Abstract
In mammals, sperm guidance in the oviduct appears essential for successful sperm arrival at the oocyte. Hitherto, three different potential sperm guidance mechanisms have been recognized: thermotaxis, rheotaxis, and chemotaxis, each of them using specific stimuli – a temperature gradient, fluid flow, and a chemoattractant gradient, respectively. Here, we review sperm behavioral in these mechanisms and indicate commonalities and differences between them.
Keywords: chemotaxis, rheotaxis, sperm behavior, sperm motility, thermotaxis
INTRODUCTION
Unlike a dogma that prevailed for decades, spermatozoa ejaculated into the mammalian female genital tract cannot reach the oocyte by coincidence. Indeed, very large numbers of spermatozoa enter the vagina or uterus (depending on the species), but very few succeed in making their way to the oocyte. In humans, roughly 300 million spermatozoa on average are ejaculated into the vagina, but only about one of every million actually enters the Fallopian tube.1,2 Upon entry, these spermatozoa apparently bind strongly to the oviductal epithelium in the isthmus, forming a sperm storage site.1,3 In this site the spermatozoa are thought to undergo capacitation,3 i.e., to acquire a state of ripening that confers on them the ability to fertilize the oocyte.4,5 Since the process of capacitation occurs asynchronously, only up to 10% of the sperm population are capacitated at any given moment,6 bringing down the number of capacitated spermatozoa in the oocyte-containing tube to the range of roughly 10–20 cells only. For reaching the oocyte at the fertilization site, this limited number of capacitated spermatozoa in the isthmus have to swim a long way full of obstacles. These facts and the tiny dimensions of the gametes relative to the dimensions of the tube make coincidental sperm arrival at the oocyte improbable7,8 and point to the need for sperm guidance.
KNOWN SPERM GUIDANCE MECHANISMS
So far three different guidance mechanisms have been proposed, on the basis of in vitro studies, to occur in the oviduct: thermotaxis – swimming up a temperature gradient (demonstrated in rabbits and humans),9 rheotaxis – swimming against a fluid flow (shown in mice and humans),1 and chemotaxis – swimming up a concentration gradient of a chemoattractant (demonstrated in humans,10 rabbits,11 and mice12). Indeed, due to obvious restrictions, all these mechanisms were demonstrated in vitro only. However, the discoveries of proper stimuli in the female strongly suggest the physiological occurrence of these mechanisms. Specifically, a temperature gradient was found to be generated within the rabbit13,14 and pig15 oviducts at ovulation, postcoitus oviductal fluid flow was discovered in female mice,1 and sperm chemoattractants were found to be secreted from the oocyte and its surrounding cumulus cells.16 As discussed below, the observations mentioned just above suggested the occurrence of these mechanisms sequentially and synergistically in vivo.
LONG- AND SHORT-RANGE MECHANISMS
At ovulation, a temperature drop at the sperm storage site creates a temperature gradient along the rabbit oviduct.14 The establishment of such an ovulation-dependent temperature gradient is not restricted to rabbits, as it has also been found in pigs,15 suggesting that this may be the rule in mammals. In addition, a constant flow of oviductal fluid all along the oviduct towards the uterus increases during estrus in rabbits and after coitus in mice.1,17 The ovulation-dependent generation of these stimuli along the oviduct and the ability of mammalian spermatozoa to respond to these stimuli strongly suggest that thermotaxis and rheotaxis are long-range sperm guidance mechanisms in mammals, guiding spermatozoa along the oviduct to the fertilization site. The existence of such a temperature gradient and an oviductal fluid flow has not been investigated in humans for obvious reasons. Since, however, human spermatozoa are thermotactically9 and rheotactically1 responsive, it is probable that such a temperature gradient and an oviductal fluid flow also exist in humans, and that thermotaxis and rheotaxis serve there as physiological long-range guidance mechanisms. The existence of two long-range guidance mechanisms implies redundancy, which is a common backup strategy for essential biological processes.18,19 It may also suggest synergy, with both mechanisms functioning together and increasing the efficiency of guidance.
Chemotaxis is a short-range mechanism, estimated to occur within the order of millimeters. This range limitation appears to be specifically relevant in the oviduct due to the contractions that the oviduct undergoes20 and the oviductal fluid flow in it,1,17 preventing generation of a long-range chemoattractant gradient. The finding that both the oocyte and its surrounding cumulus cells secrete chemoattractants, with the chemoattractant from the oocyte being a more potent one,16,21 led to the suggestion that chemotaxis is a short-range mechanism acting at the fertilization site to guide spermatozoa first to the oocyte–cumulus complex and then to the oocyte.16,22 It should be mentioned, though, that the report on decreasing concentrations of natriuretic peptide precursor A (a chemoattractant for mouse spermatozoa in vitro) along the oviduct from the ampulla to the uterotubal junction,23 raises the possibility, yet to be investigated, of multi-step chemotaxis in the oviduct, thereby extending its range.
BEHAVIORAL RESPONSES TO STIMULI
In recent years it became apparent that the basics of the behavioral mechanisms displayed by human spermatozoa are similar to those of bacteria like Escherichia coli, which swim in rather straight lines with occasional brief episodes of turning (termed tumbles), after which they swim in arbitrary new directions. They thus execute a random walk.24 When in a chemoattractant or a chemorepellent gradient, the random walk is biased towards the chemoattractant or away from the chemorepellent. Similarly, capacitated human spermatozoa occasionally exhibit episodes of hyperactivation (a vigorous motility type characterized by large amplitudes of head displacement)25 followed by arbitrary new swimming directions. As in bacteria, this random walk is biased by the stimulus – chemotactic, thermotactic, or rheotactic.
The chemotactic response
The notion of hyperactivation events and turns being major factors in directing spermatozoa in a chemoattractant gradient was clearly demonstrated both in spatial and temporal gradients. Specifically, the fraction of human spermatozoa identified as hyperactivated (equivalent to the frequency of hyperactivation events) was found smaller when the cells were in a spatial gradient of the chemoattractant progesterone, suggesting that capacitated spermatozoa maintain their course of swimming once they find the right direction (up the gradient) and turn less frequently.26 The sperm response to a temporal chemoattractant gradient is more complex, but well consistent with the observed behavior in a spatial gradient. Specifically, photorelease of the chemoattractant progesterone from its caged compound, evenly distributed in the sperm suspension, resulted in turnings and hyperactivation events, always following a short delay period (Figure 1a).26 A similar behavior was observed when cAMP or cGMP was photoreleased from their caged compounds within spermatozoa, suggesting the involvement of these cyclic nucleotides in the chemotactic response.27 This means that the attractant response consists of two phases: a delay and a turn. On the basis of these results Armon and Eisenbach26 proposed a model for sperm behavior in a spatial chemoattractant gradient (Figure 1b). According to this model, when a capacitated spermatozoon swims up the chemoattractant concentration it is continuously stimulated. This means that before the second phase commences the cell is stimulated again and again. The outcome is that only the first phase of the response occurs, meaning swimming straight ahead without turns and hyperactivation events. This situation prevails until the spermatozoon stops sensing the gradient, in which case it would adapt and restore the unstimulated mode of swimming, consisting of rather linear swimming interrupted by occasional episodes of hyperactivation, or until it happens to swim down the gradient. In that case it would exhibit turns and hyperactivation episodes to modify its direction of swimming. If so, the behavioral response of human spermatozoa to a spatial chemoattractant gradient (but not to a temporal gradient) is very similar to that of E. coli. In both cases, an increasing concentration gradient of a chemoattractant would suppress turning events, whereas a decrease would increase the frequency of such events.
Figure 1.
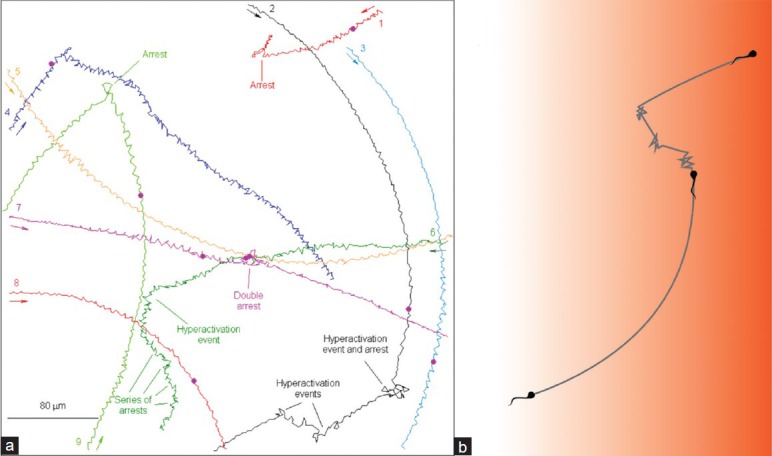
Mammalian sperm chemotaxis. (a) Tracks showing different types of responses to photorelease of the chemoattractant progesterone from its caged compound. The arrows indicate the direction of swimming. The purple dot indicates the time of the flash. (b) A model for the behavior of human spermatozoa in a spatial chemoattractant gradient. The intensity of the background color represents the chemoattractant concentration (Taken with permission from Armon and Eisenbach26).
The thermotactic response
The behavioral response of human spermatozoa to a spatial temperature gradient appears to be very similar to their response to a chemoattractant gradient, even though the responses to a temporal gradient are not identical. When spermatozoa are exposed to a fast temperature drop (i.e., to a negative temperature gradient; Figure 2a), their behavioral response consists of two components.28 One component is rather trivial – speed decrease expressed in all velocity parameters (Figure 2b). The other component is a drop in the linearity of swimming. This is reflected both in the motility parameters that represent the extent of the linearity of swimming (Figure 2c) and in the extent of side-to-side head displacement (i.e., hyperactivation; Figure 2d and 2e), resulting from higher amplitude of the flagellar wave propagation.28 The inverse response is seen when the temperature increases. It is probable that these observed changes reflect to a large extent the sperm response to the ambient temperature. However, they also reflect the response to the temperature gradient per se. This was deduced from the partial adaptation of the cells following their excitatory response (e.g., Figure 2e). In other words, spermatozoa can sense both the temperature gradient and the absolute ambient temperature. The reduced linearity and increase in hyperactivation events in response to a temperature drop led to the expansion of the model of human sperm behavior in a chemoattractant gradient (Figure 1b) to thermotaxis.28 Specifically, a capacitated spermatozoon that swims up a temperature gradient is continuously stimulated with a resultant increased velocity and linearity, due to a low level of head side-to-side displacement. When a spermatozoon swims down the gradient its velocity decreases and the frequency of turning and hyperactivation events increases until the cell happens to swim up the gradient. When a spermatozoon stops sensing the temperature gradient for a while it adapts, resuming its unstimulated swimming – a rather straight swimming with occasional turns and hyperactivation events.
Figure 2.
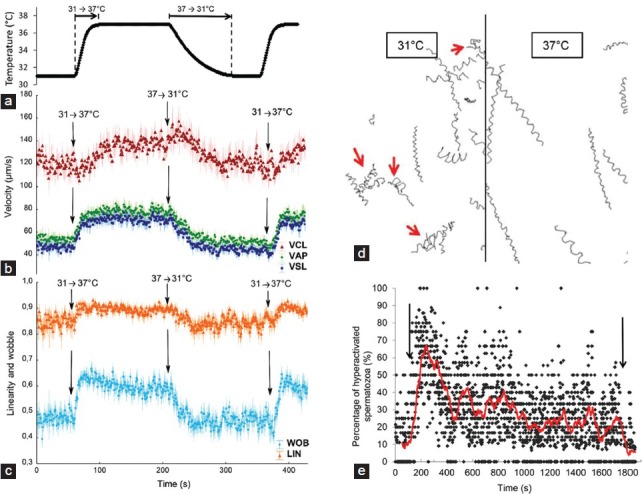
Behavioral response of human spermatozoa to temporal temperature changes. Spermatozoa were exposed to the indicated temperature changes and their motility and trajectories were recorded and analyzed. The figure shows the motility parameters curvilinear velocity (VCL), average pass velocity (VAP), straight-line velocity (VSL), linearity (LIN), wobble (WOB), percentage of hyperactivated spermatozoa and example of sperm trajectories. (a) Heating and cooling thermogram of the microscope's heating stage. (b) Temperature-jump stimulated changes in average velocity parameters. (c) Temperature-jump stimulated changes in the calculated values of linearity and wobble. (d) Representative sperm trajectories at 31°C just prior to temperature shift and at 37°C just after the temperature shift. Red arrows indicate hyperactivation events. (e) Temperature-jump stimulated changes in the percentage of hyperactivated spermatozoa (Taken with permission from Boryshpolets et al.28).
The rheotactic response
When mammalian spermatozoa (thus far demonstrated with human and mouse spermatozoa) sense a flow of fluid, about one half or more of them (both capacitated and noncapacitated spermatozoa) change their path direction and swim against the flow (Figure 3a–3d).1 When the viscosity of the medium is raised to a level that mimics the environment of the oviductal lumen, differences between capacitated and noncapacitated spermatozoa are observed. Noncapacitated spermatozoa move in a more planar path, increasing their chance to stick to the oviductal epithelium. Capacitated spermatozoa rotate around their longitude axis faster than noncapacitated spermatozoa. It was proposed that this faster rotation might enhance the detachment of capacitated spermatozoa from the oviductal surface and might enable them to swim into the main fluid current.1 When they are in the current, the spermatozoa encounter tangential forces that become stronger as they swim perpendicularly to the flow; these forces presumably reorient the spermatozoa against the flow.1 It was proposed that his reorientation is an active process due to the spiral rotation of the sperm tail.1 At least in the case of human spermatozoa, the upstream swimming involves spiral swimming against the shear flow (Figure 3e).29 Since the Ca2+ channel CatSper is apparently essential for rheotaxis, and since this channel is required for hyperactivation, it is reasonable to assume that the release of capacitated spermatozoa from the surface into the main fluid flow and their reorientation in this flow are dependent on hyperactivation.1 Because the hyperactivated flagellar waveform is both asymmetric and of larger amplitude, the flagellum receives larger tangential forces, especially in high-viscosity solution, which points the sperm into flowing solution.1 Once spermatozoa are swimming against the flow, their original rotation rate around their longitude axis is resumed and they swim more linearly (Figure 3f and 3g).
Figure 3.
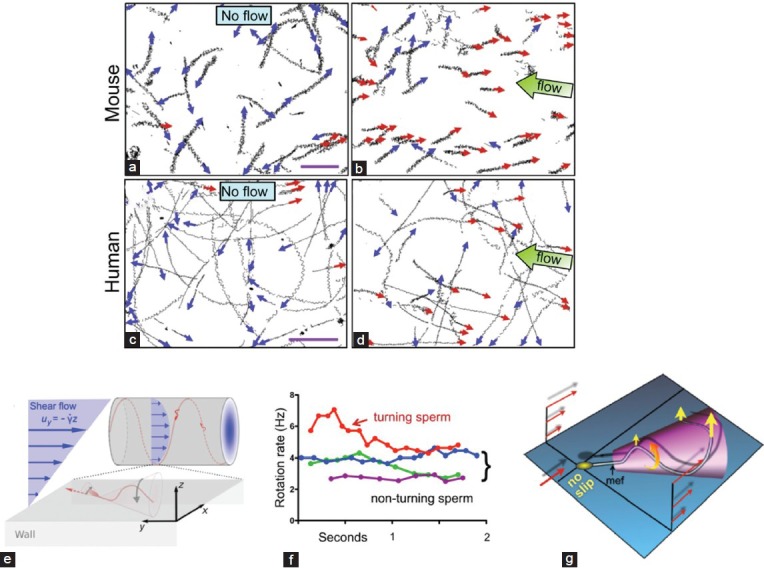
Mammalian sperm rheotaxis. (a and b) Trajectories of mouse spermatozoa in fluid flow (for 3 and 4 s, respectively), analyzed by CASA. Scale bars represent 200 μm. (c and d) Trajectories of human spermatozoa in fluid flow (5 s), analyzed by CASA. Scale bars represent 100 μm. (e) Schematic representation (not drawn to scale) describing the conical envelope of the flagellar beat that holds the spermatozoa close to the surface. The vertical flow gradient exerts a torque that turns the spermatozoa against the flow, but is counteracted by a torque from the chirality of the flagellar wave, resulting in a mean diagonal upstream motion. (f) Rotation rate of individual turning spermatozoa over time. Red line indicates a turning spermatozoon; other lines indicate sperm swimming in a straight line against fluid flow. (g) Fluid flow (red arrows) reorients a spermatozoon (yellow arrows) into the flow to reduce shear as the spermatozoon rotates (orange arrow) and propels itself upstream. Rotation maps out a three-dimensional cone shape in space, which orients spermatozoa consistently into the flow (positive rheotaxis). Tangential forces on the anterior part of the flagellum produce a clockwise force (as seen from above) whereas those on the posterior part provoke a counterclockwise force (Panels a–d, f and g are taken with permission from Miki and Clapham.1 Panel e is taken with permission from Kantsler et al.29).
Are the thermotactic and rheotactic responses linked?
The finding of Miki and Clapham,1 that spermatozoa swim against convection currents created by temperature gradients, raised the possibility that thermotaxis might be essentially rheotaxis. Even though Miki and Clapham1 were careful to say that their findings “do not mean that true thermotaxis or thermal effects on sperm motility are absent”, this possibility calls for attention. As a matter of fact, the observations reported in the literature clearly indicate that thermotaxis and rheotaxis are separate, independent processes. First, about one half or more of the spermatozoa respond by rheotaxis.1 However, thermotaxis is restricted to capacitated spermatozoa only (~10% of the sperm population).9,30 So thermotaxis and rheotaxis cannot be the same process. Second, in the modified Zigmond chamber, used to demonstrate that, in thermotaxis, spermatozoa change their swimming direction according to the temperature gradient,9 the convection was restricted to each well separately, but there was no convection in the single-layer passage between the wells (verified experimentally), where the movement of the spermatozoa was recorded. Nevertheless, only one direction was preferred by the spermatozoa – up the temperature gradient. Third, as shown above, temporal responses of individual spermatozoa to temperature shifts were clearly demonstrated.28 So clearly rheotaxis and thermotaxis are separate processes.
Are the thermotactic and chemotactic responses linked?
Saturating concentrations of chemoattractants were shown not to affect thermotaxis,31 suggesting that chemotaxis to the known stimuli and thermotaxis are independent processes. However, all the thermotaxis experiments have been carried out in media that contain buffers, and the pH of buffers is temperature sensitive. Therefore, a temperature gradient may generate a pH gradient, and spermatozoa in a thermoseparation tube30,31 could theoretically perform pH taxis rather than thermotaxis. We found that this possibility does not hold. We investigated whether thermotactically-active samples of human spermatozoa can carry out pH taxis when stimulated by ∆pH values comparable to, or higher than, the values potentially generated by the temperature dependence of the buffers in the thermoseparation tube. No pH-taxis was found in the tube, the accumulation being the same as in the control with ∆pH = 0.
COMMONALITIES AND DIFFERENCES BETWEEN THE BEHAVIORAL MECHANISMS
As can be deduced from the above descriptions of the three known guidance mechanisms, the principles of sperm guidance are common. The commonalities include: (i) the guidance is indirect in all three mechanisms. Thus, in chemotaxis and thermotaxis, spermatozoa swim up the gradient by modulating the frequency of turns and hyperactivation events, essentially making a random walk biased in the direction of the gradient. In the case of rheotaxis, spermatozoa swim into the main fluid current and there they reorient by the tangential forces. (ii) In all the mechanisms the swimming becomes more linear when spermatozoa are in the right direction (up the gradient in the case of chemotaxis and thermotaxis, and against the flow in the case of rheotaxis). (iii) All three mechanisms are only effective on capacitated spermatozoa. Indeed, noncapacitated spermatozoa can respond to a very strong chemoattractant stimulus in a temporal assay and they are rheotactically responsive, but under conditions that mimic the in vivo situation with respect to the chemoattractant gradient (in the case of chemotaxis) or to the viscosity of the medium (in the case of rheotaxis), only capacitated spermatozoa are responsive. (iv) Hyperactivation seems to be involved in all three mechanisms. (v) All the mechanisms depend on intracellular Ca2+, which affects flagellar bending and, consequently, the swimming mode. The differences between the three mechanisms are more mechanistic. Thus, in addition to the differences between the stimuli, the response of human spermatozoa to a chemotactic temporal gradient is somewhat different from that of a thermotactic temporal gradient, even though the basics are the same, and the rheotactic response is different from the former two. These differences appear to be more a matter of specificity.
COMPETING FINANCIAL INTERESTS
None declared.
REFERENCES
- 1.Miki K, Clapham DE. Rheotaxis guides mammalian sperm. Curr Biol. 2013;23:443–52. doi: 10.1016/j.cub.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harper MJ. Sperm and egg transport. In: Austin CR, Short RV, editors. Germ Cells and Fertilization. Vol. 1. Cambridge, UK: Cambridge University Press; 1982. pp. 102–27. [Google Scholar]
- 3.Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- 4.Chang MC. Fertilizing capacity of spermatozoa deposited into the Fallopian tubes. Nature. 1951;168:697–8. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- 5.Austin CR. The capacitation of the mammalian sperm. Nature. 1952;170:326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- 6.Cohen-Dayag A, Tur-Kaspa I, Dor J, Mashiach S, Eisenbach M. Sperm capacitation in humans is transient and correlates with chemotactic responsiveness to follicular factors. Proc Natl Acad Sci U S A. 1995;92:11039–43. doi: 10.1073/pnas.92.24.11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter RH. Sperm: egg ratios and putative molecular signals to modulate gamete interactions in polytocous mammals. Mol Reprod Dev. 1993;35:324–7. doi: 10.1002/mrd.1080350315. [DOI] [PubMed] [Google Scholar]
- 8.Eisenbach M, Tur-Kaspa I. Do human eggs attract spermatozoa? Bioessays. 1999;21:203–10. doi: 10.1002/(SICI)1521-1878(199903)21:3<203::AID-BIES4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 9.Bahat A, Tur-Kaspa I, Gakamsky A, Giojalas LC, Breitbart H, et al. Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat Med. 2003;9:149–50. doi: 10.1038/nm0203-149. [DOI] [PubMed] [Google Scholar]
- 10.Ralt D, Manor M, Cohen-Dayag A, Tur-Kaspa I, Ben-Shlomo I, et al. Chemotaxis and chemokinesis of human spermatozoa to follicular factors. Biol Reprod. 1994;50:774–85. doi: 10.1095/biolreprod50.4.774. [DOI] [PubMed] [Google Scholar]
- 11.Giojalas LC, Fabro G, Eisenbach M, Rovasio RA. Capacitated and chemotactic rabbit spermatozoa appear to be shortly available around ovulation. J Androl. 2001;22(Suppl 3):92. Abst 043. [Google Scholar]
- 12.Oliveira RG, Tomasi L, Rovasio RA, Giojalas LC. Increased velocity and induction of chemotactic response in mouse spermatozoa by follicular and oviductal fluids. J Reprod Fertil. 1999;115:23–7. doi: 10.1530/jrf.0.1150023. [DOI] [PubMed] [Google Scholar]
- 13.David A, Vilensky A, Nathan H. Temperature changes in the different parts of the rabbit's oviduct. Int J Gynaec Obstet. 1972;10:52–6. [Google Scholar]
- 14.Bahat A, Eisenbach M, Tur-Kaspa I. Periovulatory increase in temperature difference within the rabbit oviduct. Hum Reprod. 2005;20:2118–21. doi: 10.1093/humrep/dei006. [DOI] [PubMed] [Google Scholar]
- 15.Hunter RH, Nichol R. A preovulatory temperature gradient between the isthmus and ampulla of pig oviducts during the phase of sperm storage. J Reprod Fertil. 1986;77:599–606. doi: 10.1530/jrf.0.0770599. [DOI] [PubMed] [Google Scholar]
- 16.Sun F, Bahat A, Gakamsky A, Girsh E, Katz N, et al. Human sperm chemotaxis: both the oocyte and its surrounding cumulus cells secrete sperm chemoattractants. Hum Reprod. 2005;20:761–7. doi: 10.1093/humrep/deh657. [DOI] [PubMed] [Google Scholar]
- 17.Bishop DW. Active secretion in the rabbit oviduct. Am J Physiol. 1956;187:347–52. doi: 10.1152/ajplegacy.1956.187.2.347. [DOI] [PubMed] [Google Scholar]
- 18.Gitter A, Siegfried Z, Klutstein M, Fornes O, Oliva B, et al. Backup in gene regulatory networks explains differences between binding and knockout results. Mol Syst Biol. 2009;5:276. doi: 10.1038/msb.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krakauer DC, Plotkin JB. Redundancy, antiredundancy, and the robustness of genomes. Proc Natl Acad Sci U S A. 2002;99:1405–9. doi: 10.1073/pnas.032668599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battalia DE, Yanagimachi R. Enhanced and co-ordinated movement of the hamster oviduct during the periovulatory period. J Reprod Fertil. 1979;56:515–20. doi: 10.1530/jrf.0.0560515. [DOI] [PubMed] [Google Scholar]
- 21.Armon L, Ben-Ami I, Ron-El R, Eisenbach M. Human oocyte-derived sperm chemoattractant is a hydrophobic molecule associated with a carrier protein. Fertil Steril. 2014;102:885–90. doi: 10.1016/j.fertnstert.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Eisenbach M, Giojalas LC. Sperm guidance in mammals – An unpaved road to the egg. Nat Rev Mol Cell Biol. 2006;7:276–85. doi: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- 23.Bian F, Mao G, Guo M, Mao G, Wang J, et al. Gradients of natriuretic peptide precursor A (NPPA) in oviduct and of natriuretic peptide receptor 1 (NPR1) in spermatozoon are involved in mouse sperm chemotaxis and fertilization. J Cell Physiol. 2012;227:2230–9. doi: 10.1002/jcp.22962. [DOI] [PubMed] [Google Scholar]
- 24.Macnab RM, Koshland DE., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972;69:2509–12. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suarez SS, Ho HC. Hyperactivated motility in sperm. Reprod Domest Anim. 2003;38:119–24. doi: 10.1046/j.1439-0531.2003.00397.x. [DOI] [PubMed] [Google Scholar]
- 26.Armon L, Eisenbach M. Behavioral mechanism during human sperm chemotaxis: involvement of hyperactivation. PLoS One. 2011;6:e28359. doi: 10.1371/journal.pone.0028359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gakamsky A, Armon L, Eisenbach M. Behavioral response of human spermatozoa to a concentration jump of chemoattractants or intracellular cyclic nucleotides. Hum Reprod. 2009;24:1152–63. doi: 10.1093/humrep/den409. [DOI] [PubMed] [Google Scholar]
- 28.Boryshpolets S, Pérez-Cerezales S, Eisenbach M. Behavioral mechanism of human sperm in thermotaxis: a role for hyperactivation. Hum Reprod. 2015;30:884–92. doi: 10.1093/humrep/dev002. [DOI] [PubMed] [Google Scholar]
- 29.Kantsler V, Dunkel J, Blayney M, Goldstein RE. Rheotaxis facilitates upstream navigation of mammalian sperm cells. Elife. 2014;3:e02403. doi: 10.7554/eLife.02403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bahat A, Caplan SR, Eisenbach M. Thermotaxis of human sperm cells in extraordinarily shallow temperature gradients over a wide range. PLoS One. 2012;7:e41915. doi: 10.1371/journal.pone.0041915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahat A, Eisenbach M. Human sperm thermotaxis is mediated by phospholipase C and inositol trisphosphate receptor Ca2+channel. Biol Reprod. 2010;82:606–16. doi: 10.1095/biolreprod.109.080127. [DOI] [PubMed] [Google Scholar]


