Abstract
Chemokines are vertebrate-specific, structurally related proteins that function primarily in controlling cell movements by activating specific 7-transmembrane receptors. Chemokines play critical roles in a large number of biological processes and are also involved in a range of pathological conditions. For these reasons, chemokines are at the focus of studies in developmental biology and of clinically oriented research aimed at controlling cancer, inflammation, and immunological diseases. The small size of the zebrafish embryos, their rapid external development, and optical properties as well as the large number of eggs and the fast expansion in genetic tools available make this model an extremely useful one for studying the function of chemokines and chemokine receptors in an in vivo setting. Here, we review the findings relevant to the role that chemokines play in the context of directed single-cell migration, primarily in neutrophils and germ cells, and compare it to the collective cell migration of the zebrafish lateral line. We present the current knowledge concerning the formation of the chemokine gradient, its interpretation within the cell, and the molecular mechanisms underlying the cellular response to chemokine signals during directed migration.
Keywords: cell migration, cytokines
Introduction
Chemokines are small (typically 8–10 kD), vertebrate-specific, secreted protein ligands that bind to their cognate chemokine receptors to elicit cellular responses. The chemokine family is characterized by sequence conservation and is subdivided into four groups (CC, CXC, C, and CX3C), based on the relative positions of cysteine residues within the primary structure of the protein (Zlotnik & Yoshie, 2000). The chemokine receptors are 7-transmembrane G-protein coupled receptors (GPCRs) that are subdivided into four groups, depending on the type of chemokine they bind (and are therefore correspondingly named CCR, CXCR, XCR, and CX3CR) (Murphy et al, 2000).
Originally, chemokines (chemotactic cytokines) have been studied mainly in relation to their role in directing leukocyte trafficking in the immune system (reviewed in Baggiolini, 1998; Sallusto & Baggiolini, 2008). This has led to a classification based on the functional context of chemokine expression. Several chemokines expressed in response to infection or injury are designated as inflammatory chemokines (Baggiolini, 1998). These are mainly responsible for recruiting specific leukocyte populations to sites of inflammation. Another subset, defined as homeostatic chemokines, regulates the general mobility of leukocytes, as well as trafficking of leukocytes within and between lymphoid organs (Baggiolini, 1998). Chemokines that fulfill both roles are defined as dual chemokines (Zlotnik & Yoshie, 2012).
Correspondingly, based on the chemokines they bind, chemokine receptors are defined as inflammatory, homeostatic or dual receptors (Zlotnik & Yoshie, 2012). Members of the “regulatory” group do not control cellular signaling directly, but rather play a role in the recycling of other receptors, or in chemokine scavenging (Zlotnik & Yoshie, 2012).
Interestingly, several members of the homeostatic and regulatory chemokine groups have been found to play important roles beyond the immune system, in a diverse set of processes that include angiogenesis (Koch et al, 1992), hematopoiesis (Nagasawa et al, 1996), neural development (Zou et al, 1998), germ cell migration (Doitsidou et al, 2002), and tumor metastasis (Muller et al, 2001). In some of these processes, chemokines were shown to direct the migration of groups of cells, while in others, they were shown to guide individually migrating cells. The latter type of migration constitutes the main focus of this review.
Whereas chemokines were also shown to be involved in processes other than migration, their major function (as their name implies) concerns the control of chemotaxis: directed cell migration that is guided by a gradient of extracellular, soluble molecules (from ancient Greek chemeia: “chemistry” and taxis: “marching forward”). A related process termed haptotaxis (from haptein: “to grasp”) is usually defined as directional cell migration that is guided by a gradient of insoluble molecules, such as components of the extracellular matrix (ECM), but also ECM-bound ligands that do not freely diffuse. From the perspective of the cellular response, chemotaxis and haptotaxis in response to ECM-bound ligands are rather similar, as both involve biochemical signaling events in which an extracellular ligand activates a membrane-bound receptor. The distinction between chemotaxis and haptotaxis as broadly defined might thus be more relevant to in vitro cell migration, where a gradient of soluble ligand molecules in the absence of ECM is more easily generated. In the context of in vivo cell migration, where most if not all extracellular ligands interact to some degree with the ECM, chemotaxis in its strict sense would therefore be an exception. Alternatively, haptotaxis may be defined more stringently, setting a definition based on a threshold in adhesion energy between the cell and its surroundings (cell–ECM or cell–cell interactions). According to the latter definition of haptotaxis, chemotaxis would include all cases of cell migration in response to ECM-bound ligands.
Independent of their definitions, chemotaxis and haptotaxis can be either positive, when a cell moves toward a higher concentration of a molecule (designated a chemoattractant) or negative, when a cell migrates away from a higher concentration of a molecule (referred to then as a chemorepellent).
A significant proportion of the research concerning the molecular and cellular mechanisms of eukaryotic chemotaxis has been performed in vitro, by studying 2-dimensional (2D) migration of the slime mold Dictyostelium discoideum, and of isolated mammalian neutrophils (reviewed in Cai & Devreotes, 2011). Although the chemotactic signals are different (Konijn et al, 1967; Schroder et al, 1987; Walz et al, 1987; Yoshimura et al, 1987; Van Damme et al, 1988), the mechanisms of the chemoattractant action are similar between these two models. In the first stage, the cells sense the gradient as the chemoattractant activates a membrane-bound G-protein coupled receptor (GPCR). Differential receptor activation along the length of a cell or over time leads to the induction of cellular polarization that is then translated into directed migration.
Direct microscopic observation of migrating cells and imaging of second messenger molecules have been critical for the understanding of 2D chemotaxis in vitro. However, it has become clear that migration in a 3-dimensional (3D) environment—as occurs in vivo—can be regulated differently. For example, 3D migration of dendritic cells does not require integrins, which are essential for 2D migration in vitro (Lammermann et al, 2008). Investigating chemotaxis and the role of chemokines in this process, in the context of the intact organism or tissue, requires the use of novel model systems. The zebrafish embryo offers important advantages for studying the in vivo role and regulation of chemotaxis during development and in relation to immune system function. The optical clarity and small size of the zebrafish embryo allow direct visualization of migration processes at high temporal and spatial resolution while employing a large and expanding molecular genetics toolbox. In this review, we will discuss the recent progress made using the zebrafish embryo in studying the role of chemokines and their receptors in guiding the migration, primarily of single cells.
Zebrafish chemokines and their receptors
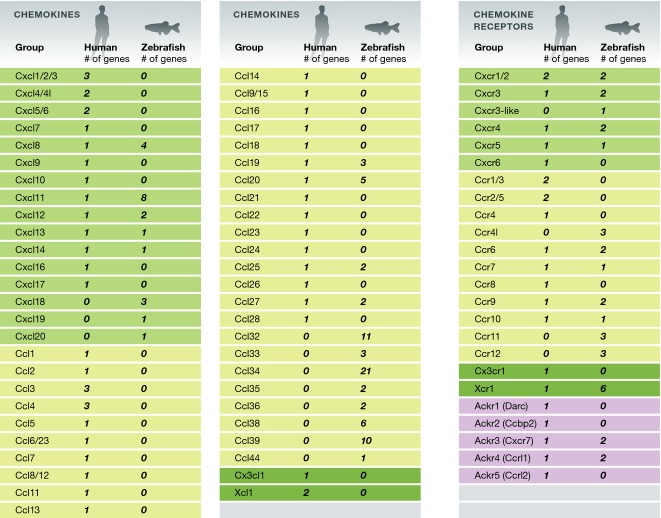
The recent sequence analysis of the zebrafish genome provided a comprehensive list of chemokine and chemokine receptor family members in this species (DeVries et al, 2006; Nomiyama et al, 2008; Bajoghli et al, 2009; Chen et al, 2013). In total, 33 zebrafish chemokine receptor and 89 chemokine genes have been identified, a number which is significantly higher than that in humans (Nomiyama et al, 2013) (Fig1). This number reflects both the additional whole-genome duplication event within the ray-finned fish, as well as several small-scale tandem gene duplications. Bona fide zebrafish orthologues have been identified for 12 out of 23 human chemokine receptor genes (Sprague et al, 2006). For the chemokine ligands, identification of orthologues proved to be more difficult, due to gene duplications within individual species both in fish and in tetrapod lineages (Bajoghli, 2013). It is important to note that although sequence similarity and conservation of gene order along the chromosome (synteny) could be employed to suggest functional chemokine–chemokine receptor interaction, genetic or biochemical characterization of these interactions should be performed to confirm the evolutionary conservation of chemokine–receptor interaction. As discussed below, such functional characterizations have been thus far performed only for Cxcl12a, Cxcl12b, Ccl19, and Cxcl8 (Il8) (Boldajipour et al, 2011; Deng et al, 2011; Wu et al, 2012; de Oliveira et al, 2013). Significantly however, in all of these examples, the corresponding receptor–ligand interactions identified in mammals have been conserved in zebrafish.
Figure 1.
Human and zebrafish chemokine and chemokine receptor genes
Chemokine genes are listed according to their subgroup (CXC (green), CC (yellow), and CX3C and XC (dark green)). Chemokine receptor genes are listed according to their specificity to chemokine subgroups, with atypical receptors in purple. Modified from Nomiyama et al (2013).
The best-characterized chemokines that function during zebrafish embryonic development are the homologs of the human homeostatic chemokine CXCL12 (also known as Stromal cell-derived factor-1, or SDF-1). The cxcl12 gene has been duplicated in the course of the whole-genome duplication during early ray-finned fish evolution (Amores et al, 1998; Postlethwait et al, 1998), giving rise to the two paralogs cxcl12a and cxcl12b. Similarly, zebrafish possess two paralogous genes of the human CXCL12-receptor CXCR4, designated cxcr4a and cxcr4b. Originally identified in forward and reverse genetics screens as essential for primordial germ cell and lateral line migration (David et al, 2002; Doitsidou et al, 2002; Knaut et al, 2003), zebrafish Cxcl12a–Cxcr4b and Cxcl12b–Cxcr4a interactions (Boldajipour et al, 2011) have since been shown to regulate the migration of a large variety of cell types. These include cell types from all germ layers, such as specific neuronal cells and axons (Gilmour et al, 2004; Knaut et al, 2005; Li et al, 2005; Sapede et al, 2005; Chalasani et al, 2007; Miyasaka et al, 2007; Palevitch et al, 2010), neural crest (Olesnicky Killian et al, 2009), endodermal progenitors (Mizoguchi et al, 2008; Nair & Schilling, 2008), neutrophils (Walters et al, 2010), endothelial cells of blood vessels, and lymphatic vessels (Siekmann et al, 2009; Bussmann et al, 2011; Fujita et al, 2011; Cha et al, 2012; Xu et al, 2014) and muscle precursors (Chong et al, 2007; Hollway et al, 2007). Some promiscuity between the paralogous gene pairs appears to exist, as interaction between Cxcl12a and Cxcr4a during angiogenesis in fin regeneration has been reported (Xu et al, 2014), as well as a possible interaction between Cxcl12b and Cxcr4b in the context of PGC migration (Boldajipour et al, 2011). In addition to CXCR4, CXCL12 binds to CXCR7, which does not activate downstream signaling (Balabanian et al, 2005; Burns et al, 2006; Naumann et al, 2010; Mahabaleshwar et al, 2012). Rather, CXCR7 acts as a decoy receptor for CXCL12 that binds the chemokine to effectively reduce the level of the molecule at certain times or in specific tissues. This function was demonstrated in vivo for Cxcr7b, one of the two CXCR7 paralogs in zebrafish (Boldajipour et al, 2008).
More recent studies demonstrated the function of the inflammatory chemokine Cxcl8 (van der Aa et al, 2010; Oehlers et al, 2010; Sarris et al, 2012; de Oliveira et al, 2013) and its receptors Cxcr1 and Cxcr2 (Oehlers et al, 2010; Deng et al, 2013) in zebrafish. Four homologs of CXCL8 named cxcl8a and cxcl8b.1, 2, and 3 are present in zebrafish. The expression of these chemokines was shown to be induced during infection, when they function in Cxcr2-dependent neutrophil recruitment.
Another recent example for chemokine function in zebrafish is that of the CC-receptor Ccr7 (Wu et al, 2012), a protein essential for proper gastrulation during early embryogenesis. In humans, CCR7 has 3 ligands, CCL19, CCL21, and CCL25, while in zebrafish, 4 homologous proteins were identified and named Ccl19.1, Ccl19.2, Ccl19.3, and Ccl21/25 (Nomiyama et al, 2008). Knockdown of Ccl19.1 results in defects very similar to those observed upon Ccr7 knockdown, consistent with the idea that Ccl19.1 is the major ligand of the four that acts through Ccr7 binding during early development (Wu et al, 2012).
Zebrafish models of chemokine-guided single-cell migration
Primordial germ cell migration
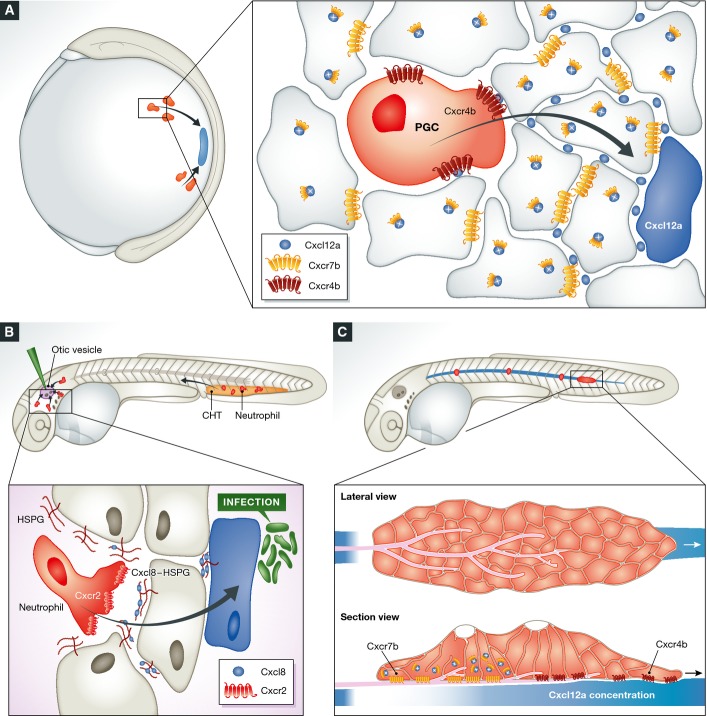
Primordial germ cells (PGCs), the progenitor of germline cells, serve as an important model for studying chemotaxis in the context of a developing embryo. Similar to PGCs in other species, zebrafish PGCs are specified away from the gonad, the position where they ultimately differentiate into sperm and eggs and therefore have to migrate to reach their final destination (reviewed in Kunwar et al, 2006; Raz & Reichman-Fried, 2006). Interestingly, zebrafish PGCs are specified at four different sites that are positioned randomly within the embryo and thus rely on a mechanism that would direct their migration toward the target from different locations (Yoon et al, 1997) (Fig2A). The principles of coordinating PGC migration are based on the dynamic expression of Cxcl12a and on the expression of its receptor Cxcr4b on the surface of PGCs (Doitsidou et al, 2002). The specificity of the guidance signal is most clearly demonstrated by the fact that the PGCs practically ignore a closely related ligand, Cxcl12b, which is produced at the time and occasionally along the route of their migration for controlling other developmental processes in the embryo (Boldajipour et al, 2011).
Figure 2.
Zebrafish models for chemokine-directed cell migration
(A) PGC (red) migration (black arrow) toward a source of Cxcl12a (blue) during somitogenesis stages. This migration process requires Cxcr4b expression in the PGCs and removal of Cxcl12b from the extracellular space by the decoy receptor Cxcr7b (magnified box). Cxcr7b expressed by somatic cells targets the chemokine to lysosomes for degradation, thereby allowing proper level and graded Cxcl12b distribution. (B) Neutrophils (red) migrate (black arrow) toward injected bacteria in the otic vesicle. The introduction of the pathogens induces Cxcl8 expression (blue), a chemokine that associates with heparan sulfate proteoglycans (HSPG) in the extracellular matrix (magnified view). Location of the caudal hematopoietic tissue (CHT, see text) where the neutrophils originate is indicated in orange. (C) Migration of the lateral line primordium (red) along a stripe of Cxcl12a (blue) requires Cxcr4b activation at the front of the cell cluster and Cxcr7b-mediated endocytosis of Cxcl12a in the back of the primordium. The magnified view is presented in lateral view (up) and in a section (bottom) to present the Cxcl12a gradient formed underneath the migrating cell cluster as a result of Cxcr7b function at the rear.
Responding to the guidance cue, the PGCs form a special type of cellular protrusions known as blebs (Blaser et al, 2006). In contrast to the intensely studied lamellipodia-based migration, blebbing is not powered by actin polymerization at the leading edge of the cell, but instead is driven by myosin contraction and hydrostatic pressure. Blebs are initiated following local myosin contraction and perturbation of the actomyosin cortex interaction with the plasma membrane. At the site of the weakened association between the cortex and the membrane, a bleb is inflated in response to the hydrostatic pressure within the cell (Charras & Paluch, 2008; Fackler & Grosse, 2008; Paluch & Raz, 2013). Subsequent reconstitution of actomyosin cortex–plasma membrane interaction within the cell protrusion, establishment of new adhesion sites at the cell front and retraction of the back of the cell jointly facilitate forward cell movement. Although the precise mechanisms are unknown (see below), activation of Cxcr4b by Cxcl12a can bias bleb localization, promoting cell migration in the direction of higher Cxcl12a expression (Blaser et al, 2006).
Neutrophil migration
The recent establishment of a diverse set of transgenic lines in which the various leukocyte lineages, such as neutrophils, macrophages, and T lymphocytes, can be traced using in vivo imaging techniques has been key to the development of zebrafish as a model for leukocyte chemotaxis (Elks et al, 2011). Facilitated by the optical clarity of the zebrafish embryos, the rapidly migrating leukocytes can be imaged at a high spatial and temporal resolution. Of the various leukocyte lineages that perform chemokine-mediated chemotaxis, neutrophils have recently gained significant attention (Henry et al, 2013). Neutrophils are a large population of leukocytes that provide the first line of defense against invading microbes. They are the first cells to be recruited to sites of infection and damaged tissues, where they kill bacteria by phagocytosis and intra- or extracellular release of antibacterial proteins (Kolaczkowska & Kubes, 2013). Similar to their human counterparts, zebrafish neutrophils appear to be recruited from distant origins to sites of infection through the induction of Cxcl8 expression after bacterial infection (Sarris et al, 2012). In zebrafish, Cxcl8 acts mainly by activating the receptor Cxcr2, which is specifically expressed by the neutrophils (Deng et al, 2013; de Oliveira et al, 2013).
Zebrafish neutrophils arise from two separate lineages, one lineage related to primitive macrophages and a second that arises during early definitive hematopoiesis, which in zebrafish occurs in the caudal hematopoietic tissue (CHT) (Le Guyader et al, 2008). Cells from both lineages reside mostly in subepidermal sites and in the CHT, with only a small proportion located in the circulation (Le Guyader et al, 2008). Neutrophils are thus recruited from the CHT, even from distant sites (Deng et al, 2013) and the chemokine that is directly or indirectly responsible for this mobilization process is Cxcl8 (Fig2B).
PGCs and neutrophils both perform long-range chemokine-directed migration within the embryo to reach their target. Differences between the two cell populations are mainly in the mode of migration, which in neutrophils involves the formation cellular protrusions in the form of pseudopodia (Yoo et al, 2010), while PGCs primarily produce blebs to translocate (Blaser et al, 2006). Below, we will discuss the distinct steps involved in chemokine-controlled migration in both cell types. These steps include chemokine gradient formation, gradient interpretation, cell polarization, and the cellular response by directed migration.
Chemokine gradient formation
Recently, chemokine gradients have been directly visualized in vivo in the context of Ccl21-mediated dendritic cell migration in mice (Weber et al, 2013) and in zebrafish, where Cxcl8 mediates neutrophil migration (Sarris et al, 2012) (Fig2B). Cxcl8 forms an extracellular, matrix-bound gradient that extends at least 100 μm around the cell that expresses the chemokine. Interestingly, Cxcl8 protein was detected beyond this local tissue gradient and was found to be enriched along the venous vasculature, which includes the CHT from which Cxcl8 meditates the mobilization of neutrophils into the vasculature (Sarris et al, 2012). Since Cxcl8 binding to the venous vasculature is also required for neutrophil arrest on the blood vessel wall and to facilitate the subsequent extravasation (Middleton et al, 1997), it appears that Cxcl8 acts at several stages of neutrophil recruitment to sites of infection.
Binding of Cxcl8 to the extracellular matrix, or more specifically to heparan sulfate proteoglycans, was found to be required for efficient neutrophil migration, indicating that zebrafish neutrophils migrate—at least in part—by haptotaxis (Sarris et al, 2012). Similarly, in vivo migration of dendritic cells in the mouse ear has recently been shown to be regulated by heparan-sulfate-bound gradients of the chemokine CCL21 (Weber et al, 2013). Whereas in the mouse model system, interfering with the heparan sulfate interaction abolished directed migration, zebrafish neutrophil migration was only partly affected by such a treatment. It is therefore possible that zebrafish neutrophils employ a combination of chemotaxis and haptotaxis (or only chemotaxis in its broader definition), conferring them with increased robustness to perturbations of this kind. An interesting open question relates to the mechanisms responsible for the migration of neutrophils away from their migration target following wound healing. In addressing this question, it would be informative to characterize the distribution and levels of the signals that attracted the cells to sites of injury and correlate these data with the behavior of the neutrophils.
An additional aspect important for gradient formation is chemokine clearance from the extracellular space. In the absence of chemokine removal, localized chemokine release and accumulation within the confined space of the tissue would lead to continuous erosion of the chemokine gradient. In the case of zebrafish PGCs, chemokine removal is mediated by the interaction of Cxcl12a with the non-signaling receptor Cxcr7b (Boldajipour et al, 2008). Cxcr7b is expressed in most somatic cells during the stages of PGC migration and mediates the endocytosis and degradation of Cxcl12a, thereby maintaining the Cxcl12a gradient at a physiological level. This sink function allows dynamic changes in the RNA expression of cxcl12a to be mirrored by a corresponding protein distribution pattern. Although the signaling activity of Cxcr7 has been debated (Rajagopal et al, 2010), it was shown that at least in the context of PGC migration, interaction of Cxcl12a with Cxcr7b leads to the localization of Cxcl12a in late endosomes. Here, Cxcr7b and Cxcl12a are separated in a β-Arrestin-dependent process, such that unbound Cxcl12a is degraded in lysosomes, whereas Cxcr7b is shuttled back to the plasma membrane (Mahabaleshwar et al, 2012). As chemokine decoy or scavenging receptors other than Cxcr7 have been identified (Borroni et al, 2008), the distribution of chemokines other than Cxcl12 could be similarly regulated. It would thus be interesting to determine the role other non-signaling chemokine receptors play in controlling chemokine-guided migration in other contexts.
In addition to its contribution to dynamic properties of the gradient, the decoy or scavenging activity of Cxcr7b plays an interesting role in another tissue. During the development of a zebrafish sensory organ, the posterior lateral line (PLL), Cxcl12a is essential for the migration of a cell cluster, the lateral line primordium (David et al, 2002; Ghysen & Dambly-Chaudiere, 2007) (Fig2C). Despite the directed migration of this group of cells, cxcl12a RNA is uniformly expressed along the migration route. Thus, a formation of a Cxcl12-encoded positional information by way of localized expression and diffusion is highly unlikely. Interestingly however, Cxcr4b signaling activity—as deduced from receptor turnover—does appear in a linear gradient within the migrating primordium (Dona et al, 2013; Venkiteswaran et al, 2013). The differences in the Cxcr4b receptor activity along the lateral line primordium, which presumably reflects differences in extracellular ligand (Cxcl12a) distribution, is critically dependent on the function of Cxcr7 at the back of the migrating cluster. There, similar to its function in the process of PGC migration, Cxcr7b removes Cxcl12a from the extracellular space. This activity effectively generates a situation where Cxcr4b expressed within cells at the front of the cluster are exposed to high levels of Cxcl12 as compared with Cxcr4b at the back of the cluster (Dona et al, 2013; Venkiteswaran et al, 2013). In this framework, Cxcr7b-expressing cells within the migrating lateral line perform a similar role to that played by the somatic cells during PGC migration as they control the chemokine level by means of ligand sequestration. Whereas this type of self-generated chemokine gradient might be unique to multicellular migration, differential receptor localization within single migrating cells could theoretically function in a similar way in directing forward migration.
Although the Cxcr4b signaling gradient extends over the entire migrating lateral line primordium (150 μm), previous experiments have shown that for driving the migration of the cluster, its signaling activity is sufficient within a few cells located in the leading front (20 μm) of the group (Haas & Gilmour, 2006). This finding suggests that principles similar to those observed in the single-cell migration of PGCs may apply to the leading cells, whose primary function would be to interpret the chemokine gradient over the length of a single cell, to polarize and to respond by directed migration. Combining computer models and experimental data, Dalle Nogare et al (2014) have recently provided evidence supporting another model. According to this work, cells at the edge of the cluster can respond to extracellular Cxcl12a by active migration, and these cells do so only when Cxcl12a levels have reached a certain threshold, rather than responding to the graded distribution of the chemokine across the cells. Although Cxcl12a levels are potentially high at different positions along the lateral line primordium cell cluster, Cxcr4b activation at the back of the cluster is blocked by Cxcr7b that reduces Cxcl12a levels. These conclusions were supported by following the behavior of fragments of the cluster that showed apolar motile behavior in groups of cells isolated from the main lateral line primordium. According to this alternative model, rather than establishing a gradient of Cxcl12a at the front of the cluster, the primary role of Cxcr7b-dependent depletion of Cxcl12a is to prevent Cxcr4b-expressing cells at the trailing edge from migrating as a result of low level of the chemokine in this location.
Gradient interpretation and polarization
In order to migrate in the correct direction, the migrating cell has to transform shallow chemokine gradients into a steep cellular polarity and perform a directed movement. To this end, the cell has to establish a front and a rear end and orient its axis with respect to the orientation of the gradient. An important observation in this context is that most chemotactic cells do not migrate in a direct straight line toward their targets, but rather exhibit only a biased movement, which over time results in the accumulation of cells at the target location. Three types of biased movements have been described: in orthotaxis, cells move with a higher velocity when migrating up the chemoattractant gradient (or down within a chemorepellent gradient) (Sarris et al, 2012). In klinotaxis, cells move more persistently when migrating in the direction of higher chemoattractant concentrations and in topotaxis, cell turning is biased toward the maximum of the chemoattractant gradient (Dickinson & Tranquillo, 1995; Ionides et al, 2004). Interestingly, the mechanisms responsible for biasing the direction of cellular movement differs dramatically between zebrafish PGCs and neutrophils. Whereas neutrophils display orthotactic behavior (Sarris et al, 2012), PGCs use a combination of klinotaxis and topotaxis (Reichman-Fried et al, 2004; Minina et al, 2007). PGCs thus exhibit higher persistence when migrating up the chemotactic gradient, coupled with an initial choice of a migration course that corresponds to the direction where the level of the chemoattractant is elevated. This difference could stem from differences in motility hallmarks between the two cell types: in contrast to neutrophils, which display a continuous movement (Sarris et al, 2012), PGCs exhibit biphasic behavior, with periods of linear movement (or run phases) followed by a stationary phase (or tumbling phases) that allow cell reorientation upon reacquisition of motility (Reichman-Fried et al, 2004).
These mechanistic differences could also stem from alternative molecular pathways employed by the two cell types in the translation of differential receptor activation into cell polarity and directed migration. In the case of Dictyostelium migration for example, differential localization of phosphatidylinositol (3,4,5)-triphosphate (PIP3) to the cell front is observed. Similarly, PIP3 is enriched at the leading edge of migrating neutrophils and inhibition of PIP3 production abolishes zebrafish neutrophil migration (Yoo et al, 2010). PIP3 recruits proteins that contain pleckstrin homology (PH) domains, thereby directing actin polymerization to the front of the cell, thus leading to cell polarization and migration. In contrast, PIP3 is uniformly distributed around the cell perimeter in PGCs and interfering with PIP3 production does not affect the directional migration of these cells (Dumstrei et al, 2004). Instead, the asymmetric calcium distribution in response to Cxcr4b signaling could potentially increase actomyosin contraction at the cell front, directing protrusions formation in the form of blebs to this aspect of the cell (Blaser et al, 2006). Nevertheless, PIP3 appears to be important for PGC migration, as reducing its level affects the extent of the cell polarity, the length of filopodia and decreases the migration speed of the cells, pointing at a role in the mechanisms promoting actual motility (Dumstrei et al, 2004). Together, orthotactic cell migration in neutrophils appears to rely on the polar distribution of PIP3 that is translated into polar actin polymerization. In the absence of polar PIP3 distribution, the topotactic migration of PGCs is based on alternative polarization pathways that may include the asymmetric distribution of calcium ions that could bias bleb formation in the direction of the chemokine gradient at the exit from the apolar tumbling phases. In the case of the lateral line organ, the translation of the distribution of the chemokine in the environment into directed migration appears in first glance as a more complex task. However, the finding that these are only the cells at the front of the cluster that are critical for this movement (Haas & Gilmour, 2006; Dalle Nogare et al, 2014) raises the option that the forward movement of the cluster in response to the chemokine relies on polarization processes that are similar to those characterized in single cells.
Cellular motility
In the absence of chemokine gradients, both PGCs and neutrophils are still highly motile cells, which efficiently polarize albeit in a random direction (Doitsidou et al, 2002; Reichman-Fried et al, 2004; Deng et al, 2013), while for directional cell migration to occur, the motility machinery has to be polarized in response to polarized chemokine receptor signaling. The coupling between directed signaling and the motility machinery is still poorly understood and constitutes a key question in the field.
At least some molecular components contributing to the motility of PGC in vivo are known. During the run phase, PGCs display an enrichment of polymerized actin at the front of the cell. This polar distribution of polymerized actin depends on the Rho-family GTPase Rac1, whose regulated function is essential for efficient PGC migration (Kardash et al, 2010). Rac1 acts downstream of heterotrimeric G-proteins in regulating general PGC motility (Xu et al, 2012), and the Rac1 family member, Rac2, is similarly required for the motility of zebrafish neutrophils (Deng et al, 2011). Importantly, interfering with Rac2 activity in neutrophils affects polarized PIP3 localization and perturbs chemotaxis in addition to general motility, whereas Rac1 in PGCs appears to regulate motility alone.
Interestingly, similar to mouse dendritic cells (Lammermann et al, 2008), zebrafish PGCs can migrate in the absence of integrin-mediated extracellular matrix adhesion in vivo (Kardash et al, 2010). Instead, PGCs may rely on E-cadherin-mediated cell–cell adhesion as an anchor in order to generate traction forces required for motility (Kardash et al, 2010). While being required to sustain efficient migration, at the onset of motility, E-cadherin level is reduced in PGCs to allow movement within the cellular environment of somatic cells (Blaser et al, 2005; Goudarzi et al, 2012).
The reduction in E-cadherin level at the onset of migration was recently shown to be controlled by the regulator of G-protein signaling (RGS) 14a (Hartwig et al, 2014). In this context, RGS14a, whose RNA is inherited by the early primordial germ cells, was shown to be responsible for maintaining the initial high level of E-cadherin in the PGCs. As the level of the RNA encoding for RGS14a declines during early development, the level of the adhesion molecule is reduced, allowing germ motility and response to the Cxcl12a attractive cues by directed migration. RGS14a thus functions as a brake that delays the onset of cell motility to stages when the guidance cue is first expressed in the environment, thereby reducing the risk of cells migrating too far from their target due to premature initiation of non-directed motility (Hartwig et al, 2014).
Cell motility needs to be suppressed also as cells approach their targets, to prevent “overshooting” and thus to ensure precise arrival and arrest at the position where they exert their function. Indeed, lower migration speed or more frequent loss of polarity could be observed for PGCs as they reached the somatic gonad and for neutrophils as they arrive at the site of inflammation (Minina et al, 2007; Sarris et al, 2012). In both cases, high chemokine levels appear to indicate arrival at the destination. Consistent with this notion is the observed reduction in migration speed under conditions of ubiquitous overexpression of the relevant chemokine (Boldajipour et al, 2008; Sarris et al, 2012). In the case of PGCs, the induction of receptor internalization triggered by high levels of Cxcl12a has been identified as a key step in the downregulation of cell motility upon arrival at the target (Minina et al, 2007).
Beyond the zebrafish
This review focuses on the use of zebrafish in studying the in vivo mechanisms of chemokine-induced migration, but some of the fundamental insights obtained using this organism have been extended to higher vertebrates. For example, in a recent study, Ulvmar et al (2014) have shown that the formation of a functional chemokine gradient within the mouse lymph node depends on a signaling and a scavenging receptor analogous to the zebrafish Cxcl12/Cxcr4/Cxcr7 system.
Part of the route that dendritic cells (DCs) take during infection is from the subcapsular sinus (SCS), a lumen lined with lymphatic endothelial cells, into the subcortical zone where they interact with T lymphocytes (Johnson & Jackson, 2014). This migration depends on the chemokines CCL19 and CCL21, which activate the receptor CCR7 on DCs. CCL21 gradient formation requires the function of the scavenging receptor ACKR4 (also known as CCRL1), which is expressed by the most cortical lymphatic endothelial cells. ACKR4-mediated endocytosis of CCL21 removes it from the SCS, thereby generating a CCL21 gradient that guides DCs toward the subcortical zone (Ulvmar et al, 2014). Since the mouse lymph node is accessible for in vivo imaging, it could be used to visualize the dynamics of chemokine gradients in a higher vertebrate. Additionally, as many chemokines could potentially interact with scavenging receptors (Nibbs & Graham, 2013), the chemokine gradient formation models identified in zebrafish germ cell and lateral line migration might be prototypical to chemokine gradient formation in other vertebrates.
Conclusions and open questions
The ability to visualize cell migration at unprecedented resolution has made the zebrafish a powerful tool for studying the molecular and cellular mechanisms of chemotaxis in vivo. PGCs and neutrophils have so far been the most intensively studied cell types, providing complementary information on the molecular mechanisms that regulate chemokine-mediated single cell migration. The comprehensive understanding achieved in these models is likely to be relevant for other cell types that rely on chemokines for directed migration as single cells. For example, lymphocyte progenitor homing to the thymus during embryonic thymopoiesis was found to be guided through the combined activity of Cxcl12a, Cxcl12b, and Ccl25a (Bajoghli et al, 2009; Hess & Boehm, 2012). Last, it would be interesting to determine the similarity and difference between the mechanism facilitating the motility and directional migration of single cells guided by chemokines and cells that respond to those signals as a group.
Acknowledgments
J.B. was supported by an Innovative Research Incentive Scheme Veni grant, financed by the Netherlands Organization for Scientific Research. E.R. was supported by an ERC advanced grant and by the German Research Foundation (DFG) Cells in Motion – Cluster of Excellence, Münster, Germany. The authors would like to thank Michal Reichman-Fried for critical reading of this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- van der Aa LM, Chadzinska M, Tijhaar E, Boudinot P, Verburg-van Kemenade BM. CXCL8 chemokines in teleost fish: two lineages with distinct expression profiles during early phases of inflammation. PLoS ONE. 2010;5:e12384. doi: 10.1371/journal.pone.0012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Bajoghli B, Aghaallaei N, Hess I, Rode I, Netuschil N, Tay BH, Venkatesh B, Yu JK, Kaltenbach SL, Holland ND, Diekhoff D, Happe C, Schorpp M, Boehm T. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell. 2009;138:186–197. doi: 10.1016/j.cell.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Bajoghli B. Evolution and function of chemokine receptors in the immune system of lower vertebrates. Eur J Immunol. 2013;43:1686–1692. doi: 10.1002/eji.201343557. [DOI] [PubMed] [Google Scholar]
- Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- Blaser H, Eisenbeiss S, Neumann M, Reichman-Fried M, Thisse B, Thisse C, Raz E. Transition from non-motile behaviour to directed migration during early PGC development in zebrafish. J Cell Sci. 2005;118:4027–4038. doi: 10.1242/jcs.02522. [DOI] [PubMed] [Google Scholar]
- Blaser H, Reichman-Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, Solnica-Krezel L, Heisenberg CP, Raz E. Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell. 2006;11:613–627. doi: 10.1016/j.devcel.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- Boldajipour B, Doitsidou M, Tarbashevich K, Laguri C, Yu SR, Ries J, Dumstrei K, Thelen S, Dorries J, Messerschmidt EM, Thelen M, Schwille P, Brand M, Lortat-Jacob H, Raz E. Cxcl12 evolution–subfunctionalization of a ligand through altered interaction with the chemokine receptor. Development. 2011;138:2909–2914. doi: 10.1242/dev.068379. [DOI] [PubMed] [Google Scholar]
- Borroni EM, Bonecchi R, Buracchi C, Savino B, Mantovani A, Locati M. Chemokine decoy receptors: new players in reproductive immunology. Immunol Invest. 2008;37:483–497. doi: 10.1080/08820130802191318. [DOI] [PubMed] [Google Scholar]
- Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann J, Wolfe SA, Siekmann AF. Arterial-venous network formation during brain vascularization involves hemodynamic regulation of chemokine signaling. Development. 2011;138:1717–1726. doi: 10.1242/dev.059881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Devreotes PN. Moving in the right direction: how eukaryotic cells migrate along chemical gradients. Semin Cell Dev Biol. 2011;22:834–841. doi: 10.1016/j.semcdb.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YR, Fujita M, Butler M, Isogai S, Kochhan E, Siekmann AF, Weinstein BM. Chemokine signaling directs trunk lymphatic network formation along the preexisting blood vasculature. Dev Cell. 2012;22:824–836. doi: 10.1016/j.devcel.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani SH, Sabol A, Xu H, Gyda MA, Rasband K, Granato M, Chien CB, Raper JA. Stromal cell-derived factor-1 antagonizes slit/robo signaling in vivo. J Neurosci. 2007;27:973–980. doi: 10.1523/JNEUROSCI.4132-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–736. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- Chen J, Xu Q, Wang T, Collet B, Corripio-Miyar Y, Bird S, Xie P, Nie P, Secombes CJ, Zou J. Phylogenetic analysis of vertebrate CXC chemokines reveals novel lineage specific groups in teleost fish. Dev Comp Immunol. 2013;41:137–152. doi: 10.1016/j.dci.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Chong SW, Nguyet LM, Jiang YJ, Korzh V. The chemokine Sdf-1 and its receptor Cxcr4 are required for formation of muscle in zebrafish. BMC Dev Biol. 2007;7:54. doi: 10.1186/1471-213X-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Nogare D, Somers K, Rao S, Matsuda M, Reichman-Fried M, Raz E, Chitnis AB. Leading and trailing cells cooperate in collective migration of the zebrafish posterior lateral line primordium. Development. 2014;141:3188–3196. doi: 10.1242/dev.106690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David NB, Sapede D, Saint-Etienne L, Thisse C, Thisse B, Dambly-Chaudiere C, Rosa FM, Ghysen A. Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc Natl Acad Sci USA. 2002;99:16297–16302. doi: 10.1073/pnas.252339399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Yoo SK, Cavnar PJ, Green JM, Huttenlocher A. Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Dev Cell. 2011;21:735–745. doi: 10.1016/j.devcel.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Sarris M, Bennin DA, Green JM, Herbomel P, Huttenlocher A. Localized bacterial infection induces systemic activation of neutrophils through Cxcr2 signaling in zebrafish. J Leukoc Biol. 2013;93:761–769. doi: 10.1189/jlb.1012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries ME, Kelvin AA, Xu L, Ran L, Robinson J, Kelvin DJ. Defining the origins and evolution of the chemokine/chemokine receptor system. J Immunol. 2006;176:401–415. doi: 10.4049/jimmunol.176.1.401. [DOI] [PubMed] [Google Scholar]
- Dickinson RB, Tranquillo RT. Transport-equations and indexes for random and biased cell-migration based on single-cell properties. Siam J Appl Math. 1995;55:1419–1454. [Google Scholar]
- Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- Dona E, Barry JD, Valentin G, Quirin C, Khmelinskii A, Kunze A, Durdu S, Newton LR, Fernandez-Minan A, Huber W, Knop M, Gilmour D. Directional tissue migration through a self-generated chemokine gradient. Nature. 2013;503:285–289. doi: 10.1038/nature12635. [DOI] [PubMed] [Google Scholar]
- Dumstrei K, Mennecke R, Raz E. Signaling pathways controlling primordial germ cell migration in zebrafish. J Cell Sci. 2004;117:4787–4795. doi: 10.1242/jcs.01362. [DOI] [PubMed] [Google Scholar]
- Elks PM, Loynes CA, Renshaw SA. Measuring inflammatory cell migration in the zebrafish. Methods Mol Biol. 2011;769:261–275. doi: 10.1007/978-1-61779-207-6_18. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Cha YR, Pham VN, Sakurai A, Roman BL, Gutkind JS, Weinstein BM. Assembly and patterning of the vascular network of the vertebrate hindbrain. Development. 2011;138:1705–1715. doi: 10.1242/dev.058776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudiere C. The lateral line microcosmos. Genes Dev. 2007;21:2118–2130. doi: 10.1101/gad.1568407. [DOI] [PubMed] [Google Scholar]
- Gilmour D, Knaut H, Maischein HM, Nusslein-Volhard C. Towing of sensory axons by their migrating target cells in vivo. Nat Neurosci. 2004;7:491–492. doi: 10.1038/nn1235. [DOI] [PubMed] [Google Scholar]
- Goudarzi M, Banisch TU, Mobin MB, Maghelli N, Tarbashevich K, Strate I, van den Berg J, Blaser H, Bandemer S, Paluch E, Bakkers J, Tolic-Norrelykke IM, Raz E. Identification and regulation of a molecular module for bleb-based cell motility. Dev Cell. 2012;23:210–218. doi: 10.1016/j.devcel.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Haas P, Gilmour D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell. 2006;10:673–680. doi: 10.1016/j.devcel.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Hartwig J, Tarbashevich K, Seggewiss J, Stehling M, Bandemer J, Grimaldi C, Paksa A, Gross-Thebing T, Meyen D, Raz E. Temporal control over the initiation of cell motility by a regulator of G-protein signaling. Proc Natl Acad Sci USA. 2014;111:11389–11394. doi: 10.1073/pnas.1400043111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KM, Loynes CA, Whyte MK, Renshaw SA. Zebrafish as a model for the study of neutrophil biology. J Leukoc Biol. 2013;94:633–642. doi: 10.1189/jlb.1112594. [DOI] [PubMed] [Google Scholar]
- Hess I, Boehm T. Intravital imaging of thymopoiesis reveals dynamic lympho-epithelial interactions. Immunity. 2012;36:298–309. doi: 10.1016/j.immuni.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Hollway GE, Bryson-Richardson RJ, Berger S, Cole NJ, Hall TE, Currie PD. Whole-somite rotation generates muscle progenitor cell compartments in the developing zebrafish embryo. Dev Cell. 2007;12:207–219. doi: 10.1016/j.devcel.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Ionides EL, Fang KS, Isseroff RR, Oster GF. Stochastic models for cell motion and taxis. J Math Biol. 2004;48:23–37. doi: 10.1007/s00285-003-0220-z. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Jackson DG. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis. 2014;17:335–345. doi: 10.1007/s10456-013-9407-0. [DOI] [PubMed] [Google Scholar]
- Kardash E, Reichman-Fried M, Maitre JL, Boldajipour B, Papusheva E, Messerschmidt EM, Heisenberg CP, Raz E. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat Cell Biol. 2010;12:47–53. doi: 10.1038/ncb2003. [DOI] [PubMed] [Google Scholar]
- Knaut H, Werz C, Geisler R, Nusslein-Volhard C. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 2003;421:279–282. doi: 10.1038/nature01338. [DOI] [PubMed] [Google Scholar]
- Knaut H, Blader P, Strahle U, Schier AF. Assembly of trigeminal sensory ganglia by chemokine signaling. Neuron. 2005;47:653–666. doi: 10.1016/j.neuron.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Konijn TM, Van De Meene JG, Bonner JT, Barkley DS. The acrasin activity of adenosine-3,5-cyclic phosphate. Proc Natl Acad Sci USA. 1967;58:1152–1154. doi: 10.1073/pnas.58.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar PS, Siekhaus DE, Lehmann R. In vivo migration: a germ cell perspective. Annu Rev Cell Dev Biol. 2006;22:237–265. doi: 10.1146/annurev.cellbio.22.010305.103337. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, Mordelet E, Zapata A, Shinomiya H, Herbomel P. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111:132–141. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- Li Q, Shirabe K, Thisse C, Thisse B, Okamoto H, Masai I, Kuwada JY. Chemokine signaling guides axons within the retina in zebrafish. J Neurosci. 2005;25:1711–1717. doi: 10.1523/JNEUROSCI.4393-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabaleshwar H, Tarbashevich K, Nowak M, Brand M, Raz E. beta-arrestin control of late endosomal sorting facilitates decoy receptor function and chemokine gradient formation. Development. 2012;139:2897–2902. doi: 10.1242/dev.080408. [DOI] [PubMed] [Google Scholar]
- Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- Minina S, Reichman-Fried M, Raz E. Control of receptor internalization, signaling level, and precise arrival at the target in guided cell migration. Curr Biol. 2007;17:1164–1172. doi: 10.1016/j.cub.2007.05.073. [DOI] [PubMed] [Google Scholar]
- Miyasaka N, Knaut H, Yoshihara Y. Cxcl12/Cxcr4 chemokine signaling is required for placode assembly and sensory axon pathfinding in the zebrafish olfactory system. Development. 2007;134:2459–2468. doi: 10.1242/dev.001958. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Verkade H, Heath JK, Kuroiwa A, Kikuchi Y. Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation. Development. 2008;135:2521–2529. doi: 10.1242/dev.020107. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nair S, Schilling TF. Chemokine signaling controls endodermal migration during zebrafish gastrulation. Science. 2008;322:89–92. doi: 10.1126/science.1160038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes HG, Rot A, Thelen M. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS ONE. 2010;5:e9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbs RJ, Graham GJ. Immune regulation by atypical chemokine receptors. Nat Rev Immunol. 2013;13:815–829. doi: 10.1038/nri3544. [DOI] [PubMed] [Google Scholar]
- Nomiyama H, Hieshima K, Osada N, Kato-Unoki Y, Otsuka-Ono K, Takegawa S, Izawa T, Yoshizawa A, Kikuchi Y, Tanase S, Miura R, Kusuda J, Nakao M, Yoshie O. Extensive expansion and diversification of the chemokine gene family in zebrafish: identification of a novel chemokine subfamily CX. BMC Genom. 2008;9:222. doi: 10.1186/1471-2164-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiyama H, Osada N, Yoshie O. Systematic classification of vertebrate chemokines based on conserved synteny and evolutionary history. Genes Cells. 2013;18:1–16. doi: 10.1111/gtc.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers SH, Flores MV, Hall CJ, OToole R, Swift S, Crosier KE, Crosier PS. Expression of zebrafish cxcl8 (interleukin-8) and its receptors during development and in response to immune stimulation. Dev Comp Immunol. 2010;34:352–359. doi: 10.1016/j.dci.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Olesnicky Killian EC, Birkholz DA, Artinger KB. A role for chemokine signaling in neural crest cell migration and craniofacial development. Dev Biol. 2009;333:161–172. doi: 10.1016/j.ydbio.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira S, Reyes-Aldasoro CC, Candel S, Renshaw SA, Mulero V, Calado A. Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J Immunol. 2013;190:4349–4359. doi: 10.4049/jimmunol.1203266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palevitch O, Abraham E, Borodovsky N, Levkowitz G, Zohar Y, Gothilf Y. Cxcl12a-Cxcr4b signaling is important for proper development of the forebrain GnRH system in zebrafish. Gen Comp Endocrinol. 2010;165:262–268. doi: 10.1016/j.ygcen.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Paluch EK, Raz E. The role and regulation of blebs in cell migration. Curr Opin Cell Biol. 2013;25:582–590. doi: 10.1016/j.ceb.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait JH, Yan YL, Gates MA, Horne S, Amores A, Brownlie A, Donovan A, Egan ES, Force A, Gong Z, Goutel C, Fritz A, Kelsh R, Knapik E, Liao E, Paw B, Ransom D, Singer A, Thomson M, Abduljabbar TS, et al. Vertebrate genome evolution and the zebrafish gene map. Nat Genet. 1998;18:345–349. doi: 10.1038/ng0498-345. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, Gerard C, Lefkowitz RJ. Beta-arrestin- but not G protein-mediated signaling by the decoy receptor CXCR7. Proc Natl Acad Sci USA. 2010;107:628–632. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz E, Reichman-Fried M. Attraction rules: germ cell migration in zebrafish. Curr Opin Genet Dev. 2006;16:355–359. doi: 10.1016/j.gde.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Reichman-Fried M, Minina S, Raz E. Autonomous modes of behavior in primordial germ cell migration. Dev Cell. 2004;6:589–596. doi: 10.1016/s1534-5807(04)00074-7. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9:949–952. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- Sapede D, Rossel M, Dambly-Chaudiere C, Ghysen A. Role of SDF1 chemokine in the development of lateral line efferent and facial motor neurons. Proc Natl Acad Sci USA. 2005;102:1714–1718. doi: 10.1073/pnas.0406382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarris M, Masson JB, Maurin D, Van der Aa LM, Boudinot P, Lortat-Jacob H, Herbomel P. Inflammatory chemokines direct and restrict leukocyte migration within live tissues as glycan-bound gradients. Curr Biol. 2012;22:2375–2382. doi: 10.1016/j.cub.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Schroder JM, Mrowietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987;139:3474–3483. [PubMed] [Google Scholar]
- Siekmann AF, Standley C, Fogarty KE, Wolfe SA, Lawson ND. Chemokine signaling guides regional patterning of the first embryonic artery. Genes Dev. 2009;23:2272–2277. doi: 10.1101/gad.1813509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague J, Bayraktaroglu L, Clements D, Conlin T, Fashena D, Frazer K, Haendel M, Howe DG, Mani P, Ramachandran S, Schaper K, Segerdell E, Song P, Sprunger B, Taylor S, Van Slyke CE, Westerfield M. The zebrafish information network: the zebrafish model organism database. Nucleic Acids Res. 2006;34:D581–D585. doi: 10.1093/nar/gkj086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvmar MH, Werth K, Braun A, Kelay P, Hub E, Eller K, Chan L, Lucas B, Novitzky-Basso I, Nakamura K, Rulicke T, Nibbs RJ, Worbs T, Forster R, Rot A. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat Immunol. 2014;15:623–630. doi: 10.1038/ni.2889. [DOI] [PubMed] [Google Scholar]
- Van Damme J, Van Beeumen J, Opdenakker G, Billiau A. A novel, NH2-terminal sequence-characterized human monokine possessing neutrophil chemotactic, skin-reactive, and granulocytosis-promoting activity. J Exp Med. 1988;167:1364–1376. doi: 10.1084/jem.167.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkiteswaran G, Lewellis SW, Wang J, Reynolds E, Nicholson C, Knaut H. Generation and dynamics of an endogenous, self-generated signaling gradient across a migrating tissue. Cell. 2013;155:674–687. doi: 10.1016/j.cell.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116:2803–2811. doi: 10.1182/blood-2010-03-276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A, Peveri P, Aschauer H, Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149:755–761. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, Luther SA, Bollenbach T, Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- Wu SY, Shin J, Sepich DS, Solnica-Krezel L. Chemokine GPCR signaling inhibits beta-catenin during zebrafish axis formation. PLoS Biol. 2012;10:e1001403. doi: 10.1371/journal.pbio.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Kardash E, Chen S, Raz E, Lin F. Gbetagamma signaling controls the polarization of zebrafish primordial germ cells by regulating Rac activity. Development. 2012;139:57–62. doi: 10.1242/dev.073924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Hasan SS, Schmidt I, Rocha SF, Pitulescu ME, Bussmann J, Meyen D, Raz E, Adams RH, Siekmann AF. Arteries are formed by vein-derived endothelial tip cells. Nat Commun. 2014;5:5758. doi: 10.1038/ncomms6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18:226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Kawakami K, Hopkins N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development. 1997;124:3157–3165. doi: 10.1242/dev.124.16.3157. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]