Abstract
Cholesterol is an essential component of the mammalian plasma membrane because it promotes membrane stability without comprising membrane fluidity. Given this important cellular role, cholesterol levels are tightly controlled at multiple levels. It has been clearly shown that cholesterol redistribution and depletion from the sperm membrane is a key part of the spermatozoon's preparation for fertilization. Some factors that regulate these events are described (e.g., bicarbonate, calcium) but the mechanisms underlying cholesterol export are poorly understood. How does a hydrophobic cholesterol molecule inserted in the sperm plasma membrane enter the energetically unfavorable aqueous surroundings? This review will provide an overview of knowledge in this area and highlight our gaps in understanding. The overall aim is to better understand cholesterol redistribution in the sperm plasma membrane, its relation to the possible activation of a cholesterol transporter and the role of cholesterol acceptors. Armed with such knowledge, sperm handling techniques can be adapted to better prepare spermatozoa for in vitro and in vivo fertilization.
Keywords: ATP binding cassette transporters, albumin, high-density lipoprotein, lipid rafts, membrane fluidity, membrane microdomains, membrane packing, oxysterols, reverse cholesterol transport, sterol transporters
INTRODUCTION
Following ejaculation into the female tract, spermatozoa must undergo a series of membrane remodeling events before they are capable of fertilizing the oocyte. This maturation process, termed capacitation, can be mimicked using a chemically defined medium for in vitro fertilization (IVF). This medium typically contains 15–25 mmol l-1 bicarbonate, 1–3 mmol l-1 calcium and 1–10 mg ml−1 fatty acid free (FAF) serum albumin. Bicarbonate and calcium initiate numerous signaling pathways, which cause a host of functional changes in the sperm population.1,2,3 Defined responses in sperm lipids include: (i) enhanced membrane fluidity (which can be measured with the fluorescent probe merocyanine 5404); (ii) a lateral redistribution of cholesterol to the apical margin of the sperm head, which can be visualized via filipin staining; followed by (iii) efflux of cholesterol from the sperm membrane to the extracellular environment in the presence of FAF albumin.5,6 The processes underlying the lateral redistribution and export of cholesterol in the sperm membrane are not well understood but appear to be critical for mammalian fertilization. This review will focus on these two capacitation-related events to provide an up-to-date overview of cholesterol behavior in the mammalian sperm membrane.
It is important to understand membrane cholesterol modulations because the sperm plasma membrane underlies its form and function.2 Sterols are a vital component of the plasma membrane in eukaryotic, but not prokaryotic, cells. It is thought that cellular sterols evolved in eukaryotic life forms to allow higher order functioning of multi-protein complexes in regionalized membrane domains such as transporters, and channels.7 The dominant cellular sterol is cholesterol of which most (approximately 90%) is located in the plasma membrane.8 Here, cholesterol is found in its free form. For intracellular storage, cholesterol must be neutralized via esterification to a fatty acid and is then stored in lipid droplets with triacylglycerol.9 Mammalian spermatozoa do not carry lipid droplets, and thus essentially lack neutral lipids such as triacylglycerol and cholesteryl esters, but other sterol forms are present. The cholesterol precursor desmosterol (for structures see Figure 1) typically accounts for about 10% of total sterols and trace amounts of their sulfated forms are also reported such as cholesterol sulfate and desmosterol sulfate.10,11,12,13
Figure 1.
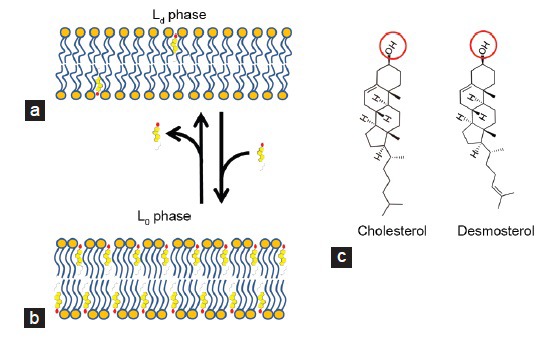
Orientation of cholesterol and desmosterol in a lipid bilayer. (a) The lipid-disordered membrane phase contains low levels of cholesterol. The membrane is fluid and has high lateral diffusion characteristics. At 4°C this membrane fraction is solubilized by detergents (detergent soluble membrane fraction). Most transmembrane proteins fit into this membrane fraction because of their α-helix transmembrane domain(s). (b) The lipid ordered phase of a membrane is still fluid but is stabilized by high levels of cholesterol. At 4°C this membrane is not solubilized by detergents and proteins and lipids can be purified in a so-called detergent-resistant membrane fraction. The exoplasmic lipid leaflet is enriched in sphingomyelin, gangliosides such as GM-1 and GPI-anchored proteins. The cytoplasmic side of this membrane fraction is characteristically enriched by the raft marker proteins caveolin and flotillin. (c) Structure of the free sterols embedded in the mammalian sperm lipid bilayer. Cholesterol and desmosterol are both oriented with the hydrophilic head group (red circle) in the polar head group region of the phospholipid bilayer and with the hydrophobic part oriented parallel to the fatty acid moieties of the phospholipid bilayer (see panels a and b). Note that biophysical studies showed that, beyond the lower lipid bilayer stability and higher fluidity, the Ld phase is also more permeable to water when compared with the Lo phase,93,94 which could be relevant for the cryopreservation of spermatozoa.
Cholesterol has a stabilizing effect on the plasma membrane by imposing conformational order on lipids (“lipid ordered”; Lo phase see Figure 1). Cholesterol fulfills this role by being inserted into the interstitial spaces of the lipid bilayer with its rigid body situated alongside the fatty acyl tail of neighboring phospholipids.14 Such conformation provides order to “lipid disordered” membranes (Ld; Figure 1), while retaining membrane fluidity and lateral diffusion of intrinsic membrane lipids and proteins. Because of the stabilizing properties of cholesterol15 variations in the cholesterol/phospholipid ratio across mammalian species has been linked to capacitation duration16 and the ability to survive cryopreservation.17 Methods to load the sperm membrane exogenously with cholesterol and thereby improve resistance to freezing have been trialed and are discussed further below.
HOW IS CHOLESTEROL TRANSPORTED FROM THE SPERM PLASMA MEMBRANE TO BIND WITH SERUM ACCEPTOR PROTEINS?
Homeostatic mechanisms controlling cholesterol are described as among the most intensely regulated biological processes and are tightly controlled on multiple levels.18 Overwhelming the system causes one of the most devastating pathologies of modern society – atherosclerosis – in which cholesterol-rich plaques accumulate in arteries.7 Cholesterol cannot be broken down inside the cell and needs to be exported to the liver to prevent excessive accumulation. In the liver, the cholesterol is taken up and metabolized to bile acids.19 This cellular cholesterol export process has three key stages. First, the export pathway is activated (e.g., through the detection of rising intracellular cholesterol levels) and specialized machinery is then stimulated to export cholesterol from the cell to the extracellular environment. Because cholesterol is hydrophobic and thus insoluble in aqueous environments, diffusible serum carrier proteins (such as lipoproteins) are required to bind the exported cholesterol and transport it around the body.20
There is a wealth of information on the reverse cholesterol transport (RCT) process in other cells, such as macrophages, because of its connection to heart disease.18,20,21 However, few studies have investigated how RCT is achieved in spermatozoa, even though, this process is a factor driving the sperm fertilization competence. We do know that some form of cholesterol acceptor is required in capacitating media to achieve capacitation, and this function is usually fulfilled by FAF albumin. However, the factors that prompt hydrophobic cholesterol molecules lodged within the sperm membrane to enter the energetically unfavorable aqueous environment surrounding the cell are largely a mystery. It is possible that this simply occurs via a passive diffusion of albumin following its contact with the sperm surface, but it is likely that much more elegant systems are involved.
ACTIVATION OF CHOLESTEROL REDISTRIBUTION AND EFFLUX IN THE SPERM PLASMA MEMBRANE
Bicarbonate and Ca2+ drive the lateral redistribution of sperm membrane lipids and proteins. In the lipid arena, this involves an enrichment of cholesterol in the apical region of the sperm head. It is here that cholesterol is exported from the sperm membrane to cholesterol acceptors in the extracellular fluid (e.g., FAF albumin). Bicarbonate has been shown to be a key effector of this efflux process because in its absence cholesterol levels are unaltered by FAF albumin. Cholesterol redistribution is driven by the bicarbonate dependent activation of cAMP production (by adenylate cyclase) and by a cAMP-dependent protein kinase A (PKA). Concomitant with the lateral translocation of sperm membrane sterols from the equatorial region of the sperm head to the apical ridge22 is the partial scrambling of the phospholipids phosphatidylethanolamine (PE) and phosphatidylserine (PS) in the same region, and the opposing retrograde movement of seminolipid (a sperm specific glycolipid) from the apical area to the equator.23,24 These phenomena are independent of free radical induced apoptosis-like exposure of PS (commonly detected using annexin-V probes) at the mid-piece.25 The exposure of PE at the sperm surface allows intercalation of the fluorescent probe m540 because this membrane type displays higher fluidity.26 It is only after activation of this pathway and the resulting lateral remodeling of the sperm membrane lipids (see Figure 2a) that FAF albumin can specifically take up cholesterol (and desmosterol) from the sperm surface.10,22,27
Figure 2.
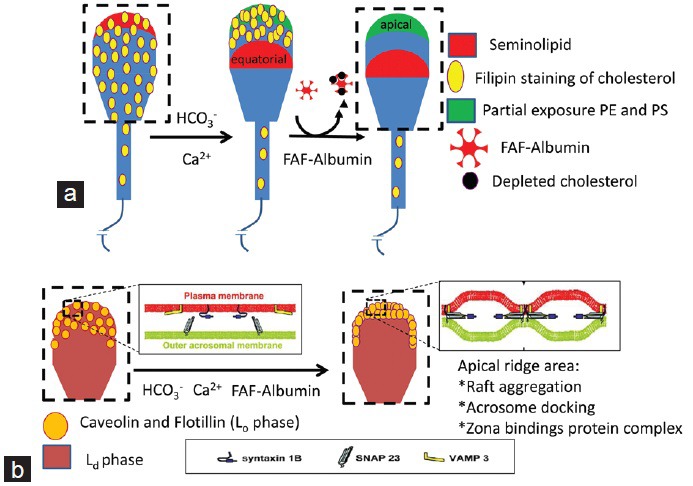
Redistribution of proteins and lipids of the sperm membrane during capacitation. (a) Lateral redistribution of cholesterol, seminolipid and partial exposure of phosphatidylethanolamine (PE) and phosphatidylserine (PS) are driven by calcium and bicarbonate ions. The nonresponsive cells are m540 negative and have lipids oriented as depicted in the sperm cartoon on the left. The responsive cells show high m540 fluorescence, a migration of seminolipid to the equatorial region, and a concentration of cholesterol in the apical area. At the apical region, a partial scrambling of PS and PE also takes place (as depicted in the middle spermatozoon). Only these responsive cells are prone to cholesterol efflux in the presence of fatty acid free-albumin and cause a loss of filipin staining as depicted in the cartoon on the right.22 (b) Capacitation is completed by the aggregation of membrane rafts in the apical ridge of the sperm head. This area also shows the formation of a trans-Soluble NSF Attachment REceptor protein complex, which facilitates the fusion of the outer acrosomal membrane to the sperm plasma membrane and the formation of a zona-binding protein complex.55
In the presence of bicarbonate and FAF albumin, a calcium-dependent aggregation of Lo membrane microdomains also occurs (Figure 2b). These aggregated microdomains are characterized by specific arrangements of proteins and lipids (such as high cholesterol levels) that create a bulging of the sperm membrane, giving them the terms membrane or lipid rafts (for an overview please see references28,29 and Figure 1). In capacitated spermatozoa, it is in these cholesterol-enriched Lo microdomains at the apical ridge area of the sperm head that a zona binding protein complex is formed.29,30,31,32
These lipid-ordered membrane microdomains can be separated from the rest of the membrane by their resistance to detergent solubilization at low temperatures (detergent resistant membrane fraction [DRM], see also Figure 1). We have demonstrated that sperm cholesterol efflux to FAF albumin under in vitro capacitating conditions result in lower amounts of cholesterol in the detergent soluble membrane fraction (DSM), while the DRM fraction had a constant amount of cholesterol27,33 and unpublished results. Nevertheless, from these observations one cannot conclude that RCT is happening exclusively in the Ld membrane area. It is also possible that RCT takes place in the Lo microdomains of the sperm head and that the depleted cholesterol is supplemented with cholesterol from the Ld microdomains (see sperm cholesterol transporters, below). In such a way, the equatorial and postequatorial membrane areas will show a net decrease in cholesterol levels.22 The differences in cholesterol between the Ld and Lo membranes and the retrograde movement of seminolipid out of the apical sperm head membrane (Figure 2a) and23,24,31 might force Lo microdomains to aggregate at the apical ridge area of the sperm head.27 Concomitantly, within the aggregated Lo microdomains a functional zona binding protein complex arises27,34,35 and stable docking of the sperm plasma membrane with the outer acrosomal membrane36,37,38,39 occurs (see Figure 2b). Note that the cholesterol redistribution-dependent raft formation and the concomitant recruitment of zona binding proteins and Soluble NSF Attachment REceptor proteins do all not take place in the equatorial segment, as this part of the sperm head is not involved in sperm-zona binding nor in acrosomal membrane fusion events. It remains intact and is the site involved in specific binding and fusion with the oolemma (fertilization).
SPERM CHOLESTEROL TRANSPORTERS
It is our view that a cholesterol transporter is probably involved in the regulation of capacitation-associated sperm cholesterol efflux. The role of this transporter would be to transfer cholesterol from the sperm membrane to an external acceptor under the correct environmental conditions. Unfortunately, few studies have investigated this hypothesis. However, there has been extensive investigation of RCT machinery in other cell types because a breakdown of this process causes atherosclerosis, the major precursor of cardiovascular disease.20 These are discussed below in a reproductive context.
ATP binding cassette transporters
ATP binding cassette (ABC) transporters are members of a large and ubiquitous transmembrane protein family that actively transport ligands across biological membranes.40 The ability of numerous ABC proteins (e.g., ABCA1–3) to export cholesterol to high-density lipoproteins (HDLs) for removal or recycling has been well described.41,42 A recent proteomic investigation targeting the bull sperm membrane43 identified multiple ABC cholesterol transporters (e.g., ABCA1, ABCA3 and ABCG2). Of these, proteins similar to ABCA14 and ABCA17 were notable for being among the top 5% most abundant based on total peptide count.43 There are very limited data available on these abundant, predominantly testis-expressed, ABC proteins. The cluster of proteins ABCA14–17 are closely related to ABCA3 and to a predominant glycoprotein of the sea urchin sperm membrane (suABCA).44 Species-specific expression patterns have been reported for the human, mouse and rat.45,46 Orthologous genes matching ABCA14–17 have been found in the dog, pig and bovine but remain poorly described.
Numerous ABC cholesterol transporters have also been detected immunologically in spermatozoa from various species, and some analysis of their ability to control sperm cholesterol levels has been undertaken. ABCA1 has been detected in both the mouse and dog.47,48 Beyond other phenotypical effects, ABCA1 gene null (-/-) mice show reduced fertility, possibly as a result of altered lipid levels, but no major morphological sperm abnormalities were noted. Antibody inhibition studies showed reduced cholesterol efflux from mouse sperm to apolipoprotein A1 (ApoA1) in the presence of anti-ABCA1, -ABCA7 and -ABCG1 antibodies.47 IVF rates were also reduced by co-incubation with these antibodies, suggesting that the transporters might contribute to the physiological regulation of capacitation-induced RCT.47 Similar antibody blocking studies were performed with ABCA17, with similar results, and HEK293 cells stably expressing ABCA17 were also shown to reduce intracellular levels of esterified lipids compared with nontransfected cells.49,50 ABCG2 was reported in epididymal and ejaculated bull spermatozoa, but is only functional in the former, with dephosphorylation stopping its activity in ejaculated spermatozoa.51
Supporting evidence can be found in the literature on the distinction between Lo and Ld domains for ABC transporters52 (Figure 3). ABC transporters on spermatozoa are predominantly present in the apical area of the head. This coincides with the area of raft aggregation (Lo) upon in vitro capacitation and the clustering and depletion of cholesterol in capacitated spermatozoa.22,53,54 This formation is dependent on bicarbonate-mediated sperm signaling, and, as both PKA and protein tyrosine kinases are activated55 it is possible that altered phosphorylation of the cholesterol transporters changes their actions in cholesterol transport.51 Interestingly, ABCG1 has been described to become tyrosine phosphorylated after interaction with caveolin-1 (a membrane raft marker), and both were required to regulate cholesterol efflux from HEK293 cells to ApoA1.56
Figure 3.
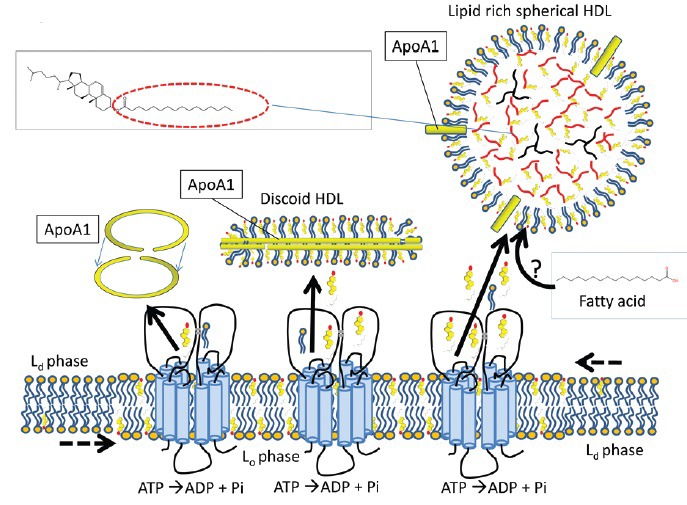
Reverse cholesterol transport (RCT) from the sperm membrane to high-density lipoproteins (HDL). ATP binding cassette (ABC) transporters can transport free sterols to acceptor proteins like HDL, which are abundant in the oviduct. The apolipoprotein A1 protein forms a circular dimer and stabilises discoidal, nascent HDL (mainly composed of phospholipids and free cholesterol). This structure can import more free cholesterol and phospholipids into its bilayer or esterify the free cholesterol with diacylglycerol to neutralize the lipids for intracellular storage (triacylglycerol in black and cholesteryl esters with the fatty acid esterified in red). The origin of the fatty acids used to esterify cholesterol in the oviduct is not known. See introduction and Figure 1 for information on Lo and Ld. Note that by analogy with other cell types, the ABC transporters are drawn in this model in the Lo microdomains.52 This model also presents the possibility that after export of cholesterol in the Lo regions, the vacant space for cholesterol is filled in by attracting cholesterol from Ld to explain why sperm RCT does not lead to disruption of aggregated Lo microdomains as observed in other cell types.95
Scavenger receptors
Other participants in the export of cholesterol are class B scavenger receptors (SR-Bs).57 SR-BI has been suggested to play a role in sperm capacitation22,58 but its presence in mature spermatozoa has not been demonstrated, although SR-BI and SR-BII have been reported in the raft domains of late spermatids.59 SR-Bs, like ABC transporters, export cholesterol to lipoproteins but – unlike ABC transporters – they can also import cholesterol. CD36 is another scavenger receptor that might mediate cholesterol homeostasis. Its expression is higher in spermatozoa from highly fertile bulls compared with bulls of lower fertility60 but a functional relationship between CD36 expression levels and RCT is yet to be established.
CHOLESTEROL ACCEPTORS
After cholesterol has been transported actively across the cell membrane, a carrier protein must be present to accept the hydrophobic molecule and carry it in the aqueous extracellular environment. Here we detail in vivo and in vitro acceptors of sperm cholesterol.
Albumin
Serum albumin is included in chemically defined media as the sterol acceptor for in vitro capacitation. Under these conditions, albumin binds free cholesterol from the sperm surface, resulting in a 20%–40% reduction in cholesterol and desmosterol levels.10 For in vitro capacitation of human samples, a FAF (<0.5% fatty acids) homologous albumin source is required, whereas bovine serum albumin (fraction V) is used for most other species. Mass spectrometry performed in our laboratory of sperm lipid efflux, and other studies, have shown that lipid-rich albumin must be depleted of fatty acids to act as a suitable acceptor of sperm surface sterols.10,61 The efflux of sterols to albumin is lipid-specific. Studies in our laboratory have shown that following in vitro capacitation the sperm pellet loses a certain proportion of sterols, but phospholipids remain at a constant level,22,62 and the albumin-containing supernatant gains sterols but not phospholipids.10
It is important to note that albumin is not the preferred cholesterol carrier for other cell types. Both albumin and lipoprotein complexes are present in the female genital tract. Both these entities can exchange lipids and are, therefore, the most likely candidates for accepting sperm surface cholesterol. However, in the oviduct it is most likely that a lipoprotein will be the dominant cholesterol acceptor (model shown for an ABC transporter and ApoA1 in Figure 3). In vivo, serum albumin is predominantly a circulatory carrier of fatty acids, not cholesterol, and its role as a cholesterol acceptor has only described during sperm capacitation. Even though the oviduct does have high concentrations of albumin,63 it is likely to exist in a form that is bound to a significant proportion of fatty acids, reducing its capacity to accept cholesterol.
High-density lipoprotein
Lipoproteins are the preferred candidates for accepting sperm surface cholesterol. HDLs, low-density lipoproteins or very low-density lipoproteins can act as sterol acceptors in the circulatory system but, of these, only HDLs are found in high concentrations in the oviduct and other fluids of the genital tract.63,64 The amount of oviductal HDL, and its sterol-carrying capacity also increased in the follicular phase, with ovulation increasing the amount of cholesterol esters and cholesterol associated with HDL.63 By contrast, no clear changes in albumin levels were observed.63
The role of HDLs in RCT is well described in the circulatory system. Both the ABC and SR-B transporters described above specifically transfer cholesterol to HDLs,21,65 but their preferred type of HDL varies. For example, ABCA1 effluxes cholesterol to apoproteins (e.g., ApoA1, ApoE, and ApoJ) of lipid-poor, nascent HDLs whereas ABCG1 effluxes cholesterol to lipid-rich, mature HDLs.66 Most cholesterol accepted by HDLs is esterified by linking the carboxylate group of a fatty acid to the hydroxyl group of cholesterol. Cholesterol esters are hydrophobic, allowing the lipid to be stored in an inert form in the HDL core (see Figure 3). Relatively small proportions of exported free cholesterol and phospholipids are used to extend the HDL lipid monolayer to increase its surface area,67 and see Figure 3. In this way, lipid-poor discoidal nascent HDL is converted to lipid-rich, spherical mature HDL. The possible source and type of fatty acids used to esterify cholesterol to cholesterol esters in oviductal HDL is unknown. We speculate that these fatty acids are delivered from albumin, the most abundant protein in oviduct fluid and a specific fatty acid transporting vehicle. If so, the ApoA1 protein at the HDL surface might esterify the incoming sperm cholesterol to the imported fatty acid from albumin. In this scenario, the sperm surface will become cholesterol depleted without any other lipid transport (e.g., concomitant phospholipid efflux), as is observed during capacitation in vitro. Alternatively, phospholipase A2 could be activated during sperm capacitation68 to provide the fatty acid source required to produce cholesterol esters in the core of HDL. If that scenario occurs in vivo, then it differs from in vitro sperm capacitation as no large differences in phospholipid composition and amounts were observed in such conditions.
The final step of capacitation-associated RCT is the receptor-mediated endocytosis of lipid binding proteins by epithelial cells of the female reproductive tract. This involves the engulfment of ApoA1- or ApoJ-containing lipoprotein complexes carrying cholesterol depleted from the sperm surface by receptor proteins on the apical surface of epithelial cells of the oviduct. High levels of two such ligands, cubalin and megalin, have been reported in this region of uterine and oviductal epithelial cells, especially during the oestrous and metoestrous phases.69 The uterine and oviductal epithelia also secrete ApoA1 and ApoJ.69 Thus, the epithelia of the female reproductive tract can regulate cholesterol efflux from spermatozoa indirectly by modulating the amounts of cholesterol and cholesterol acceptors present in the sperm's environment. However, studies assessing the interaction of sterol acceptors and endocytotic receptors at the apical surface of uterine or oviductal epithelial cells are lacking.
In this review, we propose a model of cholesterol efflux that involves (1) activation of the RCT process by bicarbonate and calcium, (2) possible cholesterol transporters, and (3) cholesterol carriers and cholesterol clearance. As cholesterol transport can be regulated by numerous means, the next section will deal with other possible regulatory mechanisms involved in RCT.
OTHER FACTORS THAT MIGHT FACILITATE STEROL TRANSPORT
β-cyclodextrins
Beta-cyclodextrins (βCDs) are cyclic oligosaccharides, which are hydrophobic on the inside and hydrophilic on the outside. These properties make them water-soluble but also able to form complexes with hydrophobic compounds. βCDs can be used to extract cholesterol from the sperm membrane in a nonphysiological dose-dependent fashion.33,62 Doses below < 0.3 mM are not sufficient to cause significant cholesterol depletion whereas higher doses (>1 mM) cause excessive cholesterol efflux that leads to cell deterioration and death. Only a very small range of concentrations with βCDs leads to successful, but quite low, fertilization rates10 as well as a concentration of zona binding proteins at the apical sperm surface.70 This in part is also because βCDs are detrimental for oocyte survival under IVF conditions.10
Interestingly, the mode of RCT elicited by FAF-albumin differs from βCD treatment as albumin leaves the aggregated raft area intact while βCD treatment causes their dispersion.62 βCDs can extract cholesterol from both the Lo and Ld phase of lipids in artificial lipid bilayers.71 In these βCD experiments, lower energy was required to extract Ld cholesterol when compared with Lo cholesterol as a consequence of the concentrated amount of polyunsaturated fatty acids (PUFAs) esterified to phospholipids in the Ld area of these artificial membranes. Esterified fatty acids in the DRM versus the DSM fraction are quite similar in spermatozoa and in both membrane fractions there is a very high degree of PUFAs, namely >5 unsaturated cis C = C bonds per phospholipid33,62 and van Gestel et al. (in preparation). Probably as a consequence of this specific high amount of PUFA-containing phospholipids in both the raft and nonraft areas, higher levels of βCDs caused not only depletion of cholesterol but also the disappearance of membrane rafts and rendered diminished DRM fractions in pig spermatozoa.62
Beta-cyclodextrins can also be preloaded with cholesterol (cholesterol-loaded cyclodextrins) and used to deliver cholesterol to the sperm surface to perturb RCT under capacitating conditions or to improve the resistance of spermatozoa to freezing.72 This method can be used to increase the ability of sperm to withstand stress imposed by cryopreservation and other sperm handling techniques.2 However, this technique should be used with caution, as more cholesterol is not always better and might retard cholesterol efflux in the female genital tract, and cause variable or reduced fertility rates.73
Binders of sperm proteins
One family of seminal plasma proteins that influence cholesterol efflux is the binders of sperm proteins (BSPs).74 The BSP family is most intensively studied in the bull and form the predominant protein fraction in seminal plasma, but are also present in the seminal plasma of other mammals.75,76 At ejaculation they bind in a rapid (half time < 1 s), and specific manner to choline head-groups of sperm membrane phospholipids (i.e., phosphatidylcholine and sphingomyelin) and thus not only directly interact with the outer lipid layer of the sperm plasma membrane but also with a phospholipid texture enriched in cholesterol.77,78 In the bull, this association has been shown to be very tight as BSP1 does not interact solely with the solvent-exposed choline group but partially inserts into the hydrophobic environment of the external leaflet of the lipid bilayer.78,79
In vitro, BSPs have been shown to cause an efflux of cholesterol from the bull sperm plasma membrane, which accelerates capacitation.80,81 Although BSP interaction in capacitating spermatozoa enhances RCT, it is not yet known how this is elicited. Possible mechanisms are: (i) BSP interacting with the outer lipid leaflet phospholipids allows it to bind cholesterol that remains attached to BSP upon its removal resulting in efflux of cholesterol from the sperm surface (direct interaction); (ii) BSP might also allow better interaction between the cholesterol transporter and either ApoA1 from HDL; or alternatively (iii) BSP might be an entity that is itself involved in transporting cholesterol either from the sperm surface or from the cholesterol transporter to HDL or FA. In the studies performed so far, no mention has been made of BSP's mode of action toward cholesterol loading of FAF-albumin. However, the capacitating effect of BSP proteins is greatly accelerated by the presence of cholesterol acceptors in follicular fluid. The causative agent was shown to be the HDL fraction of follicular fluid as depletion of this from follicular fluid or the addition of low- and very low-density lipoproteins had no effect on capacitation.82,83 It is likely that ApoA1 associated with HDL was the causative agent as ApoA1 liposomes were more stimulatory then HDL and BSP proteins, which have been shown previously to bind to purified ApoA1 plasma and HDL-associated ApoA1.84
Lipocalin-2
Lipocalin-2 is a small secretory protein from neutrophils known to be a potent factor in the innate immune defence but has recently been implicated in RCT in mouse spermatozoa. It resides in the mouse oviduct and uterus and binds to PE to induce raft aggregation in a PKA-dependent manner.85 It is possible that the partial scrambling of PE in capacitating sperm (Figure 2a)23,24 allows lipocalin-2 binding to this specific sperm surface that is selective for the apical sperm head. Lipocalin gene null (-/-) mice showed no signs of raft aggregation nor any in vivo capacitation related shedding of GPI-anchored proteins.85,86 It is also of note that lipocalin-2 levels increase in the uterotubal junction during estrus. A model representing the mode of action of lipocalin-2 in sperm surface reorganizations has been provided by Lingwood.87
Oxysterols
During capacitation, a mild production of reactive oxygen species (ROS) can be detected. This ROS source oxidizes a small portion of sperm cholesterol into oxysterols.10,88 The tenfold increase in oxysterols from 0.05% in control cells to 0.5% of total cholesterol in bicarbonate-stimulated cells is bicarbonate dependent and could be stimulated with pro-oxidants. Some oxysterol formation is regarded as a prerequisite for capacitation and inhibition with anti-oxidants (Vitamins A and E) causes reduced cholesterol depletion and impaired IVF rates.1,10
In other cell types, the formation of trace amounts of oxysterols is reported to facilitate RCT by regulating cholesterol availability in the membrane environment:89,90,91,92 for model see Figure 4. It is possible that bicarbonate induction of the oxysterol formation facilitates cholesterol efflux from sperm by one of these means. Interestingly, despite the more hydrophilic properties of oxysterols and their surface orientation they are not preferentially picked up by FAF-albumin under capacitation conditions when compared with cholesterol and desmosterol.10
Figure 4.
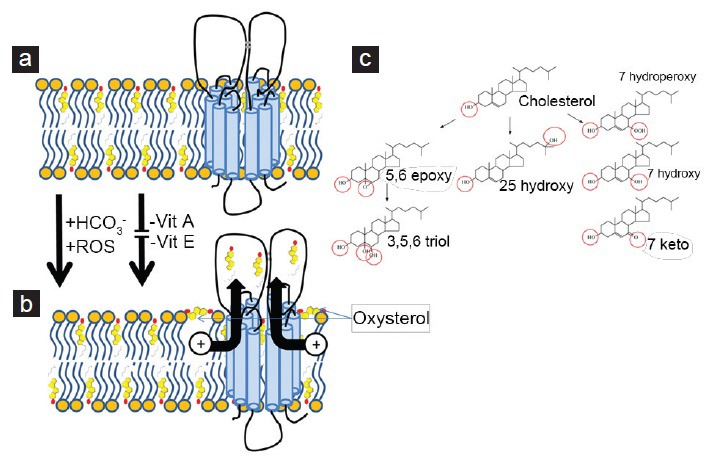
Formation of oxysterols during capacitation might enhance sperm reverse cholesterol transport. (a) Cholesterol is oriented in a parallel position to fatty acids and deeply embedded in both leaflets of the phospholipid bilayer. (b) Because of the additional hydroxyl groups of peroxidized cholesterol, the formed oxysterol no longer favors the original orientation of the parent cholesterol. Instead, it will line up the two (or three) hydroxyl groups (or derivatives thereof) in parallel with the phospholipid head groups. By doing this, the oxysterols might facilitate the transport of cholesterol either by activating the transporter directly or by lifting neighboring cholesterol partly out of the phospholipid layer91 where it is more accessible to sterol carriers (not depicted in this diagram: see Figures 2 and 3). The formation of oxysterols in the sperm membrane is dependent on bicarbonate and reactive oxygen species and can be prevented by antioxidants (Vitamin A or E). (c) Metabolic routes for the oxysterol formation in spermatozoa. The hydroxyl groups and derivatives thereof are indicated as red circles.
CONCLUSION
Fertilization is a decisive moment in life that enables the combination of genomes from two gametes to form a new organism. This event is dependent on a sophisticated spatial re-ordering of molecules of the sperm membrane, in which cholesterol depletion plays a crucial but poorly understood role. IVF protocols with the use of FAF-albumin provide a good starting point in which to investigate further aspects involved in RCT from spermatozoa. But many questions remain unanswered. What proteins are involved in sperm cholesterol transport? How are they are activated during sperm capacitation in vitro or in situ in the oviduct? Are they species specific? With the current review, we hope to provide new insights allowing relevant dedicated studies for elucidating how RCT is regulated in spermatozoa. Further insights to the factors regulating RCT would lead to more effective sperm processing techniques and culture conditions for IVF and in vivo fertilization. 95
REFERENCES
- 1.Aitken RJ, Nixon B. Sperm capacitation: a distant landscape glimpsed but unexplored. Mol Hum Reprod. 2013;19:785–93. doi: 10.1093/molehr/gat067. [DOI] [PubMed] [Google Scholar]
- 2.Leahy T, Gadella BM. Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction. 2011;142:759–78. doi: 10.1530/REP-11-0310. [DOI] [PubMed] [Google Scholar]
- 3.Reid AT, Redgrove K, Aitken RJ, Nixon B. Cellular mechanisms regulating sperm-zona pellucida interaction. Asian J Androl. 2011;13:88–96. doi: 10.1038/aja.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison RA, Ashworth PJ, Miller NG. Bicarbonate/CO2, an effector of capacitation, induces a rapid and reversible change in the lipid architecture of boar sperm plasma membranes. Mol Reprod Dev. 1996;45:378–91. doi: 10.1002/(SICI)1098-2795(199611)45:3<378::AID-MRD16>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 5.Gadella BM. Sperm membrane physiology and relevance for fertilization. Anim Reprod Sci. 2008;107:229–36. doi: 10.1016/j.anireprosci.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Martínez P, Morros A. Membrane lipid dynamics during human sperm capacitation. Front Biosci. 1996;1:d103–17. doi: 10.2741/a119. [DOI] [PubMed] [Google Scholar]
- 7.Dávalos A, Fernández-Hernando C. From evolution to revolution: miRNAs as pharmacological targets for modulating cholesterol efflux and reverse cholesterol transport. Pharmacol Res. 2013;75:60–72. doi: 10.1016/j.phrs.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luckey M. UK: Cambridge University Press; 2008. Membrane Structural Biology: with Biochemical and Biophysical Foundations. [Google Scholar]
- 9.Pol A, Gross SP, Parton RG. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J Cell Biol. 2014;204:635–46. doi: 10.1083/jcb.201311051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boerke A, Brouwers JF, Olkkonen VM, van de Lest CH, Sostaric E, et al. Involvement of bicarbonate-induced radical signaling in oxysterol formation and sterol depletion of capacitating mammalian sperm during in vitro fertilization. Biol Reprod. 2013;88:21. doi: 10.1095/biolreprod.112.101253. [DOI] [PubMed] [Google Scholar]
- 11.Lalumière G, Bleau G, Chapdelaine A, Roberts KD. Cholesteryl sulfate and sterol sulfatase in the human reproductive tract. Steroids. 1976;27:247–60. doi: 10.1016/0039-128x(76)90101-x. [DOI] [PubMed] [Google Scholar]
- 12.Langlais J, Zollinger M, Plante L, Chapdelaine A, Bleau G, et al. Localization of cholesteryl sulfate in human spermatozoa in support of a hypothesis for the mechanism of capacitation. Proc Natl Acad Sci U S A. 1981;78:7266–70. doi: 10.1073/pnas.78.12.7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zalata A, Hassan A, Christophe A, Comhaire F, Mostafa T. Cholesterol and desmosterol in two sperm populations separated on Sil-Select gradient. Int J Androl. 2010;33:528–35. doi: 10.1111/j.1365-2605.2009.00961.x. [DOI] [PubMed] [Google Scholar]
- 14.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–21. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh CJ, Chen YW, Hwang DW. Effects of cholesterol on membrane molecular dynamics studied by fast field cycling NMR relaxometry. Phys Chem Chem Phys. 2013;15:16634–40. doi: 10.1039/c3cp51739j. [DOI] [PubMed] [Google Scholar]
- 16.Davis BK. Timing of fertilization in mammals: sperm cholesterol/phospholipid ratio as a determinant of the capacitation interval. Proc Natl Acad Sci U S A. 1981;78:7560–4. doi: 10.1073/pnas.78.12.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darin-Bennett A, White IG. Influence of the cholesterol content of mammalian spermatozoa on susceptibility to cold-shock. Cryobiology. 1977;14:466–70. doi: 10.1016/0011-2240(77)90008-6. [DOI] [PubMed] [Google Scholar]
- 18.Goedeke L, Fernández-Hernando C. Regulation of cholesterol homeostasis. Cell Mol Life Sci. 2012;69:915–30. doi: 10.1007/s00018-011-0857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuipers F, Stroeve JH, Caron S, Staels B. Bile acids, farnesoid X receptor, atherosclerosis and metabolic control. Curr Opin Lipidol. 2007;18:289–97. doi: 10.1097/MOL.0b013e3281338d08. [DOI] [PubMed] [Google Scholar]
- 20.Uehara Y, Saku K. High-density lipoprotein and atherosclerosis: roles of lipid transporters. World J Cardiol. 2014;6:1049–59. doi: 10.4330/wjc.v6.i10.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luu W, Sharpe LJ, Gelissen IC, Brown AJ. The role of signalling in cellular cholesterol homeostasis. IUBMB Life. 2013;65:675–84. doi: 10.1002/iub.1182. [DOI] [PubMed] [Google Scholar]
- 22.Flesch FM, Brouwers JF, Nievelstein PF, Verkleij AJ, van Golde LM, et al. Bicarbonate stimulated phospholipid scrambling induces cholesterol redistribution and enables cholesterol depletion in the sperm plasma membrane. J Cell Sci. 2001;114(Pt 19):3543–55. doi: 10.1242/jcs.114.19.3543. [DOI] [PubMed] [Google Scholar]
- 23.Gadella BM, Gadella TW, Jr, Colenbrander B, van Golde LM, Lopes-Cardozo M. Visualization and quantification of glycolipid polarity dynamics in the plasma membrane of the mammalian spermatozoon. J Cell Sci. 1994;107(Pt 8):2151–63. doi: 10.1242/jcs.107.8.2151. [DOI] [PubMed] [Google Scholar]
- 24.Gadella BM, Lopes-Cardozo M, van Golde LM, Colenbrander B, Gadella TW., Jr Glycolipid migration from the apical to the equatorial subdomains of the sperm head plasma membrane precedes the acrosome reaction. Evidence for a primary capacitation event in boar spermatozoa. J Cell Sci. 1995;108(Pt 3):935–46. doi: 10.1242/jcs.108.3.935. [DOI] [PubMed] [Google Scholar]
- 25.Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta. 2000;1469:197–235. doi: 10.1016/s0304-4157(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 26.Harrison RA, Gadella BM. Bicarbonate-induced membrane processing in sperm capacitation. Theriogenology. 2005;63:342–51. doi: 10.1016/j.theriogenology.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 27.van Gestel RA, Brewis IA, Ashton PR, Helms JB, Brouwers JF, et al. Capacitation-dependent concentration of lipid rafts in the apical ridge head area of porcine sperm cells. Mol Hum Reprod. 2005;11:583–90. doi: 10.1093/molehr/gah200. [DOI] [PubMed] [Google Scholar]
- 28.Gadella BM, Tsai PS, Boerke A, Brewis IA. Sperm head membrane reorganisation during capacitation. Int J Dev Biol. 2008;52:473–80. doi: 10.1387/ijdb.082583bg. [DOI] [PubMed] [Google Scholar]
- 29.Nixon B, Aitken RJ. The biological significance of detergent-resistant membranes in spermatozoa. J Reprod Immunol. 2009;83:8–13. doi: 10.1016/j.jri.2009.06.258. [DOI] [PubMed] [Google Scholar]
- 30.Kongmanas K, Kruevaisayawan H, Saewu A, Sugeng C, Fernandes J, et al. Proteomic characterization of pig sperm anterior head plasma membrane reveals roles of acrosomal proteins in ZP3 binding. J Cell Physiol. 2015;230:449–63. doi: 10.1002/jcp.24728. [DOI] [PubMed] [Google Scholar]
- 31.Caballero J, Frenette G, D’Amours O, Belleannée C, Lacroix-Pepin N, et al. Bovine sperm raft membrane associated Glioma Pathogenesis-Related 1-like protein 1 (GliPr1L1) is modified during the epididymal transit and is potentially involved in sperm binding to the zona pellucida. J Cell Physiol. 2012;227:3876–86. doi: 10.1002/jcp.24099. [DOI] [PubMed] [Google Scholar]
- 32.Nixon B, Mitchell LA, Anderson AL, McLaughlin EA, O’bryan MK, et al. Proteomic and functional analysis of human sperm detergent resistant membranes. J Cell Physiol. 2011;226:2651–65. doi: 10.1002/jcp.22615. [DOI] [PubMed] [Google Scholar]
- 33.van Gestel R. PhD Thesis. Utrecht University; 2005. Membrane Characteristics of Sperm Cells During Capacitation; p. 133. [Google Scholar]
- 34.van Gestel RA, Brewis IA, Ashton PR, Brouwers JF, Gadella BM. Multiple proteins present in purified porcine sperm apical plasma membranes interact with the zona pellucida of the oocyte. Mol Hum Reprod. 2007;13:445–54. doi: 10.1093/molehr/gam030. [DOI] [PubMed] [Google Scholar]
- 35.Nixon B, Bielanowicz A, McLaughlin EA, Tanphaichitr N, Ensslin MA, et al. Composition and significance of detergent resistant membranes in mouse spermatozoa. J Cell Physiol. 2009;218:122–34. doi: 10.1002/jcp.21575. [DOI] [PubMed] [Google Scholar]
- 36.Tsai PS, Garcia-Gil N, van Haeften T, Gadella BM. How pig sperm prepares to fertilize: stable acrosome docking to the plasma membrane. PLoS One. 2010;5:e11204. doi: 10.1371/journal.pone.0011204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai PS, Brewis IA, van Maaren J, Gadella BM. Involvement of complexin 2 in docking, locking and unlocking of different SNARE complexes during sperm capacitation and induced acrosomal exocytosis. PLoS One. 2012;7:e32603. doi: 10.1371/journal.pone.0032603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai PS, De Vries KJ, De Boer-Brouwer M, Garcia-Gil N, Van Gestel RA, et al. Syntaxin and VAMP association with lipid rafts depends on cholesterol depletion in capacitating sperm cells. Mol Membr Biol. 2007;24:313–24. doi: 10.1080/09687860701228692. [DOI] [PubMed] [Google Scholar]
- 39.Ackermann F, Zitranski N, Heydecke D, Wilhelm B, Gudermann T, et al. The multi-PDZ domain protein MUPP1 as a lipid raft-associated scaffolding protein controlling the acrosome reaction in mammalian spermatozoa. J Cell Physiol. 2008;214:757–68. doi: 10.1002/jcp.21272. [DOI] [PubMed] [Google Scholar]
- 40.Linton KJ. Structure and function of ABC transporters. Physiology. 2007;22:122–30. doi: 10.1152/physiol.00046.2006. [DOI] [PubMed] [Google Scholar]
- 41.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–43. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aye IL, Singh AT, Keelan JA. Transport of lipids by ABC proteins: interactions and implications for cellular toxicity, viability and function. Chem Biol Interact. 2009;180:327–39. doi: 10.1016/j.cbi.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Byrne K, Leahy T, McCulloch R, Colgrave ML, Holland MK. Comprehensive mapping of the bull sperm surface proteome. Proteomics. 2012;12:3559–79. doi: 10.1002/pmic.201200133. [DOI] [PubMed] [Google Scholar]
- 44.Mengerink KJ, Vacquier VD. An ATP-binding cassette transporter is a major glycoprotein of sea urchin sperm membranes. J Biol Chem. 2002;277:40729–34. doi: 10.1074/jbc.M207184200. [DOI] [PubMed] [Google Scholar]
- 45.Piehler AP, Wenzel JJ, Olstad OK, Haug KB, Kierulf P, et al. The human ortholog of the rodent testis-specific ABC transporter Abca17 is a ubiquitously expressed pseudogene (ABCA17P) and shares a common 5’ end with ABCA3. BMC Mol Biol. 2006;7:28. doi: 10.1186/1471-2199-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen ZQ, Annilo T, Shulenin S, Dean M. Three ATP-binding cassette transporter genes, Abca14, Abca15, and Abca16, form a cluster on mouse Chromosome 7F3. Mamm Genome. 2004;15:335–43. doi: 10.1007/s00335-004-2281-8. [DOI] [PubMed] [Google Scholar]
- 47.Morales CR, Marat AL, Ni X, Yu Y, Oko R, et al. ATP-binding cassette transporters ABCA1, ABCA7, and ABCG1 in mouse spermatozoa. Biochem Biophys Res Commun. 2008;376:472–7. doi: 10.1016/j.bbrc.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palme N, Becher AC, Merkl M, Glösmann M, Aurich C, et al. Immunolocalization of the cholesterol transporters ABCA1 and ABCG1 in canine reproductive tract tissues and spermatozoa. Reprod Domest Anim. 2014;49:441–7. doi: 10.1111/rda.12294. [DOI] [PubMed] [Google Scholar]
- 49.Morales CR, Ni X, Smith CE, Inagaki N, Hermo L. ABCA17 mediates sterol efflux from mouse spermatozoa plasma membranes. Histol Histopathol. 2012;27:317–28. doi: 10.14670/HH-27.317. [DOI] [PubMed] [Google Scholar]
- 50.Ban N, Sasaki M, Sakai H, Ueda K, Inagaki N. Cloning of ABCA17, a novel rodent sperm-specific ABC (ATP-binding cassette) transporter that regulates intracellular lipid metabolism. Biochem J. 2005;389:577–85. doi: 10.1042/BJ20050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caballero J, Frenette G, D’Amours O, Dufour M, Oko R, et al. ATP-binding cassette transporter G2 activity in the bovine spermatozoa is modulated along the epididymal duct and at ejaculation. Biol Reprod. 2012;86:181. doi: 10.1095/biolreprod.111.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sano O, Ito S, Kato R, Shimizu Y, Kobayashi A, et al. ABCA1, ABCG1, and ABCG4 are distributed to distinct membrane meso-domains and disturb detergent-resistant domains on the plasma membrane. PLoS One. 2014;9:e109886. doi: 10.1371/journal.pone.0109886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki F, Yanagimachi R. Changes in the distribution of intramembranous particles and filipin-reactive membrane sterols during in vitro capacitation of golden hamster spermatozoa. Gamete Res. 1989;23:335–47. doi: 10.1002/mrd.1120230310. [DOI] [PubMed] [Google Scholar]
- 54.Tesarík J, Fléchon JE. Distribution of sterols and anionic lipids in human sperm plasma membrane: effects of in vitro capacitation. J Ultrastruct Mol Struct Res. 1986;97:227–37. doi: 10.1016/s0889-1605(86)80022-2. [DOI] [PubMed] [Google Scholar]
- 55.Gadella BM, Luna C. Cell biology and functional dynamics of the mammalian sperm surface. Theriogenology. 2014;81:74–84. doi: 10.1016/j.theriogenology.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Gu HM, Wang FQ, Zhang DW. Caveolin-1 interacts with ATP binding cassette transporter G1 (ABCG1) and regulates ABCG1-mediated cholesterol efflux. Biochim Biophys Acta. 2014;1841:847–58. doi: 10.1016/j.bbalip.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Nieland TJ, Shaw JT, Jaipuri FA, Duffner JL, Koehler AN, et al. Identification of the molecular target of small molecule inhibitors of HDL receptor SR-BI activity. Biochemistry. 2008;47:460–72. doi: 10.1021/bi701277x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Travis AJ, Kopf GS. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest. 2002;110:731–6. doi: 10.1172/JCI16392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casado ME, Huerta L, Ortiz AI, Pérez-Crespo M, Gutiérrez-Adán A, et al. HSL-knockout mouse testis exhibits class B scavenger receptor upregulation and disrupted lipid raft microdomains. J Lipid Res. 2012;53:2586–97. doi: 10.1194/jlr.M028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feugang JM, Rodriguez-Osorio N, Kaya A, Wang H, Page G, et al. Transcriptome analysis of bull spermatozoa: implications for male fertility. Reprod Biomed Online. 2010;21:312–24. doi: 10.1016/j.rbmo.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 61.Go KJ, Wolf DP. Albumin-mediated changes in sperm sterol content during capacitation. Biol Reprod. 1985;32:145–53. doi: 10.1095/biolreprod32.1.145. [DOI] [PubMed] [Google Scholar]
- 62.van Gestel RA, Helms JB, Brouwers JF, Gadella BM. Effects of methyl-beta-cyclodextrin-mediated cholesterol depletion in porcine sperm compared to somatic cells. Mol Reprod Dev. 2005;72:386–95. doi: 10.1002/mrd.20351. [DOI] [PubMed] [Google Scholar]
- 63.Ehrenwald E, Foote RH, Parks JE. Bovine oviductal fluid components and their potential role in sperm cholesterol efflux. Mol Reprod Dev. 1990;25:195–204. doi: 10.1002/mrd.1080250213. [DOI] [PubMed] [Google Scholar]
- 64.Langlais J, Kan FW, Granger L, Raymond L, Bleau G, et al. Identification of sterol acceptors that stimulate cholesterol efflux from human spermatozoa during in vitro capacitation. Gamete Res. 1988;20:185–201. doi: 10.1002/mrd.1120200209. [DOI] [PubMed] [Google Scholar]
- 65.Mulcahy JV, Riddell DR, Owen JS. Human scavenger receptor class B type II (SR-BII) and cellular cholesterol efflux. Biochem J. 2004;377:741–7. doi: 10.1042/BJ20030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031–43. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 67.Rye KA, Barter PJ. Regulation of high-density lipoprotein metabolism. Circ Res. 2014;114:143–56. doi: 10.1161/CIRCRESAHA.114.300632. [DOI] [PubMed] [Google Scholar]
- 68.Roldan ER, Vazquez JM. Bicarbonate/CO2 induces rapid activation of phospholipase A2 and renders boar spermatozoa capable of undergoing acrosomal exocytosis in response to progesterone. FEBS Lett. 1996;396:227–32. doi: 10.1016/0014-5793(96)01110-6. [DOI] [PubMed] [Google Scholar]
- 69.Argraves WS, Morales CR. Immunolocalization of cubilin, megalin, apolipoprotein J, and apolipoprotein A-I in the uterus and oviduct. Mol Reprod Dev. 2004;69:419–27. doi: 10.1002/mrd.20174. [DOI] [PubMed] [Google Scholar]
- 70.Bromfield EG, Nixon B. The function of chaperone proteins in the assemblage of protein complexes involved in gamete adhesion and fusion processes. Reproduction. 2013;145:R31–42. doi: 10.1530/REP-12-0316. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez SA, Gunther G, Tricerri MA, Gratton E. Methyl-ß-cyclodextrins preferentially remove cholesterol from the liquid disordered phase in giant unilamellar vesicles. J Membr Biol. 2011;241:1–10. doi: 10.1007/s00232-011-9348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Purdy PH, Graham JK. Effect of adding cholesterol to bull sperm membranes on sperm capacitation, the acrosome reaction, and fertility. Biol Reprod. 2004;71:522–7. doi: 10.1095/biolreprod.103.025577. [DOI] [PubMed] [Google Scholar]
- 73.Oliveira RR, Rates DM, Pugliesi G, Ker PG, Arruda RP, et al. Use of cholesterol-loaded cyclodextrin in donkey semen cryopreservation improves sperm viability but results in low fertility in mares. Reprod Domest Anim. 2014;49:845–50. doi: 10.1111/rda.12379. [DOI] [PubMed] [Google Scholar]
- 74.Manjunath P, Lefebvre J, Jois PS, Fan J, Wright MW. New nomenclature for mammalian BSP genes. Biol Reprod. 2009;80:394–7. doi: 10.1095/biolreprod.108.074088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leahy T, de Graaf SP. Seminal plasma and its effect on ruminant spermatozoa during processing. Reprod Domest Anim. 2012;47(Suppl 4):207–13. doi: 10.1111/j.1439-0531.2012.02077.x. [DOI] [PubMed] [Google Scholar]
- 76.Lusignan MF, Bergeron A, Crête MH, Lazure C, Manjunath P. Induction of epididymal boar sperm capacitation by pB1 and BSP-A1/-A2 proteins, members of the BSP protein family. Biol Reprod. 2007;76:424–32. doi: 10.1095/biolreprod.106.055624. [DOI] [PubMed] [Google Scholar]
- 77.Desnoyers L, Manjunath P. Major proteins of bovine seminal plasma exhibit novel interactions with phospholipid. J Biol Chem. 1992;267:10149–55. [PubMed] [Google Scholar]
- 78.Müller P, Erlemann KR, Müller K, Calvete JJ, Töpfer-Petersen E, et al. Biophysical characterization of the interaction of bovine seminal plasma protein PDC-109 with phospholipid vesicles. Eur Biophys J. 1998;27:33–41. doi: 10.1007/s002490050108. [DOI] [PubMed] [Google Scholar]
- 79.Greube A, Müller K, Töpfer-Petersen E, Herrmann A, Müller P. Influence of the bovine seminal plasma protein PDC-109 on the physical state of membranes. Biochemistry. 2001;40:8326–34. doi: 10.1021/bi010552+. [DOI] [PubMed] [Google Scholar]
- 80.Thérien I, Moreau R, Manjunath P. Bovine seminal plasma phospholipid-binding proteins stimulate phospholipid efflux from epididymal sperm. Biol Reprod. 1999;61:590–8. doi: 10.1095/biolreprod61.3.590. [DOI] [PubMed] [Google Scholar]
- 81.Thérien I, Moreau R, Manjunath P. Major proteins of bovine seminal plasma and high-density lipoprotein induce cholesterol efflux from epididymal sperm. Biol Reprod. 1998;59:768–76. doi: 10.1095/biolreprod59.4.768. [DOI] [PubMed] [Google Scholar]
- 82.Thérien I, Soubeyrand S, Manjunath P. Major proteins of bovine seminal plasma modulate sperm capacitation by high-density lipoprotein. Biol Reprod. 1997;57:1080–8. doi: 10.1095/biolreprod57.5.1080. [DOI] [PubMed] [Google Scholar]
- 83.Thérien I, Bousquet D, Manjunath P. Effect of seminal phospholipid-binding proteins and follicular fluid on bovine sperm capacitation. Biol Reprod. 2001;65:41–51. doi: 10.1095/biolreprod65.1.41. [DOI] [PubMed] [Google Scholar]
- 84.Manjunath P, Marcel YL, Uma J, Seidah NG, Chrétien M, et al. Apolipoprotein A-I binds to a family of bovine seminal plasma proteins. J Biol Chem. 1989;264:16853–7. [PubMed] [Google Scholar]
- 85.Watanabe H, Takeo T, Tojo H, Sakoh K, Berger T, et al. Lipocalin 2 binds to membrane phosphatidylethanolamine to induce lipid raft movement in a PKA-dependent manner and modulates sperm maturation. Development. 2014;141:2157–64. doi: 10.1242/dev.105148. [DOI] [PubMed] [Google Scholar]
- 86.Watanabe H, Kondoh G. Mouse sperm undergo GPI-anchored protein release associated with lipid raft reorganization and acrosome reaction to acquire fertility. J Cell Sci. 2011;124:2573–81. doi: 10.1242/jcs.086967. [DOI] [PubMed] [Google Scholar]
- 87.Lingwood D. Lipocalin 2 as a membrane-reorganizing agent. Sci Signal. 2014;7:pe19. doi: 10.1126/scisignal.2005563. [DOI] [PubMed] [Google Scholar]
- 88.Brouwers JF, Boerke A, Silva PF, Garcia-Gil N, van Gestel RA, et al. Mass spectrometric detection of cholesterol oxidation in bovine sperm. Biol Reprod. 2011;85:128–36. doi: 10.1095/biolreprod.111.091207. [DOI] [PubMed] [Google Scholar]
- 89.Massey JB. Membrane and protein interactions of oxysterols. Curr Opin Lipidol. 2006;17:296–301. doi: 10.1097/01.mol.0000226123.17629.ab. [DOI] [PubMed] [Google Scholar]
- 90.Tarling EJ, Bojanic DD, Tangirala RK, Wang X, Lovgren-Sandblom A, et al. Impaired development of atherosclerosis in Abcg1-/- Apoe-/- mice: identification of specific oxysterols that both accumulate in Abcg1-/- Apoe-/- tissues and induce apoptosis. Arterioscler Thromb Vasc Biol. 2010;30:1174–80. doi: 10.1161/ATVBAHA.110.205617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bielska AA, Olsen BN, Gale SE, Mydock-McGrane L, Krishnan K, et al. Side-chain oxysterols modulate cholesterol accessibility through membrane remodeling. Biochemistry. 2014;53:3042–51. doi: 10.1021/bi5000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bielska AA, Schlesinger P, Covey DF, Ory DS. Oxysterols as non-genomic regulators of cholesterol homeostasis. Trends Endocrinol Metab. 2012;23:99–106. doi: 10.1016/j.tem.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tong J, Briggs MM, McIntosh TJ. Water permeability of aquaporin-4 channel depends on bilayer composition, thickness, and elasticity. Biophys J. 2012;103:1899–908. doi: 10.1016/j.bpj.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rawicz W, Smith BA, McIntosh TJ, Simon SA, Evans E. Elasticity, strength, and water permeability of bilayers that contain raft microdomain-forming lipids. Biophys J. 2008;94:4725–36. doi: 10.1529/biophysj.107.121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sano O, Kobayashi A, Nagao K, Kumagai K, Kioka N, et al. Sphingomyelin-dependence of cholesterol efflux mediated by ABCG1. J Lipid Res. 2007;48:2377–84. doi: 10.1194/jlr.M700139-JLR200. [DOI] [PubMed] [Google Scholar]


