Abstract
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder, the pathophysiology of which is not completely known, although it has been shown that genetic/social learning factors, diet, intestinal microbiota, intestinal low-grade inflammation, and abnormal gastrointestinal endocrine cells play a major role. Studies of familial aggregation and on twins have confirmed the heritability of IBS. However, the proposed IBS risk genes are thus far nonvalidated hits rather than true predisposing factors. There is no convincing evidence that IBS patients suffer from food allergy/intolerance, with the effect exerted by diet seemingly caused by intake of poorly absorbed carbohydrates and fiber. Obesity is a possible comorbidity of IBS. Differences in the microbiota between IBS patients and healthy controls have been reported, but the association between IBS symptoms and specific bacterial species is uncertain. Low-grade inflammation appears to play a role in the pathophysiology of a major subset of IBS, namely postinfectious IBS. The density of intestinal endocrine cells is reduced in patients with IBS, possibly as a result of genetic factors, diet, intestinal microbiota, and low-grade inflammation interfering with the regulatory signals controlling the intestinal stem-cell clonogenic and differentiation activities. Furthermore, there is speculation that this decreased number of endocrine cells is responsible for the visceral hypersensitivity, disturbed gastrointestinal motility, and abnormal gut secretion seen in IBS patients.
Keywords: Diet, Endocrine cells, Genetic factors, Low-grade inflammation, Microbiota, Stem cells
Core tip: There are several factors that play a major role in the pathophysiology of irritable bowel syndrome (IBS). These factors are genetic disposition, diet, the intestinal microbiota, and mucosal low-grade inflammation. These factors are known to affect the gastrointestinal endocrine cells, with the densities of intestinal endocrine cells being reduced in IBS patients. The reduction in the gastrointestinal endocrine cells seems to be caused by abnormal clonogenic and differentiation activities of the intestinal stem cells. The abnormalities in the gastrointestinal endocrine cells can explain the visceral hypersensitivity, disturbed gastrointestinal motility, and abnormal gut secretion observed in IBS patients.
INTRODUCTION
Patients with irritable bowel syndrome (IBS) suffer from intermittent abdominal pain or discomfort in combination with altered bowel habits and abdominal distension/bloating[1-3]. These symptoms cause significant morbidity, with impaired quality of life and reduced work productivity[4,5], and is an economic burden to both the patients and society[6-12]. IBS patients can be divided into three subtypes according to the predominant bowel pattern: diarrhea-predominant (IBS-D), constipation-predominant (IBS-C), and both diarrhea and constipation (IBS-M)[13].
The pathophysiology of IBS is incompletely understood, and there is no diagnostic test or effective treatment for this condition[14-16]. Thus, IBS patients visit doctors more frequently, undergo more diagnostic tests and examinations, consume more medications, and are hospitalized more frequently than those without IBS[6-12]. Understanding the pathophysiology of IBS is necessary in order to develop better diagnostic methods and effective treatments, and consequently reduce the economical costs both for patients and society. New data on the pathophysiology of IBS have accumulated over the past few years, improving our understanding of this disorder[1,15-20]. The aim of this review was to account for these new data and integrate them into the current knowledge on the pathophysiology of IBS.
PATHOPHYSIOLOGY OF IBS
There is evidence that several factors play a central role in the pathophysiology of IBS, such as genetic/social learning factors, diet, the intestinal microbiota, low-grade chronic intestinal inflammation, and abnormal gastrointestinal endocrine cells[1,14-20].
Heritability and social learning
Familial aggregation: Familial clustering of IBS has been noted in everyday clinical practice, with 37% of IBS patients reportedly having a family history of the disorder[21]. Moreover, it has been shown that IBS patients are more likely (33.9%) than controls (12.6%) to have a family history of IBS[22]. In a cohort of IBS patients from Olmsted County, USA, a significant association was found between IBS patients and having a first-degree family member with IBS, but not for their non-IBS spouses[23]. The prevalence of IBS was 17% among IBS patients’ relatives, compared to 7% among their spouses’ relatives[24]. Similarly, the prevalence rates of IBS were reported to be 21%-50% and 4%-27% among relatives of IBS patients and non-IBS controls, respectively[25,26]. In a recently published, nationwide survey of the Swedish population, the risk of IBS was found to be increased in the first-, second-, and third-degree relatives of patients with IBS compared with their non-IBS counterparts, with the risk tending to be higher in more closely related relatives[27].
Twin studies: All twin studies confirm a substantial genetic component in IBS[28-31], with one exception[32]. Among 343 Australian twin pairs, IBS was found to occur at rate of 33.3% in monozygotic twins compared to 13.3% in dizygotic twins, with 56.9% of the variance being due to additive genetic factors.[28] In two studies involving 6060 and 986 American twin pairs[29,33], the first study showed that the concordance of IBS was significantly greater in monozygotic (17.2%) than in dizygotic (8.4) twins[29], and in the second study the polychoric correlation of IBS for monozygotic twins with IBS was greater than that for dizygotic twins (47% and 17%, respectively)[33]. In Scandinavia, a study conducted involving 3286 Norwegian twin pairs found that the concordance for IBS was significantly higher among monozygotic (22.4%) than dizygotic (9.1%) twins, and that the concordance was higher (48.5%) in females[31]. However, in contrast to all other twin studies, a study of 1870 British twin pairs did not reveal any significant difference in the rates of IBS between monozygotic and dizygotic twins[32].
Genetic studies: The aforementioned epidemiological and twin studies point to a potential involvement of specific genes in the pathogenesis of IBS. Most of the genetic research has concentrated on the serotonin signaling pathways, control of immune activation, bile acid synthesis, neuropeptide activity, and intestinal secretion[34-37]. More than 60 gene candidates have been proposed to play a role in the genetic predisposition to IBS, but these risk genes have yet to be validated[38]. The most important of these gene candidates are described in detail elsewhere[39]. Several studies have focused on the HTTLPR genotype, which controls the expression of the SLC6A4 (serotonin transporter protein); however, the reported association with IBS is equivocal[40-43].
The gene that is most likely to be associated with IBS, and with IBS-C in particular, is that encoding tumor necrosis factor superfamily 15 (TNFSF15). It was first described in Swedish and US patients, and was confirmed in patient cohorts in the UK and Canada[44-46]. However, in a genome-wide association study (GWAS) the association between TNFSF15 and IBS was found to be nonsignificant[47]. It was suggested that this seemingly contradictory finding can be explained by the possibility that genetic effects are diluted and more difficult to detect at the general population level[47].
In a general population GWAS, a locus at 7p22.1, which includes the genes KDELR2 and GRID2IP, showed consistent IBS risk effects[47]. KDELR2 encodes a family of receptors, the most well known of which is KDELR1 [KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 1], which is an integral membrane protein that binds the Lys-Asp-Leu-Glu and Arg-Asp-Leu-Glu amino acid motifs of target proteins and mediates their retrograde transport to the endoplasmic reticulum[48-52]. GRID2IP encodes delphilin, which is expressed in nerve-fiber-Purkinje-cell synapses in the brain[53,54].
The reasons underlying the conflicting results yielded by genetic association studies, and especially in IBS, are discussed elsewhere[38,55]. The IBS risk genes proposed so far are nonvalidated hits rather than true predisposing factors, and the studies conducted have been largely too underpowered to capture true association signals[38]. In the future, research in this field should apply the promising GWAS approach to research candidate mechanisms rather than symptom definition[38].
Environment and social learning
Parental modeling and reinforcement of illness behaviors may play a role in the pathophysiology of IBS[29,56-59]. Having a mother with IBS accounts for as much variance as having an identical twin with IBS[56]. Aggregation of IBS among spouses to IBS patients has been reported to indicate that nongenetic - and most probably environmental factors - are responsible for IBS clustering[27]. In a more recent comprehensive review, where a careful weighing of evidence was made, concluded that social learning may be one of the factors involved in the etiology of IBS[60]. Moreover, the pain caused by visceral hypersensitivity in IBS has been attributed to atypical attention to pain as a part of illness behavior[61,62].
Diet: Most IBS patients believe that certain food items trigger their symptoms[63-71]. This has resulted in IBS patients making conscious choices to avoid foodstuffs such as milk and milk products, wheat products, caffeine, cabbage, onions, peas, beans, hot spices, and fried and smoked food[63,68,72,73].The intake of energy, carbohydrates, proteins, and fats in IBS patients does not differ from that of the general population[72-78]. However, IBS patients tend to avoid several food sources that are important to their health, especially those rich in vitamins and minerals[73]. Several factors have been discussed to explain the mechanisms by which diet plays its role in the pathophysiology of IBS, such as poorly absorbed carbohydrates and fiber, food allergy/intolerance, and the comorbidity of obesity and IBS[1,17,20,79-83].
Poorly absorbed carbohydrates and fiber: Several food items contain indigestible and poorly absorbed short-chain carbohydrates, namely fermentable oligo-, di-, and monosaccharides, and polyols (FODMAPs)[1]. FODMAPs include fructose, lactose, sugar sources (sorbitol, maltitol, mannitol, xylitol, and isomalt), fructans, and galactans[1,84], and occur in a wide range of foods such as wheat, rye, vegetables, fruits, and legumes[85-87]. These unabsorbed carbohydrates enter the distal small intestine and colon, where they increase the osmotic pressure in the luminal cavity and provide a substrate for bacterial fermentation[84,88,89]. This bacterial fermentation leads to gas production, which in turn causes abdominal distension and abdominal pain/discomfort. FODMAPs have been found to trigger gastrointestinal symptoms in IBS, and a low-FODMAPs diet reduces symptoms and improves the patient’s quality of life[73,78,90-95].
Recent studies have shown that the triggering of IBS symptoms by FODMAPs is much more complicated than was originally thought. Thus, a low FODMAPs intake induces favorable changes in the intestinal microbiota[96] and gastrointestinal endocrine cells[97-100]. The change from a diet of typical Australian food to a low-FODMAPs diet was found to change the intestinal microbiota; whereas a typical Australian diet increases the relative abundance of butyrate-producing Clostridium cluster XIVa and the mucus-associated Akkermansia muciniphila, and reduces Ruminococcus torques, a low-FODMAPs diet reduces the total bacterial abundance[96]. Several endocrine cell types in the gastrointestinal tract of IBS patients are abnormal[101-120], and these abnormalities are considered to play a major role in the development of IBS symptoms and represent future targets for treatment[16,121]. Switching from a typical Norwegian diet to a low-FODMAPs diet has been shown to change the densities of endocrine cells in the stomach and large intestine toward normal levels[97-100].
Food allergy/intolerance: There is no convincing evidence to support the idea that IBS patients suffer from food allergy/intolerance[64,67,122-128]. The prevalence of nonceliac gluten sensitivity (NCGS) in the United States has been reported to range from 0.55% to 6%[129,130]. NCGS is defined as patients with gastrointestinal and extragastrointestinal IBS-like symptoms without celiac disease or wheat allergy, and with symptom relief on a gluten-free diet (GFD) and relapse on gluten challenge[130-137].
NCGS was first described more than 30 years ago[138,139], and has been the focus of several recent reports[140-144]. Contradictory results have been reported regarding whether or not NCGS patients have low-grade inflammation and abnormal intestinal permeability[141,144-151]. However, in double-blind, randomized, placebo-controlled studies[141,143,144], the positive effects on symptoms in NCGS patients were actually found to be the result of wheat withdrawal rather than gluten withdrawal[152]. In a placebo-controlled, crossover study of patients with IBS-like symptoms with self-imposed GFD[153], the gastrointestinal symptoms consistently and significantly improved when the FODMAPs intake was reduced, and these symptoms were not worsened by either a low- or high-dose challenge with gluten. It therefore seems that it is the carbohydrate content (fructans and galactans) in the wheat rather than gluten that is responsible for triggering NCGS symptoms. This conclusion is supported further by the findings that in those who believed that they had NCGS, 24% had uncontrolled symptoms despite consuming a GFD, 27% were not following a GFD alone, and 65% avoided other foods that contain high levels of FODMAPs[154].
NCGS and IBS patients experience the same symptoms that are triggered by wheat intake, and both groups have a high frequency of antigliadin antibodies (AGAs) with negative tissue transglutaminase, or deamidated gliadin peptide antibodies[133,143,155-158]. AGAs have been reported to have a good sensitivity but a low specificity for celiac disease[159], and 12%-15% of healthy subjects are reportedly positive for AGAs[155,159,160]. It is thus possible to conclude that NCGS patients are IBS patients who are self-diagnosed and have self-treated by adhering to a GFD.
Obesity and IBS: There has been some concern that the onset of symptoms upon ingesting food would result in low food intake with consequent malnutrition in patients with IBS[73,161]. However, while some studies have found an association between low body mass index (BMI) and IBS[162], others have found the predominance of normal-weight or overweight IBS patients[63]. The association between IBS and obesity was found to be controversial in a comprehensive review, and the author concluded that obesity and IBS might be linked[163].
Appetite is regulated by a large number of hormones, including those secreted by the gastrointestinal endocrine cells[164]. The densities of the following five gastrointestinal endocrine cell types that secrete hormones known to regulate appetite are abnormal in patients with IBS: ghrelin, cholecystokinin, peptide YY, enteroglucagon (oxyntomodulin), and serotonin[101,103,104,107,165-171]. Ghrelin stimulates food intake and body weight gain[172,173]. The density of this endocrine cell type is increased in IBS-D patients. The densities of endocrine cells that secrete the other four hormones, which have an anorexigenic action[174-189], are reduced in patients with IBS. This would be predictive of an increased appetite and food intake in IBS patients. BMI and appetite in IBS patients have not been fully studied, and the currently available data are controversial. It is not clear whether IBS patients have an increased appetite, which is opposed by the avoidance of eating because of worsening of symptoms upon eating, or a high BMI.
Intestinal microbiota: The role of the intestinal microbiota in the pathophysiology of IBS has been discussed in detail elsewhere[190-194]. The human intestine contains about 1014 bacteria belonging to over 1000 species[190,195,196]. These bacteria can be present in the lumen or attached to or embedded in the mucus layer of the gastrointestinal tract[197]. The number of bacteria is lower in the small intestine than in the colon, and decreases gradually toward the upper parts of the gastrointestinal tract[198-200]. The gastrointestinal microbial composition is determined by host genetic factors and environmental factors[193]. The environmental factors include mode of delivery at birth, aging, treatment with antibiotics, and sanitation status[201]. The gastrointestinal microbiota plays a role in gastrointestinal motility, gut immune defense, digestion and metabolism, inflammation, and cell proliferation[193,202].
Several studies using culture-based and culture-independent methods have shown that the microbiota - as detected in feces samples - differs between in IBS patients and healthy controls[203-229]. However, the association between IBS symptoms and specific bacterial species is uncertain[191]. Although contradictory results have been reported, decreased levels of lactobacilli and bifidobacteria, and increased levels of anaerobic bacteria such as streptococci and Escherichia coli, as well as increased ratios of Firmicutes, Bacteroidetes, and Clostridium species have been confirmed in several studies[206,226].
Low-grade inflammation: It has been suggested that the presence of colonic mucosal low-grade inflammation plays a role in the pathophysiology of IBS[18,230]. However, studies of mucosal low-grade inflammation in the colon have yielded contradictory results[231]. There are reports of increased numbers of intraepithelial immune cells, and elevated numbers of immune cells and mast cells in lamina propria of rectal biopsies taken from patients with postinfectious IBS (PI-IBS)[116,232,233]. The densities of immune and mast cells in the mucosa of patients with sporadic (nonspecific) IBS (non-PI-IBS) did not differ from those in healthy controls[234] . An increased number of intraepithelial lymphocytes has been found in studies in which no attention was paid to the previous history of gastrointestinal infection[235-237]. However, an unchanged density of mast cells was found in studies in which no distinction was made between PI-IBS and non-PI-IBS[235,238,239]. Moreover, the mast cell density was elevated in PI-IBS but not in non-PI-IBS[118,235]. Similar to the immune cells and mast cells, inconsistent findings have been reported regarding cytokines in patients with IBS[240], whereby changes in cytokines were reported in IBS patients[240-242], but not in those with non-PI-IBS[243].
The research performed to date provides compelling evidence that low-grade inflammation occurs in a subset of IBS patients, namely those with PI-IBS, but not in those with non-PI-IBS. PI-IBS represents a considerable proportion of IBS patients, with an incidence of 7%-31% among IBS patients[244-246]. Thus, low-grade inflammation plays a significant role in the pathophysiology in a subset of IBS patients.
Abnormal gastrointestinal endocrine cells
Gastrointestinal endocrine cells: The gastrointestinal tract contains at least 15 different types of endocrine cells that are spread among the epithelial cells of the mucosa[14,78,170,247-250]. These cells, which constitute about 1% of all epithelial cells in the gastrointestinal tract[247,248,251-253], have specialized sensors in the form of microvilli that project into the lumen and respond to luminal stimuli by releasing hormones[101,254-265]. The distribution, functions, and modes of action of the most important endocrine/paracrine cells are described in detail elsewhere[15,16,170]. In brief, they secrete one or more signaling substances into the lamina propria, where these substances act directly on nearby structures (autocrine/paracrine mode) and/or indirectly via an endocrine mode of action (by circulating in the blood to reach distant targets)[266]. They regulate several functions of the gastrointestinal tract such as sensation, motility, secretion, absorption, local immune defense, and food intake[1,166,170,247,248]. These cells interact and integrate with each other and with the enteric nervous system and the afferent and efferent nerve fibers of the central nervous system[1,166,170,267].
Abnormal endocrine cells have been found in both sporadic IBS and PI-IBS patients. In sporadic IBS, abnormal endocrine cells were found in the stomach, proximal small intestine (duodenum), distal small intestine (ileum), colon, and rectum[111-113,167,171,268-276]. Although the densities of endocrine cell types can vary (i.e., decrease or increase), the general trend of the entire intestinal endocrine cell population is toward a decrease in IBS. This becomes evident when intestinal biopsy samples are stained with chromogranin A, which is a common marker for endocrine cells. Thus, the densities of the total endocrine cells in the duodenum, ileum, and colon are reportedly decreased, while those of the stomach and rectum are unchanged (Figure 1)[102,269,271,272]. In contrast to sporadic IBS, the densities of intestinal endocrine cells in patients with PI-IBS tend to increase[109,113,114,116-120,277].
Figure 1.
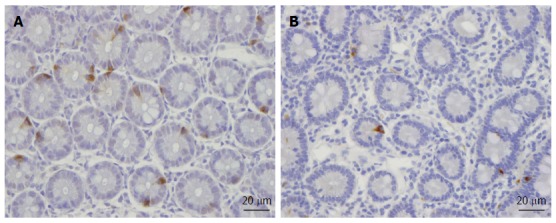
Chromogranin A-immunoreactive cells in (A) a healthy subject and (B) an irritable bowel syndrome patient.
Stem cells: Each intestinal crypt contains four to six stem cells, which originate from pluripotent stem cells of endodermal origin[247,248,278]. These cells divide into new stem cells (self-renewal; clonogeny) and into cells that differentiate into all epithelial cell types including enterocytes, goblet cells, Paneth cells, and endocrine cells (differentiation progeny)[279-293]. The differentiation progeny includes two lineages: secretory and absorptive. The secretory lineage gives rise to goblet, endocrine, and Paneth cells, and the absorptive lineage to absorptive enterocytes (Figure 2)[279-293].
Figure 2.
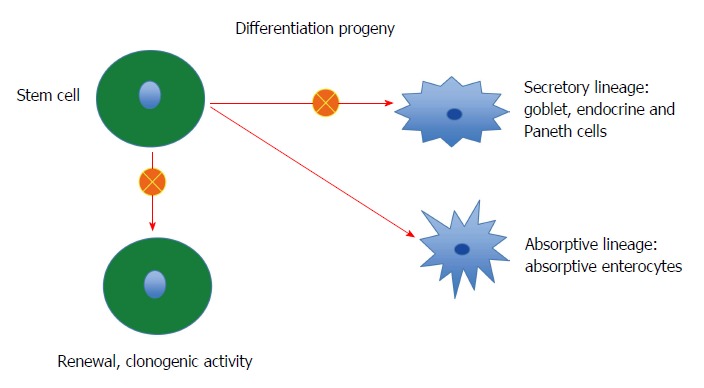
The intestinal stem cell exerts 2 activities: clonogenic activity, where it produce a copy of itself to maintain the number of stem cells constant in the crypts, and differentiation activity. The differentiation consists of 2 lineages: secretory lineage and absorptive lineage. Through a cascade of progenitors the secretory lineage give rise to goblet, endocrine and Paneth cells and the absorptive lineage to absorptive enterocytes. In IBS patients, both clonogenic and differentiation activities are abnormal.
As mentioned above, the total density of intestinal endocrine cells is reduced in sporadic IBS. A similar reduction in the density of intestinal endocrine cells has been observed in congenital malabsorptive diarrhea, and following small-intestine allograft rejection[294,295]. The decrease in the density of endocrine cells in both conditions has been found to be caused by a mutation in the gene encoding the protein neurogenin 3 (NEUROG3), which is expressed in the endocrine progenitor cells required for intestinal endocrine development, and a reduction in the progenitors of intestinal endocrine cells that express NEUROG3 and NeuroD[294,295]. It has recently been reported that the densities of cells expressing Musashi 1 (Msi-1, expressed in both stem cells and in their early progeny; Figure 3) and NEUROG3 (expressed in early endocrine cell progenitors; Figure 4)[296] were reduced in the duodenum of sporadic IBS patients[299]. It was concluded that disturbance of the clonogenic and differentiation activities of intestinal stem cells could be responsible for the reduction of intestinal endocrine cells observed in IBS patients[296].
Figure 3.
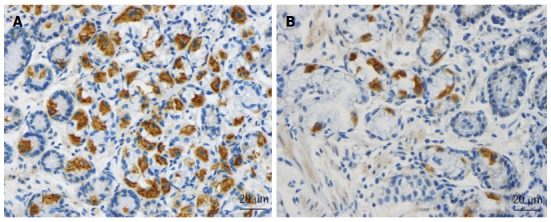
Msi-1-immunoreactive cells in duodenum of subjects from the (A) control, and (B) irritable bowel syndrome patient.
Figure 4.
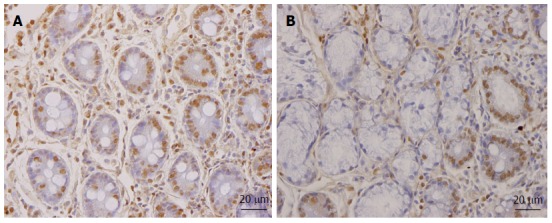
NEUROG3-immunoreactive cells in (A) a healthy subject and (B) an irritable bowel syndrome patient.
HYPOTHESIS
IBS patients exhibit visceral hypersensitivity, disturbed gastrointestinal motility, and abnormal gut secretion[297-301]. The gastrointestinal endocrine cells, as mentioned above, regulate several functions of the gut including sensation, motility, and secretion. The density of the intestinal endocrine cells is generally reduced in sporadic IBS. This reduction appears to be caused by a reduction in intestinal stem-cell self-renewal and proliferation. Intestinal stem-cell self-renewal (clonogeny) and proliferation are regulated by several signaling pathways[287]. As demonstrated in this review, heredity, diet, the intestinal microbiota, and low-grade inflammation play a major role in the pathophysiology of IBS. Changes in diet, intestinal bacterial flora, and inflammation have been reported to affect the density of endocrine cells in the gut[1,97,302]. It can be speculated that the factors that have been shown to play a major role in the pathophysiology of IBS will affect the signaling pathways for stem-cell clonogenic renewal and proliferation, resulting in abnormalities in the gastrointestinal endocrine cells with the development of IBS symptoms (Figure 5).
Figure 5.
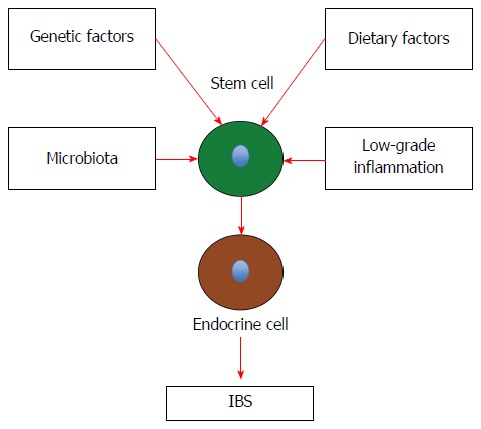
Schematic drawing to illustrate the possible pathophysiology of irritable bowel syndrome. Details are described in the text. IBS: Irritable bowel syndrome.
CONCLUSION
There is compelling evidence that genetic factors, diet, the intestinal microbiota, and mucosal low-grade inflammation play a major role in the pathophysiology of IBS. These factors are known to affect the gastrointestinal endocrine cells, with the densities of intestinal endocrine cells being reduced in IBS patients. The abnormalities in the gastrointestinal endocrine cells can explain the visceral hypersensitivity, disturbed gastrointestinal motility, and abnormal gut secretion observed in IBS patients.
The reduction in intestinal endocrine cells appears to be caused by disturbance of the clonogenic and differentiation activities of the intestinal stem cells. The clonogeny and proliferation of intestinal stem cells are regulated by several signaling pathways. It is possible that genetic factors, diet, the intestinal microbiota, and mucosal low-grade inflammation interfere with the signals regulating the clonogenic and proliferation activities of stem cells, resulting in a reduction in the density of intestinal endocrine cells. This reduction of intestinal endocrine cells can in turn result in the development of IBS symptoms.
Footnotes
Supported by Grants from Helse-Vest and Helse-Fonna, Norway.
Conflict-of-interest statement: The author declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 28, 2015
First decision: March 26, 2015
Article in press: May 21, 2015
P- Reviewer: Andrae DA, Ballou SK, Kamiya T S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151–5163. doi: 10.3748/wjg.v18.i37.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 3.Rey E, Talley NJ. Irritable bowel syndrome: novel views on the epidemiology and potential risk factors. Dig Liver Dis. 2009;41:772–780. doi: 10.1016/j.dld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Dean BB, Aguilar D, Barghout V, Kahler KH, Frech F, Groves D, Ofman JJ. Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care. 2005;11:S17–S26. [PubMed] [Google Scholar]
- 5.DiBonaventura M, Sun SX, Bolge SC, Wagner JS, Mody R. Health-related quality of life, work productivity and health care resource use associated with constipation predominant irritable bowel syndrome. Curr Med Res Opin. 2011;27:2213–2222. doi: 10.1185/03007995.2011.623157. [DOI] [PubMed] [Google Scholar]
- 6.Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology. 1995;109:1736–1741. doi: 10.1016/0016-5085(95)90738-6. [DOI] [PubMed] [Google Scholar]
- 7.Harvey RF, Salih SY, Read AE. Organic and functional disorders in 2000 gastroenterology outpatients. Lancet. 1983;1:632–634. doi: 10.1016/s0140-6736(83)91802-0. [DOI] [PubMed] [Google Scholar]
- 8.Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 9.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187.e1-3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in the irritable bowel syndrome. Arch Intern Med. 2003;163:265–274. doi: 10.1001/archinte.163.3.265. [DOI] [PubMed] [Google Scholar]
- 11.Boivin M. Socioeconomic impact of irritable bowel syndrome in Canada. Can J Gastroenterol. 2001;15 Suppl B:8B–11B. doi: 10.1155/2001/401309. [DOI] [PubMed] [Google Scholar]
- 12.Gaburri M, Bassotti G, Bacci G, Cinti A, Bosso R, Ceccarelli P, Paolocci N, Pelli MA, Morelli A. Functional gut disorders and health care seeking behavior in an Italian non-patient population. Recenti Prog Med. 1989;80:241–244. [PubMed] [Google Scholar]
- 13.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 14.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: diagnosis, pathogenesis and treatment options. New York: Nova Science Publishers, Inc; 2012. [Google Scholar]
- 15.El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. Irritable bowel syndrome: recent developments in diagnosis, pathophysiology, and treatment. Expert Rev Gastroenterol Hepatol. 2014;8:435–443. doi: 10.1586/17474124.2014.888952. [DOI] [PubMed] [Google Scholar]
- 16.El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20:384–400. doi: 10.3748/wjg.v20.i2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YJ, Park KS. Irritable bowel syndrome: emerging paradigm in pathophysiology. World J Gastroenterol. 2014;20:2456–2469. doi: 10.3748/wjg.v20.i10.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–173. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 19.Öhman L, Törnblom H, Simrén M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol. 2015;12:36–49. doi: 10.1038/nrgastro.2014.200. [DOI] [PubMed] [Google Scholar]
- 20.Soares RL. Irritable bowel syndrome: a clinical review. World J Gastroenterol. 2014;20:12144–12160. doi: 10.3748/wjg.v20.i34.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut. 1986;27:37–40. doi: 10.1136/gut.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanazawa M, Endo Y, Whitehead WE, Kano M, Hongo M, Fukudo S. Patients and nonconsulters with irritable bowel syndrome reporting a parental history of bowel problems have more impaired psychological distress. Dig Dis Sci. 2004;49:1046–1053. doi: 10.1023/b:ddas.0000034570.52305.10. [DOI] [PubMed] [Google Scholar]
- 23.Locke GR, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ. Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc. 2000;75:907–912. doi: 10.4065/75.9.907. [DOI] [PubMed] [Google Scholar]
- 24.Kalantar JS, Locke GR, Zinsmeister AR, Beighley CM, Talley NJ. Familial aggregation of irritable bowel syndrome: a prospective study. Gut. 2003;52:1703–1707. doi: 10.1136/gut.52.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito YA, Zimmerman JM, Harmsen WS, De Andrade M, Locke GR, Petersen GM, Talley NJ. Irritable bowel syndrome aggregates strongly in families: a family-based case-control study. Neurogastroenterol Motil. 2008;20:790–797. doi: 10.1111/j.1365-2982.2007.1077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito YA, Petersen GM, Larson JJ, Atkinson EJ, Fridley BL, de Andrade M, Locke GR, Zimmerman JM, Almazar-Elder AE, Talley NJ. Familial aggregation of irritable bowel syndrome: a family case-control study. Am J Gastroenterol. 2010;105:833–841. doi: 10.1038/ajg.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waehrens R, Ohlsson H, Sundquist J, Sundquist K, Zöller B. Risk of irritable bowel syndrome in first-degree, second-degree and third-degree relatives of affected individuals: a nationwide family study in Sweden. Gut. 2015;64:215–221. doi: 10.1136/gutjnl-2013-305705. [DOI] [PubMed] [Google Scholar]
- 28.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311–1317. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 29.Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 30.Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Quigley EM. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109 Suppl 1:S2–26; quiz S27. doi: 10.1038/ajg.2014.187. [DOI] [PubMed] [Google Scholar]
- 31.Bengtson MB, Rønning T, Vatn MH, Harris JR. Irritable bowel syndrome in twins: genes and environment. Gut. 2006;55:1754–1759. doi: 10.1136/gut.2006.097287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammed I, Cherkas LF, Riley SA, Spector TD, Trudgill NJ. Genetic influences in irritable bowel syndrome: a twin study. Am J Gastroenterol. 2005;100:1340–1344. doi: 10.1111/j.1572-0241.2005.41700.x. [DOI] [PubMed] [Google Scholar]
- 33.Lembo A, Zaman M, Jones M, Talley NJ. Influence of genetics on irritable bowel syndrome, gastro-oesophageal reflux and dyspepsia: a twin study. Aliment Pharmacol Ther. 2007;25:1343–1350. doi: 10.1111/j.1365-2036.2007.03326.x. [DOI] [PubMed] [Google Scholar]
- 34.Camilleri M. Serotonin in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2009;16:53–59. doi: 10.1097/med.0b013e32831e9c8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–G785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 36.Saito YA. The role of genetics in IBS. Gastroenterol Clin North Am. 2011;40:45–67. doi: 10.1016/j.gtc.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito YA, Mitra N, Mayer EA. Genetic approaches to functional gastrointestinal disorders. Gastroenterology. 2010;138:1276–1285. doi: 10.1053/j.gastro.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Amato M. Genes and functional GI disorders: from casual to causal relationship. Neurogastroenterol Motil. 2013;25:638–649. doi: 10.1111/nmo.12173. [DOI] [PubMed] [Google Scholar]
- 39.Camilleri M. Genetics of human gastrointestinal sensation. Neurogastroenterol Motil. 2013;25:458–466. doi: 10.1111/nmo.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Kerkhoven LA, Laheij RJ, Jansen JB. Meta-analysis: a functional polymorphism in the gene encoding for activity of the serotonin transporter protein is not associated with the irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:979–986. doi: 10.1111/j.1365-2036.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- 41.Jarrett ME, Kohen R, Cain KC, Burr RL, Poppe A, Navaja GP, Heitkemper MM. Relationship of SERT polymorphisms to depressive and anxiety symptoms in irritable bowel syndrome. Biol Res Nurs. 2007;9:161–169. doi: 10.1177/1099800407307822. [DOI] [PubMed] [Google Scholar]
- 42.Kohen R, Jarrett ME, Cain KC, Jun SE, Navaja GP, Symonds S, Heitkemper MM. The serotonin transporter polymorphism rs25531 is associated with irritable bowel syndrome. Dig Dis Sci. 2009;54:2663–2670. doi: 10.1007/s10620-008-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan J, Kang C, Wang M, Wang Q, Li P, Liu H, Hou Y, Su P, Yang F, Wei Y, et al. Association study of serotonin transporter SLC6A4 gene with Chinese Han irritable bowel syndrome. PLoS One. 2014;9:e84414. doi: 10.1371/journal.pone.0084414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zucchelli M, Camilleri M, Andreasson AN, Bresso F, Dlugosz A, Halfvarson J, Törkvist L, Schmidt PT, Karling P, Ohlsson B, et al. Association of TNFSF15 polymorphism with irritable bowel syndrome. Gut. 2011;60:1671–1677. doi: 10.1136/gut.2011.241877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wouters MM, Lambrechts D, Knapp M, Cleynen I, Whorwell P, Agréus L, Dlugosz A, Schmidt PT, Halfvarson J, Simrén M, Ohlsson B, Karling P, Van Wanrooy S, Mondelaers S, Vermeire S, Lindberg G, Spiller R, Dukes G, D’Amato M, Boeckxstaens G. Genetic variants in CDC42 and NXPH1 as susceptibility factors for constipation and diarrhoea predominant irritable bowel syndrome. Gut. 2014;63:1103–1111. doi: 10.1136/gutjnl-2013-304570. [DOI] [PubMed] [Google Scholar]
- 46.Swan C, Duroudier NP, Campbell E, Zaitoun A, Hastings M, Dukes GE, Cox J, Kelly FM, Wilde J, Lennon MG, et al. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFα. Gut. 2013;62:985–994. doi: 10.1136/gutjnl-2011-301213. [DOI] [PubMed] [Google Scholar]
- 47.Ek WE, Reznichenko A, Ripke S, Niesler B, Zucchelli M, Rivera NV, Schmidt PT, Pedersen NL, Magnusson P, Talley NJ, et al. Exploring the genetics of irritable bowel syndrome: a GWA study in the general population and replication in multinational case-control cohorts. Gut. 2014:Epub ahead of print. doi: 10.1136/gutjnl-2014-307997. [DOI] [PubMed] [Google Scholar]
- 48.Capitani M, Sallese M. The KDEL receptor: new functions for an old protein. FEBS Lett. 2009;583:3863–3871. doi: 10.1016/j.febslet.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 49.Hsu VW, Shah N, Klausner RD. A brefeldin A-like phenotype is induced by the overexpression of a human ERD-2-like protein, ELP-1. Cell. 1992;69:625–635. doi: 10.1016/0092-8674(92)90226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis MJ, Pelham HR. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell. 1992;68:353–364. doi: 10.1016/0092-8674(92)90476-s. [DOI] [PubMed] [Google Scholar]
- 51.Lewis MJ, Pelham HR. Sequence of a second human KDEL receptor. J Mol Biol. 1992;226:913–916. doi: 10.1016/0022-2836(92)91039-r. [DOI] [PubMed] [Google Scholar]
- 52.Wilson DW, Lewis MJ, Pelham HR. pH-dependent binding of KDEL to its receptor in vitro. J Biol Chem. 1993;268:7465–7468. [PubMed] [Google Scholar]
- 53.Matsuda K, Matsuda S, Gladding CM, Yuzaki M. Characterization of the delta2 glutamate receptor-binding protein delphilin: Splicing variants with differential palmitoylation and an additional PDZ domain. J Biol Chem. 2006;281:25577–25587. doi: 10.1074/jbc.M602044200. [DOI] [PubMed] [Google Scholar]
- 54.Miyagi Y, Yamashita T, Fukaya M, Sonoda T, Okuno T, Yamada K, Watanabe M, Nagashima Y, Aoki I, Okuda K, et al. Delphilin: a novel PDZ and formin homology domain-containing protein that synaptically colocalizes and interacts with glutamate receptor delta 2 subunit. J Neurosci. 2002;22:803–814. doi: 10.1523/JNEUROSCI.22-03-00803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Napolioni V. The relevance of checking population allele frequencies and Hardy-Weinberg Equilibrium in genetic association studies: the case of SLC6A4 5-HTTLPR polymorphism in a Chinese Han Irritable Bowel Syndrome association study. Immunol Lett. 2014;162:276–278. doi: 10.1016/j.imlet.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Levy RL, Whitehead WE, Von Korff MR, Feld AD. Intergenerational transmission of gastrointestinal illness behavior. Am J Gastroenterol. 2000;95:451–456. doi: 10.1111/j.1572-0241.2000.01766.x. [DOI] [PubMed] [Google Scholar]
- 57.Whitehead WE, Busch CM, Heller BR, Costa PT. Social learning influences on menstrual symptoms and illness behavior. Health Psychol. 1986;5:13–23. doi: 10.1037//0278-6133.5.1.13. [DOI] [PubMed] [Google Scholar]
- 58.Walker LS, Garber J, Greene JW. Somatization symptoms in pediatric abdominal pain patients: relation to chronicity of abdominal pain and parent somatization. J Abnorm Child Psychol. 1991;19:379–394. doi: 10.1007/BF00919084. [DOI] [PubMed] [Google Scholar]
- 59.Lowman BC, Drossman DA, Cramer EM, McKee DC. Recollection of childhood events in adults with irritable bowel syndrome. J Clin Gastroenterol. 1987;9:324–330. doi: 10.1097/00004836-198706000-00017. [DOI] [PubMed] [Google Scholar]
- 60.Surdea-Blaga T, Băban A, Dumitrascu DL. Psychosocial determinants of irritable bowel syndrome. World J Gastroenterol. 2012;18:616–626. doi: 10.3748/wjg.v18.i7.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chapman S, Martin M. Attention to pain words in irritable bowel syndrome: increased orienting and speeded engagement. Br J Health Psychol. 2011;16:47–60. doi: 10.1348/135910710X505887. [DOI] [PubMed] [Google Scholar]
- 62.Martin M, Chapman SC. Cognitive processing in putative functional gastrointestinal disorder: rumination yields orientation to social threat not pain. Eur J Pain. 2010;14:207–213. doi: 10.1016/j.ejpain.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Simrén M, Månsson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, Björnsson ES. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 64.Bischoff S, Crowe SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology. 2005;128:1089–1113. doi: 10.1053/j.gastro.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 65.Young E, Stoneham MD, Petruckevitch A, Barton J, Rona R. A population study of food intolerance. Lancet. 1994;343:1127–1130. doi: 10.1016/s0140-6736(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 66.Locke GR, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ. Risk factors for irritable bowel syndrome: role of analgesics and food sensitivities. Am J Gastroenterol. 2000;95:157–165. doi: 10.1111/j.1572-0241.2000.01678.x. [DOI] [PubMed] [Google Scholar]
- 67.Bischoff SC, Herrmann A, Manns MP. Prevalence of adverse reactions to food in patients with gastrointestinal disease. Allergy. 1996;51:811–818. [PubMed] [Google Scholar]
- 68.Nanda R, James R, Smith H, Dudley CR, Jewell DP. Food intolerance and the irritable bowel syndrome. Gut. 1989;30:1099–1104. doi: 10.1136/gut.30.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones VA, McLaughlan P, Shorthouse M, Workman E, Hunter JO. Food intolerance: a major factor in the pathogenesis of irritable bowel syndrome. Lancet. 1982;2:1115–1117. doi: 10.1016/s0140-6736(82)92782-9. [DOI] [PubMed] [Google Scholar]
- 70.Bhat K, Harper A, Gorard DA. Perceived food and drug allergies in functional and organic gastrointestinal disorders. Aliment Pharmacol Ther. 2002;16:969–973. doi: 10.1046/j.1365-2036.2002.01256.x. [DOI] [PubMed] [Google Scholar]
- 71.Bijkerk CJ, de Wit NJ, Stalman WA, Knottnerus JA, Hoes AW, Muris JW. Irritable bowel syndrome in primary care: the patients’ and doctors’ views on symptoms, etiology and management. Can J Gastroenterol. 2003;17:363–368; quiz 405-406. doi: 10.1155/2003/532138. [DOI] [PubMed] [Google Scholar]
- 72.Böhn L, Störsrud S, Simrén M. Nutrient intake in patients with irritable bowel syndrome compared with the general population. Neurogastroenterol Motil. 2013;25:23–30.e1. doi: 10.1111/nmo.12001. [DOI] [PubMed] [Google Scholar]
- 73.Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep. 2012;5:1382–1390. doi: 10.3892/mmr.2012.843. [DOI] [PubMed] [Google Scholar]
- 74.Jarrett M, Heitkemper MM, Bond EF, Georges J. Comparison of diet composition in women with and without functional bowel disorder. Gastroenterol Nurs. 1994;16:253–258. doi: 10.1097/00001610-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 75.Saito YA, Locke GR, Weaver AL, Zinsmeister AR, Talley NJ. Diet and functional gastrointestinal disorders: a population-based case-control study. Am J Gastroenterol. 2005;100:2743–2748. doi: 10.1111/j.1572-0241.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 76.Williams EA, Nai X, Corfe BM. Dietary intakes in people with irritable bowel syndrome. BMC Gastroenterol. 2011;11:9. doi: 10.1186/1471-230X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ligaarden SC, Lydersen S, Farup PG. Diet in subjects with irritable bowel syndrome: a cross-sectional study in the general population. BMC Gastroenterol. 2012;12:61. doi: 10.1186/1471-230X-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El-Salhy M, Ostgaard H, Gundersen D, Hatlebakk JG, Hausken T. The role of diet in the pathogenesis and management of irritable bowel syndrome (Review) Int J Mol Med. 2012;29:723–731. doi: 10.3892/ijmm.2012.926. [DOI] [PubMed] [Google Scholar]
- 79.Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108:634–641. doi: 10.1038/ajg.2013.105. [DOI] [PubMed] [Google Scholar]
- 80.Asare F, Störsrud S, Simrén M. Meditation over medication for irritable bowel syndrome? On exercise and alternative treatments for irritable bowel syndrome. Curr Gastroenterol Rep. 2012;14:283–289. doi: 10.1007/s11894-012-0268-2. [DOI] [PubMed] [Google Scholar]
- 81.Gibson PR. Food intolerance in functional bowel disorders. J Gastroenterol Hepatol. 2011;26 Suppl 3:128–131. doi: 10.1111/j.1440-1746.2011.06650.x. [DOI] [PubMed] [Google Scholar]
- 82.Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107:657–666; quiz 667. doi: 10.1038/ajg.2012.49. [DOI] [PubMed] [Google Scholar]
- 83.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Interaction between ingested nutrients and gut endocrine cells in patients with irritable bowel syndrome (review) Int J Mol Med. 2014;34:363–371. doi: 10.3892/ijmm.2014.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marcason W. What is the FODMAP diet? J Acad Nutr Diet. 2012;112:1696. doi: 10.1016/j.jand.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 85.Shepherd SJ, Gibson PR. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J Hum Nutr Diet. 2013;26:349–358. doi: 10.1111/jhn.12018. [DOI] [PubMed] [Google Scholar]
- 86.Biesiekierski JR, Rosella O, Rose R, Liels K, Barrett JS, Shepherd SJ, Gibson PR, Muir JG. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J Hum Nutr Diet. 2011;24:154–176. doi: 10.1111/j.1365-277X.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 87.Muir JG, Rose R, Rosella O, Liels K, Barrett JS, Shepherd SJ, Gibson PR. Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography (HPLC) J Agric Food Chem. 2009;57:554–565. doi: 10.1021/jf802700e. [DOI] [PubMed] [Google Scholar]
- 88.Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:707–717. doi: 10.1038/ajg.2013.96. [DOI] [PubMed] [Google Scholar]
- 89.Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, Haines ML, Shepherd SJ, Gibson PR. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 90.Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Effects of dietary guidance on the symptoms, quality of life and habitual dietary intake of patients with irritable bowel syndrome. Mol Med Rep. 2013;8:845–852. doi: 10.3892/mmr.2013.1565. [DOI] [PubMed] [Google Scholar]
- 91.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 92.Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap Adv Gastroenterol. 2012;5:261–268. doi: 10.1177/1756283X11436241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 94.Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010;25:252–258. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 95.Gibson PR, Shepherd SJ. Personal view: food for thought--western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment Pharmacol Ther. 2005;21:1399–1409. doi: 10.1111/j.1365-2036.2005.02506.x. [DOI] [PubMed] [Google Scholar]
- 96.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]
- 97.Mazzawi T, Gundersen D, Hausken T, El-Salhy M. Increased gastric chromogranin A cell density following changes to diets of patients with irritable bowel syndrome. Mol Med Rep. 2014;10:2322–2326. doi: 10.3892/mmr.2014.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mazzawi T, Gundersen D, Hausken T, El-Salhy M. Increased chromogranin a cell density in the large intestine of patients with irritable bowel syndrome after receiving dietary guidance. Gastroenterol Res Pract. 2015;2015:823897. doi: 10.1155/2015/823897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Normalization of large intestinal endocrine cells following dietary management in patients with irritable bowel syndrome. Eur J Clin Nutr. 2015:In press. doi: 10.1038/ejcn.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Effect of dietary management on the gastric endocrine cells in patients with irritable bowel syndrome. Eur J Clin Nutr. 2015;69:519–524. doi: 10.1038/ejcn.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.El-Salhy M. Ghrelin in gastrointestinal diseases and disorders: a possible role in the pathophysiology and clinical implications (review) Int J Mol Med. 2009;24:727–732. doi: 10.3892/ijmm_00000285. [DOI] [PubMed] [Google Scholar]
- 102.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Duodenal chromogranin a cell density as a biomarker for the diagnosis of irritable bowel syndrome. Gastroenterol Res Pract. 2014;2014:462856. doi: 10.1155/2014/462856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Endocrine cells in the ileum of patients with irritable bowel syndrome. World J Gastroenterol. 2014;20:2383–2391. doi: 10.3748/wjg.v20.i9.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.El-Salhy M, Gilja OH, Gundersen D, Hausken T. Endocrine cells in the oxyntic mucosa of the stomach in patients with irritable bowel syndrome. World J Gastrointest Endosc. 2014;6:176–185. doi: 10.4253/wjge.v6.i5.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El-Salhy M, Gilja OH, Hatlebakk JG, Hausken T. Stomach antral endocrine cells in patients with irritable bowel syndrome. Int J Mol Med. 2014;34:967–974. doi: 10.3892/ijmm.2014.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El-Salhy M, Gilja OH, Hausken T. Chromogranin A cells in the stomachs of patients with sporadic irritable bowel syndrome. Mol Med Rep. 2014;10:1753–1757. doi: 10.3892/mmr.2014.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.El-Salhy M, Gundersen D, Hatlebakk JG, Gilja OH, Hausken T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul Pept. 2014;188:60–65. doi: 10.1016/j.regpep.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 108.El-Salhy M, Norrgård O, Spinnell S. Abnormal colonic endocrine cells in patients with chronic idiopathic slow-transit constipation. Scand J Gastroenterol. 1999;34:1007–1011. doi: 10.1080/003655299750025110. [DOI] [PubMed] [Google Scholar]
- 109.Dizdar V, Spiller R, Singh G, Hanevik K, Gilja OH, El-Salhy M, Hausken T. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883–891. doi: 10.1111/j.1365-2036.2010.04251.x. [DOI] [PubMed] [Google Scholar]
- 110.Wendelbo I, Mazzawi T, El-Salhy M. Increased serotonin transporter immunoreactivity intensity in the ileum of patients with irritable bowel disease. Mol Med Rep. 2014;9:180–184. doi: 10.3892/mmr.2013.1784. [DOI] [PubMed] [Google Scholar]
- 111.Wang SH, Dong L, Luo JY, Gong J, Li L, Lu XL, Han SP. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041–6047. doi: 10.3748/wjg.v13.45.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park JH, Rhee PL, Kim G, Lee JH, Kim YH, Kim JJ, Rhee JC, Song SY. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:539–546. doi: 10.1111/j.1365-2982.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 113.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 114.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096–1101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coleman NS, Foley S, Dunlop SP, Wheatcroft J, Blackshaw E, Perkins AC, Singh G, Marsden CA, Holmes GK, Spiller RC. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol. 2006;4:874–881. doi: 10.1016/j.cgh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 116.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 117.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 118.Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–1694. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 119.Kim HS, Lim JH, Park H, Lee SI. Increased immunoendocrine cells in intestinal mucosa of postinfectious irritable bowel syndrome patients 3 years after acute Shigella infection--an observation in a small case control study. Yonsei Med J. 2010;51:45–51. doi: 10.3349/ymj.2010.51.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol. 2014;592:2967–2980. doi: 10.1113/jphysiol.2014.270892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Whorwell PJ. The growing case for an immunological component to irritable bowel syndrome. Clin Exp Allergy. 2007;37:805–807. doi: 10.1111/j.1365-2222.2007.02736.x. [DOI] [PubMed] [Google Scholar]
- 123.Zar S, Benson MJ, Kumar D. Food-specific serum IgG4 and IgE titers to common food antigens in irritable bowel syndrome. Am J Gastroenterol. 2005;100:1550–1557. doi: 10.1111/j.1572-0241.2005.41348.x. [DOI] [PubMed] [Google Scholar]
- 124.Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? A systematic review. Neurogastroenterol Motil. 2006;18:595–607. doi: 10.1111/j.1365-2982.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 125.Uz E, Türkay C, Aytac S, Bavbek N. Risk factors for irritable bowel syndrome in Turkish population: role of food allergy. J Clin Gastroenterol. 2007;41:380–383. doi: 10.1097/01.mcg.0000225589.70706.24. [DOI] [PubMed] [Google Scholar]
- 126.Dainese R, Galliani EA, De Lazzari F, Di Leo V, Naccarato R. Discrepancies between reported food intolerance and sensitization test findings in irritable bowel syndrome patients. Am J Gastroenterol. 1999;94:1892–1897. doi: 10.1111/j.1572-0241.1999.01226.x. [DOI] [PubMed] [Google Scholar]
- 127.McKee AM, Prior A, Whorwell PJ. Exclusion diets in irritable bowel syndrome: are they worthwhile? J Clin Gastroenterol. 1987;9:526–528. doi: 10.1097/00004836-198710000-00007. [DOI] [PubMed] [Google Scholar]
- 128.Boettcher E, Crowe SE. Dietary proteins and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:728–736. doi: 10.1038/ajg.2013.97. [DOI] [PubMed] [Google Scholar]
- 129.Pistón F, Gil-Humanes J, Barro F. Integration of promoters, inverted repeat sequences and proteomic data into a model for high silencing efficiency of coeliac disease related gliadins in bread wheat. BMC Plant Biol. 2013;13:136. doi: 10.1186/1471-2229-13-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mansueto P, Seidita A, D’Alcamo A, Carroccio A. Non-celiac gluten sensitivity: literature review. J Am Coll Nutr. 2014;33:39–54. doi: 10.1080/07315724.2014.869996. [DOI] [PubMed] [Google Scholar]
- 131.Lundin KE. Non-celiac gluten sensitivity - why worry? BMC Med. 2014;12:86. doi: 10.1186/1741-7015-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aziz I, Sanders DS. Emerging concepts: from coeliac disease to non-coeliac gluten sensitivity. Proc Nutr Soc. 2012;71:576–580. doi: 10.1017/S002966511200081X. [DOI] [PubMed] [Google Scholar]
- 133.Volta U, De Giorgio R. New understanding of gluten sensitivity. Nat Rev Gastroenterol Hepatol. 2012;9:295–299. doi: 10.1038/nrgastro.2012.15. [DOI] [PubMed] [Google Scholar]
- 134.Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, Kaukinen K, Rostami K, Sanders DS, Schumann M, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13. doi: 10.1186/1741-7015-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Catassi C, Bai JC, Bonaz B, Bouma G, Calabrò A, Carroccio A, Castillejo G, Ciacci C, Cristofori F, Dolinsek J, et al. Non-Celiac Gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5:3839–3853. doi: 10.3390/nu5103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Celiac and Non-Celiac Forms of Gluten Sensitivity: Shifting Paradigms of an Old Disease. Br Microbiol Res J. 2013;3:585–589 . doi: 10.9734/BMRJ/2013/6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Czaja-Bulsa G. Non coeliac gluten sensitivity - A new disease with gluten intolerance. Clin Nutr. 2015;34:189–194. doi: 10.1016/j.clnu.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 138.Ellis A, Linaker BD. Non-coeliac gluten sensitivity? Lancet. 1978;1:1358–1359. doi: 10.1016/s0140-6736(78)92427-3. [DOI] [PubMed] [Google Scholar]
- 139.Cooper BT, Holmes GK, Ferguson R, Thompson RA, Allan RN, Cooke WT. “Gluten-sensitive diarrhea without evidence of celiac disease”. Gastroenterology. 1981;81:192–194. [PubMed] [Google Scholar]
- 140.Campanella J, Biagi F, Bianchi PI, Zanellati G, Marchese A, Corazza GR. Clinical response to gluten withdrawal is not an indicator of coeliac disease. Scand J Gastroenterol. 2008;43:1311–1314. doi: 10.1080/00365520802200036. [DOI] [PubMed] [Google Scholar]
- 141.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O’Neill J, Carlson P, Lamsam J, Janzow D, Eckert D, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903–911.e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kaukinen K, Turjanmaa K, Mäki M, Partanen J, Venäläinen R, Reunala T, Collin P. Intolerance to cereals is not specific for coeliac disease. Scand J Gastroenterol. 2000;35:942–946. doi: 10.1080/003655200750022995. [DOI] [PubMed] [Google Scholar]
- 143.Carroccio A, Mansueto P, Iacono G, Soresi M, D’Alcamo A, Cavataio F, Brusca I, Florena AM, Ambrosiano G, Seidita A, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol. 2012;107:1898–1906; quiz 1907. doi: 10.1038/ajg.2012.236. [DOI] [PubMed] [Google Scholar]
- 144.Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, Shepherd SJ, Muir JG, Gibson PR. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–514; quiz 515. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- 145.Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–1594. doi: 10.1038/ajg.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carroccio A, Brusca I, Mansueto P, Pirrone G, Barrale M, Di Prima L, Ambrosiano G, Iacono G, Lospalluti ML, La Chiusa SM, et al. A cytologic assay for diagnosis of food hypersensitivity in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2010;8:254–260. doi: 10.1016/j.cgh.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 147.Carroccio A, Brusca I, Mansueto P, D’alcamo A, Barrale M, Soresi M, Seidita A, La Chiusa SM, Iacono G, Sprini D. A comparison between two different in vitro basophil activation tests for gluten- and cow’s milk protein sensitivity in irritable bowel syndrome (IBS)-like patients. Clin Chem Lab Med. 2013;51:1257–1263. doi: 10.1515/cclm-2012-0609. [DOI] [PubMed] [Google Scholar]
- 148.Bucci C, Zingone F, Russo I, Morra I, Tortora R, Pogna N, Scalia G, Iovino P, Ciacci C. Gliadin does not induce mucosal inflammation or basophil activation in patients with nonceliac gluten sensitivity. Clin Gastroenterol Hepatol. 2013;11:1294–1299.e1. doi: 10.1016/j.cgh.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 149.Sapone A, Lammers KM, Casolaro V, Cammarota M, Giuliano MT, De Rosa M, Stefanile R, Mazzarella G, Tolone C, Russo MI, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:23. doi: 10.1186/1741-7015-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sapone A, Lammers KM, Mazzarella G, Mikhailenko I, Cartenì M, Casolaro V, Fasano A. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol. 2010;152:75–80. doi: 10.1159/000260087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thomas KE, Sapone A, Fasano A, Vogel SN. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol. 2006;176:2512–2521. doi: 10.4049/jimmunol.176.4.2512. [DOI] [PubMed] [Google Scholar]
- 152.Nijeboer P, Bontkes HJ, Mulder CJ, Bouma G. Non-celiac gluten sensitivity. Is it in the gluten or the grain? J Gastrointestin Liver Dis. 2013;22:435–440. [PubMed] [Google Scholar]
- 153.Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320–328.e1-3. doi: 10.1053/j.gastro.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 154.Biesiekierski JR, Newnham ED, Shepherd SJ, Muir JG, Gibson PR. Characterization of Adults With a Self-Diagnosis of Nonceliac Gluten Sensitivity. Nutr Clin Pract. 2014;29:504–509. doi: 10.1177/0884533614529163. [DOI] [PubMed] [Google Scholar]
- 155.Sanders DS, Carter MJ, Hurlstone DP, Pearce A, Ward AM, McAlindon ME, Lobo AJ. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358:1504–1508. doi: 10.1016/S0140-6736(01)06581-3. [DOI] [PubMed] [Google Scholar]
- 156.Sanders DS, Patel D, Stephenson TJ, Ward AM, McCloskey EV, Hadjivassiliou M, Lobo AJ. A primary care cross-sectional study of undiagnosed adult coeliac disease. Eur J Gastroenterol Hepatol. 2003;15:407–413. doi: 10.1097/00042737-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 157.Elloumi H, El Assoued Y, Ghédira I, Ben Abdelaziz A, Yacoobi MT, Ajmi S. Immunological profile of coeliac disease in a subgroup of patients with symptoms of irritable bowel syndrome. Tunis Med. 2008;86:802–805. [PubMed] [Google Scholar]
- 158.Volta U, Tovoli F, Cicola R, Parisi C, Fabbri A, Piscaglia M, Fiorini E, Caio G. Serological tests in gluten sensitivity (nonceliac gluten intolerance) J Clin Gastroenterol. 2012;46:680–685. doi: 10.1097/MCG.0b013e3182372541. [DOI] [PubMed] [Google Scholar]
- 159.Ruuskanen A, Kaukinen K, Collin P, Huhtala H, Valve R, Mäki M, Luostarinen L. Positive serum antigliadin antibodies without celiac disease in the elderly population: does it matter? Scand J Gastroenterol. 2010;45:1197–1202. doi: 10.3109/00365521.2010.496491. [DOI] [PubMed] [Google Scholar]
- 160.Sanders DS. Celiac disease and IBS-type symptoms: the relationship exists in both directions. Am J Gastroenterol. 2003;98:707–708. doi: 10.1111/j.1572-0241.2003.07314.x. [DOI] [PubMed] [Google Scholar]
- 161.Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome-- etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60:667–672. doi: 10.1038/sj.ejcn.1602367. [DOI] [PubMed] [Google Scholar]
- 162.Kubo M, Fujiwara Y, Shiba M, Kohata Y, Yamagami H, Tanigawa T, Watanabe K, Watanabe T, Tominaga K, Arakawa T. Differences between risk factors among irritable bowel syndrome subtypes in Japanese adults. Neurogastroenterol Motil. 2011;23:249–254. doi: 10.1111/j.1365-2982.2010.01640.x. [DOI] [PubMed] [Google Scholar]
- 163.Pickett-Blakely O. Obesity and irritable bowel syndrome: a comprehensive review. Gastroenterol Hepatol (N Y) 2014;10:411–416. [PMC free article] [PubMed] [Google Scholar]
- 164.Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1187–1209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Diet and irritable bowel syndrome, with a focus on appetite-regulating hormones. In: Watson RR, editor Nutrition in the prevention and treatment of abdominal obesity, editors. San Diego: Elsevier; 2014. pp. 5–16. [Google Scholar]
- 166.Seim I, El-Salhy M, Hausken T, Gundersen D, Chopin L. Ghrelin and the brain-gut axis as a pharmacological target for appetite control. Curr Pharm Des. 2012;18:768–775. doi: 10.2174/138161212799277806. [DOI] [PubMed] [Google Scholar]
- 167.El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873–878. doi: 10.1007/s10620-011-1948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. Densities of rectal peptide YY and somatostatin cells as biomarkers for the diagnosis of irritable bowel syndrome. Peptides. 2015;67:12–19. doi: 10.1016/j.peptides.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 169.El-Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. The role of peptide YY in gastrointestinal diseases and disorders (review) Int J Mol Med. 2013;31:275–282. doi: 10.3892/ijmm.2012.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed) 2012;4:2783–2800. doi: 10.2741/e583. [DOI] [PubMed] [Google Scholar]
- 171.El-Salhy M, Lillebø E, Reinemo A, Salmelid L. Ghrelin in patients with irritable bowel syndrome. Int J Mol Med. 2009;23:703–707. doi: 10.3892/ijmm_00000183. [DOI] [PubMed] [Google Scholar]
- 172.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 173.Hosoda H, Kojima M, Kangawa K. Ghrelin and the regulation of food intake and energy balance. Mol Interv. 2002;2:494–503. doi: 10.1124/mi.2.8.494. [DOI] [PubMed] [Google Scholar]
- 174.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 175.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 176.Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- 177.Lin HC, Zhao XT, Wang L, Wong H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology. 1996;110:1491–1495. doi: 10.1053/gast.1996.v110.pm8613054. [DOI] [PubMed] [Google Scholar]
- 178.Lin HC, Zhao XT, Wang L. Intestinal transit is more potently inhibited by fat in the distal (ileal brake) than in the proximal (jejunal brake) gut. Dig Dis Sci. 1997;42:19–25. doi: 10.1023/a:1018816517404. [DOI] [PubMed] [Google Scholar]
- 179.Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep. 2006;8:367–373. doi: 10.1007/s11894-006-0021-9. [DOI] [PubMed] [Google Scholar]
- 180.Van Citters GW, Lin HC. The ileal brake: a fifteen-year progress report. Curr Gastroenterol Rep. 1999;1:404–409. doi: 10.1007/s11894-999-0022-6. [DOI] [PubMed] [Google Scholar]
- 181.Ohtani N, Sasaki I, Naito H, Shibata C, Matsuno S. Mediators for fat-induced ileal brake are different between stomach and proximal small intestine in conscious dogs. J Gastrointest Surg. 2001;5:377–382. doi: 10.1016/s1091-255x(01)80065-2. [DOI] [PubMed] [Google Scholar]
- 182.Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R, Ruggeri E, Tosetti C, Poggioli G, Morselli Labate AM, Monetti N. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology. 1993;105:733–739. doi: 10.1016/0016-5085(93)90890-o. [DOI] [PubMed] [Google Scholar]
- 183.Maljaars J, Peters HP, Masclee AM. Review article: The gastrointestinal tract: neuroendocrine regulation of satiety and food intake. Aliment Pharmacol Ther. 2007;26 Suppl 2:241–250. doi: 10.1111/j.1365-2036.2007.03550.x. [DOI] [PubMed] [Google Scholar]
- 184.Maljaars PW, Peters HP, Mela DJ, Masclee AA. Ileal brake: a sensible food target for appetite control. A review. Physiol Behav. 2008;95:271–281. doi: 10.1016/j.physbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 185.Maljaars PW, Symersky T, Kee BC, Haddeman E, Peters HP, Masclee AA. Effect of ileal fat perfusion on satiety and hormone release in healthy volunteers. Int J Obes (Lond) 2008;32:1633–1639. doi: 10.1038/ijo.2008.166. [DOI] [PubMed] [Google Scholar]
- 186.Le Quellec A, Kervran A, Blache P, Ciurana AJ, Bataille D. Oxyntomodulin-like immunoreactivity: diurnal profile of a new potential enterogastrone. J Clin Endocrinol Metab. 1992;74:1405–1409. doi: 10.1210/jcem.74.6.1592887. [DOI] [PubMed] [Google Scholar]
- 187.Ghatei MA, Uttenthal LO, Christofides ND, Bryant MG, Bloom SR. Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J Clin Endocrinol Metab. 1983;57:488–495. doi: 10.1210/jcem-57-3-488. [DOI] [PubMed] [Google Scholar]
- 188.Yu JH, Kim MS. Molecular mechanisms of appetite regulation. Diabetes Metab J. 2012;36:391–398. doi: 10.4093/dmj.2012.36.6.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fang XL, Shu G, Yu JJ, Wang LN, Yang J, Zeng QJ, Cheng X, Zhang ZQ, Wang SB, Gao P, et al. The anorexigenic effect of serotonin is mediated by the generation of NADPH oxidase-dependent ROS. PLoS One. 2013;8:e53142. doi: 10.1371/journal.pone.0053142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee KN, Lee OY. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J Gastroenterol. 2014;20:8886–8897. doi: 10.3748/wjg.v20.i27.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee KJ, Tack J. Altered intestinal microbiota in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:493–498. doi: 10.1111/j.1365-2982.2010.01482.x. [DOI] [PubMed] [Google Scholar]
- 192.Simrén M. IBS with intestinal microbial dysbiosis: a new and clinically relevant subgroup? Gut. 2014;63:1685–1686. doi: 10.1136/gutjnl-2013-306434. [DOI] [PubMed] [Google Scholar]
- 193.Doré J, Simrén M, Buttle L, Guarner F. Hot topics in gut microbiota. United European Gastroenterol J. 2013;1:311–318. doi: 10.1177/2050640613502477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ohman L, Simrén M. Intestinal microbiota and its role in irritable bowel syndrome (IBS) Curr Gastroenterol Rep. 2013;15:323. doi: 10.1007/s11894-013-0323-7. [DOI] [PubMed] [Google Scholar]
- 195.Sommer F, Bäckhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 196.Quigley EM. Probiotics and digestive health. London: Health Point Press; 1908. [Google Scholar]
- 197.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Othman M, Agüero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol. 2008;24:11–16. doi: 10.1097/MOG.0b013e3282f2b0d7. [DOI] [PubMed] [Google Scholar]
- 199.Gorbach SL. Intestinal microflora. Gastroenterology. 1971;60:1110–1129. [PubMed] [Google Scholar]
- 200.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–858. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 201.Power SE, O’Toole PW, Stanton C, Ross RP, Fitzgerald GF. Intestinal microbiota, diet and health. Br J Nutr. 2014;111:387–402. doi: 10.1017/S0007114513002560. [DOI] [PubMed] [Google Scholar]
- 202.Farrugia G, Simren M, Mawe G, Bradesi S, Bredenoord AJ. Gut microbiota and neurogastroenterology and motility: the good the bad and the ugly. Neurogastroenterol Motil. 2014;26:295. doi: 10.1111/nmo.12322. [DOI] [PubMed] [Google Scholar]
- 203.Carroll IM, Ringel-Kulka T, Keku TO, Chang YH, Packey CD, Sartor RB, Ringel Y. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301:G799–G807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 205.Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, Saarela M. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome--a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43:213–222. doi: 10.1016/j.femsim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 206.Maukonen J, Satokari R, Mättö J, Söderlund H, Mattila-Sandholm T, Saarela M. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J Med Microbiol. 2006;55:625–633. doi: 10.1099/jmm.0.46134-0. [DOI] [PubMed] [Google Scholar]
- 207.Malinen E, Krogius-Kurikka L, Lyra A, Nikkilä J, Jääskeläinen A, Rinttilä T, Vilpponen-Salmela T, von Wright AJ, Palva A. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J Gastroenterol. 2010;16:4532–4540. doi: 10.3748/wjg.v16.i36.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 209.Jeffery IB, O’Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 210.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:521–530, e248. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Carroll IM, Ringel-Kulka T, Siddle JP, Klaenhammer TR, Ringel Y. Characterization of the fecal microbiota using high-throughput sequencing reveals a stable microbial community during storage. PLoS One. 2012;7:e46953. doi: 10.1371/journal.pone.0046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Carroll IM, Chang YH, Park J, Sartor RB, Ringel Y. Luminal and mucosal-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Gut Pathog. 2010;2:19. doi: 10.1186/1757-4749-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Codling C, O’Mahony L, Shanahan F, Quigley EM, Marchesi JR. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig Dis Sci. 2010;55:392–397. doi: 10.1007/s10620-009-0934-x. [DOI] [PubMed] [Google Scholar]
- 214.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 215.Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1053–G1060. doi: 10.1152/ajpgi.00153.2011. [DOI] [PubMed] [Google Scholar]
- 216.Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Noor SO, Ridgway K, Scovell L, Kemsley EK, Lund EK, Jamieson C, Johnson IT, Narbad A. Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol. 2010;10:134. doi: 10.1186/1471-230X-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Le Gall G, Noor SO, Ridgway K, Scovell L, Jamieson C, Johnson IT, Colquhoun IJ, Kemsley EK, Narbad A. Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. J Proteome Res. 2011;10:4208–4218. doi: 10.1021/pr2003598. [DOI] [PubMed] [Google Scholar]
- 219.Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, Weidler EM, Qin X, Coarfa C, Milosavljevic A, et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology. 2011;141:1782–1791. doi: 10.1053/j.gastro.2011.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–519, e114-115. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 221.Jalanka-Tuovinen J, Salojärvi J, Salonen A, Immonen O, Garsed K, Kelly FM, Zaitoun A, Palva A, Spiller RC, de Vos WM. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737–1745. doi: 10.1136/gutjnl-2013-305994. [DOI] [PubMed] [Google Scholar]
- 222.Rigsbee L, Agans R, Shankar V, Kenche H, Khamis HJ, Michail S, Paliy O. Quantitative profiling of gut microbiota of children with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2012;107:1740–1751. doi: 10.1038/ajg.2012.287. [DOI] [PubMed] [Google Scholar]
- 223.Ponnusamy K, Choi JN, Kim J, Lee SY, Lee CH. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol. 2011;60:817–827. doi: 10.1099/jmm.0.028126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Carroll IM, Ringel-Kulka T, Ferrier L, Wu MC, Siddle JP, Bueno L, Ringel Y. Fecal protease activity is associated with compositional alterations in the intestinal microbiota. PLoS One. 2013;8:e78017. doi: 10.1371/journal.pone.0078017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Durbán A, Abellán JJ, Jiménez-Hernández N, Artacho A, Garrigues V, Ortiz V, Ponce J, Latorre A, Moya A. Instability of the faecal microbiota in diarrhoea-predominant irritable bowel syndrome. FEMS Microbiol Ecol. 2013;86:581–589. doi: 10.1111/1574-6941.12184. [DOI] [PubMed] [Google Scholar]
- 226.Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185–194. [PubMed] [Google Scholar]
- 227.Durbán A, Abellán JJ, Jiménez-Hernández N, Salgado P, Ponce M, Ponce J, Garrigues V, Latorre A, Moya A. Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome. Environ Microbiol Rep. 2012;4:242–247. doi: 10.1111/j.1758-2229.2012.00327.x. [DOI] [PubMed] [Google Scholar]
- 228.Rinttilä T, Lyra A, Krogius-Kurikka L, Palva A. Real-time PCR analysis of enteric pathogens from fecal samples of irritable bowel syndrome subjects. Gut Pathog. 2011;3:6. doi: 10.1186/1757-4749-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Si JM, Yu YC, Fan YJ, Chen SJ. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2004;10:1802–1805. doi: 10.3748/wjg.v10.i12.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51 Suppl 1:i41–i44. doi: 10.1136/gut.51.suppl_1.i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schmulson M, Chey WD. Abnormal immune regulation and low-grade inflammation in IBS: does one size fit all? Am J Gastroenterol. 2012;107:273–275. doi: 10.1038/ajg.2011.427. [DOI] [PubMed] [Google Scholar]
- 232.Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gwee KA, Collins SM, Read NW, Rajnakova A, Deng Y, Graham JC, McKendrick MW, Moochhala SM. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–526. doi: 10.1136/gut.52.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Low-grade inflammation in the rectum of patients with sporadic irritable bowel syndrome. Mol Med Rep. 2013;7:1081–1085. doi: 10.3892/mmr.2013.1320. [DOI] [PubMed] [Google Scholar]
- 235.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 236.Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 237.Akbar A, Yiangou Y, Facer P, Brydon WG, Walters JR, Anand P, Ghosh S. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut. 2010;59:767–774. doi: 10.1136/gut.2009.194449. [DOI] [PubMed] [Google Scholar]
- 238.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–1221. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- 240.Macsharry J, O’Mahony L, Fanning A, Bairead E, Sherlock G, Tiesman J, Fulmer A, Kiely B, Dinan TG, Shanahan F, et al. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467–1476. doi: 10.1080/00365520802276127. [DOI] [PubMed] [Google Scholar]
- 241.Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 242.Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 243.Chang L, Adeyemo M, Karagiannides I, Videlock EJ, Bowe C, Shih W, Presson AP, Yuan PQ, Cortina G, Gong H, et al. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol. 2012;107:262–272. doi: 10.1038/ajg.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Spiller RC. Role of infection in irritable bowel syndrome. J Gastroenterol. 2007;42 Suppl 17:41–47. doi: 10.1007/s00535-006-1925-8. [DOI] [PubMed] [Google Scholar]
- 245.Spiller R, Garsed K. Infection, inflammation, and the irritable bowel syndrome. Dig Liver Dis. 2009;41:844–849. doi: 10.1016/j.dld.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 246.Spiller R, Campbell E. Post-infectious irritable bowel syndrome. Curr Opin Gastroenterol. 2006;22:13–17. doi: 10.1097/01.mog.0000194792.36466.5c. [DOI] [PubMed] [Google Scholar]
- 247.May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323:70–75. doi: 10.1016/j.mce.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92:219–231. doi: 10.1111/j.1365-2613.2011.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tanaka-Shintani M, Watanabe M. Immunohistochemical study of enterochromaffin-like cell in human gastric mucosa. Pathol Int. 2007;57:572–583. doi: 10.1111/j.1440-1827.2007.02141.x. [DOI] [PubMed] [Google Scholar]
- 250.Lönroth H, Håkanson R, Lundell L, Sundler F. Histamine containing endocrine cells in the human stomach. Gut. 1990;31:383–388. doi: 10.1136/gut.31.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Buffa R, Capella C, Fontana P, Usellini L, Solcia E. Types of endocrine cells in the human colon and rectum. Cell Tissue Res. 1978;192:227–240. doi: 10.1007/BF00220741. [DOI] [PubMed] [Google Scholar]
- 252.Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–2644. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- 253.Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- 254.Sandström O, El-Salhy M. Ageing and endocrine cells of human duodenum. Mech Ageing Dev. 1999;108:39–48. doi: 10.1016/s0047-6374(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 255.Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. Handb Exp Pharmacol. 2012;(209):309–335. doi: 10.1007/978-3-642-24716-3_14. [DOI] [PubMed] [Google Scholar]
- 256.Lee J, Cummings BP, Martin E, Sharp JW, Graham JL, Stanhope KL, Havel PJ, Raybould HE. Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2012;302:R657–R666. doi: 10.1152/ajpregu.00345.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med. 2010;12:e1. doi: 10.1017/S146239940900132X. [DOI] [PubMed] [Google Scholar]
- 258.Raybould HE. Nutrient sensing in the gastrointestinal tract: possible role for nutrient transporters. J Physiol Biochem. 2008;64:349–356. doi: 10.1007/BF03174091. [DOI] [PubMed] [Google Scholar]
- 259.San Gabriel A, Nakamura E, Uneyama H, Torii K. Taste, visceral information and exocrine reflexes with glutamate through umami receptors. J Med Invest. 2009;56 Suppl:209–217. doi: 10.2152/jmi.56.209. [DOI] [PubMed] [Google Scholar]
- 260.Rudholm T, Wallin B, Theodorsson E, Näslund E, Hellström PM. Release of regulatory gut peptides somatostatin, neurotensin and vasoactive intestinal peptide by acid and hyperosmolal solutions in the intestine in conscious rats. Regul Pept. 2009;152:8–12. doi: 10.1016/j.regpep.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 261.Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol. 2007;292:G457–G461. doi: 10.1152/ajpgi.00411.2006. [DOI] [PubMed] [Google Scholar]
- 263.Buchan AM. Nutrient Tasting and Signaling Mechanisms in the Gut III. Endocrine cell recognition of luminal nutrients. Am J Physiol. 1999;277:G1103–G1107. doi: 10.1152/ajpgi.1999.277.6.G1103. [DOI] [PubMed] [Google Scholar]
- 264.Montero-Hadjadje M, Elias S, Chevalier L, Benard M, Tanguy Y, Turquier V, Galas L, Yon L, Malagon MM, Driouich A, et al. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J Biol Chem. 2009;284:12420–12431. doi: 10.1074/jbc.M805607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Shooshtarizadeh P, Zhang D, Chich JF, Gasnier C, Schneider F, Haïkel Y, Aunis D, Metz-Boutigue MH. The antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunity. Regul Pept. 2010;165:102–110. doi: 10.1016/j.regpep.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 266.Rindi G, Inzani F, Solcia E. Pathology of gastrointestinal disorders. Endocrinol Metab Clin North Am. 2010;39:713–727. doi: 10.1016/j.ecl.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 267.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wendelbo I, Mazzawi T, El-Salhy M. Increased serotonin transporter immunoreactivity intensity in the ileum of patients with irritable bowel disease. Mol Med Rep. 2013:in press. doi: 10.3892/mmr.2013.1784. [DOI] [PubMed] [Google Scholar]
- 269.El-Salhy M, Wendelbo IH, Gundersen D. Reduced chromogranin A cell density in the ileum of patients with irritable bowel syndrome. Mol Med Rep. 2013;7:1241–1244. doi: 10.3892/mmr.2013.1325. [DOI] [PubMed] [Google Scholar]
- 270.El-Salhy M, Vaali K, Dizdar V, Hausken T. Abnormal small-intestinal endocrine cells in patients with irritable bowel syndrome. Dig Dis Sci. 2010;55:3508–3513. doi: 10.1007/s10620-010-1169-6. [DOI] [PubMed] [Google Scholar]
- 271.El-Salhy M, Mazzawi T, Gundersen D, Hausken T. Chromogranin A cell density in the rectum of patients with irritable bowel syndrome. Mol Med Rep. 2012;6:1223–1225. doi: 10.3892/mmr.2012.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin A as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2010;45:1435–1439. doi: 10.3109/00365521.2010.503965. [DOI] [PubMed] [Google Scholar]
- 273.El-Salhy M, Hatlebakk JG, Gundersen D, Hausken T. Endocrine cells in the oxyntic mucosa of the stomach in patients with irritable bowel syndrome. World J Gastrointest Endosc. 2014;6:176–185. doi: 10.4253/wjge.v6.i5.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Chromogranin A cell density as a diagnostic marker for lymphocytic colitis. Dig Dis Sci. 2012;57:3154–3159. doi: 10.1007/s10620-012-2249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sjölund K, Ekman R, Wierup N. Covariation of plasma ghrelin and motilin in irritable bowel syndrome. Peptides. 2010;31:1109–1112. doi: 10.1016/j.peptides.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 276.El-Salhy M, Wendelbo I, Gundersen D. Serotonin and serotonin transporter in the rectum of patients with irritable bowel disease. Mol Med Rep. 2013;8:451–455. doi: 10.3892/mmr.2013.1525. [DOI] [PubMed] [Google Scholar]
- 277.El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 278.Lee CS, Kaestner KH. Clinical endocrinology and metabolism. Development of gut endocrine cells. Best Pract Res Clin Endocrinol Metab. 2004;18:453–462. doi: 10.1016/j.beem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 279.Cardoso WV, Lü J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 280.Darlington GJ. Molecular mechanisms of liver development and differentiation. Curr Opin Cell Biol. 1999;11:678–682. doi: 10.1016/s0955-0674(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 281.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 282.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- 284.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 285.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 287.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 288.Fontaine J, Le Lièvre C, Le Douarin NM. What is the developmental fate of the neural crest cells which migrate into the pancreas in the avian embryo? Gen Comp Endocrinol. 1977;33:394–404. doi: 10.1016/0016-6480(77)90055-7. [DOI] [PubMed] [Google Scholar]
- 289.Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- 290.Rawdon BB, Andrew A. Origin and differentiation of gut endocrine cells. Histol Histopathol. 1993;8:567–580. [PubMed] [Google Scholar]
- 291.Hoffman J, Kuhnert F, Davis CR, Kuo CJ. Wnts as essential growth factors for the adult small intestine and colon. Cell Cycle. 2004;3:554–557. [PubMed] [Google Scholar]
- 292.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 293.Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat. 2008;213:52–58. doi: 10.1111/j.1469-7580.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270–280. doi: 10.1056/NEJMoa054288. [DOI] [PubMed] [Google Scholar]
- 295.Fishbein TM, Novitskiy G, Lough DM, Matsumoto C, Kaufman SS, Shetty K, Zasloff M. Rejection reversibly alters enteroendocrine cell renewal in the transplanted small intestine. Am J Transplant. 2009;9:1620–1628. doi: 10.1111/j.1600-6143.2009.02681.x. [DOI] [PubMed] [Google Scholar]
- 296.El-Salhy M, Hatlebakk JG, Hausken T. The reduction in duodenal endocrine cells in IBS is associated with stem cell abnormalities. World J Gastroenterol. 2015:in press. doi: 10.3748/wjg.v21.i32.9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–1635. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 298.Gunnarsson J, Simrén M. Peripheral factors in the pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2009;41:788–793. doi: 10.1016/j.dld.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 299.Delgado-Aros S, Camilleri M. Visceral hypersensitivity. J Clin Gastroenterol. 2005;39:S194–203; discussion S210. doi: 10.1097/01.mcg.0000156114.22598.1b. [DOI] [PubMed] [Google Scholar]
- 300.Karantanos T, Markoutsaki T, Gazouli M, Anagnou NP, Karamanolis DG. Current insights in to the pathophysiology of Irritable Bowel Syndrome. Gut Pathog. 2010;2:3. doi: 10.1186/1757-4749-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Lee OY. Asian motility studies in irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:120–130. doi: 10.5056/jnm.2010.16.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.El-Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. Changes in the symptom pattern and the densities of large-intestinal endocrine cells following Campylobacter infection in irritable bowel syndrome: a case report. BMC Res Notes. 2013;6:391. doi: 10.1186/1756-0500-6-391. [DOI] [PMC free article] [PubMed] [Google Scholar]


