Abstract
Capacitation is a series of morphological and metabolic changes necessary for the spermatozoon to achieve fertilizing ability. One of the earlier happenings during mammalian sperm capacitation is the production of reactive oxygen species (ROS) that will trigger and regulate a series of events including protein phosphorylation, in a time-dependent fashion. The identity of the sperm oxidase responsible for the production of ROS involved in capacitation is still elusive, and several candidates are discussed in this review. Interestingly, ROS-induced ROS formation has been described during human sperm capacitation. Redox signaling during capacitation is associated with changes in thiol groups of proteins located on the plasma membrane and subcellular compartments of the spermatozoon. Both, oxidation of thiols forming disulfide bridges and the increase on thiol content are necessary to regulate different sperm proteins associated with capacitation. Reducing equivalents such as NADH and NADPH are necessary to support capacitation in many species including humans. Lactate dehydrogenase, glucose-6-phospohate dehydrogenase, and isocitrate dehydrogenase are responsible in supplying NAD (P) H for sperm capacitation. Peroxiredoxins (PRDXs) are newly described enzymes with antioxidant properties that can protect mammalian spermatozoa; however, they are also candidates for assuring the regulation of redox signaling required for sperm capacitation. The dysregulation of PRDXs and of enzymes needed for their reactivation such as thioredoxin/thioredoxin reductase system and glutathione-S-transferases impairs sperm motility, capacitation, and promotes DNA damage in spermatozoa leading to male infertility.
Keywords: dehydrogenases, oxidases, peroxiredoxins, reactive oxygen species, spermatozoa, thiols, thioredoxins
INTRODUCTION
Mammalian sperm capacitation is an essential process to guarantee fertilization of the mature oocyte.1,2 It is a complex process that normally occurs in the oviduct and involves biochemical and morphological changes to enable the spermatozoon competent to bind to the zona pellucida, penetrate it and finally fuse with the oolema.1,3
Biochemical changes at the level of the plasma membrane and other subcellular compartments have been associated with sperm capacitation.1,2 Early events during capacitation are the activation of adenylyl cyclase (AC) producing cAMP, activation of calcium channels, production of reactive oxygen species (ROS), cholesterol efflux from the plasma membrane, increase of intracellular pH, and activation of protein kinases among others.1,3,4
It is now well-established that mammalian sperm capacitation is an oxidative event.2,3,5 The production of ROS is an early event during the series of modifications that occur during capacitation but increasing concentrations of superoxide anion (O2•–), hydrogen peroxide (H2O2), nitric oxide (NO•), and peroxynitrite (ONOO-)6,7,8,9,10 are produced over time during capacitation. The roles of ROS during capacitation are diverse and complex and involve the activation of several targets located on the plasma membrane and in other sperm compartments. As an early event, O2• and NO• activate AC that produces cAMP during sperm capacitation.11,12 This activates protein kinase A (PKA), which is essential to support the late tyrosine phosphorylation associated with sperm capacitation in all the species studied up to now.4,13,14,15,16 In human sperm, the PKA activity is maximal at 30 min of capacitation.17,18,19
As mentioned above, the production of ROS and activation of the PKA pathway are early events during mammalian sperm capacitation. Other capacitation-associated phosphorylation events have been described – mainly in human spermatozoa – that occur later in capacitation; the necessity of the mitogen-activated protein kinase (MEK), extracellular-regulated kinase (ERK), phosphoinositide-3 kinase/Akt (PI3K/Akt pathways, and tyrosine phosphorylation are essential in guaranteeing activation of the spermatozoon during capacitation.20,21,22,23,24 Interestingly, these phosphorylation events are tightly regulated by ROS21,22 and this regulation is phosphoprotein specific; for example, capacitation by NO• is not prevented by inhibitors of the ERK pathway that inhibit capacitation induced by either bovine serum albumin or other inducers.21 The action of NO• is at the level of Ras that in turn will activate Raf, MEK, ERK and thus generate ERK substrates that are needed for capacitation-associated tyrosine phosphorylation. However, inhibitors of PI3K and Akt do not prevent NO•-induced capacitation or tyrosine phosphorylation, suggesting that this ROS acts downstream of this pathway. It is known that one of the substrates of Akt is nitric oxide synthase (NOS), thus, it is plausible that Akt phosphorylates NOS promoting the increase of cytosolic NO• levels necessary to activate Ras/MEK/ERK and finally to promote tyrosine phosphorylation.21,22
H2O2 also exerts a specific role at the time of activating kinases for sperm capacitation. This ROS is responsible for activating PKC that in turn will phosphorylate Raf to activate MEK-like proteins that are needed for triggering late tyrosine phosphorylation.20 However, H2O2 does not stimulate the ERK pathway, which is activated by NO• and O2•–.25,26 The findings presented above support the idea of crosstalk among different phosphorylation pathways that are specifically and tightly regulated by ROS in very specific manners.21,22
PROTEIN SULFHYDRYL LEVELS CHANGE DURING SPERM CAPACITATION
There are at least two forms of redox regulation. One involves the sulfhydryl/disulfide (SH/SS) pair and the second the ferrous/ferric (Fe+2/Fe+3) pair, usually present in iron-sulfur clusters. The latter is less common in animals but is found in human glutaredoxin and is involved in deglutathionylation.27 The SH/SS pairs are in balance but the redox state is dynamic and can change depending on the needs of the cell.28,29
Cellular responses to ROS include reversible redox signaling and irreversible nonenzymatic reactions,30,31 the extents of which depend on the nature and concentration of the ROS involved. The molecular action of ROS during capacitation is in part due to their reaction with SH by activating or inactivating enzymes.32,33 Protein kinases such as PKC, Ras, and other enzymes such as the already mentioned AC, among others, are susceptible to react with ROS for activation.34,35,36,37 These kinases are responsible for the increased levels of different forms of phosphorylation associated with capacitation.22,38 However, these increases can also be achieved by the complementary inactivation of protein serine/threonine or tyrosine phosphatases. It is known that these enzymes are also frequent targets of ROS,39,40 but it has yet to be confirmed whether this complementary inactivation of phosphatases by ROS occurs during mammalian capacitation.
Complex redox modifications of SH take place during sperm capacitation. There is an increase in the SH content of Triton X-100 detergent-soluble proteins, which is time-dependent occurring during the first 30–60 min of capacitation.32,33 This is a controversial phenomenon as capacitation is generally considered an oxidative process. Interestingly, cryopreservation, a process known to cause oxidative stress, induces premature capacitation41 and increases the SH content of Triton-soluble proteins along with a redistribution of these SH moieties on the sperm plasma membrane.32,42 Thus, it is possible that a rearrangement of SH-carrying proteins on the sperm plasma membrane occurs at the beginning of capacitation.3 A two-dimensional gel electrophoresis approach to human spermatozoa revealed both increases and decreases in sperm proteins during capacitation occurring in a sequence similarly to the production of O2•– and preventable by superoxide dismutase (SOD) or catalase (CAT).33
The identity of those proteins that change their SH content upon capacitation remains to be known; however, it is possible that peroxiredoxins (PRDXs) – antioxidant enzymes that have SH groups in their active sites – might show these changes during capacitation. PRDXs are key players in the modulation of ROS signaling in somatic cells.43,44,45 Some sperm proteins, with a molecular mass and isoelectric point similar to those of PRDXs,46,47 are oxidized by H2O2 during capacitation.33 The role of PRDXs in human spermatozoa is discussed in a separate section below.
SPERM OXIDASE AND SOURCES OF REACTIVE OXYGEN SPECIES FOR SPERM CAPACITATION
The identity of the sperm oxidase involved in capacitation remains elusive. Importantly, it is not clear whether the same enzyme is responsible for generating both O2•– and NO• during capacitation, depending on the species under study.
Two types of enzymes could responsible for ROS generation during sperm capacitation: NAD (P) H oxidases and NOS. One of the first candidates for O2•– generation in human spermatozoa was NOX1; however, the characteristics of this production (measured by chemiluminescence using the O2•–-specific probe MCLA) between the sperm oxidase and NOX1 from neutrophils are completely different.3,6 Thus, (1) the amounts of O2•– produced by spermatozoa during capacitation are more than three orders of magnitude lower than those of activated neutrophils. The O2•– production takes place over a period of hours in spermatozoa instead of the 30–40 min seen in leukocytes; (2) zinc (Zn2+) or semenogelin (Sg) have a greater inhibitory effect on O2•– production in spermatozoa than in neutrophils; and (3) kinases such as PKC, PTK, and ERK, which activate NOX1 have no influence in production by human spermatozoa.2,25,48 Immunocytochemistry and immunoblotting studies also confirm the absence of NOX1, NOX2, and NOX4 in human spermatozoa.49 Another plausible candidate might be NOX5, which has been identified and associated with motility in human and equine spermatozoa.49,50,51 However, because of its localization – mostly in the flagellum and midpiece and its regulation by PKC – it is unlikely that this isoform is the source of O2•– required for sperm capacitation.
As mentioned above, NO• is another important ROS involved in mammalian sperm capacitation6,7,8,9,10 and several NOS isoforms have been described in mouse and human spermatozoa that might play important roles as generators of NO•, because specific inhibitors of this enzymes such as L-NAME or L-NMMA prevent sperm capacitation in these species.52 Moreover, ROS can be induced by other forms of ROS during sperm capacitation.53 Thus, human spermatozoa incubated with DA-NONOate (a NO• donor) and SOD, a scavenger of O2•–, or with the xanthine–xanthine oxidase (X–XO) system, a well-known O2•– generator and either L-NAME or L-NMMA, were unable to undergo capacitation. Moreover, the addition of DA-NONOate triggered the production of O2•– in a dose-dependent manner and the production of NO• was stimulated by the X–XO system. This series of experiments demonstrates that the production of O2•– depends on NO•, and vice-versa. Although mammalian spermatozoa are able to produce O2•– and NO• during capacitation it is still yet to be elucidated whether the sperm oxidase is one enzyme that produces both forms of ROS, or whether oxidase/s and NOS are present in the plasma membrane and are responsible for producing the ROS necessary for capacitation.
Capacitation-associated ROS production must occur at the plasma membrane level in human and bull spermatozoa because capacitation was prevented by the addition of SOD or CAT to the incubation medium (Figure 1).8,53,54 These antioxidant enzymes act outside the spermatozoon, removing the ROS generated upon stimulation with capacitation inducers in both species.8,53,54 Noteworthy, ROS production by human spermatozoa stimulated with NADPH cannot be prevented by mitochondrial inhibitors such as antimycin A, rotenone or carbonyl cyanide m-chlorophenylhydrazone.55 From these studies, it is clear that the sperm oxidase resides in the plasma membrane and is unlikely that the mitochondria are the source of ROS for capacitation.
Figure 1.
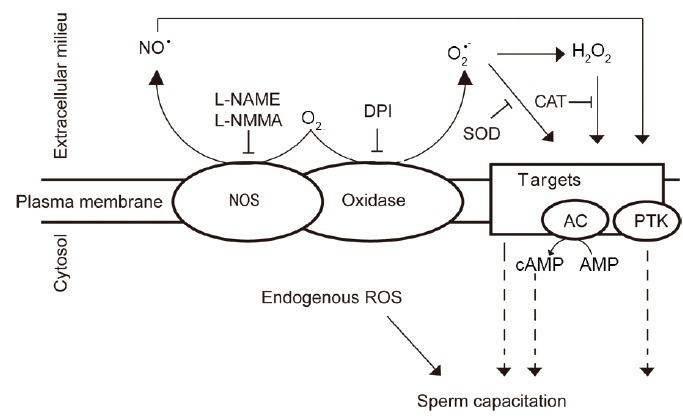
Production of ROS during mammalian sperm capacitation. Capacitation-associated production of ROS occurs on the plasma membrane of the spermatozoon. Still unrevealed a putative sperm oxidase produces O2•– that can dismutate spontaneously to form H2O2, triggering capacitation. SOD and CAT, both acting extracellularly, are able to block sperm capacitation, thus confirming that the production of these ROS forms is at the level of the plasma membrane. NO is also needed for sperm capacitation. Candidates for the source of this ROS could be a specific NOS or an oxidase with intrinsic NOS activity since L-NAME or L-NMMA–both inhibitors of NOS activity – prevent capacitation. It is also plausible that endogenous production of ROS is required for capacitation. AC: adenylyl cyclase; PTK: protein tyrosine kinase; ROS: reactive oxygen species; SOD: superoxide dismutase; NO: nitric oxide; CAT: catalase; H2O2: hydrogen peroxide; O2•–: superoxide anion; NOS: nitric oxide synthase.
Proteomics analyses of human spermatozoa revealed the presence of other oxidases like DUOX2.56 This enzyme is capable of generating H2O2 and might be responsible for the oxidative stress present in human spermatozoa in some cases of male infertility.57,58,59 It is yet to be demonstrated whether DUOX2 contributes to the generation of ROS necessary for capacitation. Because it shares characteristics with the NADPH oxidase of phagocytes,56 it is unlikely that DUOX2 has a critical role in the generation of physiological levels of ROS for capacitation.
Regardless of the identity of the sperm oxidase, it is clear that NAD (P) H is essential to generate either O2•– or NO•.60 Possible sources of these reducing equivalents were identified in studies using bull spermatozoa under capacitating conditions. The isoform C4 of lactate dehydrogenase is specific to testis and spermatozoa, and it was found in several species including the bovine and human.61,62 It generates NADH upon oxidation of lactate into pyruvate. It is found in the cytosol, in mitochondria and the plasma membrane of many species63,64,65 and this isoform represents more than 80% of LDH activity in spermatozoa.61 Because of its multiple locations, LDH-C4 is a strong candidate to generate NADH in different compartments of the spermatozoon. LDH-C4 has been associated with fertility, because of its participation in the energetic metabolism of mature spermatozoa66 and in capacitation evidenced in studies on bull and mouse spermatozoa.65,67 Moreover, a low level of LDH-C4 activity has been associated with partial or total reductions in sperm motility and concentration.68
Spermatozoa from the bull (and other species) utilize pyruvate and lactate (generated by LDH-C4) as oxidative substrates for mitochondrial respiration69 and capacitation65 (Figure 2). The cytosolic isoform of LDH-C4 converts lactate into pyruvate and NADH. Then, pyruvate can enter into the mitochondrial to be converted into acetyl-CoA by pyruvate dehydrogenase and enters into the Krebs cycle to generate reducing equivalents that will be used in the respiratory chain to generate ATP. This ATP will be used for energy purposes and to provide phosphate groups to support the series of phosphorylation events required during sperm capacitation.22,65,70 The pyruvate not used for energy purposes could be converted into lactate by mitochondrial LDH-C4 and will diffuse to the cytosol to re-feed the production of O2•– by the oxidase65 (Figure 2).
Figure 2.
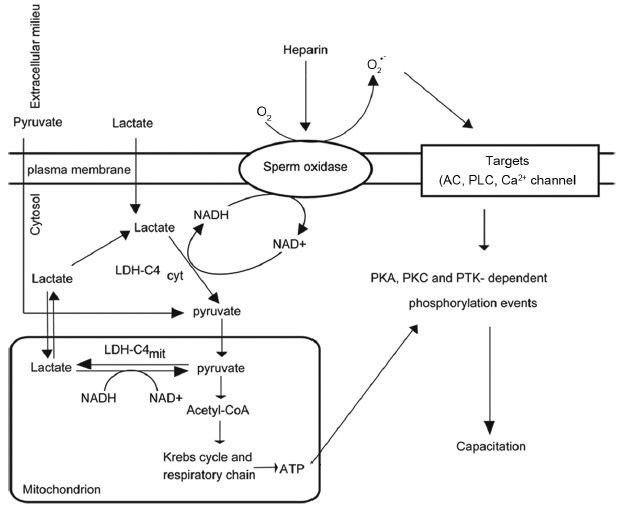
Role of lactate dehydrogenase as a supplier of NADH for bull sperm capacitation. Cytosolic and mitochondrial LDHC4 are important to supply NADH for the sperm oxidase that produces extracellular O2•– for bull sperm capacitation. Lactate is converted by the LDH-C4cyt into pyruvate and NADH that will be used as oxidases for capacitation. The pyruvate will enter into the mitochondrion to be converted into acetyl-CoA and enter into the Krebs cycle. Then, reducing equivalents will be transferred to the respiratory chain to produce ATP that will be used for energy purposes and to provide phosphate groups for a series of phosphorylation events. AC: adenylyl cyclase; PLC: phospholipase C; PKA: protein kinase A; PKC: protein kinase C; PTK: protein tyrosine kinase; O2•–: superoxide anion; LDH-C4cyt: cytosolic lactate dehydrogenase-C4.
Although the extracellular production of O2•– necessary for sperm capacitation is well-documented in different mammalian species,6,7,8,9,10 it is possible that other oxidases might exist in the spermatozoon and be involved in capacitation. The incubation of bull or human spermatozoa with NADH promotes capacitation without involving extracellular production of O2•–.65,71 The existence of a LDH-C4 in the plasma membrane of bull spermatozoa65 suggests that extracellular NADH added to the medium can be used along with pyruvate (already present in the medium) by this enzyme to produce lactate that will diffuse into the cytosol and be used by cytosolic oxidases that will generate O2•– and/or H2O2 to stimulate targets on the plasma membrane (e.g., AC) or in the cytosol (e.g., kinases) to promote capacitation (Figure 3).
Figure 3.
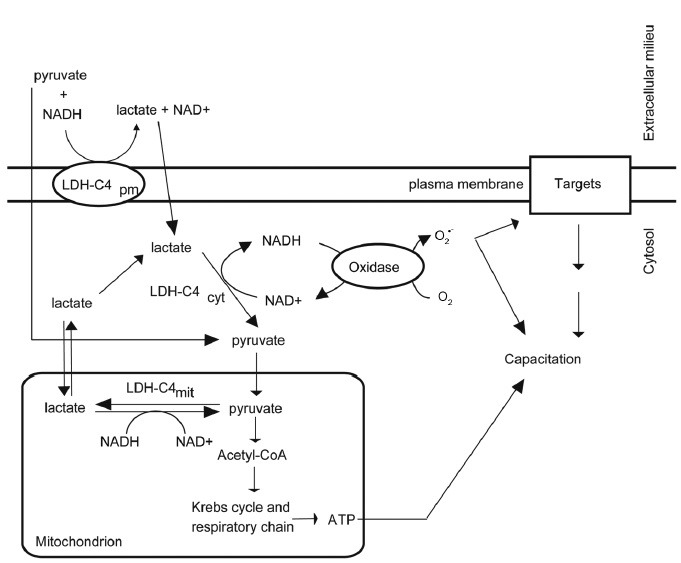
Participation of isoforms of LDH-C4 during bull sperm capacitation induced by NADH in vitro. The LDH-C4pm located at the plasma membrane utilizes exogenous pyruvate and NADH to produce lactate and NAD+. Lactate will then enter into the spermatozoon and be converted into pyruvate and NADH by the LDHcyt as described in Figure 2. Cytosolic oxidases can then utilize the NADH to generate cytosolic O2•– needed for capacitation. ROS: reactive oxygen species; LDH-C4pm: lactate dehydrogenase-C4; O2•– superoxide anion.
The addition of NADPH has been associated with the production of O2•– in human spermatozoa, which is not prevented by inhibitors of the respiratory chain.55 Even though spermatozoa are able to utilize this reducing equivalent to generate O2•–, the inability of SOD or CAT to prevent NADPH-induced capacitation, clearly indicates that extracellular NADPH is not involved in the generation of O2•– necessary for capacitation.65,71 This discrepancy has been resolved recently; thus, extracellular NADPH-induced capacitation and NO• production, which are prevented by L-NMMA (an inhibitor of NOS) but not by SOD.48
The in vivo supply of NADPH could be accomplished by two different enzymes: glucose 6-phosphate dehydrogenase (G6PDH) and isocitrate dehydrogenase (ICDH) (Figure 4). Both enzymes are present in the cytosol of spermatozoa; however, their presence will vary depending on the species under study. For instance, G6PDH is present in human72,73 but absent in bull spermatozoa.70,74 The presence of ICDH activity was found in bull spermatozoa and its inhibitor, oxalomalate, prevented sperm capacitation, suggesting an important role for this dehydrogenase to activate bull spermatozoa.70 Proteomics studies by different laboratories also confirmed the presence of ICDH in human spermatozoa;56,75,76 whether the ICDH is involved in human sperm capacitation remains unknown.
Figure 4.
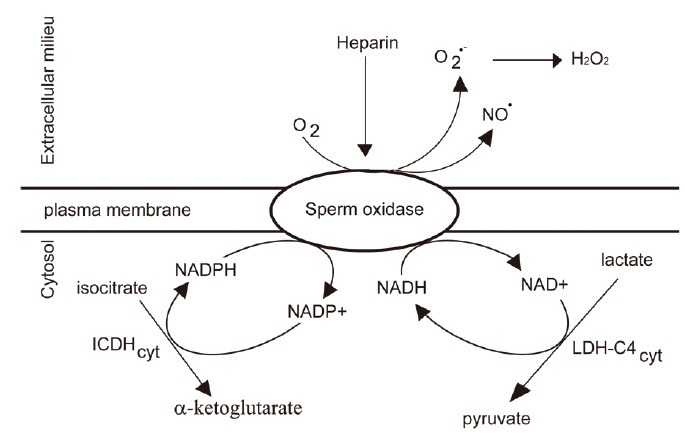
Sources of NAD(P)H for production of ROS during bovine sperm capacitation. The physiological inducer heparin promotes activation of the sperm oxidase in bull spermatozoa. The ICDHcyt and of LDH-C4cyt supply NADPH and NADH necessary for producing ROS. ROS: reactive oxygen species; ICDHcyt: cytosolic isoforms of isocitrate dehydrogenase; LDH-C4cyt: cytosolic lactate dehydrogenase C4.
The regulation of extracellular O2•– production involved in human sperm capacitation has been elucidated recently. Upon ejaculation, Sg, the major protein present in the seminal plasma and Zn2+, also abundant in this fluid, keep the sperm oxidase inactive before capacitation.77 When spermatozoa are incubated under capacitating conditions, Sg and Zn2+ are removed from the plasma membrane and thus release the blockage to the sperm oxidase, allowing the production of O2•–. However, this efficient regulatory mechanism that avoids premature capacitation before the spermatozoon reaches the oviduct cannot stop the production of this ROS once it started: thus, immediately upon production, O2•– is used and the excess dismutates to H2O2 that accumulates and becomes a potential toxic factor for sperm viability. In the following section, mechanisms to deal with ROS production and to avoid potential toxic effects of these reactive molecules in the spermatozoon will be discussed.
PEROXIREDOXINS, NEWLY DISCOVERED MODULATORS OF SPERM FUNCTION
Peroxiredoxins are acidic proteins of ~ 20–31 kDa with one or two Cys residues at the active site that are required for their enzymatic activity.78 They can reduce both organic and inorganic hydroperoxides79 and ONOO–.80,81 PRDXs have sufficient SH reactivity to be direct targets for H2O2, and this is consistent with the findings that they are readily oxidized in cells exposed to low levels of this ROS.82,83,84,85
The capability of scavenging different ROS makes PRDXs valuable candidates in the protection of spermatozoa against oxidative stress. The six members of the PRDX family are differentially localized in subcellular compartments of human spermatozoa,86,87 circumstances that allow local control of ROS levels. Within the PRDX family, PRDX6 is the most abundant isoform in human spermatozoa.86 There are decreases in the amounts of PRDX1 and PRDX6 in spermatozoa from infertile patients with clinical varicocele or idiopathic infertility.88 Interestingly, the level of SH oxidation of PRDX1 and PRDX6 is increased in spermatozoa from these patients. Along with these abnormalities in the amount or status of SH of PRDXs, these patients showed increased levels of sperm DNA fragmentation (measured by the Sperm Chromatin Structure Assay) and lower motility than healthy controls. Moreover, multiple regression analyses confirmed that the DNA fragmentation levels depend on the status of oxidation of PRDXs. Recently, it was reported that mice lacking PRDX6 are susceptible to oxidative stress showing reduced sperm motility and sperm chromatin abnormalities.89 From these studies, in human infertile patients and mice, it is clear that PRDXs play important roles in the protection of spermatozoa against oxidative stress.
An important feature of PRDXs is their ability to form complexes ranging from dimers to decamers of high molecular mass upon oxidation.90 These high molecular weight complexes can be reproduced by incubating human spermatozoa with high concentrations of H2O2, and they contain a sulfonated form of PRDX6 and presumably also of PRDX1.86 When PRDXs are sulfonated, they become chaperones to protect other proteins from oxidative stress. These complexes containing a sulfonated form of PRDX6 can be found in spermatozoa from infertile patients.88 Oxidized PRDX6 is reduced by the glutathione-S transferase pi (GSTpi) and glutathione (GSH) system.91,92 It is well-known that the concentration of GSH in spermatozoa is extremely lower than in somatic cells (0.3 mM and 10 mM, respectively),93,94 thus the recycling system exerted by GSTpi is very limited in the spermatozoon. Moreover, formation of the sulfonated form of PRDX6 is irreversible,95 in contrast to sulfonated 2-Cys PRDXs that can be reduced by sestrins or sulfiredoxin.43,96 Together, these data highlight the importance of sufficient PRDXs in the spermatozoon to assure normal function and demonstrate the high sensitivity of PRDXs to oxidative stress. Based on the evidence presented, the spermatozoon has a limited system to fight against oxidative stress, given its high sensitivity when challenged with an oxidative stress.57,97 This is because the cytosolic space where SOD and cytosolic PRDXs are located is very limited.73 2-Cys PRDXs (PRDX1–4) and PRDX5 can be reduced by the thioredoxin/thioredoxin reductase system that is present in the spermatozoa, and play important roles in protection against oxidative stress. The need for functional TRXs in the protection of spermatozoa has been demonstrated using Txndc2 and Txndc3 double knockout male mice; these animals show impaired sperm motility and elevated DNA damage and impaired chromatin in spermatozoa upon aging,98 a situation directly linked to the promotion of oxidative stress in vivo.57,98,99
Although other antioxidant enzymes such as glutathione peroxidases (GPX) and CAT may be candidates in the defense of the spermatozoon against oxidative stress, the sperm's H2O2 scavenging capacity does not involve these enzymes. CAT is present in peroxisomes and leaves the spermatozoon with the residual body during spermiogenesis.100 Moreover, its inhibition by sodium azide did not reduce H2O2 scavenging capacity nor increased lipid peroxidation in human spermatozoa treated with 1 mM H2O2.87 GPX2, 3 and 5 are not present in the human testis, seminal plasma or spermatozoa101,102 and GPX4 is inactive as an antioxidant enzyme but is important in the formation of the mitochondrial sheath.103,104,105 The possible role of GPX1 in spermatozoa is controversial because GPX1 activity was measured using cumene hydroperoxide and NADPH,106 substrates also used by PRDXs. In summary, PRDXs and the TRX/TRD system are major protectors of spermatozoa, depending on the levels of oxidative stress.
INVOLVEMENT OF PEROXIREDOXINS IN SPERM CAPACITATION
In somatic cells, PRDXs play roles not only as protectors against oxidative stress but also in modulating ROS signaling.43,44,45 In the case of the human spermatozoon, I have already explained how elevated levels of Sg and Zn2+ in seminal plasma prevent the premature capacitation.77 However, it is still to be elucidated how the spermatozoon controls the levels of the produced ROS to keep within a normal physiological range and avoid toxicity.
The PRDX family members are attractive candidates in regulating the levels of ROS for physiological functions because of their abundance and strategic localizations.86,87 Human spermatozoa incubated under capacitating conditions with fetal cord serum ultrafiltrate in the presence of thiostrepton, an inhibitor of 2-Cys PRDXs,107 showed reduced levels of tyrosine phosphorylation in a dose-dependent manner compared with controls in the absence of the inhibitor (Figure 5). These results indicate the need for active 2-Cys PRDXs to assure tyrosine phosphorylation during capacitation, possibly by regulating ROS action.
Figure 5.
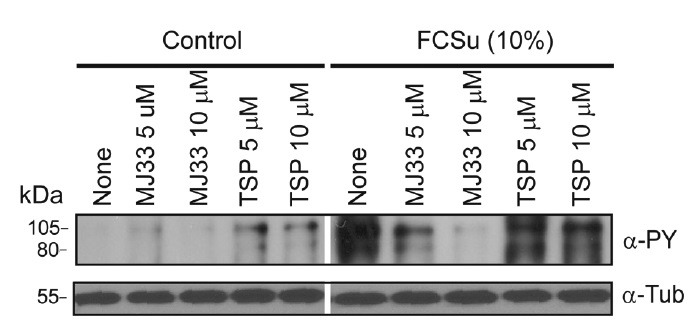
Inhibition of PRDXs and human sperm capacitation. Percoll density gradient-selected spermatozoa were incubated in BWW medium supplemented with FCSu for 4 h at 37°C in the presence or absence of TSP or MJ33, inhibitors of the 2-Cys PRDXs and of Ca2+-independent phospholipase A2 activity of PRDX6. At the end of the treatment, sperm proteins were supplemented with sample buffer containing dithiothreitol, electrophoresed and blotted with a mouse monoclonal anti-phosphotyrosine antibody (clone 4G10)(Upstate Biotechnology, Inc., (Lake Placid, NY, USA) and with an anti-tubulin antibody as a loading control. All samples are from the same gel. Blots are representative of three separate experiments using semen samples from different healthy donors. FCSu: fetal cord serum ultrafiltrate; TSP: thiostrepton; PRDXs: peroxiredoxins.
PRDX6 is the only family member with Ca2+-independent phospholipase A2 (Ca2+ iPLA2) activity.106 When we incubated spermatozoa under capacitating conditions in the presence of 1-hexadecyl-3-trifluoroethylglycero-sn-2-phosphomethanol (MJ33), an inhibitor of the Ca2+ iPLA2 activity of PRDX6,108 we also observed a reduction in tyrosine phosphorylation to a level similar to those of noncapacitated spermatozoa (Figure 5). This experiment opens new avenues for research to elucidate the regulation of phospholipids and of restructuring of plasma membrane components during sperm capacitation. From these studies, we can hypothesize that 2-Cys PRDXs are needed to control the ROS produced during capacitation to assure the signaling required to make the spermatozoon competent to recognize and fertilize the oocyte (Figure 6). This is supported by the fact that low amounts and thiol oxidation of PRDXs are associated with men infertility.88
Figure 6.
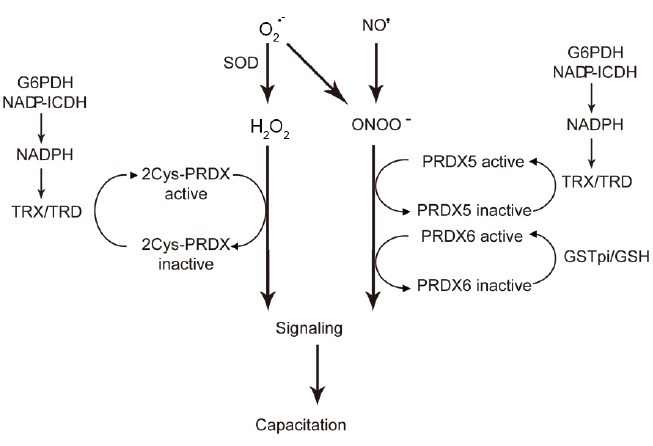
PRDXs regulate ROS signaling during human sperm capacitation. O2•–, H2O2, NO., and ONOO– are produced upon specific stimulation by capacitation inducers. 2-Cys PRDXs becomes oxidized and, therefore, inactive by reacting with H2O2. PRDX5 and PRDX6 react with either H2O2 or ONOO– and also become inactivated. This inactivation of PRDXs allows the rise of ROS in the different subcellular compartments of the spermatozoon to trigger the redox signaling necessary for capacitation. When the signal is delivered, PRDXs are reactivated by the thioredoxin–thioredoxin reductase system (for 2-Cys PRDXs and PRDX5) and by glutathione-S-transferases coupled to reduce GSH (for PRDX6). To accomplish the reactivation of PRDXs, it is necessary for a sufficient supply of NADPH (generated by G6PDH and by NAP-dependent ICDH) and of GSH. Failure to supply sufficient NADPH and GSH will reduce the ability to reactivate PRDXs and, therefore, permit the rise of ROS to toxic levels. ROS: reactive oxygen species; O2•–: superoxide anion; H2O2: hydrogen peroxide; NO•: nitric oxide; ONOO–: peroxynitrite; GSH: glutathione; G6PDH: glucose 6-phosphate dehydrogenase; ICDH: isocitrate dehydrogenase; PRDXs: peroxiredoxins.
Peroxiredoxins need to be reduced after being oxidized by ROS to keep at low levels these reactive molecules and avoid toxicity. The TRX/TRD system and/GSTpi should have sufficient supply of NADPH and of GSH, respectively, to assure the activity of PRDXs (Figure 6). Intact activity of G6PDH and of ICDH is needed to guarantee the supply of NADPH required for the reduction of oxidized TRXs. Failure to obtain sufficient amounts of NADPH or GSH by spermatozoa will promote an sustained inactivation of PRDXs that will lead to impairment of capacitation and motility by increasing redox-dependent modifications such as S-glutathionylation or tyrosine nitration as seen in human spermatozoa under conditions of oxidative stress.109,110
CONCLUSION
Mammalian sperm capacitation is an oxidative event. The production of different types of ROS is a necessary step in the promotion of this process for the spermatozoon to be able to fertilize the mature oocyte. ROS production is an enzymatic event driven by the putative sperm oxidase, although its identity is still elusive. Many research strategies have demonstrated that ROS production occurs mainly in the plasma membrane because SOD and CAT prevent capacitation, at least in human and bovine spermatozoa. However, the participation of cytosolic oxidases is possible and needs further investigation. NO• production has been also associated with capacitation. Many forms of NOS have been described in mouse and human spermatozoa, and their specific inhibitors were able to prevent capacitation, thus supporting the need for NOS activity in this process. To produce ROS, the sperm oxidase and NOS require NAD (P) H that can be supplied by dehydrogenases located both in the plasma membrane and the cytosol.
Both Sg and Zn2+ act in preventing the premature capacitation. However, how redox signaling is regulated once ROS are being produced is still unknown. PRDXs are attractive candidates to accomplish this regulation as they have not only antioxidant properties but they are also able to control the production and action of ROS in different compartments of somatic cells. Here, preliminary evidence has been presented that account for a similar regulatory mechanism in human sperm capacitation.
Redox signaling is necessary for capacitation and can be disrupted by oxidative stresses, as observed in spermatozoa from infertile men. Functional PRDXs, the TRX/TRD system and GSTpi along with sufficient concentrations of GSH and NAPDH are needed to assure redox signaling in the spermatozoon.
ACKNOWLEDGMENTS
I would like to thank Ms. Tania Morielli for her technical skill in performing the experiments and all the volunteers who donated semen samples for the experiments presented in this review. This work was supported by The Canadian Institutes of Health Research (MOP 133661).
COMPETING FINANCIAL INTERESTS
I declare no competing financial interest in this research.
REFERENCES
- 1.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill D, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 189–318. [Google Scholar]
- 2.de Lamirande E, Leclerc P, Gagnon C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol Hum Reprod. 1997;3:175–94. doi: 10.1093/molehr/3.3.175. [DOI] [PubMed] [Google Scholar]
- 3.de Lamirande E, O’Flaherty C. Sperm capacitation as an oxidative event. In: Aitken J, Alvarez J, Agawarl A, editors. Studies on Men Health and Fertility, Oxidative Stress in Applied Basic Research and Clinical Practice. New York, NY, USA: Springer Science Business Media; 2012. pp. 57–94. [Google Scholar]
- 4.Ecroyd HW, Jones RC, Aitken RJ. Endogenous redox activity in mouse spermatozoa and its role in regulating the tyrosine phosphorylation events associated with sperm capacitation. Biol Reprod. 2003;69:347–54. doi: 10.1095/biolreprod.102.012716. [DOI] [PubMed] [Google Scholar]
- 5.Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci. 1995;108(Pt 5):2017–25. doi: 10.1242/jcs.108.5.2017. [DOI] [PubMed] [Google Scholar]
- 6.de Lamirande E, Gagnon C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic Biol Med. 1995;18:487–95. doi: 10.1016/0891-5849(94)00169-k. [DOI] [PubMed] [Google Scholar]
- 7.Herrero MB, de Lamirande E, Gagnon C. Nitric oxide regulates human sperm capacitation and protein-tyrosine phosphorylation in vitro . Biol Reprod. 1999;61:575–81. doi: 10.1095/biolreprod61.3.575. [DOI] [PubMed] [Google Scholar]
- 8.O’Flaherty C, Beorlegui N, Beconi MT. Participation of superoxide anion in the capacitation of cryopreserved bovine sperm. Int J Androl. 2003;26:109–14. doi: 10.1046/j.1365-2605.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- 9.O’Flaherty C, Rodriguez P, Srivastava S. L-arginine promotes capacitation and acrosome reaction in cryopreserved bovine spermatozoa. Biochim Biophys Acta. 2004;1674:215–21. doi: 10.1016/j.bbagen.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez PC, O’Flaherty CM, Beconi MT, Beorlegui NB. Nitric oxide-induced capacitation of cryopreserved bull spermatozoa and assessment of participating regulatory pathways. Anim Reprod Sci. 2005;85:231–42. doi: 10.1016/j.anireprosci.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Belén Herrero M, Chatterjee S, Lefièvre L, de Lamirande E, Gagnon C. Nitric oxide interacts with the cAMP pathway to modulate capacitation of human spermatozoa. Free Radic Biol Med. 2000;29:522–36. doi: 10.1016/s0891-5849(00)00339-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Zheng RL. Promotion of human sperm capacitation by superoxide anion. Free Radic Res. 1996;24:261–8. doi: 10.3109/10715769609088023. [DOI] [PubMed] [Google Scholar]
- 13.Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, et al. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–37. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- 14.Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3’,5’monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod. 1996;55:684–92. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]
- 15.Roy SC, Atreja SK. Effect of reactive oxygen species on capacitation and associated protein tyrosine phosphorylation in buffalo (Bubalus bubalis) spermatozoa. Anim Reprod Sci. 2008;107:68–84. doi: 10.1016/j.anireprosci.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Galantino-Homer HL, Visconti PE, Kopf GS. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3’5’-monophosphate-dependent pathway. Biol Reprod. 1997;56:707–19. doi: 10.1095/biolreprod56.3.707. [DOI] [PubMed] [Google Scholar]
- 17.Lefièvre L, Jha KN, de Lamirande E, Visconti PE, Gagnon C. Activation of protein kinase A during human sperm capacitation and acrosome reaction. J Androl. 2002;23:709–16. [PubMed] [Google Scholar]
- 18.Harrison RA. Rapid PKA-catalysed phosphorylation of boar sperm proteins induced by the capacitating agent bicarbonate. Mol Reprod Dev. 2004;67:337–52. doi: 10.1002/mrd.20028. [DOI] [PubMed] [Google Scholar]
- 19.O’Flaherty C, de Lamirande E, Gagnon C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: modulation and protein kinase A dependency. Mol Hum Reprod. 2004;10:355–63. doi: 10.1093/molehr/gah046. [DOI] [PubMed] [Google Scholar]
- 20.O’Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species and protein kinases modulate the level of phospho-MEK-like proteins during human sperm capacitation. Biol Reprod. 2005;73:94–105. doi: 10.1095/biolreprod.104.038794. [DOI] [PubMed] [Google Scholar]
- 21.O’Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic Biol Med. 2006;40:1045–55. doi: 10.1016/j.freeradbiomed.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 22.O’Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med. 2006;41:528–40. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Luconi M, Barni T, Vannelli GB, Krausz C, Marra F, et al. Extracellular signal-regulated kinases modulate capacitation of human spermatozoa. Biol Reprod. 1998;58:1476–89. doi: 10.1095/biolreprod58.6.1476. [DOI] [PubMed] [Google Scholar]
- 24.Nauc V, De Lamirande E, Leclerc P, Gagnon C. Inhibitors of phosphoinositide 3-kinase, LY294002 and wortmannin, affect sperm capacitation and associated phosphorylation of proteins differently: Ca2+-dependent divergences. J Androl. 2004;25:573–85. doi: 10.1002/j.1939-4640.2004.tb02828.x. [DOI] [PubMed] [Google Scholar]
- 25.de Lamirande E, Gagnon C. The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol Hum Reprod. 2002;8:124–35. doi: 10.1093/molehr/8.2.124. [DOI] [PubMed] [Google Scholar]
- 26.Thundathil J, de Lamirande E, Gagnon C. Nitric oxide regulates the phosphorylation of the threonine-glutamine-tyrosine motif in proteins of human spermatozoa during capacitation. Biol Reprod. 2003;68:1291–8. doi: 10.1095/biolreprod.102.008276. [DOI] [PubMed] [Google Scholar]
- 27.Lillig CH, Berndt C, Vergnolle O, Lönn ME, Hudemann C, et al. Characterization of human glutaredoxin 2 as iron-sulfur protein: a possible role as redox sensor. Proc Natl Acad Sci U S A. 2005;102:8168–73. doi: 10.1073/pnas.0500735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–37. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 30.Halliwell B, Gutteridge J, editors. Free Radicals in Biology and Medicine. New York: Oxford University Press; 2007. Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death; pp. 187–267. [Google Scholar]
- 31.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–58. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 32.de Lamirande E, Gagnon C. Paradoxical effect of reagents for sulfhydryl and disulfide groups on human sperm capacitation and superoxide production. Free Radic Biol Med. 1998;25:803–17. doi: 10.1016/s0891-5849(98)00156-7. [DOI] [PubMed] [Google Scholar]
- 33.de Lamirande E, Gagnon C. Redox control of changes in protein sulfhydryl levels during human sperm capacitation. Free Radic Biol Med. 2003;35:1271–85. doi: 10.1016/s0891-5849(03)00501-x. [DOI] [PubMed] [Google Scholar]
- 34.Knapp LT, Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem. 2000;275:24136–45. doi: 10.1074/jbc.M002043200. [DOI] [PubMed] [Google Scholar]
- 35.Tan CM, Xenoyannis S, Feldman RD. Oxidant stress enhances adenylyl cyclase activation. Circ Res. 1995;77:710–7. doi: 10.1161/01.res.77.4.710. [DOI] [PubMed] [Google Scholar]
- 36.Gopalakrishna R, Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989;86:6758–62. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimon-Gadal S, Gerbaud P, Keryer G, Anderson W, Evain-Brion D, et al. In vitro effects of oxygen-derived free radicals on type I and type II cAMP-dependent protein kinases. J Biol Chem. 1998;273:22833–40. doi: 10.1074/jbc.273.35.22833. [DOI] [PubMed] [Google Scholar]
- 38.de Lamirande E, O’Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta. 2008;1784:106–15. doi: 10.1016/j.bbapap.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Hecht D, Zick Y. Selective inhibition of protein tyrosine phosphatase activities by H2O2 and vanadate in vitro . Biochem Biophys Res Commun. 1992;188:773–9. doi: 10.1016/0006-291x(92)91123-8. [DOI] [PubMed] [Google Scholar]
- 40.Muda M, Boschert U, Dickinson R, Martinou JC, Martinou I, et al. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J Biol Chem. 1996;271:4319–26. doi: 10.1074/jbc.271.8.4319. [DOI] [PubMed] [Google Scholar]
- 41.Cormier N, Sirard MA, Bailey JL. Premature capacitation of bovine spermatozoa is initiated by cryopreservation. J Androl. 1997;18:461–8. [PubMed] [Google Scholar]
- 42.Chatterjee S, de Lamirande E, Gagnon C. Cryopreservation alters membrane sulfhydryl status of bull spermatozoa: protection by oxidized glutathione. Mol Reprod Dev. 2001;60:498–506. doi: 10.1002/mrd.1115. [DOI] [PubMed] [Google Scholar]
- 43.Jönsson TJ, Johnson LC, Lowther WT. Structure of the sulphiredoxin-peroxiredoxin complex reveals an essential repair embrace. Nature. 2008;451:98–101. doi: 10.1038/nature06415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhee SG, Jeong W, Chang TS, Woo HA. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance. Kidney Int Suppl. 2007;106:S3–8. doi: 10.1038/sj.ki.5002380. [DOI] [PubMed] [Google Scholar]
- 45.Woo HA, Chae HZ, Hwang SC, Yang KS, Kang SW, et al. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–6. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 46.Martínez-Heredia J, Estanyol JM, Ballescà JL, Oliva R. Proteomic identification of human sperm proteins. Proteomics. 2006;6:4356–69. doi: 10.1002/pmic.200600094. [DOI] [PubMed] [Google Scholar]
- 47.Jamaluddin M, Wiktorowicz JE, Soman KV, Boldogh I, Forbus JD, et al. Role of peroxiredoxin 1 and peroxiredoxin 4 in protection of respiratory syncytial virus-induced cysteinyl oxidation of nuclear cytoskeletal proteins. J Virol. 2010;84:9533–45. doi: 10.1128/JVI.01005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Lamirande E, Lamothe G, Villemure M. Control of superoxide and nitric oxide formation during human sperm capacitation. Free Radic Biol Med. 2009;46:1420–7. doi: 10.1016/j.freeradbiomed.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, et al. NOX5 in human spermatozoa: expression, function, and regulation. J Biol Chem. 2012;287:9376–88. doi: 10.1074/jbc.M111.314955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabeur K, Ball BA. Characterization of NADPH oxidase 5 in equine testis and spermatozoa. Reproduction. 2007;134:263–70. doi: 10.1530/REP-06-0120. [DOI] [PubMed] [Google Scholar]
- 51.Richer SC, Ford WC. A critical investigation of NADPH oxidase activity in human spermatozoa. Mol Hum Reprod. 2001;7:237–44. doi: 10.1093/molehr/7.3.237. [DOI] [PubMed] [Google Scholar]
- 52.Herrero MB, Pérez Martínez S, Viggiano JM, Polak JM, de Gimeno MF. Localization by indirect immunofluorescence of nitric oxide synthase in mouse and human spermatozoa. Reprod Fertil Dev. 1996;8:931–4. doi: 10.1071/rd9960931. [DOI] [PubMed] [Google Scholar]
- 53.de Lamirande E, Lamothe G. Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic Biol Med. 2009;46:502–10. doi: 10.1016/j.freeradbiomed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 54.O’Flaherty CM, Beorlegui NB, Beconi MT. Reactive oxygen species requirements for bovine sperm capacitation and acrosome reaction. Theriogenology. 1999;52:289–301. doi: 10.1016/S0093-691X(99)00129-6. [DOI] [PubMed] [Google Scholar]
- 55.Aitken RJ, Fisher HM, Fulton N, Gomez E, Knox W, et al. Reactive oxygen species generation by human spermatozoa is induced by exogenous NADPH and inhibited by the flavoprotein inhibitors diphenylene iodonium and quinacrine. Mol Reprod Dev. 1997;47:468–82. doi: 10.1002/(SICI)1098-2795(199708)47:4<468::AID-MRD14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 56.Baker MA, Reeves G, Hetherington L, Müller J, Baur I, et al. Identification of gene products present in Triton X-100 soluble and insoluble fractions of human spermatozoa lysates using LC-MS/MS analysis. Proteomics Clin Appl. 2007;1:524–32. doi: 10.1002/prca.200601013. [DOI] [PubMed] [Google Scholar]
- 57.Tremellen K. Oxidative stress and male infertility – a clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 58.Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol. 2006;250:66–9. doi: 10.1016/j.mce.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 59.Gagnon C, Iwasaki A, De Lamirande E, Kovalski N. Reactive oxygen species and human spermatozoa. Ann N Y Acad Sci. 1991;637:436–44. doi: 10.1111/j.1749-6632.1991.tb27328.x. [DOI] [PubMed] [Google Scholar]
- 60.Griendling KK, Sorescu D, Ushio-Fukai M. NAD (P) H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 61.Zinkham WH, Blanco A, Clowry LJ., Jr An unusual isozyme of lactate dehydrogenase in mature testes: localization, ontogeny, and kinetic properties. Ann N Y Acad Sci. 1964;121:571–88. doi: 10.1111/j.1749-6632.1964.tb14227.x. [DOI] [PubMed] [Google Scholar]
- 62.Blanco A, Zinkham WH. Lactate dehydrogenases in human testes. Science. 1963;139:601–2. doi: 10.1126/science.139.3555.601. [DOI] [PubMed] [Google Scholar]
- 63.Blanco A, Burgos C, Gerez de Burgos NM, Montamat EE. Properties of the testicular lactate dehydrogenase isoenzyme. Biochem J. 1976;153:165–72. doi: 10.1042/bj1530165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burkhart JG, Ansari AA, Malling HV. Localization of cytoplasmic lactate dehydrogenase-X in spermatozoa. Arch Androl. 1982;9:115–20. doi: 10.3109/01485018208990228. [DOI] [PubMed] [Google Scholar]
- 65.O’Flaherty CM, Beorlegui NB, Beconi MT. Lactate dehydrogenase-C4 is involved in heparin- and NADH-dependent bovine sperm capacitation. Andrologia. 2002;34:91–7. doi: 10.1046/j.0303-4569.2001.00481.x. [DOI] [PubMed] [Google Scholar]
- 66.Blanco A. On the functional significance of LDH X. Johns Hopkins Med J. 1980;146:231–5. [PubMed] [Google Scholar]
- 67.Duan C, Goldberg E. Inhibition of lactate dehydrogenase C4 (LDH-C4) blocks capacitation of mouse sperm in vitro . Cytogenet Genome Res. 2003;103:352–9. doi: 10.1159/000076824. [DOI] [PubMed] [Google Scholar]
- 68.Gerez de Burgos NM, Burgos C, Coronel CE, Bertarelli de Camusso A, Pigini T, et al. Correlation of lactate dehydrogenase isoenzyme C4 activity with the count and motility of human spermatozoa. J Reprod Fertil. 1979;55:107–11. doi: 10.1530/jrf.0.0550107. [DOI] [PubMed] [Google Scholar]
- 69.Beconi MT, Beorlegui NB, Sarmiento NK, Mora NG. Phosphorylant capacity study and lactate mitochondrial oxidation in frozen bovine sperm. Life Sci. 1990;47:477–83. doi: 10.1016/0024-3205(90)90606-r. [DOI] [PubMed] [Google Scholar]
- 70.O’Flaherty C, Beorlegui N, Beconi MT. Heparin- and superoxide anion-dependent capacitation of cryopreserved bovine spermatozoa: requirement of dehydrogenases and protein kinases. Free Radic Res. 2006;40:427–32. doi: 10.1080/10715760600577856. [DOI] [PubMed] [Google Scholar]
- 71.de Lamirande E, Harakat A, Gagnon C. Human sperm capacitation induced by biological fluids and progesterone, but not by NADH or NADPH, is associated with the production of superoxide anion. J Androl. 1998;19:215–25. [PubMed] [Google Scholar]
- 72.Sarkar S, Nelson AJ, Jones OW. Glucose-6-phosphate dehydrogenase (G6PD) activity of human sperm. J Med Genet. 1977;14:250–5. doi: 10.1136/jmg.14.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aitken J, Krausz C, Buckingham D. Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol Reprod Dev. 1994;39:268–79. doi: 10.1002/mrd.1080390304. [DOI] [PubMed] [Google Scholar]
- 74.Hammerstedt RH. Tritium release from [2-3H] D-glucose as a monitor of glucose consumption by bovine sperm. Biol Reprod. 1975;12:545–51. doi: 10.1095/biolreprod12.5.545. [DOI] [PubMed] [Google Scholar]
- 75.Amaral A, Castillo J, Ramalho-Santos J, Oliva R. The combined human sperm proteome: cellular pathways and implications for basic and clinical science. Hum Reprod Update. 2014;20:40–62. doi: 10.1093/humupd/dmt046. [DOI] [PubMed] [Google Scholar]
- 76.Wang G, Guo Y, Zhou T, Shi X, Yu J, et al. In-depth proteomic analysis of the human sperm reveals complex protein compositions. J Proteomics. 2013;79:114–22. doi: 10.1016/j.jprot.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 77.de Lamirande E, Lamothe G. Levels of semenogelin in human spermatozoa decrease during capacitation: involvement of reactive oxygen species and zinc. Hum Reprod. 2010;25:1619–30. doi: 10.1093/humrep/deq110. [DOI] [PubMed] [Google Scholar]
- 78.Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- 79.Zhang P, Liu B, Kang SW, Seo MS, Rhee SG, et al. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J Biol Chem. 1997;272:30615–8. doi: 10.1074/jbc.272.49.30615. [DOI] [PubMed] [Google Scholar]
- 80.Dubuisson M, Vander Stricht D, Clippe A, Etienne F, Nauser T, et al. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett. 2004;571:161–5. doi: 10.1016/j.febslet.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 81.Peshenko IV, Shichi H. Oxidation of active center cysteine of bovine 1-Cys peroxiredoxin to the cysteine sulfenic acid form by peroxide and peroxynitrite. Free Radic Biol Med. 2001;31:292–303. doi: 10.1016/s0891-5849(01)00579-2. [DOI] [PubMed] [Google Scholar]
- 82.Baty JW, Hampton MB, Winterbourn CC. Proteomic detection of hydrogen peroxide-sensitive thiol proteins in Jurkat cells. Biochem J. 2005;389:785–95. doi: 10.1042/BJ20050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cox AG, Hampton MB. Bcl-2 over-expression promotes genomic instability by inhibiting apoptosis of cells exposed to hydrogen peroxide. Carcinogenesis. 2007;28:2166–71. doi: 10.1093/carcin/bgm093. [DOI] [PubMed] [Google Scholar]
- 84.Low FM, Hampton MB, Peskin AV, Winterbourn CC. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood. 2007;109:2611–7. doi: 10.1182/blood-2006-09-048728. [DOI] [PubMed] [Google Scholar]
- 85.Peskin AV, Low FM, Paton LN, Maghzal GJ, Hampton MB, et al. The high reactivity of peroxiredoxin 2 with H(2)O(2) is not reflected in its reaction with other oxidants and thiol reagents. J Biol Chem. 2007;282:11885–92. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- 86.O’Flaherty C, de Souza AR. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol Reprod. 2011;84:238–47. doi: 10.1095/biolreprod.110.085712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Flaherty C. Peroxiredoxins: hidden players in the antioxidant defence of human spermatozoa. Basic Clin Androl. 2014;24:1–10. doi: 10.1186/2051-4190-24-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gong S, San Gabriel MC, Zini A, Chan P, O’Flaherty C. Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. J Androl. 2012;33:1342–51. doi: 10.2164/jandrol.111.016162. [DOI] [PubMed] [Google Scholar]
- 89.Ozkosem B, O’Flaherty C. Detrimental effects of oxidative stress on spermatozoa lacking peroxiredoxin 6. Free Radic Biol Med. 2012;53:S86. [Google Scholar]
- 90.Barranco-Medina S, Lázaro JJ, Dietz KJ. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett. 2009;583:1809–16. doi: 10.1016/j.febslet.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 91.Manevich Y, Feinstein SI, Fisher AB. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc Natl Acad Sci U S A. 2004;101:3780–5. doi: 10.1073/pnas.0400181101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Noguera-Mazon V, Lemoine J, Walker O, Rouhier N, Salvador A, et al. Glutathionylation induces the dissociation of 1-Cys D-peroxiredoxin non-covalent homodimer. J Biol Chem. 2006;281:31736–42. doi: 10.1074/jbc.M602188200. [DOI] [PubMed] [Google Scholar]
- 93.Li TK. The glutathione and thiol content of mammalian spermatozoa and seminal plasma. Biol Reprod. 1975;12:641–6. doi: 10.1095/biolreprod12.5.641. [DOI] [PubMed] [Google Scholar]
- 94.Halliwell B, Gutteridge J, editors. Free Radicals in Biology and Medicine. New York: Oxford University Press; 2007. Antioxidant defences: endogenous and diet derived; pp. 79–186. [Google Scholar]
- 95.Kim SY, Jo HY, Kim MH, Cha YY, Choi SW, et al. H2O2-dependent hyperoxidation of peroxiredoxin 6 (Prd×6) plays a role in cellular toxicity via up-regulation of iPLA2 activity. J Biol Chem. 2008;283:33563–8. doi: 10.1074/jbc.M806578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Woo HA, Kang SW, Kim HK, Yang KS, Chae HZ, et al. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J Biol Chem. 2003;278:47361–4. doi: 10.1074/jbc.C300428200. [DOI] [PubMed] [Google Scholar]
- 97.Alvarez J, Aitken R. Lipid peroxidation in human spermatozoa. In: Agarwal A, Aitken R, Alvarez J, editors. Studies on Men's Health and Fertility. New York: Humana Press; 2012. pp. 119–30. [Google Scholar]
- 98.Smith TB, Baker MA, Connaughton HS, Habenicht U, Aitken RJ. Functional deletion of Txndc2 and Txndc3 increases the susceptibility of spermatozoa to age-related oxidative stress. Free Radic Biol Med. 2013;65:872–81. doi: 10.1016/j.freeradbiomed.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 99.Weir CP, Robaire B. Spermatozoa have decreased antioxidant enzymatic capacity and increased reactive oxygen species production during aging in the Brown Norway rat. J Androl. 2007;28:229–40. doi: 10.2164/jandrol.106.001362. [DOI] [PubMed] [Google Scholar]
- 100.Nenicu A, Lüers GH, Kovacs W, David M, Zimmer A, et al. Peroxisomes in human and mouse testis: differential expression of peroxisomal proteins in germ cells and distinct somatic cell types of the testis. Biol Reprod. 2007;77:1060–72. doi: 10.1095/biolreprod.107.061242. [DOI] [PubMed] [Google Scholar]
- 101.Williams K, Frayne J, Hall L. Expression of extracellular glutathione peroxidase type 5 (GPX5) in the rat male reproductive tract. Mol Hum Reprod. 1998;4:841–8. doi: 10.1093/molehr/4.9.841. [DOI] [PubMed] [Google Scholar]
- 102.Chabory E, Damon C, Lenoir A, Kauselmann G, Kern H, et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J Clin Invest. 2009;119:2074–85. doi: 10.1172/JCI38940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schneider M, Förster H, Boersma A, Seiler A, Wehnes H, et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009;23:3233–42. doi: 10.1096/fj.09-132795. [DOI] [PubMed] [Google Scholar]
- 104.Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, et al. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:1393–6. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 105.Foresta C, Flohé L, Garolla A, Roveri A, Ursini F, et al. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol Reprod. 2002;67:967–71. doi: 10.1095/biolreprod.102.003822. [DOI] [PubMed] [Google Scholar]
- 106.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med. 2005;38:1422–32. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 107.Newick K, Cunniff B, Preston K, Held P, Arbiser J, et al. Peroxiredoxin 3 is a redox-dependent target of thiostrepton in malignant mesothelioma cells. PLoS One. 2012;7:e39404. doi: 10.1371/journal.pone.0039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chatterjee S, Feinstein SI, Dodia C, Sorokina E, Lien YC, et al. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J Biol Chem. 2011;286:11696–706. doi: 10.1074/jbc.M110.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morielli T, O’Flaherty C. Oxidative stress promotes protein tyrosine nitration and S-glutathionylation impairing motility and capacitation in human spermatozoa. Free Radic Biol Med. 2012;53:S137. [Google Scholar]
- 110.Morielli T, O’Flaherty C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction. 2015;149:113–23. doi: 10.1530/REP-14-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]


