Abstract
Hyperactivation of the mechanistic target of rapamycin complex 1 (mTORC1) is a frequent event in breast cancer and current efforts are aimed at targeting the mTORC1 signaling pathway in combination with other targeted therapies. However, patients often develop drug resistance in part due to activation of the oncogenic Akt signaling and upregulation of autophagy, which protects cancer cells from apoptosis. In the present study we investigated the effects of combination therapy of rapamycin (an allosteric mTORC1 inhibitor) together with resveratrol (a phytoestrogen that inhibits autophagy). Our results show that combination of these drugs maintains inhibition of mTORC1 signaling, while preventing upregulation of Akt activation and autophagy, causing apoptosis. Additionally, this combination was effective in estrogen receptor positive and negative breast cancer cells, underscoring its versatility.
Keywords: Rapamycin, Resveratrol, mtorc1, Autophagy, Apoptosis
Breast cancer is the second leading cause of cancer related deaths affecting women, and over 200,000 new cases are diagnosed each year. Breast cancer can be classified into two major groups: estrogen receptor alpha-positive (ERα+) and ERα-negative (ERα−). About 12–24% of breast cancers are triple-negative breast cancer (TNBC) and are defined as lacking expression of human epidermal growth factor receptor 2 (HER2), ER, and progesterone receptor (PR). TNBCs are usually more aggressive and patients are more likely to relapse and develop central nervous system and visceral metastases [Schmadeka et al., 2014]. These patients are often treated with chemotherapy agents due to the lack of effective FDA-approved targeted treatments.
About two-thirds of breast cancers are ERα+ and these patients can be treated with endocrine therapy, although 40–50% fail to respond, and many develop resistance, indicating that ERα+ breast cancers are dependent on additional signaling mechanisms for survival. The mechanistic target of rapamycin (mTOR) is a serine/threonine kinase that belongs to the phosphatidylinositol 3-kinase-related kinase (PIKK) family and was discovered as a target of a naturally occurring molecule called rapamycin. mTOR regulates proliferation, cellular metabolism, protein and lipid synthesis and autophagy in response to extracellular signals such as nutrient availability and growth factors. mTOR forms two distinct complexes: mTOR Complex 1 (mTORC1) and mTORC2 and these complexes differ in their protein composition, downstream targets and sensitivity to rapamycin. mTORC1 is acutely rapamycin-sensitive and mTORC2 is rapamycin- insensitive [Alayev and Holz, 2013]. The PI3K/mTORC1 signaling pathway is hyperactivated in a variety of tumors, including breast cancer, and is important for tumor progression and resistance to endocrine therapy. This pathway is the most frequently inappropriately activated pathway in breast cancer and several alterations of the genes within the PI3K/Akt/mTOR pathway are often found in ERα+ breast cancers [Ciruelos Gil, 2014]. One of the ways by which mTORC1 signaling promotes endocrine resistance is by direct phosphorylation of ERα on Ser167 by the 40S Ribosomal S6 kinase 1 (S6K1), a major downstream effector of mTORC1, leading to ligand-independent activation of ERα [Yamnik et al., 2009, Yamnik and Holz, 2010]. ERα, in turn, transcriptionally upregulates S6K1 expression, leading to its own activation in a feed-forward loop [Holz 2012; Maruani et al., 2012]. Since it is thought that hyperactivation of PI3K/Akt/mTOR signaling is responsible for de novo and acquired drug resistance, mTOR inhibitors have emerged as a highly promising strategy for use in combination with endocrine therapy to prevent emergence and/or reverse drug resistance. Based on the results of the BOLERO-2 trial, the FDA approved the use of everolimus (an orally administered allosteric mTOR inhibitor) together with exemestane (an aromatase inhibitor) [Baselga et al., 2012]. However, one of the problems with the use of mTOR inhibitors is that they relieve the mTORC1-mediated negative-feedback loop to PI3K, causing reactivation of the oncogene Akt [Sun et al., 2005; O’Reilly et al., 2006] which consequently promotes activation of mTOR/S6K1 signaling and cell survival, a mechanism that is thought to be responsible for the emergence of resistance and cancer relapse.
Another survival mechanism used by cancer cells is upregulation of autophagy, a conserved cellular process that maintains cellular homeostasis by degrading and recycling misfolded proteins and damaged organelles. During nutrient deprivation, autophagy allows the cell to survive by reusing intracellular proteins until extracellular nutrients become available. Upon initiation of autophagy, cellular components that are targeted for degradation are sequestered in double-membrane autophagosomes, which then fuse with lysosomes and are degraded [Baehrecke, 2005; Rabinowitz and White, 2010, Rubinsztein et al., 2012]. In cancer, autophagy can either induce cancer cell death or promote cancer cell survival, contributing to cancer progression. Autophagy allows cancer cells to continue to survive by recycling intracellular components and persisting under low-nutrient or stress-induced conditions. This is a delicate balance because excessive autophagic damage will cause cell death. In breast cancer, autophagy protects cancer cells from cell death and may contribute to acquired drug resistance. mTORC1 is a central node in the signaling pathway that includes many autophagy regulating factors, such as AMPK (positive regulator [Liang et al., 2007], Akt (negative regulator [Esclatine et al., 2009] and S6K1 (positive regulator in mammals [Armour et al., 2009]. mTORC1 signaling acts to suppress autophagy, consistent with its role in supporting anabolic processes in the cell. Conversely, inhibition of mTORC1 by rapamycin potently induces autophagy [Yu et al., 2011]. Thus, use of rapamycin analogs in breast cancer treatment results in upregulation of autophagic processes that may promote breast cancer cell survival.
In this study, we investigated agents that can be used in combination with mTOR inhibitors to prevent reactivation of Akt and induction of autophagy, thus driving cells to apoptosis in both ERα+ and ERα− breast cancer cell types. Resveratrol (Trans-3,5,4’-trihydroxystilbene) is a polyphenol naturally found in red wine, grapes and peanuts that possesses disease-protective and anti-aging properties. Resveratrol is thought to act as a chemopreventative agent by attenuating autophagy, cell growth and proliferation, which are associated with cancer initiation and progression [Banerjee et al., 2002; Whitsett et al., 2006; Bishayee 2009]. Resveratrol inhibits autophagy, possibly through inhibition of S6K1, a positive regulator of autophagy [Armour et al., 2009; Demidenko and Blagosklonny, 2009]. Resveratrol is also a phytoestrogen and may be able to prevent the action of estrogen due to antagonistic activity toward estrogen receptor [Gehm et al., 1997]. In light of these data, we set out to test the effectiveness of combining rapamycin together with resveratrol to promote apoptosis of ERα+ and ERα− breast cancer cells.
MATERIALS AND METHODS
CELL CULTURE
Cells were cultured in a humidified incubator with 5% CO2 at 37°C. MCF7 and MDA-MB-231 cells were cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), while MCF10a cells were cultured in DMEM F12 media supplemented with 5% horse serum, 0.02 µg/ml epidermal growth factor (EGF), 0.5µg/ml hydrocortisone, 0.1 µg/ml cholera toxin, and 10 µg/ml insulin.
CELL TREATMENT
Cells were treated with 20nM Rapamycin (dissolved in ethanol), 100 µM Resveratrol (dissolved in DMSO) (Sigma-Aldrich, R5010) or combination of the two agents for 24 h. For control we used vehicle alone. For detection of LC3-I/II, cells were treated with 100nM Bafilomycin A1 (BafA1, B1793) for 4 h, following treatment with 20nM Rapamycin and/or 100 µM Resveratrol.
IMMUNOBLOTS
Following treatment, cells were lysed as previously described [Yamnik et al., 2009; Yamnik and Holz, 2010; Maruani et al., 2012] in ice-cold lysis buffer (10mM KPO4, 1mM EDTA, 10mM MgCl2, 50mMβ-glycerophosphate, 5mM EGTA, 0.5% Nonidet P-40 [NP-40], 0.1% Brij 35, 1mM sodium orthovanadate, 40 µg/ml phenylmethylsulfonyl fluoride, 10µg/ml leupeptin, 5 µg/ml pepstatin, pH 7.28). Lysates were cleared of insoluble material by centrifugation at 15,000g for 10 min at 4°C.
Protein concentrations in cell extracts were measured by Bradford assay (BioRAD, 500–0006) according to the manufacturer ’s protocol using Eppendorf BioPhotometer. Samples were equalized for protein concentration and denatured using 4× LDS Sample buffer (Invitrogen, B0008) and 10× Reducing agent (Invitrogen, B0009) at 70°C for 10 min. Samples were resolved using Bis-Tris Plus gels (Invitrogen, BG04120BOX) and transferred onto nitrocellulose membrane (GE Healthcare, Rahway, NJ). Membranes were probed with the following primary antibodies: p-Akt Ser473 (Cell Signaling Technologies, 9018), Akt (Cell Signaling Technologies, 4691L), p-S6K1 Thr389 (Cell Signaling Technologies, 9206), S6K1 (Cell Signaling Technologies, 2708), p-eIF4B Ser422 (Cell Signaling Technologies, 3591), p-S6 Ser240/244 (Cell Signaling Technologies, 2215), S6 (Cell Signaling Technologies, 2317S), p-PRAS40 Thr246 (Cell Signaling Technologies, 2997), PRAS40 (Cell Signaling Technologies, 2691P), p62 (Cell Signaling Technologies, 5114), LC3 (Cell Signaling Technologies, 2775), survivin (Cell Signaling Technologies, 71G4B7), and Caspase 3 (Cell Signaling Technologies, 9665); PDCD4 (Proteintech, 12587–1-AP), actin (Santa Cruz Biotechnology, sc-1615), α-tubulin (Abcam, ab7750), and PARP (Abcam, ab32071). Blots were incubated with IRDye-conjugated anti-rabbit (LI-COR, 827–08365), anti-mouse (LI-COR, 926–68070) or anti-goat (LI-COR, 926–68074) secondary antibodies and imaged using Odyssey infrared detection instrument (LI-COR). All immunoblots were performed at least thrice to ensure reproducibility.
MICROSCOPY
Microscopy was performed using an EVOS FL Auto microscope. Cells were imaged in phase under 10× magnification
PROLIFERATION ASSAY
Cells were seeded at a density of 2,500 cells/well in a 96-well plate, and allowed to attach. Cells were treated in quadruplicate with 20 nM Rapamycin and/or 100 µM Resveratrol for 48 h. To detect viable cells, cells were incubated with 100 µg/ml solution of neutral red dye in growth media for 30min at 37°C. Cells were washed and fixed in a 0.5% formalin-1% CaCl2 solution and permeabilized in 1% acetic acid-50% ethanol solution to release the incorporated neutral red reagent. Absorbance was measured at 540 nm using a microtiter plate spectrophotometer, quantified and plotted using Excel.
STATISTICAL ANALYSIS
All experiments were performed thrice to ensure reproducibility. Statistical differences were determined using a two-tailed Student’s t-test.
RESULTS
COMBINATION OF RAPAMYCIN AND RESVERATROL PREVENTS UPREGULATION OF AKT SIGNALING WHILE MAINTAINING INHIBITION OF mTORC1/S6K1
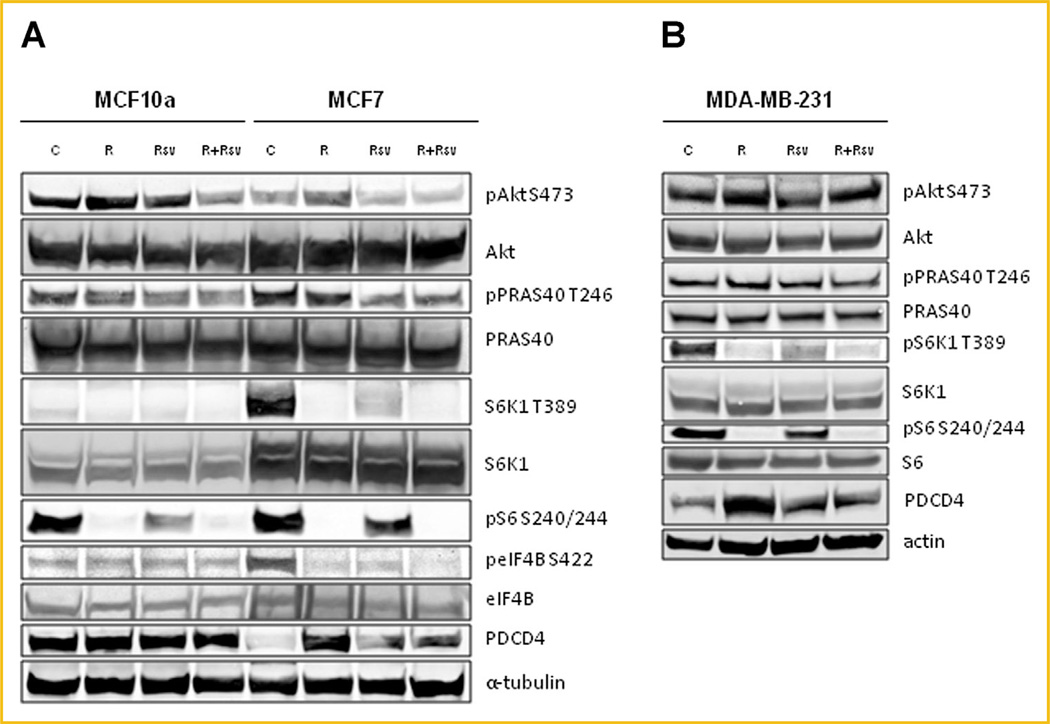
We initially tested the effect of rapamycin and resveratrol, alone or in combination on the activity of the mTORC1/Akt signaling pathway in MCF7 cells, human breast adenocarcinoma cell line, and MCF10a cells, immortalized non-transformed mammary epithelial cells (Fig. 1A). MCF7 cells have high levels of mTORC1 signaling as evidenced by increased phosphorylation of S6K1, and its substrates S6 and eIF4B, relative to MCF10a cells, and low levels of PDCD4, a negative regulator of cap-dependent protein translation initiation that is degraded by activated S6K1 signaling [Dorrello et al., 2006]. As expected, rapamycin blocked phosphorylation of S6K1 and its downstream targets. Resveratrol alone was not as efficient in blocking signaling downstream of S6K1, however, the combination of the two drugs completely inhibited the mTORC1 signaling pathway, strikingly reducing S6 and eIF4B phosphorylation, and increasing PDCD4 levels (Fig. 1A). A consequence of mTORC1 inhibition is reactivation of Akt signaling due to suppression of the mTORC1-mediated negative feedback loop to Akt, which over time re-activates mTORC1 signaling and is thought to contribute to drug resistance in patients. While treatment with rapamycin increased phosphorylation of Akt, the combination treatment of rapamycin and resveratrol was able to block activation of Akt and its downstream target PRAS40 to levels below those of untreated control (Fig. 1A).
Fig. 1.
Combination of rapamycin and resveratrol inhibits PI3K/Akt and mTOR signaling pathways in both ERα+ and TNBC cells. (A) MCF10a and MCF7 cells were treated with 20nM rapamycin and/or 100 µM resveratrol for 24 h. Cells were lysed and indicated proteins were detected by immunoblot. (B) MDA-MB-231 cells were treated as described in (A). Each experiment was performed at least thrice to ensure reproducibility.
We also tested the effectiveness of combination therapy on MDA-MB-231 triple-negative breast cancer cells (Fig. 1B). These cells lack expression of Her2, ER and PR, and while they are responsive to conventional chemotherapy, they are not sensitive to rapamycin. We found that the combination of rapamycin and resveratrol was able to robustly block mTORC1 signaling as evidenced by downregulation of S6K1 and S6 phosphorylation and increased PDCD4 levels. The combination therapy was also able to slightly downregulate Akt and PRAS40 phosphorylation compared to rapamycin treatment alone (Fig. 1B).
COMBINATION THERAPY PREVENTS RAPAMYCIN-INDUCED UPREGULATION OF AUTOPHAGY AND INDUCES APOPTOSIS
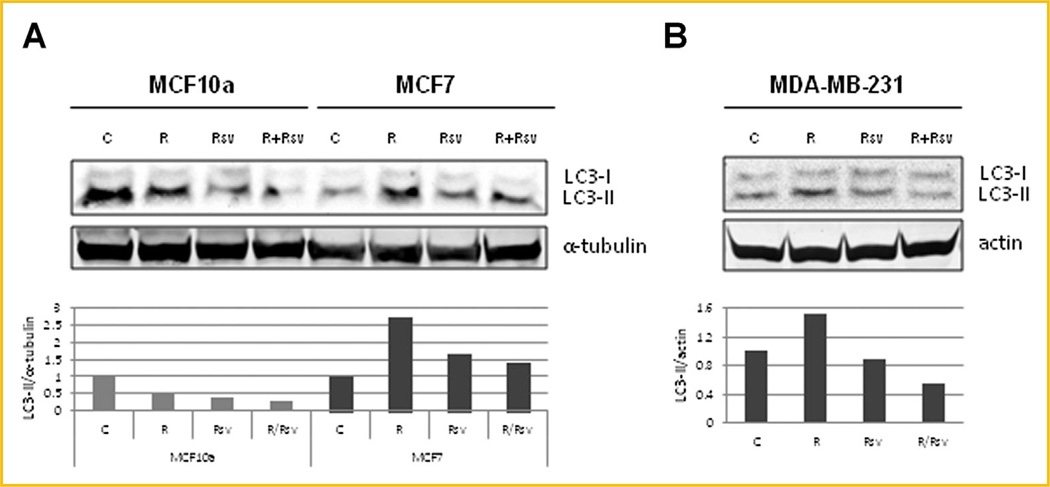
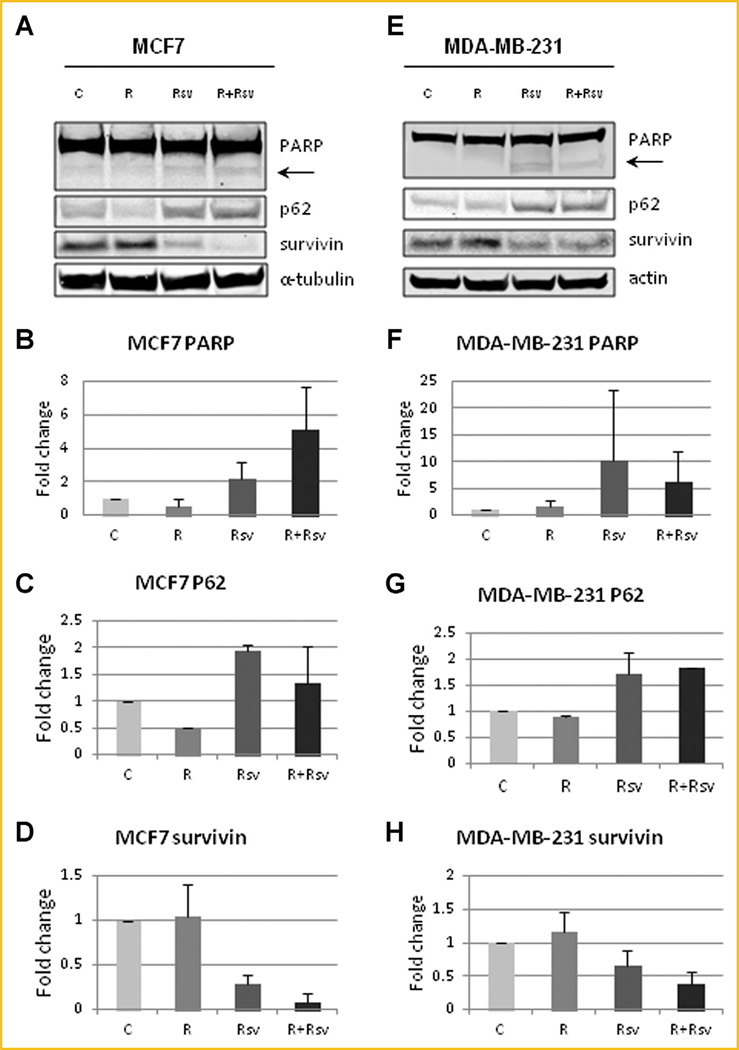
Another big challenge with the use mTORC1 inhibitors, such as rapamycin, is that rapamycin is cytostatic and not cytotoxic. mTORC1 inhibition leads to induction of autophagy, which allows cancer cells to survive and avoid apoptosis. One of the ways to measure autophagy levels is by examining LC3, whereby upon autophagy induction LC3-I is cleaved to LC3-II. The levels of LC3-II correlate with the number of autophagosomes. Since LC3-II is rapidly degraded by autophagy, addition of Bafilomycin A1 (BafA1) blocks lysosomal degradation and causes LC3-II to accumulate, therefore allowing for detection of increased autophagic flux. When we treated MCF7 cells with rapamycin, we observed a 2.7-fold increase in LC3-II expression, while addition of resveratrol reduced LC3-II levels to near control (Fig. 2A). No changes in LC3-II were observed in immortalized non-transformed MCF10a cells. Similarly, MDA-MB-231 cells treated with rapamycin demonstrated a 1.5 fold upregulation of LC3-II expression, while the addition of resveratrol reduced autophagy induction to below control (Fig. 2B). Another way of evaluating autophagy is by examining p62/SQSTM1 protein levels, which inversely correlate with autophagy induction. We observed an increase in autophagic flux in MCF7 cells treated with rapamycin alone, as evidenced by degradation of p62. Resveratrol was able to counter the effect of rapamycin by blocking autophagy induction and restoring p62 levels to above baseline (Fig. 3A,C). Similar effect on autophagy was seen in MDA-MB-231 cells (Fig. 3E,G). Another mechanism of cancer cell survival is through upregulation of survivin, an anti-apoptotic protein that inhibits apoptosis and promotes cell division. Not surprisingly, both MCF7 (Fig. 3A,D) and MDA-MB-231 (Fig. 3E,H) cells have high levels of survivin expression and these levels remain high upon rapamycin treatment. Interestingly, resveratrol treatment alone, or in combination, reduced survivin expression to levels that were lower than the untreated cells (Fig. 3A, D and E, H). Importantly, treatment of MCF7 and MDA-MB-231 cells with either resveratrol alone or in combination with rapamycin upregulated apoptosis as shown by increased cleavage of PARP (Fig. 3A,B and E,F). Thus, our results indicate that the combination treatment blocks rapamycin-induced upregulation of autophagy and induces apoptosis.
Fig. 2.
Resveratrol prevents rapamycin induced upregulation of autophagy. (A) MCF10a and MCF7 cells were treated with 20 nM rapamycin and/or 100 µM resveratrol in the presence of 100nM of BafA1 for 4 h. Cells were lysed and blotted for LC3 and α-tubulin. The histogram shows LC3-II levels (lower band) normalized to α-tubulin levels. LC3-II and α-tubulin levels were quantified using Image Studio 4.0 (Li-COR) and plotted using Excel. (B) MDA-MB-231 cells were treated with 20nM rapamycin and/or 100 µM resveratrol with 100nM of BafA1 for 4 h. Cells were lysed and blotted for LC3 and actin. The histogram shows LC3-II levels (lower band) normalized to actin levels. LC3-II and actin levels were quantified using Image Studio 4.0 (Li-COR) and plotted using Excel. Each experiment was performed at least thrice to ensure reproducibility.
Fig. 3.
Combination of rapamycin and resveratrol inhibits autophagy and induces apoptosis. (A) MCF10a and MCF7 cells were treated with 20nM rapamycin and/or 100 µM resveratrol for 24 h. Cells were lysed and probed for PARP, P62, surviving and α-tubulin. The arrow indicates cleaved PARP isoform. Quantification of cleaved PARP (B), p62 (C), and surviving (D) in MCF7 cells. (E) MDA-MB-231 cells were treated with 20nM rapamycin and/or 100 µM resveratrol for 24 h. Cells were lysed and probed for PARP, P62, surviving and actin. The arrow indicates cleaved PARP isoform. Quantification of cleaved/uncleaved PARP (F), p62 (G), and surviving (H) in MDA-MB-231 cells. Each experiment was performed at least thrice to ensure reproducibility. Quantification was performed using Image Studio 4.0 program and Excel.
COMBINATION OF RAPAMYCIN AND RESVERATROL SIGNIFICANTLY AFFECTS GROWTH OF BREAST CANCER CELLS
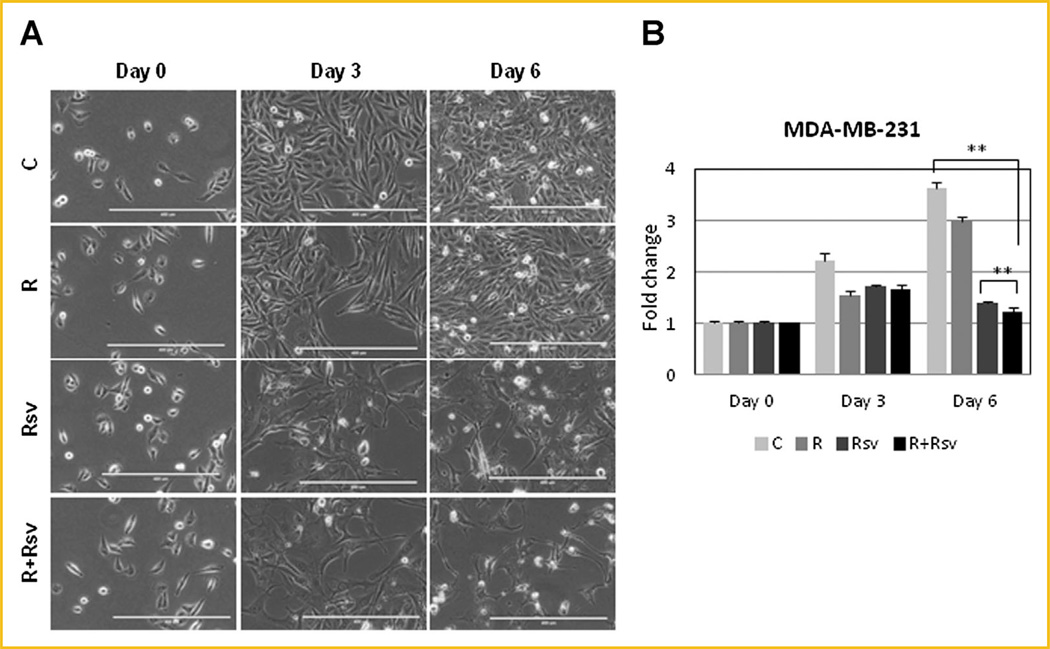
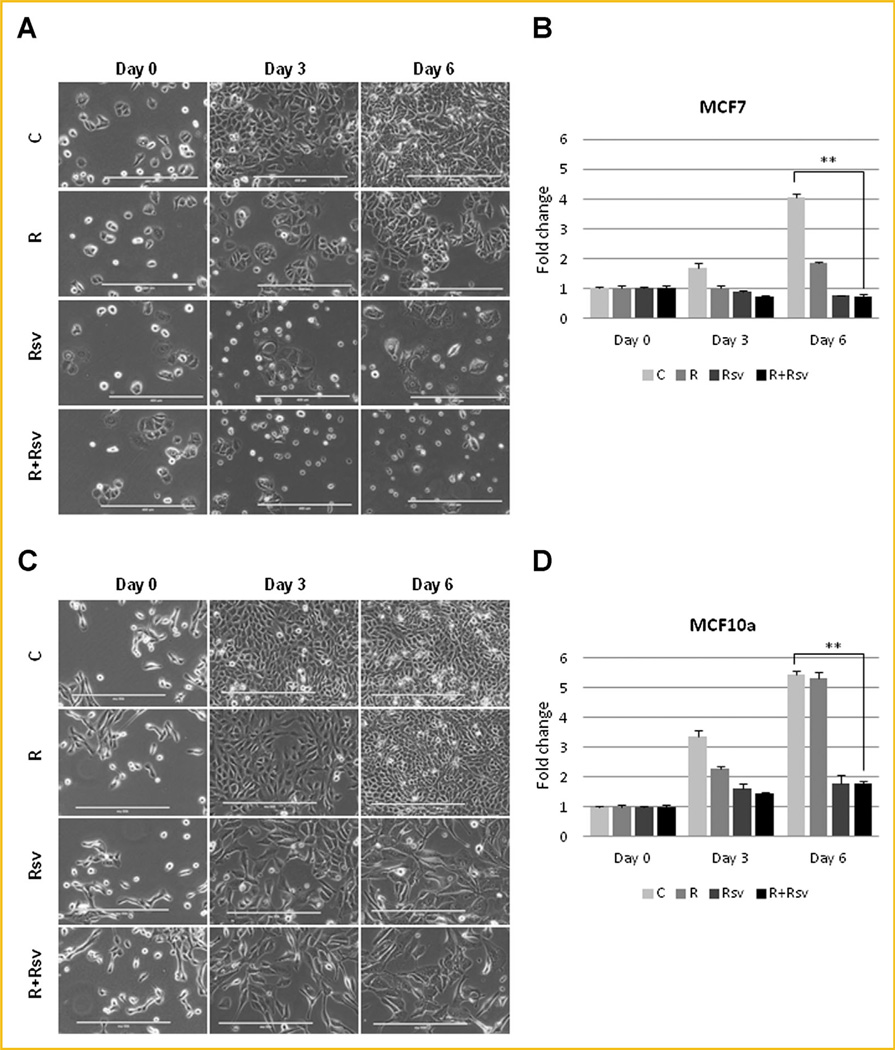
We next investigated proliferation rates and morphology of cells treated with rapamycin and/or resveratrol. Untreated MDA-MB-231 cells appeared attached and healthy; their density increasing by 3.6-fold on day 6, while cells treated with rapamycin grew slightly less. Strikingly, cells treated with resveratrol alone or in combination with rapamycin did not proliferate, remaining at about the same density by day 6. These cells did not appear healthy, contained large vacuoles, spindle-like projections, and many cells were rounded-off or dead floating cells (Fig. 4A and B). Unlike untreated MCF7 cells, cells treated with rapamycin were significantly growth inhibited, but were not undergoing cell death. In contrast, MCF7 cells treated with either resveratrol alone or in combination with rapamycin not only had reduced proliferation, but also underwent a significant amount of death, and many cells appeared rounded off and floating (Fig. 5A and B). In contrast, MCF10a cells treated with either resveratrol alone or together with rapamycin had reduced proliferation rates but appeared healthy with very few detached cells (Fig. 5C and D). Thus, our results indicate that combination treatment of rapamycin and resveratrol is selectively effective in inhibiting cell growth and inducing cell death in breast cancer cells rather than in non-transformed mammary epithelial cells.
Fig. 4.
Combination of rapamycin and resveratrol blocks proliferation of TNBC cells. (A) MDA-MB-231 cells were treated with 20nM rapamycin and/or 100µM resveratrol for 3 or 6 days and photographed using EVOS FL Auto microscope in phase using 10× magnification. Scale bar represents 400 µm. (B) MDA-MB-231 cells were with 20 nM rapamycin and/or 100 µM resveratrol for 3 or 6 days and proliferation assay was performed as described in “Materials and Methods”. ** P <0.001. Statistical analysis was performed using two-tailed Student’s t-test.
Fig. 5.
Combination of rapamycin and resveratrol blocks proliferation of ERα+ breast cancer cells. (A) MCF10a and MCF7 cells were treated with 20 nM rapamycin and/or 100 µM resveratrol for 3 or 6 days and photographed using EVOS FL Auto microscope in phase using 10× magnification. Scale bar represents 400 µm. (B) MCF10a and MCF7 cells were with 20 nM rapamycin and/or 100 µM resveratrol for 3 or 6 days and proliferation assay was performed as described in “Materials and Methods”. ** P <0.001. Statistical analysis was performed using two-tailed Student’s t-test.
DISCUSSION
Resveratrol has emerged as a subject of extensive investigation for its multi-faceted properties: anti-aging, cardioprotection, and cancer prevention. Clinical trials investigating the potential of resveratrol as an anti-cancer agent are limited, focused mainly on gastrointestinal malignancies, and indicated resveratrol’s potential in cancer prevention rather than cancer treatment. Studies testing resveratrol in animal models of breast cancer suggested potential as a chemopreventative or a cancer treatment agent, however, beneficial effects were dependent on animal and cell type [Carter et al., 2014].
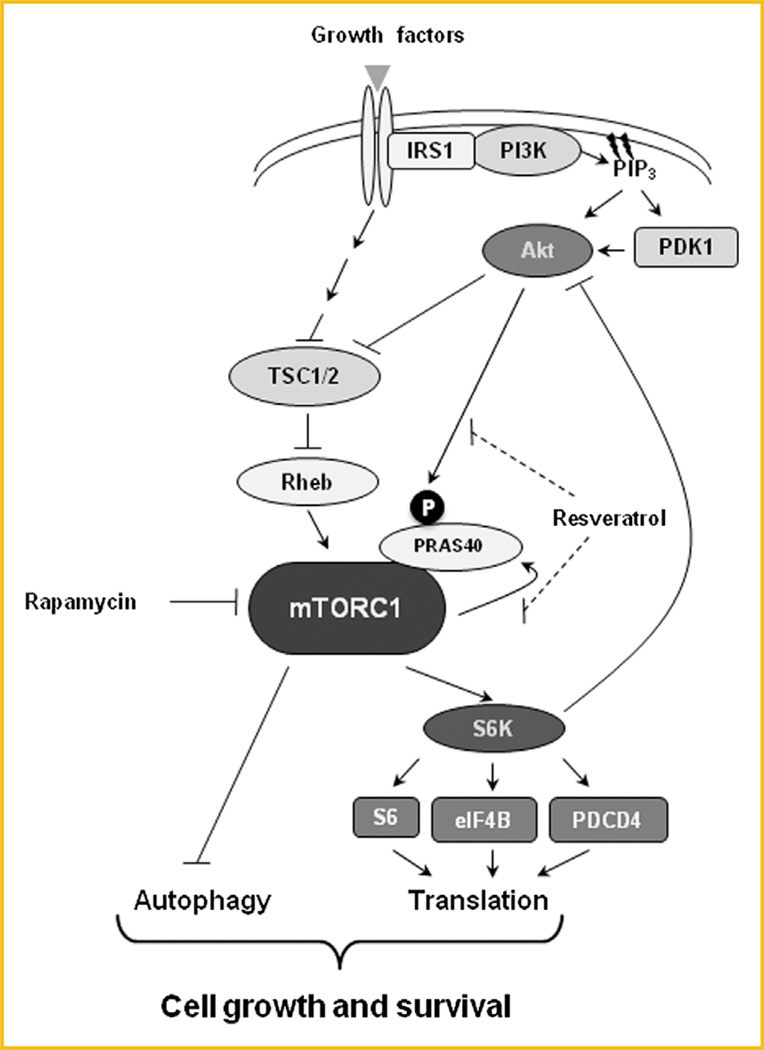
In our study, we investigated the effect of combining resveratrol with rapamycin in breast cancer cells. Hyperactivation of the PI3K/Akt/mTORC1 signaling pathway has emerged as a crucial mechanism of ERα-positive breast cancer resistance to endocrine therapy. Consequently, combination of endocrine therapy with mTORC1 inhibitors has been approved by the FDA for treatment of advanced hormone-positive breast cancer who failed to respond to adjuvant treatment. However, an important problem that arises with the use of mTORC1 inhibitors is that inhibition of the mTORC1/S6K1 signaling relieves the negative feedback loop to PI3K/Akt, which over time reactivates Akt and mTORC1 signaling and may promote cell survival and contribute to acquired resistance (Fig. 6). Our results show that resveratrol is able to prevent rapamycin-induced activation of Akt signaling in both ERα-positve and TNBC cells, although its effect on cells was more pronounced in ERα-positive cells. Additionally co-treatment of cells with rapamycin and resveratrol was able to maintain the inhibition of the mTORC1/S6K1 signaling pathway as previously described [Alayev et al., 2014a,b]. Although the effect on TNBC cells was slight in vitro, it may be more pronounced during longer treatment and/or in vivo. Currently, TNBC patients currently have no FDA-approved targeted treatment options, therefore, this combination may be effective in blocking mTORC1 signaling and may be beneficial in the clinic for this group of patients.
Fig. 6.
Effect of the combination of rapamycin and resveratrol on the PI3K/Akt/mTORC1 signaling pathway. Inhibition of mTORC1 by rapamycin induces autophagy, activates Akt signaling and promotes cell survival. Addition of resveratrol treatment prevents rapamycin-induced Akt activation and autophagy induction, promoting the progression to apoptosis.
Another challenge with the use of mTORC1 inhibitors, such as rapamycin, is that they are cytostatic rather than cytotoxic, which may be due to rapamycin-induced upregulation of autophagy. Autophagy is thought to be responsible for protecting breast cancer cells against apoptosis and may result in dormancy and cancer relapse after cessation of treatment. Importantly, in this study we showed that addition of resveratrol to rapamycin is able to prevent rapamycin-induced upregulation of autophagy and most importantly, induce apoptosis. We observed that in breast cancer cells, treatment with the combination of rapamycin and resveratrol resulted in reduced proliferation and upregulation of cell death. In contrast, in the immortalized non-transformed MCF10a cells, the combination treatment also reduced proliferation but the cells were not undergoing apoptosis. This is an important finding because it indicates that this combination may be selective for cancerous but not normal breast epithelial cells, resulting in lower acute toxicity.
To summarize, our results are very exciting for a number of reasons. First, the combination of rapamycin and resveratrol is able to block upregulation of autophagy and induce apoptosis in breast cancer cells (Fig. 6). Future work will focus on confirming these findings in animal models of breast cancer. Second, rapamycin is an FDA-approved agent with a low toxicity profile, while resveratrol is a widely-available oral natural supplement, therefore this combination is unlikely to cause severe adverse side effects in patients. That said, it is important to conduct human trials testing the safety and efficacy of this combination. Third, this combination treatment has the potential to be effective in the neoadjuvant setting, as well as in treatment of advanced disease. Thus, future work will focus on investigation of the rapamycin and resveratrol combination therapy in both animal models and human clinical studies.
ACKNOWLEDGMENTS
This work was supported by grants to MKH from the NIH (CA151112), Atol Charitable Trust, LAM Foundation (098P0113), American Cancer Society (RSG-13–287-01-TBE), fellowship support to AA from the National Cancer Center, and funding from Yeshiva University.
Grant sponsor: NIH; Grant number: CA151112; Grant sponsor: Atol Charitable Trust, LAM Foundation; Grant number: 098P0113; Grant sponsor: American Cancer Society; Grant number: RSG-13–287-01-TBE; Grant sponsor: National Cancer Center.
Footnotes
Conflicts of interest: none
REFERENCES
- 1.Alayev A, Doubleday PF, Berger SM, Ballif BA, Holz MK. Phosphoproteomics reveals resveratrol-dependent inhibition of Akt/mTORC1/S6K1 signaling. J Proteome Res. 2014 doi: 10.1021/pr500714a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alayev A, Holz MK. mTOR signaling for biological control and cancer. J Cell Physiol. 2013;228(8):1658–1664. doi: 10.1002/jcp.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alayev A, Sun Y, Snyder RB, Berger SM, Yu JJ, Holz MK. Resveratrol prevents rapamycin-induced upregulation of autophagy and selectively induces apoptosis in TSC2-deficient cells. Cell Cycle. 2014;13(3):371–382. doi: 10.4161/cc.27355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armour SM, Baur JA, Hsieh SN, Land-Bracha A, Thomas SM, Sinclair DA. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging (Albany NY) 2009;1(6):515–528. doi: 10.18632/aging.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baehrecke EH. Autophagy: Dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6(6):505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a) anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62(17):4945–4954. [PubMed] [Google Scholar]
- 7.Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishayee A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev Res (Phila) 2009;2(5):409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 9.Carter LG, D'Orazio JA, Pearson KJ. Resveratrol and cancer: Focus on in vivo evidence. Endocr Relat Cancer. 2014;21(3):R209–R225. doi: 10.1530/ERC-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014;40(7):862–871. doi: 10.1016/j.ctrv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Demidenko ZN, Blagosklonny MV. At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence. Cell Cycle. 2009;8(12):1901–1904. doi: 10.4161/cc.8.12.8810. [DOI] [PubMed] [Google Scholar]
- 12.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314(5798):467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 13.Esclatine A, Chaumorcel M, Codogno P. Macroautophagy signaling and regulation. Curr Top Microbiol Immunol. 2009;335:33–70. doi: 10.1007/978-3-642-00302-8_2. [DOI] [PubMed] [Google Scholar]
- 14.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 1997;94(25):14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holz MK. The role of S6K1 in ER-positive breast cancer. Cell Cycle. 2012;11(17):3159–3165. doi: 10.4161/cc.21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9(2):218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 17.Maruani DM, Spiegel TN, Harris EN, Shachter AS, Unger HA, Herrero-Gonzalez S, Holz MK. Estrogenic regulation of S6K1 expression creates a positive regulatory loop in control of breast cancer cell proliferation. Oncogene. 2012;31(49):5073–5080. doi: 10.1038/onc.2011.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330(6009):1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11(9):709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmadeka R, Harmon BE, Singh M. Triple-negative breast carcinoma: current and emerging concepts. Am J Clin Pathol. 2014;141(4):462–477. doi: 10.1309/AJCPQN8GZ8SILKGN. [DOI] [PubMed] [Google Scholar]
- 22.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65(16):7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 23.Whitsett T, Carpenter M, Lamartiniere CA. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. J Carcinog. 2006;5:15. doi: 10.1186/1477-3163-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem. 2009;284(10):6361–6369. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- 25.Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 2010;584(1):124–128. doi: 10.1016/j.febslet.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Parkhitko A, Henske EP. Autophagy: an ‘Achilles’ heel of tumorigenesis in TSC and LAM. Autophagy. 2011;7(11):1400–1401. doi: 10.4161/auto.7.11.17652. [DOI] [PMC free article] [PubMed] [Google Scholar]