Abstract
Understanding and sustaining biodiversity is a multi-disciplinary science that benefits highly from the creation of organized and accessible collections of biomaterials (Genome Resource Banks). Large cryo-collections are invaluable tools for understanding, cataloging, and protecting the genetic diversity of the world's unique animals and plants. Specifically, the systematic collection and preservation of semen from rare species has been developed significantly in recent decades with some biobanks now being actively used for endangered species management and propagation (including the introduction of species such as the black-footed ferret and the giant panda). Innovations emerging from the growing field of male fertility preservation for humans, livestock species, and laboratory animals are also becoming relevant to the protection and the propagation of valuable domestic and wild species. These new approaches extend beyond the “classical” methods associated with sperm freezing to include testicular tissue preservation combined with xenografting or in vitro culture, all of which have potential for rescuing vast amounts of unused germplasm. There also are other options under development that are predicted to have a high impact within the next decade (stem cell technologies, bio-stabilization of sperm cells at ambient temperatures, and the use of genomics tools). However, biobanking efforts and new fertility preservation strategies have to expand the way beyond mammalian species, which will offer knowledge and tools to better manage species that serve as valuable biomedical models or require assistance to reverse endangerment.
Keywords: conservation, cryobiology, endangered species, male fertility, spermatozoa
THE CRITICAL ROLES OF REPRODUCTIVE SCIENCES AND GENOME RESOURCE BANKING IN CONSERVATION BIOLOGY
Reproduction is essential for the continuation and evolution of living organisms on the planet. Therefore, this discipline naturally is a high priority investigative area in the general field of species conservation biology. There are two approaches, the first being in situ (in natural habitats) and the second ex situ (in captivity such as in zoos). In both settings, the goal is to maintain sustainable populations, with zoos playing a stewardship role in creating reservoirs of wildlife that are as genetically close as possible to free-living counterparts.1 Unlike with commercial livestock species, the goal of managing wildlife is to retain all existing gene diversity for at least the next century.2 This practice ensures species integrity, adaptiveness, resistance to disease, and reproductive fitness. While the theory behind ensuring sustainable populations appears straightforward, the actual practice of studying and propagating endangered species is extraordinarily complex. Importantly, there are as many mechanistic differences in reproductive biology as there are species.3,4 Challenges in studying this huge diversity include the lack of specimens, dangerous behavior by the target species, stress susceptibility, the need for genetic management and – most significantly – an enormous lack of scientific knowledge. For instance, reproductive biology is well understood for only about 0.25% of the world's 40 000 vertebrate species.5 As a result, the highest priority is for species-specific studies to establish baseline data for animals that largely have been ignored to date. Beyond the scholarly benefits of such work, the resulting data have practical use in animal conservation efforts. This might include optimizing natural breeding to enhance genetic management in zoos or to implement intensive plans to recover an endangered species for reintroduction into its native habitats.6 Additionally, characterizing biological norms has been useful to advance certain assisted reproductive technologies, including artificial insemination (AI), in vitro fertilization and embryo transfer.
In this context, establishing and using wildlife Genome Resource Banks (GRBs) – organized collections of living biomaterials – offer enormous opportunities. The value of maintaining data-rich biological samples, including microorganisms, DNA, somatic cells, tissues, blood products, germplasm and embryos, has long been recognized for human health care and agro-industries and is a fundamental component of most basic scientific research.7 However, the benefits of long-term preservation of biomaterials extend far beyond traditional animal or human health issues. For example, the storage, movement and use of genetic materials (including spermatozoa and embryos) will be key to meeting the need to double global food production by 2050 (http://www.fao.org/news). The idea that these genome banks should exist for other than humans, livestock, and crops is not new.8 First, there is the “insurance factor": that is, protecting what we have now for all species and for all existing gene diversity. Second, having repositories of biomaterials, especially germplasm, can support conservation breeding programs where the goal of producing healthy and sustainable insurance populations is only possible in the face of adequate gene diversity. Currently, such programs rely exclusively on the expensive and unsafe movement of wild animals from one zoo to another for breeding. With GRBs and assisted reproductive technologies (i.e., AI or embryo transfer), only germplasm and embryos are moved to maintain the same levels of heterozygosity. The availability of germplasm in the repository also extends the generation interval of individual animals indefinitely, to be re-derived and infused into the living population at any time, 5, 20 or more than 100 years from now. The ideal result is to decelerate natural losses in gene diversity. At the same time, managing a portion of the species as a frozen germplasm reduces space needs. For example, even partial reliance on AI with frozen semen could reduce the number of living animals required in zoos and breeding centers by as much as 50%.8
There now are real-life illustrations of using biobanks for conservation breeding. Table 1 shows examples of GRB efforts mainly based on semen freezing in Asia and Australia. Our institution is associated with these projects and is also participating in new initiatives in Kuwait and Kazakhstan to create similar biorepositories. For instance, the iconic giant panda is routinely managed in ex situ collections and on a large-scale in China using AI with fresh and frozen-thawed spermatozoa.9 The black-footed ferret, once the most endangered species in North American, has been recovered by a combination of natural mating and AI,10 including the use of semen that had been frozen for up to two decades. There also are many examples of “milestone” births using frozen–thawed spermatozoa11,12 with the incidence of success completely dependent on having an excellent understanding of the female reproductive physiology in the target species.4,13
Table 1.
Examples of genome resource banking efforts in Australasia
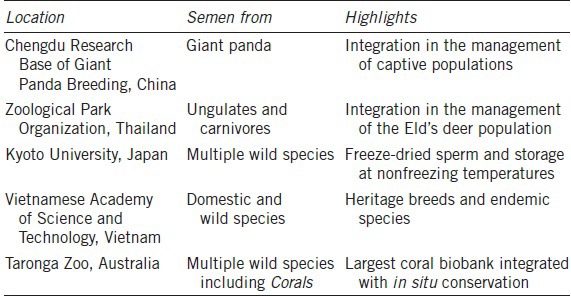
The objective of this review is to present contemporary examples of male fertility preservation in rare and endangered species. The term “fertility preservation” is tightly associated with biobanking efforts, as it refers to the preservation and propagation of valuable genomes of individuals as well as the rescue of fertile gametes from young, adult, or aged individuals dying unexpectedly. Currently, male fertility preservation is mainly based on semen collection and cryopreservation7 but other cutting-edge approaches are being developed to face the urgent need for more options to preserve and protect the fertility of any males in rare and endangered populations.
CURRENT EFFORTS ON COMPARATIVE SPERM CRYOBIOLOGY IN MAMMALIAN SPECIES
The vast majority of studies in wild mammal species have been inspired by the success in domestic bovine species in the 1950s.14 The idea of integrating semen freezing and banking into conservation projects is not new. The earliest records of semen freezing can be traced back to the Italian scientist Spallanzani in 177615 who, among many other research activities, conducted experiments with frog, human and canine spermatozoa and who also described how he carried out a successful artificial insemination of a bitch. A recent review16 provided a table of about 50 (mainly) wild species in which sperm cryopreservation had been studied, but only 11 of these had been translated into successful AI attempts. Births in new species after AI with frozen semen have increased only slightly over the past years. Also, when these reports are examined more closely, it is clear that they mostly represent small insemination trials, and few reports present evidence that the frozen semen is suitable for reliable/routine use to support conservation breeding programs. The black-footed ferret is a notable exception here,10,17 a situation in which semen that was originally frozen more than two decades ago now represents a working gene bank. The intensive efforts in China to breed giant pandas using artificial insemination have met with some success, but as most of the breeding attempts involved the combined use of natural mating and AI, with both fresh and frozen semen the value of the cryopreserved semen is difficult to estimate.18,19 Nevertheless, it is clear that banked collections of giant panda semen have been established and are now being used. Thus, to some extent they can now reasonably be regarded as constituting working genetic resource banks for the giant panda.
There has been steady progress in semen cryopreservation and banking over the past decades.7 Although semen is relatively simple to recover from many species, much more is to be learned about taxon-inherent seminal traits and sensitivity of spermatozoa to cryopreservation. While it appears obvious that small species usually produce minute ejaculate volumes (e.g., 10–50 μl for a black-footed ferret20) and gigantic animals produce large volumes (e.g., >100 ml for the African elephant21), it is well-established that sperm concentration and total sperm output are unrelated to body mass.12 For semen processing, seminal plasma osmolarity and pH dictate the composition of the required seminal extenders as well as dilution processes to retain sperm structure and function during freezing, storage and thawing. Generally, while seminal fluid osmolarity remains slightly higher (350 mOsmol l-1) than that of conspecific serum (~300 mOsmol l-1),12 there are notable exceptions; for example, the value in black-footed ferret semen can reach 790 mOsmol l-1.20 By contrast and based on evaluation of hundreds of species, pH is the least variable metric, generally remaining near neutral or only slightly alkaline.12
Of course, the initial quality of the recovered spermatozoa influences the subsequent ability of these cells to endure freezing and thawing stress. While it is relatively easy to define sperm structure, there are few data on membrane biophysical properties, even in common domestic and laboratory species. Yet this information is what allows understanding species-specific osmotic tolerances and permeability that ultimately allow formulating science-based protocols for cellular freezing and thawing.14,22 In the absence of specific biophysical data, the approach for developing sperm cryomethods has been largely empirical that is, adapting a satisfactory, “standard” protocol for the bull, ram, pig or horse14 to the species of interest. In many cases, a single cryoprotectant can be applicable widely. For example, the use of glycerol has allowed sperm recovery postthawing in diverse species and at similar volume-to-volume concentrations (4%–8%), ranging from various felid species23,24 to the Asian elephant.25,26 More recently, spermatozoa from Przewalski's horse,27 Baird's tapir28 and Indian rhinoceros29 all have been found to respond well to cryodilution and freezing protocols originally developed for the domestic horse (all members of the Perissodactyla). Thus, over the last decade, more individuals from many more species and taxa have been studied, collected, and cryobanked including wolves, primates, and equids and tapirs, as mentioned above. However, live births after AI with frozen-thawed semen have been reported only in a few new species (for instance, the Gerenuk;30 Pallas cat;31 and Persian onager32).
Among the mammals, marsupial spermatozoa present interesting differences from their eutherian counterparts. Semen cryopreservation research in marsupials33 commenced in the 1990s and, despite the best efforts of several groups of experienced cryobiologists,34 the successful insemination of marsupials with frozen semen remains only rarely successful. Diverse issues are associated with marsupial sperm cryopreservation; while motility immediately after thawing cryopreserved spermatozoa may be relatively high, around 40%–50%,35 plasma membrane integrity in the Koala decreases drastically after thawing because the sperm heads swell to several times their original volume.36 The reason for this swelling effect has so far resisted all attempts to explain it, but the problem is partly due to the unusual configuration of the chromatin. Like almost all other marsupials, koala sperm protamine 1 does not contain cysteine residues and is thus precluded from forming chromatin-stabilizing disulfide bonds. At the same time DNA in koala sperm heads naturally contains many single strand breaks, thus further reducing the stability of the chromatin under adverse conditions.37 A considerable variety of approaches to improving the successful cryopreservation of koala spermatozoa have now been investigated, but so far all have failed. The koala AI technique is now highly successful when used with fresh semen, and can be viewed seriously as an option for genetic management. In contrast, cryopreservation of macropodid (kangaroo and wallaby) spermatozoa suffers a different, but equally serious, problem.36 Extensive studies have revealed that unless cryoprotectant concentration is unusually high (e.g., >15% v/v glycerol), postthaw motility is almost never observed in macropod spermatozoa when they are viewed at 35°C. However, if the postthaw samples are viewed at temperatures below about 20°C, the postthaw motility can be as high as 70%.38 This effect is caused by a remarkably rapid destabilization of the plasma membrane when the temperature increases above a narrow threshold, typically around 22°C. In this case the action of a high glycerol concentration appears to be two-fold: it apparently protects the spermatozoa during cooling and freezing, but induces extensive damage after thawing. Exploration of alternative cryoprotectants showed that dimethylacetamide (DMA) mitigated this damage to some extent39 but not to the extent required for use with artificial insemination.
CURRENT EFFORTS ON COMPARATIVE SPERM CRYOBIOLOGY IN NONMAMMALIAN SPECIES
Studying and understanding the structure and function of sperm cells in nonmammalian species is a challenge because of the huge diversity and the lack of fundamental knowledge.12 The comparatively high cryoprotectant concentrations described above, especially with DMA, appear to benefit bird semen as well, although there are striking variations among species.12 Critical studies have been conducted by Blanco et al.40,41 who compared sperm osmotic tolerance among domestic and wild birds. For instance, Sandhill crane spermatozoa remain viable at 3000 mOsmol l-1, whereas turkey spermatozoa are damaged after exposure to 500 mOsmol l-1. Imperial eagle and Peregrine falcon spermatozoa have higher osmotic tolerance at ~800 mOsmol l-1 than those of poultry (fowl and turkey) and Golden eagle and Bonelli's eagle. Thus, in this case, species results are not aligned according to expectations for the “fowl” versus “birds-of-prey” categories but unexpectedly to more distant relatives. Although there are no studies on sperm membrane biophysical properties in birds (except data in the fowl showing clear differences from bull spermatozoa42), variations in cryo-tolerances among species (even among 17 pheasant species43) likely emanate from differing membrane biophysical properties.40 Thus, semen collection and banking in birds remain limited to a few species. Sperm processing challenges are also illustrated from amphibian and fish studies. Generally, these types of spermatozoa remain immotile in seminal plasma until released into the environment. In the case of frogs, spermatozoa are actually excreted in the urine (pH 7.5; 85 mOsmol l-1) that, in turn, naturally activates motility due to a lower osmolarity than that present in testicular tissue.44 A similar phenomenon occurs in fish spermatozoa (mostly from salmonids, sturgeons, carp, turbot, halibut and cod) that are initially immotile in seminal plasma, but then are activated by fresh or salt water.45 Spermatozoa from some fish species maintain motility for only <1 min whereas others can retain this function for several days. As a result of these characteristics, protocols for amphibian and fish spermatozoa generally focus on processing and storing inactivated cells by collecting samples into a buffered saline solution that imitates the original seminal plasma environment.46,47
Excreted amphibian spermatozoa have been successfully cryopreserved only in a couple of species (Bufo and Atelopus; unpublished) while fertilization and offspring have been produced with spermatozoa extracted from the testes of euthanized males and then frozen and thawed.44
One of the most significant differences between many terrestrial and aquatic species is the relative “flexibility” of saltwater species to cryoprotectant exposure.47 Appropriate cryoprotectant composition and osmolarity changes are crucial to ensure adequate cell dehydration and to avoid lysis. In some species, spermatozoa are highly sensitive to the cryoprotectant exposure and might require equilibration at a low temperature to reduce toxic effects, which oddly is not the case for the Cheetah that produces fragile spermatozoa.12 Thus, it is apparent that some differences are not caused by membrane properties, but rather by intrinsic mechanisms that remain to be deciphered.
More recently Hagedorn48 demonstrated the extraordinary influence of time of sperm collection on both cryo-survival as well as ability to fertilize eggs in vitro. In a study of two coral species (Elkhorn coral and Mushroom coral), >50% of conspecific eggs were fertilized in vitro with thawed spermatozoa, but only when the highest quality spermatozoa were collected during a 5-h span in an entire 5-night spawning cycle. Motile spermatozoa harvested on other nights, survived freeze–thawing only poorly and failed to fertilize. Finally, there always will be the issue of “genetics” and the well-known, but largely ignored variations that are observed among age-matched male cohorts for a given species. Inevitably, some male individuals are “good” while others are “poor” sperm freezers, as has been observed in mammalian and nonmammalian species, including bees and snakes.12
NEW ADVANCES IN MALE FERTILITY PRESERVATION APPLICABLE TO WILD SPECIES
As an alternative to mature spermatozoa, testicular tissue banking has become a promising option in vertebrates.13 Such biomaterials are sources of early stage germ cells that can be grown in vitro or grafted to an appropriate host, both for the purpose of eventually producing mature cells useful for assisted reproduction. Importantly, gametes can be rescued from adult individuals that die unexpectedly or even from prepubertal animals. Sperm samples could also be made available year-round from seasonally breeding species. However, substantial basic research is still required, largely because of the complexity of testicular tissue structure and cell heterogeneity, highlighting the similarities and differences among diverse species.13 Testicular fragments (0.5–1.0 mm3) from different mammal species have been frozen in cryovials using a programmable unit after equilibrating in glycerol (human;49) or dimethyl sulfoxide (DMSO; mouse, hamster and marmoset50). These studies have been deemed successful based on favorable postthaw histology or by measuring resumption of gametogenesis after xenografting.50 However, there appear to be significant species variance in tissue cryosensitivity. For example, we have found that felid testicular tissue survives vitrification better than the same tissue treated the same way from laboratory rodents (based on both structural and functional assays13). Interestingly, testicular tissues from ungulates (deer, gazelles, and antelopes) appear to survive slow freezing better than vitrification. Key factors are important for preserving carnivore and ungulate testes: (1) transport temperature of the freshly excised tissue to the laboratory; and (2) the need for seminiferous tubule isolation using collagenase and hyaluronidase enzyme digestion.51 A few studies have been directed at the in vitro culture of testicular tissues to produce fully formed spermatozoa that have the capacity to fertilize. We recently initiated investigations that have revealed the feasibility of using collagenase and hyaluronidase to isolate living seminiferous tubules for preservation and culture. More than 50% of germ line and somatic cells remain alive and continue to differentiate in vitro for at least 4 weeks. Such investigations should be pursued, especially given recent encouraging data from52 who demonstrated that mature mouse sperm cells could be produced in vitro. Certainly, a next high priority for wild species is to determine the mechanisms related to the acquisition of motility and centrosomal maturation in testicular spermatozoa grown in vitro, phenomena not well understood for any species yet.53 Testicular tissue from the common ferret has been xenografted into the body of immunodeficient mice that can then produce mature spermatozoa from the original donor.54 While having theoretical relevance to other wild species, the challenge can be the normally short life-span of the rodent host (much shorter than for more species) and the protracted (>35 weeks) duration required for gamete maturation from the tissue grafts. Even though testicular tissues are systematically banked, the production of mature gametes (through xenografting or long-term in vitro culture) has not transpired yet in wild mammalian species. In any case, in addition to the routine collection of semen by electroejaculation, it is highly recommended to start planning for safe collection of testicular tissue biopsies to increase the efficiency of fertility preservation in any individuals of rare and endangered populations. In amphibians, the direct cryopreservation of testicular tissue is actually more successful than preservation of the mature gametes.44 The issue is to perform biopsies on live individuals of small size. Studies conducted on birds55 and fish56 hold great promise but would need to be combined with procedures such as xenotransplantation to generate mature spermatozoa. Cryopreservation of primordial germ cells also holds promise, but would likely need to be combined with the generation of chimeras to obtain adults that can produce viable gametes.7
Stem cell technologies have a great potential for producing gametes, from spermatogonial progenitors or from differentiated cells. Characterization, isolation, and transfer of spermatogonial stem cells have been attempted in the cat and dog as models for wild carnivores with mixed results.57 In brief, this has involved isolating the spermatogonial stem cells followed by transfer into a germ cell depleted (via radiation) host. On occasion, it has been possible to recover ~ 20% of mature sperm cells derived from the donor.57 Others have transplanted germ cells from a wild felid (ocelot) into the domestic cat to produce spermatozoa successfully from the donor.58 Eventually, it probably will be more efficient to differentiate embryonic stem cells or induced pluripotent stem cells in vitro for this purpose, the latter being accomplished recently for the snow leopard.59 The striking potential of these strategies also has been demonstrated in the mouse where in vitro-differentiated embryonic stem cells have given rise to sperm-like cells60 or oocyte-like cells derived from newborn mouse skin.61
There also are exciting opportunities in preserving isolated gamete genomes via desiccation and storage at supra-zero temperatures.7 This concept is based on the anhydrobiosis phenomenon that occurs in nature (especially in Tardigrades) that allows these organisms to survive remarkably stressful environmental conditions.62 Lyophilization or freeze-drying has been proposed as an alternative method for sperm preservation and maintenance of genetic resources in different animal.63 One of the main advantages is that liquid nitrogen is no longer required for the storage and shipment of samples (at room temperature or 4°C), which considerably reduces storage and shipping costs. As mammalian spermatozoa lose their motility, viability and – at least partially – their DNA integrity when dried, they must be microinjected into an oocyte by intracytoplasmic sperm injection (ICSI). DNA fragmentation in freeze-dried spermatozoa is one of the main causes of failure of embryonic development and successful pregnancy. The use of lyophilization as a sperm preservation method does not produce satisfactory results, except in rodents. In other species, spermatozoa carry a centrosome that will participate in the fertilization process and embryonic development. We suspect that drying techniques are likely to damage the centrosome, which limits the success of this technique.53 Further studies are necessary to minimize this failure. Finally, research should be conducted to allow control of the humidity level during the storage.
LOOKING AT THE HORIZON
Regardless of traditional or new approaches (Table 2), it remains essential to thoroughly verify the integrity of anyDNA sequence and the many epigenetic factors that influence genome functionality. Certainly, rapidly emerging tools, including Next Generation Sequencing (as well as other “omics”) in association with bioinformatics will assist in providing the assurance that any new approach can preserve genomic integrity and functionality. Genomics tools enable us to better understand the origins and patterns of biological functions (even at the sperm cell level). Biodiversity genomics will soon be yet another tool to add to the assisted reproductive techniques (ART) toolbox. However, without a parallel effort in reproductive science and ART development, biogenomics applications will only continue to attract attention disproportionate to their potential for sustainably managing reproduction in endangered species. Whether it is the successful application of AI or the use of cloning to sustain an endangered living species or even “resurrect” an extinct one, success will always depend on knowledge of a species’ biology, ecology, social structure, reproductive cycle, seasonality, embryo implantation, placentation, gestation, parturition, maternal behavior, neonatal care, nutrition, disease susceptibilities, and causes of endangerment. These important factors are still poorly understood and monitored (pregnancy, for instance).
Table 2.
Advantages and challenges of cutting-edge approaches to preserve male fertility
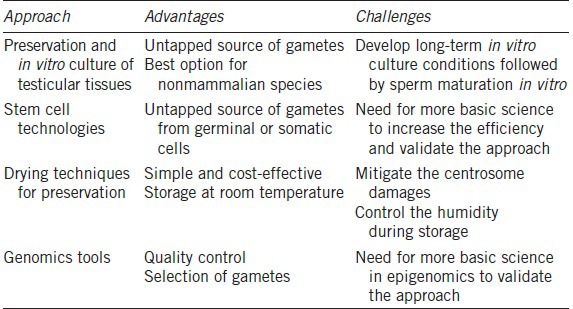
As emphasized above, success in producing new individuals with the help of semen biobanking requires a greater knowledge in basic aspects of reproductive biology and cryobiology. The complexity and diversity of reproduction in the animal kingdom are still underestimated while the ability to develop and apply semen cryobanks for wild species has been overestimated to aid reproductive management and contribute to biodiversity conservation.6,64 As seen above, the barrier to the success is not a shortage of new techniques, but rather a fundamental lack of “conservation capital” – trained scientists, sufficient numbers of research subjects, funding, and appropriate facilities designed specifically to study and manage nondomestic species. The zoo community has been slow to recognize that current management paradigms are insufficient for sustaining hundreds of species across diverse taxa.1 Likewise, conservationists have often minimized the role of zoos and resisted biotechnology when their own efforts to stem the loss of biodiversity and wild places have fallen short. Filling the gap between technology (including semen biobanking) and animal conservation is mandatory. Success will require collective and multi-disciplinary efforts to identify and fill extant limitations and fundamental gaps in knowledge, both intellectual and practical. Building more bridges with human spermatology as well as human biobanking will also help to advance the conservation field.
CONCLUSION
Fertility preservation strategies now used to ensure human reproductive health have significant application to the management of biomedical models, livestock species and conserving biodiversity, especially those animal species managed in ex situ collections that are used for “insurance,” research, public awareness and, on occasion, re-establishment or reinforcement of wild populations. With traditional cryopreservation technologies, the processing, storage and ultimate use of spermatozoa is impeded at multiple levels, mostly by a simple lack of knowledge about inherent species specificities in reproductive physiology.5,12 For certain endangered animals, our laboratory as well as other groups has learned how to deal with and circumvent this obstacle by first conducting tedious, longitudinal, fundamental studies. This often includes comparative studies, adapting techniques and knowledge about a related species to the target. And while successes have occurred, and fascinating new scholarly information has been secured, this approach is time and cost consuming and fails to provide quick assistance in cases of dire need. Thus, we see enormous benefits to looking beyond traditional cryo approaches to explore methods that can offer more widespread applications without the mandate of knowing every reproductive detail.
One of the ultimate priorities for humans as well as all animal types, becomes either customizing or finding universal solutions for preserving biomaterials, with an emphasis on achieving poststorage viability while using practical (user-friendly) technologies that also are low cost. As all stakeholders (from the biomedical, livestock and wildlife communities) could benefit from more interaction, there could be value in establishing a “fertility preservation network,” for example, to share information, tools and to even promote active collaborations and translational approaches. Finally, it is clear that the intersection between preserving fertility and the genome is linked inextricably to conventional cryobiology, or to whatever process eventually evolves that may be nondependent at extremely low temperatures. But it also is obvious that many opportunities will be lost to exploit germ cells, tissue and DNA if we simply fail to store these biomaterialsinitially. Therefore, we urge colleagues to “save everything,” or at least those tissues and cells that may or may not be valuable now, but will become so inevitably thanks to rapidly emerging stem cell and “omics” technologies. These advances will have far reaching implications in the practical management and regeneration of living populations.
COMPETING FINANCIAL INTERESTS
The author declares that he does not have any competing financial interests in relation to the work described.
REFERENCES
- 1.Monfort SL. “Mayday mayday mayday”, the millennium ark is sinking! Adv Exp Med Biol. 2014;753:15–31. doi: 10.1007/978-1-4939-0820-2_2. [DOI] [PubMed] [Google Scholar]
- 2.Ballou JD. Genetic and demographic modeling for animal colony and population management. ILAR J. 1997;38:69–75. doi: 10.1093/ilar.38.2.69. [DOI] [PubMed] [Google Scholar]
- 3.Comizzoli P, Crosier AE, Songsasen N, Gunther MS, Howard JG, et al. Advances in reproductive science for wild carnivore conservation. Reprod Domest Anim. 2009;44(Suppl 2):47–52. doi: 10.1111/j.1439-0531.2009.01373.x. [DOI] [PubMed] [Google Scholar]
- 4.Wildt DE, Comizzoli P, Pukazhenthi B, Songsasen N. Lessons from biodiversity – the value of nontraditional species to advance reproductive science, conservation, and human health. Mol Reprod Dev. 2010;77:397–409. doi: 10.1002/mrd.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wildt DE, Ellis S, Janssen D, Buff J. Toward more effective reproductive science in conservation. In: Holt WV, Pickard A, Rodger JC, Wildt DE, editors. Reproductive Sciences and Integrated Conservation. Cambridge: Cambridge University Press; 2003. pp. 2–20. [Google Scholar]
- 6.Holt WV, Brown JL, Comizzoli P. Reproductive science as an essential component of conservation biology. Adv Exp Med Biol. 2014;753:3–14. doi: 10.1007/978-1-4939-0820-2_1. [DOI] [PubMed] [Google Scholar]
- 7.Comizzoli P, Holt WV. Recent advances and prospects in germplasm preservation of rare and endangered species. Adv Exp Med Biol. 2014;753:331–56. doi: 10.1007/978-1-4939-0820-2_14. [DOI] [PubMed] [Google Scholar]
- 8.Wildt DE, Rall WF, Critser JK, Monfort SL, Seal US. Genome resource banks: living collections for biodiversity conservation. Bioscience. 1997;47:689–98. [Google Scholar]
- 9.Howard JG, Zhang Z, Li D, Huang Y, Zhang M, et al. Male reproductive biology in giant pandas in breeding programmes in China. In: Wildt DE, Zhang A, Zhang H, Janssen DL, Ellis S, editors. Giant Pandas: Biology, Veterinary Medicine and Management. Cambridge, United Kingdom: Cambridge University Press; 2006. pp. 159–97. [Google Scholar]
- 10.Howard JG, Wildt DE. Approaches and efficacy of artificial insemination in felids and mustelids. Theriogenology. 2009;71:130–48. doi: 10.1016/j.theriogenology.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 11.Saragusty J. Genome banking for vertebrates wildlife conservation. In: Katkov I, editor. Current Frontiers in Cryobiology. Croatia: InTech; 2012. pp. 293–368. 574. [Google Scholar]
- 12.Comizzoli P, Songsasen N, Hagedorn M, Wildt DE. Comparative cryobiological traits and requirements for gametes and gonadal tissues collected from wildlife species. Theriogenology. 2012;78:1666–81. doi: 10.1016/j.theriogenology.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Comizzoli P, Wildt DE. Mammalian fertility preservation through cryobiology: value of classical comparative studies and the need for new preservation options. Reprod Fertil Dev. 2013;26:91–8. doi: 10.1071/RD13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leibo SP, Songsasen N. Cryopreservation of gametes and embryos of non-domestic species. Theriogenology. 2002;57:303–26. doi: 10.1016/s0093-691x(01)00673-2. [DOI] [PubMed] [Google Scholar]
- 15.Spallanzani L. Modena: 1776. Animal and vegetal physiology acts. II. Observations and experiences about the spermatozoa in human and animals. [Google Scholar]
- 16.Fickel J, Wagener A, Ludwig A. Semen cryopreservation and the conservation of endangered species. Eur J Wildl Res. 2007;53:81–9. [Google Scholar]
- 17.Howard JG, Marinari PE, Wildt DE. Black-footed ferret: model for assisted reproductive technologies contributing to in situ conservation. In: Holt WV, Pickard AR, Rodger JC, Wildt DE, editors. Reproductive Science and Integrated Conservation. Vol. 8. Cambridge: Cambridge University Press; 2003. pp. 249–66. [Google Scholar]
- 18.Huang Y, Li D, Zhou Y, Zhou Q, Li R, et al. Factors affecting the outcome of artificial insemination using cryopreserved spermatozoa in the giant panda (Ailuropoda melanoleuca) Zoo Biol. 2012;31:561–73. doi: 10.1002/zoo.20421. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Zhang H, Li D, Zhang G, Wei R, et al. Relationship of the estrogen surge and multiple mates to cub paternity in the giant panda (Ailuropoda melanoleuca): implications for optimal timing of copulation or artificial insemination. Biol Reprod. 2012;87:112. doi: 10.1095/biolreprod.112.102970. [DOI] [PubMed] [Google Scholar]
- 20.Santymire RM, Marinari PE, Kreeger JS, Wildt DE, Howard J. Sperm viability in the black-footed ferret (Mustela nigripes) is influenced by seminal and medium osmolality. Cryobiology. 2006;53:37–50. doi: 10.1016/j.cryobiol.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Kiso WK, Brown JL, Siewerdt F, Schmitt DL, Olson D, et al. Liquid semen storage in elephants (Elephas maximus and Loxodonta africana): species differences and storage optimization. J Androl. 2011;32:420–31. doi: 10.2164/jandrol.110.011460. [DOI] [PubMed] [Google Scholar]
- 22.Gosálvez J, López-Fernández C, Fernández JL, Gouraud A, Holt WV. Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Mol Reprod Dev. 2011;78:951–61. doi: 10.1002/mrd.21394. [DOI] [PubMed] [Google Scholar]
- 23.Crosier AE, Pukazhenthi BS, Henghali JN, Howard J, Dickman AJ, et al. Cryopreservation of spermatozoa from wild-born Namibian cheetahs (Acinonyx jubatus) and influence of glycerol on cryosurvival. Cryobiology. 2006;52:169–81. doi: 10.1016/j.cryobiol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Stoops MA, Bond JB, Bateman HL, Campbell MK, Levens GP, et al. Comparison of different sperm cryopreservation procedures on post-thaw quality and heterologous in vitro fertilisation success in the ocelot (Leopardus pardalis) Reprod Fertil Dev. 2007;19:685–94. doi: 10.1071/rd06078. [DOI] [PubMed] [Google Scholar]
- 25.Saragusty J, Hildebrandt TB, Behr B, Knieriem A, Kruse J, et al. Successful cryopreservation of Asian elephant (Elephas maximus) spermatozoa. Anim Reprod Sci. 2009;115:255–66. doi: 10.1016/j.anireprosci.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Thongtip N, Mahasawangkul S, Thitaram C, Pongsopavijitr P, Kornkaewrat K, et al. Successful artificial insemination in the Asian elephant (Elephas maximus) using chilled and frozen-thawed semen. Reprod Biol Endocrinol. 2009;7:75. doi: 10.1186/1477-7827-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pukazhenthi BS, Johnson A, Guthrie HD, Songsasen N, Padilla LR, et al. Improved sperm cryosurvival in diluents containing amides versus glycerol in the Przewalski's horse (Equus ferus przewalskii) Cryobiology. 2014;68:205–214. doi: 10.1016/j.cryobiol.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Pukazhenthi BS, Togna GD, Padilla L, Smith D, Sanchez C, et al. Ejaculate traits and sperm cryopreservation in the endangered Baird's tapir (Tapirus bairdii) J Androl. 2011;32:260–70. doi: 10.2164/jandrol.110.011833. [DOI] [PubMed] [Google Scholar]
- 29.Stoops MA, Atkinson MW, Blumer ES, Campbell MK, Roth TL. Semen cryopreservation in the Indian rhinoceros (Rhinoceros unicornis) Theriogenology. 2010;73:1104–15. doi: 10.1016/j.theriogenology.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Penfold LM, L Monfort S, Wolfe BA, Citino SB, Wildt DE. Reproductive physiology and artificial insemination studies in wild and captive gerenuk (Litocranius walleri walleri) Reprod Fertil Dev. 2005;17:707–14. doi: 10.1071/rd05077. [DOI] [PubMed] [Google Scholar]
- 31.Swanson WF. Application of assisted reproduction for population management in felids: the potential and reality for conservation of small cats. Theriogenology. 2006;66:49–58. doi: 10.1016/j.theriogenology.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Schook MW, Wildt DE, Weiss RB, Wolfe BA, Archibald KE, et al. Fundamental studies of the reproductive biology of the endangered persian onager (Equus hemionus onager) result in first wild equid offspring from artificial insemination. Biol Reprod. 2013;89:41. doi: 10.1095/biolreprod.113.110122. [DOI] [PubMed] [Google Scholar]
- 33.Molinia FC, Rodger JC. Pellet-freezing spermatozoa of two marsupials: the tammar wallaby, Macropus eugenii, and the brushtail possum, Trichosurus vulpecula. Reprod Fertil Dev. 1996;8:681–4. doi: 10.1071/rd9960681. [DOI] [PubMed] [Google Scholar]
- 34.Johnston SD, Holt WV. Germplasm conservation in marsupials. In: Watson PF, Holt WV, editors. Cryobanking the Genetic Resource. Wildlife Conservation for the Future. London: Taylor and Francis; 2001. pp. 203–25. [Google Scholar]
- 35.Zee YP, Holt WV, Gosalvez J, Allen CD, Nicolson V, et al. Dimethylacetamide can be used as an alternative to glycerol for the successful cryopreservation of koala (Phascolarctos cinereus) spermatozoa. Reprod Fertil Dev. 2008;20:724–33. doi: 10.1071/rd08036. [DOI] [PubMed] [Google Scholar]
- 36.Johnston SD, Satake N, Zee Y, López-Fernández C, Holt WV, et al. Osmotic stress and cryoinjury of koala sperm: an integrative study of the plasma membrane, chromatin stability and mitochondrial function. Reproduction. 2012;143:787–97. doi: 10.1530/REP-11-0436. [DOI] [PubMed] [Google Scholar]
- 37.Zee YP, López-Fernández C, Arroyo F, Johnston SD, Holt WV, et al. Evidence that single-stranded DNA breaks are a normal feature of koala sperm chromatin, while double-stranded DNA breaks are indicative of DNA damage. Reproduction. 2009;138:267–78. doi: 10.1530/REP-09-0021. [DOI] [PubMed] [Google Scholar]
- 38.Holt WV, Penfold LM, Johnston SD, Temple-Smith P, McCallum C, et al. Cryopreservation of macropodid spermatozoa: new insights from the cryomicroscope. Reprod Fertil Dev. 1999;11:345–53. doi: 10.1071/rd99076. [DOI] [PubMed] [Google Scholar]
- 39.McClean R, Zee YP, Holt WV, Johnston SD. Cryopreservation of kangaroo spermatozoa using alternative approaches that reduce cytotoxic exposure to glycerol. Cryobiology. 2008;57:304–7. doi: 10.1016/j.cryobiol.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Blanco JM, Long JA, Gee G, Wildt DE, Donoghue AM. Comparative cryopreservation of avian spermatozoa: benefits of non-permeating osmoprotectants and ATP on turkey and crane sperm cryosurvival. Anim Reprod Sci. 2011;123:242–8. doi: 10.1016/j.anireprosci.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Blanco JM, Long JA, Gee G, Donoghue AM, Wildt DE. Osmotic tolerance of avian spermatozoa: influence of time, temperature, cryoprotectant and membrane ion pump function on sperm viability. Cryobiology. 2008;56:8–14. doi: 10.1016/j.cryobiol.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Watson PF, Kunze E, Cramer P, Hammerstedt RH. A comparison of critical osmolality and hydraulic conductivity and its activation energy in fowl and bull spermatozoa. J Androl. 1992;13:131–8. [PubMed] [Google Scholar]
- 43.Saint Jalme M, Lecoq R, Seigneurin F, Blesbois E, Plouzeau E. Cryopreservation of semen from endangered pheasants: the first step towards a cryobank for endangered avian species. Theriogenology. 2003;59:875–88. doi: 10.1016/s0093-691x(02)01153-6. [DOI] [PubMed] [Google Scholar]
- 44.Clulow J, Trudeau VL, Kouba AJ. Amphibian declines in the twenty-first century: why we need assisted reproductive technologies. Adv Exp Med Biol. 2014;753:275–316. doi: 10.1007/978-1-4939-0820-2_12. [DOI] [PubMed] [Google Scholar]
- 45.Cabrita E, Robles V, Herráez P. London, UK: 2008. Nature. Methods in Reproductive Aquaculture: Marine and Freshwater Species; p. 549. [Google Scholar]
- 46.Kouba AJ, Vance CK. Applied reproductive technologies and genetic resource banking for amphibian conservation. Reprod Fertil Dev. 2009;21:719–37. doi: 10.1071/RD09038. [DOI] [PubMed] [Google Scholar]
- 47.Tiersch TR, Yang H, Jenkins JA, Dong Q. Sperm cryopreservation in fish and shellfish. Soc Reprod Fertil Suppl. 2007;65:493–508. [PubMed] [Google Scholar]
- 48.Hagedorn M, Spindler R. The reality, use and potential for cryopreservation of coral reefs. Adv Exp Med Biol. 2014;753:317–29. doi: 10.1007/978-1-4939-0820-2_13. [DOI] [PubMed] [Google Scholar]
- 49.Hovatta O, Foudila T, Siegberg R, Johansson K, von Smitten K, et al. Pregnancy resulting from intracytoplasmic injection of spermatozoa from a frozen-thawed testicular biopsy specimen. Hum Reprod. 1996;11:2472–3. doi: 10.1093/oxfordjournals.humrep.a019140. [DOI] [PubMed] [Google Scholar]
- 50.Ehmcke J, Schlatt S. Animal models for fertility preservation in the male. Reproduction. 2008;136:717–23. doi: 10.1530/REP-08-0093. [DOI] [PubMed] [Google Scholar]
- 51.Comizzoli P, Wildt DE. On the horizon for fertility preservation in domestic and wild carnivores. Reprod Domest Anim. 2012;47(Suppl 6):261–5. doi: 10.1111/rda.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato T, Katagiri K, Yokonishi T, Kubota Y, Inoue K, et al. In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat Commun. 2011;2:472. doi: 10.1038/ncomms1478. [DOI] [PubMed] [Google Scholar]
- 53.Comizzoli P, Wildt DE. Centrosomal functions and dysfunctions in cat spermatozoa. Centrosome research. In: Schatten H, editor. The Centrosome. New York, NY: Humana Press; 2012b. pp. 49–58. [Google Scholar]
- 54.Gourdon JC, Travis AJ. Spermatogenesis in ferret testis xenografts: a new model. Comp Med. 2011;61:145–9. [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, Cheng KM, Silversides FG. Production of live offspring from testicular tissue cryopreserved by vitrification procedures in Japanese quail (Coturnix japonica) Biol Reprod. 2013;88:124. doi: 10.1095/biolreprod.113.108951. [DOI] [PubMed] [Google Scholar]
- 56.Majhi SK, Hattori RS, Rahman SM, Strüssmann CA. Surrogate production of eggs and sperm by intrapapillary transplantation of germ cells in cytoablated adult fish. PLoS One. 2014;9:e95294. doi: 10.1371/journal.pone.0095294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Travis AJ, Kim Y, Meyers-Wallen V. Development of new stem cell-based technologies for carnivore reproduction research. Reprod Domest Anim. 2009;44(Suppl 2):22–8. doi: 10.1111/j.1439-0531.2009.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silva RC, Costa GM, Lacerda SM, Batlouni SR, Soares JM, et al. Germ cell transplantation in felids: a potential approach to preserving endangered species. J Androl. 2012;33:264–76. doi: 10.2164/jandrol.110.012898. [DOI] [PubMed] [Google Scholar]
- 59.Verma R, Holland MK, Temple-Smith P, Verma PJ. Inducing pluripotency in somatic cells from the snow leopard (Panthera uncia), an endangered felid. Theriogenology. 2012;77:220–8. doi: 10.1016/j.theriogenology.2011.09.022. 228.e1. [DOI] [PubMed] [Google Scholar]
- 60.Nayernia K, Nolte J, Michelmann HW, Lee JH, Rathsack K, et al. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11:125–32. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Dyce PW, Liu J, Tayade C, Kidder GM, Betts DH, et al. In vitro and in vivo germ line potential of stem cells derived from newborn mouse skin. PLoS One. 2011;6:e20339. doi: 10.1371/journal.pone.0020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crowe JH, Crowe LM, Wolkers WF, Oliver AE, Ma X, et al. Stabilization of dry Mammalian cells: lessons from nature. Integr Comp Biol. 2005;45:810–20. doi: 10.1093/icb/45.5.810. [DOI] [PubMed] [Google Scholar]
- 63.Gil L, Olaciregui M, Luño V, Malo C, González N, et al. Current status of freeze-drying technology to preserve domestic animals sperm. Reprod Domest Anim. 2014;49(Suppl 4):72–81. doi: 10.1111/rda.12396. [DOI] [PubMed] [Google Scholar]
- 64.Holt WV, Lloyd RE. Artificial insemination for the propagation of CANDES: the reality! Theriogenology. 2009;71:228–35. doi: 10.1016/j.theriogenology.2008.09.003. [DOI] [PubMed] [Google Scholar]


