Abstract
One of the most common lesions present in the spermatozoa of human infertility patients is an idiopathic failure of sperm-egg recognition. Although this unique cellular interaction can now be readily by-passed by assisted reproductive strategies such as intracytoplasmic sperm injection (ICSI), recent large-scale epidemiological studies have encouraged the cautious use of this technology and highlighted the need for further research into the mechanisms responsible for defective sperm-egg recognition. Previous work in this field has established that the sperm domains responsible for oocyte interaction are formed during spermatogenesis prior to being dynamically modified during epididymal maturation and capacitation in female reproductive tract. While the factors responsible for the regulation of these sequential maturational events are undoubtedly complex, emerging research has identified the molecular chaperone, heat shock protein A2 (HSPA2), as a key regulator of these events in human spermatozoa. HSPA2 is a testis-enriched member of the 70 kDa heat shock protein family that promotes the folding, transport, and assembly of protein complexes and has been positively correlated with in vitro fertilization (IVF) success. Furthermore, reduced expression of HSPA2 from the human sperm proteome leads to an impaired capacity for cumulus matrix dispersal, sperm-egg recognition and fertilization following both IVF and ICSI. In this review, we consider the evidence supporting the role of HSPA2 in sperm function and explore the potential mechanisms by which it is depleted in the spermatozoa of infertile patients. Such information offers novel insights into the molecular mechanisms governing sperm function.
Keywords: egg, fertilization, heat shock protein A2, molecular chaperone, sperm, sperm-egg interactions
INTRODUCTION
Male infertility afflicts at least 1 in 20 men of reproductive age.1 Notwithstanding a small percentage of male patients who exhibit azoospermia,2,3 the majority of infertile men produce sufficient numbers of spermatozoa to fertilize an ovum in vivo. However, the quality of these individuals’ gametes is compromised to the point that fertilization and the initiation of normal embryonic development are not possible. One of the most frequent functional defects in these cells is an inability to recognize and adhere to the outer vestments of the egg, a structure known as the zona pellucida (ZP), and subsequently engage in the complex cascade of cellular processes that culminate in fertilization.4 Accordingly, bioassays of sperm-ZP interaction can accurately predict male infertility in vivo.5 Indeed, assessment of sperm-ZP binding with the hemizona assay provides the highest discriminatory power for fertilization success/failure of any sperm parameter assessed.6,7
Despite the biological importance of ZP binding, the fact that this barrier can now be readily breached through the advent of assisted reproductive technologies such as intracytoplasmic sperm injection (ICSI) has meant that the molecular basis of sperm-ZP recognition remains poorly characterized.8 This is particularly alarming in view of large-scale epidemiological studies that have documented an increased risk of birth defects in children conceived via ICSI, but not necessarily by conventional in vitro fertilization.9 Such findings raise the prospect that the human ZP may possess the ability to select superior quality spermatozoa, a notion supported by recent demonstrations that the ZP selectively binds sperm with normal morphology and nuclear chromatin DNA.10 Furthermore, biological selection of sperm for ICSI on the basis of their ZP binding affinity has been shown to produce higher quality embryos and contribute to improved implantation and clinical pregnancy rate compared to sperm selected by conventional subjective approaches.11,12,13 Thus, in spite of the major advance ICSI has provided for the alleviation of male-factor infertility, there is a pressing need for basic research into physiopathology of sperm-ZP interactions.
Research into this cell-specific and tightly regulated interaction has revealed that it is coordinated by specialized sperm domains overlying the anterior region of the sperm head. These domains are formed during the latter phases of spermatogenesis before being dynamically modified upon passage through both the male and female reproductive tracts.14 Thus, freshly ejaculated spermatozoa cannot recognize the egg; only after these cells have undergone a complex process of functional maturation, known as capacitation, do they express any affinity for the ZP.15,16 The ZP ligands that mediate sperm-egg recognition are currently being actively debated, with models centered on the importance of ZP2 and/or ZP3/4 under consideration.17,18,19,20 Similarly, the identity of the ZP receptor(s) on the surface of mammalian spermatozoa remains elusive. While a variety of candidates have been described, gene deletion studies have failed to confirm the exclusive significance of any of these molecules in mediating sperm-egg recognition.21 An alternative concept founded on the basis of studies by Asquith et al.,16 suggests that the biological importance of this event is so great that no single molecular entity has sole responsibility for mediating sperm-ZP binding. Rather, sperm-egg recognition is proposed to be a highly redundant process mediated by several ZP receptors that are brought to the cell surface and/or assembled into functional complexes during capacitation under the influence of molecular chaperones.22,23,24
Although this model was originally developed on the basis of studies conducted in the mouse, more recent work supports its relevance to ZP recognition in the human.25,26 Among the chaperones that have been implicated in this process in human spermatozoa, a testis-enriched member of the heat shock protein (HSP) 70 family, HSPA2 (Hspa2), has emerged as a key candidate. In the current review, we consider the established and rapidly emerging roles of HSPA2 in promoting the morphological differentiation of the male gamete during spermatogenesis and the subsequent functional transformation of these cells during capacitation. Such data serve to highlight the potential of HSPA2 as a clinically useful marker of sperm quality and emphasize the need for further analysis of this chaperone as a means of providing important insights into some of the most challenging questions concerning the molecular mechanisms regulating sperm function.
BACKGROUND TO THE HSP70 FAMILY OF MOLECULAR CHAPERONES
Molecular chaperones constitute a large family of structurally diverse proteins that are ubiquitously expressed in all organisms.27 More than 20 chaperone families, differing primarily with regard to their molecular weight and structural characteristics, have been described. Due to their ability to confer cellular resistance to environmental stressors, the majority of these chaperone families are referred to as cell stress response or, more commonly, HSPs.28 In mammalian species, the HSPs are divided into the HSP100 (HSPH), HSP90 (HSPC), HSP70 (HSPA), HSP60 (HSPD), HSP40 and HSP27 (HSPB) families.29 Several members of each gene family are represented within the human genome,30 a redundancy that may either relate to differences in intra-organelle compartmentalization and/or tissue/development-specific expression patterns.31 The overlapping expression of many different molecular chaperone families highlights the importance of their specialized functions, which extend from archetypical protective roles through to regulation of normal cellular functions, including: metabolism, growth, differentiation and apoptosis.32 Molecular chaperones can fulfill these diverse functions by virtue of their ability to selectively recognize and interact with exposed hydrophobic domains in their client proteins. Such interactions prevent inappropriate association or aggregation and direct the proteins into productive folding, transport or degradation pathways.33
The 70 kDa HSPs (HSP70) are among the most highly abundant and conserved members of the chaperone family, with at least 13 members represented in the human genome.30 These folding catalysts possess a modular architecture comprising three major functional domains: a conserved N-terminal ATPase domain, a substrate-binding domain and a C-terminal domain that acts as a lid for the substrate binding domain.34,35 The substrate binding and release cycle of HSP70s is commonly regulated by co-chaperones from the family of J-domain proteins (primarily HSP40 in eukaryotes) that target these chaperones to their respective substrates, and is further fine-tuned by nucleotide exchange factors.36 The primary function of HSP70s centers on their ability to transiently bind to partially synthesized or denatured peptide sequences, thereby preventing their aggregation and allowing them to (re)fold into a functional state. However, by virtue of their ability to stabilize client proteins in a partially folded state, HSP70s also aid in the transmembrane transport of proteins, and in their assembly into functional complexes35 (Figure 1). A novel, testis-enriched member of this HSP70 family, known as HSPA2, has emerged as a key regulator of several phases of sperm development and maturation.26,37,38
Figure 1.

Functional roles of heat shock proteins (HSP). The evolutionarily conserved molecular chaperones of the HSP70 (HSPA) family fulfill an essential role in maintaining protein quality control in a variety of cell types. Such protective activities center on the ability of the chaperones to assist the correct (re)folding of nascent and denatured proteins, thereby preventing their unwanted aggregation and functional inactivation. However, HSP70s also play an important role in facilitating protein-protein interactions, the assembly of multimeric protein complexes, and in the transport of proteins across intracellular membranes.
THE ROLE OF HEAT SHOCK PROTEIN A2 IN MOUSE SPERMATOZOA
Heat shock protein A2 (HSPA2) was originally identified in experiments designed to assess the effects of heat shock on protein synthesis in the germ cells of male mice.39,40,41 Subsequent work revealed that Hspa2 mRNA transcripts42 and protein43 displayed an expression profile that was both testis-enriched44 and developmentally regulated.45 Thus, gene expression was initiated in early meiosis43,45 and immediately followed by protein synthesis in leptotene-zygotene spermatocytes.46 Targeted mutation of the Hspa2 gene47 revealed that the chaperone is indispensable for the transition of spermatogenic cells through the late meiotic stages of spermatogenesis.48 Specifically, it has been shown that Hspa2 null males are infertile due to the combined effects of arrested spermatogenic cell development coinciding with the G2–M-phase transition of meiosis I prophase and the apoptotic elimination of late stage pachytene spermatocytes.48,49 Such a pronounced phenotype has been attributed to two primary roles for HSPA2 in these cells. Firstly, HSPA2 supports the formation of a heterodimeric complex between CDC2 and cyclin B1,50 and secondly, HSPA2 appears to act as a component of the synaptonemal complex.48 More recent work has shown that such functions may be augmented by the interaction of HSPA2 with an additional suite of testis enriched proteins, including: SHC SH2 domain-binding protein 1-like protein,51 the nuclear autoantigenic sperm protein52 and, the putative DExD-box helicase MOV10-like-1 that is essential for safeguarding the genetic information in the male germline.53
Interestingly, the stability of the HSPA2 protein during this critical phase of germ cell development is also influenced by its interaction with BAT3 (HLA-B associated transcript 3; also known as BCL2-associated athanogene 6, BAG6),54 a chaperone-like protein that appears to be important for the folding and activity of apoptotic signaling molecules.55 In this context, it has been shown that Bat3 deficiency leads to the poly-ubiquitination and subsequent degradation of HSPA2 protein.54 As anticipated, the loss of HSPA2 in Bat3 deficient mice arrests meiosis at prophase I and induces apoptosis in late pachytene spermatocytes, thereby resulting in complete male infertility.54 Such findings identify BAT3 as a critical regulator of HSPA2 in spermatogenesis and raise the prospect that it may represent a molecular target in idiopathic male infertility.
In addition to its fundamental roles in the completion of meiosis, the abundant expression of HSPA2 in postmeiotic germ cells has encouraged speculation that the protein fulfills additional function(s) during spermiogenesis. This notion is supported by evidence that, after the completion of meiosis, HSPA2 acquires a new role as a chaperone of spermatid-specific DNA packaging transition proteins.38 These transition proteins serve as an intermediary, replacing histones before themselves being replaced by protamines during the nuclear condensation that accompanies spermiogenesis.56 Owing to its ability to escort the transition proteins and mediate their assembly into DNA packaging structures, HSPA2 is thereby able to act as a major regulator of genome reorganization in differentiating spermatids.38 Further studies have also implicated the chaperoning activity of HSPA2 in the correct folding, assembly or trafficking of the subunits comprising the CatSper ion channel that is required for sperm cell hyperactivation and male fertility.57 Nevertheless, the HSPA2 protein has yet to be ascribed any specific functional role in mature mouse spermatozoa.
THE ROLE OF HEAT SHOCK PROTEIN A2 IN HUMAN SPERMATOZOA
Following its original identification in the mouse testes, immunoreactive HSPA2 protein homologs have since been reported in the testes of diverse phyla thus raising the prospect that it may play a highly conserved functional role during spermatogenesis.37 In support of this concept, the human and mouse Hspa2 homologs possess 91.7% identity in the nucleotide coding sequence and 98.2% in the corresponding amino acid sequence.58 Examination of the human HSPA2 protein has revealed significant expression in normal testes, with immunoreactivity being detected in spermatocytes and spermatids.59 Subsequent work confirmed that Hspa2 is selectively expressed in a biphasic pattern during human spermatogenesis.60 Thus, the first wave of Hspa2 expression occurs in spermatocytes where it is predicted to support meiosis. In contrast, the second wave occurs in elongating spermatids during spermiogenesis.60 Importantly, however, there is presently no direct evidence that human HSPA2 is involved in the dissociation of the synaptonemal complex or in the chaperoning of cyclin-CDK complexes as has been reported in the mouse.
Nevertheless, the importance of HSPA2 in the production of male germ cells has been highlighted by the demonstration that down-regulation of Hspa2 gene expression is strongly correlated with significant reductions in sperm concentration. Indeed, in the case of both oligozoospermic ART patients61 and those individuals suffering from complete azoospermia associated with spermatocyte arrest or Sertoli cell-only syndrome,62 the relative levels of Hspa2 gene expression are significantly lower than that of fertile controls. Similarly, aberrant HSPA2 protein expression has also been reported in immature human spermatozoa, which fail to complete normal spermiogenesis. This defect results in the production of spermatozoa with excessive cytoplasmic retention60,63 and a reduced ability to engage in interactions with both the ZP and cumulus matrices.64,65 Accordingly, HSPA2 has also been identified among a small number of proteins that are under-represented in defective spermatozoa with lesions in egg recognition.26 However, a key difference of this latter comparative proteomic study was that it focused on a subset of infertile donors whose spermatozoa exhibited an isolated lesion in their ability bind to the ZP without any accompanying defects in sperm motility or morphology.
On the basis of these conflicting data, at least two models have emerged to account for the role of HSPA2 in promoting ZP binding and cumulus matrix penetration. The first of these has been pioneered by Huszar and colleagues who postulate that the chaperoning activity of HSPA2 (originally described as a variant of creatine kinase M) is restricted to sperm development within the testis where it is required to facilitate major cycles of protein transport that drive cytoplasmic extrusion and plasma membrane remodeling during spermiogenesis.60,64,65,66,67 Such events are believed to not only underpin the formation of the ZP-binding domains but also those that are responsible for binding of the hyaluronic acid rich matrix of the cumulus mass. Consequently, the levels of HSPA2 remaining in mature human spermatozoa and the capacity of these cells to bind hyaluronic acid polymers, have both been reported to provide a robust discriminative index for fertilizing potential.64,65,68
An alternative model suggests that HSPA2 may instead play an important functional role in mature spermatozoa following their morphological differentiation within the testes. This model draws on evidence that HSPA2 is retained in mature spermatozoa and is ideally positioned in the peri-acrosomal region to participate in oocyte interactions.26 However, this model is not without controversy given that the protein has been variously reported to be constitutively expressed on the human sperm plasma membrane,69 to undergo a significant, albeit modest, increase in surface expression following the induction of capacitation in spermatozoa from fertile donors (i.e., 6.15% ±1.35 vs 13.1% ±2.69; P = 0.017),70 or to remain permanently within an intracellular location.25,26 Whether such controversy simply reflects the use of different methods of detection and/or antibodies71 has yet to be fully resolved, but through the combined use of ultrastructural, immunolocalization, and flow cytometry analyses our evidence favors HSPA2 occupying an intracellular location and thus playing an indirect role in mediating sperm-egg recognition.26 Specifically, we posit that HSPA2 facilitates the assembly and/or presentation of zona recognition complexes on the sperm surface.25,26,72
Support for this hypothesis rests with our demonstration that HSPA2 stably interacts with a number of multimeric complexes, with aggregate molecular weights of greater than 150 kDa, in spermatozoa from fertile normozoospermic individuals.26 In proof-of-concept studies we have established that one of the major HSPA2 complexes harbors two additional proteins, sperm adhesion molecule 1 (SPAM1) and arylsulfatase A (ARSA), both of which have been implicated in interactions with the cumulus-oocyte complex.73,74,75,76,77,78,79 Interestingly, this complex undergoes a marked, capacitation-associated translocation leading to the repositioning of ARSA to the outer leaflet of the sperm surface, a location compatible with a role in the mediation of sperm-ZP interactions. Conversely, SPAM1 appears to reorient away from the sperm surface, possibly reflecting its primary role in cumulus matrix dispersal preceding sperm-ZP recognition.25,26 In addition to aligning perfectly with the functional requirements of spermatozoa engaged in the process of fertilization, this regulated shift in surface expression is commensurate with the observation that spermatozoa in the advanced stages of capacitation lose their ability to bind hyaluronic acid.80
In recent unpublished studies, we have shown that the dynamic, capacitation-associated translocation of proteins appears to extend to additional HSPA2 client proteins thus raising the possibility that the chaperone holds a key role in priming the sperm surface architecture in advance of their interaction with the cumulus-oocyte complex. Such activity, in turn, appears to be driven by the tyrosine phosphorylation of HSPA2 during the latter stages of capacitation and can be completely abolished by incubation of spermatozoa in broad spectrum tyrosine kinase inhibitors.25 Taken together, these results offer a rational explanation for why HSPA2 expression, hyaluronic acid binding and sperm-zona interaction are functionally linked and why they are all associated with male infertility; without HSPA2, neither the hyaluronidase receptor, nor the zona receptor(s), would be expressed in the coordinated manner needed to achieve fertilization.
MECHANISMS UNDERPINNING THE LOSS OF HEAT SHOCK PROTEIN A2 FROM THE SPERMATOZOA OF INFERTILE PATIENTS
A major goal of our ongoing investigations has been to determine how the incorporation of HSPA2 into the differentiating gamete becomes so dramatically disrupted in cases of infertility. Among the various possibilities that could account for the selective loss of HSPA2, genetic mutations in the encoding Hspa2 gene, epigenetic regulation of Hspa2 gene expression, and/or perturbations in protein expression/stability arising from exposure of developing germ cells to oxidative stress are currently under consideration. While the former explanation cannot be entirely ruled out, it appears to contradict evidence from transgenic mouse models in which the targeted ablation of the Hspa2 gene leads to complete spermatogenic arrest (see Section “THE ROLE OF HEAT SHOCK PROTEIN A2 IN MOUSE SPERMATOZOA”). Similarly, although there is evidence that the methylation status of the Hspa2 gene correlates with its transcription level in human somatic cells, these findings are not without controversy (reviewed by71). In contrast, oxidative stress is well known to play a fundamental role in the etiology of male infertility by negatively affecting sperm quality and function.81
As outlined previously, it is known that the HSPA2 protein is translated from an early stage in spermatogenesis and serves multiple functions being both necessary for the progression of meiosis and a major marker for the quality of spermiogenesis.60 A defining characteristic of spermiogenesis is that it is extremely sensitive to oxidative damage owing to the fact that it is driven by the differential translation of proteins from long-lived mRNA species. While attention is usually focused on the damage that free radicals can inflict on DNA, RNA is equally vulnerable to oxidative attack, as is the process of protein translation.82 In this light, the round spermatid may be uniquely vulnerable to oxidative attack since this cell type is replete with the mRNA species it will need to build a spermatozoon and is responsible for carefully orchestrating the movement of these mRNAs from ribonucleoprotein particles (RNP) to polysomes to affect their differential translation.83 It is therefore possible that the reason HSPA2 expression is reduced in defective spermatozoa lacking the ability to bind to the ZP is that either the mRNA for this chaperone or the mechanisms for its translation have been oxidatively damaged during spermiogenesis.
An attack on mRNA integrity is suggested by previous studies revealing low levels of Hspa2 mRNA expression in the defective spermatozoa of patients exhibiting oligoteratozoospermia or oligozoospermia associated with varicocele.61,84 A particular role for oxidative stress is supported by the strong positive correlations that have been established between defects in sperm binding to hyaluronic acid polymers (mediated in part by SPAM1, one of the binding partners for HSPA2) and increased levels of peroxidative damage to the sperm plasma membrane.85,86 The possibility that such an oxidative attack would strike late in spermatogenesis, when sperm differentiation is occurring, is suggested by a highly significant under-representation of phosphoglycerate kinase (PGK), mirroring the loss of HSPA2, in spermatozoa exhibiting an inability to bind to the ZP.26 The significance of this finding is that the PGK isoform in spermatozoa (PGK2) is transcribed late in spermatogenesis to become the major PGK in spermatozoa.87 The loss of this protein is therefore consistent with the disruption of protein translation during spermiogenesis, as the PGK2 mRNA migrates from RNP to polysomes, because of oxidative damage to the mRNA and/or disruption of the translation machinery itself. If this is the case then a number of other mRNA species that show the same orchestrated movement from RNPs to polysomes during spermatogenesis, should also be affected.88 The assessment of the mRNA profiles of spermatozoa exhibiting a failure of sperm-egg interaction could, therefore, prove to be a valuable tool in evaluating sperm reproductive capacity and functional competence in infertile men.
Finally, as an alternative to mRNA damage, it is also possible that the lack of HSPA2 seen in spermatozoa of infertile patients may arise from a mechanism involving the targeted destruction of the protein itself. Consistent with this notion, recent work from our laboratory has shown that electrophilic aldehydes, such as 4-hydroxynonenal (4HNE), generated as a result of reactive oxygen species-induced lipid peroxidation are readily capable of adducting proteins localized within the head of human spermatozoa.89 Furthermore, peptides belonging to HSP70 family members have been identified among the major 4HNE alkylated targets in these damaged cells.89 While the primary impact of 4HNE covalently binding to proteins in mature spermatozoa is likely to involve conformational changes and/or aggregation leading to disruption of their functionality,90 it may have a more pronounced effect in developing germ cells (Figure 2). Indeed in tissues such as the testis, which possess an intrinsic ubiquitin-proteasome system, such insults commonly lead to the activation of a protein degradation cascade that selectively eliminates damaged proteins in an effort to mitigate the impact of oxidative injury.91,92,93 Alternative, ubiquitin-dependent lysosomal degradation mechanisms have also recently been reported for 4HNE-modified proteins.94 Whether such mechanisms underpin the loss of susceptible proteins such as HSPA2 from maturing spermatozoa remains to be established. Similarly, it also has yet to be investigated whether aberrant expression of BAT3 might contribute to this phenotype through accelerated degradation of HSPA254 during human spermatogenesis.
Figure 2.
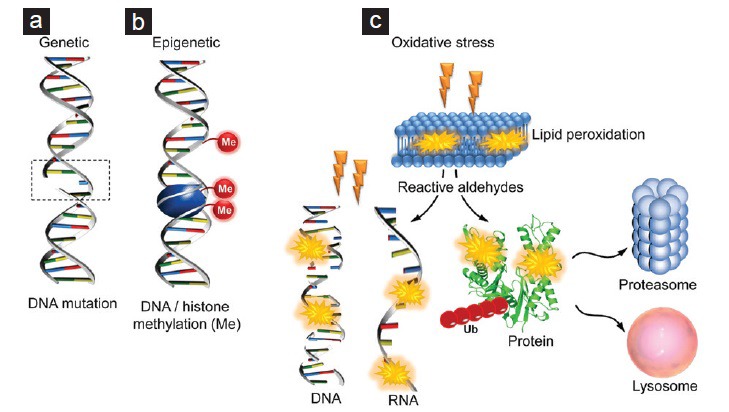
Mechanisms underpinning the loss of heat shock protein A2 (HSPA2) from the spermatozoa of infertile patients. Although the mechanism(s) underpinning the selective loss of HSPA2 from the differentiating gametes of infertile patients is currently unknown, several possibilities have been raised. These include: (a) genetic mutations in the encoding Hspa2 gene, (b) epigenetic regulation of Hspa2 gene expression, and/or (c) perturbations in protein expression/stability arising from exposure of developing germ cells to oxidative stress. With regard to the latter it is possible the oxidative attack could act directly to damage the Hspa2 gene or mRNA transcript. Alternatively, the HSPA2 protein itself may be targeted for destruction following adduction by electrophilic aldehydes generated as a result of reactive oxygen species-induced lipid peroxidation. Such insults are known to lead ubiquitin (Ub)-dependent degradation via proteasomal or lysosomal pathways.
CONCLUSIONS
An idiopathic failure of sperm-egg recognition ranks among the major reproductive lesions experienced in male infertility patients. Data from a number of independent laboratories suggest that this process is commonly impaired because of an underrepresentation of a key molecular chaperone, HSPA2, in pathologically defective spermatozoa. These findings accord with the view that molecular chaperones are critically involved in conferring upon spermatozoa the potential to interact with the oocyte during the sequential phases of sperm maturation.22, 95 These studies open up new research questions concerning the incidence of HSPA2 insufficiency in the patient population, the pathways by which this chaperone is incorporated into the differentiating gamete, how such incorporation becomes so dramatically disrupted in cases of infertility and the mechanisms by which HSPA2 regulates the differential surface expression of molecules involved in recognition of the oocyte-cumulus complex. Addressing these questions will have important implications for the diagnosis, treatment and prevention of infertility, and, in so doing, answer the long-standing call for evidence-based medicine in andrological practice.
REFERENCES
- 1.McLachlan RI, de Kretser DM. Male infertility: the case for continued research. Med J Aust. 2001;174:116–7. doi: 10.5694/j.1326-5377.2001.tb143180.x. [DOI] [PubMed] [Google Scholar]
- 2.Cedenho AP. Evaluation of the subfertile male. In: Oehninger S, Kruger T, editors. Male Infertility, Diagnosis and Treatment. UK: Inferma UK Ltd; 2007. pp. 117–40. [Google Scholar]
- 3.Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142:62–5. doi: 10.1016/s0022-5347(17)38662-7. [DOI] [PubMed] [Google Scholar]
- 4.Liu DY, Baker HW. Defective sperm-zona pellucida interaction: a major cause of failure of fertilization in clinical in-vitro fertilization. Hum Reprod. 2000;15:702–8. doi: 10.1093/humrep/15.3.702. [DOI] [PubMed] [Google Scholar]
- 5.Arslan M, Morshedi M, Arslan EO, Taylor S, Kanik A, et al. Predictive value of the hemizona assay for pregnancy outcome in patients undergoing controlled ovarian hyperstimulation with intrauterine insemination. Fertil Steril. 2006;85:1697–707. doi: 10.1016/j.fertnstert.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 6.Oehninger S, Morshedi M, Franken D. The hemizona assay for assessment of sperm function. Methods Mol Biol. 2013;927:91–102. doi: 10.1007/978-1-62703-038-0_9. [DOI] [PubMed] [Google Scholar]
- 7.Oehninger S, Mahony M, Ozgür K, Kolm P, Kruger T, et al. Clinical significance of human sperm-zona pellucida binding. Fertil Steril. 1997;67:1121–7. doi: 10.1016/s0015-0282(97)81449-5. [DOI] [PubMed] [Google Scholar]
- 8.Franken DR, Oehninger S. The clinical significance of sperm-zona pellucida binding: 17 years later. Front Biosci. 2006;11:1227–33. doi: 10.2741/1875. [DOI] [PubMed] [Google Scholar]
- 9.Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–13. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- 10.Liu DY, Baker HW. Human sperm bound to the zona pellucida have normal nuclear chromatin as assessed by acridine orange fluorescence. Hum Reprod. 2007;22:1597–602. doi: 10.1093/humrep/dem044. [DOI] [PubMed] [Google Scholar]
- 11.Liu DY. Could using the zona pellucida bound sperm for intracytoplasmic sperm injection (ICSI) enhance the outcome of ICSI? Asian J Androl. 2011;13:197–8. doi: 10.1038/aja.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu F, Qiu Y, Zou Y, Deng ZH, Yang H, et al. Use of zona pellucida-bound sperm for intracytoplasmic sperm injection produces higher embryo quality and implantation than conventional intracytoplasmic sperm injection. Fertil Steril. 2011;95:815–8. doi: 10.1016/j.fertnstert.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Paes Almeida Ferreira de Braga D, Iaconelli A, Jr, Cássia Sávio de Figueira R, Madaschi C, Semião-Francisco L, et al. Outcome of ICSI using zona pellucida-bound spermatozoa and conventionally selected spermatozoa. Reprod Biomed Online. 2009;19:802–7. doi: 10.1016/j.rbmo.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Reid AT, Redgrove K, Aitken RJ, Nixon B. Cellular mechanisms regulating sperm-zona pellucida interaction. Asian J Androl. 2011;13:88–96. doi: 10.1038/aja.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell LA, Nixon B, Aitken RJ. Analysis of chaperone proteins associated with human spermatozoa during capacitation. Mol Hum Reprod. 2007;13:605–13. doi: 10.1093/molehr/gam043. [DOI] [PubMed] [Google Scholar]
- 16.Asquith KL, Baleato RM, McLaughlin EA, Nixon B, Aitken RJ. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J Cell Sci. 2004;117:3645–57. doi: 10.1242/jcs.01214. [DOI] [PubMed] [Google Scholar]
- 17.Gupta SK, Bhandari B, Shrestha A, Biswal BK, Palaniappan C, et al. Mammalian zona pellucida glycoproteins: structure and function during fertilization. Cell Tissue Res. 2012;349:665–78. doi: 10.1007/s00441-011-1319-y. [DOI] [PubMed] [Google Scholar]
- 18.Clark GF. The role of carbohydrate recognition during human sperm-egg binding. Hum Reprod. 2013;28:566–77. doi: 10.1093/humrep/des447. [DOI] [PubMed] [Google Scholar]
- 19.Avella MA, Baibakov B, Dean J. A single domain of the ZP2 zona pellucida protein mediates gamete recognition in mice and humans. J Cell Biol. 2014;205:801–9. doi: 10.1083/jcb.201404025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avella MA, Xiong B, Dean J. The molecular basis of gamete recognition in mice and humans. Mol Hum Reprod. 2013;19:279–89. doi: 10.1093/molehr/gat004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dun MD, Mitchell LA, Aitken RJ, Nixon B. Sperm-zona pellucida interaction: molecular mechanisms and the potential for contraceptive intervention. Handb Exp Pharmacol. 2010;198:139–78. doi: 10.1007/978-3-642-02062-9_9. [DOI] [PubMed] [Google Scholar]
- 22.Dun MD, Aitken RJ, Nixon B. The role of molecular chaperones in spermatogenesis and the post-testicular maturation of mammalian spermatozoa. Hum Reprod Update. 2012;18:420–35. doi: 10.1093/humupd/dms009. [DOI] [PubMed] [Google Scholar]
- 23.Dun MD, Smith ND, Baker MA, Lin M, Aitken RJ, et al. The chaperonin containing TCP1 complex (CCT/TRiC) is involved in mediating sperm-oocyte interaction. J Biol Chem. 2011;286:36875–87. doi: 10.1074/jbc.M110.188888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redgrove KA, Anderson AL, Dun MD, McLaughlin EA, O’Bryan MK, et al. Involvement of multimeric protein complexes in mediating the capacitation-dependent binding of human spermatozoa to homologous zonae pellucidae. Dev Biol. 2011;356:460–74. doi: 10.1016/j.ydbio.2011.05.674. [DOI] [PubMed] [Google Scholar]
- 25.Redgrove KA, Anderson AL, McLaughlin EA, O’Bryan MK, Aitken RJ, et al. Investigation of the mechanisms by which the molecular chaperone HSPA2 regulates the expression of sperm surface receptors involved in human sperm-oocyte recognition. Mol Hum Reprod. 2013;19:120–35. doi: 10.1093/molehr/gas064. [DOI] [PubMed] [Google Scholar]
- 26.Redgrove KA, Nixon B, Baker MA, Hetherington L, Baker G, et al. The molecular chaperone HSPA2 plays a key role in regulating the expression of sperm surface receptors that mediate sperm-egg recognition. PLoS One. 2012;7:e50851. doi: 10.1371/journal.pone.0050851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis RJ. The molecular chaperone concept. Semin Cell Biol. 1990;1:1–9. [PubMed] [Google Scholar]
- 28.Ritossa F. Discovery of the heat shock response. Cell Stress Chaperones. 1996;1:97–8. doi: 10.1379/1466-1268(1996)001<0097:dothsr>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14:630–42. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–11. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–11. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- 32.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–51. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Saibil HR. Chaperone machines in action. Curr Opin Struct Biol. 2008;18:35–42. doi: 10.1016/j.sbi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Mayer MP. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem Sci. 2013;38:507–14. doi: 10.1016/j.tibs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–84. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–92. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eddy EM. Role of heat shock protein HSP70-2 in spermatogenesis. Rev Reprod. 1999;4:23–30. doi: 10.1530/ror.0.0040023. [DOI] [PubMed] [Google Scholar]
- 38.Govin J, Caron C, Escoffier E, Ferro M, Kuhn L, et al. Post-meiotic shifts in HSPA2/HSP70.2 chaperone activity during mouse spermatogenesis. J Biol Chem. 2006;281:37888–92. doi: 10.1074/jbc.M608147200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen RL, O’Brien DA, Jones CC, Rockett DL, Eddy EM. Expression of heat shock proteins by isolated mouse spermatogenic cells. Mol Cell Biol. 1988;8:3260–6. doi: 10.1128/mcb.8.8.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen RL, O’Brien DA, Eddy EM. A novel hsp70-like protein (P70) is present in mouse spermatogenic cells. Mol Cell Biol. 1988;8:828–32. doi: 10.1128/mcb.8.2.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien DA. Stage-specific protein synthesis by isolated spermatogenic cells throughout meiosis and early spermiogenesis in the mouse. Biol Reprod. 1987;37:147–57. doi: 10.1095/biolreprod37.1.147. [DOI] [PubMed] [Google Scholar]
- 42.Zakeri ZF, Wolgemuth DJ, Hunt CR. Identification and sequence analysis of a new member of the mouse HSP70 gene family and characterization of its unique cellular and developmental pattern of expression in the male germ line. Mol Cell Biol. 1988;8:2925–32. doi: 10.1128/mcb.8.7.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosario MO, Perkins SL, O’Brien DA, Allen RL, Eddy EM. Identification of the gene for the developmentally expressed 70 kDa heat-shock protein (P70) of mouse spermatogenic cells. Dev Biol. 1992;150:1–11. doi: 10.1016/0012-1606(92)90002-x. [DOI] [PubMed] [Google Scholar]
- 44.Murashov AK, Wolgemuth DJ. Distinct transcripts are recognized by sense and antisense riboprobes for a member of the murine HSP70 gene family, HSP70.2, in various reproductive tissues. Mol Reprod Dev. 1996;43:17–24. doi: 10.1002/(SICI)1098-2795(199601)43:1<17::AID-MRD3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 45.Dix DJ, Rosario-Herrle M, Gotoh H, Mori C, Goulding EH, et al. Developmentally regulated expression of Hsp70-2 and a Hsp70-2/lacZ transgene during spermatogenesis. Dev Biol. 1996;174:310–21. doi: 10.1006/dbio.1996.0076. [DOI] [PubMed] [Google Scholar]
- 46.Dix DJ, Allen JW, Collins BW, Poorman-Allen P, Mori C, et al. HSP70-2 is required for desynapsis of synaptonemal complexes during meiotic prophase in juvenile and adult mouse spermatocytes. Development. 1997;124:4595–603. doi: 10.1242/dev.124.22.4595. [DOI] [PubMed] [Google Scholar]
- 47.Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, et al. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci U S A. 1996;93:3264–8. doi: 10.1073/pnas.93.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mori C, Nakamura N, Dix DJ, Fujioka M, Nakagawa S, et al. Morphological analysis of germ cell apoptosis during postnatal testis development in normal and Hsp 70-2 knockout mice. Dev Dyn. 1997;208:125–36. doi: 10.1002/(SICI)1097-0177(199701)208:1<125::AID-AJA12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 49.Allen JW, Dix DJ, Collins BW, Merrick BA, He C, et al. HSP70-2 is part of the synaptonemal complex in mouse and hamster spermatocytes. Chromosoma. 1996;104:414–21. doi: 10.1007/BF00352265. [DOI] [PubMed] [Google Scholar]
- 50.Zhu D, Dix DJ, Eddy EM. HSP70-2 is required for CDC2 kinase activity in meiosis I of mouse spermatocytes. Development. 1997;124:3007–14. doi: 10.1242/dev.124.15.3007. [DOI] [PubMed] [Google Scholar]
- 51.Liu M, Shi X, Bi Y, Qi L, Guo X, et al. SHCBP1L, a conserved protein in mammals, is predominantly expressed in male germ cells and maintains spindle stability during meiosis in testis. Mol Hum Reprod. 2014;20:463–75. doi: 10.1093/molehr/gau014. [DOI] [PubMed] [Google Scholar]
- 52.Alekseev OM, Richardson RT, O’Rand MG. Linker histones stimulate HSPA2 ATPase activity through NASP binding and inhibit CDC2/Cyclin B1 complex formation during meiosis in the mouse. Biol Reprod. 2009;81:739–48. doi: 10.1095/biolreprod.109.076497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frost RJ, Hamra FK, Richardson JA, Qi X, Bassel-Duby R, et al. MOV10L1 is necessary for protection of spermatocytes against retrotransposons by Piwi-interacting RNAs. Proc Natl Acad Sci U S A. 2010;107:11847–52. doi: 10.1073/pnas.1007158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasaki T, Marcon E, McQuire T, Arai Y, Moens PB, et al. Bat3 deficiency accelerates the degradation of Hsp70-2/HspA2 during spermatogenesis. J Cell Biol. 2008;182:449–58. doi: 10.1083/jcb.200802113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thress K, Song J, Morimoto RI, Kornbluth S. Reversible inhibition of Hsp70 chaperone function by Scythe and Reaper. EMBO J. 2001;20:1033–41. doi: 10.1093/emboj/20.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimmins S, Sassone-Corsi P. Chromatin remodelling and epigenetic features of germ cells. Nature. 2005;434:583–9. doi: 10.1038/nature03368. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Xia J, Cho KH, Clapham DE, Ren D. CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J Biol Chem. 2007;282:18945–52. doi: 10.1074/jbc.M701083200. [DOI] [PubMed] [Google Scholar]
- 58.Bonnycastle LL, Yu CE, Hunt CR, Trask BJ, Clancy KP, et al. Cloning, sequencing, and mapping of the human chromosome 14 heat shock protein gene (HSPA2) Genomics. 1994;23:85–93. doi: 10.1006/geno.1994.1462. [DOI] [PubMed] [Google Scholar]
- 59.Son WY, Hwang SH, Han CT, Lee JH, Kim S, et al. Specific expression of heat shock protein HspA2 in human male germ cells. Mol Hum Reprod. 1999;5:1122–6. doi: 10.1093/molehr/5.12.1122. [DOI] [PubMed] [Google Scholar]
- 60.Huszar G, Stone K, Dix D, Vigue L. Putative creatine kinase M-isoform in human sperm is identifiedas the 70-kilodalton heat shock protein HspA2. Biol Reprod. 2000;63:925–32. doi: 10.1095/biolreprod63.3.925. [DOI] [PubMed] [Google Scholar]
- 61.Cedenho AP, Lima SB, Cenedeze MA, Spaine DM, Ortiz V, et al. Oligozoospermia and heat-shock protein expression in ejaculated spermatozoa. Hum Reprod. 2006;21:1791–4. doi: 10.1093/humrep/del055. [DOI] [PubMed] [Google Scholar]
- 62.Son WY, Han CT, Hwang SH, Lee JH, Kim S, et al. Repression of hspA2 messenger RNA in human testes with abnormal spermatogenesis. Fertil Steril. 2000;73:1138–44. doi: 10.1016/s0015-0282(00)00496-9. [DOI] [PubMed] [Google Scholar]
- 63.Huszar G, Sbracia M, Vigue L, Miller DJ, Shur BD. Sperm plasma membrane remodeling during spermiogenetic maturation in men: relationship among plasma membrane beta 1,4-galactosyltransferase, cytoplasmic creatine phosphokinase, and creatine phosphokinase isoform ratios. Biol Reprod. 1997;56:1020–4. doi: 10.1095/biolreprod56.4.1020. [DOI] [PubMed] [Google Scholar]
- 64.Ergur AR, Dokras A, Giraldo JL, Habana A, Kovanci E, et al. Sperm maturity and treatment choice of in vitro fertilization (IVF) or intracytoplasmic sperm injection: diminished sperm HspA2 chaperone levels predict IVF failure. Fertil Steril. 2002;77:910–8. doi: 10.1016/s0015-0282(02)03073-x. [DOI] [PubMed] [Google Scholar]
- 65.Huszar G, Ozkavukcu S, Jakab A, Celik-Ozenci C, Sati GL, et al. Hyaluronic acid binding ability of human sperm reflects cellular maturity and fertilizing potential: selection of sperm for intracytoplasmic sperm injection. Curr Opin Obstet Gynecol. 2006;18:260–7. doi: 10.1097/01.gco.0000193018.98061.2f. [DOI] [PubMed] [Google Scholar]
- 66.Huszar G, Ozenci CC, Cayli S, Zavaczki Z, Hansch E, et al. Hyaluronic acid binding by human sperm indicates cellular maturity, viability, and unreacted acrosomal status. Fertil Steril. 2003;79(Suppl 3):1616–24. doi: 10.1016/s0015-0282(03)00402-3. [DOI] [PubMed] [Google Scholar]
- 67.Kovanci E, Kovacs T, Moretti E, Vigue L, Bray-Ward P, et al. FISH assessment of aneuploidy frequencies in mature and immature human spermatozoa classified by the absence or presence of cytoplasmic retention. Hum Reprod. 2001;16:1209–17. doi: 10.1093/humrep/16.6.1209. [DOI] [PubMed] [Google Scholar]
- 68.Huszar G, Jakab A, Sakkas D, Ozenci CC, Cayli S, et al. Fertility testing and ICSI sperm selection by hyaluronic acid binding: clinical and genetic aspects. Reprod Biomed Online. 2007;14:650–63. doi: 10.1016/s1472-6483(10)61060-7. [DOI] [PubMed] [Google Scholar]
- 69.Naaby-Hansen S, Herr JC. Heat shock proteins on the human sperm surface. J Reprod Immunol. 2010;84:32–40. doi: 10.1016/j.jri.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motiei M, Tavalaee M, Rabiei F, Hajihosseini R, Nasr-Esfahani MH. Evaluation of HSPA2 in fertile and infertile individuals. Andrologia. 2013;45:66–72. doi: 10.1111/j.1439-0272.2012.01315.x. [DOI] [PubMed] [Google Scholar]
- 71.Scieglinska D, Krawczyk Z. Expression, function, and regulation of the testis-enriched heat shock HSPA2 gene in rodents and humans. Cell Stress Chaperones. 2014;20:221–35. doi: 10.1007/s12192-014-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nixon B, Mitchell LA, Anderson AL, McLaughlin EA, O’bryan MK, et al. Proteomic and functional analysis of human sperm detergent resistant membranes. J Cell Physiol. 2011;226:2651–65. doi: 10.1002/jcp.22615. [DOI] [PubMed] [Google Scholar]
- 73.Xu H, Liu F, Srakaew N, Koppisetty C, Nyholm PG, et al. Sperm arylsulfatase A binds to mZP2 and mZP3 glycoproteins in a nonenzymatic manner. Reproduction. 2012;144:209–19. doi: 10.1530/REP-11-0338. [DOI] [PubMed] [Google Scholar]
- 74.Wu A, Anupriwan A, Iamsaard S, Chakrabandhu K, Santos DC, et al. Sperm surface arylsulfatase A can disperse the cumulus matrix of cumulus oocyte complexes. J Cell Physiol. 2007;213:201–11. doi: 10.1002/jcp.21113. [DOI] [PubMed] [Google Scholar]
- 75.Tantibhedhyangkul J, Weerachatyanukul W, Carmona E, Xu H, Anupriwan A, et al. Role of sperm surface arylsulfatase A in mouse sperm-zona pellucida binding. Biol Reprod. 2002;67:212–9. doi: 10.1095/biolreprod67.1.212. [DOI] [PubMed] [Google Scholar]
- 76.Carmona E, Weerachatyanukul W, Soboloff T, Fluharty AL, White D, et al. Arylsulfatase a is present on the pig sperm surface and is involved in sperm-zona pellucida binding. Dev Biol. 2002;247:182–96. doi: 10.1006/dbio.2002.0690. [DOI] [PubMed] [Google Scholar]
- 77.Zhou C, Kang W, Baba T. Functional characterization of double-knockout mouse sperm lacking SPAM1 and ACR or SPAM1 and PRSS21 in fertilization. J Reprod Dev. 2012;58:330–7. doi: 10.1262/jrd.2011-006. [DOI] [PubMed] [Google Scholar]
- 78.Martin-Deleon PA. Germ-cell hyaluronidases: their roles in sperm function. Int J Androl. 2011;34:e306–18. doi: 10.1111/j.1365-2605.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 79.Kimura M, Kim E, Kang W, Yamashita M, Saigo M, et al. Functional roles of mouse sperm hyaluronidases, HYAL5 and SPAM1, in fertilization. Biol Reprod. 2009;81:939–47. doi: 10.1095/biolreprod.109.078816. [DOI] [PubMed] [Google Scholar]
- 80.Cayli S, Sakkas D, Vigue L, Demir R, Huszar G. Cellular maturity and apoptosis in human sperm: creatine kinase, caspase-3 and Bcl-XL levels in mature and diminished maturity sperm. Mol Hum Reprod. 2004;10:365–72. doi: 10.1093/molehr/gah050. [DOI] [PubMed] [Google Scholar]
- 81.Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl. 2014;16:31–8. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Avery SV. Molecular targets of oxidative stress. Biochem J. 2011;434:201–10. doi: 10.1042/BJ20101695. [DOI] [PubMed] [Google Scholar]
- 83.Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech. 2010;73:241–78. doi: 10.1002/jemt.20783. [DOI] [PubMed] [Google Scholar]
- 84.Lima SB, Cenedeze MA, Bertolla RP, Filho PA, Oehninger S, et al. Expression of the HSPA2 gene in ejaculated spermatozoa from adolescents with and without varicocele. Fertil Steril. 2006;86:1659–63. doi: 10.1016/j.fertnstert.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 85.Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod. 1998;13:1429–36. doi: 10.1093/humrep/13.6.1429. [DOI] [PubMed] [Google Scholar]
- 86.Huszar G, Vigue L. Correlation between the rate of lipid peroxidation and cellular maturity as measured by creatine kinase activity in human spermatozoa. J Androl. 1994;15:71–7. [PubMed] [Google Scholar]
- 87.Danshina PV, Geyer CB, Dai Q, Goulding EH, Willis WD, et al. Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol Reprod. 2010;82:136–45. doi: 10.1095/biolreprod.109.079699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iguchi N, Tobias JW, Hecht NB. Expression profiling reveals meiotic male germ cell mRNAs that are translationally up- and down-regulated. Proc Natl Acad Sci U S A. 2006;103:7712–7. doi: 10.1073/pnas.0510999103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, et al. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem. 2012;287:33048–60. doi: 10.1074/jbc.M112.366690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu W, Akhand AA, Kato M, Yokoyama I, Miyata T, et al. 4-hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J Cell Sci. 1999;112(Pt 14):2409–17. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- 91.Grune T, Davies KJ. The proteasomal system and HNE-modified proteins. Mol Aspects Med. 2003;24:195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 92.Pickering AM, Davies KJ. Degradation of damaged proteins: the main function of the 20S proteasome. Prog Mol Biol Transl Sci. 2012;109:227–48. doi: 10.1016/B978-0-12-397863-9.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shringarpure R, Grune T, Davies KJ. Protein oxidation and 20S proteasome-dependent proteolysis in mammalian cells. Cell Mol Life Sci. 2001;58:1442–50. doi: 10.1007/PL00000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marques C, Pereira P, Taylor A, Liang JN, Reddy VN, et al. Ubiquitin-dependent lysosomal degradation of the HNE-modified proteins in lens epithelial cells. FASEB J. 2004;18:1424–6. doi: 10.1096/fj.04-1743fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bromfield EG, Nixon B. The function of chaperone proteins in the assemblage of protein complexes involved in gamete adhesion and fusion processes. Reproduction. 2013;145:R31–42. doi: 10.1530/REP-12-0316. [DOI] [PubMed] [Google Scholar]


