Abstract
Importin proteins were originally characterized for their central role in protein transport through the nuclear pores, the only intracellular entry to the nucleus. This vital function must be tightly regulated to control access by transcription factors and other nuclear proteins to genomic DNA, to achieve appropriate modulation of cellular behaviors affecting cell fate. Importin-mediated nucleocytoplasmic transport relies on their specific recognition of cargoes, with each importin binding to distinct and overlapping protein subsets. Knowledge of importin function has expanded substantially in regard to three key developmental systems: embryonic stem cells, muscle cells and the germ line. In the decade since the potential for regulated nucleocytoplasmic transport to contribute to spermatogenesis was proposed, we and others have shown that the importins that ferry transcription factors into the nucleus perform additional roles, which control cell fate. This review presents key findings from studies of mammalian spermatogenesis that reveal potential new pathways by which male fertility and infertility arise. These studies of germline genesis illuminate new ways in which importin proteins govern cellular differentiation, including via directing proteins to distinct intracellular compartments and by determining cellular stress responses.
Keywords: cell fate, cell stress, importin, karyopherin, nucleocytoplasmic transport, spermatid, spermatocyte, spermatogenesis
MAMMALIAN SPERMATOGENESIS – A SERIES OF TIGHTLY REGULATED DEVELOPMENTAL SWITCHES
Formation of spermatozoa
Spermatogenesis requires a highly coordinated and tightly regulated sequence of cellular processes in order for the diploid male germ cell precursors to mature into spermatozoa, as reviewed briefly here. In the adult mammalian testis, this progression involves spermatogonial mitoses, spermatocyte meiosis and the structural transformation of haploid spermatids. Complete male germline maturation occurs while these cells are embedded in the columnar epithelium formed by nondividing Sertoli cells. This epithelium where spermatogenesis takes place constitutes the coiled seminiferous tubules of the testes; these are completely encased by peritubular myoid cells and embedded in a complex interstitial tissue containing testosterone-producing Leydig cells, macrophages, fibroblasts and both lymphatic and blood vessels.1
Development of the adult testis depends on events that are initiated in early embryonic life. A small subset of proximal epiblast cells undergo germline specification and commitment in response to signaling molecules, such as the bone morphogenetic proteins BMP4 and BMP8b, to form the initial primordial germ cell (PGC) population.2 These cells migrate to the bipotential genital ridge where they undergo sex determination, becoming gonocytes as they commit to a male fate in response to factors produced by the pre-Sertoli cells that fully enclose them in the nascent testis cords. The gonocytes proliferate, then are quiescent until after birth. The onset of spermatogenesis involves gonocyte re-entry into the cell cycle and their radial migration to contact the seminiferous cord basement membrane. In this position, the first spermatogonial cohort forms both spermatogonial stem cells (SSCs) and differentiating spermatogonia.3 The latter first proliferate, then enter meiosis and complete the maturation sequence described briefly above for adult spermatogenesis. A proportion of the SSCs must undergo self-renewing mitotic divisions throughout life to sustain male fertility. Another appropriate fraction of these divisions also yields the next generation of spermatogonia that commence differentiation; these are distinguished from SSCs by their ongoing maintenance of cytoplasmic bridges between clonal siblings until the time when they are released as spermatozoa into the tubular lumen. Spermatogonia first undergo a sequence of amplifying divisions as undifferentiated subtypes and then as a distinct subpopulation of differentiating spermatogonia that are committed to enter meiosis. Upon forming primary spermatocytes, the tetraploid male germ cells undergo two successive nuclear meiotic divisions to form haploid round spermatids. During the period termed spermiogenesis, the sperm flagellum and acrosome are formed as the spermatid elongates. Nuclear remodeling during spermiogenesis includes distal repositioning of nuclear pore complexes4 and the replacement of histones, first with transition proteins and then with protamines,5 both critical steps for compaction of the male genome.6
Regulated entry of transcription factors into the nucleus controls cellular differentiation
The progressive transformation of a pluripotent embryonic cell into highly specialized spermatozoa is controlled by the milieu of extrinsic signals that facilitate their specification, promote their survival and proliferation, and govern the pace of their maturation. Several reviews have highlighted the critical roles of particular signaling pathways7,8 and provide information about the transcriptional changes that accompany these transitions.9,10 Of particular relevance is an observation we first made many years ago: that many key signaling molecules, transcription factors in particular, undergo movement between the cytoplasm and the nucleus at the times of key transitions between spermatogenic differentiation states.11 Such moieties include the pluripotency factor Oct3/4, the TGFβ superfamily transcription factors, Smads, and the Hedgehog pathway transcription factor, Gli1. We proposed that the developmental switches that control male germline cell differentiation are mediated by regulating entry of these important transcription factors into the nucleus. Our initial research hypothesis was that synthesis of both an appropriate transcription factor and its specific nucleocytoplasmic transport mediator would be required to effect each developmental transition. Studies over the past decade have provided strong evidence for this phenomenon in embryonic stem cells.12,13,14 Our analyses of the mammalian testis have also yielded support for this hypothesis and revealed new ways in which the importin proteins, originally identified as key nucleocytoplasmic transport factors, contribute to sperm formation.
IMPORTIN PROTEINS – CENTRAL TO NUCLEOCYTOPLASMIC TRANSPORT
The nuclear pore: gatekeeper to the nucleus
As the defining organelle of eukaryotic cells, the nucleus confines genomic DNA within a phospholipid double bilayer termed the nuclear envelope (NE). Nuclear pores are the only portals through the NE. Nucleocytoplasmic transport through nuclear pore complexes (NPCs) is tightly regulated and essential for eukaryotic cell survival and for normal development. Molecules smaller than ~40 kDa, including water, ions and proteins can transit NPCs passively via diffusion; molecules larger than ~40 kDa require a carrier molecule to move through the NPC in either direction. Active transport of proteins through the nucleus typically utilizes a transporter molecule that recognizes a transport signal on cargoes, either a nuclear localization signal (NLS) for nuclear import or a nuclear export signal (NES) for export.15,16 Each pore is comprised of ~30 different nucleoporin proteins (NUPs) arranged in a cylindrical form with octagonal symmetry.17 Each NPC has NUP filaments that extend into both the cytoplasm and the nucleoplasm and line the pore openings.18 Several NUPs are involved directly in transport through the NPC, as they bind the proteins that ferry molecular cargo between the nucleus and cytoplasm.
Importin proteins constitute a key class of nucleocytoplasmic transport molecules
The general name for the class of soluble proteins that transports cargo through the NPC is karyopherins. These are commonly considered as three separate groups: importins, which mediate transport into the nucleus; exportins, which move proteins out of the nucleus; and transportins, which can confer movement in either direction. Importins are the most widely studied molecules responsible for transporting proteins into the nucleus.15
The importins are further categorized into importin αs and importin βs, based on their structural and functional features. In classical nucleocytoplasmic transport, an importin α protein functions as an adapter by linking cargo proteins to an importin β1 molecule. This complex forms in the cytoplasm in an environment of high RanGDP levels. Each importin α functions by recognizing a nuclear localization signal (NLS) in cargoes, typically one or two stretches of 8–10 amino acids containing a high proportion of basic residues, termed monopartite and bipartite NLSs, respectively (Figure 1ai). The binding of a cargo molecule by importin α allows it to bind to importin β1, creating a trimeric complex (Figure 1aii). Importin β1 binds to the NUPs lining the nuclear pore to mediate translocation of this complex into the nucleus. In the presence of high levels of RanGTP within the nucleus, the transport complex is dissociated (Figure 1aiii). Both importin α and β1 proteins are recycled to the cytoplasm by independent means, in readiness to complete another round of transport, after the cargo is released to perform its nuclear function.
Figure 1.
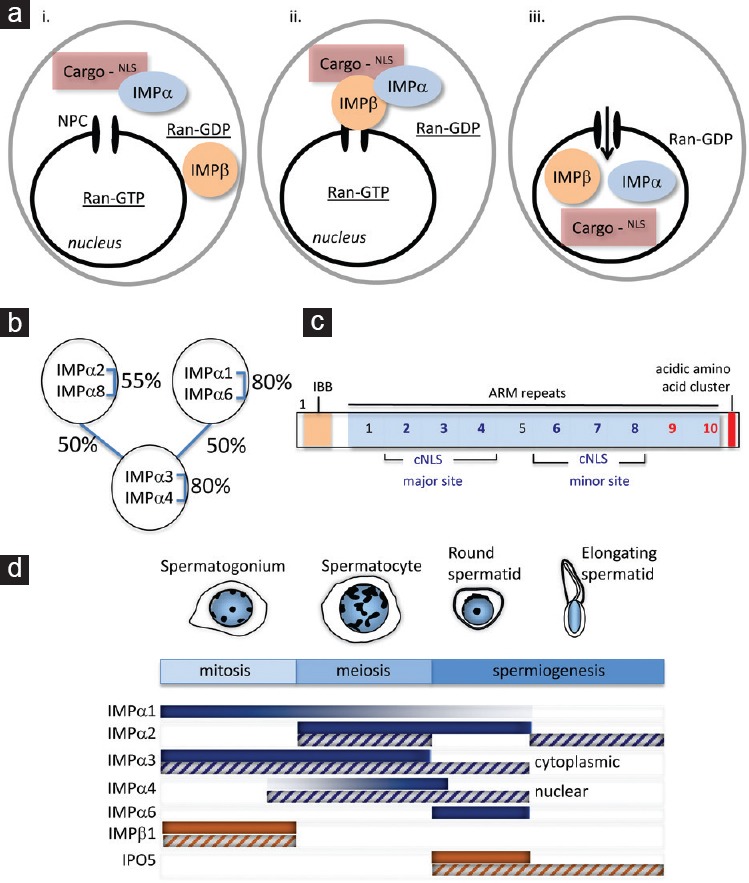
Synthesis of importin proteins that function in nucleocytoplasmic transport is developmentally regulated during spermatogenesis. (a) Classical nucleocytoplasmic transport. Proteins larger than 40 kDa that undertake key functions in the nucleus require active transport through the nuclear pore complex (NPC). For many proteins, such movement is mediated by members of the importin family. (i) Importin α (IMPα) proteins act as receptors that recognize nuclear localization signal (NLS) motifs in the cargo proteins. (ii) Complex formation with importin β1 (IMPβ) allows the passage of cargo molecules through the nuclear pore. Other IMPβ family members, including IPO5, can mediate IMPα-independent transport. (iii) Once in the nucleus, the complex is dissociated, releasing the cargo protein to play its nuclear role, and the importin proteins are recycled back into the cytoplasm. Maintenance of the RanGDP/GTP gradient, in which RanGTP is high in the nucleus, is critical for regulated nucleocytoplasmic transport. (b) Three clades of importin α proteins. The importin α (IMPα) proteins are classified into three distinct subgroups based on percentage identity, as indicated. Despite this classification, there is significant (50%) identity across subgroups. (c) IMPα proteins harbor multiple domains that mediate cargo binding. The classical localization signal (cNLS) classes recognized by the six murine IMPa cargo proteins correspond to either mono- or bipartite sequences of 8–10 basic residue-enriched amino acids. These sequences occur in the major and/or minor binding grooves within the ARM repeat motifs and can be identified with reasonable accuracy by the cNLS Mapper algorithm.42 However, cargo binding to a C-terminal “acidic” domain has been identified.13 This domain was recently examined through a new algorithm designed specifically to identify common amino acid sequence patterns within the set of binding proteins that mediate binding to the C-terminus of IMPα proteins. This newly defined motif has been designated iCBS, for ‘IMPα C-terminal binding segment’.32 IBB, importin β binding domain. ARM, armadillo. (d) Importins are highly regulated at both the RNA and protein level, and each has a specific pattern of expression that correlates with spermatogenic progression. The major developmental stages of spermatogenesis are shown, with the mitotic, meiotic, and spermiogenic phases indicated. Based on studies in rodent models, importin transcript (solid bars) and protein (hatched bars) levels are presented by shading intensity, indicating a potential role as drivers of germ cell development through adult spermatogenesis. IMPα3 and IMPα4 are noted, as despite their high degree of similarity (80%), they are each detected in different stages of spermatogenesis, and in different subcellular compartments.
To date, six importin α proteins have been identified in the mouse, with each known by several aliases that often create confusion when comparing various publications: e.g., importin α1/Kpna1/S1, importin α2/Kpna2/P1, importin α3/Kpna3/Q2, importin α4/Kpna4/Q1, importin α6/Kpna6/S2 and importin α8/Kpna7. A complete summary of gene names for mouse and human nuclear transport machinery has been published.16 The importin α proteins are classified into three conserved subfamilies defined by their structural relatedness: clade α1 (α1/α6), clade α2 (α2/α8) or clade α3 (α3/α4)19 (Figure 1bi). All contain several common functional motifs (Figure 1bii): an N-terminal domain for importin β binding (IBB), internal cargo cNLS binding sites, 10 armadillo (ARM) repeats including the 10th ARM repeat required for importin α nuclear export via CAS protein binding, and a short C-terminal acidic domain.20,21 The IBB domain of importin α contains a sequence of basic residues (KRR) that is highly similar to a cNLS. It confers auto-inhibition of cargo molecule binding: in the absence of bound importin β1, the IBB domain competes with the cargo for binding to the importin α, causing the cargo to remain within the nucleus.20 The second through fourth ARM repeats constitute the major cNLS binding site for monopartite NLSs and for the longer cluster of amino acids in bipartite cNLSs. Repeats 6–8 correspond to what is termed the minor binding site, for interactions with the shorter segment of a bipartite cNLSs.21
Importin β proteins can bind to a cargo molecule either directly to mediate transport or, in the case of importin β1, to associate indirectly via an adapter importin α. The flexible arrangement of a repeating motif in importin βs, termed the HEAT repeat, that undergoes structural changes upon binding to cargoes, NPCs and Ran,22,23 is a feature most likely important for the multiplicity of functions performed by members of this family.
As a general class of proteins, importins also have been studied in a wide variety of model systems and found to serve non transport-related functions involved in controlling transcription of genes,24,25 embryonic stem cell pluripotency,12,13,14 adaption to cellular stress,24,25,26,27 nuclear envelope assembly,28 spindle formation29 and lamin assembly.30 Their involvement in functions other than nucleocytoplasmic transport depends on where importins become localized, for example whether they are directed to a particular organelle or retained in the cytoplasm. This is an area of intense interest as it provides insights not only into how proteins can be sequestered within different cellular compartments but it also delineates processes that influence the pace or nature of cargo nuclear entry and hence regulate changes in cell fate.
IMPORTIN SYNTHESIS AND SUBCELLULAR LOCALIZATION IS DYNAMIC DURING MALE GERMLINE DEVELOPMENT
Changing importin levels influence cellular functions
There is accumulating evidence to show that each mammalian importin α protein (six identified in mouse, seven in human) selectively binds unique and overlapping cargo subsets.19,31,32 This has lead to the understanding that each importin α performs distinct functions by ferrying unique subsets of factors into the nucleus. Thus regulated importin α mRNA synthesis is predicted to drive altered nuclear protein content by changing importin α protein levels; this will of course lead to changes in gene expression and ultimately alter cell behavior and fate.
The finding that individual importins and exportins have dynamic expression levels during development was identified in several systems, including embryonic stem cells, muscle cells and during spermatogenesis.12,13,14,16,33 This has been most extensively documented throughout the course of male germ cell differentiation, from fetal life through adulthood, using several techniques including quantitative reverse transcription-polymerase chain reaction (PCR), in situ hybridization, GEOprofiles dataset analysis (NCBI website https://www.ncbi.nlm.nih.gov/geoprofiles/), immunohistochemistry and western blot analysis,32,34,35,36,37 as summarized below and illustrated in Figure 1c.
Importin β proteins in spermatogenesis
The first importin for which a testicular expression profile was reported was an importin β molecule, IPO5 (originally named RanBP5 and Importin β3), identified in a search for genes preferentially expressed in germ cells of the mouse testis using differential display technology.38 The relatively high abundance of this mRNA in the mouse testis was identified by northern blotting, and its prevalent cellular site of expression in the adult was shown to be pachytene spermatocytes and round spermatids. Extensive cellular localization profiling during rodent testis development for both IPO5 and importin β1 mRNAs and proteins has illustrated their distinct patterns of highly cell-specific production.34 In the adult testis, importin β1 mRNA and protein were found to be most abundant in early-stage germ cells (spermatogonia and spermatocytes), while the IPO5 transcript and protein were detected only the later, haploid stages. The IPO5 protein immunohistochemistry signal was strongest in elongating spermatids, emerging after the peak transcript signal in round spermatids. The expression of these two importins also differed in the fetal testis.35,37 Expression of the gene Kpnb1 and cytoplasmic importin β1 protein were detected in mitotic Sertoli and male germ cells at all fetal ages, whereas the IPO5 protein was observed only within germ cells. IPO5 protein was predominantly located in the nucleus in both male and female germ cells at embryonic day (E) 11.5. In oogonia, the signal became exclusively nuclear at E12.5 and E13.5 but then was entirely cytoplasmic at E14.5 and barely detectable at E16.5; IPO5 protein movement from the nucleus into the cytoplasm was concordant with oogonial entry into meiosis.37 In contrast, the signal in male germ cells shifted from cytoplasmic at E12.5–E14.5 to nuclear in E16.5 gonocytes, transitioning at the stage when fetal male germ cells exit mitosis to become quiescent. The precise functional significance of this profound gender-specific shift in IPO5 subcellular localization within fetal germ cells remains to be elucidated, but these movements are clearly associated with particular cellular transitions.
An additional importin β family member, importin 13 (IPO13) is also highly expressed in murine germ cells, with its transcript levels being highest in both male and female cells at the pachytene stage, in adults for males and in the fetal ovary for females.39 Of the two IPO13 splice variants, synthesis of the longer isoform correlated with UBC9 nuclear localization in both male and female germ cells. UBC9 is required for correct formation/function of the synaptonemal complex, and short interfering (si) RNA knockdown of the longer, but not the shorter, IPO13 transcript in fetal ovaries reduced UBC9 nuclear localization in oogonia. This study demonstrated that IPO13 production is needed for germline progression through meiosis.39 It will be interesting to identify whether other nuclear cargo involved in meiosis require IPO13 and to ascertain the functional significance of the shorter transcript present only in spermatogenic cells; this variant lacks the N-terminal RanGTP binding domain required for correct binding of cargo and its release in the nucleus.
Importin α proteins
Examination of individual importin α mRNAs has also revealed dynamic and cell-specific expression profiles during mammalian spermatogenesis. An early study examined mRNAs encoding five of the six murine importin αs in the developing and adult rodent testis.35 Transcripts for importins α1, α2, α3 and α6 were readily detected by reverse transcription-PCR in the fetal testis, while that encoding α4 was not. In the postnatal testis, importins α1 and α4 displayed the greatest degree of difference when tested at ages ranging from birth (day 0) to adulthood, with these appearing more abundant in the adult testis than in the newborn and juvenile (ages 5–15 days) testis. Intriguingly, the importins that are most structurally related differ most markedly in their sites of cellular synthesis in the adult testis (Figure 1c). For example, the importin α1 mRNA signal is detected in spermatogonia and spermatocytes by in situ hybridization, whereas importin α6 is observed only in round spermatids. Knowledge of how importin gene transcription is controlled by growth factors and hormonal signaling is lacking, including in the context of spermatogenesis.
These distinct patterns of transcript production manifest as differences in protein abundance and are accompanied by distinct patterns of subcellular localization reported for the importin α proteins for which antibodies are available to perform immunolocalization. Although they exhibit an overall sequence similarity of 80%, importin α3 appears as exclusively cytoplasmic in developing germ cells, while importin α4 is nuclear in spermatocytes and in round and elongating spermatids, as observed using immunohistochemistry19,36,37 (Figure 1c). As stated earlier, importin α proteins are typically cytoplasmic to bind their cargo molecules, and their residence in the nucleus is considered transient. The apparent sequestration of importin α4 within the germ cell nucleus suggests a distinct function for this protein that will be discussed below.
Downregulation of importin α2 levels in embryonic stem cells is required for their differentiation into a neural lineage.13 This occurs through the release of Oct6 from cytoplasmic retention mediated by its binding to the importin α2 C-terminus (Figure 1bii). We have recently discovered that a reduction of importin α2 levels is also a feature of male germline differentiation. In the adult mouse testis, importin α2 protein is most readily observed in the cytoplasm and nucleus of spermatocytes and in the cytoplasm of elongating spermatids, while the cytoplasm of round spermatids exhibits a distinctly weaker signal.19 This qualitative indication that importin α2 levels are reduced during the transition of male germ cells from meiosis into the haploid state was reinforced when direct measurements were made in isolated rat germ cells. The mass of importin α2 per cell was substantially lower (~2.5-fold) in haploid spermatids compared with pachytene spermatocytes, while the levels of importin α3 (1.5-fold lower), importin α4 (unchanged) and importin β1 (1.25-fold higher) varied less.32
The differences in importin subtype levels and subcellular localizations are consistent with our understanding that importins serve many functions other than nucleocytoplasmic transport. Our measurements of importin α and β1 levels revealed that the stoichiometry of the importin α:β molecular ratio is far from being 1:1, as would be required for classical nuclear import roles. Nuclear transport by importin β1 alone has also been documented15 and this can be inhibited by importin α protein, in the case of the Snail transcription factor.40 The levels of importins α2, α3 and α4 are ~6-fold higher in spermatocytes and ~3-fold higher in spermatids than those of importin β1. This provides a clear indication that importin α proteins function independently of β1 and provide impetus for studies that could reveal such roles, including those outlined below.
FINDING IMPORTIN CARGOES
The “α-importome”
To determine how and where importin α proteins influence male germline fate, it has been critical to delineate the “α-importome”. Summarized in Figure 2a, our studies have employed several distinct unbiased screening approaches to interrogate either whole rodent testes or isolated germ cells (spermatocytes and round spermatids). A yeast two-hybrid (Y2H) screen for importin α2 binding partners in the adult mouse testis identified and validated several nuclear proteins.41 These include Homologous protein 2 (Hop2), which is essential for fertility because of its role in promoting pairing between homologous chromosomes during meiosis. Two other binding partners were visualized as distinct nuclear speckles: androgen receptor interacting protein 3 (Arip3) was identified in promyelocytic leukemia (PML) bodies and was associated with SUMO1, while the Cysteine and histidine rich protein (Chrp) variant t4 colocalized with the U1snRNP in the spermatocyte model GC2 cell line, thereby indicating a possible role in RNA processing in male germ cells.41 A Y2H screen of the fetal mouse testis identified an additional importin α2 binding partner that is integral to nuclear paraspeckles.42
Figure 2.
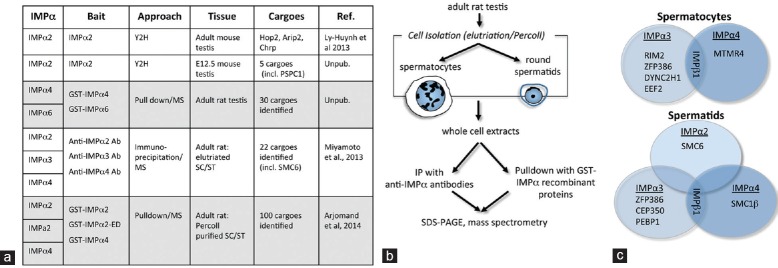
Identification of importin-binding proteins of relevance to spermatogenic differentiation. (a) Table outlining the different approaches used to identify IMPα-specific cargoes. Yeast 2 hybrid (Y2H) screening was used to identify over five specific, validated IMPα2 cargoes. Around 130 protein cargoes were identified using recombinant IMPα proteins tagged with GST in pulldown assays from lysates of whole adult rat testis or from Percoll-purified spermatocytes (SC) and spermatids (ST). An antibody-based immunoprecipitation (IP) approach, followed by mass spectrometry (MS) led to identification of up to 22 proteins specific for the different IMPαs. (b) Schematic of isolation processes leading to identification of cargoes from isolated germ cell subtypes. The most comprehensive studies to date have used pulldown assays with GST-conjugated recombinant IMPα proteins or immunoprecipitation from isolated spermatocyte or round spermatid lysates, followed by cargo identification through mass spectrometry.19,32 Around 130 IMPα binding proteins were identified using this approach. (c) Immunoprecipitation from spermatocytes and spermatids reveals different cargoes bound by each IMPa protein at specific stages of spermatogenesis.19 Examples listed include both nuclear and non-nuclear proteins. IMPα3 and IMPα4 both bind to IMPβ in spermatids and in spermatocytes, as expected, but each binds cargo subsets that are distinct in each germ cell type. This approach also identified common IMPα-specific cargoes: for example ZFP386 was identified as an IMPα3 cargo in both spermatocyte and spermatid lysates.
More extensive analyses have incorporated immunoprecipitation or pulldown studies with recombinant importin α proteins using whole adult rat testes or isolated rat spermatocytes and round spermatids followed by mass spectrometry (Figure 2b). These approaches identified over 150 proteins with diverse structures and functions. In addition, known importin α interactors such as importin β1, and a high proportion of nuclear proteins involved in meiosis, transcriptional regulation and RNA metabolism were identified, including the Structural maintenance of chromosomes 6-like 1 (SMC6; Figure 2c), Structural maintenance of chromosomes 1β (SMC1β), Centrosomal protein 350 kDa (CEP350), and the Y box binding proteins 1 and 2.19,32 Interrogation of 100 candidate importin α binding partners for the presence of a consensus importin α binding sequence (i.e., a monopartite or bipartite NLS) using the cNLS Mapper program based on yeast-derived sequence motifs43 indicated that many do not contain an obvious stretch of basic amino acids that could constitute a canonical importin α binding motif.32 This highlighted two obvious possibilities: (1) that their binding to importin α could be indirect and/or (2) that a distinct structural configuration–perhaps context-dependent–would be required for binding between an importin α protein and a particular cargo molecule. A better understanding of how individual importin αs interact with binding partners is required, particularly in the mammalian system, and this is an area of intense interest.20,21,44 The information in our studies of spermatogenesis may provide a useful starting point, as indicated below.
Many of the importin binding partners we identified are not nuclear proteins, including Dynein cytoplasmic 2 heavy chain 1 (DYNC2H1), Myotubularin-related protein 4 (MTMR4) and Phosphatidylethanolamine binding protein 1 (PEBP1).19,32 It is predominantly from studies of somatic cells that we have come to understand the diversity of their cytoplasmic localizations, such as in the endoplasmic reticulum and at the plasma membrane, and the range of their functions, including cilia formation, protein sorting in the endoplasmic reticulum and modulation of intracellular signaling. A few become highly localized to a specific cytoplasmic site during the course of spermatogenesis. One candidate binding partner has been implicated in exocytotic functions at the neural synapse as well as in the sperm acrosome. Regulating synaptic membrane exocytosis 2 (RIM2), identified as binding to importin α in isolated rat germ cells19 (Figure 2c), is involved in the membrane docking events required for human sperm acrosomal exocytosis through its interactions with Rab3 and MUNC1345 and is considered to itself be a scaffolding protein.
Other importin binding proteins in developing germline cells
Some other studies have identified intriguing roles for importins in governing sperm formation. The SubH2Bv histone variant protein is localized on the acrosome during rodent and bovine spermatogenesis and–although it contains a bipartite NLS motif–it does not enter the nucleus in germ cells.46 Instead, this sequence, which is sufficient to enable nuclear accumulation of overexpressed SubH2Bv in somatic cells, mediates its binding to importin α6. Localization of SubH2Bv to the subacrosomal perinuclear theca has been proposed to mediate expansion of the developing acrosomal cap across the nucleus, with importin α6 providing the key physical link between the acrosome and nuclear envelope. Using immunolocalization in murine and bovine testes sections, Tran et al. showed that importin α6 is present on the outer surface of acrosomal vesicles in early steps of spermiogenesis, but moves progressively to the region between the inner acrosomal membrane and nuclear envelope.46 This implicates importin α6 in trafficking of acrosomal vesicles from the Golgi to the nuclear envelope during spermiogenesis and is consistent with outcomes from our importin α binding partner screenings that identified several proteins associated with vesicle trafficking.19,32
Importin 4 is an importin β family member identified as functioning during spermatid nucleus reorganization. Its transcript is highly expressed in spermatocytes and spermatids, and the protein has been implicated as contributing to the movement of the transition protein 2 (TP2) into the nucleus.47 Whether any of the other germline-specific histone variants selectively associate with importin proteins remains to be established, and it will be informative to determine the contributions of individual importin proteins to key processes in spermiogenesis such as formation of the species-specific shape of the nucleus and acrosomal vesicle. We have much to learn about how transport of materials required for DNA packaging and repackaging during meiosis and spermiogenesis is accomplished through a nuclear membrane that is itself undergoing major structural modifications.4,5,6
DISCOVERING HOW IMPORTINS CONTRIBUTE TO CELLULAR PHYSIOLOGY IN MAMMALIAN SPERMATOGENESIS
Nuclear roles for importins in cellular stress during gametogenesis
A crucial feature enabling importins to function in nucleocytoplasmic transport is their relocation to the cytoplasm after binding a cargo molecule and releasing it into the nucleus (Figure 1a). This normally occurs because a high Ran-GTP: RanGDP gradient between the nucleus and cytoplasm is maintained by cytoplasmic RanGAP1/RanBP1 and nuclear-localized RCC1.16 This gradient is lost following exposure of cells to stressors such as H2O2, ultraviolet irradiation or heat shock, and importin α proteins accumulate in the nucleus (Figure 3a).26 We recently identified that one functional outcome of this unusual localization of importin α is altered transcription of a discrete subset of genes, including the downregulation those encoding several replication-dependent histones.25 Given that both importin α2 and α4 are nuclear-localized in postmitotic germ cells, with importin α4 appearing to be exclusively nuclear, we considered that this unusual location might relate to the need for germ cells to manage oxidative and other stressors during their development within the seminiferous epithelium. Through analysis of two unique mouse models (Figure 3b), we obtained evidence that importin α4 levels directly and selectively determine the capacity for spermatids to resist oxidative stress.27 Haploid germ cells from transgenic male mice overexpressing importin α4 had a greater resistance (>30%) to transient H2O2 exposure, while a higher proportion (~30%) of spermatids exhibited decreased viability from importin α4 gene knockout mice compared with their littermate controls. The mechanisms underpinning this outcome remain to be determined, but we propose that genes that are downstream targets of nuclear importin α proteins in spermatogenesis are crucial for enabling male germline cells to recover from exposure to stress.
Figure 3.
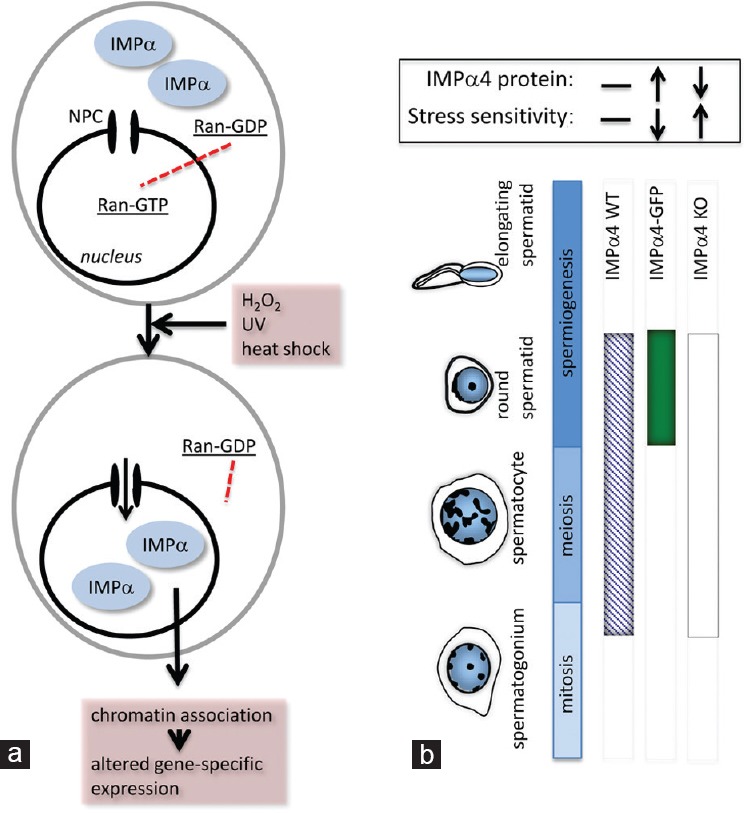
Importin function in cellular stress. (a) There is a well-defined association between changes in importin protein localization and function and the responses of cells to stress. In cultured cells, exposure to stresses that include oxidative stress (H2O2), ultraviolet light (UV) and heat shock, leads to rapid IMPα nuclear accumulation. This altered IMPα subcellular localization is considered to result from the collapsing RanGTP–GDP gradient, which results from the stress-induced decrease in cellular ATP levels. In the absence of high nuclear RanGTP, IMPα-mediated nuclear export and import halt. Significantly, these nuclear-located IMPa proteins associate strongly with chromatin, and are thought to directly induce specific alterations in gene expression.25,26 (b) Examination of mouse models with altered importin expression revealed a role for IMPα4 protein in haploid germ cell stress responses. Three mouse strains were compared to assess this: wild type (IMPα4 WT; presence of IMPα4 protein indicated as a blue hatched band), transgenic mice overexpressing IMPα4 with enhanced green fluorescent protein (EGFP) under the protamine promoter for exclusive expression in post-meiotic germ cells (IMPα4-EGFP; solid green band), and an IMPα4 complete knockout line (IMPα4-KO; white band). When testicular germ cells were exposed to high levels of oxidative stress through H2O2, the haploid germ cells–which express IMPα4-EGFP– exhibited increased resistance to stress-induced cell death. Conversely, the absence of IMPα4 led to increased sensitivity in this population. This indicates that IMPα4 aids in protecting the male germline against stress.27
Discovery of the iCBS – a new binding domain implicated in cell fate control
Our quest to delineate the putative spermatogenic α-importome described above highlighted the need to better understand the nature of the binding between importin α proteins and their nuclear and cytoplasmic cargoes. A short acidic domain in the importin α2 C-terminus was recently identified as a binding site for the neural lineage transcription factor, Oct6 (Figure 1bii). This interaction mediates Oct6 cytoplasmic retention in undifferentiated embryonic stem cells, with downregulation of importin α2 synthesis, enabling Oct6 nuclear translocation via binding to importin α1 in the conventional major binding groove.13 Having measured a 2.5-fold drop in importin α2 levels in germ cells progressing from meiosis into the haploid state, we searched our list of 100 candidate binding partners for sequences possibly mediating binding to the importin α2 C-terminus. We successfully identified and confirmed a motif present in two of the proteins, Smarca4 and Setx, which could mediate binding to this region which we have termed the iCBS, or importin α C-terminal binding segment32 iCBS is shown in Figure 1bii. This information and related approaches will enable further refinement of our understanding of how cell fate changes are effected through changes to intracellular levels of individual importins.
Domains within spermatozoa
As highlighted in previous sections, there is increasing evidence that importins contribute to the formation of distinct subcellular domains in both the nucleus and cytoplasm. In addition to the discovery that importin α6 is associated with the developing acrosome, our preliminary indirect immunofluorescence analyses suggest that each importin localizes to a discrete region in epididymal mouse spermatozoa (Figure 4a), and several independent proteomic analyses have shown that importins are present in post-testicular germ cells (e.g.,48). It remains to be established whether importins play active roles in delivering proteins to the correct domain, whether they merely restrict their movements between different compartments by binding to them, and whether they are integral to the formation of intracellular complex formations by serving as molecular scaffolds.
Figure 4.
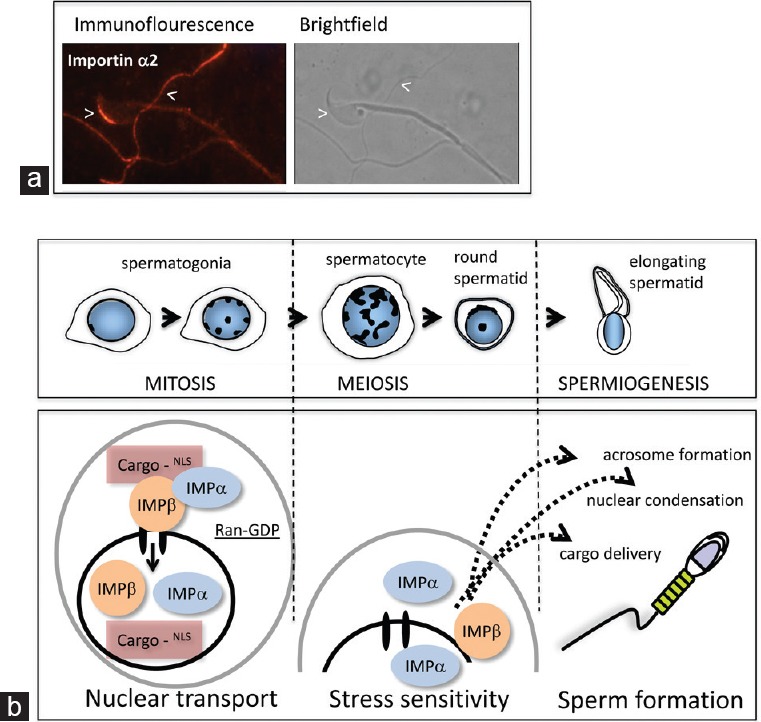
Importins contribute to many stages of mammalian sperm formation. (a) Importin α2 protein is present in mature mouse spermatozoa. Detection of importin α2 in sperm isolated from the mouse cauda epididymis using direct immunofluorescence revealed that it is localized predominantly to the anterior region of the acrosome, with additional signals detected most readily in the principle piece of the tail. (b) Schematic illustration of phases of spermatogenesis in which distinct roles for importin proteins have been identified or postulated through the studies outlined in this review. The established role in nucleocytoplasmic transport is likely to function at all stages. The presence of nuclear-localized importins α2 and α4 suggests that they have a role in physiological processes related to cellular stress behaviors in post-mitotic male germline cells. The contribution of importins to formation of the acrosome and other subcellular domains in the highly organized mature spermatid is evident from published and ongoing work.
APPLICATIONS OF THIS KNOWLEDGE TO IMPROVING HUMAN HEALTH AND FERTILITY
The highly conserved structures and functional roles of importins led us to investigate their expression profiles in the adult human testis, where patterns similar to those documented in rodents were observed.36 In addition, we documented that the nuclear localization of human importin α3 (termed importin α4 in rodent nomenclature) in spermatocytes occurs when exportin-1, also known as XPO1 and CRM1, is cytoplasmically located, providing evidence that nuclear transport processes are significantly different during meiosis. The nuclear export protein, exportin-1 controls the intracellular localization of over 200 proteins containing a nuclear export signal, and amongst these are tumor suppressors such as p21. In the nucleus, p21 acts predominantly to restrict cell cycle progression; when in the cytoplasm, it can inhibit apoptosis. This latter situation, in which exportin-1 is upregulated, is linked with a variety of cancers, and the development of small molecule inhibitors of exportin-1 is considered as an important therapeutic goal for treatment of kidney, pancreatic and cervical cancers.49,50 In the developing mouse testis, the exportin-1 transcript is highest in spermatogonia and in the juvenile testis and is substantially lower in meiotic and haploid germ cells.16 Thus, the relative amount of exportin-1 is dynamic during germ cell maturation, and identification of its cargoes using approaches employed for importin α proteins might reveal key elements of progression through differentiation. The existence of additional mechanisms for protein nuclear export in developing spermatozoa remains to be explored.
Functional studies of human germline maturation are problematic because of ethical considerations that limit access to material and the technical challenge of maintaining germ cells in vitro. However, it remains highly desirable to identify what underlies the high frequency of male infertility that is of unknown etiology. The disruption to gametogenesis documented for worms, flies and mice when individual importins are absent51 indicates that mutations affecting importin expressions or functions during gametogenesis will be detrimental to male fertility. Elucidation of the physiological roles of nuclear-localized importin α proteins in normal cells will aid our understanding of why they also exhibit upregulation and nuclear accumulation in certain neoplastic cells, as has been extensively documented for importin α2.52 Our studies should provide a foundation for better understanding of what goes awry when sperm are malformed, have impaired motility, cannot undergo an appropriate acrosome reaction or do not recognize the oocyte to achieve fertilization. Knowledge from studies of spermatogenesis that address how hormonal and growth factor signaling modulates importin α protein levels will yield great opportunities for controlling cancer and manipulating cell fate in vitro.
ACKNOWLEDGMENTS
The work described in this manuscript received research project and fellowship support (COE#348239 and #DPO878102) and fellowship support from the NHMRC (ID 545916 and ID 334013).
COMPETING FINANCIAL INTERESTS
The authors have no competing interests to declare.
REFERENCES
- 1.Kerr JB, Loveland KL, O’Bryan MK, de Kretser DM. Cytology of the testis and intrinsic control mechanisms. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. USA: Elsevier Science; 2006. pp. 827–94. [Google Scholar]
- 2.Ewen KA, Koopman P. Mouse germ cell development: from specification to sex determination. Mol Cell Endocrinol. 2010;323:76–93. doi: 10.1016/j.mce.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–7. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho HC. Redistribution of nuclear pores during formation of the redundant nuclear envelope in mouse spermatids. J Anat. 2010;216:525–32. doi: 10.1111/j.1469-7580.2009.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Govin J, Caron C, Lestrat C, Rousseaux S, Khochbin S. The role of histones in chromatin remodelling during mammalian spermiogenesis. Eur J Biochem. 2004;271:3459–69. doi: 10.1111/j.1432-1033.2004.04266.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction. 2010;139:287–301. doi: 10.1530/REP-09-0281. [DOI] [PubMed] [Google Scholar]
- 7.Barakat B, Itman C, Mendis SH, Loveland KL. Activins and inhibins in mammalian testis development: new models, new insights. Mol Cell Endocrinol. 2012;359:66–77. doi: 10.1016/j.mce.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today. 2009;87:1–26. doi: 10.1002/bdrc.20142. [DOI] [PubMed] [Google Scholar]
- 9.Song HW, Wilkinson MF. Transcriptional control of spermatogonial maintenance and differentiation. Semin Cell Dev Biol. 2014;30:14–26. doi: 10.1016/j.semcdb.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92:577–95. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogarth C, Itman C, Jans DA, Loveland KL. Regulated nucleocytoplasmic transport in spermatogenesis: a driver of cellular differentiation? Bioessays. 2005;27:1011–25. doi: 10.1002/bies.20289. [DOI] [PubMed] [Google Scholar]
- 12.Young JC, Major AT, Miyamoto Y, Loveland KL, Jans DA. Distinct effects of importin α2 and α4 on Oct3/4 localization and expression in mouse embryonic stem cells. FASEB J. 2011;25:3958–65. doi: 10.1096/fj.10-176941. [DOI] [PubMed] [Google Scholar]
- 13.Yasuhara N, Yamagishi R, Arai Y, Mehmood R, Kimoto C, et al. Importin alpha subtypes determine differential transcription factor localization in embryonic stem cells maintenance. Dev Cell. 2013;26:123–35. doi: 10.1016/j.devcel.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Yasuhara N, Shibazaki N, Tanaka S, Nagai M, Kamikawa Y, et al. Triggering neural differentiation of ES cells by subtype switching of importin-alpha. Nat Cell Biol. 2007;9:72–9. doi: 10.1038/ncb1521. [DOI] [PubMed] [Google Scholar]
- 15.Kimura M, Imamoto N. Biological significance of the importin-ß family-dependent nucleocytoplasmic transport pathways. Traffic. 2014;15:727–48. doi: 10.1111/tra.12174. [DOI] [PubMed] [Google Scholar]
- 16.Major AT, Whiley PA, Loveland KL. Expression of nucleocytoplasmic transport machinery: clues to regulation of spermatogenic development. Biochim Biophys Acta. 2011;1813:1668–88. doi: 10.1016/j.bbamcr.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Wente SR, Rout MP. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol. 2010;2:a000562. doi: 10.1101/cshperspect.a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran EJ, King MC, Corbett AH. Macromolecular transport between the nucleus and the cytoplasm: advances in mechanism and emerging links to disease. Biochim Biophys Acta. 2014;1843:2784–95. doi: 10.1016/j.bbamcr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto Y, Baker MA, Whiley PA, Arjomand A, Ludeman J, et al. Towards delineation of a developmental α-importome in the mammalian male germline. Biochim Biophys Acta. 2013;1833:731–42. doi: 10.1016/j.bbamcr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Lott K, Cingolani G. The importin ß binding domain as a master regulator of nucleocytoplasmic transport. Biochim Biophys Acta. 2011;1813:1578–92. doi: 10.1016/j.bbamcr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marfori M, Mynott A, Ellis JJ, Mehdi AM, Saunders NF, et al. Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim Biophys Acta. 2011;1813:1562–77. doi: 10.1016/j.bbamcr.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Kappel C, Zachariae U, Dölker N, Grubmüller H. An unusual hydrophobic core confers extreme flexibility to HEAT repeat proteins. Biophys J. 2010;99:1596–603. doi: 10.1016/j.bpj.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forwood JK, Lange A, Zachariae U, Marfori M, Preast C, et al. Quantitative structural analysis of importin-ß flexibility: paradigm for solenoid protein structures. Structure. 2010;18:1171–83. doi: 10.1016/j.str.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto Y, Loveland KL, Yoneda Y. Nuclear importin α and its physiological importance. Commun Integr Biol. 2012;5:220–2. doi: 10.4161/cib.19194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasuda Y, Miyamoto Y, Yamashiro T, Asally M, Masui A, et al. Nuclear retention of importin α coordinates cell fate through changes in gene expression. EMBO J. 2012;31:83–94. doi: 10.1038/emboj.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto Y, Saiwaki T, Yamashita J, Yasuda Y, Kotera I, et al. Cellular stresses induce the nuclear accumulation of importin alpha and cause a conventional nuclear import block. J Cell Biol. 2004;165:617–23. doi: 10.1083/jcb.200312008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young JC, Ly-Huynh JD, Lescesen H, Miyamoto Y, Browne C, et al. The nuclear import factor importin α4 can protect against oxidative stress. Biochim Biophys Acta. 2013;1833:2348–56. doi: 10.1016/j.bbamcr.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Hachet V, Köcher T, Wilm M, Mattaj IW. Importin alpha associates with membranes and participates in nuclear envelope assembly in vitro . EMBO J. 2004;23:1526–35. doi: 10.1038/sj.emboj.7600154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schatz CA, Santarella R, Hoenger A, Karsenti E, Mattaj IW, et al. Importin alpha-regulated nucleation of microtubules by TPX2. EMBO J. 2003;22:2060–70. doi: 10.1093/emboj/cdg195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adam SA, Sengupta K, Goldman RD. Regulation of nuclear lamin polymerization by importin alpha. J Biol Chem. 2008;283:8462–8. doi: 10.1074/jbc.M709572200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedrich B, Quensel C, Sommer T, Hartmann E, Köhler M. Nuclear localization signal and protein context both mediate importin alpha specificity of nuclear import substrates. Mol Cell Biol. 2006;26:8697–709. doi: 10.1128/MCB.00708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arjomand A, Baker MA, Li C, Buckle AM, Jans DA, et al. The α-importome of mammalian germ cell maturation provides novel insights for importin biology. FASEB J. 2014;28:3480–93. doi: 10.1096/fj.13-244913. [DOI] [PubMed] [Google Scholar]
- 33.Hall MN, Griffin CA, Simionescu A, Corbett AH, Pavlath GK. Distinct roles for classical nuclear import receptors in the growth of multinucleated muscle cells. Dev Biol. 2011;357:248–58. doi: 10.1016/j.ydbio.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loveland KL, Hogarth C, Szczepny A, Prabhu SM, Jans DA. Expression of nuclear transport importins beta 1 and beta 3 is regulated during rodent spermatogenesis. Biol Reprod. 2006;74:67–74. doi: 10.1095/biolreprod.105.042341. [DOI] [PubMed] [Google Scholar]
- 35.Hogarth CA, Calanni S, Jans DA, Loveland KL. Importin alpha mRNAs have distinct expression profiles during spermatogenesis. Dev Dyn. 2006;235:253–62. doi: 10.1002/dvdy.20569. [DOI] [PubMed] [Google Scholar]
- 36.Whiley PA, Miyamoto Y, McLachlan RI, Jans DA, Loveland KL. Changing subcellular localization of nuclear transport factors during human spermatogenesis. Int J Androl. 2012;35:158–69. doi: 10.1111/j.1365-2605.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- 37.Hogarth CA, Jans DA, Loveland KL. Subcellular distribution of importins correlates with germ cell maturation. Dev Dyn. 2007;236:2311–20. doi: 10.1002/dvdy.21238. [DOI] [PubMed] [Google Scholar]
- 38.Anway MD, Li Y, Ravindranath N, Dym M, Griswold MD. Expression of testicular germ cell genes identified by differential display analysis. J Androl. 2003;24:173–84. doi: 10.1002/j.1939-4640.2003.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi YL, Tanaka SS, Yasuda K, Matsui Y, Tam PP. Stage-specific Importin13 activity influences meiosis of germ cells in the mouse. Dev Biol. 2006;297:350–60. doi: 10.1016/j.ydbio.2006.04.465. [DOI] [PubMed] [Google Scholar]
- 40.Sekimoto T, Miyamoto Y, Arai S, Yoneda Y. Importin alpha protein acts as a negative regulator for Snail protein nuclear import. J Biol Chem. 2011;286:15126–31. doi: 10.1074/jbc.M110.213579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ly-Huynh JD, Lieu KG, Major AT, Whiley PA, Holt JE, et al. Importin alpha2-interacting proteins with nuclear roles during mammalian spermatogenesis. Biol Reprod. 2011;85:1191–202. doi: 10.1095/biolreprod.111.091686. [DOI] [PubMed] [Google Scholar]
- 42.Major AT, Hogarth CA, Miyamoto Y, Sarraj MA, Smith CL, et al. Specific interaction with the nuclear transporter importin alpha 2 can modulate paraspeckle protein 1 delivery to nuclear paraspeckles. Mol Biol Cell. 2015;26:1543–58. doi: 10.1091/mbc.E14-01-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kosugi S, Hasebe M, Tomita M, Yanagawa H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc Natl Acad Sci U S A. 2009;106:10171–6. doi: 10.1073/pnas.0900604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chook YM, Süel KE. Nuclear import by karyopherin-ßs: recognition and inhibition. Biochim Biophys Acta. 2011;1813:1593–606. doi: 10.1016/j.bbamcr.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bello OD, Zanetti MN, Mayorga LS, Michaut MA. RIM, Munc13, and Rab3A interplay in acrosomal exocytosis. Exp Cell Res. 2012;318:478–88. doi: 10.1016/j.yexcr.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran MH, Aul RB, Xu W, van der Hoorn FA, Oko R. Involvement of classical bipartite/karyopherin nuclear import pathway components in acrosomal trafficking and assembly during bovine and murid spermiogenesis. Biol Reprod. 2012;86:84. doi: 10.1095/biolreprod.111.096842. [DOI] [PubMed] [Google Scholar]
- 47.Pradeepa MM, Manjunatha S, Sathish V, Agrawal S, Rao MR. Involvement of importin-4 in the transport of transition protein 2 into the spermatid nucleus. Mol Cell Biol. 2008;28:4331–41. doi: 10.1128/MCB.00519-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang G, Guo Y, Zhou T, Shi X, Yu J, et al. In-depth proteomic analysis of the human sperm reveals complex protein compositions. J Proteomics. 2013;79:114–22. doi: 10.1016/j.jprot.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Wettersten HI, Landesman Y, Friedlander S, Shacham S, Kauffman M, et al. Specific inhibition of the nuclear exporter exportin-1 attenuates kidney cancer growth. PLoS One. 2014;9:e113867. doi: 10.1371/journal.pone.0113867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Senapedis WT, Baloglu E, Landesman Y. Clinical translation of nuclear export inhibitors in cancer. Semin Cancer Biol. 2014;27:74–86. doi: 10.1016/j.semcancer.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto Y, Boag PR, Hime GR, Loveland KL. Regulated nucleocytoplasmic transport during gametogenesis. Biochim Biophys Acta. 2012;1819:616–30. doi: 10.1016/j.bbagrm.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 52.Christiansen A, Dyrskjøt L. The functional role of the novel biomarker karyopherin α 2 (KPNA2) in cancer. Cancer Lett. 2013;331:18–23. doi: 10.1016/j.canlet.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


