Abstract
This study compares the family financial and employment impacts of having a child with fragile X syndrome (FXS), autism spectrum disorder (ASD), or intellectual disabilities (ID). Data from a 2011 national survey of families of children with FXS were matched with data from the National Survey of Children with Special Health Care Needs 2009–2010 to form four analytic groups: children with FXS (n = 189), children with special health care needs with ASD only (n = 185), ID only (n = 177), or both ASD and ID (n = 178). Comparable percentages of parents of children with FXS (60%) and parents of children with both ASD and ID (52%) reported that their families experienced a financial burden as a result of the condition, both of which were higher than the percentages of parents of children with ASD only (39%) or ID only (29%). Comparable percentages of parents of children with FXS (40%) and parents of children with both ASD and ID (46%) reported quitting employment because of the condition, both of which were higher than the percentages of parents of children with ID only (25%) or ASD only (25%). In multivariate analyses controlling for cooccurring conditions and functional difficulties and stratified by age, adjusted odds ratios for the FXS group aged 12–17 years were significantly elevated for financial burden (2.73, 95% CI 1.29–5.77), quitting employment (2.58, 95% CI 1.18–5.65) and reduced hours of work (4.34, 95% CI 2.08–9.06) relative to children with ASD only. Among children aged 5–11 years, the adjusted odds ratios for the FXS group were elevated but statistically insignificant for financial burden (1.63, 95% CI 0.85–3.14) and reducing hours of work (1.34, 95% CI 0.68–2.63) relative to children with ASD only. Regardless of condition, cooccurring anxiety or seizures, limits in thinking, reasoning, or learning ability, and more irritability were significantly associated with more caregiver financial and employment impacts. Proper management of anxiety or seizures and functional difficulties of children with FXS or other developmental disabilities may be important in alleviating adverse family caregiver impacts.
Keywords: Fragile X syndrome, Intellectual disability, Autism spectrum disorders, Caregiver impacts
1. Introduction
Fragile X syndrome (FXS) occurs in persons with a full mutation in the FMR1 (fragile X mental retardation 1) gene. The prevalence of FXS is estimated at 1/4000 males and 1/8000 females (Coffee et al., 2009; Peprah, 2012). FXS is characterized by cognitive and behavioral problems in affected males and, to a lesser degree, in affected females (Saul & Tarleton, 1993). FXS is the most common inherited cause of intellectual disability (ID) (Cornish, Turk, & Hagerman, 2008). FXS is one of the principal single-gene disorders associated with autism. Approximately 20% to 50% of persons with FXS meet full diagnostic criteria for autism (Moss & Howlin, 2009).
There are a variety of factors that play a role in how having a child with a disability such as FXS affects the family. These factors include characteristics of the child (e.g., age, severity of disability, extent of behavior problems), the family status (e.g., parental education, parent mental health, maternal genetic status, financial resources, social support systems, number of children with a disability), educational and employment opportunities for the child with FXS, and life events not directly associated with FXS (death of a parent, divorce, job layoff or transition). These factors inevitably interact in complex ways to shape adaptation in both positive and negative ways.
Despite the complexity of these causative influences on family adaptation, a persistent and largely unanswered question is whether families who have a child with one form of disability as a group are more or less affected by their child's particular condition than families who have a child with another form of disability. The literature on the family financial and employment impacts of caring for children with disabilities has primarily focused on autism (Cidav, Marcus, & Mandell, 2012; Kogan et al., 2008; Montes & Halterman, 2008a, 2008b) and ID (McGrath, Stransky, Cooley, & Moeschler, 2011; Schieve, Boulet, Kogan, Van Naarden-Braun, & Boyle, 2011). It has been shown that caregiver financial and employment impacts are greater in families with children with special health care needs (CSHCN) and autism compared to other CSHCN (Kogan et al., 2008). Among families of children with ID, such impacts appear to be greater among families of children with autism, cerebral palsy, hearing or vision impairment (Schieve et al., 2011). There are far fewer published studies of the impacts for families of FXS, perhaps because of the challenges in collecting needed data for rare conditions like FXS. These studies are based on convenience samples and have shown that families affected by FXS experienced a significant negative employment and financial impact (Bailey et al., 2012; Ouyang, Grosse, Raspa, & Bailey, 2010), as well as elevated rates of maternal depression, anxiety, stress, and lowered quality of life (Bailey, Sideris, Roberts, & Hatton, 2008).
Despite the documented association between FXS, ID, and autism, fine-grained analysis has revealed very different developmental, behavioral and cognitive profiles of FXS from those found in persons with idiopathic autism (Lewis et al., 2006; Moss & Howlin, 2009). Family impacts of FXS may be greater than ASD or ID alone because of the complex nature of FXS. Studying the family impact of FXS compared with ASD or ID could help put into context the needs of families affected by FXS, and inform the broader discussion on early and differentiated diagnosis, care, and services for FXS.
In addition to the major diagnoses of FXS, ID, or ASD, we also take into consideration varying functional difficulties and cooccurring conditions when investigating family caregiver impacts. To fully address the consequences of a condition, it is important to know the functional difficulties that exist, beyond receiving a clinical diagnosis (Lollar, Hartzell, & Evans, 2012). Parents caring for persons with ID consider the psychiatric or behavioral problems of their child to be an extra burden (Irazabal et al., 2012; Maes, Broekman, Dosen, & Nauts, 2003; Martorell, Gutierrez-Recacha, Irazabal, Marsa, & Garcia, 2011). The numbers of co-occurring conditions and problem behaviors such as irritability have been shown to be major contributors of family impact of FXS (Bailey et al., 2012; Ouyang et al., 2010). Identifying functional difficulties that have the greatest impact can help design appropriate management strategies and services that meet the needs of affected families.
This study aims to compare the family caregiver financial and employment impacts of having children with FXS to children with ASD and ID, ASD only, or ID only, using similar questions asked in an FXS caregiver survey and National survey of children with special health care needs (NS-CSHCN) 2009–2010. We test the hypotheses that familial caregiver economic impacts of children with FXS are similar to those of children with both ID and ASD, but greater than those with ID or ASD alone. We also investigate the role of affected children's functional limitations (learning, communication, socialization) and co-occurring conditions (depression, anxiety, and seizures) on financial and employment impacts.
2. Methods
2.1. Data source and sample
The sample of persons with FXS was a convenience sample that came from a caregiver survey administered during the year 2011 to families having a child with FXS aged 5 years or older who were enrolled in a research registry (https://www.ourfragileXworld.org). The registry hosted by RTI International, is designed to administer surveys about the nature and consequences of FXS. RTI International has partnered with two fragile X foundations, researchers, and clinicians for survey design and enrollment. Surveys from the registry have provided new knowledge about fragile X and proved that parents are a valuable source of information (Bailey, Raspa & Olmsted, 2011). Of the 508 families invited by mail to participate in the study, 350 respondents (68.9%) completed the survey. Survey respondents were parents of an individual with the full mutation or premutation of FXS, which was determined through parent-reported FXS testing results. Most (92.6%) of the respondents completed the survey online with the remainder completing the survey by phone. A detailed description of the survey can be found elsewhere (Bailey et al., 2012). We restricted the FXS sample to respondents who had a son or daughter with the full mutation who were 17 years or younger for comparison with the NS-CSHCN 2009–2010, which was administered to families with children aged 17 years or younger. The FXS sample before matching consisted of 193 individuals, aged 5–17 years, 81% of whom were males. The surveyed parents were mostly female (91%), white (85%), and married (86%). The surveyed parents averaged 43 years of age, ranging from 26 to 76 years.
The comparison groups were drawn from the NS-CSHCN 2009–2010 which was designed and funded by the Maternal and Child Health Bureau (MCHB) and conducted by the Centers for Disease Control and Prevention's National Center for Health Statistics. Independent random samples of US households were identified through a random-digit dial telephone survey and subsequently screened to include households with CSHCN <18 years old by using a 5-item screener (Bethell et al., 2002). The CSHCN screener is designed to reflect MCHB's consequences-based definition of CSHCN: children with special health care needs are those who have a chronic physical, developmental, behavioral, or emotional condition and who also require health and related services of a type or amount beyond that required by children generally. Detailed interviews by parental reports were completed for 40,242 CSHCN between July 2009 and March 2011. The interview completion rate, defined as the proportion of households known to include CSHCN that completed all sections of the interview, was 80.8%. (Centers for Disease Control & Prevention, 2011).
2.2. Construction of comparison groups
Using the NS-CSHCN, we constructed three comparison groups: children with parent-reported physician-diagnosed ASD and ID, children with physician-diagnosed ASD but no ID, and children with physician-diagnosed ID but no autism. Families from the NS-CSHCN and the FXS survey were matched in a 1:1 ratio based on the social demographic profile of the FXS survey respondents and surveyed child using coarsened exact matching (CEM) (Blackwell, Iacus, King, & Porro, 2009; Iacus, King, & Porro, 2012). Three separate matches were performed for each reference group of ASD and ID, ASD only and ID only. Specifically, CEM temporarily coarsens/categorizes each variable by recoding so that substantively indistinguishable values are grouped and assigned the same numerical value. Then, the exact matching is applied to the coarsened data to determine the matches and to prune unmatched units. Thus, the sample size of each group for further analysis can be reduced based on the number of matched and unmatched units. Conditional regressions are then conducted using the uncoarsened values of the matched dataset. CEM is more flexible than exact matching, which typically yields few matches because the requirements on data grow exponentially with the dimensionality on matching variables. Compared to propensity score matching or other approximate matching methods, CEM provides stricter matching criteria and eliminates the need for iterations in balance checking and rematching. In our preliminary analyses, propensity score matching yielded more imbalances between the comparison groups than did CEM. The propensity score matched samples had close propensity scores but not close values on matching variables. Since our primary concern was to remove the imbalance between comparison groups from different surveys, CEM was chosen for its ability to reduce imbalance.
The variables we used in CEM are social demographic variables in both surveys, including those that have been shown to be associated with caregiver work loss (Okumura, Van Cleave, Gnanasekaran, & Houtrow, 2009). They include: parental education (high school or below/above high school), family structure (two-parent/single mother/Other), family income relative to the federal poverty level (below 100%/100%–200%/200%–400%/above 400%), parental relationship to the surveyed child (mother/father/other), child race/ethnicity (non-Hispanic White/non-Hispanic Black/Hispanic/Other, which includes Asian, American Indian or Unknown), child sex (female/male), and child age (5–7/8–12/13–17).
2.3. Caregiver financial and employment impact outcome variables
Both surveys asked similarly phrased questions about caregiver employment and financial impacts. Questions from both surveys are listed in Table 1. Questions on family financial burden referred to the condition under investigation. In the FXS caregiver survey, the answers to the financial burden question were 4-point scale (0: not at all; 1: a little bit; 2: somewhat; 3 a great deal). For purpose of comparison to answers in NS-CSHCN (No/Yes), we recoded the answers to No (not at all or a little bit) and Yes (somewhat or a great deal). This was consistent with Bailey et al., 2012. Questions on family caregiver employment impact include both questions on quitting work and questions on reducing work hours.
Table 1.
Questions used to construct variables in Fragile X Syndrome caregiver survey 2011 and National survey of children with special health care needs 2009–2010.
| FXS caregiver survey | National survey of children with special health care needs | |
|---|---|---|
| Caregiver employment and time use | ||
| Financial burden | 1. “To what extent, if at all, has having a son or daughter with fragile X caused a financial burden on your family?” (not at all, a little bit, somewhat, a great deal); | Have [S.C.j's health conditions caused financial problems for your family? (No/Yes) |
| No – not at all/a little bit | ||
| Yes – somewhat/a great deal | ||
| Quit working | 2. As a result of having a son or daughter with fragile X has anyone in your family ever had to quit working to care for your child? | Have you or other family members stopped working because of [S.C.j's health conditions? (No/Yes) |
| (No/Yes) | ||
| Change work hours | 3. As a result of having a son or daughter with fragile X has anyone in your family had to change work hours? (No/Yes) | Have you or other family members cut down on the hours you work because of [S.C.j's health conditions? (No/Yes) |
| Co-occurring conditions | Has your child ever been diagnosed with or treated by a medical professional for any of the following conditions? | Has a doctor or other health care provider ever told you that the child had |
| No/Yes | No/Yes | |
| Depression | Depression | |
| Anxiety problems | Anxiety | |
| Autism | Autism, Asperger's disorder, pervasive developmental disorder, or other autism spectrum disorder | |
| Epilepsy or seizure disorder | Seizures | |
| Child ability and behavior | ||
| Learning, understanding, or paying attention | How would you describe your child's ability to listen and pay attention to others, overall thinking, reasoning, and learning abilities | Compared to other [CHSCN_AGEj-year-old children, would you say [he/she] experiences a lot, a little, or no difficulty learning, understanding, or paying attention? |
| 1 – Fair or poor | 1 – A lot of difficulty | |
| 2 – Very good or good | 2 – A little or no difficulty | |
| Speech or communication | Inappropriate speech subscale (talks excessively; repetitive speech; talks to self loudly; repeats a word or phrase over and over) | Compared to other [CHSCN_AGEj-year-old children, would you say [he/she] experiences a lot, a little, or no difficulty speaking, communicating, or being understood? |
| 1 – 5 or higher | 1 – A lot of difficulty | |
| 2 – 0 to 4 | 2 – A little or no difficulty | |
| Behavior/irritability | Irritability subscale (injures self on purpose; aggressive to others verbally or physically; inappropriately nosy or rough; screams inappropriately; temper tantrums/outbursts; irritable and whiny; disobedient, difficult to control; yells at inappropriate times; disrupt others; uncooperative; deliberately hurts oneself; does physical violence to self) | Compared to other [CSHCN_AGEj-year-old children, would you say [he/shej experiences a lot, a little, or no difficulty with behavior problems, such as acting-out, fighting, bullying, or arguing? |
| 1 – 11 or higher | 1 – A lot of difficulty | |
| 2 – 0 to 10 | 2 – A little or no difficulty | |
| Social avoidance | Social avoidance (seeks isolation from others; withdrawn, prefers solitary activities; isolates himself from other children or adults; prefers to be alone) | Compared to other [CSHCN_AGEj-year-old children, would you say [he/she] experiences a lot, a little, or no difficulty making and keeping friends? |
| 1 – 4 or higher | 1 – A lot of difficulty | |
| 2 – 0 to 3 | 2 – A little or no difficulty | |
2.4. Functional difficulties and co-occurring conditions variables
In addition to indicator variables of whether the surveyed child had FXS, ASD, or ID, we controlled for functional difficulties and co-occurring conditions in multivariate analysis of familial impacts. Both surveys included questions on functional difficulties, although the wording was not exactly the same (Table 1). We constructed four variables: (1) difficulty with learning, understanding, or paying attention, (2) difficulty with speaking, (3) behavior problems, and (4) social difficulties. The last three constructed variables from the FXS survey were subscales from the Aberrant Behavior Checklist-Community version (ABC-C) (Aman & Singh, 1994; Bailey et al., 2012). The ABC-C is a 58-item measure of behavior problems that uses a 4-point Likert-type scale ranging from 0 (not at all a problem) to 3 (the problem is severe in degree). We also included indicators on physician or other health care provider reported conditions of depression, anxiety and seizures. Although attention-deficit/hyperactivity disorder (ADHD) is a major co-occurring condition for FXS, the questions asked in the fragile X caregiver survey were separate on attention problems and hyperactive problems, making it incompatible with the questions on ADHD in the NS-CSHCN that did not distinguish between attention or hyperactive problems. Thus, we excluded this information out of concern of measurement error.
2.5. Statistical analysis
Demographic characteristics, caregiver impacts, co-occurring conditions, and functional limitations were compared among the four analytical groups (FXS, ASD only, ID only, ASD and ID) using chi-squared proportion test. Multivariate logistic regression modeling stratified by age were used on the matched pooled families with FXS and families with ASD, ID, or both to assess associations between children's FXS status and caregiver impacts. The effect of FXS group status was shown with the reference group being ASD only, ID only, or ASD and ID from three separate logistic regressions. Multivariable logistic regression results were reported for FXS group status, the co-occurring conditions (depression, anxiety, and seizures), and functional or activity limitations as odds ratios adjusted for covariates and 95% confidence intervals. The logistic regression models also control for race, gender, highest household education, survey respondent's marital status, and household income relative to federal poverty line. A P-value of < 0.05 was considered statistically significant.
3. Results
After matching, we were able to retain 177 matched children with ID only, 185 matched children with ASD only, and 178 matched children with both ID and ASD. Out of the 193 children with FXS before matching, 189 of them were able to find a match in at least one of the three comparison groups. We had a total of 729 children (Table 2). The comparison groups had the same distribution as the FXS group with respect to child age (about 50% of the overall sample were aged 5–11 years), race/ ethnicity (about 85% were non-Hispanic whites), gender (over 80% were male), highest household education (over 90% had above high school education), relationship to surveyed child (over 90% were mothers), family structure (over 85% were two-parent household), and household income (about 20% were below 200% of the poverty level).
Table 2.
Demographic characteristics of children with fragile X syndrome (FXS) and matched comparison groups of autism spectrum disorders (ASD) and intellectual disability (ID), ASD only and ID only: matched sample from FXS caregiver survey 2011 and National survey of children with special health care needs 2009–2010.
| Variables | Overall n (%) (N =729) |
N (%) by matched comparison groupsa |
|||
|---|---|---|---|---|---|
| FXS (n = 189) | ASD and ID (n = 178) | ASD only (n = 185) | ID only (n = 177) | ||
| Age category (years) | |||||
| 5–11 | 372 (51.0) | 101 (53.4) | 94 (52.8) | 94 (50.8) | 83 (46.9) |
| 12–17 | 357 (49.0) | 88 (46.6) | 84 (47.2) | 91 (49.2) | 94 (53.1) |
| Race/ethnicity in four categories | |||||
| White | 639 (87.7) | 164 (86.8) | 157 (88.2) | 162 (87.6) | 156 (88.1) |
| Black-non-Hispanic | 25 (3.4) | 7 (3.7) | 5 (2.8) | 6 (3.2) | 7 (4.0) |
| Hispanic | 35 (4.8) | 10 (5.3) | 9 (5.1) | 9 (4.9) | 7 (4.0) |
| Other | 30 (4.1) | 8 (4.2) | 7 (3.9) | 8 (4.3) | 7 (4.0) |
| Child gender | |||||
| Male | 592 (81.2) | 153 (81.0) | 146 (82.0) | 152 (82.2) | 141 (79.7) |
| Female | 137 (18.8) | 36 (19.0) | 32 (18.0) | 33 (17.8) | 36 (20.3) |
| Highest household education | |||||
| High school | 49 (6.7) | 14 (7.4) | 10 (5.6) | 12 (6.5) | 13 (7.3) |
| Above high school | 680 (93.3) | 175 (92.6) | 168 (94.4) | 173 (93.5) | 164 (92.7) |
| Relationship to target child | |||||
| Mother | 664 (91.1) | 172 (91.0) | 163 (91.6) | 168 (90.8) | 161 (91.0) |
| Father | 59 (8.1) | 15 (7.9) | 14 (7.9) | 15 (8.1) | 15 (8.5) |
| Other | 6 (0.8) | 2 (1.1) | 1 (0.6) | 2 (1.1) | 1 (0.6) |
| Marital status of survey respondent | |||||
| Other | 114 (15.6) | 27 (14.3) | 26 (14.6) | 33 (17.8) | 28 (15.8) |
| Married | 615 (84.4) | 162 (85.7) | 152 (85.4) | 152 (82.2) | 149 (84.2) |
| Household income relative to federal poverty level | |||||
| < 100% | 87 (11.9) | 22 (11.6) | 24 (13.5) | 22 (11.9) | 19 (10.7) |
| 100%–≤200% | 90 (12.3) | 23 (12.2) | 19 (10.7) | 23 (12.4) | 25 (14.1) |
| 200%–≤300% | 155 (21.3) | 41 (21.7) | 40 (22.5) | 39 (21.1) | 35 (19.8) |
| 300%–≤400% | 132 (18.1) | 34 (18.0) | 29 (16.3) | 34 (18.4) | 35 (19.8) |
| >400% | 265 (36.4) | 69 (36.5) | 66 (37.1) | 67 (36.2) | 63 (35.6) |
| Family structure | |||||
| Two parents | 625 (85.7) | 159 (84.1) | 154 (86.5) | 158 (85.4) | 154 (87.0) |
| Single mom | 86 (11.8) | 25 (13.2) | 20 (11.2) | 23 (12.4) | 18 (10.2) |
| Other | 18 (2.5) | 5 (2.6) | 4 (2.2) | 4 (2.2) | 5 (2.8) |
Demographic characteristics between the four analytical groups were compared using chi-squared proportion test. After matching, there were no statistical differences in the demographic characteristics among these four analytical groups.
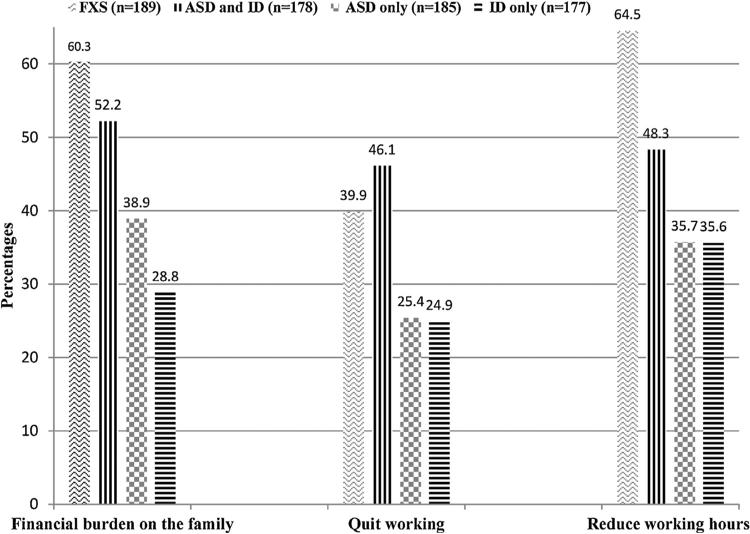
Significant differences were observed in reported financial and employment impacts (Fig. 1). Overall, more than 60% of caregivers of children with FXS reported that their child's condition caused an excessive financial burden on their families, compared with 52% of parents of children with ASD and ID, 39% of parents of children with ASD only, and 29% for ID only. When the answers from the FXS caregiver survey were coded as No (not at all) and Yes (a little bit, somewhat or a great deal), the differences in reported financial burdens between caregivers of FXS and other families were even greater. With regard to the most serious caregiver employment impact when a parent or other family member feels obliged to quit paid employment because of the child's condition, approximately 40% of caregivers of children with FXS said that they or another family member had quit working, as did 46% of parents of children with both ASD and ID and 25% of children with either ASD only or ID only. Almost 64% of caregivers of children with FXS reported changes in work hours, as did 48% of caregivers of children with ASD and ID and 36% of caregivers of children with ASD only or ID only.
Fig. 1.
Percentages of families that reported financial burden, quitting working, and reducing working hours: children with Fragile X Syndrome (FXS) and matched comparison groups of autism spectrum disorders (ASD) and intellectual disability (ID), ASD only, and ID only. Differences across groups (FXS, ASD and ID, ASD, ID) are statistically significant (P < 0.001) by chi-squared proportion test.
In pairwise comparisons, all differences in caregiver outcomes between groups were significant except the following: there were no statistically significant differences between the FXS group and the group with both ASD and ID regarding the percentages of reporting financial burden or quitting work. In addition, there were no statistically significant differences between the ASD only group and ID only group regarding quitting working or reducing working hours.
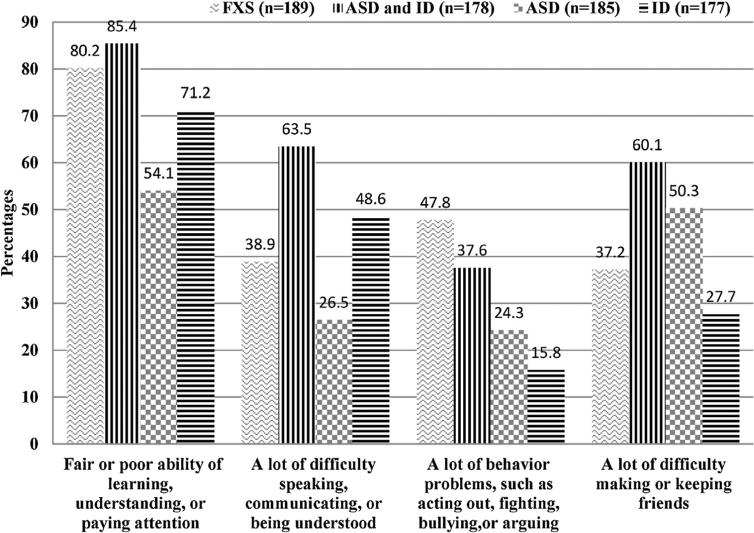
Fig. 2 compares reported functional difficulties for children with FXS and comparison groups. Significantly higher percentages of children with FXS (80.2%) and children with both ASD and ID (85.4%) were reported as having fair or poor overall abilities in comparison to ASD only (54.1%) or ID only (71.2%). A higher percentage of children with FXS (47.8%) reported behavioral problems than other groups (range: 15.8–37.6%). A lower percentage of children with FXS (37.2%) were reported as having a lot of difficulty making or keeping friends compared to children with ASD only (50.3%) or children with both ASD and ID (60.1%).
Fig. 2.
Percentage of reported functional difficulties of children with Fragile X Syndrome (FXS) and matched comparison groups of autism spectrum disorders (ASD) and intellectual disability (ID), ASD only, and ID only. Differences across groups (FXS, ASD and ID, ASD, ID) are statistically significant (P < 0.001) by chi-squared proportion test.
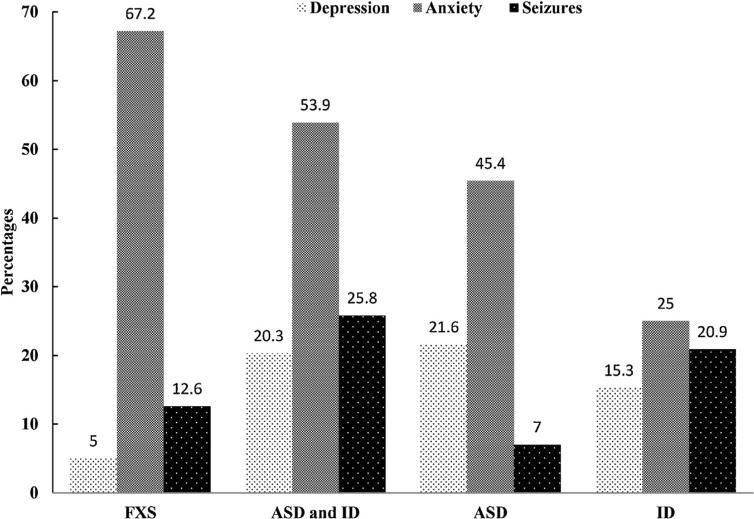
Fig. 3 compares reported co-occurring conditions for children with FXS and comparison groups. As high as 67.2% of children with FXS were reported as diagnosed with anxiety, significantly higher than any other comparison group (range: 25.0–53.9%). In contrast, only 5% of children with FXS were reported as diagnosed with depression, significantly lower than any other comparison group (range: 15.3–21.6%). About 12.6% of children with FXS were reported as diagnosed with seizures, higher than children with ASD only (7.0%), but lower than children with ID and ASD (25.8%) or ID without ASD (20.9%).
Fig. 3.
Percentage of reported co-occurring conditions of depression, anxiety, and seizures among children with Fragile X Syndrome (FXS) and matched comparison groups of autism spectrum disorders (ASD) and intellectual disability (ID), ASD only, and ID only. Differences across groups (FXS, ASD and ID, ASD, ID) are statistically significant (P < 0.001) by chi-squared proportion test.
Multivariate logistic regression results stratified by age (5–11 years and 12–17 years) are shown in Table 3. The results indicate the independent associations of condition type, co-occurring conditions, and functional difficulties. After controlling for co-occurring conditions, functional impacts of the child's condition, and family socioeconomic background, having a child with FXS was still significantly associated with caregiver financial burden and reduced working hours in reference to any of the other three comparison groups for children aged 12–17 years. For example, the odds of reporting financial burden for having a child with FXS was 2.61 times as large as the odds of reporting financial burden for having a child with ID only (OR = 2.61; 95% CI 1.59–4.29). In contrast, there was essentially no difference across ASD, ID, or both in terms of reported financial burden or change in working hours. For children aged 5–11 years, having a child with FXS was significantly associated with caregiver financial burden and reduced working hours in reference to the ID only group, but the odds ratios were insignificantly elevated relative to the ASD only group or ID and ASD group.
Table 3.
Logistic regression analyses (adjusted odds ratios and 95% confidence intervals) of financial burden and employment impact by FXS status, co-occurring conditions, and functional limitations, stratified by child's age.
| Children aged 5–11 years |
Children aged 12–17 years |
|||||
|---|---|---|---|---|---|---|
| Financial burden | Quit job | Reduce working hours | Financial burden | Quit job | Reduce working hours | |
| Reference: ASD only | 1.63 (0.85–3.14) |
0.99 (0.50–1.96) |
1.34 (0.68–2.63) |
2.73*** (1.29–5.77) |
2.58** (1.18–5.65) |
4.34*** (2.08–9.06) |
| Reference: intellectual disability only | 2.30** (1.11–4.77) |
1.38 (0.64–2.95) |
2.31** (1.09–4.90) |
2.82*** (1.36–5.83) |
0.99 (0.49–1.99) |
4.53*** (2.20–9.30) |
| Reference: intellectual disability and ASD | 1.32 (0.66–2.64) |
0.75 (0.37–1.51) |
1.59 (0.79–3.20) |
3.80*** (1.82–7.93) |
1.15 (0.58–2.30) |
3.79*** (1.86–7.72) |
| Co-occurring conditions | ||||||
| Depression | 0.59 (0.26–1.38) |
0.64 (0.26–1.56) |
0.94 (0.40–2.17) |
2.17** (1.17–4.05) |
0.89 (0.48–1.67) |
1.37 (0.75–2.49) |
| Anxiety | 1.47 (0.87–2.50) |
1.21 (0.70–2.10) |
1.21 (0.70–2.08) |
1.83** (1.06–3.17) |
1.4 (0.81–2.42) |
1.06 (0.63–1.80) |
| Seizures | 1.17 (0.61–2.26) |
1.57 (0.83–2.95) |
1.7 (0.88–3.28) |
2.11** (1.11–4.01) |
2.20*** (1.21–4.00) |
1.19 (0.65–2.19) |
| Child ability and behavior | ||||||
| Fair or poor ability to learn, understand, or pay attention | 2.17*** (1.23–3.83) |
1.07 (0.60–1.91) |
2.51*** (1.37–4.60) |
1.54 (0.83–2.86) |
1.74* (0.92–3.30) |
1.18 (0.66–2.12) |
| Difficulty with speech or communication | 0.77 (0.46–1.27) |
1.52 (0.91–2.54) |
0.74 (0.44–1.26) |
1.39 (0.81–2.38) |
0.91 (0.54–1.53) |
1.24 (0.74–2.08) |
| Irritability | 1.3 (0.78–2.18) |
1.04 (0.61–1.76) |
1.90** (1.12–3.21) |
1.4 (0.79–2.46) |
1.35 (0.77–2.35) |
1.27 (0.73–2.20) |
| Difficulty with making or keeping friends | 1.66* (0.99–2.78) |
0.94 (0.55–1.59) |
1.16 (0.68–1.96) |
0.9 (0.53–1.52) |
1.32 (0.79–2.22) |
1.09 (0.66–1.81) |
Three logistic regression models were run to show the effect of FXS group status with the reference group being ASD only, ID only, or ASD and ID. Besides co-occurring conditions and child ability and behavior listed here, all regressions control for race, gender, highest household education, survey respondent's marital status, and household income relative to federal poverty line.
P < 0.05.
P < 0.01.
P < 0.001.
Functional difficulties had mixed patterns of associations with the financial and employment outcome variables. In the older group of children aged 12–17 years, none of the associations was significant. Among children aged 5–11 years, caregivers of children with fair or poor ability in learning/understanding/paying attention were more likely to report financial burden (OR = 2.17; 95% CI 1.23–3.83) and more likely to report reducing working hours (OR = 2.51; 95% CI 1.37–4.60). Those caregivers who reported their child had higher levels of irritability were more likely to report reduced working hours (OR = 1.90; 95% CI 1.12–3.21). Neither social avoidance nor difficulty with speech or communication was associated with financial or employment impact.
Co-occurring conditions also had mixed patterns of associations with the financial and employment outcome variables. Among the younger children aged 5–11 years, none of the co-occurring conditions were significantly associated with any of the outcome measures. Among children aged 12–17 years, all three co-occurring conditions were significantly associated with perceived financial burden. Most of the associations with changes in parental employment were not significant. The one exception is that seizures in the child were significantly independently associated with quitting employment by the caregiver (OR = 2.20; 95% CI 1.21–4.00).
4. Discussion
Compared to family caregivers of CSHCN with diagnoses of ASD only or ID only, higher percentages of caregivers of children with FXS reported a negative family financial and employment impact. In contrast, the percentages of reported negative caregiver or family outcomes were mostly similar for families of children with FXS and families of children with both ASD and ID. In multivariate analyses, having a child with FXS aged 12–17 years was associated with higher likelihood of financial burden and reduced working hours relative to having a child with ASD or ID. Children with FXS were more likely to be reported as having behavioral problems/irritability and more likely to have parent-reported anxiety than children with ASD or ID. Factors associated with family impact included fair or poor thinking/reasoning/learning ability, more irritability, and co-occurring seizures or anxiety.
Both factors related to children with FXS and factors related to their caregivers may be associated with greater family impact in children with FXS. Children with FXS may have more functional limitations, complex health care and service needs and unmet needs than those with ASD or ID only. Our study showed that a higher percentage of children with FXS had irritability or fair or poor ability to learn/understand/pay attention, which were important factors in family impacts. Another potential reason for more negative financial and employment impacts is that family caregivers, usually the mothers of children with FXS, may have more functional limitations and care needs than other caregivers. Mothers of children with FXS are carriers (a premutation status) of FXS or may themselves be affected if they have the full mutation. Bailey et al. (2012) showed that one third of caregivers for children with FXS had seen a professional for anxiety, stress, or depression during the past year, and one fourth were taking medication to help with these symptoms. We do not have data to investigate health care and service needs of both children and their caregivers of FXS, in comparison to ASD or ID, which are important topics for future research.
Our results point to the importance of acknowledging the interactions of cognitive, behavioral, and psychosocial problems when investigating potential consequences for families of individuals with conditions such as FXS. Our findings are consistent with those of Martorell et al. (2011), who showed that the interaction of ID and co-occurring mental health problems resulted in a higher degree of burden on families than when just one of these conditions was present. Our findings are also consistent with a previous study that documented substantially higher overall health care costs for children with diagnoses of both ASD and ID than for children with diagnoses of ASD only or ID only (Peacock, Amendah, Ouyang, & Grosse, 2012). It has been suggested that persons with both ID and ASD have different needs from persons with ID or ASD alone (Matson & Shoemaker, 2009).
Our findings on the role of anxiety or seizures in family impacts expand on a previous report by Ouyang et al. (2010) indicating the associations between co-occurring conditions in FXS and their impact on the family. A report of physician-diagnosed anxiety was present in over 67% of children with FXS in our sample. Much lower frequencies of anxiety were reported for children with ASD only or ID only. This finding of high rates of anxiety disorders was consistent with Cordeiro, Ballinger, Hagerman and Hessl (2011) who documented that in comparison to idiopathic ID and the general population, FXS had significantly higher rates of anxiety disorders, ranging from 75% to 90% using appropriate clinical diagnostic criteria. In light of our finding that anxiety is independently and significantly associated with family financial burden in multivariate analyses, thorough clinical assessment and treatment of anxiety could be an important care component for individuals living with FXS. Although seizures in individuals with FXS are not as frequent as ID or ID and ASD, seizures stand out in multivariate analyses as a factor associated with family financial burden and caregivers quitting work. Studies suggested that seizures appeared to be associated with developmental-behavioral comorbidity that impacts function in FXS (Berry-Kravis et al., 2010). Future research is needed to further understand the impact of seizure in individuals with FXS and their families.
The study has several limitations. Although we used a matching method to construct social demographically similar comparison groups, there may still be unobserved differences across different surveys that could lead to the reported differences in caregiver impacts. For example, the majority of the FXS survey respondents completed the survey online while NS-CSHCN was a telephone based survey. Different survey responding methods may introduce bias. However, our study did not find responses to investigated questions differ systematically by surveys, which suggested that bias introduced by combing surveys, if existent, is not systematic and should not compromise our findings. Second, the questions on employment, time use, co-morbid conditions, and functional limitations are similar but not identical in these two surveys, which could be another source of bias. We tried to minimize such bias by selecting only those questions that solicit objective information and are similarly phrased. We suggest that surveys targeting particular population use tested questions from population based national surveys whenever possible for validity of comparison. Third, the sample of caregivers from the FXS survey was relatively well educated and had high family income, as were the comparison groups through matching. Although NS-CSHCN is nationally representative, the matching to a convenience sample no longer retains the representativeness of the comparison groups from NS-CSHCN. The caregiver impact may differ by the socioeconomic status of the household, and our results cannot be generalized to the general population of caregivers of FXS, ID, or ASD. A fourth limitation is that we did not examine differences among FXS families by whether the parents of children with FXS reported a physician diagnosis of autism, who comprised about 41% of the FXS sample, due to small sample size. In analyses not reported here, autism was associated with higher percentages of FXS families reporting negative financial or employment impacts, but we were not able to find statistically significant differences in financial or employment impacts by autism status in bivariate or regression analyses. Future studies distinguishing among FXS individuals, with or without autism, using a larger sample with more clinical details may better describe the spectrum of phenotypes in FXS and the implications for families.
In summary, this study shows that family caregivers affected by FXS are significantly more likely to report financial burden and to quit their job or cut working hours because of the condition than family caregivers affected by ASD only or ID only. Co-occurring conditions such as anxiety and seizure and functional limitations in learning or behavior are important factors in these family impacts. These findings may inform other discussions such as the importance of timely diagnosis and proper management of FXS. Health care and other services need to be responsive to the complex needs of children with FXS and their families. Additional research on access to care and unmet needs for children with FXS and their caregivers is needed in order to gain a deeper understanding of factors contributing to the elevated family financial burden and employment impacts of FXS.
Acknowledgement
The FXS survey from which these data were drawn was funded by a contract from Novartis Pharmaceutical Corporation (AFQ056B-5001) to RTI International.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease and Control and Prevention.
References
- Aman MG, Singh NB. Aberrant behavior checklist – Community, supplementary manual. Slosson Educational Publications; East Aurora, New York: 1994. [Google Scholar]
- Bailey DB, Raspa M, Olmsted MG. Using a parent survey to advance knowledge about the nature and consequences of fragile X syndrome. American Journal on Intellectual and Developmental Disabilities. 2011;115:447–460. doi: 10.1352/1944-7558-115.6.447. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Bishop E, Mitra D, Martin S, Wheeler A, Sacco P. Health and economic consequences of fragile X syndrome for caregivers. Journal of Developmental and Behavioral Pediatrics. 2012;33:705–712. doi: 10.1097/DBP.0b013e318272dcbc. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Sideris J, Roberts J, Hatton D. Child and genetic variables associated with maternal adaptation to fragile X syndrome: A multidimensional analysis. American Journal of Medical Genetics, Part A. 2008;146A:720–729. doi: 10.1002/ajmg.a.32240. [DOI] [PubMed] [Google Scholar]
- Bethell CD, Read D, Stein RE, Blumberg SJ, Wells N, Newacheck PW. Identifying children with special health care needs: Development and evaluation of a short screening instrument. Ambulatory Pediatrics. 2002;2:38–48. doi: 10.1367/1539-4409(2002)002<0038:icwshc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in fragile X syndrome: Characteristics and comorbid diagnoses. American Journal on Intellectual and Developmental Disabilities. 2010;115:461–472. doi: 10.1352/1944-7558-115.6.461. [DOI] [PubMed] [Google Scholar]
- Blackwell M, Iacus S, King G, Porro G. cem: Coarsened exact matching in Stata. Stata Journal. 2009;9:524L–546. [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Health Statistics, State and Local Area Integrated Telephone Survey 2009–2010 National Survey of Children with Special Health Care Needs Frequently Asked Questions. 2011 http://www.cdc.gov/nchs/slaits/cshcn.htm.
- Cidav Z, Marcus SC, Mandell DS. Implications of childhood autism for parental employment and earnings. Pediatrics. 2012;129:617–623. doi: 10.1542/peds.2011-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, Warren ST. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. American Journal of Human Genetics. 2009;85:503–514. doi: 10.1016/j.ajhg.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: Prevalence and characterization. Journal of Neurodevelopmental Disorders. 2011;3:57–67. doi: 10.1007/s11689-010-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Turk J, Hagerman R. The fragile X continuum: New advances and perspectives. Journal of Intellectual Disability Research. 2008;52:469–482. doi: 10.1111/j.1365-2788.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- Iacus SM, King G, Porro G. Causal inference without balance checking: Coarsened exact matching. Political Analysis. 2012;20:1–24. [Google Scholar]
- Irazabal M, Marsa F, Garcia M, Gutierrez-Recacha P, Martorell A, Salvador-Carulla L, Ochoa S. Family burden related to clinical and functional variables of people with intellectual disability with and without a mental disorder. Research in Developmental Disabilities. 2012;33:796–803. doi: 10.1016/j.ridd.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Kogan MD, Strickland BB, Blumberg SJ, Singh GK, Perrin JM, van Dyck PC. A national profile of the health care experiences and family impact of autism spectrum disorder among children in the United States, 2005–2006. Pediatrics. 2008;122:e1149–e1158. doi: 10.1542/peds.2008-1057. [DOI] [PubMed] [Google Scholar]
- Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, Schroeder S. Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. Journal of Intellectual Disability Research. 2006;50:532–545. doi: 10.1111/j.1365-2788.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- Lollar DJ, Hartzell MS, Evans MA. Functional difficulties and health conditions among children with special health needs. Pediatrics. 2012;129:e714–e722. doi: 10.1542/peds.2011-0780. [DOI] [PubMed] [Google Scholar]
- Matson JL, Shoemaker M. Intellectual disability and its relationship to autism spectrum disorders. Research in Developmental Disabilities. 2009;30:1107–1114. doi: 10.1016/j.ridd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Maes B, Broekman TG, Dosen A, Nauts J. Care giving burden of families looking after persons with intellectual disability and behavioural or psychiatric problems. Journal of Intellectual Disability Research. 2003;47:447–455. doi: 10.1046/j.1365-2788.2003.00513.x. [DOI] [PubMed] [Google Scholar]
- Martorell A, Gutierrez-Recacha P, Irazabal M, Marsa F, Garcia M. Family impact in intellectual disability, severe mental health disorders and mental health disorders in ID. A comparison. Research in Developmental Disabilities. 2011;32:2847–2852. doi: 10.1016/j.ridd.2011.05.021. [DOI] [PubMed] [Google Scholar]
- McGrath RJ, Stransky ML, Cooley WC, Moeschler JB. National profile of children with Down syndrome: Disease burden, access to care, and family impact. Journal of Pediatrics. 2011;159:535–540 e532. doi: 10.1016/j.jpeds.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Montes G, Halterman JS. Association of childhood autism spectrum disorders and loss of family income. Pediatrics. 2008a;121:e821–e826. doi: 10.1542/peds.2007-1594. [DOI] [PubMed] [Google Scholar]
- Montes G, Halterman JS. Child care problems and employment among families with preschool-aged children with autism in the United States. Pediatrics. 2008b;122:e202–e208. doi: 10.1542/peds.2007-3037. [DOI] [PubMed] [Google Scholar]
- Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: Implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. Journal of Intellectual Disability Research. 2009;53:852–873. doi: 10.1111/j.1365-2788.2009.01197.x. [DOI] [PubMed] [Google Scholar]
- Okumura MJ, Van Cleave J, Gnanasekaran S, Houtrow A. Understanding factors associated with work loss for families caring for CSHCN. Pediatrics. 2009;124(Suppl. 4):S392–S398. doi: 10.1542/peds.2009-1255J. [DOI] [PubMed] [Google Scholar]
- Ouyang L, Grosse S, Raspa M, Bailey D. Employment impact and financial burden for families of children with fragile X syndrome: Findings from the National Fragile X Survey. Journal of Intellectual Disability Research. 2010;54:918–928. doi: 10.1111/j.1365-2788.2010.01320.x. [DOI] [PubMed] [Google Scholar]
- Peacock G, Amendah D, Ouyang L, Grosse SD. Autism spectrum disorders and health care expenditures: The effects of co-occurring conditions. Journal of Developmental and Behavioral Pediatrics. 2012;33:2–8. doi: 10.1097/DBP.0b013e31823969de. [DOI] [PubMed] [Google Scholar]
- Peprah E. Fragile X syndrome: The FMR1 CGG repeat distribution among world populations. Annals of Human Genetics. 2012;76:178–191. doi: 10.1111/j.1469-1809.2011.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul RA, Tarleton JC. FMR1-related disorders. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews. University of Washington; Seattle, WA: 1993. [Google Scholar]
- Schieve LA, Boulet SL, Kogan MD, Van Naarden-Braun K, Boyle CA. A population-based assessment of the health, functional status, and consequent family impact among children with Down syndrome. Disability and Health Journal. 2011;4:68–77. doi: 10.1016/j.dhjo.2010.06.001. [DOI] [PubMed] [Google Scholar]