Abstract
We compared hospital encounters between adolescents and young adults with fragile X syndrome (FXS) to peers with intellectual disability (ID) from other causes, autism spectrum disorder (ASD), and a comparison group without these conditions matched by gender, age, and insurance coverage. Those with FXS, ASD, or ID were more likely to have had hospital encounters. In terms of age groups, we found mental illness hospitalizations decreased during adulthood as compared to adolescence for those with FXS, and we found that for conditions unrelated to FXS (e.g., respiratory, genitourinary, gastroenteritis, and pneumonia) adolescents had higher rates of hospitalization compared to their peers with FXS, ID, or ASD. We analyzed epilepsy, common among people with FXS and designated as an ambulatory care sensitive condition that can be treated outside the hospital, and found that people with FXS, ID, and ASD had higher odds of hospitalization due to epilepsy in both age groups than did the comparison group.
Keywords: intellectual disability, fragile X syndrome, adolescents, health care, health status
Introduction
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability (ID), with a reported prevalence of 1/3600–4000 males and 1/4000–6000 females (Coffee et al., 2009; McLennan, Polussa, Tassone, & Hagerman, 2011; Tarleton & Saul, 1993; Turner, Webb, Wake, & Robinson, 1996). In general, males are more severely affected by this disorder than females. Most males with FXS have mild to moderate ID, while only about one-third of affected females have ID (Belmonte & Bourgeron, 2006; Hagerman & Hagerman, 2002; Loesch, Huggins, & Hagerman, 2004). The health care needs of children, but not necessarily adults, with FXS have been described in the research literature, and guidelines have been established for evaluation, monitoring, and treatment. The guidelines emphasize features of FXS, including hypotonia and hypermobile joints, scoliosis, pes planus, inguinal hernias, recurrent otitis media and sinusitis, gastro-esophageal reflux, strabismus, refraction errors, sleep disturbances, macro-orchidism, cardiac murmur, behavioral problems, and epilepsy (Hagerman et al., 2009; Hersh et al., 2011). The literature also contains documentation of self-reported health conditions from nonrepresentative samples of the adult population with FXS (Bailey et al., 2008; Berry-Kravis et al., 2010; Hartley et al., 2011; Raspa, Bailey, Bishop, Holiday, & Olmsted, 2010). Other studies have used surveys (Berry-Kravis et al., 2010; Kronk et al., 2010) or clinical record review (Berry-Kravis, 2002; Musumeci et al., 1999) to confirm comorbid conditions. Since studies that rely on self-reporting and clinical record review can be biased, we conducted a literature search for studies that examined comorbid conditions and used community or large insurance plans for data. We only found a Finnish study that reported no difference in overall cancer prevalence between 302 persons with FXS and the rest of the population during a 21-year follow-up period (Sund, Pukkala, & Patja, 2009).
FXS is one cause of ID and it is unknown whether the health care utilization of people with FXS differs from that of people in the broader category of ID of any cause. In addition, although some individuals might have both FXS and autism spectrum disorder (ASD), some individuals with FXS may be misdiagnosed as having ASD when delays in development are coupled with behavioral issues. The positive diagnosis of FXS relies on a DNA test that became available over 20 years ago (Garber, Visootsak, & Warren, 2008). In South Carolina, where the current study was conducted, it has been the policy of the South Carolina Department of Disabilities and Special Needs (DDSN) to offer free genetic testing to anyone who receives services from the agency for ID or ASD to identify the cause of each individual’s condition. For those individuals who consented to the DDSN-sponsored genetic testing, we were able to confirm whether they were diagnosed with the full mutation FXS; however, it is likely that some people with FXS received DDSN services for ID but did not elect to be tested, and others with FXS did not receive DDSN services.
The health status of people with ID and/or ASD is affected by comorbid conditions associated with their primary disability, the social determinants of health, and the health care system. The transition from pediatric to adult health care is one area of concern and the issues include reported lapses in care between the last pediatric visit and the first visit with an adult health care provider, less guidance with health care decisions from providers upon entering the adult system, reduced access to physicians, longer waiting periods between appointments, and reduced coordination between health care professionals (American Academy of Pediatrics, 2002; Blum, 2002; Geenen, Powers, & Sells, 2003; Peter, Forke, Ginsburg, & Schwartz, 2009; Reiss and Gibson, 2002). The transition has not been described for individuals with FXS, but a consensus statement of the American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, and American Society of Internal Medicine, supports the policy of having a written health care transition plan in place for all adolescents with special health care needs by the age of 14 years (American Academy of Pediatrics, 2002). In general, the transition from pediatric to adult care for all people is determined on an individual basis, but typically occurs between the ages of 18 and 21 (American Academy of Pediatrics, 2011).
The literature on the health and illness experience of adolescents and young adults with FXS is limited and does not address emergency department (ED) visits and inpatient hospitalizations (IPH), the two types of hospital encounters. The purpose of this study is to describe the patterns of hospital encounters among adolescents and young adults with FXS using a population-based approach. FXS is a specific cause of ID so the comparison of hospital encounters is between adolescents and young adults with FXS and the larger independent age-specific groups of people with ID (and no FXS or ASD), those with ASD and no ID, and a matched comparison group with none of these conditions. Any IPH that originated through the ED was categorized as an IPH to avoid double counting. We addressed four hypotheses:
Adolescents and young adults with FXS, ASD, or ID have a higher probability of encounters with EDs and IPH services when compared to a matched group of adolescents and young adults without these conditions.
Young adults with FXS have a higher probability of hospital encounters than adolescents with FXS.
Compared to a matched group of unaffected individuals, hospital encounters are more likely in the adolescent and young adult FXS groups for conditions previously reported to be more prevalent among people with FXS (e.g., seizures and epilepsy; mental illness conditions; neurologic conditions; and ear, nose, and throat conditions), though age modifies the difference in the frequency of these encounters.
There are no differences in the probabilities of hospital encounters between individuals with FXS and a comparison group of unaffected individuals for ambulatory care sensitive conditions (ACSCs) that are not associated with FXS (e.g., bacterial pneumonia, gastroenteritis, genitourinary system conditions, and respiratory conditions).
Methods
We used a statewide population-based cross-sectional design to make comparisons about hospital encounters between adolescents and young adults with FXS, ASD, or ID and a group without these conditions matched by gender, age, and length of insurance coverage. Matching was used to select a comparison group for three reasons: (1) there are differences in health care experience by gender and FXS is predominately a male condition, (2) the study was designed to document the transition from pediatric to adult medical care and the typical age of transfer is 18 years, and (3) insurance coverage influences care patterns. Four independent groups of adolescents and young adults were identified as follows: (1) FXS (International Classification of Diseases, 9th edition [ICD9] code 759.83), (2) ASD (ICD9 code 299), (3) other causes of ID (ICD9 codes 317–319), and (4) a matched group free of these conditions.
We compared the probability of hospital encounters related to four broad disease categories and four selected ACSCs (Ansari, Laditka, & Laditka, 2006). The four broad categories included two that were associated with FXS—all mental illness (International Classification of Diseases, 9th edition-Clinical Modification [ICD9CM] code 290–319) and all neurological conditions (ICD9CM code 320–389)—as well as two that were not associated with FXS: all respiratory conditions (ICD9CM code 460–519) and all genitourinary conditions (ICD9CM code 580–629). ACSCs are defined as conditions that can be managed effectively in the ambulatory setting; therefore, ED visits or IPHs due to these conditions may often be attributed to inadequate primary care. The Agency for Healthcare Research and Quality (AHRQ) has developed a list of ACSCs, with the acknowledgement that other factors outside the direct control of the health care system, such as lack of patient adherence to recommendations and environmental conditions, can result in hospitalization (AHRQ, 2004). The ACSCs that we investigated included epilepsy (ICD9 code 345 and 780.3) and conditions of the ear, nose and throat (ICD9 codes 382, 462, 463, 465, 472.1), because they are more common in people with FXS (Bailey et al., 2008; Hersh et al., 2011). We also included the ACSCs of gastroenteritis (ICD9 code 558.9), and pneumonia (ICD9 codes 481, 482.2, 482.3, 482.9, 483, 485, 486), which are not specifically linked to FXS. Overall, if adolescents and young adults with FXS do not differ from their matched gender, age, and similarly insured peers on hospital encounters related to ACSCs, then their outpatient primary care and their medical management would be viewed as being as satisfactory as that of the general population. Also, if the rate of ED or IPH visits for ACSCs increases between the 15–19 years age group and that of the 20–24 years age group, then the transition from pediatric to adult care for individuals with FXS would be the suspected cause.
The statewide data used in this study were housed at the South Carolina Budget and Control Board’s Division for Research and Statistics (DRS), the central repository for the state’s health and human services data. Health encounter data included two insurance sources: Medicaid and the State Health Plan. Linkages to the South Carolina Department of Social Services (DSS) and the South Carolina Department of Education (SDE) provided additional data elements. Through a series of statutes and agreements, agencies and organizations entrust their data systems to the DRS while retaining control of their information at all times. To “link across” data sources from multiple providers, the DRS developed a series of algorithms using source-specific personal identifiers to create global unique identifiers. Using global identifiers in lieu of personal identifiers enables staff to protect confidentiality. Data usage and linkage approvals for the project were obtained from participating providers from whom the data originated. This study is part of a larger project investigating the transition from pediatric to adult services for adolescents and young adults with rare conditions. The methods used to merge data across sources for the larger project are described in detail in a methods paper (Royer et al., 2014). For this study, we included people who were enrolled for all 12 months of each year between 2000 and 2010; often the same individuals were included in multiple years as long as they were between the ages of 15 and 24 years during this period. We used age as a dichotomous variable: adolescents (15–19 years) and young adults (20–24 years), as the study was designed to analyze the transition from pediatric to adult medical care.
Case Identification
People with FXS were identified by searching for ICD9 code 759.83 from a variety of electronic sources. We first identified FXS using confirmatory DNA analysis at the laboratory that identifies most cases of FXS in South Carolina. These data were provided through DDSN and were sent to DRS with a flag indicating a positive result. The second approach to identifying FXS involved the search for the ICD9 code in multiple data sources among individuals who were 15–24 years old during the time period 2000–2010, inclusive. When it was available, we searched administrative data for diagnostic codes when people were younger. This was done for all 15–24-year-olds with ID or ASD to determine if they had been given an ICD9 code for FXS at younger ages. Thus, we were able to ascertain FXS from multiple sources using a number of probing strategies. We required two separate instances of FXS coding to identify a case as FXS.
Comparison Groups Identification
To select the two comparison groups of individuals with ASD or ID without FXS, we first searched for the ICD9 codes for these conditions in the Medicaid, State Health Plan, and Hospital Discharge Dataset (HDDS) files. Due to the fact that many physicians and other health care providers often code for the presenting diagnosis and not for any underlying disabilities, we also searched previous years in our data sources to identify whether people had been coded for ASD or ID earlier in life. Again, we required coding in two separate instances to classify someone with ID or ASD, and we excluded those individuals who were classified as FXS on two or more occasions, regardless of whether they had ever also been coded as having ID and/or ASD. (These excluded individuals were included in the FXS case group.) For the remainder of the group, we classified those who were coded with both ID and ASD as having ASD, and the rest as having ID.
We obtained the third comparison group from the State Health Plan exclusively, to ensure that members of that group did not have any of the three conditions. The State Health Plan dataset includes families in which at least one person is working for a local or state agency or organization. It does not include information on race; thus, we coded the race variable as missing for this comparison group. We selected two individuals without a disability from the State Health Plan dataset for each individual with a disability (FXS, ID, or ASD) identified in all three datasets, and matched all groups by age, gender, and years of insurance coverage.
Independent Variables and Model Building
The variables considered from the Medicaid and State Health Plan datasets included the ICD9CM primary diagnosis code or the main reason for the ED visit or IPH, as recorded by hospital coders; age group; gender; race (if available); and county of residence type (dichotomized as urban or rural based on U.S. Census rural-urban commuting area codes). A linkage with the SDE provided data on most recent grade level attained and codes for being placed in special education services. These codes were assigned according to the Education Finance Act (EFA) and include the following categories: Educable Mentally Handicapped, Trainable Mentally Handicapped, Profound Mentally Handicapped, Learning Disability (which includes Developmentally Delayed and Other Health Impaired), Orthopedically Handicapped (which includes Traumatic Brain Injury), ASD, Emotionally Handicapped, Hearing Handicapped, and Visually Handicapped. Finally, we obtained data on supplemental nutrition assistance (food stamps) from DSS, which we used as a proxy for socioeconomic status.
For some individuals studied, there were multiple observations at different ages. Assuming that observations from the same person were more alike than observations from different people, we used generalized estimating equation (GEE) models to compare the affected groups (FXS, ASD, ID) among themselves and to an unaffected comparison group (Hardin & Hilbe, 2012; Liang & Zeger, 1986). Logistic GEE models allow analysis of relationships between repeated binary outcome models in which, in addition to the main effects of the three conditions (FXS, ASD, ID), we included the potential confounders of age group (15–19 and 20–24 years); race (Caucasian, African American, Other); gender (male, female); food stamps enrollment (yes, no); grade 12 attainment or higher (yes, no); placed in special education (yes, no); and county type (rural, urban).
Because our interest was to test whether the transition from adolescence to young adulthood had any influence on the probability of hospital encounters between condition and comparison groups, we added terms to the model for the interaction of each condition with each age group. If any interaction term was statistically significant, the key results for conditions were reported and stratified by age. Results from our models estimated the odds of having a hospital encounter given that the person has the condition versus the odds of having a hospital encounter given that the person does not have the condition (odds ratio), after adjusting for potential confounders. The adjusted odds ratio was then converted into the probability of having a hospital encounter for people with and without the condition. We also used the fitted GEE model to estimate adjusted probabilities of hospital encounters for each group, controlling for the same confounders as mentioned above. Because the length of enrollment in the insurance plans was variable for different individuals, we used person-years of enrollment to standardize the rates of hospital encounters to a common denominator. We used Statistical Analysis System (SAS) 9.3 in all the analyses.
Results
For the period of 2000 to 2010, we selected individuals aged 15 through 24 years, with at least 1 year of eligibility for Medicaid or State Health Plan insurance, and we identified 125 individuals with FXS, 2,592 individuals with ASD, 10,685 with ID, and a comparison group (2 to 1 match) of 26,804 peers unaffected by any of these conditions. Forty-one individuals with FXS (32.8%) were confirmed by DNA testing. Table 1 shows the basic characteristics of these four groups. The gender-specific prevalence for FXS in our sample was 8.8 per 10,000 males and 1.9 per 10,000 females, aged 15–24 years in the Medicaid or State Health Plan databases with at least 1 year of eligibility. Extrapolating from the 2005 South Carolina population (United States Census Bureau, 2005), the gender-specific prevalence in the state was 2.8 per 10,000 males and 1.2 per 10,000 females, aged 15–24 years.
Table 1.
Distribution of the Number of Adolescents and Young Adults From South Carolina According to Condition and Several Demographic and Socioeconomic Characteristics (2000–2010)
| Fragile X Syndrome |
Autism Spectrum Disorder |
Intellectual Disability |
Comparison Groupa |
|
|---|---|---|---|---|
| Totalb | 125 |
2,592 | 10,685 | 26,804 |
| Sex | ||||
| Male | 89 (71.2%) | 1978 (76.3%) | 6,220 (58.2%) | 16,528 (61.7%) |
| Female | 36 (28.8%) | 614 (23.7%) | 4,465 (41.8%) | 10,276 (38.3%) |
| Age groupc | ||||
| 15–19 years | 103 (82.4%) | 2,283 (88.1%) | 8,801 (82.4%) | 22,063 (82.3%) |
| 20–24 years | 88 (70.4%) | 1,349 (52.0%) | 6,629 (62.0%) | 14,829 (55.3%) |
| Race | ||||
| Caucasian | 65 (52.0%) | 947 (36.5%) | 3,204 (30.0%) | - |
| African American | 35 (28.0%) | 848 (32.7%) | 5,961 (55.8%) | - |
| Other/Missing | 25 (20.0%) | 797 (30.8%) | 1,520 (14.2%) | - |
| County description | ||||
| Urban | 85 (68.0%) | 1,987 (76.7%) | 7,303 (68.4%) | 19,526 (72.9%) |
| Rural | 40 (32.0%) | 605 (23.3%) | 3,382 (31.6%) | 7,278 (27.1%) |
| Food stamp use | ||||
| Yes | 18 (14.4%) | 390 (15.0%) | 1,791 (16.8%) | 690 (2.6%) |
| No | 107 (85.6%) | 2,202 (85.0%) | 8,894 (83.2%) | 26,114 (97.4%) |
| Attained grade 12 or higher | ||||
| Yes | 76 (60.8%) | 1,604 (61.9%) | 6,422 (60.1%) | 21,001 (78.4%) |
| No/Missing | 49 (39.2%) | 988 (38.1%) | 4,263 (39.9%) | 5,803 (21.6%) |
| Placed in special education | ||||
| Yes | 112(89.6%) | 2,374 (91.6%) | 9,943 (93.1%) | 2,426 (9.0%) |
| No/Missing | 13 (10.4%) | 218 (8.4%) | 742 (6.9%) | 24,378 (91.0%) |
Data retrieved from State Health Plan records, which did not record race.
Total = unduplicated number of persons with at least 1 full year of eligibility to Medicaid or State Health Plan.
Age groups sum to a number greater than overall total. Age category numbers are based on number of persons with at least 1 full year of eligibility in that age group. Therefore, individuals could be in both age categories.
In our results, adolescents and young adults with FXS, ASD, and ID were all more likely to be male, consistent with the higher prevalence of these conditions in males. Table 1 also shows that the proportion of African Americans in our FXS study group was 28%; it was about 33% in the group of people with ASD and about 56% of those with ID. For the state as a whole, approximately 28% of the population is African American (United States Census Bureau, 2005). Also, 68% of those with FXS, 77% of those with ASD, 68% of those with ID, and 73% of those in the comparison group lived in urban counties; similarly, 72% of the state population lived in urban areas in 2005 (United States Census Bureau, 2005).
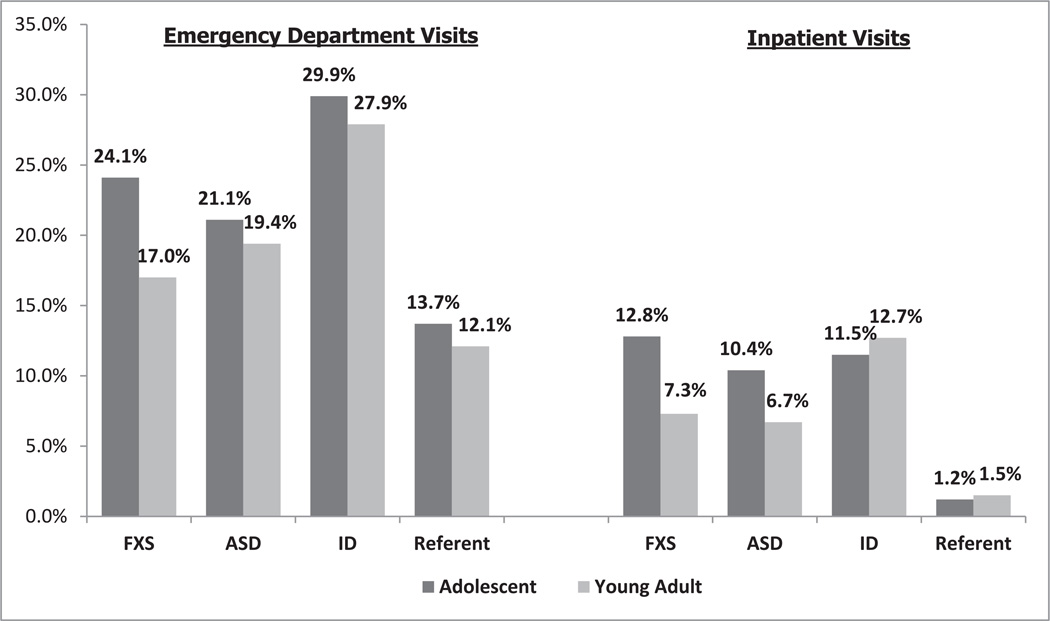
To test our first hypothesis, we compared the adjusted probabilities of any hospital encounters (ED visits and IPHs) for adolescents and young adults with FXS, ASD, and ID to the unaffected comparison group. We found that the adjusted probabilities per person-year for those with FXS were 27.1% for adolescents and 18.6% for young adults; for people with ASD it was 24.7% for adolescents and 21.8% for young adults; for those with ID it was 33.8% for adolescents and 32.3% for young adults; and for the unaffected group it was 14.5% for adolescents and 13.1% for young adults. Overall, the probabilities of hospital visits were statistically higher for adolescents and young adults with FXS, ASD, and ID, as compared to the unaffected group. Figure 1 shows the adjusted probabilities for the FXS, ASD, ID, and comparison groups by type of visit (ED or IPH). The adjusted probability of ED visits was higher for adolescents with FXS compared to adolescents in the unaffected group (24.1% vs. 13.7%). The adjusted probabilities of ED visits were also higher for young adults with FXS versus unaffected young adults (17.0% vs. 12.1%). For the other two conditions, ASD and ID, the adjusted rates of ED visits were also higher than the rates in the unaffected comparison group for both adolescents (ASD = 21.1%, ID = 29.9%) and young adults (ASD = 19.4%, ID = 27.9%). Regarding the probability of IPHs, adolescents with FXS, ASD, or ID showed much higher adjusted rates of visits than the unaffected comparison group. Among young adults, the group with ID showed a higher probability than the comparison group (12.7% vs. 1.5%); although for those with either FXS and/or ASD, the probability was close to 7%. Similarly, among adolescents, the probability of IPHs for people with any of the conditions (ASD = 10.4%, ID = 11.5%, FXS = 12.8%) was higher than it was for the comparison group (1.2%).
Figure 1.
Adjusted predicted probabilities from the fitted models of emergency department and inpatient stays by age, for those with FXS, ASD, ID, and comparison group. Note that the confounders are age group and its interactions with each of the three conditions, gender, food stamp use, grade 12 or higher attainment, placed in special education, the interaction between attaining grade 12 or higher and placed in special education, and urban/rural county type. FXS = fragile X syndrome; ASD = autism spectrum disorder; ID = intellectual disability.
Our second hypothesis was that young adults with FXS have higher probabilities of hospital encounters (ED visits and IPHs) than do adolescents with FXS. In our study, the overall probability of hospital encounters was actually lower for young adults (18.6%) as compared to adolescents (27.1%), but the difference was not statistically significant. The probabilities for ED visits of adolescents and young adults with FXS were not statistically different either (p = 0.0600), with rates of 24.1% and 17.0%, respectively. Similarly, for IPHs of adolescents with FXS versus young adults with FXS, the respective rates were 12.8% and 7.3%, and the difference was not statistically significant (p = 0.0639).
Table 2 shows the adjusted odds ratios for differences in hospital encounters between individuals with FXS, ASD, or ID and confidence intervals relative to a comparison with a group of unaffected peers. In general, the odds of an ED visit for adolescents with any of the three conditions were between 1.7 and 2.7 times the odds for adolescents without these conditions. Except for the odds ratio for young adults with FXS, all of the odds ratios for the young adults were statistically significant. Similarly, the odds of an IPH among adolescents with FXS, ASD, or ID were between 9.4 and 11.8 times the odds for the comparison group. All of these odds ratios were statistically significant for adolescents.
Table 2.
Age-Specific Odds Ratios for Emergency Department, Inpatient Hospital Visits and Visits Associated and Not Associated With FXS Among Adolescents and Young Adults With FXS, ASD, or ID, Controlling for Key Confounders
| 15–19 |
20–24 |
||||
|---|---|---|---|---|---|
| Age (years) | N | OR (95% CI) | N | OR (95% CI) | p-value |
| Emergency Department | |||||
| FXS | 161 | 2.0 (1.4, 2.8) | 89 | 1.3 (0.8, 2.0) | 0.0600 |
| ASD | 2990 | 1.7 (1.5, 1.9) | 1590 | 1.5 (1.3, 1.7) | 0.0570 |
| ID | 19464 | 2.7 (2.5, 2.9) | 15812 | 2.4 (2.2, 2.6) | <0.0001 |
| Inpatient Hospitalization | |||||
| FXS | 51 | 11.8 (7.6,18.4) | 25 | 6.3 (3.5,11.4) | 0.0639 |
| ASD | 953 | 9.4(7.6,11.5) | 308 | 5.7 (4.5,7.3) | <0.0001 |
| ID | 4595 | 10.4 (8.7,12.5) | 3667 | 11.7 (9.7,14.0) | 0.0009 |
| Conditions Associated With FXS | |||||
| All Mental Illness | |||||
| FXS | 58 | 108.4 (55.4, 212.1) | ≤10 | 33.2 (12.8, 85.9) | 0.0101 |
| ASD | 976 | 85.6 (54.8, 133.7) | 437 | 71.1 (44.4, 114.0) | 0.0716 |
| ID | 2614 | 57.8 (37.1, 90.1) | 2002 | 53.3 (34.1, 83.4) | 0.1377 |
| All Neurological Conditions | |||||
| FXS | 17 | 5.8 (2.5, 13.3) | ≤10 | 5.6 (2.0, 15.6) | 0.9581 |
| ASD | 303 | 6.1 (4.4, 8.5) | 131 | 5.1 (3.5, 7.4) | 0.1703 |
| ID | 1200 | 6.2 (4.5, 8.5) | 913 | 6.1 (4.4, 8.4) | 0.8754 |
| ACS-Epilepsy/Seizures | |||||
| FXS | 29 | 36.1 (13.9, 93.7) | 11 | 20.8 (5.3, 80.6) | 0.2680 |
| ASD | 392 | 26.8 (14.8, 48.8) | 171 | 22.4 (11.9,42.4) | 0.1919 |
| ID | 4013 | 19.3 (10.5, 35.5) | 2766 | 20.3 (11.1, 37.1) | 0.4835 |
| ACSC-Severe ENT | |||||
| FXS | ≤10 | 1.7 (0.8, 4.1) | ≤10 | 1.7 (0.5, 5.5) | 0.9838 |
| ASD | 295 | 2.0 (1.5, 2.7) | 102 | 1.3 (0.9, 1.7) | 0.0124 |
| ID | 1179 | 4.2 (3.4, 5.2) | 950 | 3.4 (2.7, 4.1) | <0.0001 |
| Conditions Not Associated With FXS | |||||
| All Respiratory Conditions | |||||
| FXS | 11 | 1.9 (1.1, 3.4) | 14 | 1.7 (0.6, 4.4) | 0.8216 |
| ASD | 293 | 1.9 (1.5, 2.4) | 129 | 1.7 (1.3, 2.2) | 0.2558 |
| ID | 2776 | 4.2 (3.5, 4.9) | 1966 | 3.7 (3.1, 4.4) | 0.0100 |
| All Genitourinary Conditions | |||||
| FXS | ≤10 | 5.1 (2.1, 12.7) | ≤10 | 2.3 (0.7, 6.9) | 0.2769 |
| ASD | 93 | 2.3 (1.5, 3.3) | 49 | 2.2 (1.5, 3.4) | 0.9527 |
| ID | 1380 | 5.8 (4.3, 7.6) | 1241 | 7.0 (5.3, 9.2) | 0.0004 |
| ACSC-Gastroenteritis | |||||
| FXS | ≤10 | 2.8 (0.7, 11.6) | ≤10 | 1.7 (0.2, 12.3) | 0.6897 |
| ASD | 30 | 1.9 (1.2, 3.2) | 14 | 1.6 (0.8, 2.9) | 0.5224 |
| ID | 256 | 3.9 (2.7, 5.6) | 193 | 3.9 (2.7, 5.7) | 0.9344 |
| ACSC-Pneumonia | |||||
| FXS | ≤10 | 6.2 (1.8, 21.0) | ≤10 | 4.8 (1.1, 20.6) | 0.7693 |
| ASD | 39 | 3.0 (1.7, 5.4) | 18 | 2.9 (1.5, 5.7) | 0.8932 |
| ID | 221 | 4.6 (2.8, 7.5) | 170 | 5.2 (3.1, 8.7) | 0.2848 |
Note. Sex, race, county type (urban/rural), food stamp use, attained grade 12 or higher, and placed in special education are the key confounders. The age-specific odds ratios and associated confidence intervals are interpretable relative to the referent group. The matched group unaffected by FXS, ASD, or ID was set as the referent group. The p-value is a comparison of the two age-specific odds ratios relative to each other.
FXS = fragile X syndrome; ASD = autism spectrum disorder; ID = intellectual disability; ACSC = Ambulatory Care Sensitive Conditions; ENT = ear, nose, and throat.
Odds ratios for covariates are not shown in Table 2 but are summarized here. Males had increased odds of hospital encounters (p < 0.0001), food stamps enrollment was not associated with ED visits, but not attaining grade 12 was associated with higher odds of ED visits (p < 0.0001). For IPHs, not attaining grade 12 (p < 0.0001), being male (p < 0.0001), living in an urban area (p = 0.0051), and being placed in special education (p < 0.0001) were all associated with an increased rate. ED visits did not differ significantly between individuals residing in urban versus rural counties.
Our third hypothesis was that, compared to a matched group of unaffected individuals, hospital encounters were more frequent in the FXS group for conditions previously reported to be more prevalent among people with FXS (e.g., seizures and epilepsy; mental illness conditions; neurologic conditions; and ear, nose, and throat conditions), but we expected that age would have modified the difference in the frequency of these encounters. This third hypothesis was tested concurrently with our fourth hypothesis, which stated that there were no differences in the rates of hospital encounters between individuals with FXS and a comparison group of unaffected individuals for ACSCs not associated with FXS.
Table 2 displays, for individuals with FXS, the adjusted odds ratios of having had a hospital encounter for several types of conditions according to their association with FXS by age. Regarding conditions associated with FXS, the odds of an adolescent with FXS to have had a hospital encounter related to mental illness were about 108.4 times higher than the odds for an individual without FXS. These odds were reduced to 33.2 times for young adults with FXS. For this case only, the effect of interaction with age was statistically significant (p = 0.0101). Also, for an individual with FXS compared to an individual in the comparison group, the odds of having had a hospital encounter related to a neurological condition were about 5.8 times and 5.6 times the odds for an adolescent and a young adult without FXS, respectively. In this case, the interaction with age was not statistically significant. Table 2 also shows that, for ACSCs, the odds of individuals with FXS to have had a hospital encounter related to epilepsy or seizures were much higher than the odds for unaffected individuals. As for conditions not associated with FXS, individuals with FXS had higher odds of hospital encounters related to genitourinary, gastrointestinal, and respiratory conditions than did unaffected individuals. In some cases, the differences were statistically significant. The interaction with age was not statistically significant for any of these conditions.
We also calculated the system-specific odds for hospital encounters for people with ID and those with ASD. We found that adolescents with ID and ASD had statistically significantly higher odds of hospital encounters, compared to young adults with the following conditions: all mental illness; all neurological illness; ear, nose, and throat illness; and all respiratory illness. The conditions that were not statistically significantly different for the two age groups, for either ID or ASD, were epilepsy and genitourinary conditions. However, it should be noted that the odds ratio of hospital encounters due to epilepsy for people with ASD was 26.8 (95%CI: 14.8–48.8, p < .0001) for 15- to 19-year-olds and 22.4 (95% CI: 11.87–42.4, p < .0001) for 20- to 24-year-olds when compared to the group with none of the conditions. The odds ratio of hospital encounters due to epilepsy for people with ID was 19.3 (95%CI: 10.5–35.5, p < .0001) for 15- to 19-year-olds and 20.3 (95% CI: 11.1–37.1, p < .0001) for 20- to 24-year-olds when compared to the unaffected group. Obviously, these odds ratios for hospital encounters reflect the high epilepsy prevalence for these groups.
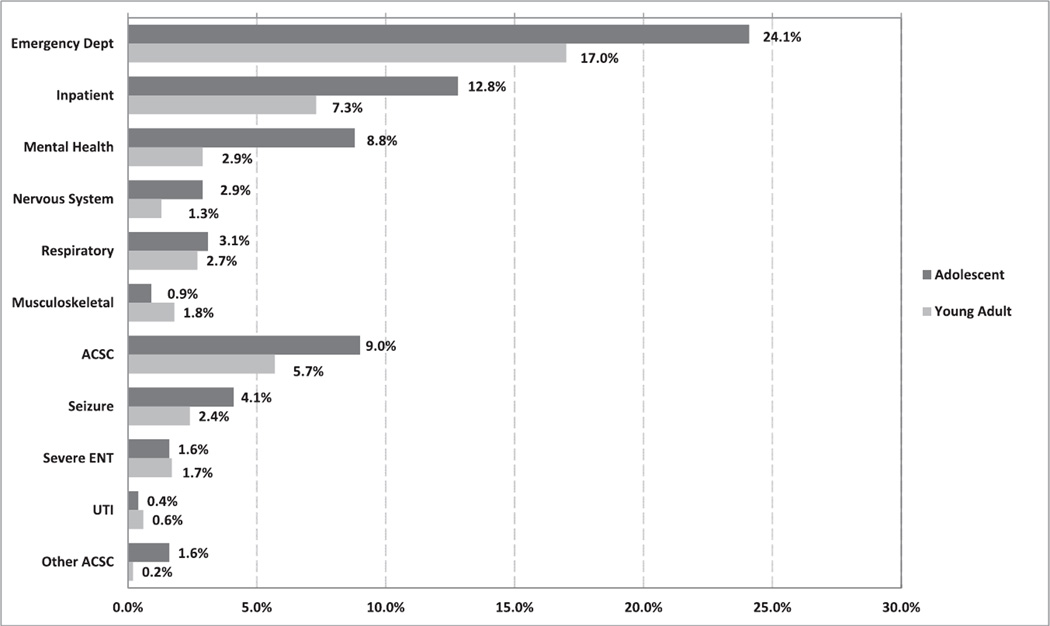
Figure 2 shows the adjusted rates from the fitted models for the two age groups with FXS. The confounders were gender, food stamps, whether the individual attained grade 12, whether the individual was placed in special education, the interaction of attaining grade 12 and receiving special education, and the rural/urban composition of the county of residence. Figure 2 also shows the adjusted rates and standard errors when comparing adolescents and young adults for a number of hospital encounter types. We found that there was a significant difference between the two age groups for all mental illness. We hypothesized that young adults with FXS have a higher probability of hospital encounters than adolescents with FXS, but our analyses did not demonstrate a significant difference in hospital encounters between the two age groups.
Figure 2.
Adjusted predicted probabilities from the fitted models of visits by age, for those with FXS. Note that the confounders are gender, food stamp use, grade 12 attainment, placed in special education, the interaction between grade 12 attainment and special education placement, and urban/rural county type. FXS = fragile X syndrome; ACSC = ambulatory care sensitive conditions; ENT = ear, nose, and throat; UTI = urinary tract infection.
Discussion
Using population-based data, we estimated the prevalence of FXS in the general population of South Carolina and found that our estimate was comparable to the often-referenced estimate of 1/ 3600 (Turner et al., 1996). The prevalence among members of the two insurance plans we used in our analyses was higher than estimated for the general population, because Medicaid includes a disproportionately high number of people who have ID and fewer people without disability in the age groups studied.
This study confirmed our first hypothesis that adolescents and young adults with FXS have higher odds of having IPH or ED visits than the comparison group, but the hospitalization rates were similar to the groups with ID and ASD. Our second hypothesis, that there would be more IPH and ED visits for young adults with FXS as compared to adolescents with FXS, was not confirmed, with the exception of people with FXS who required care for mental illness. This hypothesis relates to the transition from pediatric to adult medical care, a topic that has received substantial attention. It is important to note the IPH odds ratio and mental illness hospital use (see Table 2) were statistically significantly higher for adolescents as compared to young adults when we controlled for known confounders. The premise of concerns about transition is that IPHs will increase after adolescents leave pediatric care; however, we found that inpatient stays and combined ED visits and IPHs for mental illness were actually higher for adolescents as compared to young adults. We assume this was due to the age of onset of psychiatric conditions, typically occurring during adolescence, followed by medication and stabilization during early adulthood. Previous research has suggested the possibility that some of these adolescent ED and IPH visits could have been prevented if outpatient care during adolescence was intensified (Bindman, Chaltopadhyay, Osmond, Huen, & Bacchetti, 2005; Oster & Bindman, 2003); however, this could not be investigated using the data from this study. There was no statistically significant difference in hospital utilization for adolescents and young adults with FXS for other diagnoses. For the segment of the study population that was insured by Medicaid and the State Health Plan, it appears that the transition out of pediatrics was not associated with increased risk for IPH and ED visits related to ACSCs.
Our third hypothesis, that system-specific visits are more prevalent among people with FXS than among the comparison group for conditions reported in the literature as being common among those with FXS, was confirmed. All mental illness (OR 108.4: 95% CI 55.4–212.1), all neurologic conditions (OR 5.8: 95% CI 2.5–13.3), and the ACSC for seizures/epilepsy (OR 36.1: 95% CI 13.9–93.7), were statistically significantly higher for those with FXS, in contrast to the comparison group, after controlling for potential confounders. It is well established that seizure disorders are more common among people with neurodevelopmental disorders, including FXS, ASD, and ID, which could have driven the observed differences in ED/IPH utilization rates for those problems (Centers for Disease Control and Prevention, 2008; Deykin & MacMahon, 1979; Hart & Shorvon, 1995; Kotsopoulos, van Merode, Kessels, de Krom, & Knottnerus, 2002; McDermott et al., 2005). We explored the frequency of an epilepsy diagnosis among the three groups in our sample, and found that the prevalence of any diagnosis of epilepsy was, in fact, substantially higher among those with any of the three disabilities (24.8% for FXS, 36.7% for ASD, and 27.1% for ID versus 1.4% for the comparison group). The point estimates for the rate of seizures are similar, although a little higher, than those noted in the literature (Berry-Kravis et al., 2010; Centers for Disease Control and Prevention, 2008; Kotsopoulos et al., 2002; McDermott et al., 2005). The higher rate of occurrence is probably the result of misdiagnoses of related neurologic conditions such as tic disorders, stereotypes, and medication effects, which could not be clarified using the secondary data sources. Nonetheless, optimizing ambulatory care has the potential to reduce the need for emergency room treatment and hospitalization in adolescents and young adults who have FXS, ASD, or ID and also have a seizure disorder. Clinicians who provide care for people with these conditions should focus on finding effective ways to prevent the overutilization of emergency or inpatient treatment for these patients, when effective care can be provided in the ambulatory setting. These results need confirmation from other studies, because the 95% confidence intervals were wide. If confirmed, the high rate of IPH and ED use for all neurologic conditions and seizures/epilepsy by adolescents and young adults with FXS is worrisome because it suggests that the knowledge of the high prevalence of these conditions for people with FXS is not sufficient for primary care/ambulatory providers to prevent ED visits and IPHs, which are more intensive and expensive.
Finally, our fourth hypothesis, that the rate of hospital encounters for conditions not reported to be more prevalent among people with FXS is similar between people with FXS and the comparison group, was only partially confirmed. The conditions for which there was no difference when the comparison was made with young adults included all respiratory conditions, genitourinary conditions, and gastroenteritis. The rate of hospital use for adolescents, compared to their age peers with FXS were elevated for all respiratory conditions (OR 1.9: 95% CI 1.1–3.4), genitourinary conditions (OR 5.1: 95% CI 2.1–12.7), and pneumonia (OR 6.2: 95% CI 1.8–21.0). Pneumonia hospitalizations were also higher for young adults with FXS (OR 4.8: 95% CI 1.1–20.6), compared to unaffected peers. The explanation for higher rates of hospitalization for these conditions was not revealed by this study, although it should be noted that the genitourinary condition result might be spurious because of the small sample size (less than 10 individuals in this group; Table 2). This finding suggests that there might be a disparity in the general access to primary/ambulatory care for adolescents with FXS. This is in keeping with our previous finding for certain common conditions among people with FXS, such as seizure disorders and epilepsy, for which primary care seems to be inadequate to prevent ED utilization and hospitalizations. The results may be at least partly attributable to sample size, as we had a relatively small number of individuals with FXS in our study.
We relied on secondary data to classify our cases and comparison groups; thus, the groups were not precise. We used all ICD9 codes under 299, meaning that all the codes 299.0–299.9 were included. We could not identify all cases of FXS, as some people who refused genetic testing through DDSN were probably included in the ID or ASD group. In addition, when we could not distinguish the primary disabling condition, we classified people with both ID and ASD diagnoses in the ASD group. We did have service records from the DDSN and public school placements; thus, whenever ID or ASD services were recorded, we used the information to classify cases, but we acknowledge imprecision in the ID and ASD classification. This imprecision prevented us from specifying the severity of the condition. We acknowledge that children who first present with ASD prior to their FXS diagnosis are more likely to have a severe presentation of FXS; however, our data did not allow us to classify severity.
For the individuals in this population-based study, we had access to some potentially important confounding variables such as food stamp use (a proxy for socioeconomic status), attained level of education, placement in special education, and rural or urban county of residence. None of these variables contributed significantly in all of the GEE models shown in Table 2 and, in the case of county type, the direction was different for mental health visits as compared to all other ACSC visits. Living in a rural county increased the odds of having IPH/ED utilization for mental health conditions, and being in a rural county increased the odds of all other ACSC visits (results not shown in the table). We did not have access to other potential confounders, such as functional status, severity of the disability, and receipt of social- and health-related supports and services.
Our findings did not distinguish whether patients were experiencing substantial health problems that could not be treated in primary care, if they were not trying to access primary or outpatient specialty care, if they were having difficulty accessing ambulatory care, or whether there were gaps in the quality of care provided. In the case of seizures/epilepsy for people with FXS, the underlying condition might have required ED or IPH visits despite good outpatient care. We do not have data to address these concerns; thus, additional research, probably with a larger sample, is needed to better describe the factors influencing IPH and ED care and ACSC visits for mental illness, neurologic problems, and epilepsy/seizures. Also, research on individuals with FXS and their primary care providers regarding potential barriers to early treatment is needed, as well as research on a wider age group. Clearly, our study focus on 15–24-year-olds limits our ability to describe the full range of events during young adulthood. We are only capturing the early years of adulthood, and the hospital utilization pattern might substantially change if the age group were expanded. Finally, quantitative research using systematic reviews of clinical records could assess the adequacy of care provided to individuals with FXS and identify areas that would improve it. Such reviews could lead to the development of new care guidelines that would be helpful in reducing the need for ACSC visits.
Overall, our finding that both adolescents and young adults with FXS, ASD, and ID had higher rates of hospital visits, when compared to people without these conditions, can be used to raise awareness among health care providers about efficacious treatment for seizures/epilepsy, other nervous system conditions, mental illness conditions, and genitourinary conditions in this relatively young age group in outpatient settings. We found that, for the study group with FXS, there were no conditions for which hospital use was statistically significantly increased during adulthood as compared to adolescence, suggesting that, in the dimension of hospital utilization at least, the transition from pediatric to adult care is occurring smoothly.
Acknowledgments
This research was supported by a cooperative agreement from the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, the South Carolina Budget and Control Board Division of Research and Statistics, the South Carolina Department of Health and Human Services, the South Carolina Public Employee Benefit Authority, the South Carolina Department of Education, or the South Carolina Department of Social Services.
Contributor Information
Suzanne McDermott, Arnold School of Public Health, University of South Carolina.
James W. Hardin, Arnold School of Public Health, University of South Carolina
Julie A. Royer, South Carolina Revenue and Fiscal Affairs Office
Joshua R. Mann, School of Medicine, University of South Carolina
Xin Tong, Arnold School of Public Health, University of South Carolina.
Orgul D. Ozturk, Moore School of Business, University of South Carolina
Lijing Ouyang, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention.
References
- Agency for Healthcare Research and Quality. AHRQ Quality Indicators – Prevention Quality Indicators: Software Documentation, Version 2.1 – SAS. Rockville, MD: Agency for Healthcare Research and Quality; 2004. [Google Scholar]
- American Academy of Pediatrics. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110:1304–1306. [PubMed] [Google Scholar]
- American Academy of Pediatrics. Clinical report—Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182–200. doi: 10.1542/peds.2011-0969. [DOI] [PubMed] [Google Scholar]
- Ansari Z, Laditka JN, Laditka SB. Access to health care and hospitalization for ambulatory care sensitive conditions. Medical Care Research and Review. 2006;63(6):719–741. doi: 10.1177/1077558706293637. http://dx.doi.org/10.1177/1077558706293637. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics Part A. 2008;146A:2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nature Neuroscience. 2006;9(10):1221–1225. doi: 10.1038/nn1765. http://dx.doi.org/10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E. Epilepsy in fragile X syndrome. Developmental Medicine & Child Neurology. 2002;44:724–728. doi: 10.1017/s0012162201002833. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in fragile X syndrome: Characteristics and comorbid diagnoses. American Journal on Intellectual and Developmental Disabilities. 2010;115:461–472. doi: 10.1352/1944-7558-115.6.461. [DOI] [PubMed] [Google Scholar]
- Bindman AB, Chattopadhyay A, Osmond DH, Huen W, Bacchetti P. The impact of medicaid managed care on hospitalizations for ambulatory care sensitive conditions. Health Services Research. 2005;40:19–37. doi: 10.1111/j.1475-6773.2005.00340.x. http://dx.doi.Org/10.1111/j.1475-6773.2005.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum RW. Improving transition for adolescents with special health care needs from pediatric to adult-centered health care—Introduction. Pediatrics. 2002;110:1301–1303. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Epilepsy surveillance among adults— 19 states, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveillance Summaries. 2008;57:20. [PubMed] [Google Scholar]
- Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, Warren ST. Incidence of fragile X syndrome by newborn screening for Methylated FMR1 DNA. The American Journal of Human Genetics. 2009;85(4):503–514. doi: 10.1016/j.ajhg.2009.09.007. http://dx.doi.org/10.1016/j.ajhg.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deykin EY, MacMahon B. The incidence of seizures among children with autistic symptoms. The American Journal of Psychiatry. 1979;136:1310–1312. doi: 10.1176/ajp.136.10.1310. [DOI] [PubMed] [Google Scholar]
- Garber KB, Visootsak J, Warren ST. Fragile X syndrome. European Journal of Human Genetics. 2008;16(6):666–672. doi: 10.1038/ejhg.2008.61. http://dx.doi.org/10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geenen SJ, Powers LE, Sells W. Understanding the role of health care providers during the transition of adolescents with, disabilities and special health care needs. Journal of Adolescent Health. 2003;32(3):225–233. doi: 10.1016/s1054-139x(02)00396-8. http://dx.doi.org/10.1016/S1054-139X(02)00396-8. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. http://dx.doi.org/10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ. Fragile X syndrome: Diagnosis, treatment, and research. Baltimore, MD: Johns Hopkins University Press; 2002. [Google Scholar]
- Hardin JW, Hilbe J. Generalized estimating equations. 2nd edition. Boca Raton, FL: Chapman & Hall/CRC; 2012. [Google Scholar]
- Hart YM, Shorvon SD. The nature of epilepsy in the general population. II. Medical care. Epilepsy Research. 1995;21:51–58. doi: 10.1016/0920-1211(95)00008-x. http://dx.doi.org/10.1016/0920-1211(95)00008-X. [DOI] [PubMed] [Google Scholar]
- Hartley SL, Seltzer MM, Raspa M, Olmstead M, Bishop E, Bailey DB. Exploring the adult life of men and women with fragile X syndrome: Results from a national survey. American Journal on Intellectual and Developmental Disabilities. 2011;116:16–35. doi: 10.1352/1944-7558-116.1.16. http://dx.doi.org/10.1352/1944-7558-116.L16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh JH, Saul RA, Saal HM, Braddock SR, Enns GM, Gruen JR, Spire P. Clinical report—Health supervision for children with fragile X syndrome. Pediatrics. 2011;127:994–1006. doi: 10.1542/peds.2010-3500. [DOI] [PubMed] [Google Scholar]
- Kotsopoulos IA, van Merode T, Kessels FG, de Krom MC, Knottnerus JA. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia. 2002;43(11):1402–1409. doi: 10.1046/j.1528-1157.2002.t01-1-26901.x. http://dx.doi.org/10.1046/j.1528-1157.2002.t01-1-26901.x. [DOI] [PubMed] [Google Scholar]
- Kronk R, Bishop EE, Raspa M, Bickel JO, Mandel DA, Bailey DB., Jr Prevalence, nature, and correlates of sleep problems among children with fragile X syndrome based on a large scale parent survey. Sleep. 2010;33:679–687. doi: 10.1093/sleep/33.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. http://dx.doi.org/10.2307/2336267. [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Mental Retardation and Developmental Disabilities Research Review. 2004;10:31–41. doi: 10.1002/mrdd.20006. http://dx.doi.org/10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- McDermott S, Moran R, Platt T, Wood H, Isaac T, Dasari S. Prevalence of epilepsy in adults with mental retardation and related disabilities in primary care. American Journal on Mental Retardation. 2005;110:48–56. doi: 10.1352/0895-8017(2005)110<48:POEIAW>2.0.CO;2. http://dx.doi.org/10.1352/0895-8017(2005)110%3C48:POEIAW%3E2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- McLennan Y, Polussa J, Tassone F, Hagerman R. Fragile X syndrome. Current Genomics. 2011;12(3):216–224. doi: 10.2174/138920211795677886. http://dx.doi.org/10.2174/138920211795677886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci SA, Hagerman RJ, Ferri R, Bosco P, Dalla Bernardina B, Tassinari CA, Elia M. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia. 1999;40(8):1092–1099. doi: 10.1111/j.1528-1157.1999.tb00824.x. http://dx.doi.org/10.1111/j.1528-1157.1999.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Oster A, Bindman AB. Emergency department visits for ambulatory care sensitive conditions—Insights into preventable hospitalizations. Medical Care. 2003;41(2):198–207. doi: 10.1097/01.MLR.0000045021.70297.9F. http://dx.doi.org/10.1097/01.MLR.0000045021.70297.9F. [DOI] [PubMed] [Google Scholar]
- Peter NG, Forke CM, Ginsburg KR, Schwarz DF. Transition from pediatric to adult care: Internists’ perspectives. Pediatrics. 2009;123(2):417–423. doi: 10.1542/peds.2008-0740. http://dx.doi.org/10.1542/peds.2008-0740. [DOI] [PubMed] [Google Scholar]
- Raspa M, Bailey DB, Bishop E, Holiday D, Olmsted M. Obesity, food selectivity, and physical activity in individuals with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities. 2010;115(6):482–495. doi: 10.1352/1944-7558-115.6.482. http://dx.doi.org/10.1352/1944-7558-115.6.482. [DOI] [PubMed] [Google Scholar]
- Reiss J, Gibson R. Health care transition: Destinations unknown. Pediatrics. 2002;110:1307–1314. [PubMed] [Google Scholar]
- Royer JA, Hardin JW, McDermott S, Ouyang L, Mann JR, Ozturk OD, Bolen J. Use of state administrative data sources to study adolescents and young adults with rare conditions. Journal of General Internal Medicine. 2014;29(3):732–738. doi: 10.1007/s11606-014-2925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistical Analysis System (Version 9.3) [Computer software] Cary, NC: SAS Institute, Inc; [Google Scholar]
- Sund R, Pukkala E, Patja K. Cancer incidence among persons with fragile X syndrome in Finland: A population-based study. Journal of Intellectual Disability Research. 2009;53:85–90. doi: 10.1111/j.1365-2788.2008.01116.x. http://dx.doi.org/10.1111/j.1365-2788.2008.01116.x. [DOI] [PubMed] [Google Scholar]
- Tarleton JC, Saul RA. Molecular genetic advances in fragile X syndrome. Journal of Pediatrics. 1993;122:169–185. doi: 10.1016/s0022-3476(06)80110-1. [DOI] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. American Journal of Medical Genetics. 1996;64:196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. http://dx.doi.org/10.1002/(SICI)1096-8628(19960712)64:1%3C196::AID-AJMG35%3E3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau. State and county quickfacts. 2005 Retrieved from http://www.census.gov/quickfacts/table/PST045214/45,00. [Google Scholar]