Abstract
This chapter explores the possibility that capacitation and apoptosis are linked processes joined by their common dependence on the continued generation of reactive oxygen species (ROS). According to this model capacitation is initiated in spematozoa following their release into the female reproductive tract as a consequence of intracellular ROS generation, which stimulates intracellular cAMP generation, inhibits tyrosine phosphatase activity and enhances the formation of oxysterols prior to their removal from the sperm surface by albumin. The continued generation of ROS by capacitating populations of spermatozoa eventually overwhelms the limited capacity of these cells to protect themselves from oxidative stress. As a result the over-capacitation of spermatozoa leads to a state of senescence and the activation of a truncated intrinsic apoptotic cascade characterized by enhanced mitochondrial ROS generation, lipid peroxidation, motility loss, caspase activation and phosphatidylserine externalization. The latter may be particularly important in instructing phagocytic leukocytes that the removal of senescent, moribund spermatozoa should be a silent process unaccompanied by the generation of proinflammatory cytokines. These observations reveal the central role played by redox chemistry in defining the life and death of spermatozoa. A knowledge of these mechanisms may help us to engineer novel solutions to both support and preserve the functionality of these highly specialized cells.
Keywords: apoptosis, reactive oxygen species, sperm capacitation
INTRODUCTION
Despite its obvious biological importance, we still understand very little of the molecular mechanisms responsible for the acquisition of sperm function during the differentiation of these cells in the male and female reproductive tracts. Similarly, we understand very little of the processes by which these mechanisms are disrupted in cases of male infertility. Studies in animal models such as the nematode worm, Caenorhabditis elegans, have clearly implicated errors in posttranslational processes such as phosphorylation, dephosphorylation and preoteolysis in the aetiology of infertility in this species.1 However, our understanding of the molecular basis of infertility in our own species, or even commercially important domestic species such as cattle or horses, is still highly fragmented. This is strategically important because impaired reproductive competence commonly involves some element of functional deficiency on the part of the spermatozoa, to the extent that defective sperm function has been heralded as the single most important, defined cause of infertility in our species.2 There is a general appreciation that male infertility is an extremely complex phenomenon, involving failures of sperm motility and/or defects in the ability of spermatozoa to engage with the oocyte.3,4,5,6 Furthermore, in the wake of studies revealing the important role that spermatozoa play in the postfertilization induction of normal embryo development, attention has recently focused on processes that extend beyond the act of fertilization itself to influence the entire health trajectory of the offspring.5,6 The molecular basis of these defects is still very poorly understood, although there is a growing consensus that one of the major factors contributing to impaired sperm function is oxidative stress. Oxidative stress has been acknowledged as a cause of defective sperm function since the pioneering studies of Tosic and Walton7 who demonstrated the toxic effect of endogenously generated H2O2 on bovine sperm metabolism and motility. While this observation was made more than half a century ago we are still just beginning to understand the causes and consequences of oxygen metabolite generation in the male germ line. This review will summarize our current understanding of this field and explore the possibility that capacitation and the intrinsic apoptotic cascade represent opposite ends of a metabolic spectrum driven by the generation of reactive oxygen metabolites.
LIFE AND DEATH IN THE GERM LINE
Traditionally, studies in the area of sperm cell biology focus on the molecular mechanisms that underpin the acquisition and expression of functionality in viable cells. However, of equal importance are the biochemical pathways that control the way in which spermatozoa die in vivo. Spermatozoa are one of the two most important cells in biology yet a great majority of these cells will never deliver on their biological purpose of fertilizing the oocyte. For most of these cells, life will end with a quiet death in the male and female reproductive tracts. Spermatozoa are first generated by the germinal epithelium of the testes long after immunological tolerance has been established and, as a result, these cells are potentially capable of eliciting immunological responses that can affect semen quality8 as well as male and female fertility.9 Within the testes, dead and moribund spermatozoa may be protected from the immune system by a physical blood-testes barrier. However, once these cells enter the rete testes and epididymis they are no longer sequestered behind an anatomical barrier and are open to attack by phagocytes. Indeed, there is a growing body of evidence (see chapters in this Symposium volume by Sutovsky and Da Silva) for a quality control system operating at the level of the epididymis, which allows abnormal spermatozoa, immature germ cells and apoptotic epithelial cells, to be recognized within the epididymal lumen and selectively removed by phagocytosis.10,11,12,13 Even greater levels of phagocytic activity occur in the female reproductive tract (cervix or uterus, depending on the mode of insemination) following insemination.14,15,16 In all species studied, semen deposition elicits a massive leukocytic invasion, followed by phagocytosis of spermatozoa, aided by the formation of neutrophil extracellular traps.17 The purpose of this postinsemination leukocytic infiltration is clearly to remove all of the dead and dying spermatozoa, as well other contaminating cell types such as pathogenic bacteria, from the female tract following insemination. In addition to physically removing unwanted cells from the female tract, this leukocytic infiltration also serves to program the uterus, inducing a tolerogenic response to the presence semen and supporting embryonic development and implantation.18,19
The immunological response to insemination is therefore centre stage in the biology of conception. However, it is a process that has to be controlled. While it is essential that phagocytes in the male and female reproductive tract are capable of recognizing nonviable spermatozoa and effecting their phagocytic removal, it is also essential that this phagocytosis is silent, in the sense that it will not be accompanied by a full blown oxidative burst and generation of pro-inflammatory cytokines. If this did occur, it could have very profound consequences for the integrity of the female reproductive tract, particularly in species with multiple mating systems, such as the chimpanzee, where a female in estrus may be mated multiple times by multiple males during the period of maximal tumescence.20
There are several components to the mechanisms by which the reproductive tract is protected from the immunological consequences of insemination. Firstly male seminal fluid is a potent source of the Treg cell-inducing agents, TGFβ and prostaglandin E.21 Prostaglandins (PG) of the E series (PGE and, in humans, 19-hydroxy PGE) also raise intracellular cAMP in leukocytes thereby suppressing lymphocyte proliferation, natural killer (NK) cell activity and suppressing cytokine release from antigen presenting cells.22 Seminal plasma also protects cells from the ROS generated by activated leukocytes because it is richly endowed with antioxidants capable of scavenging any free radicals generated in the immediate vicinity of spermatozoa.23,24 These antioxidants consist of enzymes such as superoxide dismutase, glutathione peroxidase and catalase as well as small molecular mass scavengers including tyrosine, melatonin and uric acid.25,26,27,28 Furthermore, seminal plasma contains factors that induce neutrophil migration,29 and modulate the local cytokine profile,30 in such a manner as to generate a tolerogenic state towards sperm antigens.31 Critically, spermatozoa are also recognized by phagocytes as being a cell that should be phagocytosed in the absence of an oxidative burst. Thus, when human spermatozoa were coated with anti-sperm antibodies and complement and co-incubated with neutrophils, phagocytosis was observed but in the complete absence of an oxidative burst.32 Cytological examination of the cells revealed a limited generation of ROS at the site of sperm-leukocyte contact but thereafter no propagation of the response was observed.32 Clearly spermatozoa that are sufficiently moribund to warrant phagocytosis express surface signals that suppress the generation of ROS by activated leukocytes. What could these surface signals be?
Sperm apoptosis
In other cellular systems where ‘silent phagocytosis’ has been observed, the trigger is the expression of surface markers indicating that the target cells have experienced an apoptotic death. Thus when macrophages consume neutrophils during the resolution of the inflammatory response, the phagocytosis is silent and no pro-inflammatory cytokines are released.33 In this context, the phagocytosis of apoptotic neutrophils by macrophages represents an active and highly regulated process that not only serves to remove potentially damaging cells from the inflammatory milieu, but also directs the phenotype of the phagocytic cell to be anti-inflammatory.33 The mechanism by which an apoptotic cell communicates its state of innocence to marauding phagocytes is complex and thought to involve the surface exposure of factors signifying that an apoptotic death has occurred.34 While a variety of such signals may exist, phosphatidylserine exposure on the cell surface is universally recognized as a marker of apoptosis, and this is certainly true of apoptotic spermatozoa.
Apoptosis in spermatozoa is a poorly understood process that is significantly different from the phenomenon observed in somatic cells. There are two distinguishing characteristics of sperm apoptosis that should be considered, which include (i) the nature of the process itself and (ii) the factors that trigger its induction. One of the earliest events observed during the intrinsic apoptotic cascade in spermatozoa is a sudden increase in the generation of mitochondrial ROS and a concomitant loss of sperm motility.35 The mitochondrial production of ROS, in turn, initiates a lipid peroxidation cascade that results in the generation of cytotoxic lipid aldehydes such as 4-hydroxynonenal (4HNE) and acrolein as a result of free radical attacks on the polyunsaturated fatty acids that abound in spermatozoa.36 These lipid peroxides are powerful electrophiles that bind to the nucleophilic centres of proteins in the immediate vicinity.36 Some of the immediate protein targets are molecules associated the mitochondrial electron transport chain, including succinic acid dehydrogenase.36 As a result of such protein adduction, electron transport within the mitochondria is dysregulated, causing the leakage of electrons which are consumed by the universal electron acceptor, oxygen, to generate superoxide anion, which rapidly dismutates to H2O2, which then triggers yet more lipid peroxidation. In this manner, the oxidative stress initiated by the mitochondria becomes a self-propagating process that forces these cells down the intrinsic apoptotic pathway.35 The motility loss associated with oxidative stress is probably a result of both ATP depletion and the binding of aldehydes to key proteins in the sperm axoneme such as dynein heavy chain.37
The next events observed during the intrinsic apoptotic cascade are the activation of caspases in the sperm cytosol and the surface expression of phosphatidylserine, as detected by annexin V binding. Both of these features have been repeatedly observed in spermatozoa undergoing apoptosis within several species including man,38 rabbit,39 buffalo,40 bull,41 dog,42 boar,43 silver fox,44 stallion45 and ram.46 In light of observations in other cell types in seems reasonable to propose that the phagocytosis of moribund spermatozoa is triggered by the surface expression of phosphatidylserine in weakly motile, apoptotic spermatozoa. This phagocytosis is presumably silent because the phagocytes recognize phosphatidylserine on the sperm surface, as the signature of an apoptotic, nonthreatening cell. It is at this early stage of apoptosis that cells would be phagocytosed in vivo, long before DNA damage in the sperm nucleus becomes evident.34 At this stage of apoptosis, the process deviates in spermatozoa and somatic cells. In somatic cells endonucleases released from the mitochondria or activated in the cytoplasm move into the cell nucleus to deliver the coup de grace by cutting up the DNA into internucleosomal fragments that generate the characteristic laddering pattern when the nuclei of apoptotic cells are analysed by agarose gel electrophoresis. While such laddering is typical of apoptotic somatic cells, it is never seen in spermatozoa for two reasons. First, sperm chromatin contains very few nucleosomes, most of these structures having been removed from germ cell nuclei during spermiogenesis. Secondly, the nucleases activated and released during apoptosis are unable to physically penetrate the sperm nucleus because, uniquely in this cell type, the nuclear DNA in the sperm head, is physically separated from the mitochondria and most of the cytoplasm in the sperm midpiece. Indeed, the need to protect sperm chromatin from nuclease attack may have been a major factor determining the characteristic architecture of spermatozoa. While the details of sperm morphology may vary massively between vertebrate species, the physical separation of the sperm nucleus from the mitochondria, is a consistent feature of these cells.
As a consequence of their structure, the only product of apoptosis that can move from the sperm midpiece to the nucleus in the sperm head is the H2O2 generated following the dismutation of mitochondrial superoxide anion. This is why most DNA damage in spermatozoa is oxidatively induced and why the oxidative base adduct, 8-hydroxy-2’-deoxyguanosine (8OHdG) is such a powerful and effective biomarker of sperm quality (Figure 1).47
Figure 1.
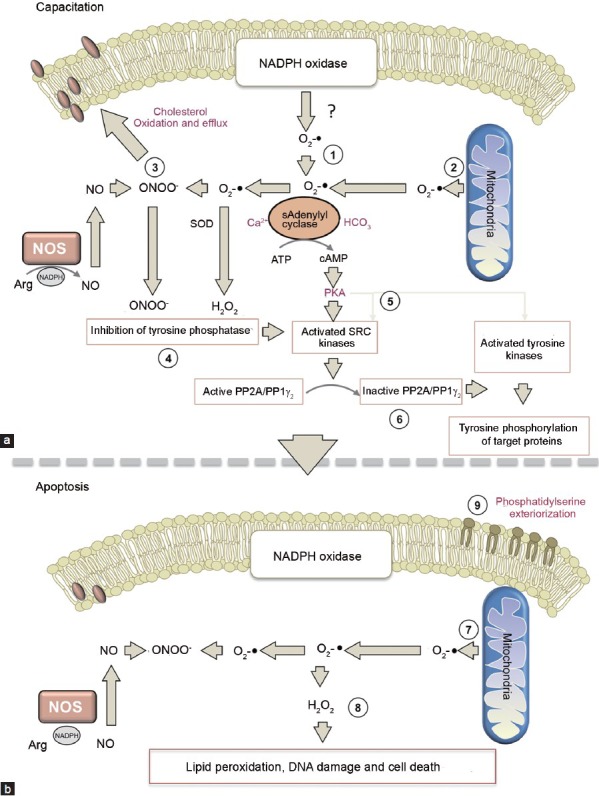
Proposed pathways mediating the redox regulation of sperm capacitation and the initiation of apoptosis. (a) (1) In capacitating spermatozoa ROS generation is possibly elevated because of an increase in NADPH oxidase activity subsequent to activation of the hexose monophosphate shunt activity and the enhanced availability of NADPH.76 (2) The second source of O2-• in mammalian spermatozoa are the mitochondria in the sperm midpiece which generate a low level of ROS during steady state respiration. (3) The O2-• generated from these sources is thought to combine with NO produced as a consequence of nitric oxide synthase activity to generate the powerful oxidant, peroxynitrite (ONOO-), which mediates the oxidation of sterols that then exit the plasma membrane generating a dramatic increase in membrane fluidity as a consequence. (4) The combined action of ONOO- and H2O2 (generated as a result of superoxide dismutase activity), leads to the inhibition of tyrosine phosphatase activity, thereby facilitating tyrosine phosphorylation. (5) The combination of O2-•, HCO3- and Ca2+, activates soluble adenylyl cyclase stimulating cAMP production and the activation of PKA. The latter both directly activates SRC kinases (pp60cSRC and cABL) and simultaneously suppresses an inhibitor of SRC, C-terminal SRC kinase; as a result, SRC activity is significantly increased. (6) SRC then phosphorylates and inactivates a protein phosphatase permitting the dramatic upregulation of tyrosine phosphorylation that characterizes the capacitated state.77 (b) (7) When the oxidative stress associated with capacitation overwhelms the limited antioxidant defences offered by spermatozoa the cells enter the intrinsic apoptotic cascade, one of the early features of which is an increase in mitochondrial ROS. (8) Mitochondrial ROS generation induces the formation of small molecular mass aldehydes such as 4HNE which bind to the mitochondrial electron transport chain and stimulate yet more ROS generation in a self-perpetuating cascade. This further enhances the oxidative damage to the cell impairing motility and activating caspases. (9) During the advanced stages of apoptosis, phosphatidylserine appears on the exofacial surface of the spermatozoa and acts as a signal promoting the silent phagocytosis of these cells by dendritic cells, macrophages and neutrophils following insemination. ROS: reactive oxygen species; PKA: protein kinase A.
Occasionally papers have appeared describing the use of the TUNEL assay for the diagnosis of apoptosis-like DNA fragmentation in spermatozoa.48 On the surface of it this would seem to be evidence for nuclease-mediated DNA cleavage since this is the property measured by this assay. There are two problems with this interpretation: (i) as indicated above, nucleases activated during apoptosis are physically prevented from moving from the midpiece, where they are generated, to the nucleus to effect DNA fragmentation, and (ii) the TUNEL assay depends on the ability of terminal deoxynucleotidyl transferase to catalyze the addition of labelled bases to the site of DNA nicks. The activity of this enzyme depends on the detection of 3’hydroxy ends at the site of DNA strand breaks. The creation of 3’hydroxy ends at sites of DNA fragmentation is the responsibility of a nuclease, APE1, which spermatozoa do not possess.49 The only time that spermatozoa become TUNEL positive is when they are in the perimortem and DNA fragmentation is extensive.49,50 Thus, in a recent study, the TUNEL assay could not detect oxidative DNA damage in spermatozoa consequent to H2O2 exposure, while in the same cells, intracellular and extracellular 8OHdG could be clearly identified in a manner that was highly correlated with the outcome of the sperm chromatin structure assay (SCSA).49
THE INDUCTION OF APOPTOSIS
If apoptosis is such an important process as far as spermatozoa are concerning what factors are responsible for inducing this process? In reality, this is probably the wrong question. Although apoptosis can be induced in vitro, with wortmannin for example, this is the exception. Most of the classical activators of apoptosis in somatic cells, such as lipopolysaccharide or staurosporine, are not effective, or are only weakly effective, with spermatozoa.48 A possible explanation is that in vivo, apoptosis may never be actively induced in spermatozoa, rather, it is their default position. All spermatozoa (bar the lucky few that get to participate in fertilization) are destined to undergo an apoptotic death in the male or female reproductive tract – this is their fate. The real question is therefore not what induces apoptosis in spermatozoa but what prevents it from occurring. In this context, we have recently demonstrated that the survival of mammalian spermatozoa depends heavily on the activity of phosphoinositide 3- (PI3) kinase. This enzyme generates the phosphoinositide, PtdIns (3,4,5) P3, which binds to another kinase AKT1 effecting the transport of the latter to the plasma membrane where it, in turn, becomes phosphorylated. Activated AKT1 is critical for the maintenance of sperm viability. As long as AKT1 is phosphorylated, an array of pro-survival downstream targets such as the apoptosis inducing factor, Bad, are also phosphorylated and the spermatozoa survive.35 By contrast, as soon as AKT1 becomes dephosphorylated, spermatozoa are immediately directed towards the intrinsic apoptotic cascade. Thus the key to sperm survival is maintaining the phosphorylation status of AKT1 and the key to that objective is keeping PI3 kinase in a phosphorylated activated state.
In most cells the ability of PI3K to generate PtdIns (3,4,5) P3 is in dynamic equilibrium with a phosphatase that negatively regulates the bioavailability of the phosphoinositide by catalysing its dephosphorylation to the corresponding bisphosphate, PIP2 [PtdIns (4,5) P2]. This phosphatase is known as PTEN. Immunocytochemical localization of PTEN revealed that the phosphorylated, stabilized, form of this enzyme (pSer380) is localized in the equatorial segment of the sperm head, distant from the PI3K in the principal piece of the tail. This physical separation of PI3K and phosphorylated PTEN is absolutely unique to spermatozoa and would ensure that the former is free to generate PtdIns (3,4,5) P3 without any interference from PTEN phosphatase activity.
If PTEN is not a key regulator of PtdIns (3,4,5) P3 availability in spermatozoa, then the only other point of control for cell survival would be the activation of PI3 kinase itself. We therefore propose that in vivo the prolonged survival of spermatozoa in the male and female reproductive tracts is mediated by pro-survival factors which serve to maintain PI3 kinase in an activated phosphorylated state. It is the absence of such factors that limits the lifespan of mammalian spermatozoa in vitro to a matter of hours whereas in vivo they last for days in both the epididymis and, postinsemination, in the isthmic region of the Fallopian tubes. Identifying these prosurvival factors is clearly the key to engineering in vitro storage media that will keep spermatozoa in a viable functional state for prolonged periods of time. Our search for such prosurvival factors was originally guided by a proteomic analysis of spermatozoa, which highlighted the presence of receptors in the sperm plasma membrane which are known to be coupled to PI3 kinase and could therefore reasonably fulfil a prosurvival role.51 On this basis the prosurvival factors we have identified to date are prolactin and insulin;52 we are certain there are others and there may well be different prosurvival factors in different species. The identification of these molecules will greatly facilitate our capacity to design the sperm reservation media of the future without the need to revert to low temperature strategies.
ROS CONTROL SPERM CAPACITATION AS WELL AS CELL DEATH
Reactive oxygen metabolites are not only fundamental to the apoptotic process that ends the life of sperm, they are also heavily involved in the capacitation of these cells when they are in their functional prime. The notion that there is a link between ROS, and sperm capacitation has its origins in a series of studies dating back more than 20 years indicating that the generation of ROS is fundamental to the attainment of a capacitated state. Thus pioneering studies by Claude Gagnon's group in McGill University, Canada, demonstrated that complex biological fluids (an ultrafiltrate of fetal chord serum) could induce biological features of capacitation, such as hyperactivated movement, via mechanisms that could be effectively reversed by the presence of antioxidant enzymes such as superoxide dismutase.53 In a separate series of studies we demonstrated that the tyrosine phosphorylation events associated with human sperm capacitation were also stimulated by ROS via mechanisms that could be reversed with various antioxidants of including catalase.54,55 ROS are thought to exert a positive influence on tyrosine phosphorylation in capacitating spermatozoa through their ability to enhance intracellular levels of cAMP and inhibit tyrosine phosphatase activity.56 Indeed the case for ROS involvement in cAMP generation and tyrosine phosphorylation has now been made for human,54,55,56,57 rat,58 mouse,59 bull,60 stallion,61 and boar62 spermatozoa (Figure 1).
In terms of the specific reactive oxygen metabolites responsible for the induction of sperm capacitation, a pivotal role for H2O2 generation has been suggested by experiments demonstrating that direct addition of this oxidant to suspensions of human, hamster or bovine spermatozoa, leads to the stimulation of tyrosine phosphorylation and capacitation.55,60,63,64,65 Similarly, the artificial creation of oxidizing conditions by exposing spermatozoa to extracellularly-generated ROS using the glucose oxidase or xanthine oxidase systems, has been shown to stimulate capacitation and tyrosine phosphorylation in several species (man, hamster, bull and horse) via mechanisms that can be reversed by the addition of catalase.56 The biological importance of H2O2 has been further emphasized by the ability of catalase to inhibit the spontaneous induction of tyrosine phosphorylation in capacitating mammalian spermatozoa.54 and to suppress sperm functions such as hyperactivation, the acrosome reaction and sperm-oocyte fusion that are all ultimately dependent on the attainment of a capacitated state.54,56,63,66
In addition to H2O2, evidence has been presented suggesting that a variety of alternative ROS can stimulate sperm capacitation including O2-•, NO and ONOO-.67,68,69,70,71 In vivo there will probably be so much interconversion of these oxygen metabolites during sperm capacitation that it would be possible for all of these ROS to participate in this process. If it is the oxidizing power of these molecules which is critical for their biological impact on capacitation, then we can be predict that the ultimate drivers of this process will be H2O2 and ONOO-. The latter, in particular, is known to elicit many of the features of capacitating spermatozoa including the inhibition of tyrosine phosphatase activity72 and the activation of tyrosine kinases of the Src family.73 In addition, ONOO- and other ROS may be involved in the capacitation dependent oxidation of sterols,74 which serves to facilitate the loss of these stabilizing molecules from the sperm plasma membrane (Figure 1).62
CONCLUSION
Since both capacitation and apoptosis are redox-regulated, it is tempting to speculate that they are part of a continuous process. ROS generation is clearly critical for capacitation to occur however it represents a relatively risky strategy for this cell type because spermatozoa are uniquely susceptible to oxidative stress due to their relative lack of antioxidant protection and manifest abundance of oxidizable substrates in the form of polyunstaturated fatty acids, proteins and DNA. Thus, a tipping point is likely to arise during capacitation when the intrinsic generation of ROS overwhelms the spermatozoa's capacity to defend themselves against oxidative attack. When this occurs, the spermatozoa become oxidatively stressed and start to generate cytotoxic aldehydes like 4HNE which accelerate the generation of mitochondrial ROS and disrupt motility by forming adducts with proteins that are essential for flagellar movement such as dynein (Figure 1). Motility loss is then followed by the surface expression of phosphatidylserine, which serves as a trigger for silent phagocytosis by leukocytes entering the female reproductive tract following insemination. In this context, spermatozoa that have been through the process of capacitation and have entered a postcapacitation state, are known to be particularly vulnerable to phagocytosis.75 Oxidative DNA damage occurs around this time and results in the creation of abasic sites as a consequence of the involvement of OGG1 (8-oxoguanine DNA glycosylase) the first enzyme in the base excision repair pathway. Such abasic sites can influence the stability of the DNA leading to single strand breaks. Finally as the cells begin to lose their vitality there is a marked increase in DNA fragmentation associated with the generation of positive signals in the TUNEL assay. This cascade of changes following the activation of ROS generation by spermatozoa, highlight the exquisite sensitivity of these cells to changes in their redox status; the cellular generation of ROS is essential for the functionality of these cells but ultimately seals their apoptotic fate.
AUTHORS’ CONTRIBUTIONS
RJA generated the first draft of the manuscript, which was then carefully reviewed and modified by MAB and BN.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Miao L, L’Hernault SW. Role of posttranslational modifications in C. elegans and ascaris spermatogenesis and sperm function. Adv Exp Med Biol. 2014;759:215–39. doi: 10.1007/978-1-4939-0817-2_10. [DOI] [PubMed] [Google Scholar]
- 2.Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, et al. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–7. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aitken RJ, Irvine DS, Wu FC. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am J Obstet Gynecol. 1991;164:542–51. doi: 10.1016/s0002-9378(11)80017-7. [DOI] [PubMed] [Google Scholar]
- 4.Franken DR, Oehninger S, Burkman LJ, Coddington CC, Kruger TF, et al. The hemizona assay (HZA): a predictor of human sperm fertilizing potential in in vitro fertilization (IVF) treatment. J In Vitro Fert Embryo Transf. 1989;6:44–50. doi: 10.1007/BF01134581. [DOI] [PubMed] [Google Scholar]
- 5.McPherson NO, Owens JA, Fullston T, Lane M. Preconception diet or exercise interventions in obese fathers normalizes sperm microRNA profile and metabolic syndrome in female offspring. Am J Physiol Endocrinol Metab. 2015 Feb 17; doi: 10.1152/ajpendo.00013.2015. doi: 10.1152/ajpendo.00013.2015 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Aitken RJ. Human spermatozoa: revelations on the road to conception. F1000Prime Rep. 2013;5:39. doi: 10.12703/P5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tosic J, Walton A. Formation of hydrogen peroxide by spermatozoa and its inhibitory effect of respiration. Nature. 1946;158:485. doi: 10.1038/158485a0. [DOI] [PubMed] [Google Scholar]
- 8.Cui D, Han G, Shang Y, Liu C, Xia L, et al. Antisperm antibodies in infertile men and their effect on semen parameters: a systematic review and meta-analysis. Clin Chim Acta. 2015;444:29–36. doi: 10.1016/j.cca.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Chamley LW, Clarke GN. Antisperm antibodies and conception. Semin Immunopathol. 2007;29:169–84. doi: 10.1007/s00281-007-0075-2. [DOI] [PubMed] [Google Scholar]
- 10.Smith TB, Cortez-Retamozo V, Grigoryeva LS, Hill E, Pittet MJ, et al. Mononuclear phagocytes rapidly clear apoptotic epithelial cells in the proximal epididymis. Andrology. 2014;2:755–62. doi: 10.1111/j.2047-2927.2014.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da Silva N, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, et al. A dense network of dendritic cells populates the murine epididymis. Reproduction. 2011;141:653–63. doi: 10.1530/REP-10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos-Ibeas P, Pericuesta E, Fernández-González R, Ramírez MA, Gutierrez-Adan A. Most regions of mouse epididymis are able to phagocytose immature germ cells. Reproduction. 2013;146:481–9. doi: 10.1530/REP-13-0145. [DOI] [PubMed] [Google Scholar]
- 13.Sutovsky P, Moreno R, Ramalho-Santos J, Dominko T, Thompson WE, et al. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J Cell Sci. 2001;114(Pt 9):1665–75. doi: 10.1242/jcs.114.9.1665. [DOI] [PubMed] [Google Scholar]
- 14.Aitken RJ, Baker MA. Oxidative stress, spermatozoa and leukocytic infiltration: relationships forged by the opposing forces of microbial invasion and the search for perfection. J Reprod Immunol. 2013;100:11–9. doi: 10.1016/j.jri.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Matthijs A, Engel B, Woelders H. Neutrophil recruitment and phagocytosis of boar spermatozoa after artificial insemination of sows, and the effects of inseminate volume, sperm dose and specific additives in the extender. Reproduction. 2003;125:357–67. [PubMed] [Google Scholar]
- 16.Troedsson MH, Loset K, Alghamdi AM, Dahms B, Crabo BG. Interaction between equine semen and the endometrium: the inflammatory response to semen. Anim Reprod Sci. 2001;68:273–8. doi: 10.1016/s0378-4320(01)00164-6. [DOI] [PubMed] [Google Scholar]
- 17.Katila T. Post-mating inflammatory responses of the uterus. Reprod Domest Anim. 2012;47(Suppl 5):31–41. doi: 10.1111/j.1439-0531.2012.02120.x. [DOI] [PubMed] [Google Scholar]
- 18.Schjenken JE, Robertson SA. Seminal fluid and immune adaptation for pregnancy – comparative biology in mammalian species. Reprod Domest Anim. 2014;49(Suppl 3):27–36. doi: 10.1111/rda.12383. [DOI] [PubMed] [Google Scholar]
- 19.Robertson SA, Moldenhauer LM. Immunological determinants of implantation success. Int J Dev Biol. 2014;58:205–17. doi: 10.1387/ijdb.140096sr. [DOI] [PubMed] [Google Scholar]
- 20.Goodall J. Cambridge (MS): Belknap Press; 1986. The Chimpanzees of Gombe; p. 673. [Google Scholar]
- 21.Robertson SA, Prins JR, Sharkey DJ, Moldenhauer LM. Seminal fluid and the generation of regulatory T cells for embryo implantation. Am J Reprod Immunol. 2013;69:315–30. doi: 10.1111/aji.12107. [DOI] [PubMed] [Google Scholar]
- 22.Kelly RW. Immunosuppressive mechanisms in semen: implications for contraception. Hum Reprod. 1995;10:1686–93. doi: 10.1093/oxfordjournals.humrep.a136156. [DOI] [PubMed] [Google Scholar]
- 23.Jones R, Mann T, Sherins R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril. 1979;31:531–7. doi: 10.1016/s0015-0282(16)43999-3. [DOI] [PubMed] [Google Scholar]
- 24.Rhemrev JP, van Overveld FW, Haenen GR, Teerlink T, Bast A, et al. Quantification of the nonenzymatic fast and slow TRAP in a postaddition assay in human seminal plasma and the antioxidant contributions of various seminal compounds. J Androl. 2000;21:913–20. [PubMed] [Google Scholar]
- 25.Kratz EM, Piwowar A, Zeman M, Stebelová K, Thalhammer T. Decreased melatonin levels and increased levels of advanced oxidation protein products in the seminal plasma are related to male infertility. Reprod Fertil Dev. 2014 Sep 12; doi: 10.1071/RD14165. doi: 10.1071/RD14165 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Maleki BH, Tartibian B, Vaamonde D. The effects of 16 weeks of intensive cycling training on seminal oxidants and antioxidants in male road cyclists. Clin J Sport Med. 2014;24:302–7. doi: 10.1097/JSM.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 27.van Overveld FW, Haenen GR, Rhemrev J, Vermeiden JP, Bast A. Tyrosine as important contributor to the antioxidant capacity of seminal plasma. Chem Biol Interact. 2000;127:151–61. doi: 10.1016/s0009-2797(00)00179-4. [DOI] [PubMed] [Google Scholar]
- 28.Aitken RJ, Buckingham DW, Brindle J, Gomez E, Baker HW, et al. Analysis of sperm movement in relation to the oxidative stress created by leukocytes in washed sperm preparations and seminal plasma. Hum Reprod. 1995;10:2061–71. doi: 10.1093/oxfordjournals.humrep.a136237. [DOI] [PubMed] [Google Scholar]
- 29.Assreuy AM, Alencar NM, Cavada BS, Rocha-Filho DR, Feitosa RF, et al. Porcine spermadhesin PSP-I/PSP-II stimulates macrophages to release a neutrophil chemotactic substance: modulation by mast cells. Biol Reprod. 2003;68:1836–41. doi: 10.1095/biolreprod.102.013425. [DOI] [PubMed] [Google Scholar]
- 30.Denison FC, Grant VE, Calder AA, Kelly RW. Seminal plasma components stimulate interleukin-8 and interleukin-10 release. Mol Hum Reprod. 1999;5:220–6. doi: 10.1093/molehr/5.3.220. [DOI] [PubMed] [Google Scholar]
- 31.Remes Lenicov F, Varese A, Merlotti A, Geffner J, Ceballos A. Prostaglandins in semen compromise the immune response against sexually transmitted pathogens. Med Hypotheses. 2014;83:208–10. doi: 10.1016/j.mehy.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 32.D’Cruz OJ, Wang BL, Haas GG., Jr Phagocytosis of immunoglobulin G and C3-bound human sperm by human polymorphonuclear leukocytes is not associated with the release of oxidative radicals. Biol Reprod. 1992;46:721–32. doi: 10.1095/biolreprod46.4.721. [DOI] [PubMed] [Google Scholar]
- 33.Hart SP, Dransfield I, Rossi AG. Phagocytosis of apoptotic cells. Methods. 2008;44:280–5. doi: 10.1016/j.ymeth.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–72. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 35.Koppers AJ, Mitchell LA, Wang P, Lin M, Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J. 2011;436:687–98. doi: 10.1042/BJ20110114. [DOI] [PubMed] [Google Scholar]
- 36.Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, et al. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem. 2012;287:33048–60. doi: 10.1074/jbc.M112.366690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker MA, Weinberg A, Hetherington L, Villaverde AI, Velkov T, et al. Defining the mechanisms by which the reactive oxygen species by-product, 4-hydroxynonenal, affects human sperm cell function. Biol Reprod. 2015 Feb 11; doi: 10.1095/biolreprod.114.126680. doi: biolreprod.114.126680 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.You L, Wang YX, Zeng Q, Li M, Huang YH, et al. Semen phthalate metabolites, spermatozoa apoptosis, and DNA damage: a cross-sectional study in China. Environ Sci Technol. 2015;49:3805–12. doi: 10.1021/acs.est.5b00588. [DOI] [PubMed] [Google Scholar]
- 39.Vasicek J, Pivko J, Chrenek P. Reproductive performance of New Zealand White rabbits after depletion of apoptotic spermatozoa. Folia Biol (Krakow) 2014;62:109–17. doi: 10.3409/fb62_2.109. [DOI] [PubMed] [Google Scholar]
- 40.Mohan R, Atreja SK. 'soya milk Tris-based phytoextender reduces apoptosis in cryopreserved buffalo (Bubalus bubalis) spermatozoa’. Reprod Domest Anim. 2014;49:797–805. doi: 10.1111/rda.12371. [DOI] [PubMed] [Google Scholar]
- 41.Zhao XM, Ren JJ, Zhao SJ, Cui LS, Hao HS, et al. Apoptosis-like events and in vitro fertilization capacity of sex-sorted bovine sperm. Reprod Domest Anim. 2014;49:543–9. doi: 10.1111/rda.12305. [DOI] [PubMed] [Google Scholar]
- 42.Yu IJ. Canine sperm cryopreservation using glucose in glycerol-free Tris. Cryo Letters. 2014;35:101–7. [PubMed] [Google Scholar]
- 43.Zeng C, Tang K, He L, Peng W, Ding L, et al. Effects of glycerol on apoptotic signaling pathways during boar spermatozoa cryopreservation. Cryobiology. 2014;68:395–404. doi: 10.1016/j.cryobiol.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Kostro K, Krakowski L, Lisiecka U, Jakubczak A, Zmuda A, et al. Flow cytometric evaluation of sperm apoptosis in semen of silver foxes in the breeding period. Anim Reprod Sci. 2014;144:54–8. doi: 10.1016/j.anireprosci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Johannisson A, Morrell JM, Thorén J, Jönsson M, Dalin AM, et al. Colloidal centrifugation with Androcoll-E prolongs stallion sperm motility, viability and chromatin integrity. Anim Reprod Sci. 2009;116:119–28. doi: 10.1016/j.anireprosci.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Martí E, Pérez-Pé R, Colás C, Muiño-Blanco T, Cebrián-Pérez JA. Study of apoptosis-related markers in ram spermatozoa. Anim Reprod Sci. 2008;106:113–32. doi: 10.1016/j.anireprosci.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 47.De Iuliis GN, Thomson LK, Mitchell LA, Finnie JM, Koppers AJ, et al. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2’-deoxyguanosine, a marker of oxidative stress. Biol Reprod. 2009;81:517–24. doi: 10.1095/biolreprod.109.076836. [DOI] [PubMed] [Google Scholar]
- 48.Weil M, Jacobson MD, Raff MC. Are caspases involved in the death of cells with a transcriptionally inactive nucleus? Sperm and chicken erythrocytes. J Cell Sci. 1998;111(Pt 18):2707–15. doi: 10.1242/jcs.111.18.2707. [DOI] [PubMed] [Google Scholar]
- 49.Smith TB, Dun MD, Smith ND, Curry BJ, Connaughton HS, et al. The presence of a truncated base excision repair pathway in human spermatozoa that is mediated by OGG1. J Cell Sci. 2013;126(Pt 6):1488–97. doi: 10.1242/jcs.121657. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell LA, De Iuliis GN, Aitken RJ. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: development of an improved methodology. Int J Androl. 2011;34:2–13. doi: 10.1111/j.1365-2605.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 51.Baker MA, Reeves G, Hetherington L, Müller J, Baur I, et al. Identification of gene products present in Triton X-100 soluble and insoluble fractions of human spermatozoa lysates using LC-MS/MS analysis. Proteomics Clin Appl. 2007;1:524–32. doi: 10.1002/prca.200601013. [DOI] [PubMed] [Google Scholar]
- 52.Pujianto DA, Curry BJ, Aitken RJ. Prolactin exerts a prosurvival effect on human spermatozoa via mechanisms that involve the stimulation of Akt phosphorylation and suppression of caspase activation and capacitation. Endocrinology. 2010;151:1269–79. doi: 10.1210/en.2009-0964. [DOI] [PubMed] [Google Scholar]
- 53.Leclerc P, de Lamirande E, Gagnon C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med. 1997;22:643–56. doi: 10.1016/s0891-5849(96)00379-6. [DOI] [PubMed] [Google Scholar]
- 54.Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci. 1995;108(Pt 5):2017–25. doi: 10.1242/jcs.108.5.2017. [DOI] [PubMed] [Google Scholar]
- 55.Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine DS. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci. 1998;111(Pt 5):645–56. doi: 10.1242/jcs.111.5.645. [DOI] [PubMed] [Google Scholar]
- 56.Aitken RJ, Nixon B. Sperm capacitation: a distant landscape glimpsed but unexplored. Mol Hum Reprod. 2013;19:785–93. doi: 10.1093/molehr/gat067. [DOI] [PubMed] [Google Scholar]
- 57.Villegas J, Kehr K, Soto L, Henkel R, Miska W, et al. Reactive oxygen species induce reversible capacitation in human spermatozoa. Andrologia. 2003;35:227–32. doi: 10.1046/j.1439-0272.2003.00564.x. [DOI] [PubMed] [Google Scholar]
- 58.Lewis B, Aitken RJ. A redox-regulated tyrosine phosphorylation cascade in rat spermatozoa. J Androl. 2001;22:611–22. [PubMed] [Google Scholar]
- 59.Ecroyd HW, Jones RC, Aitken RJ. Endogenous redox activity in mouse spermatozoa and its role in regulating the tyrosine phosphorylation events associated with sperm capacitation. Biol Reprod. 2003;69:347–54. doi: 10.1095/biolreprod.102.012716. [DOI] [PubMed] [Google Scholar]
- 60.Rivlin J, Mendel J, Rubinstein S, Etkovitz N, Breitbart H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol Reprod. 2004;70:518–22. doi: 10.1095/biolreprod.103.020487. [DOI] [PubMed] [Google Scholar]
- 61.Baumber J, Sabeur K, Vo A, Ball BA. Reactive oxygen species promote tyrosine phosphorylation and capacitation in equine spermatozoa. Theriogenology. 2003;60:1239–47. doi: 10.1016/s0093-691x(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 62.Boerke A, Brouwers JF, Olkkonen VM, van de Lest CH, Sostaric E, et al. Involvement of bicarbonate-induced radical signaling in oxysterol formation and sterol depletion of capacitating mammalian sperm during in vitro fertilization. Biol Reprod. 2013;88:21. doi: 10.1095/biolreprod.112.101253. [DOI] [PubMed] [Google Scholar]
- 63.Bize I, Santander G, Cabello P, Driscoll D, Sharpe C. Hydrogen peroxide is involved in hamster sperm capacitation in vitro . Biol Reprod. 1991;44:398–403. doi: 10.1095/biolreprod44.3.398. [DOI] [PubMed] [Google Scholar]
- 64.O’Flaherty C, de Lamirande E, Gagnon C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic Biol Med. 2006;40:1045–55. doi: 10.1016/j.freeradbiomed.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 65.O’Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med. 2006;41:528–40. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 66.Griveau JF, Renard P, Le Lannou D. An in vitro promoting role for hydrogen peroxide in human sperm capacitation. Int J Androl. 1994;17:300–7. doi: 10.1111/j.1365-2605.1994.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 67.de Lamirande E, Gagnon C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int J Androl. 1993;16:21–5. doi: 10.1111/j.1365-2605.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 68.Herrero MB, de Lamirande E, Gagnon C. Tyrosine nitration in human spermatozoa: a physiological function of peroxynitrite, the reaction product of nitric oxide and superoxide. Mol Hum Reprod. 2001;7:913–21. doi: 10.1093/molehr/7.10.913. [DOI] [PubMed] [Google Scholar]
- 69.Herrero MB, de Lamirande E, Gagnon C. Nitric oxide is a signaling molecule in spermatozoa. Curr Pharm Des. 2003;9:419–25. doi: 10.2174/1381612033391720. [DOI] [PubMed] [Google Scholar]
- 70.Thundathil J, de Lamirande E, Gagnon C. Nitric oxide regulates the phosphorylation of the threonine-glutamine-tyrosine motif in proteins of human spermatozoa during capacitation. Biol Reprod. 2003;68:1291–8. doi: 10.1095/biolreprod.102.008276. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez PC, Beconi MT. Peroxynitrite participates in mechanisms involved in capacitation of cryopreserved cattle. Anim Reprod Sci. 2009;110:96–107. doi: 10.1016/j.anireprosci.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 72.Takakura K, Beckman JS, MacMillan-Crow LA, Crow JP. Rapid and irreversible inactivation of protein tyrosine phosphatases PTP1B, CD45, and LAR by peroxynitrite. Arch Biochem Biophys. 1999;369:197–207. doi: 10.1006/abbi.1999.1374. [DOI] [PubMed] [Google Scholar]
- 73.Serafini M, Mallozzi C, Di Stasi AM, Minetti M. Peroxynitrite-dependent upregulation of SRC kinases in red blood cells: strategies to study the activation mechanisms. Methods Enzymol. 2005;396:215–29. doi: 10.1016/S0076-6879(05)96020-5. [DOI] [PubMed] [Google Scholar]
- 74.Patel RP, Diczfalusy U, Dzeletovic S, Wilson MT, Darley-Usmar VM. Formation of oxysterols during oxidation of low density lipoprotein by peroxynitrite, myoglobin, and copper. J Lipid Res. 1996;37:2361–71. [PubMed] [Google Scholar]
- 75.Oren-Benaroya R, Kipnis J, Eisenbach M. Phagocytosis of human post-capacitated spermatozoa by macrophages. Hum Reprod. 2007;22:2947–55. doi: 10.1093/humrep/dem273. [DOI] [PubMed] [Google Scholar]
- 76.Urner F, Sakkas D. Involvement of the pentose phosphate pathway and redox regulation in fertilization in the mouse. Mol Reprod Dev. 2005;70:494–503. doi: 10.1002/mrd.20222. [DOI] [PubMed] [Google Scholar]
- 77.Battistone MA, Da Ros VG, Salicioni AM, Navarrete FA, Krapf D, et al. Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Mol Hum Reprod. 2013;19:570–80. doi: 10.1093/molehr/gat033. [DOI] [PMC free article] [PubMed] [Google Scholar]


