Abstract
Chemical reactivity and stability of highly epitaxial mixed-conductive LaBaCo2O5.5+δ (LBCO) thin films on (001) LaAlO3 (LAO) single-crystalline substrates, fabricated by using pulsed laser deposition system, were systematically investigated. Microstructure studies from x-ray diffraction indicate that the films are c-axis oriented with the interface relationship of [100]LBCO//[100]LAO and (001)LBCO//(001)LAO. LBCO thin films can detect the ethanol vapor concentration as low as 10ppm and the response of LBCO thin film to various ethanol vapor concentrations is very reliable and reproducible with the switch between air and ethanol vapor. Moreover, the fast response of the LBCO thin film, as the p-type gas sensor, is better than some n-type oxide semiconductor thin films and comparable with some nanorods and nanowires. These findings indicate that the LBCO thin films have great potential for the development of gas sensors in reducing/oxidizing environments.
Cobalt-based oxides have attracted scientists and engineers much attention due to their unique magnetic, electrical transport and optical properties, which have been used to design various new concept devices, such as solid state fuel cell, energy harvest, gas sensors, etc1,2,3,4,5,6,7,8,9,10. Among them, the oxygen-deficient double perovskite cobaltates, LaBaCo2O5.5+δ (LBCO), show particularly interesting phenomena with the A-site order, nanoscale order, and disorder structures, such as, different spin state configurations, giant MR effect, etc11,12,13,14,15,16. To under these physics nature, single crystalline LBCO thin films are required. Recently, highly epitaxial single crystalline LBCO thin films were fabricated on various substrates, such as (001) LaAlO3 (LAO)16, (001) SrTiO317,18,19, (001) MgO20,21, and (110) NdGaO322. The LBCO films not only exhibit a much larger magnetoresistance value than those from various phases of its bulk material, but also possess an extraordinary sensitivity to H2 and O2, especially an exceedingly fast redox reaction at high temperature and a superfast oxygen vacancy exchange diffusion chemical dynamics16,23. Therefore, the LBCO thin films are one promising candidate for the development of the gas sensors in reducing/oxidizing environments, which is one of the critical research issues in the public safety and energy security.
Gas-sensing materials using the n-type oxide semiconductor have been intensively studied, such as ZnO24,25, TiO226,27,28, SnO29,30,31, WO332, Fe2O333,34,35, In2O336, etc. However, there are very few reports on the gas-sensing materials using p-type oxide semiconductor, such as NiO37,38,39, CuO40, LaFeO341, Co3O4,42,43,44,45,46,47,48,49,50,51,52,53,54,55 LBCO16,23. Compared with n-type oxide semiconductor for gas-sensing, the p-type oxide semiconductors with distinctive surface reactivity and oxygen adsorption can enhance gas selectivity, decrease the dependence of humidity to negligible level and improve the recovery speed, reviewed by Kim and Lee46. Also, it is a good catalyst to promote selective oxidation of various volatile organic compounds47,48,49,50.
Various oxidation states (Co2+ /Co3+ /Co4+) of cobalt in LBCO thin film make it to be a good candidate as p-type gas sensor. The conductivity is strongly dependent on the valence states, which can change the LBCO from good conductive to insulate. Ideally, when the valence states are all Co+3, the LBCO should be insulate. And when the valence states are mixed with Co+3 and Co+4 or Co+2 and Co+3, the LBCO should be semiconductor or semimetallic, respectively. Since the LBCO films were grown in the pure oxygen of 250 mTorr, the films are good conductive. Moreover, the Co+4 is not as stable as Co+3. Therefore, the valence states should be a mixed state of Co+3 and Co+4. When LBCO thin film is exposed to the reducing gas, parts of Co+4 changed into Co+3 and the resistance increases. In the contrast, the gas change to oxidizing gas, parts of Co+3 changed into Co+4 and the resistance decreases51,52. Ethanol vapor is safer and more active than other gas, such as H2, ammonia, hydrogen sulfide, which is used as the representative of reducing gases. In this paper, we explore here the transient responses of the LBCO thin films to various ethanol vapor concentrations at different operating temperatures. It was found that even though ethanol vapor concentration is as low as 10 ppm, the LBCO thin film still can detect the transient response. Moreover, the response time can be reduced to ~24 s under the ethanol vapor concentration of 400 ppm with the recovery time of only ~10 s at 375 oC. These results indicate that the LBCO thin films have great potential for the development of the gas sensor applications in reducing/oxidizing environments, expect the applications on giant magnetoresistance and cathode for solid oxide fuel cell.
Results and Discussion
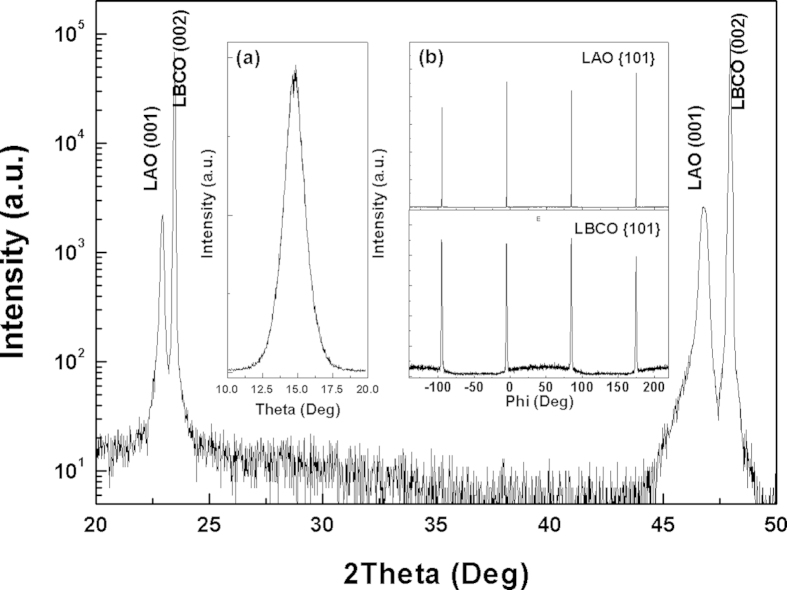
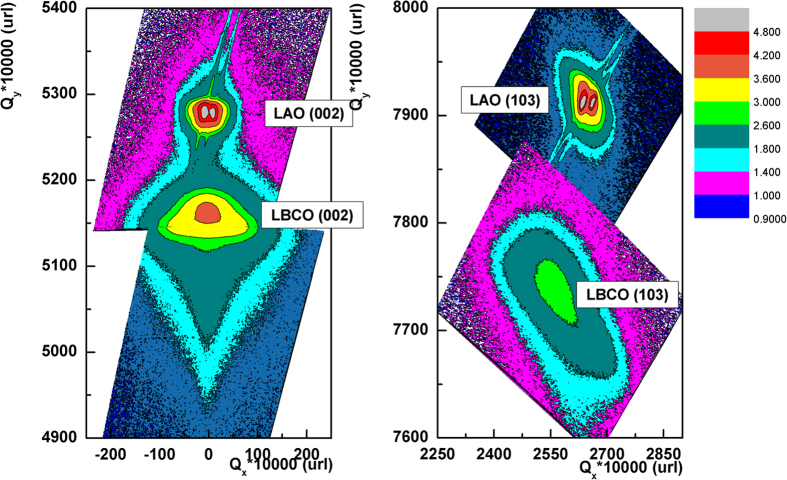
The crystalline quality of the LBCO thin films was characterized by the XRD θ−2θ scan, rocking curve, φ scan and reciprocal space mappings (RSMs). Figure 1 is a typical θ−2θ pattern for the LBCO thin films on (001) LAO substrates showing that only the (00l) peaks can be detected for the LBCO thin films. It is revealed that the films are c-axis oriented or the c-axis normal to the substrate surface. The rocking curve measurements from the (002) reflections for the LBCO films show that the Full Width of Half Maximum (FWHM) is ~0.92°, as shown in the inset (a) of Fig. 1, indicating that the LBCO films on LAO substrates have good crystalline quality. To understand the in-plane interface relationships between the LBCO films and the LAO substrates, the φ scans measurements have been performed. The inset (b) of Fig. 1 is the φ scans taken around from the {101} reflections of the LBCO films and LAO substrates. The four-fold symmetry and sharp peaks in the φ scan suggest that the films have good epitaxial nature. Therefore, the orientation relationships between the thin films and the substrates can be determined to be [100]LBCO//[100]LAO and (001)LBCO//(001)LAO. RSMs is a very effective method to study the microstructure information, such as lattice parameters, defect density, domain structure, etc. in order to obtain the lattice parameters and interface strain of LBCO films on LAO substrates, the RSMs have been performed taken around from the symmetric (002) and asymmetric (103) reflections of the LBCO films and LAO substrates, as shown in the Fig. 2. According to the Bragg law and the angles’ relationship between these crystalline planes, the in-plane (a & b) abd out-of–plane (c) lattice parameters of LBCO films on LAO substrates can be calculated from the RSMs. The in-plane and out-of-plane lattice parameter is 3.90 Å and 3.88 Å, respectively. It is shown that this LBCO film on LAO is full relaxation state.
Figure 1. Typical XRD pattern of the LBCO films grown on (001) LAO substrates.
The inset is (a) rocking curve from the (002) reflection for the LBCO film and (b) the φ scans taken around the {101} diffraction of the LBCO film and LAO substrate.
Figure 2.
Reciprocal space mappings taken around from the symmetric (002) and asymmetric (103) reflections of the LBCO films and LAO substrates.
In order to identify the optimum working temperature for the LBCO gas sensors, the sensing transient response of the LBCO films to 1000 ppm ethanol vapor exposure were investigated as a function of sensor temperature from 250 oC to 450 oC, as shown in the Fig. 3(a). It is clearly seen that the resistance of LBCO thin film increase when it is exposed to the ethanol vapor (reducing gas), then the resistance decrease when the gas change from 1000 ppm ethanol vapor to air, which is in agreement with our previous studies under the gas switch between H2 and O216,23. In fact, the LBCO thin films will generate a high density of oxygen vacancies when it is exposed to the reducing gas53, such as ethanol vapor gas. This chemical processing can be represented by:
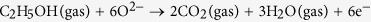 |
Figure 3.
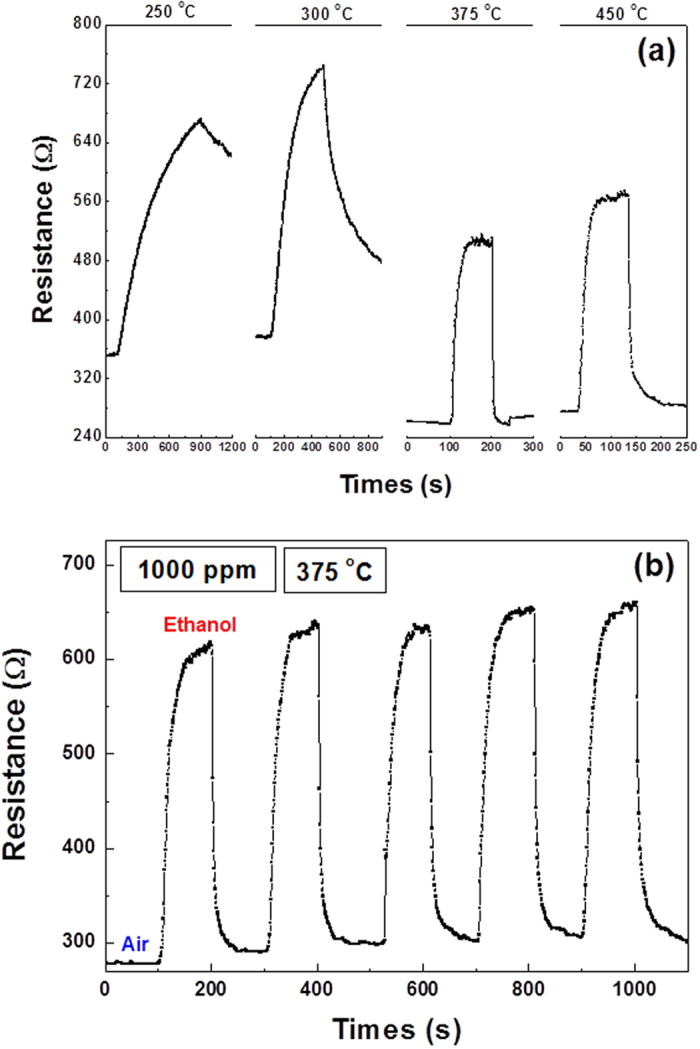
(a) Transient responses at different temperatures from 250 to 450 °C, (b) transient response vs. number of exposure tests during the change of air to 1000 ppm ethanol vapor concentration at 375 oC.
The electrons are released into the LBCO films through the oxidation reactions with ethanol vapor reducing gas. Thus, the increased electronic density will result in the increase of the resistance due to the LBCO films are p-type semiconductors, On the other hand, the resistances of the LBCO thin films will decrease when exposed to oxidizing gas, such as air. The response time from air to 1000 ppm ethanol vapor decrease with the temperature increase. Especially, the response time rapidly drop and become faster when the temperature exceed to 375 oC. Although the LBCO thin films can detect ethanol gas at the temperature of 250 oC and 300 oC, the resistance of LBCO thin film can’t recovery completely in short time when it exposed to air. Thus, the working temperature above 300 °C is better for the excellent performance as ethanol/reducing gas sensor. Meanwhile, the stability of the LBCO thin film as ethanol/reducing gas sensor has been examined, as shown in Fig. 3(b). The response and recovery repeatability of the LBCO thin film is examined under 1000 ppm ethanol vapor at 375 °C. It shows that the resistance change of LBCO thin film can keep its initial value after circle operation, revealing that the LBCO thin film as ethanol/reducing gas sensor have good reproducibility.
To investigate the sensitivity of LBCO thin film as ethanol gas sensor, the transient response or the resistance switching behavior during the change between air and various ethanol vapor concentrations at 375 oC was examined. As shown in the Fig. 4, the LBCO thin films have reliable and reproducible responses during the change between air and various ethanol vapor concentrations. Even at very low concentration of 10 ppm, the LBCO films still can detect transient response. The ratio of resistance switching between air to ethanol vapor is strongly dependent upon the ethanol vapor concentrations, or linearly-like increases with the increase of the ethanol vapor partial pressures from 10 ppm to 5000 ppm, as seen in the inset of Fig. 4. The sensitivity (S) of the LBCO thin film as the ethanol gas sensor increase form 1.04 at 10 ppm to 2.92 at 5000 ppm. The S determined by using the formula S = Rg/Ra, where Ra and Rg are resistance of the gas sensor in air and in testing gas atmosphere, respectively. The sensitivity can be enhanced by replacing the air with oxygen gas.
Figure 4.
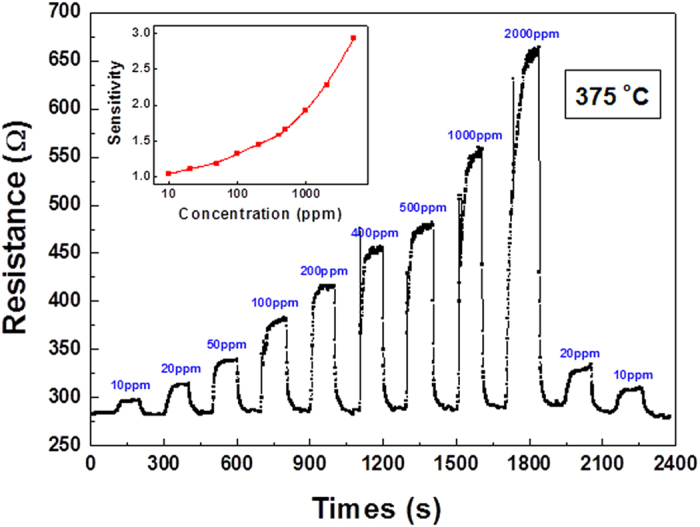
Transient responses and the sensitivity (inset) of the LBCO gas sensors during the change of air to various ethanol vapor concentrations at 375 °C.
It is known that the response and recovery times are the two important quantity factors for the gas sensors, As shown in the inset of Fig. 5, the response time is the time taken by the resistance change from Rair to Rair+[Rgas-Rair]*90%, and the recovery time is the time taken by the resistance change from Rgas to Rgas– [Rgas-Rair]*90%. The response and recovery factors for the LBCO thin films as ethanol gas sensors are shown in Fig. 5 and Table 1. The response time decreases from ~34 s (10 ppm) to ~24 s (400 ppm) with the increase of ethanol vapor concentrations. Then, the response time increases with the increase of the ethanol vapor partial pressures from 500 ppm to 2000 ppm. Therefore, the optimum response time is ~24 s at the concentration of 400 ppm. The reason for the phenomena is still unclear and will be explored in the following work. There is no transition for the recovery time, which decreases from ~29 s (10 ppm) to ~6 s (2000 ppm) with the increase of the ethanol vapor concentration. Compared with some ethanol sensors, epitaxial LBCO thin film exhibits better quality factors. Table 2 gives the as-reported response times of various ethanol sensors at intermediate temperature ranges. Obviously, the response (recovery) time of the LBCO epitaxial thin films is less than that from the n-type gas sensors based on the NiO thin films, SnO2 thin film and TiO2 thick films. Normally, the response of p-type oxide semiconductor gas sensor is slower than n-type semiconductor gas sensor54. Moreover, it is comparable with the ZnO nanorods, CuO microsphere, hollow sea urchin-like structure α-Fe2O3 and various nanostructure Co3O4 listed in the Table 2. Nanorods and nanowires normally have fast response due to the large reactivity area. The comparable fast response in LBCO thin films indicates that epitaxial LBCO thin films will be an good gas sensor for reducing gas, especially for ethanol vapor. The response and recovery time definitely can be improved by adjusting the nanostructure of LBCO, like LBCO nanorods and nanowires. Therefore, LBCO materials have great potential for the development of the application of gas sensors in reducing/oxidizing environments.
Figure 5.
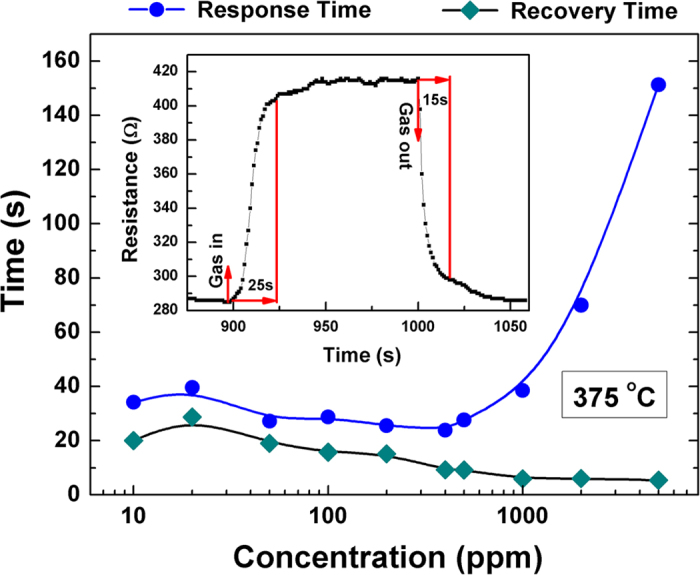
The plot of response time and recovery time of the LBCO gas sensors during the change of air to various ethanol vapor concentrations at 375 °C, the inset shows the definition of the response and recovery times.
Table 1. Sensitivity, response time and recovery time for the LBCO film gas sensors under during the change of air to various ethanol vapor concentrations at 375 oC.
| Ethanol vapor concentrations(ppm) | Sensitivity | Response time(s) | Recovery time(s) |
|---|---|---|---|
| 10 | 1.04 | 34 | 20 |
| 20 | 1.11 | 40 | 29 |
| 50 | 1.19 | 27 | 19 |
| 100 | 1.33 | 29 | 16 |
| 200 | 1.45 | 26 | 15 |
| 400 | 1.58 | 24 | 9 |
| 500 | 1.65 | 28 | 9 |
| 1000 | 1.92 | 38 | 6 |
| 2000 | 2.28 | 70 | 6 |
| 5000 | 2.92 | 151 | 5.5 |
Table 2. Comparison of Various Oxide Semiconductors in Ethanol Vapor Sensing.
| Materials | Ethanol Concentration(ppm) | Working Temperature(oC) | Response Time(s) | Recovery Time(s) | Reference |
|---|---|---|---|---|---|
| LBCO Thin Films | 100 | 375 | 29 | 16 | This paper |
| Co3O4 Nanofibers | 100 | 336 | 22.7 | 2.4 | 43 |
| Co3O4: Nanosheets, Nanorods, Nanocubes | 100 | 400 | 66, 29, 49 | 10–13 | 45 |
| α-Fe2O3 Hollow Sea Urchin-like Structures | 21–2100 | 350 | 7–21 | 11–14 | 35 |
| CuO Microsphere | 200 | 400 | 9–17 | 4–17 | 40 |
| NiO: Thin Films, Hollow Hemispheres | 100 | 300 | 62, 49 | 47, 42 | 39 |
| ZnO Nanorods | 100 | 370 | 22 | 27 | 25 |
| TiO2 Thick Films | 300 | 350 | 128 | 348 | 28 |
| SnO2 Thin Film | 1250 | 350 | 42 | 88 | 31 |
In summary, the sensing transient response of the epitaxial LBCO thin films during the changes of air to various ethanol vapor exposure have been investigated in the temperature range of 250 oC to 450 oC. It is shown that the LBCO thin films have reliable and reproducible responses to ethanol vapor, even at very low concentration of 10 ppm. The optimum response time is ~24 s under the ethanol vapor concentration of 400 ppm with the recovery time of ~10 s at 375 oC. The performance of epitaxial LBCO thin film as the ethanol vapor sensor is better than that of some n-type oxide semiconductor thin films and comparable to that of the nanorods and nanowires structure. It is revealing that the LBCO thin films can be a promising candidate as gas sensor in reducing/oxidizing environments.
Methods
Film preparation
A KrF excimer pulsed laser deposition system with a wavelength 248 nm was used to fabricate the LBCO thin films on (001) LAO single-crystalline substrates. Details of the processing conditions can be found in the related literatures17. Briefly, the films were grown under an oxygen pressure of 250 mTorr at 700 °C with the laser energy density of about 2.0 J /cm2 at 10 Hz. The typical film thickness is about ~200 nm with the growth rate of ~13.3 nm/min. After growth the films were annealed at 700 oC for 15 min in pure oxygen (200 Torr) and then cooled down to room temperature at the rate of 30 oC/min.
Structural and electrical characterization
The crystalline quality and epitaxial behavior of the LBCO thin films were characterized by high-resolution x-ray diffraction (HRXRD) using PANalytical X’Per MRD. The LBCO thin films have been used for the gas sensing measurements. The ethanol sensing properties of the LBCO thin films were systematically studied using the GS-1TP intelligent gas sensing analysis system (Beijing Elite Tech. Co. Ltd., China)55. This analysis system consists of several parts: a heating system, gas distribution system, probe system, vacuum system, measurement system and related control software. The samples can be heated up to 500 °C with a precision of 1 °C. In order to obtain the accurate data, all the samples will be preheated at the working temperature for ~20 min. Once the LBCO gas sensors were stable, the ethanol gas will be injected into the test chamber. After the resistance of the LBCO gas sensor reaches a new constant value, we will open the test chamber to recover LBCO sensor into the air atmosphere.
Additional Information
How to cite this article: Liu, M. et al. Gas Sensing Properties of Epitaxial LaBaCo2O5.5+δ Thin Films. Sci. Rep. 5, 10784; doi: 10.1038/srep10784 (2015).
Acknowledgments
This research was supported by the Natural Science Foundation of China (Nos. 51202185, 51390472, 51471169 and 11028409), the Fundamental Research Funds for the Central University, the National Science Foundation under NSF-NIRT-0709293, the U.S. Department of Energy under DE-FE0003780, and the State of Texas through the Texas Center for Superconductivity at the University of Houston. Moreover, M. Y. wants to acknowledge the Foundation of Shaanxi Educational Committee (14JK1331). The ethanol sensing properties of the LBCO thin films were measured using the GS-1TP intelligent gas sensing analysis system at Beijing Elite Tech. Co. Ltd., China, the authors also thank Ms. Dongmei Xu and Dr. Qi Qi for their help in gas-sensing property measurements.
Footnotes
The authors declare no competing financial interests.
Author Contributions M.L. and C.C. designed and setup the research, M.L. and S.R. prepared film samples, M.L., S.R. and R.Z. performed the structural characterization by HRXRD, C.M., Z.X., X.X., M.Y. and S.B. performed the electrical characterization. M.L., C.M. and C.C. analyzed the data and co-wrote the paper. All contributed discussion.
References
- Jacobson A. J. Materials for Solid Oxide Fuel Cells. Chem. Mater. 22, 660–674 (2010). [Google Scholar]
- DeSouza R. A. & Kilner J. A. Oxygen Transport in La1-xSrxMn1-yCoyO3-δ Perovskites - Part I. Oxygen Tracer Diffusion. Solid State Ion. 106, 175–187 (1998). [Google Scholar]
- Post M. L. et al. Material Chemistry of Perovskite Compounds as Chemical Sensors. Sens. Actuators B: Chem. 59, 190–194 (1999). [Google Scholar]
- Wang S. et al. The Effect of the Magnitude of the Oxygen Partial Pressure Change in Electrical Conductivity Relaxation Measurements: Oxygen Transport Kinetics in La0.5Sr0.5CoO3-δ Solid State Ion. 140, 125–131 (2001). [Google Scholar]
- Luo G. P. et al. Electrical and Magnetic Properties of La0.5Sr0.5CoO3 Thin Films. Appl. Phys. Lett. 76, 1908–1910 (2000). [Google Scholar]
- Taskin A. A. et al. Achieving Fast Oxygen Diffusion in Perovskites by Cation Ordering. Appl. Phys. Lett. 86, 091910 (2005). [Google Scholar]
- Kim G. et al. Oxygen Exchange Kinetics of Epitaxial PrBaCo2O5+δ Thin Films. Appl. Phys. Lett. 88, 024103 (2006). [Google Scholar]
- Kim G. et al. Rapid Oxygen Ion Diffusion and Surface Exchange Kinetics in PrBaCo2O5+x with A Perovskite Related Structure and Ordered A Cations. J. Mater. Chem. 17, 2500–2505 (2007). [Google Scholar]
- Yuan Z. et al. Epitaxial Behavior and Transport Properties of PrBaCo2O5 Thin Films on (001) SrTiO3. Appl. Phys. Lett. 90, 212111 (2007). [Google Scholar]
- Fauth F. et al. Interplay of Structural, Magnetic and Transport Properties in The Layered Co-based Perovskite LnBaCo2O5 (Ln = Tb, Dy, Ho). Eur. Phys. J. B 21, 163–174 (2001). [Google Scholar]
- Fauth F. et al. Intermediate Spin State of Co3+ and Co4+ Ions in La0.5Ba0.5CoO3 Evidenced by Jahn-Teller Distortions. Phys. Rev. B 65, 060401/1–4 (2002). [Google Scholar]
- Nakajima T. et al. New A-site Ordered Perovskite Cobaltite LaBaCo2O6: Synthesis, Structure, Physical Property and Cation Order-Disorder Effect. J. Phys. Soc. Jpn. 74, 1572–1577 (2005). [Google Scholar]
- Kundu A. K. et al. Spin-Locking Effect in The Nanoscale Ordered Perovskite Cobaltite LaBaCo2O6. Phys. Rev. B 76, 184432 (2007). [Google Scholar]
- Rautama E. L. et al. Cationic Ordering and Microstructural Effects in The Ferromagnetic Perovskite La0.5Ba0.5CoO3: Impact Upon Magnetotransport Properties. Chem. Mater. 20, 2742–2750 (2008). [Google Scholar]
- Rautama E. L. et al. New Member of the “112” Family, LaBaCo2O5.5: Synthesis, Structure, and Magnetism. Chem. Mater. 21, 102–109 (2009). [Google Scholar]
- Liu J. et al. Epitaxial Nature and Transport Properties in (LaBa)Co2O5+δ Thin Films. Chem. Mater. 22, 799–802 (2010). [Google Scholar]
- Liu M. et al. Magnetic and Transport Properties of Epitaxial (LaBa)Co2O5.5+δ Thin Films on (001) SrTiO3. Appl. Phys. Lett. 96, 132106 (2010). [Google Scholar]
- Ma C. R. et al. M. H. Magnetic and Electrical Transport Properties of LaBaCo2O5.5+δ Thin Films on Vicinal (001) SrTiO3 Surfaces. ACS Appl. Mater. Interfaces 5, 451–455 (2013). [DOI] [PubMed] [Google Scholar]
- Zou Q. et al. Step Terrace Tuned Anisotropic Transport Properties of Highly Epitaxial LaBaCo2O5.5+δ Thin Films on Vicinal SrTiO3 Substrates. ACS Appl. Mater. Interfaces 6, 6704–6708 (2014). [DOI] [PubMed] [Google Scholar]
- Liu M. et al. Giant Magnetoresistance and Anomalous Magnetic Properties of Highly Epitaxial Ferromagnetic (LaBa)Co2O5.5+δ Thin Films on (001) MgO. ACS Appl. Mater. Interfaces 4, 5524–5528 (2012). [DOI] [PubMed] [Google Scholar]
- Ma C. R. et al. Interface Effects on the Electronic Transport Properties in Highly Epitaxial LaBaCo2O5.5+δ Films. ACS Appl. Mater. Interfaces 6, 2540–2545 (2014). [DOI] [PubMed] [Google Scholar]
- Liu M. et al. Strain-Induced Anisotropic Transport Properties of (LaBa)Co2O5+δ Thin Films on NdGaO3 Substrates. ACS Appl. Mater. Interfaces 6, 8526–8530 (2014). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. PO2 Dependant Resistance Switch Effect in Highly Epitaxial (LaBa)Co2O5+δ Thin Films. Appl. Phys. Lett. 97, 094101 (2010). [Google Scholar]
- Tarwal N. L. et al. A Selective Ethanol Gas Sensor Based on Spray-Derived Ag-ZnO Thin Films. J. Mater. Sci. 48, 7274–7282 (2013). [Google Scholar]
- Yin M. L. et al. Development of an Alcohol Sensor Based on ZnO Nanorods Synthesized Using a Scalable Solvothermal Method. Sens. Actuators B: Chem. 185, 735–742 (2013). [Google Scholar]
- Nisar J. et al. TiO2-Based Gas Sensor: A Possible Application to SO2. ACS Appl. Mater. Interfaces 5, 8516–8522 (2013). [DOI] [PubMed] [Google Scholar]
- Hu P. G. et al. Enhancement of Ethanol Vapor Sensing of TiO2 Nanobelts by Surface Engineering. ACS Appl. Mater. Interfaces 11, 3263–3269 (2010). [DOI] [PubMed] [Google Scholar]
- Cheng X. L. et al. Ag Nanoparticles Modified TiO2 Spherical Heterostructures with Enhanced Gas-Sensing Performance. Sens. Actuators B: Chem. 155, 716–722 (2011). [Google Scholar]
- Yuasa M. et al. Preparation of a Stable Sol Suspension of Pd-Loaded SnO2 Nanocrystals by a Photochemical Deposition Method for Highly Sensitive Semiconductor Gas Sensors. ACS Appl. Mater. Interfaces 4, 4231–4236 (2012). [DOI] [PubMed] [Google Scholar]
- Li X. B. et al. Controllable Low-Temperature Chemical Vapor Deposition Growth and Morphology Dependent Field Emission Property of SnO2 Nanocone Arrays with Different Morphologies. ACS Appl. Mater. Interfaces 5, 3033–3041 (2013). [DOI] [PubMed] [Google Scholar]
- Basu S. et al. Fast Response Time Alcohol Gas Sensor Using Nanocrystalline F-Doped SnO2 Films Derived via Sol-Gel Method. Bull. Mater. Sci. 36, 521–533 (2013). [Google Scholar]
- Moon H. G. et al. Extremely Sensitive and Selective NO Probe Based on Villi-like WO3 Nanostructures for Application to Exhaled Breath Analyzers. ACS Appl. Mater. Interface 5, 10591–10596 (2013). [DOI] [PubMed] [Google Scholar]
- Wang L. L. et al. Three-Dimensional Hierarchical Flowerlike α-Fe2O3 Nanostructure: Synthesis and Ethanol-Sensing Properties. ACS Appl. Mater. Interfaces 3, 4689–4694 (2011). [DOI] [PubMed] [Google Scholar]
- Lou Z. et al. Branch-like Hierarchical Heterostructure (α-Fe2O3/TiO2): A Novel Sensing Material for Trimethylamine Gas Sensor. ACS Appl. Mater. Interfaces 5, 12310–12316 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang F. H. et al. Controlled Synthesis and Gas-Sensing Properties of Hollow Sea Urchin-like α-Fe2O3 nanostructures andα-Fe2O3 nanocubes. Sens. Actuators B: Chem. 141, 381–389 (2009). [Google Scholar]
- Singh N. et al. Gold-Nanoparticle-Functionalized In2O3 Nanowires as CO Gas Sensors with a Significant Enhancement in Response. ACS Appl. Mater. Interfaces 3, 2246–2252 (2011). [DOI] [PubMed] [Google Scholar]
- Fu J. C. et al. Enhance Gas Sensing Performance of Electrospun Pt-Functionalized NiO Nanotubes with Chemical and Electronic Sensitization. ACS Appl. Mater. Interfaces 5, 7410–7416 (2013). [DOI] [PubMed] [Google Scholar]
- Zhu G. X. et al. Platelet-like Nickle Hydroxide: Synthesis and the Transferring to Nickel Oxide as A Gas Sensor. Sens. Actuators B: Chem. 412, 100–106 (2013). [DOI] [PubMed] [Google Scholar]
- Cho N. G. et al. Gas Sensing Properties of p-type Hollow NiO Hemispheres Prepared by Polymeric Colloidal Templating Method. Sens. Actuators B: Chem. 155, 366–371 (2011). [Google Scholar]
- Zhu G. X. et al. Facile Fabrication and Enhanced Sensing Properties of Hierarchically Porous CuO Architectures. ACS Appl. Mater. Interfaces 4, 744–751 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang Y. M. et al. Improvement of Response to Formaldehyde st Ag-LaFeO3 Based Gas Sensors Through Incorporation of SWCNTs. Sens. Actuators B: Chem. 195, 509–514 (2014). [Google Scholar]
- Lv Y. Y. et al. MOF-Templated Synthesis of Porous Co3O4 Concave Nanocubes with High Specific Surface Area and Their Gas Sensing Properties. ACS Appl. Mater. Interfaces 6, 4186–4195 (2014). [DOI] [PubMed] [Google Scholar]
- Yoon J.–W. et al. Design of a Highly Sensitive and Selective C2H5OH Sensor Using p-Type Co3O4 Nanofiber. Sens. Actuators B: Chem. 161, 570–577 (2012). [Google Scholar]
- Wen Z. et al. Rhombus-Shaped Co3O4 Nanorod Arrays for High-Performance Gas Sensor. Sens. Actuators B: Chem. 186, 172–179 (2013). [Google Scholar]
- Choi K. I. et al. C2H5OH Sensing Characteristics of Various Co3O4 Nanostructures Prepared by Solvothermal. Sens. Actuators B: Chem. 146, 183–189 (2010). [Google Scholar]
- Kim H. J. & Lee J. H. Highly Sensitive and Selective Gas Sensors Using p-type Oxide Semiconductors: Overreview. Sens. Actuators B: Chem. 192, 607–627 (2014). [Google Scholar]
- Pirkanniemi K. & Sillanpää M. Heterogeneous water phase catalysis as an environmental application: a review. Chemosphere, 48, 1047–1060 (2002). [DOI] [PubMed] [Google Scholar]
- Bettahar M. M. et al. On the partial oxidation of propane and propylene on mixed metal oxide catalysts. Appl.Catal. A: Gen. 145, 1–48 (1996). [Google Scholar]
- Motooka Y. & Ozaki A. Regularities in catalytic properties of metal oxides in propylene oxidation. J. Catal. 5, 116–124 (1966). [Google Scholar]
- Kaye S. S. & Long J. R. Hydrogen storage in the dehydrated Prussian blue analogues M-3[Co(CN)(6)](2) (M = Mn, Fe, Co, Ni, Cu, Zn). J. Am. Chem. Soc. 127, 6506–6507 (2005). [DOI] [PubMed] [Google Scholar]
- Bao S. B. et al. Ultrafast Atomic Layer-by-Layer Oxygen Vacancy-Exchange Diffusion in Double-Perovskite LnBaCo2O5.5+δ Thin Films. Sci. Rep. 4, 4726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. B. et al. Ultrafast Chemical Dynamic Behavior in Highly Epitaxial LaBaCo2O5+δ Thin Films. J.Mater. Chem. C 2, 5660–5666 (2014). [Google Scholar]
- Liu M. et al. Effects of Annealing Ambient on Electrical Properties of LaBaCo2O5.5+δ Thin Films. J. Nano Research 27, 25–30 (2014). [Google Scholar]
- Hübner M. & Simion C. E. Influence of Humidity on CO Sensing with p-type CuO Thick Film Gas Sensors. Sens. Actuators B: Chem. 153, 347–353 (2011). [Google Scholar]
- Guo Y. et al. Fabrication of (Calcein-ZnS)(n) Ordered Ultrathin Films on the Basis of Layered Double Hydroxide and Its Ethanol Sensing Behavior. Ind. Eng. Chem. Res. 51, 8966–8973 (2012). [Google Scholar]