Abstract
AIM: To determine the incidence and predictors of thiopurine-related adverse events.
METHODS: Subjects with Crohn’s disease who were followed in the Alberta Inflammatory Bowel Disease Consortium patient database registry were identified. Retrospective chart review was conducted between August 5th, 2010 and June 1st, 2012. We collected data on: age at diagnosis; sex; disease location and behaviour at time of prescribing thiopurine; perianal fistulising disease at or prior to thiopurine prescription; smoking status at time of thiopurine prescription, use of corticosteroid within 6 mo of diagnosis; dosage, age at onset, and cessation of 5-aminosalicyclic acid (5-ASA); anti-tumour necrosis factor medication exposure and intestinal resection before thiopurine prescription. The primary outcome of interest was the first adverse event that led to discontinuation of the first thiopurine medication used. Logistic regression models were used to associate clinical characteristics with outcomes after adjusting for potential confounders. Risk estimates were presented as odds ratios (OR) with 95% CI. Effect modification by age and sex were explored.
RESULTS: Our cohort had a median follow-up duration of 5.8 years [interquartile range (IQR 25th-75th) 2.7-9.1]. Thiopurine therapy was discontinued in 31.3% of patients because of: hypersensitivity reactions (7.1%), acute pancreatitis (6.2%), gastrointestinal intolerance (5.4%), leucopenia (3.7%), hepatotoxicity (3.4%), infection (1.1%) and other reasons (4.3%). A higher incidence of thiopurine withdrawal was observed in patients over the age of 40 (39.4%, P = 0.007). A sex-by-age interaction (P = 0.04) was observed. Females older than 40 years of age had an increased risk of thiopurine discontinuation due to an adverse event (age above 40 vs age below 40, adjusted OR = 2.8; 95%CI: 1.4-5.6). In contrast, age did not influence thiopurine withdrawal in males (age above 40 vs below 40, adjusted OR = 0.9; 95%CI: 0.4-2.1). Other clinical variables (disease location and phenotype, perianal disease, smoking history, history of intestinal resection and prior 5-ASA or corticosteroid use) were not associated with an increased risk an adverse event leading to therapy cessation.
CONCLUSION: Thiopurine withdrawal due to adverse events is commoner in women over the age of 40 at prescription. These findings need to be replicated in other cohorts.
Keywords: Thiopurines, Azathioprine, Mercaptopurine, Adverse events
Core tip: In Crohn’s disease, adverse events to thiopurines are a common occurrence leading to discontinuation of therapy in 1 in 3 patients in this referral centre cohort. Adverse events leading to discontinuation of the drug were significantly more common in female patients over the age of 40 years at drug prescription. These findings should be replicated in other centres, in other clinical indications for thiopurine use and correlated to thiopurine 6-methyltransferase genotype, activity and thiopurine metabolities.
INTRODUCTION
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) characterized by chronic and relapsing intestinal inflammation that can affect any segment of the gastrointestinal tract. The aetiology of CD is multifactorial consisting of an interplay of altered immune responses to environmental stimuli such as the gut microbiota in genetically predisposed individuals[1-3]. Most patients with CD require chronic immune-suppressing medications to control their disease, and when these drugs fail intestinal resections are required[4].
Thiopurine analogs consist of mercaptopurine (MP) and its pro-drug azathioprine (AZA). Thiopurines have been shown to reduce corticosteroid use and maintain remission in patients with CD[5,6], but this evidence has been questioned by more recent data from two randomised controlled trials[7,8] in CD patients with early disease, precluding a widespread usage of thiopurines in all patients with early CD. Treatment paradigms have evolved in the last decade with the introduction of anti-tumour necrosis factor (TNF) therapy[9-11]. Emerging evidence suggests that the combination of thiopurines with anti-TNF therapy may be associated with greater efficacy for moderate to severe CD when compared to monotherapy with anti-TNF agents[12-14]. However, balancing efficacy against adverse events associated with immunosuppressive medications remains a persistent challenge in IBD management.
Adverse events lead to discontinuation of thiopurines in 9%-25% of cases[15,16]. In a previous meta-analysis reporting on studies between 1966 to 1994, thiopurine withdrawal due to an adverse event was described in 8.9% of the cases[17]. Reported rates have varied over the years but large referral-centre studies have shown higher discontinuation rates than previously reported (11%[18], 15%[19], 18.3%[20], 39%[21], 28%[22], 31%[23], 25.9%[24], 27.4%[25]). Thiopurine discontinuation and adverse events in the era of anti-TNF therapy has not been well described. Further, clinical variables that predict adverse events when prescribing thiopurines are not available. Genetic polymorphisms of the thiopurine 6-methyltransferase (TPMT) have been shown to correlate with subnormal enzyme activity and myelotoxicity. The effect of TPMT polymorphism on gastrointestinal toxicity is still unclear with an earlier study[26] showing a dis-concordance between TPMT heterozygosity and gastrointestinal intolerances. More recent data however has shown a significant association[27] between TPMT polymorphism and gastrointestinal intolerances. It is unclear what is the effect of gender on TPMT activity, with some studies showing an increased activity in males[28-31], decreased activity in females[32] or no effect of gender at all[33]. Patient age[30,31], has no effect on TPMT activity but combination treatment with 5-ASA therapy might increase 6-thioguanine nucleotide levels due to a negative effect of 5-ASA therapy on TPMT activity. Earlier reports had shown no effect of 5-ASA co-administration[30,34,35] on TPMT activity but more recent data has indicated 5-ASA therapy increases 6-thioguanine nucleotide levels[36,37]. Due to inconsistent data, identifying a clinical phenotype associated with thiopurine intolerance may facilitate in the decision-making process when prescribing a thiopurine and thus enable improved patient experiences and outcomes.
Thus, we evaluated thiopurine discontinuation due to adverse events in a cohort of CD patients and investigated clinical characteristics associated with adverse events.
MATERIALS AND METHODS
Study population
Subjects with CD who were followed in the Alberta Inflammatory Bowel Disease (IBD) Consortium patient database registry were identified[38]. Retrospective chart review was conducted between August 5th, 2010 and June 1st, 2012. We identified all patients with CD in our registry who had a current or previous prescription of a thiopurine agent (AZA or MP). Patients with no history of thiopurine therapy or with a diagnosis of ulcerative colitis, IBD unspecified, or microscopic colitis at time of chart review were not included in this study. We identified 366 CD patients with a current or prior prescription of a thiopurine. The clinical scenarios that patients with CD were prescribed a thiopurine are described in Figure 1. Fifteen subjects were excluded because the reason for withdrawal was unavailable (n = 12) or the status of thiopurine prescription was not obvious from the chart review (n = 3).
Figure 1.
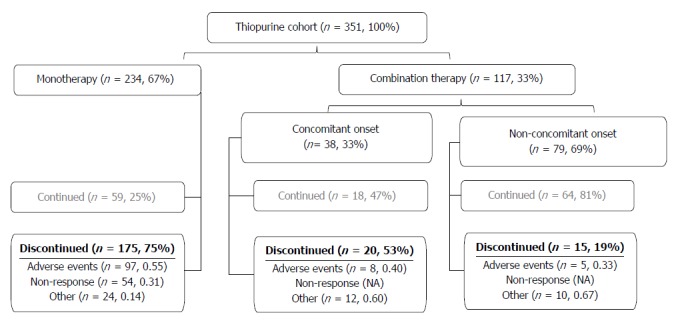
Patient disposition. Disposition of the whole study cohort. Drug disposition is described in the materials and methods section. Thiopurines were discontinued due to adverse events, non-response or other non-clinical reasons.
Outcomes
The primary outcome of interest was the first adverse event that led to discontinuation of the first thiopurine medication (AZA or MP) used. The diagnosis of the adverse event was based on the clinical opinion of the prescribing gastroenterologist in conjunction with investigations (e.g., elevated lipase for pancreatitis) when available. Adverse events were defined as: acute pancreatitis as defined by relevant clinical symptoms (e.g., epigastric pain) and serum lipase > 3 times above the reference range; leucopenia defined as a white blood cell count of less than 3500/mL; gastrointestinal intolerance (GI) as defined by a gastroenterologist recording symptoms of nausea, vomiting or non-specific abdominal pain in the absence of any other cause; hepatotoxicity as defined by a rise in either (1) alanine transaminase levels more than three times of upper limit of normal; (2) alkaline phosphatase levels more than twice upper limit of normal; or (3) total bilirubin level more than twice upper limit of normal (not including Gilbert’s syndrome) when associated with increased alanine transaminase or alkaline phosphatase[39,40]; infection and hypersensitivity reactions including arthralgias, myalgias, rash, fever and flu-like reaction alone or in combination as diagnosed by a gastroenterologist[41]. Uncommon adverse events (e.g., alopecia, photosensitivity, skin cancer) were grouped as other. Data on the date of the adverse event and if the adverse event led to drug withdrawal were recorded.
Variables
Information extracted using a comprehensive chart and electronic health record review included demographic data, laboratory studies, microbiology results, diagnostic imaging, operative and pathology reports, dictation notes, discharge summaries, and medication profiles. Data extraction was conducted independently by two trained clinicians (GM and MFD). Data extracted included: age at diagnosis (A1 ≤ 16 years, A2 17-40 years, A3 > 40 years); sex; location of disease (L1 ileal disease, L2 colonic disease, L3 ileocolonic disease and L4 upper gastrointestinal disease) by Montreal Classification[42] at time of prescribing thiopurine; disease behaviour by Montreal Classification[42] at thiopurine prescription (B1 inflammatory, B2 fibrostenotic, B3 penetrating disease); perianal fistulising disease at or prior to thiopurine prescription; smoking status at time of thiopurine prescription, which was classified as current smoking, past history of smoking, never smoker, or unknown; use of corticosteroid within 6 mo of diagnosis; dosage, age at onset, and cessation of 5-aminosalicyclic acid (5-ASA); thiopurines (AZA and MP) and anti-TNF (infliximab and adalimumab); combination therapy; and intestinal resection before thiopurine prescription. Based on their thiopurine exposure, patients were categorized into three groups; (1) thiopurine monotherapy, defined as patients naive to anti-TNF therapy or suffering an adverse event that led to discontinuation of the thiopurine prior to exposure to anti-TNF therapy; (2) non-concomitant onset combination therapy defined as anti-TNF therapy started more than 3 mo after thiopurine onset; and (3) concomitant onset combination therapy defined as concomitant prescription of thiopurine and anti-TNF within 3 mo. In the last two groups, the adverse event occurred while the patient was being exposed to combination therapy.
Statistical analysis
The primary outcome was cessation of the first thiopurine used (AZA or MP) due to an adverse event. An adverse event was defined as one or more of the following: acute pancreatitis; leucopenia; GI; hepatotoxicity; infection; and hypersensitivity reactions[41]. The frequency distribution for categorical variables and median with interquartile range (IQR) for continuous variables were calculated, and their comparisons were based on the Fisher exact test and Wilcoxon rank-sum test, respectively. Logistic regression was performed to evaluate associations between clinical factors and discontinuing a thiopurine for an adverse event. Risk estimates were presented as OR with 95%CI. The clinical factors that were a priori included into the model included: disease phenotype at the onset of thiopurine as described by age, location, behavior (B), and perianal fistulising disease; intestinal resection prior to thiopurine prescription, history of smoking, corticosteroids within 6 mo of diagnosis, and drug utilization patterns prior to thiopurine prescription for 5-ASA and anti-TNF. An interaction term between age (defined as ≤ or > 40 years) and sex was modeled to assess for effect modification. All statistical analyses were conducted using SAS version 9.2 (SAS Institute, Inc, Cary, NC). P values < 0.05 were considered to be statistically significant.
RESULTS
The study consisted of 351 subjects with a median follow-up duration of 5.8 years (IQR 25th-75th, 2.7-9.1]. Two patients were first initiated on MP while 349 patients were first treated with AZA. Most (n = 234, 66.7%) patients received thiopurine monotherapy, with the rest were exposed to anti-TNF therapy. The median dose of AZA prescribed was 2.1 mg/kg (IQR 25th-75th, 1.9-2.4 mg/kg). The median dose of MP prescribed was 1.2 mg/kg (IQR, 25th-75th 0.9-1.3 mg/kg). Drug disposition of the whole study group is described in Figure 1. Patient characteristics stratified by adverse events are shown in Table 1.
Table 1.
Cohort demographics n (%)
| Variables | Total (n = 351) | Discontinued due to adverse event (n = 110) | Continued therapy (n = 241) | P value |
| Gender | 0.36 | |||
| Female | 185 (52.7) | 62 (56.3) | 123 (51.1) | |
| Male | 166 (47.3) | 48 (43.6) | 118 (48.9) | |
| Age at diagnosis (A) (yr) | 0.05 | |||
| < 17 | 56 (16.0) | 13 (11.8) | 43 (17.8) | |
| 17-40 | 233 (66.4) | 70 (63.3) | 163 (67.6) | |
| > 40 | 62 (17.7) | 27 (24.5) | 35 (14.5) | |
| Age at thiopurine (yr) | 0.007 | |||
| < 17 | 21 (6.5) | 5 (4.5) | 16 (6.6) | |
| 17-40 | 195 (60.0) | 44 (40.0) | 151 (62.7) | |
| > 40 | 109 (33.5) | 43 (39.1) | 66 (27.4) | |
| L4 (upper gastrointestinal disease) | 0.52 | |||
| Yes | 27 (7.7) | 10 (9.1) | 17 (7.1) | |
| No | 324 (92.3) | 100 (90.1) | 224 (92.9) | |
| Behaviour (B) (%) | 0.91 | |||
| B1 (inflammatory) | 183 (56.0) | 55 (50.0) | 128 (53.1) | |
| B2 (fibrostenotic) | 62 (19.0) | 20 (18.2) | 42 (17.4) | |
| B3 (penetrating) | 82 (25.1) | 26 (23.6) | 56 (23.2) | |
| Perianal disease before thiopurine | 0.69 | |||
| Yes | 82 (23.4) | 24 (21.8) | 58 (24.1) | |
| No | 269 (76.6) | 86 (78.2) | 183 (75.9) | |
| Corticosteroid at diagnosis | 0.35 | |||
| Yes | 142 (52.8) | 39 (35.5) | 103 (42.7) | |
| No | 127 (47.2) | 42 (38.2) | 85 (35.3) | |
| Pre-thiopurine intestinal resection | 0.81 | |||
| Yes | 140 (39.9) | 45 (40.9) | 95 (39.4) | |
| No | 211 (60.1) | 65 (59.1) | 146 (60.6) | |
| Disease duration before thiopurines (yr) | 0.22 | |||
| < 1 | 100 (30.8) | 22 (20.0) | 78 (32.4) | |
| 1-5 | 81 (24.9) | 22 (20.0) | 59 (24.5) | |
| 5-10 | 63 (19.4) | 23 (20.9) | 40 (16.6) | |
| > 10 | 81 (24.9) | 25 (22.7) | 56 (23.2) | |
| 5-ASA exposure before thiopurine | 66 (24.8) | 18 (16.4) | 48 (19.9) | 0.47 |
| Anti-TNFα exposure1 | < 0.0001 | |||
| Thiopurine monotherapy | 234 (66.7) | 97 (88.2) | 137 (56.9) | |
| Non-con. Comb. therapy | 79 (22.5) | 5 (4.5) | 74 (30.7) | |
| Con. Comb therapy | 38 (10.8) | 8 (7.3) | 30 (12.4) | |
| Smoking history | 0.12 | |||
| Never | 180 (51.3) | 48 (43.6) | 132 (54.8) | |
| History of smoking | 126 (35.9) | 49 (44.5) | 77 (32.0) | |
| Unknown | 45 (12.8) | 13 (11.8) | 32 (13.2) |
Thiopurine monotherapy, defined as patients naive to anti-TNF therapy or suffering an adverse event that led to discontinuation of the thiopurine prior to exposure to anti-TNF therapy; non-concomitant onset combination therapy defined as anti-TNF therapy started more than 3 mo after thiopurine onset; concomitant onset combination therapy defined as concomitant prescription of thiopurine and anti-TNF within 3 mo. Clinical characteristics of the 351 CD patients treated with a thiopurine stratified by an adverse event requiring withdrawal of thiopurines. Anti-TNF: Anti-tumour necrosis factor; ASA: Aminosalicylic acid; CD: Crohn's disease.
Adverse events
Adverse events leading to thiopurine discontinuation occurred in 110 patients out of the total cohort of 351 and were distributed as follows (Table 2): hypersensitivity reactions (n = 25, 7.1%); acute pancreatitis (n = 22, 6.2%); GI toxicity (n = 19, 5,4%); leucopenia (n = 13, 3.7%); hepatotoxicity (n = 12, 3.4%) and infection (n = 4, 1.1%). Fifteen patients (4.3%) stopped medication for other adverse events (Table 2). Multiple adverse events were not recorded in a single patient as first adverse event leading to therapy cessation was the primary end point of this study. The four infections described were two intra-abdominal abscesses, one case of molluscum contagiosum and one case of pulmonary coccidioidomycosis. These patients were not leukopenic. Details regarding the types of adverse events were missing in the medical charts of four patients (3.6%).
Table 2.
Adverse events n (%)
| Variables | Hypersensitivity | Pancreatitis | GI | Leucopenia | Hepatotoxicity | Infection |
| patients | 25 (7.1) | 22 (6.2) | 19 (5.4) | 13 (3.7) | 12 (3.4) | 4 (1.1) |
| Gender | ||||||
| Males | 16 (64.0) | 9 (40.9) | 7 (36.8) | 4 (30.8) | 4 (33.3) | 2 (50.0) |
| Females | 9 (36.0) | 13 (59.1) | 12 (63.2) | 9 (69.2) | 8 (66.7) | 2 (50.0) |
| Age at thiopurine (yr) | 37.6 (32.3, 47.9) | 43.9 (32.2, 48.8) | 26.5 (22.8, 47.1) | 29.6 (23.6, 47.7) | 49 (41.8, 57.9) | 24.3 (19.3, 27.1) |
| Time from prescription to withdrawal of thiopurine (d) | 31 (29.0, 65.0) | 29 (14.5, 30.0) | 17 (7.0, 26.0) | 347.5 (159.0, 866.0) | 51 (30.0, 70,0) | 1907 (603.0, 2718.0) |
| AZA dose (mg/kg) | 2.2 (1.6, 2.4) | 2.3 (2.0, 2.4) | 2 (1.7, 2.2) | 1.6 (0.8, 2.3) | 1.3 (0.8, 2.0) | 2.1 (2.0, 3.1) |
Main reasons leading to discontinuation of thiopurine therapy in 351 patients due to an adverse event. No multiple reasons were recorded. Data are given as median and IQR unless otherwise stated. Other (n = 15, 13.6%) causes for discontinuation were alopecia (n = 3), photosensitivity (n = 1), basal cell carcinoma (n = 2), mood disturbance (n = 1), syncopal episodes (n = 1), fatigue (n = 1), headache (n = 1), eye problems (n = 1) and unknown (n = 4). AZA: Azathioprine; IQR: Interquartile range; GI: Gastrointestinal intolerance.
The median time from initiation to cessation of therapy for patients with a hypersensitivity reaction, acute pancreatitis, and gastrointestinal intolerance were of 31.0 (IQR 29.0, 65.0), 29.0 (IQR 14.5, 30.0) and 17.0 (IQR 7.0, 26.0) d respectively. In contrast, leucopenia resulted in drug cessation after a median of 347.5 d (IQR 159.0, 866.0) (P < 0.0001). Moreover, median AZA dosages were lower in patients who had to discontinue drug therapy due to leucopenia (1.6 mg/kg, IQR 0.8, 2.3) and hepatotoxicity (1.3 mg/kg, IQR 0.8, 2.0) (P = 0.04).
Clinical predictors of adverse events
Patients over the age of 40 when they started a thiopurine were more likely to discontinue the drug (P = 0.007) as compared to patients under the age of 40 (Table 1). In the multivariate analysis, effect modification was identified by age at thiopurine prescription for sex (P < 0.05, Wald test). In patients over 40 years of age at thiopurine prescription, females had a 4.0-fold (adjusted OR = 4.0, 95%CI: 1.9-8.3) increased risk of discontinuing therapy due to an adverse event than females under the age of 40. In contrast, age did not influence thiopurine withdrawal (adjusted OR = 1.31, 95%CI: 0.59-2.9) in males (Table 3).
Table 3.
Multivariate analysis
| Adjust variables | Crude analysis | Adjusted analysis |
| Total (n = 351) | ||
| Age starting thiopurine older than 40 vs 40 or younger | OR (95%CI) | |
| Female | 3.6 (1.9-7.1) | 4.0 (1.9-8.3) |
| Male | 1.2 (0.6-2.6) | 1.3 (0.6-3.0) |
Predictors of thiopurine-related adverse events as determined by multivariate analysis. A significant age-by-gender interaction was observed in both crude and adjusted analyses, P-value = 0.03 and 0.04 respectively.
In the stratified multivariate analysis by sex, female gender with an age of over 40 at thiopurine prescription was associated with a significantly increased risk of thiopurine discontinuation due to an adverse event (adjusted OR = 2.8, 95%CI: 1.4-5.6). This risk was not seen in male patients (adjusted OR = 0.9, 95%CI: 0.4-2.1). The other modeled clinical factors (smoking history, pre-thiopurine intestinal resection, disease behaviour and location, perianal disease and previous corticosteroid or 5-ASA use) were not associated with thiopurine discontinuation secondary to an adverse event (Table 4).
Table 4.
Stratified multivariate analysis
| Adjusted variables |
Adjusted OR (95%CI) |
||
| All cohort (n = 351) | Female (n = 185) | Male (n = 166) | |
| Age at Thiopurine prescription > 40 (sex-by-age interaction, P = 0.04) | 2.4 (1.4-4.2) | 2.8 (1.4-5.6) | 0.9 (0.4-2.1) |
| Female vs male (referent) | 1.2 (0.7-2.0) | NA | NA |
| Smoking history (current/past) | 1.5 (0.9-2.5) | 1.5 (0.8-3.1) | 1.6 (0.8-3.6) |
| Pre-thiopurine intestinal resection vs no resection (referent) | 0.86 (0.43-1.71) | 0.7 (0.3-1.8) | 1.0 (0.4-2.8) |
| L1 vs L2 (referent) | 1.4 (0.6-3.2) | 1.2 (0.4-3.6) | 3.1 (0.9-10.4) |
| L3 vs L2 (referent) | 1.5 (0.7-3.0) | 1.1 (0.4-2.7) | 2.1 (0.7-6.3) |
| B2 vs B1 (referent) | 1 (0.5-2.2) | 1.0 (0.3-2.8) | 0.7 (0.2-2.4) |
| B3 vs B1 (referent) | 1.1 (0.5-2.5) | 1.0 (0.3-2.8) | 0.8 (0.3-2.5) |
| Pre-thiopurine perianal disease vs no perianal disease (referent) | 0.7 (0.4-1.3) | 1.1 (0.5-2.7) | 0.7 (0.3-1.8) |
| 5-ASA at time of thiopurine vs past or never (referent) | 0.8 (0.4-1.5) | 0.7 (0.3-1.8) | 1.0 (0.4-2.4) |
| Corticosteroid at diagnosis vs no exposure at diagnosis (referent) | 0.9 (0.5-1.6) | 0.7 (0.3-1.5) | 0.9 (0.4-2.2) |
| L4 vs L2 (referent) | 1.8 (0.7-4.3) | 3.4 (0.9-12.2) | 0.9 (0.3-3.1) |
Predictors of thiopurine discontinuation in all cohort and as determined by this stratified multivariate analysis by sex. Demographic data suggests a higher incidence of adverse events in female patients over the age of 40 years. An age-by-gender interaction is seen as described in this multivariate analysis of thiopurine-exposed patients in female subjects over the age of 40 years. ASA: Aminosalicylic acid; NA: Not available.
DISCUSSION
Thiopurine antimetabolite drugs are effective therapy in IBD. Thiopurines are commonly used first line immunosuppressive therapy in subjects with moderate-severe CD. Thiopurines are also prescribed in combination with anti-TNF therapy to optimize effectiveness[42]. However, widespread use of thiopurines is hampered by potential adverse events that can lead to drug cessation. Our study highlights that intolerance to thiopurines is prevalent; particularly, among women with CD who are over the age of 40. This study demonstrates real-life clinical practice that suggests that combination strategies with anti-TNF therapy and long-term thiopurine monotherapy are therapeutic aims that might not be easy to achieve for patients with CD.
Nearly two-thirds of patients discontinued thiopurine therapy after a median follow-up of 5.8 years. This finding was comparable to prior studies[15,43]. However, a prospective cohort study of 394 patients exposed to thiopurine therapy, reported a lower frequency of withdrawals (16%) from thiopurines due to adverse events[44].
Leucopenia led to treatment cessation in 13 out of the 351 subjects with a median duration of treatment before discontinuation of 348 d. Thiopurine-induced myelotoxicity has a cumulative incidence of 7% with an incident rate of 3% per patient per year[43]. Our study was limited because of the lack of TPMT genotype and activity from our study population. However, TPMT polymorphisms explain leukopenia in only a quarter of cases[45].
The mean overall prevalence of thiopurine-induced liver toxicity was 3.4% in our study, which was similar to the prevalence of 3.4% described in a systematic review including 3485 patients[46]. Only 4 infectious events were described in this cohort of 351 patients on thiopurines. Although this study was not designed to assess the risk of infectious adverse events in CD patients exposed to thiopurines, our data is in line with previous studies[47] and is similar to previous findings in the TREAT registry that did not identify and increased incidence of sepsis in subjects on immunomodulators[48].
Pancreatitis is an idiosyncratic reaction. Pancreatitis was reported in 22 out of the 351 subjects (6.3%) which is slightly higher than the incident rate reported elsewhere (1.4%-3.3%)[49-51]. As expected from rarity of the reported incidence of lymphomatous adverse events in thiopurine-exposed subjects (0.9/1000 patient-years in the literature[52]), no cases were reported in our cohort.
We have shown after adjusting for different factors that females over the age of 40 years are at a higher risk of adverse events secondary to thiopurines as compared to women prescribed the drug below the age of 40 years. In contrast, age did not modify this association among men. This is novel finding has a potential clinical implication, if further research validates this finding in other cohorts. The higher risk of toxicity in older women may alter the decision to prescribe a thiopurine when compared against a different treatment option. Disease phenotype, smoking history, surgery or previous corticosteroid or 5-ASA usage were not found to be significantly associated with adverse events. Similar findings have been previously described in a small IBD Spanish cohort[53]. These findings could possibly be explained by recent data showing a significantly lower TPMT activity in females as compared to males[29-31,54].
We showed that patients exposed to an anti-TNF had significantly less episodes of drug cessation due to an adverse event. This variable was not included in our multivariate analysis as we did not feel this would be clinically useful. We would not advocate initiating anti-TNF therapy in CD patients to decrease the incidence of thiopurine discontinuation due to adverse events. Moreover, we feel that persistence with a thiopurine in patients on combination therapy is an indication of disease severity in an otherwise sick CD population with already a number of disease-related symptoms.
There are several strengths to this study. This is a large referral centre that follows a large cohort of CD patients. Fine phenotyping was conducted by a select group of trained physicians who performed random audits for quality assurance. Limitations to the study should be noted. This is a retrospective study that relied on a chart review process and thus, some clinical data were not comprehensively captured. Cessation of thiopurine therapy due to an adverse event was left at the discretion of the primary caring physician. As this was the primary inclusion criteria, this inherent variability might explain some of the noted differences in the adverse events incidence data. Patients were followed in a referral centre and thus our study population may have been skewed towards CD patients with a more complicated disease course. Also, data on TPMT genotype, activity and thiopurine metabolites were not available to explain these clinical findings in our study population. Finally, our results are exclusive to CD. We aimed to carry these analyses in a CD cohort in order to allow the multivariate analysis to identify a clinically useful phenotype in this population significantly related to a thiopurine adverse event. Entering other clinical parameters related to other inflammatory bowel diseases might have produced a more composite but less clinically meaningful outcome.
Thiopurines are effective therapy in certain CD phenotypes. Significantly more adverse events have been noted in female patients over the age of 40 years. Although our findings should not preclude this group from the thiopurine class of drugs, clinicians should be aware of the possible increased risk of toxicity in this patient cohort. Further work is needed to validate our findings in different patient populations and to try and explain the aetiology of this novel finding.
ACKNOWLEDGMENTS
We acknowledge the Alberta Inflammatory Bowel Disease Consortium and the Alberta Innovates Health Solutions for funding support for this project.
COMMENTS
Background
Thiopurine medication is effective therapy in the management of inflammatory bowel disease. Their clinical effectiveness is hampered by the incidence of related adverse events leading to drug discontinuation. A large recent retrospective Spanish cohort study indicates that a quarter of patients suffer an adverse event when exposed to thiopurine therapy leading to discontinuation of therapy in 17%. Multiple variables have been associated with the onset of adverse events including age, gender, the type of inflammatory bowel disease, co-administration with 5-aminosalicylic acid and thiopurine 6-methyltransferase activity (TMPT). Although a higher TMPT activity is noticed in infants and young children, this is unaffected by age in adulthood. Gender does seem to have an effect on thiopurine metabolism with some reports showing a disparity in TMPT activity in between gender with a lower TMPT activity being described in women. These findings are not universal with some reports finding no difference in thiopurine metabolism between males and females. Co-administration of thiopurine and 5-aminosalicylic acid therapy does increase the incidence of adverse events due to a negative effect of 5-aminosalicylic acid therapy on TPMT activity with a consequent rise of 6-thioguanine levels leading to adverse events. Despite these observations, measurements of TPMT activity and thiopurine metabolites are not routinely carried out in most healthcare systems. Most regions in Canada do not support these expensive tests. Similarly in the United Kingdom, despite TPMT measurement prior to therapy initiation is endorsed by the British Society of Gastroenterology, a substantial number of clinical commissioning groups do not financially support this test. Similar limitations are seen across other parts of the world. Moreover, in most cases adverse thiopurine-related adverse events are not explained by TPMT deficiencies. Identifying a clinical phenotype that could potentially predict adverse events to thiopurine in a real-life practice would be inexpensive and clinically useful.
Research frontiers
Thiopurine-related adverse events are common. Some may be explained by TPMT deficiency, though in most cases (including myelosuppression), it is clinically impossible to predict which patients will be intolerant to this medication. The current research hotspot is to identify a clinical phenotype associated with increased adverse event. This would be clinically useful as it would inform decision-making when starting immunosuppressive therapy.
Innovations and breakthroughs
To this date, the authors try and predict adverse events to thiopurine therapy by measuring TPMT activity prior to first prescription. Measuring thiopurine metabolites may be useful to try and optimise therapy and decrease adverse events. It is not clinically possible as yet to predict other adverse events. Furthermore, these expensive tests are not routinely available in large parts of the world. The authors hereby describe a clinical phenotype that significantly predicts an increased risk to discontinuation of thiopurine therapy due to an adverse event. This is a clinically useful finding that will improve decision-making when prescribing immunosuppressive therapy.
Applications
Patients of a female gender prescribed a thiopurine at any age over 40 years are at an increased risk to discontinuation of therapy due to an adverse event.
Terminology
TPMT is an enzyme involved in the breakdown of azathioprine/mercaptopurine to the active metabolite 6-thioguanine. A low TPMT activity is associated with increased levels of 6-thioguanine which enhances the clinical efficacy of any prescribed dose but also leads to a higher incidence of myelosuppression.
Peer-review
This is a retrospective study on chart review dealing with adverse events among patients with Crohn’s disease followed in the Alberta inflammatory bowel disease Consortium patient database. The manuscript reveals frequencies of a number of adverse events observed in a referral centre cohort including that of women older than 40 years have an increased risk for adverse events and discontinuation of thiopurine therapy.
Footnotes
Ethics approval: The study was approved by the Conjoint Health Research Ethics Board of the University of Calgary.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: Gordon W Moran has received consultancy fees from Abbvie and financial support for educational activities from Abbvie, MSD, Merck Sharp and Dohme Ltd and Ferring. Gilaad G Kaplan has served as a speaker for Merck, Schering-Plough, Jansen, and Abbott. He has participated in advisory board meetings for Abbott, Merck, Schering-Plough, Shire, Jansen, and UCB Pharma. Dr Kaplan has received research support from Abbott, Merck, and Shire. Subrata Ghosh has served as a speaker for Merck, Schering-Plough, Centocor, Abbott, UCB Pharma, Pfizer, Ferring, and Procter and Gamble. He has participated in ad-hoc advisory board meetings for Centocor, Abbott, Merck, Schering-Plough, Proctor and Gamble, Shire, UCB Pharma, Pfizer, and Millennium. He has received research funding from Procter and Gamble, Merck, and Schering-Plough. Remo Panaccione has served as a speaker, a consultant and an advisory board member for Abbott Laboratories, Merck, Schering-Plough, Shire, Centocor, Elan Pharmaceuticals, and Procter and Gamble. He has served as a consultant and speaker for Astra Zeneca. He has served as a consultant and an advisory board member for Ferring and UCB. He has served as a consultant for Glaxo-Smith Kline and Bristol Meyers Squibb. He has served as a speaker for Byk Solvay, Axcan, Jansen, and Prometheus. He has received research funding from Merck, Schering-Plough, Abbott Laboratories, Elan Pharmaceuticals, Procter and Gamble, Bristol Meyers Squibb, and Millennium Pharmaceuticals. He has received educational support from Merck, Schering-Plough, Ferring, Axcan, and Jansen. The remaining authors declare no conflict of interest.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 29, 2014
First decision: December 11, 2014
Article in press: February 11, 2015
P- Reviewer: Fries W, Nguyen DL, Nielsen OH, Stocco G S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 2.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Frolkis A, Dieleman LA, Barkema HW, Panaccione R, Ghosh S, Fedorak RN, Madsen K, Kaplan GG. Environment and the inflammatory bowel diseases. Can J Gastroenterol. 2013;27:e18–e24. doi: 10.1155/2013/102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, Frolkis T, Barkema HW, Rioux KP, Panaccione R, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn‘s disease. Cochrane Database Syst Rev. 2010;(6):CD000545. doi: 10.1002/14651858.CD000545.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Prefontaine E, Sutherland LR, Macdonald JK, Cepoiu M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2009;(1):CD000067. doi: 10.1002/14651858.CD000067.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Cosnes J, Bourrier A, Laharie D, Nahon S, Bouhnik Y, Carbonnel F, Allez M, Dupas JL, Reimund JM, Savoye G, Jouet P, Moreau J, Mary JY, Colombel JF; Groupe d’Etude Th rapeutique des Affections Inflammatoires du Tube Digestif (GETAID) Early administration of azathioprine vs conventional management of Crohn’s Disease: a randomized controlled trial. Gastroenterology. 2013;145:758–765.e2; quiz e14-15. doi: 10.1053/j.gastro.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 8.Panés J, López-Sanromán A, Bermejo F, García-Sánchez V, Esteve M, Torres Y, Domènech E, Piqueras M, Gomez-García M, Gutiérrez A, et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn’s disease. Gastroenterology. 2013;145:766–774.e1. doi: 10.1053/j.gastro.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 9.D’Haens GR, Panaccione R, Higgins PD, Vermeire S, Gassull M, Chowers Y, Hanauer SB, Herfarth H, Hommes DW, Kamm M, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199–212; quiz 213. doi: 10.1038/ajg.2010.392. [DOI] [PubMed] [Google Scholar]
- 10.Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Talley NJ, Abreu MT, Achkar JP, Bernstein CN, Dubinsky MC, Hanauer SB, Kane SV, Sandborn WJ, Ullman TA, Moayyedi P. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol. 2011;106 Suppl 1:S2–25; quiz S26. doi: 10.1038/ajg.2011.58. [DOI] [PubMed] [Google Scholar]
- 12.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 13.D’Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, De Vos M, van Deventer S, Stitt L, Donner A, Vermeire S, Van de Mierop FJ, Coche JC, van der Woude J, Ochsenkühn T, van Bodegraven AA, Van Hootegem PP, Lambrecht GL, Mana F, Rutgeerts P, Feagan BG, Hommes D; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–667. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 14.Melmed GY, Spiegel BM, Bressler B, Cheifetz AS, Devlin SM, Harrell LE, Irving PM, Jones J, Kaplan GG, Kozuch PL, et al. The appropriateness of concomitant immunomodulators with anti-tumor necrosis factor agents for Crohn’s disease: one size does not fit all. Clin Gastroenterol Hepatol. 2010;8:655–659. doi: 10.1016/j.cgh.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Lakatos PL, Kiss LS. Current status of thiopurine analogues in the treatment in Crohn’s disease. World J Gastroenterol. 2011;17:4372–4381. doi: 10.3748/wjg.v17.i39.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel CA. Review article: explaining risks of inflammatory bowel disease therapy to patients. Aliment Pharmacol Ther. 2011;33:23–32. doi: 10.1111/j.1365-2036.2010.04489.x. [DOI] [PubMed] [Google Scholar]
- 17.Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995;123:132–142. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- 18.López-Martín C, Chaparro M, Espinosa L, Bejerano A, Maté J, Gisbert JP. Adverse events of thiopurine immunomodulators in patients with inflammatory bowel disease. Gastroenterol Hepatol. 2011;34:385–392. doi: 10.1016/j.gastrohep.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 19.de Jong DJ, Derijks LJ, Naber AH, Hooymans PM, Mulder CJ. Safety of thiopurines in the treatment of inflammatory bowel disease. Scand J Gastroenterol Suppl. 2003;(239):69–72. doi: 10.1080/00855920310002726. [DOI] [PubMed] [Google Scholar]
- 20.Saibeni S, Virgilio T, D’Incà R, Spina L, Bortoli A, Paccagnella M, Peli M, Sablich R, Meucci G, Colombo E, et al. The use of thiopurines for the treatment of inflammatory bowel diseases in clinical practice. Dig Liver Dis. 2008;40:814–820. doi: 10.1016/j.dld.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Jharap B, Seinen ML, de Boer NK, van Ginkel JR, Linskens RK, Kneppelhout JC, Mulder CJ, van Bodegraven AA. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16:1541–1549. doi: 10.1002/ibd.21221. [DOI] [PubMed] [Google Scholar]
- 22.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485–489. doi: 10.1136/gut.50.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hindorf U, Lindqvist M, Hildebrand H, Fagerberg U, Almer S. Adverse events leading to modification of therapy in a large cohort of patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24:331–342. doi: 10.1111/j.1365-2036.2006.02977.x. [DOI] [PubMed] [Google Scholar]
- 24.Gearry RB, Barclay ML, Burt MJ, Collett JA, Chapman BA. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2004;13:563–567. doi: 10.1002/pds.926. [DOI] [PubMed] [Google Scholar]
- 25.Costantino G, Furfaro F, Belvedere A, Alibrandi A, Fries W. Thiopurine treatment in inflammatory bowel disease: response predictors, safety, and withdrawal in follow-up. J Crohns Colitis. 2012;6:588–596. doi: 10.1016/j.crohns.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Schwab M, Schäffeler E, Marx C, Fischer C, Lang T, Behrens C, Gregor M, Eichelbaum M, Zanger UM, Kaskas BA. Azathioprine therapy and adverse drug reactions in patients with inflammatory bowel disease: impact of thiopurine S-methyltransferase polymorphism. Pharmacogenetics. 2002;12:429–436. doi: 10.1097/00008571-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Ansari A, Arenas M, Greenfield SM, Morris D, Lindsay J, Gilshenan K, Smith M, Lewis C, Marinaki A, Duley J, et al. Prospective evaluation of the pharmacogenetics of azathioprine in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:973–983. doi: 10.1111/j.1365-2036.2008.03788.x. [DOI] [PubMed] [Google Scholar]
- 28.Klemetsdal B, Wist E, Aarbakke J. Gender difference in red blood cell thiopurine methyltransferase activity. Scand J Clin Lab Invest. 1993;53:747–749. doi: 10.3109/00365519309092580. [DOI] [PubMed] [Google Scholar]
- 29.Karas-Kuzelicki N, Milek M, Mlinaric-Rascan I. MTHFR and TYMS genotypes influence TPMT activity and its differential modulation in males and females. Clin Biochem. 2010;43:37–42. doi: 10.1016/j.clinbiochem.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Gisbert JP, Gomollón F, Cara C, Luna M, González-Lama Y, Pajares JM, Maté J, Guijarro LG. Thiopurine methyltransferase activity in Spain: a study of 14,545 patients. Dig Dis Sci. 2007;52:1262–1269. doi: 10.1007/s10620-006-9119-z. [DOI] [PubMed] [Google Scholar]
- 31.Cooper SC, Ford LT, Berg JD, Lewis MJ. Ethnic variation of thiopurine S-methyltransferase activity: a large, prospective population study. Pharmacogenomics. 2008;9:303–309. doi: 10.2217/14622416.9.3.303. [DOI] [PubMed] [Google Scholar]
- 32.Tamm R, Oselin K, Kallassalu K, Magi R, Anier K, Remm M, Metspalu A. Thiopurine S-methyltransferase (TPMT) pharmacogenetics: three new mutations and haplotype analysis in the Estonian population. Clin Chem Lab Med. 2008;46:974–979. doi: 10.1515/CCLM.2008.187. [DOI] [PubMed] [Google Scholar]
- 33.Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 34.Loit E, Tricco AC, Tsouros S, Sears M, Ansari MT, Booth RA. Pre-analytic and analytic sources of variations in thiopurine methyltransferase activity measurement in patients prescribed thiopurine-based drugs: A systematic review. Clin Biochem. 2011;44:751–757. doi: 10.1016/j.clinbiochem.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Booth RA, Ansari MT, Tricco AC, Loit E, Weeks L, Doucette S, Skidmore B, Hoch JS, Tsouros S, Sears M, et al. Assessment of thiopurine methyltransferase activity in patients prescribed azathioprine or other thiopurine-based drugs. Evid Rep Technol Assess (Full Rep) 2010;(196):1–282. [PMC free article] [PubMed] [Google Scholar]
- 36.Gao X, Zhang FB, Ding L, Liu H, Wang XD, Chen BL, Bi HC, Xiao YL, Zhao LZ, Chen MH, et al. The potential influence of 5-aminosalicylic acid on the induction of myelotoxicity during thiopurine therapy in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2012;24:958–964. doi: 10.1097/MEG.0b013e3283545ae3. [DOI] [PubMed] [Google Scholar]
- 37.Hande S, Wilson-Rich N, Bousvaros A, Zholudev A, Maurer R, Banks P, Makrauer F, Reddy S, Burakoff R, Friedman S. 5-aminosalicylate therapy is associated with higher 6-thioguanine levels in adults and children with inflammatory bowel disease in remission on 6-mercaptopurine or azathioprine. Inflamm Bowel Dis. 2006;12:251–257. doi: 10.1097/01.MIB.0000206544.05661.9f. [DOI] [PubMed] [Google Scholar]
- 38.Moran GW, Dubeau MF, Kaplan GG, Yang H, Seow CH, Fedorak RN, Dieleman LA, Barkema HW, Ghosh S, Panaccione R. Phenotypic features of Crohn’s disease associated with failure of medical treatment. Clin Gastroenterol Hepatol. 2014;12:434–42.e1. doi: 10.1016/j.cgh.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 39.Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272–276. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 40.Mumoli N, Cei M, Cosimi A. Drug-related hepatotoxicity. N Engl J Med. 2006;354:2191–2193; author reply 2191-2193. [PubMed] [Google Scholar]
- 41.Meggitt SJ, Anstey AV, Mohd Mustapa MF, Reynolds NJ, Wakelin S. British Association of Dermatologists’ guidelines for the safe and effective prescribing of azathioprine 2011. Br J Dermatol. 2011;165:711–734. doi: 10.1111/j.1365-2133.2011.10575.x. [DOI] [PubMed] [Google Scholar]
- 42.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 43.Gisbert JP, Gomollón F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103:1783–1800. doi: 10.1111/j.1572-0241.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- 44.Gisbert JP, Niño P, Rodrigo L, Cara C, Guijarro LG. Thiopurine methyltransferase (TPMT) activity and adverse effects of azathioprine in inflammatory bowel disease: long-term follow-up study of 394 patients. Am J Gastroenterol. 2006;101:2769–2776. doi: 10.1111/j.1572-0241.2006.00843.x. [DOI] [PubMed] [Google Scholar]
- 45.Colombel JF, Ferrari N, Debuysere H, Marteau P, Gendre JP, Bonaz B, Soulé JC, Modigliani R, Touze Y, Catala P, et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn’s disease and severe myelosuppression during azathioprine therapy. Gastroenterology. 2000;118:1025–1030. doi: 10.1016/s0016-5085(00)70354-4. [DOI] [PubMed] [Google Scholar]
- 46.Gisbert JP, González-Lama Y, Maté J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2007;102:1518–1527. doi: 10.1111/j.1572-0241.2007.01187.x. [DOI] [PubMed] [Google Scholar]
- 47.Siegel CA, Sands BE. Review article: practical management of inflammatory bowel disease patients taking immunomodulators. Aliment Pharmacol Ther. 2005;22:1–16. doi: 10.1111/j.1365-2036.2005.02520.x. [DOI] [PubMed] [Google Scholar]
- 48.Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Price S, Langholff W, Londhe A, Sandborn WJ. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012;107:1409–1422. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miheller P, Lakatos PL. Thiopurines in Crohn’s disease, is there something new? Expert Opin Drug Metab Toxicol. 2010;6:1505–1514. doi: 10.1517/17425255.2010.525505. [DOI] [PubMed] [Google Scholar]
- 50.van Geenen EJ, de Boer NK, Stassen P, Linskens RK, Bruno MJ, Mulder CJ, Stegeman CA, van Bodegraven AA. Azathioprine or mercaptopurine-induced acute pancreatitis is not a disease-specific phenomenon. Aliment Pharmacol Ther. 2010;31:1322–1329. doi: 10.1111/j.1365-2036.2010.04287.x. [DOI] [PubMed] [Google Scholar]
- 51.Bermejo F, Lopez-Sanroman A, Taxonera C, Gisbert JP, Pérez-Calle JL, Vera I, Menchén L, Martín-Arranz MD, Opio V, Carneros JA, et al. Acute pancreatitis in inflammatory bowel disease, with special reference to azathioprine-induced pancreatitis. Aliment Pharmacol Ther. 2008;28:623–628. doi: 10.1111/j.1365-2036.2008.03746.x. [DOI] [PubMed] [Google Scholar]
- 52.Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 53.Martínez F, Nos P, Pastor M, Garrigues V, Ponce J. Adverse effects of azathioprine in the treatment of inflammatory bowel disease. Rev Esp Enferm Dig. 2001;93:769–778. [PubMed] [Google Scholar]
- 54.Klemetsdal B, Tollefsen E, Loennechen T, Johnsen K, Utsi E, Gisholt K, Wist E, Aarbakke J. Interethnic difference in thiopurine methyltransferase activity. Clin Pharmacol Ther. 1992;51:24–31. doi: 10.1038/clpt.1992.4. [DOI] [PubMed] [Google Scholar]


