Abstract
Capacitation and the acrosome reaction are key phenomena in mammalian fertilization. These phenomena were found more than 60 years ago. However, fundamental questions regarding the nature of capacitation and the timing of the acrosome reaction remain unsolved. Factors were postulated over time, but as their roles were not verified by gene-disruption experiments, widely accepted notions concerning the mechanism of fertilization are facing modifications. Today, although in vitro fertilization systems remain our central research tool, the importance of in vivo observations must be revisited. Here, primarily focusing on our own research, I summarize how in vivo observations using gene-manipulated animals have elucidated new concepts in the mechanisms of fertilization.
Studies of the mechanisms of fertilization date back to Aristotle (384-322 BCE), who thought that the woman provided fertile ground for the male seed to grow. By the 17th century, however, it was recognized that females produce eggs. Leeuwenhoek's microscope provided the next insight, making it possible to visualize the spermatozoa in semen. Using this microscopic observation, Hartsoeker (one of the first spermatologists) claimed that he could observe a small person residing in the head of spermatozoa. Then in 1876, Hertwig found that the nuclei of the sperm and egg fuse during fertilization in sea urchin.1 In the 1950s, mammalian spermatozoa were found to undergo a physiological change called capacitation2,3 and a subsequent morphological change known as the acrosome reaction.4 Thus, when we look back the history, the comprehension of the mechanisms of fertilization sometimes went in the wrong direction, but gradually nearing the true figure by modifying or abandoning old notions. In this process, the evolution of experimental tools such as light microscopy, antibodies, electron microscopy, etc., played important roles. Today, powerful investigative aids such as transgenic animals and/or gene-disrupted KO animals have become available. We can create an animal deficient in a given gene of interest or one with a “designer gene.” For example, the latter includes spermatozoa with a green fluorescent protein (GFP) in their acrosome to report acrosomal integrity. These gene-manipulated animals give us deeper insight into the mechanisms of fertilization. In the present article, I describe the new findings, most of which have depended on the use of gene-manipulated animals.
THE IN VITRO FERTILIZATION SYSTEM
After the discovery of capacitation2,3 and the acrosome reaction,4 it took more than 15 years until Yanagimachi and Chang reported in vitro fertilization (IVF) in hamsters,5 and for mice, it required another 15 years until an efficient fertilization system became available.6 A few years later, human IVF was successfully achieved, and the first test tube baby was born, which led Robert Edwards receiving a Nobel Prize in 2010. IVF was supplemented by another discovery that fertilization could be achieved by injecting sperm directly into the egg cytoplasm by a pipette (Intra-Cytoplasmic Sperm Injection).7,8 These findings boosted assisted fertilization for infertile couples, and today, a significant number of IVF babies are born worldwide.
Although IVF showed great clinical success, it had weaknesses as a probe to study the mechanisms of fertilization. One reason may be that a suitable medium for mouse fertilization did not emerge until 20 years after the discovery of capacitation. Even fertile spermatozoa failed to fertilize eggs unless they were incubated in a proper medium. Moreover, there is no consensus as to which currently-used media is the best during IVF. For example, once we learned that frozen C57BL/6 sperm were prone to lose their fertilizing ability in IVF, Takeo et al. developed a medium for these spermatozoa allowing them to penetrate eggs by the addition of methyl-beta-cyclodextrin.9 This indicates that IVF results are significantly affected by the constitution of the medium. It also implies that the addition of various factors in the IVF medium may affect the results of IVF.
THE EMERGENCE OF A NEW TECHNIQUE – KNOCKOUT MICE
After the discovery and establishment of pluripotent embryonic stem cells (ES cells) from the inner cell mass of a blastocyst,10 Capecchi11 and Smithies12 independently demonstrated that a gene of interest could be disrupted by homologous recombination using ES cells. Their finding became a powerful tool in analyzing the role of genes in living mice.
Before describing the results of gene-disruption experiments, I would like to mention the drawbacks of this technique.
Existence of cumulatively functioning genes
If no phenotype is seen after gene disruption, one may conclude that the gene of interest is not essential to the phenomenon one is studying. However, when some genes are paired with others and cumulatively form an essential gene set, a single gene disruption may not result in an apparent phenotype. G1 cyclins in yeast are an example of this. These proteins (CLNI, CLN2 and CldV3) are encoded by three individual genes and are expressed in the G1 phase of the cell cycles, but cells mutant for any two of the three genes are phenotypically wild type and G1 arrest could be observed only in the triple mutant yeasts.13
Effects on neighboring genes
When myogenic regulatory factor 4 (Mrf4), a basic helix-loop-helix Mrf family member, was disrupted, Braun and Arnold declared that the mice die at birth,14 Zhang et al. indicated that the mice survive,15 and Patapoutian et al. reported that the mice occasionally die.16 Afterward, it was found that insertion of a neo gene was detrimental to the neighboring Myf5 gene and that Mrf4 disruption was not the cause of the neonatal death.17 A similar case was reported in the disruption of the prion gene, which is responsible for bovine spongiform encephalopathy. Some groups reported the disruption caused an ataxia phenotype, whereas others claimed they found no phenotype. The difference was that when some of the targeting vectors were used, it caused an exon skip and connected the prion gene to the neighboring doppel gene to express an aberrant fusion protein ectopically.18
Involvement of microRNAs
MicroRNAs (miRNAs) often reside in the intron area of certain genes, and it is known that the disruption of miRNA (s) sometimes causes a severe phenotype in the mouse.19 Therefore, when we design the targeting vector, we must be careful not to eliminate miRNA (s) unintentionally from the modified area.20
Subtle effects
When we observe the phenotype of KO mice, the experimental time frame is limited. Although the gene disruption may not show a significant phenotype, the mice might have a subtle disadvantage. To discover a 5% fitness reduction, the corresponding sample size should be over 2000 and if it were 1%, it might require 600,000.21 In other words, it is difficult to clarify the subtle effect (s) of gene disruption in normal experimentation. However, these subtle differences could ultimately affect the life of a species from an evolutionary point of view.
In this article, I neglected to describe most genes showing subtle differences and classified them as “nonessential” for the sake of simplicity in describing the fundamental mechanisms of fertilization.
VERIFICATION OF VARIOUS FACTORS IN KO MOUSE LINES
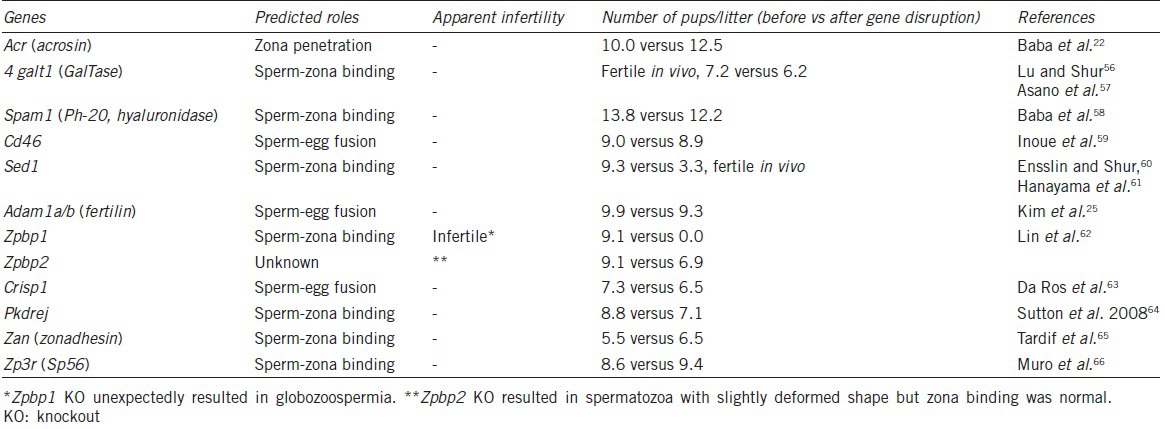
After IVF had become available in mice, various fertilization-related factors were identified using the IVF systems. These factors were subjected to gene KO experiments, and their respective roles were verified in vivo. The first gene examined in the KO mouse system in the field of fertilization research was acrosin, a sperm acrosomal enzyme. Acrosin was widely thought to play an important role in sperm penetration of the zona pellucida. Thus, acrosin-null spermatozoa were believed to become fertilization incapable. However, to everyone's surprise, acrosin KO mice were fertile, although a slight delay was observed in zona penetration.22
Another example was “fertilin,” which attracted the attention of many researchers.23 Fertilin is a heterodimer consisting of two subunits: Adam1b and Adam2. Initially, fertilin was disrupted by eliminating Adam2, and the fertilin-disrupted male mice showed an infertile phenotype.24 Fertilin was thought to be a fusion protein, but strangely, the phenotype was loss of zona binding ability of the spermatozoa. As also shown in this example, gene function in vivo does not necessarily correspond to expectations. Later, when fertilin was disrupted by eliminating Adam1b instead of Adam2, the fertilin-null males showed normal fertility.25 As mentioned above, when a KO mouse showed two different phenotypes, the wild-type phenotype was normally the true phenotype and any others were caused by disruption of an unrelated factor (s). In this particular case, it was learned that Adam2 was essentially required in testis (not in spermatozoa) to make fertile spermatozoa by forming a heterodimer with Adam1a.26 Other factors, demonstrated not to be essential using KO mice, are summarized in Table 1.
Table 1.
Most gene KO mice showed no, subtle or unexpected phenotypes
ESSENTIAL FACTORS FOUND BY KO MOUSE LINES
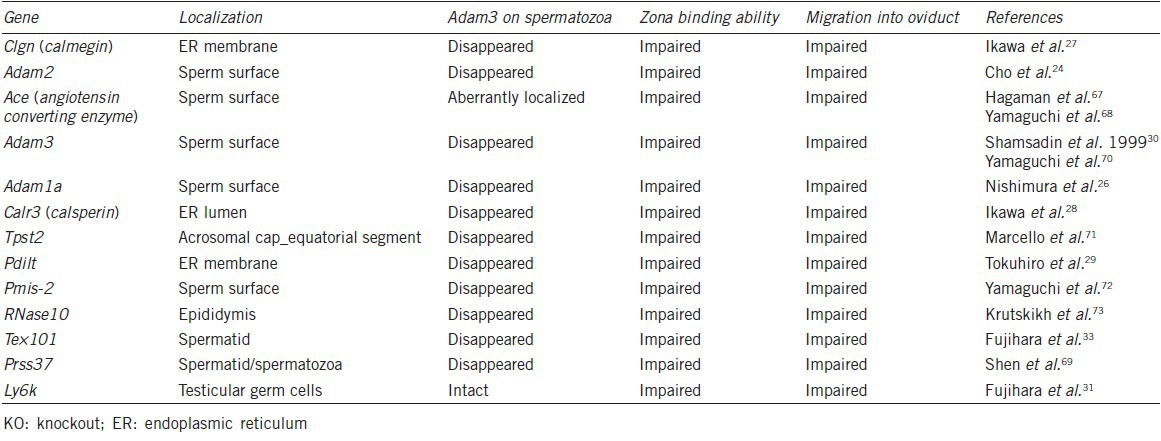
Although various genes predicted to be important for IVF experiments were shown to be dispensable in vivo, others were serendipitously found as essential factors for fertilization. The first case was the calmegin KO. Calmegin is a testis-specific molecular chaperone, which is expressed mainly in pachytene stage spermatocytes and disappears from spermatozoa upon spermiation. We expected a phenotype in spermatogenesis, but no abnormality was found in calmegin KO mice. However, we discovered that the males were infertile despite having normal spermatozoa in terms of number and motility.27 Further investigation revealed that the spermatozoa lost their zona-binding ability. We made two more testis-specific molecular chaperone KO mouse lines, calsperin KO and Pdilt KO. Lacking these genes, the spermatozoa again became incapable of binding to zona.28,29 If these genes were only expressed during spermatogenesis, how then was sperm-zona binding affected? As of now, we are aware of at least 13 genes involved in the formation of sperm zona-binding ability, and in all 13 cases, the spermatozoa lack Adam3 (or have aberrant Adam3). Since the Adam3-disrupted male mice are infertile30 without affecting other gene products, Adam3 could be an ultimately essential factor in all of the gene-disrupted mouse lines as shown in Table 2. Interestingly, these gene KO mouse lines shared common phenotypes, with (i) no migration into the oviduct and (ii) aberrant zona-binding ability in vitro.
Table 2.
KO mice with impaired zona binding ability
AN INCONVENIENT TRUTH
Although the data in Table 2 indicated Adam3 on spermatozoa as a key protein in the fertilization process, Adam3 is surprisingly a pseudogene in humans. Therefore, to place Adam3 in the center of the general fertilization scheme may not be appropriate. Do humans have a completely different mechanism of fertilization from mice? Considering the fact that most of the genes in Table 2 are conserved in human, we could assume the general schema is similar in humans and mice. Our current hypothesis is that we are still missing the ultimate factors contributing to sperm-zona binding. In this context, Ly6k is very interesting as spermatozoa from the Ly6k KO mice lost zona-binding ability while Adam3 remains present on spermatozoa.31 However, Ly6k could not be the ultimate key molecule, as it disappears from mature spermatozoa even in wild-type mice. I think we are coming closer to the ultimate factors, but the process of spermatozoa-egg encounters requires further investigation.
IS “SPERM-ZONA BINDING” DISPENSABLE?
In mice, the uterus and oviduct meet in a structure called the uterotubal junction (UTJ), which significantly reduces the number of spermatozoa reaching the eggs. In order to elucidate the mechanisms of UTJ penetration by spermatozoa, we produced chimeric mice that ejaculate both wild-type spermatozoa and GFP-tagged, calmegin-disrupted spermatozoa, and we mated them with wild-type females. We found that only wild-type spermatozoa migrated into the oviduct, while the equally motile calmegin-disrupted spermatozoa remained in the uterus.32 This indicated that some unknown recognition mechanisms function in the UTJ region. Although spermatozoa from the gene-disrupted mouse lines in Table 2 fail to migrate into the oviduct, we do not know the reason why the zona-binding ability is always associated with UTJ penetrating ability. What would happen if spermatozoa were directly injected into the oviduct, bypassing the UTJ? We tried this experiment using Pdilt,29 Tex10133 and Ly6k31 KO mouse spermatozoa. To our surprise, the spermatozoa of these three KO mouse lines fertilized the eggs. In other words, spermatozoa could fertilize eggs in the oviduct without the so-called “zona-binding ability.” A similar case was reported in Adam1a −/− mice; the sperm from Adam1a −/− mice could fertilize eggs in vitro when they were covered with cumulus layers.26
SHOULD THE “ZONA-INDUCED ACROSOME REACTION” BE RENOUNCED?
Many reports indicated that the acrosome reaction was induced upon contact with the zona pellucida, and many researchers considered that spermatozoa undergoing the acrosome reaction before zona contact had no fertilizing ability.34 In this context, zona-binding proteins were assumed to initiate the signaling cascade leading to the acrosome reaction.35 We made a transgenic mouse line that expressed GFP in the acrosome. This allowed us to observe the moment of the acrosome reaction. Spermatozoa on the zona pellucida were observed, but zona-binding spermatozoa did not acrosome react under a live imaging system.36,37 In addition, a recent study by Jin et al. indicated that most of the fertilizing spermatozoa were acrosome-reacted before reaching the zona pellucida.38 The experiments using gene-manipulated animals renounce the “zona-induced acrosome reaction” theory, at least in the mouse.
What about acrosomal exocytosis? If the acrosomal enzymes were released before spermatozoa approach the zona pellucida, it would be difficult for released enzymes to facilitate zona penetration. This question was also investigated using gene-manipulated animals. We previously generated Izumo139 and Cd9 KO mouse lines.40,41,42 Spermatozoa from the Izumo1 KO line and eggs from the Cd9 KO line were not able to fuse with wild-type gametes of the opposite sex. Therefore, we could observe many spermatozoa from Izumo1 KO males in wild-type eggs or wild-type spermatozoa inside the perivitelline space of Cd9 KO eggs. We recovered both of these acrosome-reacted and zona-penetrated spermatozoa from the perivitelline space by cracking the zona with a piezo-driven micropipette. The spermatozoa swam out from the perivitelline space and were added to freshly recovered cumulus covered eggs. We found that these spermatozoa could penetrate egg investments (cumulus layers and zona pellucida) a second time and, in the case of wild-type spermatozoa recovered from Cd9 KO eggs, fuse with the eggs.43 Thus, the timing of the acrosome reaction before zona binding seemed to be considerably flexible. This re-penetration experiment indicated that if enzymes are released from the sperm during the acrosome reaction, all enzymes are dispensable for the sperm penetration of the zona pellucida. If enzymes were involved in zona penetration, they might not be the kind released from the acrosome; rather, they remained on the spermatozoa even after the acrosome reaction. In the mouse, it was reported that the acrosomal matrix proteins remain associated with the sperm for prolonged periods of time following the induction of acrosomal exocytosis.44,45 If acrosomal enzymes (s) were involved, they should have remained on the sperm surface even after zona penetration, sperm recovery, and during the repeated penetration of the fresh egg investments.
In any case, the timing of the acrosome reaction is flexible, as indicated long ago in the rabbits.46 These findings also indicated that the significant “sperm-zona binding” must occur between acrosome-reacted spermatozoa and the zona pellucida, while most of the classical “sperm-zona binding” assays were observing binding between acrosome-intact spermatozoa and the zona pellucida47 (Figure 1).
Figure 1.
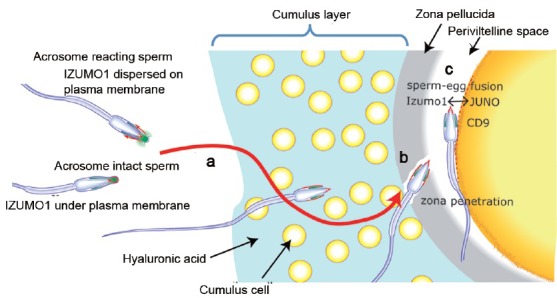
The mechanisms of fertilization, elucidated by gene-manipulated animals. (a) Spermatozoa that present Adam3 (or some unknown factor(s)) can migrate into the oviduct and reach the vicinity of the eggs. Acrosome reaction is induced before spermatozoa reach the zona pellucida and the fusion-related sperm protein Izumo1 on the outer acrosomal membrane migrates out to sperm surface (indicated by red color). (b) Spermatozoa bind to zona pellucida when mixed with cumulus-free oocytes.74 However, this binding (mostly observed between the acrosome-intact spermatozoa and zona pellucida) was dispensable. The spermatozoa that lost the so-called “zona-binding” ability remained able to fertilize eggs in vivo once the oviduct migration step was bypassed.29,31,33 Moreover, the timing of the acrosome reaction is flexible, as acrosome-reacted spermatozoa recovered from the perivitelline space could penetrate the zona pellucida a second time and fertilize eggs.43 The mechanism of sperm penetration of zona pellucida is largely unknown. (c) Only acrosome-reacted spermatozoa can fuse with eggs. Spermatozoa without Izumo1 never fused with eggs.39 Cd9 on the egg played an important role in fertilization,40,41,42 but Cd9-disrupted females were not completely infertile. In addition, no direct interaction between Cd9 and Izumo1 was observed. This led us to predict a real counterpart for Izumo1. Using the newly established AVEXIS assay, JUNO was recently found to be a counterpart for Izumo1 on the egg.51 Modified from review.75
FACTORS ESSENTIAL FOR SPERM-EGG FUSION
The first fusion-related factor, Cd9, was discovered serendipitously. A tetraspanin protein coding Cd9 was initially disrupted by researchers in other fields to examine its role in immunology. However, the Cd9-disrupted females were infertile, due to the eggs requiring Cd9 for sound fusion ability with spermatozoa.40,41,42 On the sperm side, we had a monoclonal antibody OBF13, which inhibited sperm-egg fusion.48 This was one of the fertilization inhibitory antibodies as shown in Table 1. While most of the factors in Table 1 are shown to be nonessential as a result of KO experiments, the role of the OBF13 antigen remained unexamined by KO experiments for a long time. This was due to OBF13 being an IgM class antibody; therefore, there were technical difficulties in identifying the antigen. Once western blot sensitivity improved, we could finally identify the antigen and succeeded in cloning the gene. From its sequence, it was found to be a member of the immunoglobulin superfamily with a single Ig-like domain. We named this gene Izumo1 based on a Japanese shrine dedicated to marriage. As mentioned in an earlier section, the Izumo1-disrupted spermatozoa could acrosome react and penetrate both cumulus and zona pellucida layers, but were unable to fuse with eggs as we expected.39
The fusing ability of Cd9-disrupted eggs was severely impaired, but it was not entirely lost, differing from the complete infertility seen in Izumo1 disruption. In addition, the binding of the putative functional fragment of Izumo1 in the N-terminus region (Izumo1: 57–113) to the egg surface was not altered by disruption of Cd9.49 Thus, Izumo1 binding to a protein other than Cd9 was expected on the egg surface. However, as the number of eggs that we can use for the experiment is quite limited, the purification of Izumo1 binding protein from eggs seemed difficult by conventional means. However, a method called the AVEXIS assay (avidity-based extracellular interaction screen) was invented.50 Using this method, a soluble, biochemically active, highly avid recombinant mouse Izumo1 ectodomain was prepared and the reactivity against HEK293 cells transfected with a normalized mouse oocyte cDNA expression library was analyzed and Bianchi et al. successfully identified the Izumo1 binding protein on the egg and named it JUNO after the goddess of marriage.51 The Juno-disrupted female mice were completely infertile. Now that interacting components Izumo1 and JUNO have been found, rapid progress in the elucidation of the sperm-egg fusion mechanism is expected to follow (Figure 2).
Figure 2.
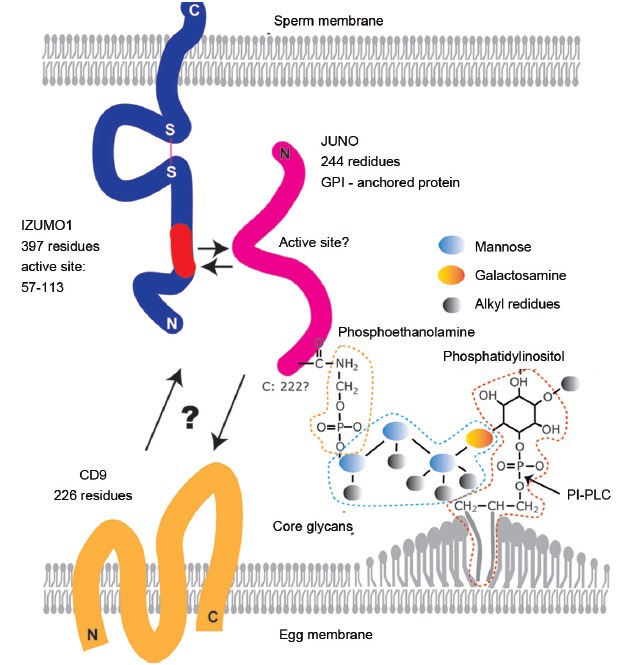
Factors involved in sperm-egg fusion. Izumo1, migrated outward from the outer acrosomal membrane to the sperm surface, tending to localize in the equatorial segment of spermatozoa. Various segments of Izumo1 were examined for their binding ability to eggs and residue 57–113 was indicated to contain an active binding site.49 Using the AVEXIS assay, JUNO was identified as an Izumo1 binding protein and its role in fusion was verified by gene-disruption experiments. JUNO is a 244-residue protein but is cleaved at 222 to form a GPI (glycosylphosphatidylinositol)-anchored protein. GPI-anchored proteins are initially formed on the cytosolic side and flipped over to the outer membrane side in the final maturation stage. The next helpful piece of information will be the elucidation of the active site of JUNO. Since Izumo1 (57–113) bound to Cd9-disrupted eggs normally, the elucidation of Cd9's role(s) will offer further clarification.
LIVE IMAGING OF FERTILIZATION
Observation of fertilization using gene-manipulated animals has given us a new insight. To investigate the role of Izumo1 in fusion, we made a transgenic mouse line containing the Izumo1-mCherry fusion protein and visualized the dynamic movement of Izumo1 during the fertilization process.52
Although OBF13 was a monoclonal antibody, various staining patterns were obtained in spermatozoa before and after the acrosome reaction.53 Our long-standing question was how Izumo1 changed its localization from under the plasma membrane to the sperm surface during the acrosome reaction. Two possibilities were postulated: (i) migration via two steep curves in the equatorial sheath and (ii) Re-adsorption of the antigen after acrosomal vesiculation. However, both hypotheses had their own shortcomings.54 Moreover, the exact localization of Izumo1 in live spermatozoa was unclear because it resided under the plasma membrane. First, the red fluorescent protein-tagged Izumo1-bearing spermatozoa were observed under a confocal microscope where it was revealed that Izumo1 was in the acrosomal cap area of both the inner and outer acrosomal membrane. The migration of Izumo1 upon acrosome reaction was then imaged in live cells. Apparently, Izumo1 migrated on the sperm surface, not by adsorption of vesicles formed by the acrosome reaction. It was further confirmed that Izumo1 did not migrate via the acrosomal sheath. This introduced the new hypothesis that Izumo1 migrated out from the outer acrosomal membrane to the plasma membrane at the beginning of the acrosome reaction when the two membranes fused making tiny holes (Figure 1a). Izumo1 migrated out to the plasma membrane and spread all over the head, but tended to associate in the equatorial segment.52
The dynamic movement of Izumo1 at fusion was also observed using the same transgenic mouse line. Izumo1 mainly localized to the equatorial segment dispersed in the first step of sperm-egg fusion. However, Izumo1 on the inner acrosomal membrane did not disperse but was incorporated into the cytoplasm of the egg, together with the inner acrosomal membrane structure. These Izumo1 movements were recorded in real time.52 In conjunction with electron microscopic observations reported by many researchers, we realized that the sperm-egg fusion is apparently divided into two different phases as explained in Figure 3.
Figure 3.
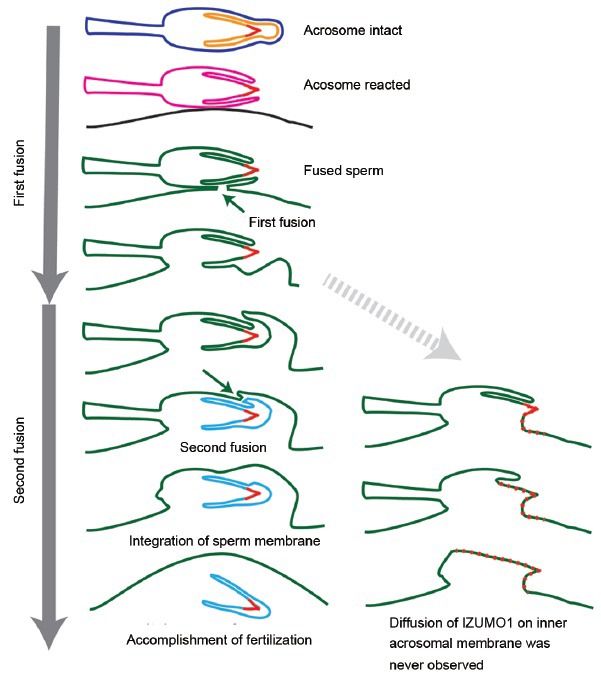
Fertilization requires two independent fusions. Intact spermatozoa have a plasma membrane (blue) and an acrosomal membrane (orange). After the acrosome reaction, these two membranes fuse and form a new sperm membrane (pink). The first fusion takes place between the pink membrane and egg plasma membrane (black). After the first fusion, egg and sperm membrane form a new consecutive membrane (green). If fusion is accomplished in this step, Izumo1 on the acrosomal cap of the inner acrosomal membrane (indicated by red) should spread on the newly-formed egg surface (green). However, the second fusion (invagination) follows the first fusion that separates the acrosomal cap and acrosomal sheath areas (light blue) from the fused membrane (green). Thus, Izumo1 on the inner acrosomal membrane is invaginated into the cytoplasm of the eggs. From live imaging, Izumo1 seems to be required for the first fusion. The nature of the second fusion remains totally unknown.
CONCLUSION
Observation of fertilization using gene-manipulated animals has brought us a new schematic diagram in mammalian fertilization (Figure 1). Note that the classical theories of the zona-induced acrosome reaction are not included in the figure. In order to understand the molecular mechanisms of fertilization, we apparently need more information. Reflecting on the progress in fertilization research, the role of gene-manipulated animals seems all the more important. Fortunately, the Crisper/Cas9 system has opened a new (wide) door for gene-disruption experiments.55 The method is both quick and easy and applicable to mammals, fish, insects, and even to plants. In one sense, gene disruption is easier than antibody production. Use of gene-manipulated animals will soon become as routine as gel-electrophoresis.
ACKNOWLEDGMENTS
I am grateful to Dr. Martin M Matzuk and Ms. Samantha Young for helpful discussion and critical reading of the manuscript. This work was supported in part by grants from the Ministry of Education, Science, Sports, Culture and Technology of Japan.
COMPETING FINANCIAL INTERESTS
The authors would declare no competing interests, or other interests that might be perceived to influence the results and/or discussion reported in this article.
REFERENCES
- 1.Clift D, Schuh M. Restarting life: fertilization and the transition from meiosis to mitosis. Nat Rev Mol Cell Biol. 2013;14:549–62. doi: 10.1038/nrm3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581–96. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- 3.Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–8. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- 4.Dan J. Studies on the acrosome reaction. I. Reaction to egg water and other stimuli. Biol Bull. 1952;103:54–66. [Google Scholar]
- 5.Yanagimachi R, Chang MC. Fertilization of hamster eggs in vitro. Nature. 1963;200:281–2. doi: 10.1038/200281b0. [DOI] [PubMed] [Google Scholar]
- 6.Toyoda Y, Yokoyama M, Hoshi T. Studies on the fertilization of mouse eggs in vitro. Jpn J Anim Reprod. 1971;16:147–57. [Google Scholar]
- 7.Uehara T, Yanagimachi R. Microsurgical injection of spermatozoa into hamster eggs with subsequent transformation of sperm nuclei into male pronuclei. Biol Reprod. 1976;15:467–70. doi: 10.1095/biolreprod15.4.467. [DOI] [PubMed] [Google Scholar]
- 8.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 9.Takeo T, Hoshii T, Kondo Y, Toyodome H, Arima H, et al. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod. 2008;78:546–51. doi: 10.1095/biolreprod.107.065359. [DOI] [PubMed] [Google Scholar]
- 10.Robertson E, Bradley A, Kuehn M, Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986;323:445–8. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- 11.Capecchi MR. Generating mice with targeted mutations. Nat Med. 2001;7:1086–90. doi: 10.1038/nm1001-1086. [DOI] [PubMed] [Google Scholar]
- 12.Smithies O. Forty years with homologous recombination. Nat Med. 2001;7:1083–6. doi: 10.1038/nm1001-1083. [DOI] [PubMed] [Google Scholar]
- 13.Reed SI. G1-specific cyclins: in search of an S-phase-promoting factor. Trends Genet. 1991;7:95–9. doi: 10.1016/0168-9525(91)90279-Y. [DOI] [PubMed] [Google Scholar]
- 14.Braun T, Arnold HH. Inactivation of Myf-6 and Myf-5 genes in mice leads to alterations in skeletal muscle development. EMBO J. 1995;14:1176–86. doi: 10.1002/j.1460-2075.1995.tb07101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Behringer RR, Olson EN. Inactivation of the myogenic bHLH gene MRF4 results in up-regulation of myogenin and rib anomalies. Genes Dev. 1995;9:1388–99. doi: 10.1101/gad.9.11.1388. [DOI] [PubMed] [Google Scholar]
- 16.Patapoutian A, Yoon JK, Miner JH, Wang S, Stark K, et al. Disruption of the mouse MRF4 gene identifies multiple waves of myogenesis in the myotome. Development. 1995;121:3347–58. doi: 10.1242/dev.121.10.3347. [DOI] [PubMed] [Google Scholar]
- 17.Olson EN, Arnold HH, Rigby PW, Wold BJ. Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell. 1996;85:1–4. doi: 10.1016/s0092-8674(00)81073-9. [DOI] [PubMed] [Google Scholar]
- 18.Rossi D, Cozzio A, Flechsig E, Klein MA, Rülicke T, et al. Onset of ataxia and Purkinje cell loss in PrP null mice inversely correlated with Dpl level in brain. EMBO J. 2001;20:694–702. doi: 10.1093/emboj/20.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasuwa H, Ueda J, Ikawa M, Okabe M. miR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science. 2013;341:71–3. doi: 10.1126/science.1237999. [DOI] [PubMed] [Google Scholar]
- 20.Osokine I, Hsu R, Loeb GB, McManus MT. Unintentional miRNA ablation is a risk factor in gene knockout studies: a short report. PLoS Genet. 2008;4:e34. doi: 10.1371/journal.pgen.0040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brookfield J. Can genes be truly redundant? Curr Biol. 1992;2:553–4. doi: 10.1016/0960-9822(92)90036-a. [DOI] [PubMed] [Google Scholar]
- 22.Baba T, Azuma S, Kashiwabara S, Toyoda Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem. 1994;269:31845–9. [PubMed] [Google Scholar]
- 23.Blobel CP, Wolfsberg TG, Turck CW, Myles DG, Primakoff P, et al. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature. 1992;356:248–52. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- 24.Cho C, Bunch DO, Faure JE, Goulding EH, Eddy EM, et al. Fertilization defects in sperm from mice lacking fertilin beta. Science. 1998;281:1857–9. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- 25.Kim E, Yamashita M, Nakanishi T, Park KE, Kimura M, et al. Mouse sperm lacking ADAM1b/ADAM2 fertilin can fuse with the egg plasma membrane and effect fertilization. J Biol Chem. 2006;281:5634–9. doi: 10.1074/jbc.M510558200. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura H, Kim E, Nakanishi T, Baba T. Possible function of the ADAM1a/ADAM2 Fertilin complex in the appearance of ADAM3 on the sperm surface. J Biol Chem. 2004;279:34957–62. doi: 10.1074/jbc.M314249200. [DOI] [PubMed] [Google Scholar]
- 27.Ikawa M, Wada I, Kominami K, Watanabe D, Toshimori K, et al. The putative chaperone calmegin is required for sperm fertility. Nature. 1997;387:607–11. doi: 10.1038/42484. [DOI] [PubMed] [Google Scholar]
- 28.Ikawa M, Tokuhiro K, Yamaguchi R, Benham AM, Tamura T, et al. Calsperin is a testis-specific chaperone required for sperm fertility. J Biol Chem. 2011;286:5639–46. doi: 10.1074/jbc.M110.140152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokuhiro K, Ikawa M, Benham AM, Okabe M. Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility [corrected] Proc Natl Acad Sci U S A. 2012;109:3850–5. doi: 10.1073/pnas.1117963109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shamsadin R, Adham IM, Nayernia K, Heinlein UA, Oberwinkler H, et al. Male mice deficient for germ-cell cyritestin are infertile. Biol Reprod. 1999;61:1445–51. doi: 10.1095/biolreprod61.6.1445. [DOI] [PubMed] [Google Scholar]
- 31.Fujihara Y, Okabe M, Ikawa M. GPI-anchored protein complex, LY6K/TEX101, is required for sperm migration into the oviduct and male fertility in mice. Biol Reprod. 2014;90:60. doi: 10.1095/biolreprod.113.112888. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi T, Isotani A, Yamaguchi R, Ikawa M, Baba T, et al. Selective passage through the uterotubal junction of sperm from a mixed population produced by chimeras of calmegin-knockout and wild-type male mice. Biol Reprod. 2004;71:959–65. doi: 10.1095/biolreprod.104.028647. [DOI] [PubMed] [Google Scholar]
- 33.Fujihara Y, Tokuhiro K, Muro Y, Kondoh G, Araki Y, et al. Expression of TEX101, regulated by ACE, is essential for the production of fertile mouse spermatozoa. Proc Natl Acad Sci U S A. 2013;110:8111–6. doi: 10.1073/pnas.1222166110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bleil JD, Beall CF, Wassarman PM. Mammalian sperm-egg interaction: fertilization of mouse eggs triggers modification of the major zona pellucida glycoprotein, ZP2. Dev Biol. 1981;86:189–97. doi: 10.1016/0012-1606(81)90329-8. [DOI] [PubMed] [Google Scholar]
- 35.Gong X, Dubois DH, Miller DJ, Shur BD. Activation of a G protein complex by aggregation of beta-1,4-galactosyltransferase on the surface of sperm. Science. 1995;269:1718–21. doi: 10.1126/science.7569899. [DOI] [PubMed] [Google Scholar]
- 36.Nakanishi T, Ikawa M, Yamada S, Parvinen M, Baba T, et al. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 1999;449:277–83. doi: 10.1016/s0014-5793(99)00433-0. [DOI] [PubMed] [Google Scholar]
- 37.Baibakov B, Gauthier L, Talbot P, Rankin TL, Dean J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development. 2007;134:933–43. doi: 10.1242/dev.02752. [DOI] [PubMed] [Google Scholar]
- 38.Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, et al. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci U S A. 2011;108:4892–6. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–8. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 40.Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, et al. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet. 2000;24:279–82. doi: 10.1038/73502. [DOI] [PubMed] [Google Scholar]
- 41.Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287:319–21. doi: 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- 42.Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287:321–4. doi: 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]
- 43.Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci U S A. 2011;108:20008–11. doi: 10.1073/pnas.1116965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardy DM, Oda MN, Friend DS, Huang TT., Jr A mechanism for differential release of acrosomal enzymes during the acrosome reaction. Biochem J. 1991;275(Pt 3):759–66. doi: 10.1042/bj2750759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim KS, Foster JA, Gerton GL. Differential release of guinea pig sperm acrosomal components during exocytosis. Biol Reprod. 2001;64:148–56. doi: 10.1095/biolreprod64.1.148. [DOI] [PubMed] [Google Scholar]
- 46.Valdivia M, Sillerico T, De Ioannes A, Barros C. Proteolytic activity of rabbit perivitelline spermatozoa. Zygote. 1999;7:143–9. doi: 10.1017/s0967199499000507. [DOI] [PubMed] [Google Scholar]
- 47.Gahlay G, Gauthier L, Baibakov B, Epifano O, Dean J. Gamete recognition in mice dependes on the cleavage status of an egg's zona pellucida protein. Scinece. 2010;329:216–9. doi: 10.1126/science.1188178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okabe M, Yagasaki M, Oda H, Matzno S, Kohama Y, et al. Effect of a monoclonal anti-mouse sperm antibody (OBF13) on the interaction of mouse sperm with zona-free mouse and hamster eggs. J Reprod Immunol. 1988;13:211–9. doi: 10.1016/0165-0378(88)90002-2. [DOI] [PubMed] [Google Scholar]
- 49.Inoue N, Hamada D, Kamikubo H, Hirata K, Kataoka M, et al. Molecular dissection of IZUMO1, a sperm protein essential for sperm-egg fusion. Development. 2013;140:3221–9. doi: 10.1242/dev.094854. [DOI] [PubMed] [Google Scholar]
- 50.Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–7. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–7. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satouh Y, Inoue N, Ikawa M, Okabe M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J Cell Sci. 2012;125(Pt 21):4985–90. doi: 10.1242/jcs.100867. [DOI] [PubMed] [Google Scholar]
- 53.Okabe M, Adachi T, Takada K, Oda H, Yagasaki M, et al. Capacitation-related changes in antigen distribution on mouse sperm heads and its relation to fertilization rate in vitro . J Reprod Immunol. 1987;11:91–100. doi: 10.1016/0165-0378(87)90014-3. [DOI] [PubMed] [Google Scholar]
- 54.Toshimori K. Dynamics of the mammalian sperm membrane modification leading to fertilization: a cytological study. J Electron Microsc (Tokyo) 2011;60(Suppl 1):S31–42. doi: 10.1093/jmicro/dfr036. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–8. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Q, Shur BD. Sperm from beta 1, 4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development. 1997;124:4121–31. doi: 10.1242/dev.124.20.4121. [DOI] [PubMed] [Google Scholar]
- 57.Asano M, Furukawa K, Kido M, Matsumoto S, Umesaki Y, et al. Growth retardation and early death of beta-1,4-galactosyltransferase knockout mice with augmented proliferation and abnormal differentiation of epithelial cells. EMBO J. 1997;16:1850–7. doi: 10.1093/emboj/16.8.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baba D, Kashiwabara S, Honda A, Yamagata K, Wu Q, et al. Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J Biol Chem. 2002;277:30310–4. doi: 10.1074/jbc.M204596200. [DOI] [PubMed] [Google Scholar]
- 59.Inoue N, Ikawa M, Nakanishi T, Matsumoto M, Nomura M, et al. Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol Cell Biol. 2003;23:2614–22. doi: 10.1128/MCB.23.7.2614-2622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ensslin MA, Shur BD. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell. 2003;114:405–17. doi: 10.1016/s0092-8674(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 61.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–50. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 62.Lin YN, Roy A, Yan W, Burns KH, Matzuk MM. Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis. Mol Cell Biol. 2007;27:6794–805. doi: 10.1128/MCB.01029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, et al. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1) Dev Biol. 2008;320:12–8. doi: 10.1016/j.ydbio.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutton KA, Jungnickel MK, Florman HM. A polycystin-1 controls postcopulatory reproductive selection in mice. Proc Natl Acad Sci U S A. 2008;105:8661–6. doi: 10.1073/pnas.0800603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tardif S, Wilson MD, Wagner R, Hunt P, Gertsenstein M, et al. Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida. J Biol Chem. 2010;285:24863–70. doi: 10.1074/jbc.M110.123125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muro Y, Buffone MG, Okabe M, Gerton GL. Function of the acrosomal matrix: zona pellucida 3 receptor (ZP3R/sp56) is not essential for mouse fertilization. Biol Reprod. 2012;86:1–6. doi: 10.1095/biolreprod.111.095877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hagaman JR, Moyer JS, Bachman ES, Sibony M, Magyar PL, et al. Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci U S A. 1998;95:2552–7. doi: 10.1073/pnas.95.5.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamaguchi R, Yamagata K, Ikawa M, Moss SB, Okabe M. Aberrant distribution of ADAM3 in sperm from both angiotensin-converting enzyme (Ace)- and calmegin (Clgn)-deficient mice. Biol Reprod. 2006;75:760–6. doi: 10.1095/biolreprod.106.052977. [DOI] [PubMed] [Google Scholar]
- 69.Shen C, Kuang Y, Liu J, Feng J, Chen X, et al. Prss37 is required for male fertility in the mouse. Biol Reprod. 2013;88:123. doi: 10.1095/biolreprod.112.107086. [DOI] [PubMed] [Google Scholar]
- 70.Yamaguchi R, Muro Y, Isotani A, Tokuhiro K, Takumi K, et al. Disruption of ADAM3 impairs the migration of sperm into oviduct in mouse. Biol Reprod. 2009;81:142–6. doi: 10.1095/biolreprod.108.074021. [DOI] [PubMed] [Google Scholar]
- 71.Marcello MR, Jia W, Leary JA, Moore KL, Evans JP. Lack of tyrosylprotein sulfotransferase-2 activity results in altered sperm-egg interactions and loss of ADAM3 and ADAM6 in epididymal sperm. J Biol Chem. 2011;286:13060–70. doi: 10.1074/jbc.M110.175463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaguchi R, Fujihara Y, Ikawa M, Okabe M. Mice expressing aberrant sperm-specific protein PMIS2 produce normal-looking but fertilization-incompetent spermatozoa. Mol Biol Cell. 2012;23:2671–9. doi: 10.1091/mbc.E11-12-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krutskikh A, Poliandri A, Cabrera-Sharp V, Dacheux JL, Poutanen M, et al. Epididymal protein Rnase10 is required for post-testicular sperm maturation and male fertility. FASEB J. 2012;26:4198–209. doi: 10.1096/fj.12-205211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hartmann JF, Gwatkin RB, Hutchison CF. Early contact interactions between mammalian gametes in vitro: evidence that the vitellus influences adherence between sperm and zona pellucida. Proc Natl Acad Sci U S A. 1972;69:2767–9. doi: 10.1073/pnas.69.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okabe M. The cell biology of mammalian fertilization. Development. 2013;140:4471–9. doi: 10.1242/dev.090613. [DOI] [PubMed] [Google Scholar]


