Abstract
Intestinal stem cells (ISCs) replenish and regenerate several types of cells in the gut, both during normal homeostasis and in response to various insults such as infections. Although gut structure and complexity vary across phyla, two functional categories of differentiated cell types are always present: absorptive cells and those of the secretory lineage. A series of studies in Drosophila and mouse published in The EMBO Journal, including one in this issue, identifies conserved roles for the Snail family of zinc finger transcription factors in regulating self-renewal and differentiation of ISCs (Korzelius et al, 2014; Loza-Coll et al, 2014; Horvay et al, 2015).
See also: K Horvay et al (May 2015), J Korzelius et al (December 2014) and MA Loza-Coll et al (December 2014)
The mouse gut is made up of enterocytes (ECs) and several types of secretory intestinal cells, enteroendocrine (EE), Paneth and goblet cells, while Drosophila guts only contain ECs and absorptive EE cells. In both species, the decision to commit to absorptive (EC) or secretory (EE) fate depends on the activity of the conserved Notch (N) signalling pathway: low N during differentiation leads to EE fate, while high N specifies EC fate (Ohlstein & Spradling, 2007).
The Drosophila gene escargot (esg) is a member of the Snail family, along with snail and worniu (Nieto, 2002), and has been used as ISC marker. In addition to ISCs, Esg is also found in enteroblasts (EBs), the daughter cells of ISC that remain undifferentiated under non-injured conditions. The mouse genome also encodes three Snail family members: Snai1 (or Snail), Snai2 (or Slug) and Snai3 (or Smuc) (Nieto, 2002). Strikingly, mouse Snai1 is also expressed in intestinal stem cells known as crypt base columnar cells (CBCs) and their undifferentiated progeny, which are transit amplifying cells that have not yet chosen a final fate (Horvay et al, 2011).
Two groups have now established the central role of Esg in regulating Drosophila ISC maintenance (Korzelius et al, 2014; Loza-Coll et al, 2014). Loss of Esg in the stem cells leads to their differentiation, resulting in the depletion of the ISC pool. As a consequence, the regenerative ability of the gut following infection is lost with dire consequences on survival (Korzelius et al, 2014). Similarly, loss of Esg in the EBs also leads to their rapid differentiation (Korzelius et al, 2014; Loza-Coll et al, 2014). Thus, Esg maintains the undifferentiated state both in the stem cells and in their multipotent daughters. Similarly, in the mouse gut, conditional knockout of Snai1 leads to the loss of CBCs (Horvay et al, 2015). Further analysis showed that CBCs die by apoptosis after Snai1 ablation, indicating that although Snai1 and Esg play roles in maintaining ISCs, they may do so in different ways. As in the case of flies, the regenerative ability of mouse guts lacking Esg is severely impaired following insult, in this case radiation. However, loss of Snai1 does not appear to affect the transit amplifying population in the mouse. Moreover, to further emphasise the role of Esg/Snai1 in maintaining ISCs, over-expression of Esg/Snai1 in mouse and fly ISCs leads to excessive stem cell accumulation and a lack of differentiated cells (Korzelius et al, 2014; Horvay et al, 2015). This also results in an inability of the gut to respond to challenges, as the prevention of differentiation means that cells lost to damage are not replenished. Finally, expressing Esg in ECs leads to partial de-differentiation into stem cells (Korzelius et al, 2014).
How does Snai1/Esg act in stem cells to specify their fate? This family of transcription factors is thought to act mainly by repressing expression of its targets (Nieto, 2002). The two fly groups used DamID and expression profiling to identify promoters bound by Esg and genes whose expression depends on Esg. Cross-referencing these lists led to the identification of candidate genes likely to be the direct targets of Esg, and they fell into one of two classes: genes downregulated or upregulated by Esg (Korzelius et al, 2014). The former reflects Esg’s expected repressor function. Many of the genes required for differentiation into ECs and EEs are repressed by Esg, including pdm (also called nubbin), which encodes a POU and homeodomain transcription factor that the authors show can trigger EC fate, as well as the EC marker Myo31-DF (also called MyoIa), the cell proliferation inhibitor tribbles and many other differentiation markers, such as immune genes and gut enzymes. This wide set of Esg-bound genes indicates that Esg acts in a very pleiotropic manner to inhibit several aspects of differentiation at once. Similar results in mouse muscle, where Snai1 prevents the binding of MyoD on the enhancers of differentiation genes, establish Esg repression of a wide range of differentiation genes as a paradigm for its function (Soleimani et al, 2012). The second class of targets were unexpectedly upregulated by Esg. They contain Esg-binding regions and encode components of several signalling pathways required for ISC self-renewal. This suggests that, in addition to acting as a repressor of differentiation genes, Esg may play a role as a transcriptional activator in specifying ISC maintenance. Although surprising, there is evidence that other members of the Snail family can act as activators of transcription, depending on the enhancer context (Rembold et al, 2014). Horvay et al performed genome-wide expression analysis to identify Snai1-regulated genes in CBCs. They found that Snai1 directly regulates expression of the apoptosis inhibitor SerinC3 transcriptionally, and speculate that this may explain the death of CBCs lacking Snai1.
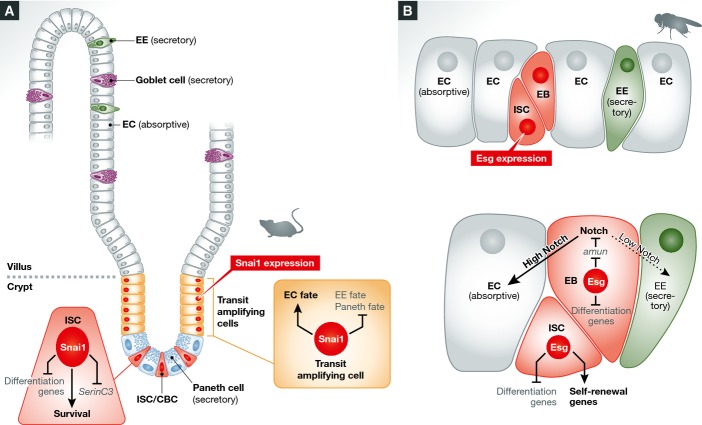
Figure 1.
Model of Snai1 action in the mouse gut and of Esg in fly ISCs
(A) Snai1 is expressed in CBCs and transit amplifying cells. It regulates CBC survival and differentiation of transit amplifying cells to the secretory or absorptive lineage. (B) Esg is found in ISCs and EBs in the fly gut, where it acts to repress differentiation genes. Moreover, it may activate self-renewal genes in ISCs. Finally, amun is a target of Esg in EBs, where it affects N signalling to determine EC versus EE fate.
In addition to its roles in maintaining ISCs, Loza-Coll and Horvay also identified a requirement for Esg/Snai1 in regulating the fate of differentiating cells in the intestine (Loza-Coll et al, 2014; Horvay et al, 2015). esg-deficient cells in the gut gave rise to many more EEs than normal, and Snai1 mutants had many more EEs and Paneth cells. In both cases, this increase was at the expense of the EC lineage. Conversely, over-expression of Snai1 led to a decrease in EE and Paneth cells, suggesting that Snai1 and Esg bias cells towards absorptive fates at the expense of secretory fates. Loza-Coll et al (2014) explored the relationship between Esg and N, a known inhibitor of secretory fate specification. They found that N activity was reduced in the absence of Esg and that this was sufficient to explain the bias in cell fate. Using DamID, they identified amun, a known modulator of N signalling, as transcriptional target band being repressed by Esg. They observed that amun derepression led to the inhibition of N, which was responsible for the EE accumulation observed in Esg knockdowns. Thus, by regulating multiple targets, Esg can play many roles both in stem cell maintenance and in biasing daughter cell fate.
Altogether, these studies provide insight into the cell-intrinsic actions of a transcription factor that controls stem cell self-renewal. However, stem cells reside in niches and depend on niche-derived signals for their continued maintenance, and the relationship between these extrinsic maintenance cues and intrinsic ones like Snail genes is an enduring question. Horvay et al (2011) have previously shown that Wnt signalling is required for both Snai1 expression and its nuclear localisation in CBCs, suggesting a direct link between extrinsic niche signalling and Snai1 activity within the stem cells. The mechanisms controlling esg transcription in the fly gut are not known; however, it is tempting to postulate a similar regulation by niche signals. Moreover, Korzelius et al (2014) suggest that differentiation factors such as Pdm1 are responsible for repressing esg in differentiated cells, pointing to the existence of stable genetic states in which either stem cell or differentiation genes are expressed.
Finally, these studies add to the growing body of work that implicates epithelial–mesenchymal transition (EMT)-regulating transcription factors in stem cell biology. EMT is characterised by loss of epithelial organisation and adhesion, and adoption of mesenchymal features; it is thought to be essential for metastasis to occur. EMT factors, including the Snail family and two others, Twist and Zeb, are expressed in many different stem cells. For instance, Snail is present in mammalian epidermal stem cells, Snai2 in mammary and haematopoietic stem cells (Perez-Losada et al, 2002; Guo et al, 2012; De Craene et al, 2014), while both Snai1 and Snai2 are required to prevent differentiation in muscle progenitors (Soleimani et al, 2012). In flies, cyst stem cells (CySCs) in the testis require both Esg and the Zeb homologue, Zfh1, for self-renewal (Leatherman & Dinardo, 2008; Voog et al, 2014) while neuroblasts require Worniu to prevent differentiation (Lai et al, 2012). Sequencing data from Korzelius et al indicate that snail, worniu, Zfh1 and its paralogue Zfh2 are all expressed in ISCs. However, the relationship between the roles of these genes in self-renewal and EMT is not clear. Indeed, fly and mouse ISCs express E-cadherin (Snippert et al, 2010; Korzelius et al, 2014), which is normally repressed during EMT, as do testis CySCs, which require E-cadherin for self-renewal (Voog et al, 2008). Nonetheless, Korzelius et al do find that genes required for septate junctions are repressed by Esg. Recent work suggests that the levels of the EMT transcription factor Twist required to regulate proliferation and ‘stemness’ are lower than those required to induce EMT (Beck et al, 2015). This could apply also to other EMT transcription factors and could be a safeguard mechanism to ensure that stem cells that acquire too much of a self-renewal transcription factor are detached from their niche due to decreased adhesion, thereby reducing their exposure to self-renewal factors.
References
- Beck B, Lapouge G, Rorive S, Drogat B, Desaedelaere K, Delafaille S, Dubois C, Salmon I, Willekens K, Marine JC, Blanpain C. Different levels of Twist1 regulate skin tumor initiation, stemness, and progression. Cell Stem Cell. 2015;16:67–79. doi: 10.1016/j.stem.2014.12.002. [DOI] [PubMed] [Google Scholar]
- De Craene B, Denecker G, Vermassen P, Taminau J, Mauch C, Derore A, Jonkers J, Fuchs E, Berx G. Epidermal Snail expression drives skin cancer initiation and progression through enhanced cytoprotection, epidermal stem/progenitor cell expansion and enhanced metastatic potential. Cell Death Differ. 2014;21:310–320. doi: 10.1038/cdd.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zurrer-Hardi U, Bell G, Tam WL, Mani SA, van Oudenaarden A, Weinberg RA. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvay K, Casagranda F, Gany A, Hime GR, Abud HE. Wnt signaling regulates Snai1 expression and cellular localization in the mouse intestinal epithelial stem cell niche. Stem Cells Dev. 2011;20:737–745. doi: 10.1089/scd.2010.0188. [DOI] [PubMed] [Google Scholar]
- Horvay K, Jarde T, Casagranda F, Perreau VM, Haigh K, Nefzger CM, Akhtar R, Gridley T, Berx G, Haigh JJ, Barker N, Polo JM, Hime GR, Abud HE. Snai1 regulates cell lineage allocation and stem cell maintenance in the mouse intestinal epithelium. EMBO J. 2015;34:1319–1335. doi: 10.15252/embj.201490881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzelius J, Naumann SK, Loza-Coll MA, Chan JS, Dutta D, Oberheim J, Glasser C, Southall TD, Brand AH, Jones DL, Edgar BA. Escargot maintains stemness and suppresses differentiation in Drosophila intestinal stem cells. EMBO J. 2014;33:2967–2982. doi: 10.15252/embj.201489072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Miller MR, Robinson KJ, Doe CQ. The Snail family member Worniu is continuously required in neuroblasts to prevent Elav-induced premature differentiation. Dev Cell. 2012;23:849–857. doi: 10.1016/j.devcel.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loza-Coll MA, Southall TD, Sandall SL, Brand AH, Jones DL. Regulation of Drosophila intestinal stem cell maintenance and differentiation by the transcription factor Escargot. EMBO J. 2014;33:2983–2996. doi: 10.15252/embj.201489050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Perez-Losada J, Sanchez-Martin M, Rodriguez-Garcia A, Sanchez ML, Orfao A, Flores T, Sanchez-Garcia I. Zinc-finger transcription factor Slug contributes to the function of the stem cell factor c-kit signaling pathway. Blood. 2002;100:1274–1286. [PubMed] [Google Scholar]
- Rembold M, Ciglar L, Yanez-Cuna JO, Zinzen RP, Girardot C, Jain A, Welte MA, Stark A, Leptin M, Furlong EE. A conserved role for Snail as a potentiator of active transcription. Genes Dev. 2014;28:167–181. doi: 10.1101/gad.230953.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Soleimani VD, Yin H, Jahani-Asl A, Ming H, Kockx CE, van Ijcken WF, Grosveld F, Rudnicki MA. Snail regulates MyoD binding-site occupancy to direct enhancer switching and differentiation-specific transcription in myogenesis. Mol Cell. 2012;47:457–468. doi: 10.1016/j.molcel.2012.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voog J, D’Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–1136. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voog J, Sandall SL, Hime GR, Resende LP, Loza-Coll M, Aslanian A, Yates JRIII, Hunter T, Fuller MT, Jones DL. Escargot restricts niche cell to stem cell conversion in the Drosophila testis. Cell Rep. 2014;7:722–734. doi: 10.1016/j.celrep.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]