Abstract
During angiogenesis, endothelial cell migration is coordinated by integrin-mediated contact with the extra-cellular matrix (ECM), coupled with receptor tyrosine kinase signalling to regulate dynamic cytoskeletal and plasma membrane reorganization. A recent paper by Vitorino et al (2015) defined a new MAP4K4–moesin–talin–β1-integrin pathway that could be therapeutically exploited to suppress pathologic angiogenesis.
See also: P Vitorino et al (March 2015)
Angiogenesis, the process of new vessel formation from pre-existing vasculature, is a vital physiological event during development as well as an important mechanism contributing to tumour growth and metastasis. Efficient angiogenesis requires appropriate interaction between migrating vascular endothelial cells and the surrounding ECM (Doyle et al, 2013). The migrating endothelial cells termed the tip cells develop branched actin-rich protrusions that extend out from the cell membrane, to constantly probe the ECM and enable a rapid cellular response to changes in the microenvironment (Phng et al, 2013). The integrin family of cell surface adhesion receptors are key integrators of ECM-initiated signals, providing a physical link between the ECM and the cellular cytoskeleton as well as being established regulators of angiogenic growth factor signalling (Ivaska & Heino, 2011). The fibronectin-binding integrins α4β1 and α5β1 and the αv receptors are the most extensively studied, and investigations into these receptors have resulted in the development of integrin antagonists as potential anti-angiogenic cancer therapies (Reardon & Cheresh, 2011). However, so far none of these inhibitors have entered the clinic, and optimal strategies to target integrins for inhibition of pathological angiogenesis need to be further explored.
Integrins on the cell surface switch between inactive (low affinity), primed (active but unengaged) and fully active (ligand-bound) states that determine the cellular response to the chemical, mechanical and topological features of the ECM (Byron et al, 2009). In adherent cells, integrin activity is under tight spatio-temporal control, in turn modulating the local attachment in different subcellular regions, for example in the leading edge of lamellipodia during cell migration (Bouvard et al, 2013). Integrin activation is an allosteric process whereby the critical step involves binding of the integrin activator talin to the integrin β-subunit cytoplasmic domain. The talin head domain interacts with the integrin tail and the plasma membrane, while the talin rod domain couples the active adhesion receptor to the actin cytoskeleton and promotes recruitment of other adhesome components (Shattil et al, 2010). The transition of integrins from an active to an inactive receptor is much less well understood. Nevertheless, several integrin inactivating molecules, SHARPIN, ICAP-1, DOK-1 and filamin, have been described, and they all function directly or indirectly by displacing talin from the integrin cytoplasmic domain (Bouvard et al, 2013). In contrast, the molecular mechanisms that determine the balance between active and inactive integrins in specific subcellular locations remain poorly defined.
In an attempt to characterize new pathways regulating endothelial cell migration, the authors screened chemical inhibitors against known targets in a 3-dimensional HUVEC sprouting assay. GNE-220, a kinase inhibitor, was found to induce longer subcellular protrusions in HUVECs. However, although the number and length of protrusions were increased in GNE-220-treated cells, the endothelial cell sprouts exhibited an abnormally thick and shorter morphology compared to untreated cells. Functional analysis showed that among the few known kinases sensitive to GNE-220, only MAP4K4 loss of function phenocopied GNE-220 treatment. More detailed analysis revealed that MAP4K4 inhibition or silencing results in the formation of long, thin subcellular protrusions and a loss in membrane retractions. A similar phenotype was also observed when Map4k4 was conditionally deleted from the mouse endothelium during embryonic development in Tie2-Cre mice, or following ubiquitous postnatal deletion in the Rosa26.CreERT2 mouse model. Mice with heterozygous deletion of Map4k4 in the endothelium were viable and exhibited no obvious defects, whereas homozygous endothelial deletion of Map4k4 during embryonic development resulted in haemorrhage and oedema at E14.5 and embryonic lethality at E16.5. A closer analysis of the developing vessels at an earlier time point revealed delayed development of the head vasculature, which extends from the periphery towards the apex of the skull in a stereotypic pattern, between E13 and E15. The ubiquitous deletion of Map4k4, initiated at postnatal day P1, resulted in delayed outward of retinal vessels at P7 in Map4k4fl/fl:Rosa26.CreERT2 pups. Both embryonic and postnatal vascular growth in a Map4k4-deficient background were characterized by the appearance of long subcellular protrusions at the growing vascular front, whereas endothelial cell number or proliferation remained unaffected, suggesting a specific role for MAP4K4 in endothelial cell migration.
The accumulation of thin membrane protrusions in vitro and in vivo was associated with increased integrin-mediated adhesion and impaired focal adhesion disassembly (Fig1). The authors observed that in a normal sprouting endothelium, there is an absence of active β1-integrin receptor within the tips of retracting membrane protrusions. Instead, active β1-integrin appears to be confined to focal adhesion structures at the base of the retraction fibre localized close to the cell body. In contrast, in MAP4K4-inhibited cells, active β1-integrin accumulated along the long narrow protrusions, indicating aberrant activation of the receptor in these poorly retracting structures. In line with this notion, inhibition of (fibronectin-binding) α5β1-integrin function restored the membrane retraction in MAP4K4-deficient endothelial cells. Furthermore, α5β1-integrin blocking antibodies reduced the number of long protrusions in the retinal vascular front at P7 in Map4k4-deleted pups.
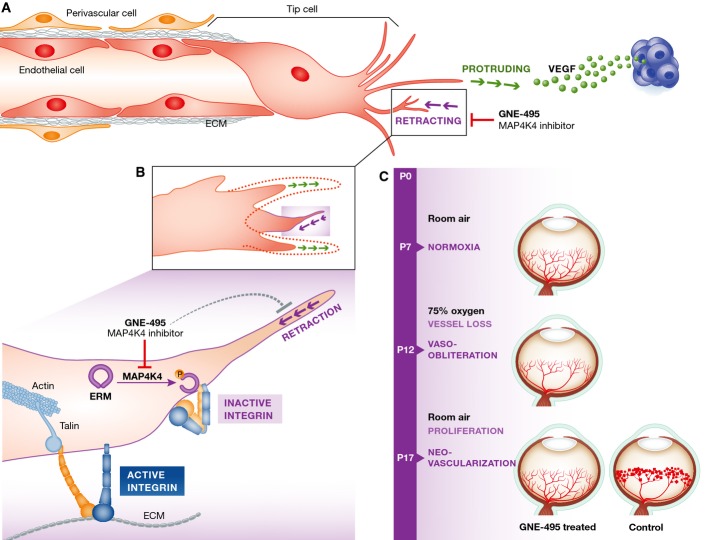
Figure 1.
MAP4K4 inhibitors block sprouting angiogenesis by interfering with integrin inhibition
(A) The angiogenic sprouting of endothelial cells (EC) is controlled by the balance between pro-angiogenic signals, such as vascular endothelial growth factor (VEGF) produced, for example by hypoxic tumour cells (in blue), and integrin-mediated contacts with the extracellular matrix (ECM) molecules. During sprouting, endothelial cells compete for the ‘tip cell’ position in a process regulated by the VEGF/VEGFR and the Notch signalling systems. A migrating ‘tip cell’ followed by proliferating stalk cells guides the direction of sprout formation and invades the surrounding tissue by extending numerous protrusions. Dynamic cycles of tip cell protrusion and retraction are important for angiogenesis. The retraction phase is sensitive to MAP4K4 inhibitor GNE-495 in vitro and in vivo. (B) Integrins are locally inactivated at the tip of the retracting subcellular protrusions. MAP4K4 phosphorylates and activates ERM protein moesin on T558. Active moesin competes with integrin activator talin for the binding to the β1-integrin cytoplasmic tail (yellow) and switches the integrin to a bent inactive conformation. This supports retraction of the protrusion whereas closer to the cell body integrins remain active and talin bound in ECM binding focal adhesion. GNE-495 inhibits MAP4K4 and thus perturbs the retraction that is necessary for normal sprouting. (C) GNE-495 is efficient in vivo to antagonize retinal neovascularization in the oxygen-induced retinopathy model (OIR).
MAP4K4 has previously been demonstrated to phosphorylate moesin on threonine residue 558, leading to the release of moesin autoinhibition (Baumgartner et al, 2006) and protein activation. Vitorino and colleagues observed that silencing of this ERM protein was sufficient to recapitulate the effects of MAP4K4 inhibition, suggesting that moesin is necessary for MAP4K4-mediated integrin inhibition. Importantly, expression of a constitutively active phospho-mimetic mutant of moesin was sufficient to rescue the long protrusions generated upon MAP4K4 inhibition, placing moesin downstream of MAP4K4 in the pathway. Similarly to talin, moesin contains an integrin tail binding FERM domain and activation of moesin exposes this FERM domain to facilitate protein–protein interactions. Interestingly, in vitro assays demonstrated that moesin can displace the talin head FERM domain from integrin β-tails. Due to the structural differences between the moesin and talin FERM domains (Elliott et al, 2010), moesin–integrin interaction does not activate the receptor. Instead, MAP4K4-dependent moesin activity appears to inhibit integrin function by disrupting talin binding to the integrin β-tail.
Following the identification of a MAP4K4-dependent mechanism for integrin inactivation, Vitorino and colleagues developed a small-molecule antagonist directed against this kinase as a potentially novel anti-angiogenic therapy. The MAP4K4 antagonist alleviated pathological angiogenesis in a model of oxygen-induced retinopathy, which mimics key features of retinopathy of prematurity and proliferative diabetic retinopathy, by reducing the number of vascular tufts and haemorrhages. Furthermore, the ubiquitous deletion of Map4k4 in adult mice inhibited the growth of murine tumours and resulted in vascular alterations including reduced tumour perfusion. However, the potential side effects of MAP4K4 loss of function, using the MAP4K4 antagonist or upon Map4k4 deletion, on the resting mature vasculature or other MAP4K4-expressing cells were not investigated in this study.
The findings of Vitorino and colleagues are conceptually novel and exciting for several reasons. Firstly, the authors describe a detailed MAP4K4-dependent molecular mechanism that drives local integrin inactivation in adherent cells in vitro and in vivo. Furthermore, the fine balance between integrin activation states is important for the regulation of vascular endothelial cell sprouting during angiogenesis. This work therefore introduces a novel concept that opposes the use of integrin inhibitors for blocking angiogenesis. Specifically, it proposes that preventing localized integrin inactivation may offer a viable anti-angiogenic therapy.
The mechanism of localized β1-integrin inactivation identified in this study could also be important for therapy. While blocking all β1-integrins is problematic due to the likely adverse side effects, inhibition of MAP4K4 could specifically block adhesion site dynamics in sprouting angiogenesis. Furthermore, integrins can regulate the signals and trafficking of vascular endothelial growth factor receptor 2 (VEGFR2), and integrin inhibition may result in increased VEGFR2 activity (Mattila et al, 2008) or cell surface availability (Reynolds et al, 2009). Thus, pro- versus anti-angiogenic integrin effects are strictly context dependent. Novel mechanistic insights and specific drugs will enable to explore the full potential of modulating localized receptor activity for the treatment of vascular disease.
References
- Baumgartner M, Sillman AL, Blackwood EM, Srivastava J, Madson N, Schilling JW, Wright JH, Barber DL. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc Natl Acad Sci USA. 2006;103:13391–13396. doi: 10.1073/pnas.0605950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard D, Pouwels J, De Franceschi N, Ivaska J. Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat Rev Mol Cell Biol. 2013;14:430–442. doi: 10.1038/nrm3599. [DOI] [PubMed] [Google Scholar]
- Byron A, Humphries JD, Askari JA, Craig SE, Mould AP, Humphries MJ. Anti-integrin monoclonal antibodies. J Cell Sci. 2009;122:4009–4011. doi: 10.1242/jcs.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AD, Petrie RJ, Kutys ML, Yamada KM. Dimensions in cell migration. Curr Opin Cell Biol. 2013;25:642–649. doi: 10.1016/j.ceb.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott PR, Goult BT, Kopp PM, Bate N, Grossmann JG, Roberts GC, Critchley DR, Barsukov IL. The Structure of the talin head reveals a novel extended conformation of the FERM domain. Structure. 2010;18:1289–1299. doi: 10.1016/j.str.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J, Heino J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu Rev Cell Dev Biol. 2011;27:291–320. doi: 10.1146/annurev-cellbio-092910-154017. [DOI] [PubMed] [Google Scholar]
- Mattila E, Auvinen K, Salmi M, Ivaska J. The protein tyrosine phosphatase TCPTP controls VEGFR2 signalling. J Cell Sci. 2008;121:3570–3580. doi: 10.1242/jcs.031898. [DOI] [PubMed] [Google Scholar]
- Phng LK, Stanchi F, Gerhardt H. Filopodia are dispensable for endothelial tip cell guidance. Development. 2013;140:4031–4040. doi: 10.1242/dev.097352. [DOI] [PubMed] [Google Scholar]
- Reardon DA, Cheresh D. Cilengitide: a prototypic integrin inhibitor for the treatment of glioblastoma and other malignancies. Genes Cancer. 2011;2:1159–1165. doi: 10.1177/1947601912450586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC, Tucker GC, Hodivala-Dilke KM. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorino P, Yeung S, Crow A, Bakke J, Smyczek T, West K, McNamara E, Eastham-Anderson J, Gould S, Harris SF, Ndubaku C, Ye W. MAP4K4 regulates integrin-FERM binding to control endothelial cell motility. Nature. 2015;519:425–430. doi: 10.1038/nature14323. [DOI] [PubMed] [Google Scholar]