 and BSA vs. 329–363 (F = 89.55, P = 0.0001). In B, P < 0.05 for BSA100 vs. TOE1100, and P < 0.0001 for BSA500 vs. TOE1500 (F = 49.60, P = 0.0001). In C, P < 0.001 for C vs. 329–363100, and P < 0.0001 for C vs. 329–363500 (F = 22.01, P = 0.0001).
TOE1 Can Bind Directly to the HIV-1 TAR Element.
Tat exerts its modulatory effects by specifically binding to an RNA region in the viral LTR called the TAR element through a basic domain of nine amino acids (21). Because TOE1 was able to inhibit Tat activity and also harbors a similar basic domain we postulated that TOE1 might achieve this inhibition through binding the TAR element. To test this hypothesis, we prepared a fluorescently labeled TAR RNA probe and performed RNA gel shift analyses using either the recombinant 329–363 TOE1 fragment, or a synthetic peptide (ED35) comprising the identical amino acid sequence, but lacking the 6×His tag present in the recombinant peptide. Fig. 6A, Upper Left and Right, shows specific binding of both recombinant 329–363 TOE1 and the corresponding synthetic peptide to the TAR RNA probe, respectively. Binding specificity was demonstrated using an unlabeled TAR RNA as competitor, with complete competition observed at 10-fold molar excess. Because the TOE1 ED35 synthetic peptide comprises a highly basic domain, it remains possible that binding to TAR occurs nonspecifically. To test whether TOE1 binds specifically to the TAR, we constructed a TAR probe with a three-ribonucleotide deletion designed to eliminate the TAR bulge structure. This region of the TAR has been previously shown to be necessary for Tat binding (27). Our results show that TOE1 was not able to bind to this TAR mutant (Fig. 6A, Lower Left) and that this mutant was not able to compete for TOE1 binding to a WT TAR probe (Fig. 6A, Lower Right). From these results, we concluded that TOE1 is an RNA-binding protein capable of specific binding to the TAR element in the HIV-1 LTR.
Fig. 6.
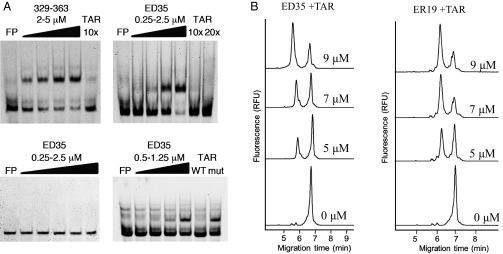
TOE1 peptides directly interact with HIV-1 TAR sequence. (A) RNA EMSA of a fluorescent TAR RNA probe in the presence of TOE1 peptides. Increasing amounts of either purified recombinant His-tagged 329–363 (Upper Left) or a synthetic peptide ED35 consisting of amino acids 329–363 of TOE1 (Upper Right) were incubated for 15 min at room temperature with TAR and then run on native 6% acrylamide gels. Specific competitor unlabeled RNA was added at the molar excess indicated. EMSA performed with a mutant TAR lacking the UCU bulge (Lower Left) and with WT TAR in the presence of mutant or WT unlabeled competitor (Lower Right) at 10× molar excess. (B) Electropherograms of labeled TAR with TOE1 synthetic peptides separated by capillary electrophoresis and detected by laser induced fluorescence after incubation at 75 °C for 5 min followed by a 5-min incubation at room temperature. The ER19 synthetic peptide consists of amino acids 329–347. (Left) TAR incubated with ER19 (0, 5, 7, and 9 µM). (Right) TAR incubated with ED35 (5, 7, and 9 µM). The complex between each peptide and TAR is observed as a peak at 6 min, and free TAR is observed at 7 min. All samples were also incubated with BSA and yeast tRNA and were separated under 500 V/cm in a 60-cm-long capillary in 25 mM Borax buffer at 22 °C.
We proceeded to assess the affinity of TOE1 for the TAR element by capillary electrophoresis (CE), using the same fluorescently labeled TAR probe and the 35-amino-acid synthetic peptide TOE1 fragments described above, or a smaller 19-amino-acid peptide including the NLS (ER-19). Fig. 6B shows the electropherograms from CE experiments wherein increasing amounts of TOE1 peptides were incubated in binding reactions with a constant amount of labeled TAR probe. With increasing TOE1 peptide, an increase in the peak eluting at ∼6 min was observed. From these results, TOE1 binding to the TAR was confirmed, and an affinity binding constant of about 4 μM was calculated for the 19-amino-acid peptide.
TOE1 Can Inhibit Replication of HIV-1 in Infected CD4+ T Cells.
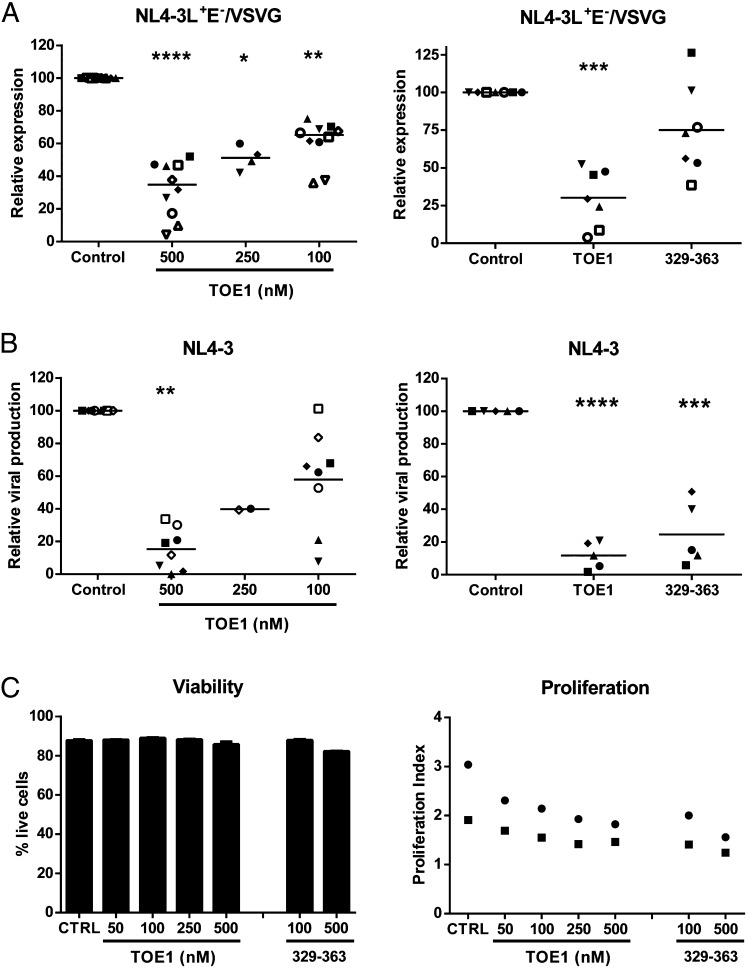
We next wanted to test whether TOE1 would be capable of reproducing the observed antiviral activity in primary human CD4+ T cells infected with HIV-1. First, we analyzed the inhibition of HIV-1 LTR transcriptional activity in activated primary human CD4+ T cells infected with a luciferase reporter virus [i.e., NL4-3Luc+Env− pseudotyped with the vesicular stomatitis virus G (VSVG) protein envelope]. Incubation with recombinant full-length TOE1 induced a dose-dependent inhibition of HIV-1 LTR-driven expression, ranging from 40% at 100 nM to 70% at 500 nM TOE1 (Fig. 7A, Left). In addition, the TOE1 peptide consisting of residues 329–363 was chosen for further evaluation because it corresponds to the smallest active peptide capable of inhibiting HIV-1-LTR activity. Our results also showed a reproducible inhibition of HIV-1 expression by the 329–363 peptide, although statistical significance was not achieved for this peptide due to the very high variability between donors for this experiment (Fig. 7A, Right). From these experiments, we confirmed that both TOE1 and its GraB-derived proteolytic fragment 329–363 could inhibit Tat transactivation of HIV-1 expression following virus infection.
Fig. 7.
TOE1 inhibits HIV-1 expression and replication in CD4+ T cells. Primary human CD4+ T cells were first activated and inoculated with luciferase-encoding, VSVG-pseudotyped viral particles (A) or fully infectious NL4-3 viruses (B), washed, and then incubated with recombinant TOE1 or 329–363 peptide at the indicated concentrations. Luciferase activity was determined after 3 d, and viral production was evaluated by measuring the p24 content after 6 d. Results from 5 to 10 individual experiments with different blood donors are shown; each symbol represents one donor and the horizontal line shows the mean. Results are presented as viral expression (A) or viral production (B) relative to the untreated control. (C) The effect of TOE1 and peptide 329–363 on CD4+ T-cell survival and proliferation were evaluated for two donors as described in Materials and Methods.
We also determined the effect on HIV-1 replication, using a fully infectious X4-tropic virus (i.e., NL4-3), and found the dose-dependent effect of TOE1 addition was even more potent, with a 40–85% inhibition of HIV-1 replication at a TOE1 concentration of 100–500 nM (Fig. 7B, Left). The 329–363 peptide also showed a very potent, reproducible, and highly significant inhibition of viral production ranging from 60% to 95% inhibition for individual donors (Fig. 7B, Right).
Viability assays revealed very little toxicity for TOE1 and the 329–363 peptide in CD4+ T cells with a 5–6% increase in mortality observed at the highest tested concentration (Fig. 7C, Left). However, both full-length TOE1 and the 329–363 peptide showed evidence of some inhibition of cell proliferation, with variability seen among the donor samples tested (Fig. 7C, Right). This diminished proliferation could be partly responsible for the reduced viral production, but cannot account for the drastic reduction in viral expression. These results indicated that TOE1 was able to not only inhibit HIV-1 transcriptional activity but was also effective in reducing replication of whole infectious HIV-1.
Discussion
We report here a previously unidentified function for the nuclear protein TOE1 as an inhibitor of HIV-1 replication acting at the level of transcription. We also describe the previously unsuspected property of TOE1 secretion from activated T cells and an independent ability to readily cross the plasma membrane from the extracellular milieu and incorporate into cells. Furthermore, we demonstrate the direct interaction of TOE1 with the HIV-1 TAR element and suggest that this might contribute, at least in part, to the ability of TOE1 to inhibit virus replication.
Using two independent assays, expression of a luciferase reporter gene in both transfected and primary human cells, and modulation of virus replication in CD4+ T cells, we demonstrated an inhibitory activity of TOE1 on HIV-1. We showed that TOE1 expression, as well as uptake of purified protein administered to the extracellular milieu, can interfere with Tat transactivation of its target sequence TAR. As this is a necessary step for the efficient elongation of the viral RNA, it is likely that TOE1 repression of HIV-1 replication is a direct consequence of its inhibitory effect on LTR expression. Relevant to this activity, TOE1 has been shown to harbor a deadenylation domain that was found to be functional in vitro (3). Therefore, it could be argued that TOE1 expression may affect viral RNA expression as a result of a nonspecific decrease in mRNA stability. However, the anti–HIV-1 activity of TOE1 could be restricted to a 35-amino-acid region of TOE1 encompassing the NLS that is not included in the deadenylation domain. Accordingly, it is unlikely that the effect of TOE1 on HIV-1 LTR transactivation can be accounted for solely by a deadenylase catalytic activity.
In parallel, we observed an increase in TOE1 expression in CD8+ T lymphocytes stimulated by activation of the T-cell receptor, indicating that TOE1 could have a function in the immune response driven by CD8+ T cells, which are considered to be pivotal in the resistance to HIV-1 infection and its progression to AIDS (14, 28). Moreover, TOE1 was both secreted and proteolytically processed by GraB (Figs. 2 and 3). The identification of TOE1 as a GraB substrate not only marks the definition of a previously unidentified substrate for this protease but also links TOE1 to the CD8+ T-cell–mediated immune response. Although we also observed induction and secretion of TOE1 from activated CD4+ T cells, we did not detect any TOE1 fragmentation in these cells consistent with GraB being responsible for TOE1 cleavage.
Although GraB is better known for its role in apoptosis with caspase-3 and other proapoptotic molecules being recognized substrates (29, 30), it has recently been shown that it can also have a role in signaling inflammation by inducing a cleavage of IL-1α that potentiates its activation of the IL-1 receptor (31). Thus, GraB can participate in noncytotoxic responses to pathogens, and this function may also involve processing of TOE1. Interestingly, protease inhibitors have been found to interfere with CAF activity (32). However, two separate studies ruled out both GraA and B as direct effectors of noncytotoxic anti–HIV-1 activity, as well as the presence of CAF in cytotoxic granules (33, 34). These reports do not exclude, however, that CAF could be a substrate for GraB.
It is noteworthy that, similarly to Tat, TOE1 harbors a long Lys/Arg-rich NLS composed of a largely uninterrupted sequence of 12 basic residues (Table 1). The basic region in Tat is indispensable for the recognition of the TAR sequence and is necessary for efficient elongation of the viral transcript and therefore is crucial for viral replication. Additionally, this sequence is responsible for the cell-penetrating activity of Tat. Among the fragments generated by GraB processing of TOE1, the 329–363, containing the Lys/Arg-rich NLS, retained both cell-penetrating capability and inhibition of viral replication (Figs. 4 and 5). Therefore, we suggest a previously unidentified function for TOE1 as a signaling, cytokine-like molecule functioning as an adjuvant antiviral involved in the immune response. At present, we do not know if other cell types, immune or otherwise, can also produce and release TOE1 in response to specific stimuli.
Currently, it is not clear what the function of TOE1 processed by GraB is or if it is related to its antiviral activity. Both full-length and the 329–363 truncated form displayed similar levels of inhibition of HIV-1 LTR in cell lines and primary human CD4+ T cells, and a comparable effect on viral replication in HIV-1–infected CD4+ T cells. An obvious difference between the full-length and 329–363 fragment was observed in the cell-penetrating ability, with the full-length TOE1 appearing to follow a Tat-like mode of uptake in cells and localized primarily in endosomal-like structures. In contrast, the 329–363 truncated form exhibited a diffuse pattern following uptake over the same time frame. Clearly, to have any function on the LTR domain, TOE1 must be released from these structures. By eliminating this requirement, the 329–363 fragment might be able to immediately exert its effects on the LTR permitting a more rapid inhibitory response. Moreover, it could be argued that the uptake of the whole TOE1 protein could have additional effects on the targeted cell's transcriptome, given that TOE1 could affect RNA metabolism. Therefore, a potential advantage of the GraB cleavage of TOE1 might lie in the production of a more directed antiviral molecule. Although this difference may have only a negligible impact in culture experiments, it could be of significance in the economy of the immune response to a viral infection in the whole organism.
The ability of a protein to independently cross the plasma membrane of cells in culture was first reported in 1988 for HIV-1 Tat (25, 26). Since then, other cell-penetrating peptide (CPP)-containing proteins have been described. In recent years, these peptides have been the focus of intense study aimed at the use of these sequences as tools to deliver a therapeutic cargo (35, 36). Unfortunately, endosomal entrapment is often a limitation associated with CPPs, particularly for cationic Tat-like CPPs. This report presents data showing that the TOE1 peptide consisting of residues 329–363 appears to escape this constraint and could be an important asset in the further development of a TOE1-based anti–HIV-1 therapeutic or as a transporter for the delivery of drugs or other cargos into cells.
Mechanistically, we demonstrated that the TOE1 domain containing the Lys/Arg-rich NLS interacts directly with HIV-1 TAR using two independent methods, i.e., RNA gel-shift and capillary electrophoresis assays (Fig. 6). These results support a hypothesis whereby TOE1 functions by competition with Tat for the binding to TAR to achieve inhibition of viral RNA transcription. We have shown that TOE1 binding to the TAR stem loop can be eliminated by deleting the side bulge structure and that TOE1 did not appear to interfere with Tat/Rev activity on the RRE. However, we did note that TOE1 had an effect on the activation of the Mo-MLV LTR, albeit less than that of the HIV-1 LTR, which may indicate that TOE1 could bind additional viral RNA stem loop structures.
We suggest that the direct interaction between TOE1 and the TAR is involved in the mechanism of inhibition. Shown here are functional similarities between TOE1 and the previously described CAF, including an inhibitory activity at the level of LTR transcription, secretion from CD8+ T cells, and participation of a protease in its activity. Despite these similarities, it is not known at present whether TOE1 shares additional features with CAF including enhanced production from the CD8+ T cells of HIV-1–infected long-term nonprogressors. These and other studies relating to the mechanism of TOE1 action await further investigation.
Materials and Methods
Cell Culture.
293T and HeLa cells were cultured in DMEM high glucose supplemented with 10% (vol/vol) FBS and 1% Pen/Strep. NK92 cells were cultures in α-MEM supplemented with 10% FBS, 1% Pen/Strep, and 0.1 mM β-mercaptoethanol.
Reagents and Antibodies.
Cell-Mask Deep Red Plasma membrane stain was obtained from Life Technologies.
Anti-TOE1 antibodies used were as follows: Ab-86 is a homemade rabbit polyclonal antibody directed toward the C terminus of TOE1; other TOE1 antibodies used were a mouse monoclonal from Abnova (#00114034) and a rabbit polyclonal from GeneTex (#GTX117545). Anti–β-actin antibody was from SIGMA. α-FLAG agarose resin was from Rockland Immunologicals. Human recombinant GraB and the inhibitor Ac-IETD-CHO were from Enzo Life Science. zVAD-fmk was from EMD Millipore. LipofectAmine 2000 was from Life Technologies and JetPrime was from Polyplus Transfection. Synthetic peptides were purchased from Biomatik.
Luciferase Assays.
293T cells were seeded at 5 × 104 in 24-well plates. The following day, 100 ng DNA were transfected in each well using Lipofectamine 2000. At 16–18 h after transfection, cells were lysed and assayed following injection of 100 μL luciferase assay buffer. Constructs used here were pLTRX-LUC, containing the reporter luciferase gene under the control of the HIV-1 LTR (−644 to +78 fragment from HIV-1 Bru), pCEP4-Tat, and pcDNA3-TOE1 (containing a FLAG tag) and included pcDNA3 (used as carrier DNA). A plasmid encoding the Tat/Rev fusion was kindly provided by Bryan Cullen, Duke University, Durham, NC. The RRE LTR luciferase reporter was constructed by replacing the TAR element in the HIV LTR (kindly provided by Bryan Cullen), with the RRE. The Moloney-MLV LTR luciferase reporter construct was a kind gift from Stephen Goff, Columbia University, New York.
LDH Assay.
The LDH assay was based on the method of Koh and Choi with some modifications; 100 μL culture medium from each sample were assayed as described previously (37). Briefly, 100 μL media from 293T cells transfected with the indicated amount of TOE1 were collected 24 h after transfection, and LDH activity was measured. The medium from cells lysed was used as a positive control for the LDH assay.
In Vitro GraB Cleavage.
One microgram recombinant TOE1 was incubated with 100 nM GraB in the GraB buffer (50 mM Hepes, pH 7.5, 75 mM NaCl, 0.01% CHAPS, and 2 mM DTT) for the indicated times up to 2 h. TOE1 or TOE1 mutants were obtained by immunoprecipitation from 293T-transfected cells with either FLAG-tagged TOE1 or TOE1 carrying point mutations in selected aspartate residues using the transfection reagent JetPrime. The day after transfection, cells were lysed in Nonidet P-40 Lysis buffer (50 mM Hepes, pH 7.5, 250 mM NaCl, 0.1% Nonidet P-40, and 1 mM EDTA), and TOE1 was immunoprecipitated using α-FLAG M2 agarose resin and washed in GraB buffer (−DTT) before reactions. GraB reactions were then performed as above.
Expression and Purification of Recombinant Proteins.
TOE1 and its deletion mutants were subcloned into the pET vector system (Novagen) for bacterial expression including a 6×His tag in the C-terminal end. EGFP was inserted at the C-terminal end of TOE1 in fusion proteins for microscopy. BL21 DE-transformed bacteria were grown for 16 h induced with 0.2 mM isopropyl-beta-d-thiogalactopyranoside (IPTG) for 3 h and then resuspended in PBS + 0.05% Triton X-100 +1 mM PMSF. After two rounds of sonication consisting of eight pulses of 30 s followed by a 30-s pause, Triton X-100 was adjusted to 0.5%, and the lysates were cleared by centrifugation. His-tagged recombinant proteins were purified on a TALON metal affinity resin (Clontech) for transfection and luciferase assay experiments or on a NiNTA affinity resin (Qiagen) for EGFP fusion and microscopy experiments. Fractions of eluates were collected and analyzed by both Coomassie staining and Western blot to assess purity. Fractions highly enriched (>80–90% purity) in TOE1 proteins were collected and dialyzed against PBS. After dialysis, proteins were quick frozen and stored at −80 °C with the addition of 10% glycerol.
Live Cell Confocal Microscopy.
HeLa cells were seeded into Lab-Tek II Chambers Coverglass 1.5 (Nunc; 155409). The day after, cells were incubated with EGFP or TOE1-EGFP fusion recombinant proteins for 1 h at 37 °C. Following incubation, the cells were washed twice with PBS and once with PBS containing 0.25 mg/mL heparin. Cells were incubated with 0.25 μg/mL CellMask Deep Red Plasma membrane stain (Life Technologies; C10046) for 5 min at room temperature and then imaged on a Zeiss LSM 510 Meta confocal microscope.
Primary Cell Isolation and Culture.
All primary cells were isolated from normal healthy donors following approved protocols from the Institutional Bioethics Committee from the Ottawa Hospital Research Institute and Centre Hospitalier Universitaire de Québec, with signed informed consent from all donors. For Western blot and Immunoprecipitation purposes, peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll-Hypaque gradients, and CD8+ T cells were isolated using an autoMACS system and CD8 microbeads (Miltenyi Biotec). Alternatively, CD4+ T cells were isolated using CD4 targeting autoMACS beads. After purification, the cells were cultured in RPMI-1640 supplemented with 20% (vol/vol) FBS and 1% Pen/Strep at 2.5 × 106 cells/mL. The following day, the cells were passed and cultured for 24–72 h with 10% (vol/vol) FBS containing recombinant human IL-2 (100 U/mL; Peprotech). Stimulated samples were cultured with the addition of beads preloaded with α-CD3 and α-CD28 antibodies, whereas control samples were treated with unloaded beads (Miltenyi Biotec). For virus infection studies, CD4+ T cells were purified with EasySep CD4+ T-cell enrichment kit (Stemcell). Cells were then activated with the mitogenic agent PHA-L (1 µg/mL) for 3 d prior to their use and maintained in complete RPMI-1640 culture medium supplemented with recombinant human IL-2 (30 U/mL) at a density of 2 × 106 cells/mL.
HIV-1 Infection Studies.
Fully infectious virions were generated by transient transfection of 293T cells with pNL4-3, whereas luciferase encoding, single-cycle pseudotyped HIV-1 particles were produced by cotransfection of 293T cells with pNL4-3 Luc+E-R+ and pHCMV-G. Virus stocks were normalized for virion content using an in-house sensitive double antibody sandwich ELISA specific for the major core viral p24 protein. Activated CD4+ T cells were inoculated with the luciferase reporter virus NL4-3Luc+Env− pseudotyped with VSVG envelope, or the fully infectious X4-tropic NL4-3 HIV-1, used at 10 ng p24 per 105 cells. Cells were incubated with viruses for 3 h and thoroughly washed and resuspended in complete RPMI + IL-2 at 106/mL in 96-well plates. Recombinant full length TOE1 or derived peptides were then added. Luciferase activity was assessed after 3 d, whereas viral production was quantified after 3 and 6 d by ELISA against the HIV-1 p24 Gag protein. Cell viability was evaluated using the 7-aminoactinomycin D (7-AAD) exclusion test. The effect of TOE1 treatment on cell proliferation was analyzed using the carboxyfluorescein succinimidyl ester (CFSE) dilution assay after a 3-d treatment. Cells were analyzed by flow cytometry, and the proliferation index was calculated using the FCS express 4 software. All experiments were repeated with 5–10 different donors for CD4+ T cells, and each figure combines the results obtained with all of the different donors. The statistical significance of the difference between groups was determined by comparing raw data using a one-way ANOVA with Dunnet multiple comparison test. Data were log-transformed to stabilize variances when necessary. Calculations were made with Prism version 6.00 (GraphPad Software). P < 0.05 was considered statistically significant.
RNA Gel Shift.
Recombinant TOE1 proteins or synthetic peptides were incubated at the indicated concentrations and the following carboxyfluorescein (FAM)- or Cy5-labeled TAR probes: WT TAR, 5′-GGCCAGAUCUGAGCCUGGGAGCUCUCUGGCC-3′, or mutant bulgeless TAR, 5′-GGCCAGAGAGCCUGGGAGCUCUCUGGCC-3′, in a buffer containing 10 mM Tris, pH 8.0/50 mM KCl/2.5 mM MgCl2/1.0 mM DTT/10% glycerol/1 mg/mL BSA/0.1 mg/mL yeast tRNA for 15 min at room temperature. Binding competition was performed using the indicated fold molar excess of a TAR probe equivalent to that described above without the FAM label. TAR/TOE1 complexes were visualized on a BioRad GelDoc+ apparatus with ImageLab software following separation on 6% acrylamide gels.
Capillary Electrophoresis.
Capillary electrophoresis was performed as previously described in ref. 38.
For further details, see SI Text.
Supplementary Material
Acknowledgments
This work was supported by the Canadian Institutes of Health Research Grant HOP-103233 (to M.J.T. and I.d.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7888.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1500857112/-/DCSupplemental.
References
- 1.de Belle I, Wu JX, Sperandio S, Mercola D, Adamson ED. In vivo cloning and characterization of a new growth suppressor protein TOE1 as a direct target gene of Egr1. J Biol Chem. 2003;278(16):14306–14312. doi: 10.1074/jbc.M210502200. [DOI] [PubMed] [Google Scholar]
- 2.Sperandio S, Tardito S, Surzycki A, Latterich M, de Belle I. TOE1 interacts with p53 to modulate its transactivation potential. FEBS Lett. 2009;583(13):2165–2170. doi: 10.1016/j.febslet.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Wagner E, Clement SL, Lykke-Andersen J. An unconventional human Ccr4-Caf1 deadenylase complex in nuclear cajal bodies. Mol Cell Biol. 2007;27(5):1686–1695. doi: 10.1128/MCB.01483-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong KW, et al. Whole-genome screening identifies proteins localized to distinct nuclear bodies. J Cell Biol. 2013;203(1):149–164. doi: 10.1083/jcb.201303145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz LS, Stone MR, Mackewicz CE, Levy JA. Differential gene expression in CD8+ cells exhibiting noncytotoxic anti-HIV activity. Virology. 2003;311(2):400–409. doi: 10.1016/s0042-6822(03)00177-6. [DOI] [PubMed] [Google Scholar]
- 6.Levy JA. The search for the CD8+ cell anti-HIV factor (CAF) Trends Immunol. 2003;24(12):628–632. doi: 10.1016/j.it.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Levy JA, Mackewicz CE, Barker E. Controlling HIV pathogenesis: The role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol Today. 1996;17(5):217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 8.Katz BZ, et al. Differential gene expression of soluble CD8+ T-cell mediated suppression of HIV replication in three older children. J Med Virol. 2011;83(1):24–32. doi: 10.1002/jmv.21933. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Mariño B, Foster H, Hao Y, Levy JA. Differential gene expression in CD8(+) cells from HIV-1-infected subjects showing suppression of HIV replication. Virology. 2007;362(1):217–225. doi: 10.1016/j.virol.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CH, Weinhold KJ, Bartlett JA, Bolognesi DP, Greenberg ML. CD8+ T lymphocyte-mediated inhibition of HIV-1 long terminal repeat transcription: A novel antiviral mechanism. AIDS Res Hum Retroviruses. 1993;9(11):1079–1086. doi: 10.1089/aid.1993.9.1079. [DOI] [PubMed] [Google Scholar]
- 11.Mackewicz CE, Blackbourn DJ, Levy JA. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc Natl Acad Sci USA. 1995;92(6):2308–2312. doi: 10.1073/pnas.92.6.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. International HIV Controllers Study (2010) The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330(6010):1551–1557. [DOI] [PMC free article] [PubMed]
- 13.Pereyra F, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197(4):563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 14.Walker BD, Yu XG. Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol. 2013;13(7):487–498. doi: 10.1038/nri3478. [DOI] [PubMed] [Google Scholar]
- 15.Chang TL, François F, Mosoian A, Klotman ME. CAF-mediated human immunodeficiency virus (HIV) type 1 transcriptional inhibition is distinct from alpha-defensin-1 HIV inhibition. J Virol. 2003;77(12):6777–6784. doi: 10.1128/JVI.77.12.6777-6784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trapani JA. Granzymes: A family of lymphocyte granule serine proteases. Genome Biol. 2001;2(12):S3014. doi: 10.1186/gb-2001-2-12-reviews3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullen SP, Martin SJ. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15(2):251–262. doi: 10.1038/sj.cdd.4402244. [DOI] [PubMed] [Google Scholar]
- 18.Andrade F. Non-cytotoxic antiviral activities of granzymes in the context of the immune antiviral state. Immunol Rev. 2010;235(1):128–146. doi: 10.1111/j.0105-2896.2010.00909.x. [DOI] [PubMed] [Google Scholar]
- 19.Afonina IS, Cullen SP, Martin SJ. Cytotoxic and non-cytotoxic roles of the CTL/NK protease granzyme B. Immunol Rev. 2010;235(1):105–116. doi: 10.1111/j.0105-2896.2010.00908.x. [DOI] [PubMed] [Google Scholar]
- 20.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science. 1999;285(5433):1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 21.Romani B, Engelbrecht S, Glashoff RH. Functions of Tat: The versatile protein of human immunodeficiency virus type 1. J Gen Virol. 2010;91(Pt 1):1–12. doi: 10.1099/vir.0.016303-0. [DOI] [PubMed] [Google Scholar]
- 22.Kuppuswamy M, Subramanian T, Srinivasan A, Chinnadurai G. Multiple functional domains of Tat, the trans-activator of HIV-1, defined by mutational analysis. Nucleic Acids Res. 1989;17(9):3551–3561. doi: 10.1093/nar/17.9.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou M, et al. The Tat/TAR-dependent phosphorylation of RNA polymerase II C-terminal domain stimulates cotranscriptional capping of HIV-1 mRNA. Proc Natl Acad Sci USA. 2003;100(22):12666–12671. doi: 10.1073/pnas.1835726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richard JP, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278(1):585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 25.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 26.Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55(6):1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 27.Roy S, Delling U, Chen CH, Rosen CA, Sonenberg N. A bulge structure in HIV-1 TAR RNA is required for Tat binding and Tat-mediated trans-activation. Genes Dev. 1990;4(8):1365–1373. doi: 10.1101/gad.4.8.1365. [DOI] [PubMed] [Google Scholar]
- 28.Walker CM, Moody DJ, Stites DP, Levy JA. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 29.Quan LT, et al. Proteolytic activation of the cell death protease Yama/CPP32 by granzyme B. Proc Natl Acad Sci USA. 1996;93(5):1972–1976. doi: 10.1073/pnas.93.5.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry M, et al. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol Cell Biol. 2000;20(11):3781–3794. doi: 10.1128/mcb.20.11.3781-3794.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afonina IS, et al. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1α. Mol Cell. 2011;44(2):265–278. doi: 10.1016/j.molcel.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackewicz CE, Craik CS, Levy JA. The CD8+ cell noncytotoxic anti-HIV response can be blocked by protease inhibitors. Proc Natl Acad Sci USA. 2003;100(6):3433–3438. doi: 10.1073/pnas.0630379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackewicz CE, et al. Lack of the CD8+ cell anti-HIV factor in CD8+ cell granules. Blood. 2003;102(1):180–183. doi: 10.1182/blood-2002-10-3034. [DOI] [PubMed] [Google Scholar]
- 34.Mackewicz CE, Lieberman J, Froelich C, Levy JA. HIV virions and HIV infection in vitro are unaffected by human granzymes A and B. AIDS Res Hum Retroviruses. 2000;16(4):367–372. doi: 10.1089/088922200309241. [DOI] [PubMed] [Google Scholar]
- 35.Snyder EL, Dowdy SF. Cell penetrating peptides in drug delivery. Pharm Res. 2004;21(3):389–393. doi: 10.1023/B:PHAM.0000019289.61978.f5. [DOI] [PubMed] [Google Scholar]
- 36.Shi NQ, Qi XR, Xiang B, Zhang Y. A survey on “Trojan Horse” peptides: Opportunities, issues and controlled entry to “Troy”. J Control Release. 2014;194:53–70. doi: 10.1016/j.jconrel.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Sperandio S, et al. Paraptosis: Mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ. 2004;11(10):1066–1075. doi: 10.1038/sj.cdd.4401465. [DOI] [PubMed] [Google Scholar]
- 38.Krylov SN. Nonequilibrium capillary electrophoresis of equilibrium mixtures (NECEEM): A novel method for biomolecular screening. J Biomol Screen. 2006;11(2):115–122. doi: 10.1177/1087057105284339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.