Abstract
Biomarker-based sperm analysis elevates the treatment of human infertility and ameliorates reproductive performance in livestock. The negative biomarker-based approach focuses on proteins and ligands unique to defective spermatozoa, regardless of their morphological phenotype, lending itself to analysis by flow cytometry (FC). A prime example is the spermatid specific thioredoxin SPTRX3/TXNDC8, retained in the nuclear vacuoles and superfluous cytoplasm of defective human spermatozoa. Infertile couples with high semen SPTRX3 are less likely to conceive by assisted reproductive therapies (ART) and more prone to recurrent miscarriage while low SPTRX3 has been associated with multiple ART births. Ubiquitin, a small, proteolysis-promoting covalent posttranslational protein modifier is found on the surface of defective posttesticular spermatozoa and in the damaged protein aggregates, the aggresomes of spermiogenic origin. Semen ubiquitin content correlates negatively with fertility and conventional semen parameters, and with sperm binding of lectins LCA (Lens culinaris agglutinin; reveals altered sperm surface) and PNA (Arachis hypogaea/peanut agglutinin; reveals acrosomal malformation or damage). The Postacrosomal Sheath WWI Domain Binding Protein (PAWP), implicated in oocyte activation during fertilization, is ectopic or absent from defective human and animal spermatozoa. Consequently, FC-parameters of PAWP correlate with ART outcomes in infertile couples and with fertility in bulls. Assays based on the above biomarkers have been combined into multiplex FC semen screening protocols, and the surface expression of lectins and ubiquitin has been utilized to develop nanoparticle-based bull semen purification method validated by field artificial insemination trials. These advances go hand-in-hand with the innovation of FC-technology and genomics/proteomics-based biomarker discovery.
Keywords: artificial insemination, biomarker, fertility, fertilization, flow cytometry, infertility, nanotechnology, oocyte activation, Postacrosomal Sheath WWI Domain Binding Protein, sperm, SPTRX3, thioredoxin, ubiquitin
INTRODUCTION
Conventional, light microscopy-based semen analysis provides a useful baseline of information on sperm count, motility and morphology of semen samples collected to perform human assisted reproductive therapy (ART), reproduce livestock by artificial insemination (AI), or evaluate male fertility of model animals used in biomedical research and toxicology such as rodents and nonhuman primates. However, due to their limitation to obvious morphological and physiological sperm defects visible at the relatively low magnification and resolution of a conventional light microscope, only a limited degree of correlation is observed between conventional semen parameters and sperm phenotypes and the actual fertility of an individual. This gap is even more evident when only one or few collections are used to evaluate the fertility of an individual, be a suspected infertile man or a production animal used for agricultural production.
The first step beyond conventional andrological evaluation coincided with the adaptation of innovative morphometric and fluorometric instruments into human and animal andrology. Computer-assisted semen analysis (CASA) and related Integrated Visual Optical System technology have allowed for highly accurate analysis of sperm count, morphometry, and motility,1 and are currently being endowed with capabilities to measure molecular-level sperm defects such as reduced sperm viability and abnormal chromatin packaging associated with sperm DNA fragmentation (SCA CASA; http://www.micropticsl.com/). The andrologist's tool box has been greatly expanded by the development of flow cytometry (FC) based semen analysis and concomitant acceleration of the research and development efforts in the area of fluorescent probes. In its initial stage, FC-based analysis multiplied the throughput, speed, sensibility, replicability, accuracy and informative value of semen analysis by introducing probes for sperm viability, acrosomal integrity and mitochondrial membrane potential, and probes sensing sperm DNA damage, ATP production and capacitation state.2,3,4,5,6 The next logical step in the expansion and dissemination of automated semen analysis is the validation of the ever increasing arsenal associated with normal or deviant sperm function, structure and molecular make-up.
Biomarker based sperm quality evaluation adds value to conventional and computer-aided semen analysis for the treatment of human male infertility and amelioration of male reproductive performance in livestock species. The “negative” biomarker-based approach to andrological evaluation focuses on proteins and lectin ligands that are detectable predominantly or exclusively in defective spermatozoa. Consequently, such biomarkers are considered “negative” indicators of normal sperm morphology and function, and as such have been associated with reduced male fertility or complete sterility.7 On the flip side of this approach, biomarkers known to be present in phenotypically normal, morphologically intact, viable, progressively motile spermatozoa may be down-regulated, ectopically expressed, posttranslationally modified or completely missing from defective spermatozoa.8 Contrary to conventional light microscopic semen analysis, the negative biomarker approach detects sperm defects at the molecular level, regardless of whether or not they are manifested in a visible morphological phenotype or diminished sperm motility.7 Such analysis lends itself to automated, objective testing by FC, correlates with field AI fertility in livestock and reflects the outcomes of ART in infertile human couples. The present review is focused on three major sperm proteins, SPTRX3/TXNDC8, ubiquitin, and Postacrosomal Sheath WWI Domain Binding Protein (PAWP) (Figure 1), recently validated as negative biomarkers of human and/or animal male fertility by a combination of laboratory and epidemiological approaches.
Figure 1.
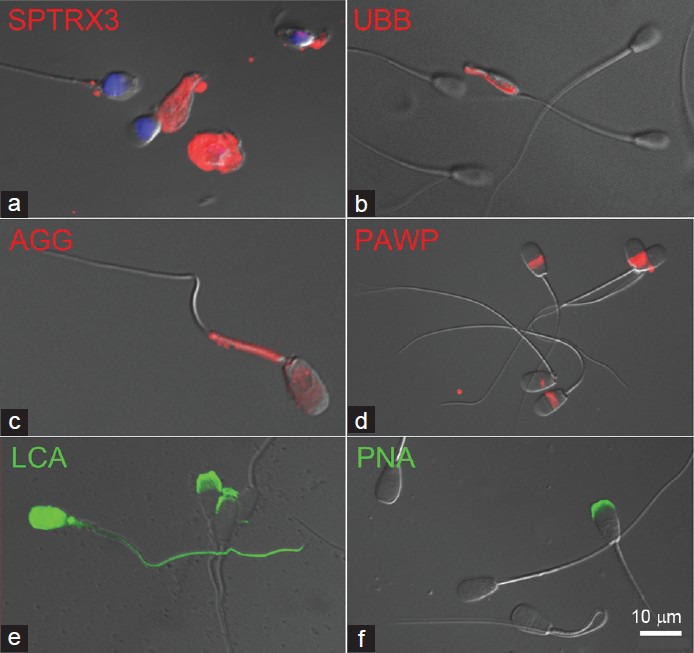
Fluorescent labeling of negative sperm quality biomarkers in human and animal spermatozoa. (a) Testis-specific thioredoxin SPTRX3 (red) is retained in the superfluous cytoplasm of defective human spermatozoa. (b) Ubiquitin (red) coats the surface of a defective bull spermatozoon with its flagellum coiled around the head but is undetectable in morphologically normal spermatozoa. (c) Aggresomes (red) are stress-induced aggregates of ubiquitinated proteins, here detected by ProteoStat kit in the mitochondrial sheath and head of a defective boar spermatozoon. (d) Sperm head postacrosomal protein PAWP (red) is detected at varied intensities in bull spermatozoa. (e) Lectin LCA (green) binds exclusively to the acrosomes of phenotypically normal bull spermatozoa but to the whole head and tail surface in the defective ones (segmental aplasia of the mitochondrial sheath is shown). (f) Lectin PNA binds to damaged/ruffled acrosomes, but not the intact ones in live bull spermatozoa. DNA in panel (a) was counterstained blue with DAPI. Epifluorescence micrographs are superimposed over parfocal images taken with DIC optics. PAWP: postacrosomal Sheath WWI Domain Binding Protein; LCA: Lens culinaris agglutinin; PNA: peanut agglutinin; DIC: differential interference contrast.
THIOREDOXINS AND PEROXIREDOXINS
A prime example of a negative sperm quality biomarker in humans is the spermatid specific thioredoxin 3 (SPTRX3/TXNDC8), present in testicular round spermatids of most mammalian species studied, but retained exclusively in the nuclear vacuoles and superfluous midpiece cytoplasm of defective human spermatozoa.9 Redoxin research and redox regulation have become increasingly important to many aspects of sperm biology.10 In most organisms, redox homeostasis is maintained by the thioredoxin and glutathione/glutaredoxin systems.11 Interestingly, in addition to the more ubiquitous members, mammals possess a number of proteins belonging to these two redox systems specifically or preferentially expressed during spermatogenesis and/or in mature spermatozoa. Indeed, the thioredoxin system is particularly enriched in proteins expressed in the male reproductive system. Closely related to SPTRX3, two other sperm-specific thioredoxins, SPTRX1/TXNDC212 and SPTRX2/TXNDC313 are found in close association with the sperm tail fibrous sheath. However, while SPTRX1 is expressed only during the assembly of the longitudinal columns of the fibrous sheath and is absent from mature spermatozoa,14 SPTRX2 is an integral component of both the transversal ribs and longitudinal columns of the sperm tail fibrous sheath, and remains present in the mature spermatozoa.15 This temporally and spatially restricted expression of SPTRX1 and SPTRX2 in a structural component of the spermatozoa tail anticipated an essential role of these two proteins in sperm motility and fertility. Surprisingly, mice harboring targeted inactivating mutations in both Sptrx1 and Sptrx2 genes are fully viable with no overt phenotypes regarding sperm function, except for an age-dependent increase in reactive oxygen species (ROS) generation and loss of motility.16 Another two thioredoxin proteins have been identified in mammalian spermatozoa. TXL2/TXNDC6 is a microtubule-binding protein that is found in the manchette and flagellar axoneme of developing spermatids and in the cilia in the lung epithelium.17 Finally, the aforementioned SPTRX3/TXNDC8 is first expressed in the Golgi apparatus of pachytene spermatocytes, and in round and elongating spermatids with a transient association to the developing acrosome.9
Importantly, several of the above sperm thioredoxins are involved in different pathologies relevant to male fertility. Hence, SPTRX2 and SPTRX3 have been found to be postobstructive sperm autoantigens in vasectomized rats9,15 and mutations in SPTRX2 have been reported to cause primary ciliary dyskinesia.18 Moreover, TXL2 is overexpressed in colon cancer and promotes tumor metastasis.19 Highly relevant to the topic of this review, SPTRX3 was found to be present in abnormal spermatozoa of infertile men.9 Using image-based ImageStream/FlowSight flow cytometry (www.amnis.com), we have shown that human spermatozoa that register high SPTRX3-induced fluorescence in a flow cytometric measurement are the ones retaining SPTRX3 in the nuclear vacuoles and superfluous cytoplasm surrounding the sperm tail connecting piece, both of which are considered defects in human andrology.20 A comprehensive study of 239 infertile couples from a general infertility clinic population was conducted to validate SPTRX3 as a diagnostic marker of human male infertility. The couples in which men displayed elevated semen content of SPTRX3 produced fewer normal zygotes by ART and had a significantly reduced chance of conceiving by ART. On the opposite end of the spectrum, men from couples that delivered twins after multiple embryo transfer had the lowest overall semen SPTRX3 levels.21 Follow-up studies are under way to determine if high semen levels of SPTRX3 predispose couples to recurrent miscarriage and whether couples with low SPTRX3 are more prone to multiple births after multiple embryo transfer. If such trends are confirmed, SPTRX3 based semen screening could be useful for unambiguous diagnosis of male infertility, treatment decision-making and management of established pregnancies after ART.
Thioredoxins are maintained in their reduced active form by thioredoxin reductases.22 In addition to the cytosolic and mitochondrial thioredoxin reductase enzymes, mammals have a thioredoxin/glutathione reductase (TGR/TXNRD3) composed of an N-terminal glutaredoxin domain followed by a thioredoxin reductase module.23 TGR is mainly expressed in elongating spermatids at the time when the mitochondrial sheath is formed but is later absent from mature spermatozoa. Consistent with this localization, TGR is found to interact with glutathione peroxidase GPX4,23 a “moonlight” enzyme that transitions from a soluble form in developing spermatids to an insoluble, enzymatically inactive form in mature spermatozoa where it becomes the major constituent of the mitochondrial capsule.24 Both TGR and GPX4 are selenoproteins, highlighting the important role of selenium in male fertility.25 Indeed, deletion of the mitochondrial form of GPX4 in mice causes male infertility due to impaired sperm quality and severe structural abnormalities of sperm midpiece.26 In addition to TGR and GPX4, a testis-specific isoform of glutaredoxin GRX2 is exclusively expressed in murine spermatids.27
Peroxiredoxins are another class of redox proteins that impact on mammalian sperm function by acting as hydrogen peroxide reductases, with thioredoxins as the electron donors.28,29 Peroxiredoxin 2 (PRDX2) was identified in boar and mouse spermatozoa,30 with localization patterns including acrosome, mitochondrial sheath, and cytoplasmic droplet. Among all peroxiredoxins, one isoform of PRDX4 has been found to be specifically expressed in mouse spermatids,31 and PPRDX4 knockout mice display increased cell death in spermatids caused by high levels of oxidative stress.32 Consistent with the role of peroxiredoxins as redox sensors,28,29 decreased levels, and a highly oxidized status of PRDX4 have been reported in the spermatozoa of infertile men.33 The related protein, PRDX1, was found at reduced levels in the seminal plasma of men with idiopathic asthenozoospermia, possibly contributing to the increased ROS levels and reduced sperm motility typical of this cohort of patients.34
UBIQUITIN AND UBIQUITIN-LIKE PROTEIN MODIFIERS
Ubiquitin is a small (76 amino acid residues) proteolysis-promoting posttranslational protein modifier. Ubiquitin employs a highly regulated enzymatic cascade termed ubiquitin-proteasome system (UPS) to bind covalently, in a tandem fashion, to outlived or otherwise damaged protein molecules destined for recycling by the 26S proteasome, a multi-subunit ubiquitin-specific protease complex.35 Defective bull spermatozoa and sperm fragments become ubiquitinated on their entire surface posttesticularly, during epididymal passage.36 The sperm ubiquitome, (i.e., the species cohort of proteins that are ubiquitinated in epididymal spermatozoa) changes between individual epididymal compartments, as described in the domestic cat.37 Ubiquitin as well as other components of the UPS are secreted by the principal epididymal epithelial cells (EECs).38 Such protein secretion occurs through a peculiar apocrine secretion mechanism involving the detachment and rupture of large segments of apical EEC cytoplasm, the apical blebs, that have the ability to sequester UPS components and other proteins lacking obvious secretory tags so they can be exported to the epididymal lumen and became incorporated in the epididymal fluid (EF).38,39 Proteasomes are present in the EF38,40 and the UPS-associated ubiquitin-activating, ubiquitin-conjugating, deubiquitinating and proteolytic activities are detectable by zymological and fluorometric assays.38 Furthermore, the turnover of epididymal proteins may be contributed by UPS in conjunction with protein aggregation and formation of amyloid structures in the EF,41 extending the concept of epididymal extracellular quality control from spermatozoa to proteins.42
Ubiquitinated bull spermatozoa display increased sperm DNA fragmentation detectable by TUNEL assay,43 as well as increased affinity for lentil agglutinin Lens culinaris agglutinin (LCA)44 and a lack of surface platelet activating factor receptor (PAFR),8 altogether suggestive of possible apoptotic and/or oxidative damage to sperm plasma membrane. Coating with lysine-rich ubiquitin may give the defective spermatozoa and sperm fragments a positive charge to promote their agglutination. Surface ubiquitination could at the same time protect the epididymis from innate autoimmune response induced by sperm-specific antigens no longer concealed by an intact plasma membrane, which could lead to autoimmune infertility due to self-production of anti-sperm antibodies. In addition, ubiquitination was proposed to facilitate the removal of defective spermatozoa during epididymal passage, a hypothesis supported by the observation of the cultured EEC phagocytizing dead spermatozoa and ubiquitin-coated microspheres.36 Early after publication, attempts were made to refute the proposed role of defective sperm phagocytosis in epididymal sperm quality control.45 However, recent studies identified an extensive network of phagocytosis-competent dendritic cells in the initial segment of the mouse epididymis.46 Newest whole animal studies show that dendritic cells are capable of phagocytizing sloughed epithelial cells in a ligated epididymis,47 as well as spermatozoa and microspheres in situ (Da Silva, data presented at the World Congress of Spermatology, Newcastle, NSW, Australia, to which the present Special Issue is dedicated).
Elevated semen ubiquitin content correlates with fertility outcomes and conventional semen parameters in humans and animals, and can be measured by FC using anti-ubiquitin antibodies43,48 and fluorochromes with affinity to aggresomes,44 the cellular stress-induced aggregates of nonrecycled ubiquitinated proteins most likely of spermiogenic origin. In domestic bull, sperm surface ubiquitination correlates positively with DNA damage43 and frequency of acrosomal damage49 and negatively with conventional semen parameters (sperm count, sperm motility, % normal morphology) and scrotal circumference,8 as well as with conception rates in AI service.44 Increased white blood cell contamination revealed by immunolabeling of PAFR correlated positively with semen ubiquitin level in breeding bulls undergoing breeding soundness evaluation but the sperm surface expression of PAFR actually appeared diminished in ubiquitinated spermatozoa.8 The latter observation is consistent with the proposed role of PAF and its receptor in sperm function.50 Similar to PAFR, sperm content and expression and patterns of signaling protein PAWP (discussed below) and seminal plasma binder of sperm BSP5 are altered in ubiquitinated spermatozoa.44,51 Currently under investigation is the relationship of bull sperm ubiquitination to reproductive seasonality and to the “dilutability” of bull semen (i.e., the possibility of lowering sperm number per AI dose in high fertility bulls with presumably lower semen content of ubiquitinated spermatozoa). Sperm surface ubiquitination showed the seasonality in both stallions and boars, having a negative correlation with semen parameters and fertility in both species and a positive correlation with the incidence of sperm cytoplasmic droplets in boars.52,53,54,55
Similar to bulls, increased sperm ubiquitination coincides with increased DNA damage and reduced motility in humans.56 Increased human sperm ubiquitination is associated with teratospermia and asthenozoospermia in general infertility clinic population,23,48 as well as in individuals with self-reported reprotoxic occupational exposure57 and in infertile men with heritable male infertility syndromes such as dysplasia of the fibrous sheath (stump tail syndrome).58 Some ubiquitinated proteins, albeit not those on the sperm surface, are intrinsic to normal spermatozoa, and their detection and quantity may thus correlate positively with various indicators of male fertility, including but not limited to sperm quality, resistance to cryodamage and assisted fertilization outcomes in humans and animals.59,60,61,62
POSTACROSOMAL SHEATH WWI DOMAIN BINDING PROTEIN
The PAWP has been implicated in the induction of oocyte activation during fertilization in humans and other mammals, and validated as a sperm quality/fertility biomarker in bulls and humans. The discovery of PAWP was based on a proteomic search of the perinuclear theca (PT), the cytoskeletal capsule protecting the sperm nucleus, selectively extracted from isolated sperm-heads. Of all the PT proteins identified, only one had the characteristic of a signal transduction protein.63 Because this protein resides exclusively in the postacrosomal sheath of the PT (PAS-PT) and contains a functional PPXY consensus binding site (where X represents any residue) for group I WW (WWI) domain containing proteins it was named PAWP. The Pawp gene, also known as WW domain binding protein 2 N-Terminal Like (Wbp2nl) codes for a sperm-specific ~32 kDa protein in human which shares sequence similarity with its paralogue, WBP2 (GeneTree ENSGT00530000063718). WBP2 is traceable to Drosophila and is detectable in its spermatozoa as well as the spermatozoa of several eutherian mammals we examined (R. Oko, unpublished). Unlike PAWP, it is extractable from mammalian spermatozoa in nonionic detergents but its subcellular localization is not known. PAWP, on the other hand, appears to be first detected in fish. In their N-terminal halves, PAWP and WBP2 contain a GRAM domain, shown in other proteins to be involved in membrane-coupling via phosphoinositides, while in their C-terminal halves, they share PPXY motifs and have a relatively high proline content. Depending on species, PAWP contains one (e.g., humans) to several (e.g., porcine) PPXY motifs which in eutherian mammals are interspersed among repeating YGXPPXG motifs of unknown significance.
The PAWP protein is synthesized during the latter half of spermiogenesis, when spermatids gain the capacity to activate oocytes and is assembled over the caudal half of the elongating spermatid nucleus, as part of PAS-PT.64,65,66 PAWP mRNA is first detected in mid-pachytene spermatocytes; its level of expression increases in round spermatids and eventually declines during spermatid elongation. PAWP protein resides exclusively in the PAS-PT of human, mouse, rhesus monkey, bull, boar and rabbit spermatozoa, and is most concentrated in proximity to the equatorial segment.63 During fertilization, PAWP is retained on the sperm head after both the acrosome reaction, and zona pellucida binding and penetration; it is among the first components dispersed from sperm head to the oocyte cytoplasm at the time of gamete fusion.63,65,67
Microinjection of recombinant PAWP protein or PAWP complementary RNA (cRNA) into human, mouse, and Xenopus oocytes triggers the release of calcium from oocyte endoplasmic reticulum and results in the initiation of oocyte activation including meiotic resumption, pronuclear formation and embryo cleavage.63,68,69 The mimicking of the sperm-induced oocyte activation process by PAWP highly supported its role as a sperm-borne oocyte activating factor (SOAF) or a component thereof. To provide proof for this hypothesis, individual spermatozoa were microinjected into the oocytes together with PAWP-specific antibodies or synthetic peptides derived from the PPXY motif of PAWP. These PAWP-derived competitive inhibitors and antibodies were able to block the sperm-induced intracellular calcium release and oocyte activation in mammalian (human, mouse, swine) and nonmammalian (Xenopus) oocytes,63,68,69 suggesting that PAWP was not only sufficient but required for oocyte activation. Such a trial, which has never been documented for other candidate SOAFs, implicated PAWP as a potential diagnostic marker for screening male infertility in the ART clinics and livestock AI Centers.
In the quest to find the clinical application of PAWP, its expression was investigated in the spermatozoa of men, presenting with idiopathic male infertility, who underwent ART by intracytoplasmic sperm injection (ICSI).70 Sperm PAWP levels, measured by FC, highly correlated with the success of fertilization after ICSI, independent of other factors such as male/female age, and sperm count, morphology, motility and DNA fragmentation. Concurrently, sperm PAWP levels were found to be significantly correlated with successful early embryonic development after ART and lower numbers of arrested embryos within 3–5 days post-ICSI.70 This suggests an important role for PAWP in not only initiation of calcium oscillations at fertilization but also in sustaining the normal embryonic development since the pattern of calcium oscillations can impact preimplantation embryonic development in mice. The FC-based measurements also suggested that sperm sub-population with heterogeneous PAWP levels may be detected in one individual semen sample, highlighting the necessity of screening the sperm sample and ideally choosing the spermatozoon with normal PAWP level for ICSI.
The findings in human are in line with observations in livestock species where semen collections with defective PAWP levels demonstrated poor AI fertility and abnormal sperm phenotypes. Proper postacrosomal sheath localization and FC-measured relative quantity of PAWP correlated with field AI fertility in a large study of 298 fertile AI sires with extensive records of AI conception rates.44 In this study, semen PAWP content appeared predictive of sires’ field AI fertility in a multiplex flow cytometric test also including probing of ubiquitin, aggresome, and lectin LCA and peanut agglutinin (PNA) binding sites, as will be discussed below. Similar to our SPTRX3 studies in humans,20 the image-based FC combining high throughout single cell fluorometry with multi-channel single cell imaging was applied to connect the relative PAWP-induced fluorescence levels with morphological phenotypes of individual spermatozoa. It was found that spermatozoa with severely malformed sperm heads had the lowest overall PAWP levels, and often lacked PAWP altogether. Spermatozoa with near-median levels of PAWP were mostly normal morphologically and displayed the typical wide band of PAWP labeling in the PAS, as seen in motile spermatozoa purified by sperm swim-up. Finally, spermatozoa with highest PAWP included macrocephalic spermatozoa as well as those with ectopic PAWP localization to sperm tail and superfluous cytoplasm trapped in and around coiled flagella.44 In addition to showing that missing, ectopic or abnormally high expression of PAWP is associated with abnormal sperm phenotypes, this analysis demonstrated the power of image-based FC, which eliminates extrapolation between fluorometry and cell imaging.
LECTINS AND MULTIPLEX FLOW CYTOMETRIC ASSAYS
Improved throughput of FC based on new technology such as capillary flow [Millipore EasyCyte Plus flow cytometer;49] allows for simultaneous assessment of multiple sperm parameters and biomarkers. In our recent trial, we have combined ubiquitin and PAWP based biomarker assays with those based on the affinity of plant lectins to sperm glycans/glycoproteins with organelle-specific localization.44,49
Binding of LCA, a lentil lectin with affinity for α-linked manosyl saccharide residues, to the sperm head and tail surfaces has been documented in human,71,72 boar,73 and mouse74 spermatozoa. We were the first to describe a differential pattern of LCA binding between the surface-ubiquitinated and nonubiquitinated bull spermatozoa.75 In phenotypically normal spermatozoa, the fluorescently conjugated LCA binds exclusively to acrosomal surfaces; on the contrary, LCA adheres to the entire sperm head and tail surface of defective spermatozoa. Such ubiquitin-and-LCA double-labeled spermatozoa also lacked the immunoreactivity to antibodies recognizing arylsulfatase A,75 a sperm surface protein implicated in the fertilization process.76 Consequently, LCA has been incorporated into a multiplex flow cytometric assay of bull spermatozoa. In a study of 298 fertile AI bulls, LCA showed a positive correlation with the percentages of ubiquitinated spermatozoa and lectin PNA-binding, acrosome-damaged spermatozoa, as well as with the percentage of spermatozoa with abnormally high content of PAWP protein.44 Lectin LCA by itself did not appear to be highly predictive of conception rates or nonreturn rates after AI.
Lectin PNA (Arachis hypogaea/peanut agglutinin) has an affinity toward disaccharides with terminal galactoses, including those present in the sperm acrosomal matrix. Consequently, lectin PNA has been known for a long time to bind to the acrosomal membranes/matrix of the spermatozoa with malformed or damaged acrosomes, and used for flow cytometric assays of acrosomal integrity [e.g.,77,78]. We have validated a dual ubiquitin-PNA assay for bull spermatozoa sperm samples from two fertile AI bulls collected in the course of a scrotal insulation trial. Using a conventional flow cytometer and a sperm-analysis dedicated capillary flow cytometer EasyCyte Plus, we found a statistically significant positive correlation between sperm contents of ubiquitinated and acrosome-damaged spermatozoa.49 Sperm ubiquitination assessed by anti-ubiquitin antibodies, in the absence of PNA-monitored acrosomal damage, correlated with the percentage of morphologically normal spermatozoa.49 In a larger study of 298 AI bulls with extensive fertility records, cited above,44 PNA was combined with LCA, ubiquitin, PAWP and aggresome assays. Summary data from two trials including all 298 bulls showed that PNA fluorescence correlated with sperm levels of PAWP. The PNA along with PAWP and aggresome were among the three most informative sperm parameters overall (including conventional semen parameters) in a step-wise regression analysis of a subgroup of 102 sires.
SEMEN NANOPURIFICATION METHOD DERIVED FROM BIOMARKER RESEARCH
Nanotechnologies are increasingly being implemented in all areas of life sciences, including agriculture and biomedicine.79 Semen nanopurification can be used to lower the sperm number per AI semen dose, allowing for increased production of semen doses per collection from sires with valuable genomes. An added benefit of nanopurfication may be provided by the removal of decaying moribund spermatozoa that are a source of harmful ROS. In human ART, sperm preparation for ICSI could be nanopurified to eliminate spermatozoa with potentially corrupted DNA. Target spermatozoa in this case would be the ones that do not bind nanoparticles designed to adhere to defective sperm surfaces. Thus, potential concerns for nano-reprotoxicity associated with purification methods targeting ligands on the surfaces of normal spermatozoa, would be avoided.
The ability of anti-ubiquitin antibodies and PNA lectin to bind the surfaces of defective spermatozoa has recently been utilized by our group to develop nanoparticle-based bull semen purification method validated by field AI trials. In pretrails, we tested various types of antibody and lectin coated magnetic spheres in micron diameter range but found that the efficiency of defective sperm binding to such large round surfaces was very low. We thus developed ferritin nanoparticles with uneven surfaces that maximize cell-particle contact area and are now commonly used for somatic or stem cell purification. In pretrials using bovine in vitro fertilization with cryopreserved spermatozoa nanopurified after thawing, the anti-ubiquitin antibody coated nanoparticles showed the most improvement in postpurification sperm viability and oocyte fertilization rates in vitro. However, the PNA particles were more efficient than the ubiquitin-binding ones when used for field AI with semen purified immediately after collection, that is, prior to final extension and cryopreservation.51 A total of 798 cows were inseminated with control semen at a nominal dose of 20 million spermatozoa/dose, or with a half dose of control and nanopurifed semen. Pregnancy rates in cows inseminated with a half dose PNA-nanopurified semen were similar, even slightly higher than those attained with a full dose of control semen and statistically significantly higher than pregnancy rates in cows inseminated with a half dose of control or ubiquitin-nanopurified semen.51 Thus, it appears that it is possible to double the number of AI doses per semen collection by removing 25%–30% of spermatozoa. The nanopurification procedure uses a simple magnetic separator bar placed alongside or under a test tube, requires no equipment with moving parts is easily incorporated in the workflow of semen collection and processing and would be inexpensive on an industrial scale. Consequently, no deleterious side effects were observed in the inseminated cows or in the calves born in the described trial.
CONCLUSIONS AND FUTURE OUTLOOK
Data from our and others’ laboratories illustrate how biomarker-based technologies allow for unbiased diagnosis of human male infertility and informed clinical treatment decision-making. In livestock reproduction, biomarker-based flow cytometric analysis correlates with conventional semen parameters and conception rates in AI service, and may even have a predictive value for future fertility of young sires, thus reducing the time and costs expended on extensive progeny testing. Probing of “negative,” infertility associated sperm quality biomarkers is inclusive of spermatozoa with obvious abnormal spermatozoa and those that are morphologically normal but carry detrimental or even embryo-lethal molecular defects like DNA fragmentation. In addition to biomarkers prevalent in defective spermatozoa, proteins, and ligands important for normal sperm function may either be undetectable or over-expressed/ectopically localized in defective spermatozoa and thus captured only by the biomarker-based semen analysis.
Advances in biomarker identification and validation go hand-in-hand with innovation in flow cytometric technology such as the introduction of dedicated bench-top sperm flow cytometers and flow cytometers with single cell imaging capabilities, eliminating the need for extrapolation between biomarker quantification and light-microscopic analysis of sperm phenotypes. Recently introduced biomarker-based semen nanopurification in livestock has a dual benefit of increasing the dilutability and thus AI dose number per semen collection, and removing decaying spermatozoa, a potential source of ROS harmful to viable spermatozoa within the collection. Consequently, semen nanopurification could find its way to human infertility clinics, primarily as a tool for preselecting the fittest spermatozoa for ART. Beyond infertility diagnostics and gamete preparation, recombinant proteins such as PAWP with its ability to activate human oocytes68 could be applied directly as a treatment, in this case to alleviate oocyte activation failure after ART by ICSI.80
Genomics will undoubtedly figure prominently in sperm quality analysis and biomarker discovery in the very near future. A genotype-to-phenotype approach currently being developed in our laboratory is aimed at discovering polymorphisms associated with particular sperm phenotypes. Thus far, efforts have been unsuccessful to identify polymorphisms associated with heritable sperm defects such as the dysplasia of the fibrous sheath or globozoospermia in humans81 and presumed heritable noncompensable sperm defects in livestock species.82
ACKNOWLEDGMENTS
Work summarized in this review was supported by grants 2013-67015-20961 and 2011-67015-20025 from USDA-NIFA, award #1R21HD066333-01 from NIH-NICHD and award #13324-2007 from Missouri Life Science Research Board, and by seed funding from the F21C Program, University of Missouri to PS. AMV was supported by the Instituto de Salud Carlos III [Projects PI050065 and PI080557, co-financed by the Fondo Social Europeo, FEDER] and Junta de Andalucía [Projects P07-CVI-02697 and P08-CVI-03629], Spain. This study was also supported by a Canadian Institutes of Health Research grant (MOP-84440 to R.O.).
REFERENCES
- 1.Farrell P, Trouern-Trend V, Foote RH, Douglas-Hamilton D. Repeatability of measurements on human, rabbit, and bull sperm by computer-assisted sperm analysis when comparing individual fields and means of 12 fields. Fertil Steril. 1995;64:208–10. [PubMed] [Google Scholar]
- 2.Robles V, Martínez-Pastor F. Flow cytometric methods for sperm assessment. Methods Mol Biol. 2013;927:175–86. doi: 10.1007/978-1-62703-038-0_16. [DOI] [PubMed] [Google Scholar]
- 3.Petrunkina AM, Harrison RA. Fluorescence technologies for evaluating male gamete (dys) function. Reprod Domest Anim. 2013;48(Suppl 1):11–24. doi: 10.1111/rda.12202. [DOI] [PubMed] [Google Scholar]
- 4.Garner DL, Johnson LA, Yue ST, Roth BL, Haugland RP. Dual DNA staining assessment of bovine sperm viability using SYBR-14 and propidium iodide. J Androl. 1994;15:620–9. [PubMed] [Google Scholar]
- 5.Troiano L, Granata AR, Cossarizza A, Kalashnikova G, Bianchi R, et al. Mitochondrial membrane potential and DNA stainability in human sperm cells: a flow cytometry analysis with implications for male infertility. Exp Cell Res. 1998;241:384–93. doi: 10.1006/excr.1998.4064. [DOI] [PubMed] [Google Scholar]
- 6.Evenson DP, Melamed MR. Rapid analysis of normal and abnormal cell types in human semen and testis biopsies by flow cytometry. J Histochem Cytochem. 1983;31:248–53. [PubMed] [Google Scholar]
- 7.Sutovsky P, Lovercamp K. Molecular markers of sperm quality. Soc Reprod Fertil Suppl. 2010;67:247–56. doi: 10.7313/upo9781907284991.021. [DOI] [PubMed] [Google Scholar]
- 8.Sutovsky P, Plummer W, Baska K, Peterman K, Diehl JR, et al. Relative levels of semen platelet activating factor-receptor (PAFr) and ubiquitin in yearling bulls with high content of semen white blood cells: implications for breeding soundness evaluation. J Androl. 2007;28:92–108. doi: 10.2164/jandrol.106.000216. [DOI] [PubMed] [Google Scholar]
- 9.Jiménez A, Zu W, Rawe VY, Pelto-Huikko M, Flickinger CJ, et al. Spermatocyte/spermatid-specific thioredoxin-3, a novel Golgi apparatus-associated thioredoxin, is a specific marker of aberrant spermatogenesis. J Biol Chem. 2004;279:34971–82. doi: 10.1074/jbc.M404192200. [DOI] [PubMed] [Google Scholar]
- 10.Fujii J, Tsunoda S. Redox regulation of fertilisation and the spermatogenic process. Asian J Androl. 2011;13:420–3. doi: 10.1038/aja.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bindoli A, Rigobello MP. Principles in redox signaling: from chemistry to functional significance. Antioxid Redox Signal. 2013;18:1557–93. doi: 10.1089/ars.2012.4655. [DOI] [PubMed] [Google Scholar]
- 12.Miranda-Vizuete A, Ljung J, Damdimopoulos AE, Gustafsson JA, Oko R, et al. Characterization of Sptrx, a novel member of the thioredoxin family specifically expressed in human spermatozoa. J Biol Chem. 2001;276:31567–74. doi: 10.1074/jbc.M101760200. [DOI] [PubMed] [Google Scholar]
- 13.Sadek CM, Damdimopoulos AE, Pelto-Huikko M, Gustafsson JA, Spyrou G, et al. Sptrx-2, a fusion protein composed of one thioredoxin and three tandemly repeated NDP-kinase domains is expressed in human testis germ cells. Genes Cells. 2001;6:1077–90. doi: 10.1046/j.1365-2443.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 14.Yu Y, Oko R, Miranda-Vizuete A. Developmental expression of spermatid-specific thioredoxin-1 protein: transient association to the longitudinal columns of the fibrous sheath during sperm tail formation. Biol Reprod. 2002;67:1546–54. doi: 10.1095/biolreprod.102.004838. [DOI] [PubMed] [Google Scholar]
- 15.Miranda-Vizuete A, Tsang K, Yu Y, Jiménez A, Pelto-Huikko M, et al. Cloning and developmental analysis of murid spermatid-specific thioredoxin-2 (SPTRX-2), a novel sperm fibrous sheath protein and autoantigen. J Biol Chem. 2003;278:44874–85. doi: 10.1074/jbc.M305475200. [DOI] [PubMed] [Google Scholar]
- 16.Smith TB, Baker MA, Connaughton HS, Habenicht U, Aitken RJ. Functional deletion of Txndc2 and Txndc3 increases the susceptibility of spermatozoa to age-related oxidative stress. Free Radic Biol Med. 2013;65:872–81. doi: 10.1016/j.freeradbiomed.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Sadek CM, Jiménez A, Damdimopoulos AE, Kieselbach T, Nord M, et al. Characterization of human thioredoxin-like 2. A novel microtubule-binding thioredoxin expressed predominantly in the cilia of lung airway epithelium and spermatid manchette and axoneme. J Biol Chem. 2003;278:13133–42. doi: 10.1074/jbc.M300369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duriez B, Duquesnoy P, Escudier E, Bridoux AM, Escalier D, et al. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc Natl Acad Sci U S A. 2007;104:3336–41. doi: 10.1073/pnas.0611405104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Zhao X, Li K, Luo G, Nie Y, et al. Thioredoxin-like protein 2 is overexpressed in colon cancer and promotes cancer cell metastasis by interaction with ran. Antioxid Redox Signal. 2013;19:899–911. doi: 10.1089/ars.2012.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckman C, George TC, Friend S, Sutovsky M, Miranda-Vizuete A, et al. High throughput, parallel imaging and biomarker quantification of human spermatozoa by ImageStream flow cytometry. Syst Biol Reprod Med. 2009;55:244–51. doi: 10.3109/19396360903056224. [DOI] [PubMed] [Google Scholar]
- 21.Buckman C, Ozanon C, Qiu J, Sutovsky M, Carafa JA, et al. Semen levels of spermatid-specific thioredoxin-3 correlate with pregnancy rates in ART couples. PLoS One. 2013;8:e61000. doi: 10.1371/journal.pone.0061000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396:120–4. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 23.Ozanon C, Chouteau J, Sutovsky P. Clinical adaptation of the sperm ubuquitin tag immunoassay (SUTI): relationship of sperm ubiquitylation with sperm quality in gradient-purified semen samples from 93 men from a general infertility clinic population. Hum Reprod. 2005;20:2271–8. doi: 10.1093/humrep/dei013. [DOI] [PubMed] [Google Scholar]
- 24.Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, et al. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:1393–6. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 25.Boitani C, Puglisi R. Selenium, a key element in spermatogenesis and male fertility. Adv Exp Med Biol. 2008;636:65–73. doi: 10.1007/978-0-387-09597-4_4. [DOI] [PubMed] [Google Scholar]
- 26.Schneider M, Förster H, Boersma A, Seiler A, Wehnes H, et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009;23:3233–42. doi: 10.1096/fj.09-132795. [DOI] [PubMed] [Google Scholar]
- 27.Hudemann C, Lönn ME, Godoy JR, Zahedi Avval F, Capani F, et al. Identification, expression pattern, and characterization of mouse glutaredoxin 2 isoforms. Antioxid Redox Signal. 2009;11:1–14. doi: 10.1089/ars.2008.2068. [DOI] [PubMed] [Google Scholar]
- 28.Rhee SG, Woo HA. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid Redox Signal. 2011;15:781–94. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 29.Hanschmann EM, Godoy JR, Berndt C, Hudemann C, Lillig CH. Thioredoxins, glutaredoxins, and peroxiredoxins – molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid Redox Signal. 2013;19:1539–605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manandhar G, Miranda-Vizuete A, Pedrajas JR, Krause WJ, Zimmerman S, et al. Peroxiredoxin 2 and peroxidase enzymatic activity of mammalian spermatozoa. Biol Reprod. 2009;80:1168–77. doi: 10.1095/biolreprod.108.071738. [DOI] [PubMed] [Google Scholar]
- 31.Yim SH, Kim YJ, Oh SY, Fujii J, Zhang Y, et al. Identification and characterization of alternatively transcribed form of peroxiredoxin IV gene that is specifically expressed in spermatids of postpubertal mouse testis. J Biol Chem. 2011;286:39002–12. doi: 10.1074/jbc.M111.257220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iuchi Y, Okada F, Tsunoda S, Kibe N, Shirasawa N, et al. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem J. 2009;419:149–58. doi: 10.1042/BJ20081526. [DOI] [PubMed] [Google Scholar]
- 33.Gong S, San Gabriel MC, Zini A, Chan P, O’Flaherty C. Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. J Androl. 2012;33:1342–51. doi: 10.2164/jandrol.111.016162. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Liu N, Zeng H. Peroxiredoxin I in sperm and reactive oxygen species in seminal plasma in patients with idiopathic asthenozoospermia. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39:842–8. doi: 10.3969/j.issn.1672-7347.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 36.Sutovsky P, Moreno R, Ramalho-Santos J, Dominko T, Thompson WE, et al. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J Cell Sci. 2001;114:1665–75. doi: 10.1242/jcs.114.9.1665. [DOI] [PubMed] [Google Scholar]
- 37.Vernocchi V, Morselli MG, Varesi S, Nonnis S, Maffioli E, et al. Sperm ubiquitination in epididymal feline semen. Theriogenology. 2014;82:636–42. doi: 10.1016/j.theriogenology.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Baska KM, Manandhar G, Feng D, Agca Y, Tengowski MW, et al. Mechanism of extracellular ubiquitination in the mammalian epididymis. J Cell Physiol. 2008;215:684–96. doi: 10.1002/jcp.21349. [DOI] [PubMed] [Google Scholar]
- 39.Hermo L, Jacks D. Nature's ingenuity: bypassing the classical secretory route via apocrine secretion. Mol Reprod Dev. 2002;63:394–410. doi: 10.1002/mrd.90023. [DOI] [PubMed] [Google Scholar]
- 40.Jones R. Sperm survival versus degradation in the Mammalian epididymis: a hypothesis. Biol Reprod. 2004;71:1405–11. doi: 10.1095/biolreprod.104.031252. [DOI] [PubMed] [Google Scholar]
- 41.Cornwall GA, Von Horsten HH, Whelly S. Cystatin-related epididymal spermatogenic aggregates in the epididymis. J Androl. 2011;32:679–85. doi: 10.2164/jandrol.111.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornwall GA, von Horsten HH, Swartz D, Johnson S, Chau K, et al. Extracellular quality control in the epididymis. Asian J Androl. 2007;9:500–7. doi: 10.1111/j.1745-7262.2007.00309.x. [DOI] [PubMed] [Google Scholar]
- 43.Sutovsky P, Neuber E, Schatten G. Ubiquitin-dependent sperm quality control mechanism recognizes spermatozoa with DNA defects as revealed by dual ubiquitin-TUNEL assay. Mol Reprod Dev. 2002;61:406–13. doi: 10.1002/mrd.10101. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy CE, Krieger KB, Sutovsky M, Xu W, Vargovic P, et al. Protein expression pattern of PAWP in bull spermatozoa is associated with sperm quality and fertility following artificial insemination. Mol Reprod Dev. 2014;81:436–49. doi: 10.1002/mrd.22309. [DOI] [PubMed] [Google Scholar]
- 45.Cooper TG, Yeung CH, Jones R, Orgebin-Crist MC, Robaire B. Rebuttal of a role for the epididymis in sperm quality control by phagocytosis of defective sperm. J Cell Sci. 2002;115:5–7. doi: 10.1242/jcs.115.1.5. [DOI] [PubMed] [Google Scholar]
- 46.Da Silva N, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, et al. A dense network of dendritic cells populates the murine epididymis. Reproduction. 2011;141:653–63. doi: 10.1530/REP-10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith TB, Cortez-Retamozo V, Grigoryeva LS, Hill E, Pittet MJ, et al. Mononuclear phagocytes rapidly clear apoptotic epithelial cells in the proximal epididymis. Andrology. 2014;2:755–62. doi: 10.1111/j.2047-2927.2014.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutovsky P, Terada Y, Schatten G. Ubiquitin-based sperm assay for the diagnosis of male factor infertility. Hum Reprod. 2001;16:250–8. doi: 10.1093/humrep/16.2.250. [DOI] [PubMed] [Google Scholar]
- 49.Odhiambo JF, Sutovsky M, DeJarnette JM, Marshall C, Sutovsky P. Adaptation of ubiquitin-PNA based sperm quality assay for semen evaluation by a conventional flow cytometer and a dedicated platform for flow cytometric semen analysis. Theriogenology. 2011;76:1168–76. doi: 10.1016/j.theriogenology.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Roudebush WE. Role of platelet-activating factor in reproduction: sperm function. Asian J Androl. 2001;3:81–5. [PubMed] [Google Scholar]
- 51.Odhiambo JF, DeJarnette JM, Geary TW, Kennedy CE, Suarez SS, et al. Increased conception rates in beef cattle inseminated with nanopurified bull semen. Biol Reprod. 2014;91:97. doi: 10.1095/biolreprod.114.121897. [DOI] [PubMed] [Google Scholar]
- 52.Sutovsky P, Turner RM, Hameed S, Sutovsky M. Differential ubiquitination of stallion sperm proteins: possible implications for infertility and reproductive seasonality. Biol Reprod. 2003;68:688–98. doi: 10.1095/biolreprod.102.005306. [DOI] [PubMed] [Google Scholar]
- 53.Lovercamp KW, Safranski TJ, Fischer KA, Manandhar G, Sutovsky M, et al. Arachidonate 15-lipoxygenase and ubiquitin as fertility markers in boars. Theriogenology. 2007;67:704–18. doi: 10.1016/j.theriogenology.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 54.Gamboa S, Ramalho-Santos J. SNARE proteins and caveolin-1 in stallion spermatozoa: possible implications for fertility. Theriogenology. 2005;64:275–91. doi: 10.1016/j.theriogenology.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 55.Kuster CE, Hess RA, Althouse GC. Immunofluorescence reveals ubiquitination of retained distal cytoplasmic droplets on ejaculated porcine spermatozoa. J Androl. 2004;25:340–7. doi: 10.1002/j.1939-4640.2004.tb02798.x. [DOI] [PubMed] [Google Scholar]
- 56.Hodjat M, Akhondi MA, Al-Hasani S, Mobaraki M, Sadeghi MR. Increased sperm ubiquitination correlates with abnormal chromatin integrity. Reprod Biomed Online. 2008;17:324–30. doi: 10.1016/s1472-6483(10)60215-5. [DOI] [PubMed] [Google Scholar]
- 57.Sutovsky P, Hauser R, Sutovsky M. Increased levels of sperm ubiquitin correlate with semen quality in men from an andrology laboratory clinic population. Hum Reprod. 2004;19:628–38. doi: 10.1093/humrep/deh131. [DOI] [PubMed] [Google Scholar]
- 58.Rawe VY, Olmedo SB, Benmusa A, Shiigi SM, Chemes HE, et al. Sperm ubiquitination in patients with dysplasia of the fibrous sheath. Hum Reprod. 2002;17:2119–27. doi: 10.1093/humrep/17.8.2119. [DOI] [PubMed] [Google Scholar]
- 59.Purdy PH. Ubiquitination and its influence in boar sperm physiology and cryopreservation. Theriogenology. 2008;70:818–26. doi: 10.1016/j.theriogenology.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 60.Eskandari-Shahraki M, Tavalaee M, Deemeh MR, Jelodar GA, Nasr-Esfahani MH. Proper ubiquitination effect on the fertilisation outcome post-ICSI. Andrologia. 2013;45:204–10. doi: 10.1111/j.1439-0272.2012.01330.x. [DOI] [PubMed] [Google Scholar]
- 61.Zarei-Kheirabadi M, Shayegan Nia E, Tavalaee M, Deemeh MR, Arabi M, et al. Evaluation of ubiquitin and annexin V in sperm population selected based on density gradient centrifugation and zeta potential (DGC-Zeta) J Assist Reprod Genet. 2012;29:365–71. doi: 10.1007/s10815-011-9689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muratori M, Marchiani S, Forti G, Baldi E. Sperm ubiquitination positively correlates to normal morphology in human semen. Hum Reprod. 2005;20:1035–43. doi: 10.1093/humrep/deh678. [DOI] [PubMed] [Google Scholar]
- 63.Wu AT, Sutovsky P, Manandhar G, Xu W, Katayama M, et al. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem. 2007;282:12164–75. doi: 10.1074/jbc.M609132200. [DOI] [PubMed] [Google Scholar]
- 64.Wu AT, Sutovsky P, Xu W, van der Spoel AC, Platt FM, et al. The postacrosomal assembly of sperm head protein, PAWP, is independent of acrosome formation and dependent on microtubular manchette transport. Dev Biol. 2007;312:471–83. doi: 10.1016/j.ydbio.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 65.Oko R, Sutovsky P. Biogenesis of sperm perinuclear theca and its role in sperm functional competence and fertilization. J Reprod Immunol. 2009;83:2–7. doi: 10.1016/j.jri.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 66.Tovich PR, Sutovsky P, Oko RJ. Novel aspect of perinuclear theca assembly revealed by immunolocalization of non-nuclear somatic histones during bovine spermiogenesis. Biol Reprod. 2004;71:1182–94. doi: 10.1095/biolreprod.104.030445. [DOI] [PubMed] [Google Scholar]
- 67.Sutovsky P, Manandhar G, Wu A, Oko R. Interactions of sperm perinuclear theca with the oocyte: implications for oocyte activation, anti-polyspermy defense, and assisted reproduction. Microsc Res Tech. 2003;61:362–78. doi: 10.1002/jemt.10350. [DOI] [PubMed] [Google Scholar]
- 68.Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, et al. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J. 2014;28:4434–40. doi: 10.1096/fj.14-256495. [DOI] [PubMed] [Google Scholar]
- 69.Aarabi M, Qin Z, Xu W, Mewburn J, Oko R. Sperm-borne protein, PAWP, initiates zygotic development in Xenopus laevis by eliciting intracellular calcium release. Mol Reprod Dev. 2010;77:249–56. doi: 10.1002/mrd.21140. [DOI] [PubMed] [Google Scholar]
- 70.Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, et al. Sperm content of postacrosomal WW binding protein is related to fertilization outcomes in patients undergoing assisted reproductive technology. Fertil Steril. 2014;102:440–7. doi: 10.1016/j.fertnstert.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Kallajoki M, Malmi R, Virtanen I, Suominen J. Glycoconjugates of human sperm surface. A study with fluorescent lectin conjugates and Lens culinaris agglutinin affinity chromatography. Cell Biol Int Rep. 1985;9:151–64. doi: 10.1016/0309-1651(85)90089-x. [DOI] [PubMed] [Google Scholar]
- 72.Lee MC, Damjanov I. Lectin binding sites on human sperm and spermatogenic cells. Anat Rec. 1985;212:282–7. doi: 10.1002/ar.1092120310. [DOI] [PubMed] [Google Scholar]
- 73.Fàbrega A, Puigmulé M, Dacheux JL, Bonet S, Pinart E. Glycocalyx characterisation and glycoprotein expression of Sus domesticus epididymal sperm surface samples. Reprod Fertil Dev. 2012;24:619–30. doi: 10.1071/RD11064. [DOI] [PubMed] [Google Scholar]
- 74.Wu SC, Yang HT, Liu M. Biochemical identification and characterisation of changes associated with capacitation of mannosylated glycoproteins in murine sperm. Andrologia. 2012;44(Suppl 1):747–55. doi: 10.1111/j.1439-0272.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 75.Baska KM, Sutovsky P. Protein modification by ubiquitination and is consequences for spermatogenesis, sperm maturation, fertilization and pre-implantation embryonic development. In: Tokumoto T, editor. New Impact on Protein Modifications in the Regulation of Reproductive System. Kerala: Research Signpost; 2005. pp. 83–114. [Google Scholar]
- 76.Xu H, Liu F, Srakaew N, Koppisetty C, Nyholm PG, et al. Sperm arylsulfatase A binds to mZP2 and mZP3 glycoproteins in a nonenzymatic manner. Reproduction. 2012;144:209–19. doi: 10.1530/REP-11-0338. [DOI] [PubMed] [Google Scholar]
- 77.Nagy S, Jansen J, Topper EK, Gadella BM. A triple-stain flow cytometric method to assess plasma- and acrosome-membrane integrity of cryopreserved bovine sperm immediately after thawing in presence of egg-yolk particles. Biol Reprod. 2003;68:1828–35. doi: 10.1095/biolreprod.102.011445. [DOI] [PubMed] [Google Scholar]
- 78.Graham JK, Kunze E, Hammerstedt RH. Analysis of sperm cell viability, acrosomal integrity, and mitochondrial function using flow cytometry. Biol Reprod. 1990;43:55–64. doi: 10.1095/biolreprod43.1.55. [DOI] [PubMed] [Google Scholar]
- 79.Sutovsky P, Kennedy CE. Biomarker-based nanotechnology for the improvement of reproductive performance in beef and dairy cattle. Ind Biotechnol. 2013;9:24–30. [Google Scholar]
- 80.Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reprod Biomed Online. 2014;28:560–71. doi: 10.1016/j.rbmo.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 81.Francavilla S, Cordeschi G, Pelliccione F, Bocchio M, Francavilla F. Isolated teratozoospermia: a cause of male sterility in the era of ICSI? Front Biosci. 2007;12:69–88. doi: 10.2741/2049. [DOI] [PubMed] [Google Scholar]
- 82.Chenoweth PJ. Genetic sperm defects. Theriogenology. 2005;64:457–68. doi: 10.1016/j.theriogenology.2005.05.005. [DOI] [PubMed] [Google Scholar]


