Abstract
In this experimental prospective study, we aimed to analyze the effect of transient scrotal hyperthermia on the male reproductive organs, from the perspective of sperm parameters, semen plasma biochemical markers, and oxidative stress, to evaluate whether different frequencies of heat exposure cause different degrees of damage to spermatogenesis. Two groups of volunteers (10 per group) received testicular warming in a 43°C water bath 10 times, for 30 min each time: group 1: 10 consecutive days; group 2: once every 3 days. Sperm parameters, epididymis and accessory sex gland function, semen plasma oxidative stress and serum sex hormones were tested before treatment and in the 16-week recovery period after treatment. At last, we found an obvious reversible decrease in sperm concentration (P = 0.005 for Group 1 and P= 0.008 for Group 2 when the minimums were compared with baseline levels, the same below), motility (P = 0.009 and 0.021, respectively), the hypoosmotic swelling test score (P = 0.007 and 0.008, respectively), total acrosin activity (P = 0.018 and 0.009, respectively), and an increase in the seminal plasma malondialdehyde concentration (P = 0.005 and 0.017, respectively). The decrease of sperm concentration was greater for Group 2 than for Group 1 (P = 0.031). We concluded that transient scrotal hyperthermia seriously, but reversibly, negatively affected the spermatogenesis, oxidative stress may be involved in this process. In addition, intermittent heat exposure more seriously suppresses the spermatogenesis compared to consecutive heat exposure. This may be indicative for clinical infertility etiology analysis and the design of contraceptive methods based on heat stress.
Keywords: hyperthermia, oxidative stress, seminal plasma biochemical markers, sperm parameters, spermatogenesis
INTRODUCTION
The testes of most animals, including humans, are found in the scrotum outside the main body cavity and are thus 2–8°C below the core body temperature.1,2 In addition, a countercurrent heat exchange system between the pampiniform plexus and the testicular artery effectively regulates the temperature within the testes, ensuring normal spermatogenesis. Epidemiological investigations have shown that the semen quality of car drivers, welders, and sauna enthusiasts was poorer than that of normal people.3,4,5 Previous studies have shown that cryptorchidism and varicocele can seriously affect spermatogenesis, mainly due to the elevated temperature of the scrotum.6,7 However, those studies were mainly retrospective. The heat stress intensity and exposure time lacked accurate quantification, and the relationship between heat exposure frequency and the degree of spermatogenesis damage was not explored.
Epididymis and accessory glands secrete several factors that play critical roles in sperm physiology. Neutral alpha-glucosidase (NAG), fructose, and zinc are traditionally used as markers of the functional activity of the epididymis, seminal vesicles, and the prostate. The epididymis has been shown to play an important role in sperm maturation, including the gaining of motility. Several studies have found positive correlations between the seminal levels of NAG and sperm motility.8,9 Fructose has been shown to be the main source of energy and metabolism of spermatozoa, and positive correlations have also been demonstrated between the seminal levels of fructose and sperm motility.10 Zinc also plays a critical role in sperm function, mainly by maintaining chromatin stability.11 Studies have also shown a positive effect of zinc content on the percentage of motile sperm.12,13 As these markers are critical factors affecting sperm function, especially sperm motility, we were interested in whether transient scrotal hyperthermia can affect the function of the epididymis and male accessory glands, and whether this is associated with spermatogenesis damage caused by heat stress. There has been no research on this question reported to date.
Oxidative stress is a key aspect of pathogenesis in many diseases. Studies have found that the increase in testicular temperature observed in cryptorchidism and varicocele has been associated with an increase in testicular oxidative stress.14,15 Experimental models have also shown that transient mild testicular hyperthermia can destroy the balance between oxidative capacity and antioxidant capacity.16,17 The human spermatozoa are highly susceptible to oxidative stress, high levels of free radicals, and reactive oxygen species (ROS), including the superoxide anion and hydrogen peroxide (H2O2).18,19 Oxidative stress can be deleterious and cause oxidative damage to the sperm plasma membrane and DNA fragmentation of both the nuclear and mitochondrial genomes.20 Although oxidative stress has been investigated in animal models and cryptorchidism and varicocele patients, we wondered whether oxidative and antioxidant capacity varied in the human testes when they are exposed to mild hyperthermia.
In summary, sperm parameters can largely reflect spermatogenesis, and epididymis and accessory sex gland function can obviously affect sperm parameters. Oxidative stress may be a key factor in the damage of spermatogenesis. The aim of this study was to analyze the effect of transient scrotal hyperthermia on male reproductive organs from the perspective of semen parameters, the epididymis and accessory sex gland functions, and semen plasma oxidative stress, to evaluate whether different frequencies of heat exposure could cause different degrees of damage to the spermatogenesis process.
MATERIALS AND METHODS
Subjects
This study was approved by our Local Ethics Committee. Volunteers were recruited through poster and bulletin board advertisements. The inclusion criteria were as follows, male, 22–50 years old, married and having fathered at least one child, no plan to father another child, good health without hypertension or trauma. Exclusion criteria were as follows: not married or have no children, plan to have another child, cryptorchidism or varicocele, severe heart, brain or renal disease, could not commit to finishing the experiment. Finally, a total of 20 healthy Chinese male volunteers between the ages of 22 and 45 were eligible and enrolled into the study after they had completed a written informed consent. The subjects had no significant medical history, and all had normal results from a physical examination carried out during the recruitment. They had normal sexual hormone levels (follicle-stimulating hormone [FSH], luteinizing hormone [LH], sex hormone-binding globulin [SHBG], estradiol [E2], testosterone [T], free T) and two consecutive normal semen analyses at 2 weeks intervals (semen volume ≥ 1.5 ml, sperm concentration ≥ 15 million ml−1, progressive sperm ≥ 32%) according to the World Health Organization (WHO) criteria (5th edition).21
Study design
Subjects were initially randomized into one of the two groups, each group consisting of 10 volunteers. All of the subjects underwent testicular warming at 43°C in a water bath 10 times, for 30 min each time. In brief, the lower half body of each subject was soaked in the bathtub in which the water was regulated to be 43°C. To maintain the water temperature constant, we continuously added the adjusted hot water (43°C) into the bathtub, and we also drained the water from the bathtub by the same flow rate. For subjects in Group 1, this was carried out once a day for 10 consecutive days while for subjects in Group 2, it was once every 3 days, 10 times. The treatment phase was followed by a recovery phase of 16 weeks. Blood was collected before and every 3 weeks after the water bath treatment, a total of 6 times. Semen samples were collected twice before the water bath treatment and every 2 weeks after, for a total of 10 times.
Semen sample collection and processing
Semen samples were obtained from each subject by masturbation after between 3 and 7 days of sexual abstinence and collected in sterile containers. After liquefaction at 37°C, samples were examined for semen volume, pH, sperm concentration, viability, and normal morphology according to the WHO guidelines for the examination and processing of human semen (5th edition).21 A duplicate reading was performed by different operators and the results are the mean of these determinations.
Conventional semen analysis
Semen volume was evaluated by semen weight, assuming a density of 1.0 g ml−1. The container was weighed before and after sample collection, and the difference between the weights was recorded as the volume. The pH value was measured using pH paper and compared with the calibration strip to determine the value. For the assessment of sperm concentration and motility, 10 μl of well-mixed semen was placed in a clean Makler chamber (which had been held at 37°C), covered with a coverslip, and immediately examined at a total magnification of ×400. Ten of the 100 squares in the microscope field were randomly scanned, and the sperm count was recorded using a cytometer. With the help of an ocular grid, the proportion of each of three motility categories was assessed, progressive sperm, nonprogressive sperm, and immotile sperm. Total sperm count was calculated by multiplying sperm concentration by semen volume.
Sperm function assay
The hypo-osmotic swelling (HOS) test was performed according to the method described by Vivas et al.22 In brief, a HOS solution containing 1.351 g of fructose and 0.735 g of sodium citrate was made up to 100 mL with distilled water, frozen at −20°C in several aliquots, then thawed and mixed well before use. One milliliter of HOS solution was placed at 37°C for 10 min. The semen sample (0.1 ml) was added to the HOS solution (an initial count of coiled tails was noted beforehand) and incubated for 30 min at 37°C. A drop of the mixture was placed on a glass slide and covered with a slip. The swell of spermatozoa tails was observed under a phase contrast microscope at a magnification of ×400. The percentages of reacted sperm (swollen tails) were assessed by counting a minimum of 200 spermatozoa. A duplicate observation was made, and the mean of the two was taken.
Total acrosin activity assay was measured using a commercially available kit (HuaKang, Shenzhen, China), which was a modification of the Kennedy method.23 Seven and a half million spermatozoa were obtained from each semen sample and centrifuged to remove semen plasma. A detergent buffer containing N-α-benzoyl-dl-arginine p-nitroanilide hydrochloride (BAPNA) was used for activation of proacrosin into enzymatically active acrosin. The sperm pellet was then suspended in 1 ml of detergent buffer for 1 h at 24°C. In this procedure, BAPNA was hydrolyzed by acrosin and converted to a chromophoric product (4-nitroaniline) that was detected at 410 nm using a V-1100 spectrophotometer (Mapada, Shanghai, China). Total acrosin activity (μIU 10−6 sperm) was calculated according to the kit instructions.
Hormone assays
Blood samples were obtained, centrifuged to separate the plasma, and stored at −20°C until used in the hormone assay. Plasma concentrations of FSH, LH, T, free T, E2, and SHBG were all tested using a chemiluminescent immunoassay method on the automated UniCel DxI 800 analyzer (Beckman Coulter, Brea, USA). The commercial kits were also provided by Beckman Coulter, Inc. All of the hormone assays were carried out by the same specialized technician, to minimize the effect of between-assay variability.
Biochemical marker analysis of the epididymis and accessory sex glands
Semen samples were centrifuged for 10 min at 3000 × g after semen analysis and seminal plasma was decanted and stored at −20°C until analysis for biochemical markers was carried out. Levels of seminal plasma NAG were measured according to the photometric method described by Vivas-Acevedo et al.22 The substrate (paranitrophenyl a-p-nitrophenolglucopyranoside) can be hydrolyzed by NAG into paranitrophenyl, the latter can be measured by spectrophotometer at a wavelength of 405 nm after a 2-h incubation at 37°C, pH 6.8. The acid isoenzyme originates in the prostate can be selectively inhibited by sodium dodecyl sulfate to allow the measurement of NAG activity, the use of castanospermine can inhibit the nonglucosidase-related substrate breakdown and make the assay more sensitive. The results were expressed as mU ml−1.
Levels of the fructose were measured according to the method described by Henkel et al.24 In brief, samples were mixed and diluted (5 μl seminal plasma plus 50 μl distilled water) in an Eppendorf cup. Afterward, 12.5 μl 63 μmol l−1 ZnSO4 and 12.5 μl 0.1 mol l−1 NaOH were added and then centrifuged to deproteinize. Then 50 μl of the supernatant were collected and mixed with 50 μl indole reagent (2 mmol l−1 indole in 16 mM benzoic acid), 500 μl 32% HCl added, covered with parafilm, and heated for 20 min at 50°C in a water bath. After all, the samples were cooled in ice water and tested at 470 nm in a spectrophotometer. At last, the fructose concentrations were calculated and expressed as μmol ml−1.
Seminal plasma zinc concentration was measured using a commercially available kit (Bred Co. Ltd., Shenzhen, China) according to the manufacturer's instructions, essentially as previously described.25 The proteins in the sample were precipitated with trichloroacetic acid, the supernatant mixed with a water-soluble pyridylazo dye (5-Br-PAPS) and the absorbance measured at 560 nm.
Oxidative stress assay
Semen samples were centrifuged for 10 min at 3000 × g after semen analysis, and seminal plasma was decanted and stored at −20°C until analysis for oxidative stress was carried out. Superoxide dismutase (SOD), catalase (CAT) activity and malondialdehyde (MDA) levels were determined using commercial kits (Beyotime, Haimen, China). SOD activity was measured using the inhibition of nitroblue tetrazolium (NBT) reduction by the combination xanthine–xanthine oxidase as described by Zini et al.26 The amount of seminal plasma able to diminish the reduction of NBT by 50% was set as one unit of SOD activity. The result was finally expressed as U ml−1.
CAT activity was determined by the reduction in concentration of exogenous hydrogen peroxide (H2O2) after incubation with the test samples, using the method described previously.27 Briefly, samples were treated with excess H2O2 hydrogen peroxide. Decomposition by catalase CAT occurred for a specified time, and the remaining H2O2 hydrogen peroxide coupled with a substrate was treated with peroxidase, to generate a red product, N-4-antipyryl-3-chloro-5-sulfonate-p-benzoquinonemonoimine that absorbs maximally at 520 nm. The activity of CAT was finally presented as U ml−1.
For the lipid peroxidation level, MDA was tested as described by Kang et al.28 A 200 μl aliquot of thiobarbituric acid reagent was added to 100 μl of the centrifuged seminal plasma. The mixture was treated in a boiling water bath for 15 min. After cooling, the suspension was centrifuged (1000 × g, 10 min), the supernatant was separated, and the absorbance was measured at 530 nm. The MDA content was finally expressed as μmol ml−1.
The protein content in the supernatant of the seminal plasma was estimated by the biuret method,29 using bovine serum albumin as a standard, and finally expressed as mg ml−1. SOD, CAT, and MDA content were then converted to U mg−1 protein, U mg−1 protein, and μmol mg−1 protein, respectively.
Statistical analysis
All of the results were expressed as mean ± s.e.m. Characteristics of subjects between the two groups were compared using Wilcoxon test, data at each time point after hyperthermia were compared with data before hyperthermia (baseline) also using Wilcoxon test, due to skewness in the distributions of some of these measurements. The two sperm or semen plasma test results before hyperthermia were averaged as the baseline level. To evaluate the effect of the degree of hyperthermia on spermatogenesis, the minimum of sperm concentration in the two groups were compared using an analysis of covariance, the baseline value was regarded as a covariate. Comparisons of rates of oligozoospermia or severe oligozoospermia in the two groups were made using Fisher Exact test. Statistical analysis was performed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered significant.
RESULTS
All 20 subjects completed the clinical study voluntarily. There were no significant differences in age, body mass index, or sperm parameters between the subjects in the two groups before hyperthermia (Table 1).
Table 1.
Characteristics of subjects in the both treatment groups
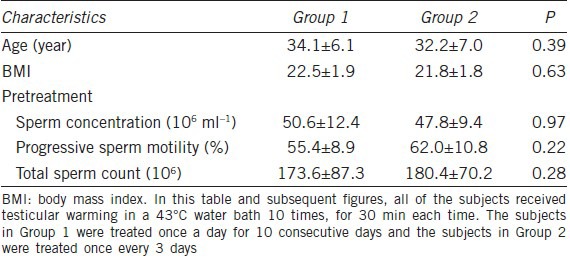
Conventional sperm parameters
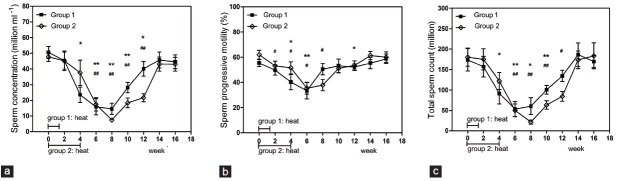
The sperm concentration and total sperm count of both groups showed reversible decreases. The minimum levels of sperm concentration observed at week eight after treatment were significantly different from baseline levels (P = 0.005 for Group 1 and P = 0.008 for Group 2). The minimum levels of total sperm count observed at week 6 or 8 after treatment were also significantly different from baseline levels (P = 0.009 for Group 1 and P = 0.005 for Group 2). The sperm concentration of Group 2 (week 8: 15.5% of baseline value) decreased more drastically than that of Group 1 (week 8: 28.8% of baseline value), and the Group 2 concentrations recovered more slowly (Figures 1 and 2). The magnitude of sperm concentration reduction for Group 2 was greater than for Group 1 (P = 0.031). Sperm progressive motility decreased in both groups and followed a similar pattern to that of sperm concentration, with the lowest value recorded at 6 weeks after hyperthermia treatment, the minimum in both groups were significantly different from baseline levels (P = 0.009 for Group 1 and P = 0.021 for Group 2) (Figure 1). Furthermore, 7 and 4 subjects in Group 1 reached oligozoospermia (less than 15 million ml−1) and severe oligozoospermia (less than 5 million ml−1), respectively; 9 and 4 subjects in Group 2 reached oligozoospermia and severe oligozoospermia respectively, no significant difference existed (Figure 2).
Figure 1.
Mean (±s.e.m.) sperm concentration (a), sperm progressive motility (b) and total sperm count (c) before and after treatment in the 2 groups of subjects. *P < 0.05 when compared with baseline in Group 1; **P < 0.01 when compared with baseline in Group 1; #P < 0.05 when compared with baseline in Group 2; ##P < 0.01 when compared with baseline in Group 2.
Figure 2.
Number of subjects in each treatment group in which the sperm concentration was suppressed to severe oligozoospermia (less than 5 million ml−1; black bars) and oligozoospermia (less than 15 million ml−1; black bars plus dark hatched bars).
The hypo-osmotic swelling test
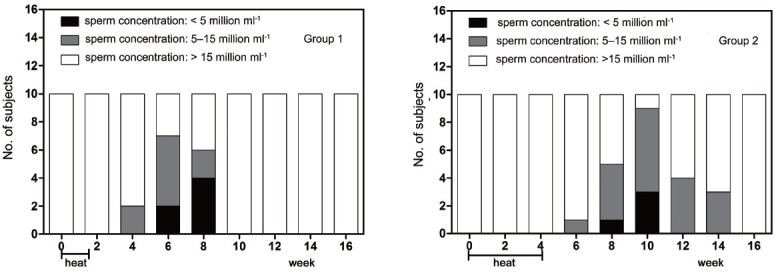
The mean value of the tail swelling rate for Group 1 decreased significantly from week 4 to 8 compared with the baseline level (P = 0.007 when the minimum was compared with baseline level), recovering at week 10. The value for group 2 decreased significantly from week 6 to 10, compared with the baseline level (P = 0.008 when the minimum was compared with the baseline level), recovering at week 12. The lowest values for both groups appeared at week 6 (Figure 3a).
Figure 3.
Mean (±s.e.m.) sperm hypo-osmotic swelling rate (a), sperm total acrosin activity (b) before and after treatment in the 2 groups of subjects. There were 3, 3 and 1 values missing at week 6, 8 and 10 respectively in Group 1 due to low value of total sperm count; there were 1, 5, 8, 1 and 1 values missing at week 4, 6, 8, 10 and 12 respectively in Group 2 due to low value of total sperm count. *P < 0.05 when compared with baseline in Group 1; **P < 0.01 when compared with baseline in Group 1; #P < 0.05 when compared with baseline in Group 2; ##P < 0.01 when compared with baseline in Group 2.
Total acrosin activity assay
The total acrosin activity assay required at least 15 × 106 sperm, according to the test kit instructions. However, the total sperm count of some subjects decreased severely and was insufficient to carry out the assay, thus some tests at the middle time points were missing. Our results showed that the total acrosin activity for Group 1 decreased significantly from week 4 to 10, compared with the baseline level (P = 0.018 when the minimum was compared with the baseline level). The value for Group 2 decreased significantly at week 2 compared with the baseline level (P = 0.009) (Figure 3b).
Hormone assays
Follicle-stimulating hormone, LH, E2, T, free T, and SHBG were tested at each time point. The results showed that none of the hormone levels changed significantly (P > 0.05), except for E2 of group 1 at week 2, compared with the baseline level (P = 0.042) (Figure 4).
Figure 4.
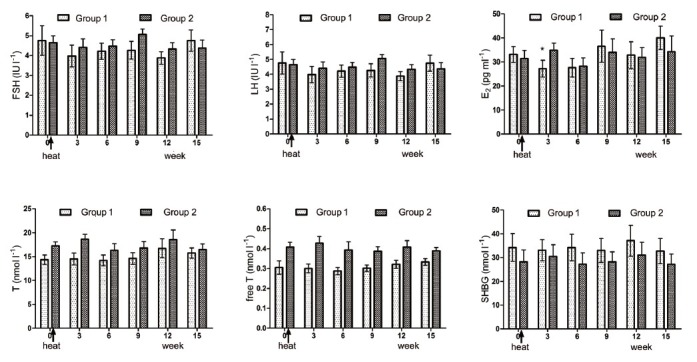
Mean (±s.e.m.) serum follicle-stimulating hormone, luteinizing hormone, estradiol (E2), testosterone (t), free T and sex hormone-binding globulin before and after treatment in the 2 groups of subjects. *P < 0.05 when compared with baseline in Group 1. All P > 0.05 when compared with baseline levels except for E2 test at week 3 in Group 1 (P = 0.037).
Biochemical marker analysis of the epididymis and accessory sex glands
Seminal plasma NAG, fructose, and zinc concentrations showed no significant difference compared with the baseline level in both groups at each time point (P > 0.05) (Figure 5).
Figure 5.
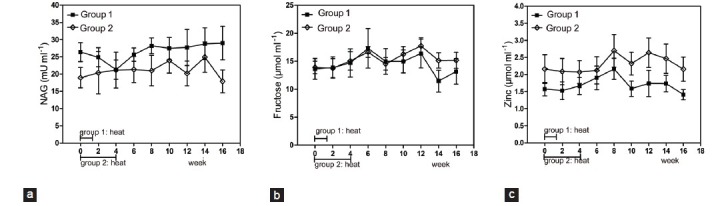
Mean (±s.e.m.) seminal plasma neutral a-glucosidase (a), fructose (b) and zinc (c) concentration before and after hyperthermia in the 2 groups of subjects. All P > 0.05 when compared with baseline levels.
Oxidative stress assays (superoxide dismutase, catalase, malondialdehyde)
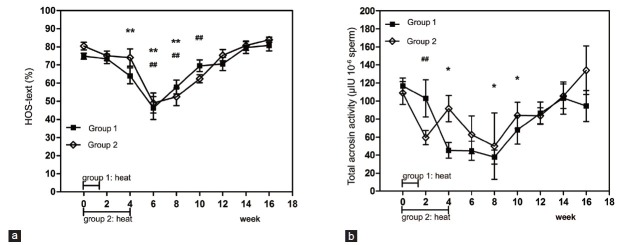
Seminal plasma SOD showed no obvious variation at each time point compared with the baseline levels for both groups (P > 0.05). CAT also showed no obvious change except that the value tested at week 4 significantly decreased compared with the baseline level in Group 1 (P = 0.017). MDA levels for Group 1 increased significantly from week 2 to week 10, compared with the baseline level, with the highest value reached at week 8 (P = 0.005 when the maximum was compared with baseline level). MDA levels for group 2 increased significantly at week 8 compared with the baseline level (P = 0.017) (Figure 6).
Figure 6.
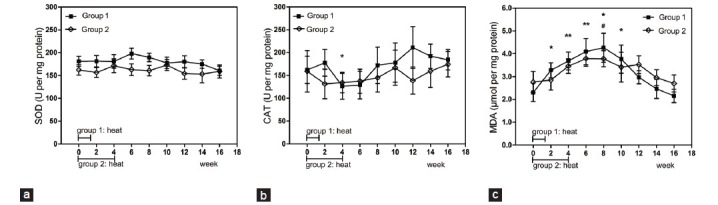
Mean (±s.e.m.) seminal plasma SOD (a), CAT (b) and MDA (c) content before and after hyperthermia in the 2 groups of subjects. *P < 0.05 when compared with baseline in Group 1; **P < 0.01 when compared with baseline in Group 1; #P < 0.05 when compared with baseline in Group 2; ##P < 0.01 when compared with baseline in Group 2. SOD: superoxide dismutase; CAT: catalase; MDA: malondialdehyde.
DISCUSSION
Many studies have been carried out to investigate the effect of hyperthermia on spermatogenesis, and the corresponding mechanism, using animal models.30,31,32 However, clinical studies are relatively rare. This study was carried out to investigate the effect of heat stress on the male reproductive organs. We monitored the dynamic changes in sperm parameters, accessory sex gland function, and semen plasma oxidative stress over a 16-week period, which was long enough to overlap with an entire spermatogenic cycle.
Consistent with other reports on the effect of hyperthermia on spermatogenesis in rats,33 monkeys,34,35 and humans,5,36 we showed a significant decrease and a good reversibility in sperm concentration and total sperm count (Figure 1). The minimum sperm concentration and total sperm count were recorded 6 or 8 weeks after treatment for both groups. The sperm concentration of Group 2 (15.5% of pretreatment value) decreased more drastically than that of Group 1 (28.8% of pretreatment value). Based on widely accepted concepts of duration of human spermatogenesis, a complete spermatogenic cycle (i.e. spermatogonia to spermatozoa) in men usually last for almost 74 days, it can be divided into 4 periods: spermatogonia mitosis (about 28 days), spermatocytes meiosis period I (about 23 days), spermatocytes meiosis period II (about 1 day) and spermiogenesis (about 22 days).37 It takes about 12 days (1–22 days) for the spermatozoa to pass through the epididymis and vas deferens and to reach the ejaculate.38 According to this phenomenon, for Group 1, sperm collected at week 8 were at the spermatocyte stage (meiosis) during the whole period of hyperthermia. For Group 2, those collected at this point were at the spermatocyte stage (meiosis) and elongated spermatid stage (spermiogenesis) at an early and late period of hyperthermia, respectively. Previous studies have shown that in heat stress-induced stage-specific damage to germ cells, those at the spermatocyte and spermatid stages were more susceptible than cells at other stages while spermatogonia were not sensitive to heat stress at all.39,40 The minimum sperm concentration and count recorded at week 8 in Group 1 may be due to serious impact on the spermatocytes. Due to “double hit,” hit to the spermatocyte and the spermatid, the sperm concentration and total sperm count of Group 2 were more severely reduced than for Group 1. The sperm output at week 10 and week 12 recovered gradually, possibly due to the relatively low sensitivity to heat stress of spermatogonian mitosis (about 58–86 days before ejaculation), a conclusion that has been drawn in previous studies.41,42 Output recovered to the baseline level by weeks 14–16, which was long enough to overlap with an entire spermatogenic cycle. Furthermore, more subjects were induced to oligozoospermia in Group 2 than Group 1, even though the difference was not significant (Figure 2). The results of our study indicated that intermittent hyperthermia could more seriously affect spermatogenesis than consecutive hyperthermia, probably because intermittent hyperthermia affects multiple heat-sensitive stages of spermatogenesis. This may be indicative for clinical infertility etiology analysis and the design of contraceptive methods based on heat stress. Progressive sperm motility also showed a reversible reduction. This variation may be the result of two factors. Hyperthermia may affect the formation of the sperm movement apparatus. As the process of spermiogenesis is susceptible to heat stress, this question is being researched by our group. Alternatively, heat stress may alter the normal functioning of the epididymis, which may lead to faster sperm epididymal transit, thus reducing the time required for spermatozoa maturation and resulting in a large number of immotile spermatozoa in the ejaculate. This has been reviewed elsewhere.43,44
The modifications of sperm parameters were not paralleled by changes in hormone levels. The finding that there were no significant differences in LH, FSH, E2, T, free T, and SHBG pre- and post-treatment was consistent with other studies (Figure 4).5,34,35,36 We know that the Leydig cells are the main cells that synthesize and secrete T. The stability of T and free T indicates that the function of the Leydig cells may not be damaged by short exposure to moderate heat.
It is well-known that the epididymis and accessory sex glands play a critical role in the functional status of sperm, especially their motility.13,45 In this study, we found that there was no significant variation in the three biochemical markers, seminal NAG, fructose, and zinc concentration, before and after heat treatment (all P values > 0.05) (Figure 5). Namely that epididymis and accessory sex gland functions were not severely affected, this indicated that the deterioration of semen quality were not caused by posttesticular factors, but testicular factors.
The HOS test is a functional test of sperm membrane integrity. Those sperm with damaged or chemically inactive plasma membranes do not show cytoplasmic swelling and their tails remain uncurled when they are in hypoosmotic swelling fluid. Sperm membrane integrity is important for its metabolism and specific changes in the dynamics of the membrane are required for the successful union of the male and female gametes, that is, for sperm capacitation, the acrosome reaction, and binding of the spermatozoa to the egg surface.46 Acrosin activity is a suitable marker for the fertilizing capacity of human spermatozoa, as it is involved in the acrosome reaction and assists fertilization by acting as a secondary zona pellucida binding protein.47,48 Our results revealed that hyperthermia can seriously affect sperm membrane integrity (Figure 3a) and fertilization capacity (Figure 3b), this may be one aspect of male infertility caused by heat stress.
Oxidative stress may be a critical factor leading to damage of sperm function. Our results showed that the seminal plasma antioxidants, SOD and CAT, showed no significant change except that CAT tested at week 4 significantly decreased from baseline in Group 1. However, a marker of lipid peroxidation, MDA, increased significantly before recovering to the baseline level in both groups (Figure 6). Therefore, the seminal plasma antioxidant capacity may not change severely, and ROS may be obviously increased when exposed to moderate heat. High levels of ROS may be generated by abnormal spermatozoa and leukocytes,49 and can damage membrane intergrity,50,51 decrease sperm motility,52,53 influence acrosomal functionality,54 and elevate levels of DNA damage.55 The consensus that scrotal heat stress could cause oxidative stress damage has been reached. Paul et al.16 reported that mild, transient scrotal heat stress causes the down-regulation of antioxidant enzyme expression in mouse testes. Tawadrous et al.15 found infertile men with varicocele have a relatively low level of seminal plasma antioxidants and high level of lipid peroxidation. However, our results showed that the antioxidative capacity did not decrease severely even though lipid peroxidation increased. This is in agreement with another study, which reported that hot summer can significantly increase bull seminal plasma lipid peroxidation while antioxidant enzyme activity remained stable.56 These differing conclusions may be due to different periods of exposure, and to different response intensity to heat stress among different species.
Subjects in the two groups were exposed to different frequencies, but the same total intensity of heat treatment. We found that the damage to spermatogenesis, sperm function, and oxidative stress in the two groups were similar. In general, both exhibited a good security and reversibility. Previous studies based on animal models have revealed that the most significant consequence of heat stress on the testes is the loss of germ cells via apoptosis,30,34,57 with the mitochondria-dependent pathway the key apoptotic pathway involved.30 Oxidative stress can cause serious injury to the mitochondria, initiating apoptosis and leading ultimately to cell death.58,59 Although we have no data on testicular tissue morphology or pathology, we can speculate that oxidative stress participates in the suppression of spermatogenesis in the circumstance of human scrotal heat stress, leading to a significant decrease in sperm concentration, motility, and sperm function. This viewpoint has been demonstrated in other studies.7,16
CONCLUSIONS
This study was a preliminary investigation on sperm and semen plasma parameters in humans affected by an accurate intensity of heat stress. The results indicated that sperm parameters and function reversibly decreased while serum sexual hormone levels, epididymis, and accessory sex gland function were not affected. Oxidative stress may participate in the suppression of spermatogenesis. In addition, intermittent heat exposure can more seriously damage spermatogenesis than consecutive heat exposure. This may be guiding significance to analyze the clinical etiology of heat-induced semen quality decline, and also indicative for the design of contraceptive methods based on heat stress. Further study should be carried out on how to control the heat exposure intensity and frequency, to achieve a realistic contraceptive effect.
AUTHOR CONTRIBUTIONS
MR participated in the design of the study, carried out the semen analysis and sexual hormone test, as well as drafted the manuscript. XLZ carried out a test of semen plasma biochemical markers and revised the manuscript. JY carried out the oxidative stress assay. SFH recruited the volunteers. HL performed the statistical analysis. WX and CHZ designed and conceived of the study.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the National Science and Technology Support Program of the Ministry of Science and Technology (No. 2012BAI31B08), the National Natural Science Foundation of China (No. 31171380).
REFERENCES
- 1.Danno S, Itoh K, Matsuda T, Fujita J. Decreased expression of mouse Rbm3, a cold-shock protein, in Sertoli cells of cryptorchid testis. Am J Pathol. 2000;156:1685–92. doi: 10.1016/S0002-9440(10)65039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivell R. Lifestyle impact and the biology of the human scrotum. Reprod Biol Endocrinol. 2007;5:15. doi: 10.1186/1477-7827-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thonneau P, Ducot B, Bujan L, Mieusset R, Spira A. Effect of male occupational heat exposure on time to pregnancy. Int J Androl. 1997;20:274–8. doi: 10.1046/j.1365-2605.1997.d01-303.x. [DOI] [PubMed] [Google Scholar]
- 4.Hjollund NH, Bonde JP, Jensen TK, Olsen J. Diurnal scrotal skin temperature and semen quality. The Danish First Pregnancy Planner Study Team. Int J Androl. 2000;23:309–18. doi: 10.1046/j.1365-2605.2000.00245.x. [DOI] [PubMed] [Google Scholar]
- 5.Garolla A, Torino M, Sartini B, Cosci I, Patassini C, et al. Seminal and molecular evidence that sauna exposure affects human spermatogenesis. Hum Reprod. 2013;28:877–85. doi: 10.1093/humrep/det020. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Li X. Molecular basis of cryptorchidism-induced infertility. Sci China Life Sci. 2010;53:1274–83. doi: 10.1007/s11427-010-4072-7. [DOI] [PubMed] [Google Scholar]
- 7.Shiraishi K, Takihara H, Matsuyama H. Elevated scrotal temperature, but not varicocele grade, reflects testicular oxidative stress-mediated apoptosis. World J Urol. 2010;28:359–64. doi: 10.1007/s00345-009-0462-5. [DOI] [PubMed] [Google Scholar]
- 8.Viljoen MH, Bornman MS, van der Merwe MP, du Plessis DJ. Alpha-glucosidase activity and sperm motility. Andrologia. 1990;22:205–8. doi: 10.1111/j.1439-0272.1990.tb01967.x. [DOI] [PubMed] [Google Scholar]
- 9.Fourie MH, du Toit D, Bornman MS, van der Merwe MP, du Plessis DJ. alpha-Glucosidase, sperm ATP concentrations, and epididymal function. Arch Androl. 1991;26:139–41. doi: 10.3109/01485019108987636. [DOI] [PubMed] [Google Scholar]
- 10.Patel SM, Skandhan KP, Mehta YB. Seminal plasma fructose and glucose in normal and pathological conditions. Acta Eur Fertil. 1988;19:329–32. [PubMed] [Google Scholar]
- 11.Kvist U, Björndahl L, Kjellberg S. Sperm nuclear zinc, chromatin stability, and male fertility. Scanning Microsc. 1987;1:1241–7. [PubMed] [Google Scholar]
- 12.Chia SE, Ong CN, Chua LH, Ho LM, Tay SK. Comparison of zinc concentrations in blood and seminal plasma and the various sperm parameters between fertile and infertile men. J Androl. 2000;21:53–7. [PubMed] [Google Scholar]
- 13.Elzanaty S, Richthoff J, Malm J, Giwercman A. The impact of epididymal and accessory sex gland function on sperm motility. Hum Reprod. 2002;17:2904–11. doi: 10.1093/humrep/17.11.2904. [DOI] [PubMed] [Google Scholar]
- 14.Peltola V, Huhtaniemi I, Ahotupa M. Abdominal position of the rat testis is associated with high level of lipid peroxidation. Biol Reprod. 1995;53:1146–50. doi: 10.1095/biolreprod53.5.1146. [DOI] [PubMed] [Google Scholar]
- 15.Tawadrous GA, Aziz AA, Mostafa T. Seminal soluble fas relationship with oxidative stress in infertile men with varicocele. Urology. 2013;82:820–3. doi: 10.1016/j.urology.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Paul C, Teng S, Saunders PT. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol Reprod. 2009;80:913–9. doi: 10.1095/biolreprod.108.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pino JA, Osses N, Oyarzún D, Farías JG, Moreno RD, et al. Differential effects of temperature on reactive oxygen/nitrogen species production in rat pachytene spermatocytes and round spermatids. Reproduction. 2013;145:203–12. doi: 10.1530/REP-12-0330. [DOI] [PubMed] [Google Scholar]
- 18.Tremellen K. Oxidative stress and male infertility – A clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 19.Gavella M, Lipovac V. Protective effects of exogenous gangliosides on ROS-induced changes in human spermatozoa. Asian J Androl. 2013;15:375–81. doi: 10.1038/aja.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol. 2006;250:66–9. doi: 10.1016/j.mce.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 21.5th ed. Geneva: World Health Organization; 2010. WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 22.Vivas-Acevedo G, Lozano-Hernández R, Camejo MI. Varicocele decreases epididymal neutral a-glucosidase and is associated with alteration of nuclear DNA and plasma membrane in spermatozoa. BJU Int. 2014;113:642–9. doi: 10.1111/bju.12523. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy WP, Kaminski JM, Van der Ven HH, Jeyendran RS, Reid DS, et al. A simple, clinical assay to evaluate the acrosin activity of human spermatozoa. J Androl. 1989;10:221–31. doi: 10.1002/j.1939-4640.1989.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 24.Henkel R, Maass G, Schuppe HC, Jung A, Schubert J, et al. Seasonal changes of neutral alpha-glucosidase activity in human semen. J Androl. 2006;27:34–9. doi: 10.2164/jandrol.05064. [DOI] [PubMed] [Google Scholar]
- 25.Makino T, Saito M, Horiguchi D, Kina K. A highly sensitive colorimetric determination of serum zinc using water-soluble pyridylazo dye. Clin Chim Acta. 1982;120:127–35. doi: 10.1016/0009-8981(82)90083-3. [DOI] [PubMed] [Google Scholar]
- 26.Zini A, Fischer MA, Mak V, Phang D, Jarvi K. Catalase-like and superoxide dismutase-like activities in human seminal plasma. Urol Res. 2002;30:321–3. doi: 10.1007/s00240-002-0283-0. [DOI] [PubMed] [Google Scholar]
- 27.He Z, Sun X, Mei G, Yu S, Li N. Nonclassical secretion of human catalase on the surface of CHO cells is more efficient than classical secretion. Cell Biol Int. 2008;32:367–73. doi: 10.1016/j.cellbi.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Kang X, Xie Q, Zhou X, Li F, Huang J, et al. Effects of hepatitis B virus S protein exposure on sperm membrane integrity and functions. PLoS One. 2012;7:e33471. doi: 10.1371/journal.pone.0033471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–66. [PubMed] [Google Scholar]
- 30.Vera Y, Diaz-Romero M, Rodriguez S, Lue Y, Wang C, et al. Mitochondria-dependent pathway is involved in heat-induced male germ cell death: lessons from mutant mice. Biol Reprod. 2004;70:1534–40. doi: 10.1095/biolreprod.103.024661. [DOI] [PubMed] [Google Scholar]
- 31.Cai H, Ren Y, Li XX, Yang JL, Zhang CP, et al. Scrotal heat stress causes a transient alteration in tight junctions and induction of TGF-ß expression. Int J Androl. 2011;34:352–62. doi: 10.1111/j.1365-2605.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 32.Li XX, Chen SR, Shen B, Yang JL, Ji SY, et al. The heat-induced reversible change in the blood-testis barrier (BTB) is regulated by the androgen receptor (AR) via the partitioning-defective protein (Par) polarity complex in the mouse. Biol Reprod. 2013 doi: 10.1095/biolreprod.113.109405. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Crespo M, Pintado B, Gutiérrez-Adán A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol Reprod Dev. 2008;75:40–7. doi: 10.1002/mrd.20759. [DOI] [PubMed] [Google Scholar]
- 34.Lue YH, Lasley BL, Laughlin LS, Swerdloff RS, Hikim AP, et al. Mild testicular hyperthermia induces profound transitional spermatogenic suppression through increased germ cell apoptosis in adult cynomolgus monkeys (Macaca fascicularis) J Androl. 2002;23:799–805. [PubMed] [Google Scholar]
- 35.Lue Y, Wang C, Liu YX, Hikim AP, Zhang XS, et al. Transient testicular warming enhances the suppressive effect of testosterone on spermatogenesis in adult cynomolgus monkeys (Macaca fascicularis) J Clin Endocrinol Metab. 2006;91:539–45. doi: 10.1210/jc.2005-1808. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Cui YG, Wang XH, Jia Y, Sinha Hikim A, et al. transient scrotal hyperthermia and levonorgestrel enhance testosterone-induced spermatogenesis suppression in men through increased germ cell apoptosis. J Clin Endocrinol Metab. 2007;92:3292–304. doi: 10.1210/jc.2007-0367. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad G, Moinard N, Esquerré-Lamare C, Mieusset R, Bujan L. Mild induced testicular and epididymal hyperthermia alters sperm chromatin integrity in men. Fertil Steril. 2012;97:546–53. doi: 10.1016/j.fertnstert.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 38.Rowley MJ, Teshima F, Heller CG. Duration of transit of spermatozoa through the human male ductular system. Fertil Steril. 1970;21:390–6. [PubMed] [Google Scholar]
- 39.Yin Y, Hawkins KL, DeWolf WC, Morgentaler A. Heat stress causes testicular germ cell apoptosis in adult mice. J Androl. 1997;18:159–65. [PubMed] [Google Scholar]
- 40.Lue YH, Hikim AP, Swerdloff RS, Im P, Taing KS, et al. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology. 1999;140:1709–17. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- 41.Bergh A, Damber JE. Local regulation of Leydig cells by the seminiferous tubules. Effect of short-term cryptorchidism. Int J Androl. 1984;7:409–18. doi: 10.1111/j.1365-2605.1984.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 42.Kong WH, Zheng G, LU JN, Tso JK. Temperature dependent expression of cdc2 and cyclin B1 in spermatogenic cells during spermatogenesis. Cell Res. 2000;10:289–302. doi: 10.1038/sj.cr.7290056. [DOI] [PubMed] [Google Scholar]
- 43.Bedford JM. Effects of elevated temperature on the epididymis and testis: experimental studies. Adv Exp Med Biol. 1991;286:19–32. doi: 10.1007/978-1-4684-5913-5_3. [DOI] [PubMed] [Google Scholar]
- 44.Bedford JM. The status and the state of the human epididymis. Hum Reprod. 1994;9:2187–99. doi: 10.1093/oxfordjournals.humrep.a138416. [DOI] [PubMed] [Google Scholar]
- 45.Gonzales GF, Kortebani G, Mazzolli AB. Leukocytospermia and function of the seminal vesicles on seminal quality. Fertil Steril. 1992;57:1058–65. doi: 10.1016/s0015-0282(16)55025-0. [DOI] [PubMed] [Google Scholar]
- 46.Carrell DT, Aston KI. Methods in Molecular Biology. New York: Humana Press; 2013. The hypo-osmotic swelling test for evaluation of sperm membrane integrity. Spermatogenesis, Methods and Protocols; p. 21. [DOI] [PubMed] [Google Scholar]
- 47.Tesarik J, Drahorad J, Testart J, Mendoza C. Acrosin activation follows its surface exposure and precedes membrane fusion in human sperm acrosome reaction. Development. 1990;110:391–400. doi: 10.1242/dev.110.2.391. [DOI] [PubMed] [Google Scholar]
- 48.Navaeian-Kalat E, Deemeh MR, Tavalaee M, Abasi H, Modaresi M, et al. High total acrosin activity in varicocele individuals. Andrologia. 2012;44(Suppl 1):634–41. doi: 10.1111/j.1439-0272.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- 49.Athayde KS, Cocuzza M, Agarwal A, Krajcir N, Lucon AM, et al. Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. J Androl. 2007;28:613–20. doi: 10.2164/jandrol.106.001966. [DOI] [PubMed] [Google Scholar]
- 50.Christova Y, James PS, Jones R. Lipid diffusion in sperm plasma membranes exposed to peroxidative injury from oxygen free radicals. Mol Reprod Dev. 2004;68:365–72. doi: 10.1002/mrd.20084. [DOI] [PubMed] [Google Scholar]
- 51.Brouwers JF, Boerke A, Silva PF, Garcia-Gil N, van Gestel RA, et al. Mass spectrometric detection of cholesterol oxidation in bovine sperm. Biol Reprod. 2011;85:128–36. doi: 10.1095/biolreprod.111.091207. [DOI] [PubMed] [Google Scholar]
- 52.Aitken RJ, Curry BJ. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal. 2011;14:367–81. doi: 10.1089/ars.2010.3186. [DOI] [PubMed] [Google Scholar]
- 53.Aitken RJ, Gibb Z, Mitchell LA, Lambourne SR, Connaughton HS, et al. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol Reprod. 2012;87:110. doi: 10.1095/biolreprod.112.102020. [DOI] [PubMed] [Google Scholar]
- 54.Henkel R, Ludwig M, Schuppe HC, Diemer T, Schill WB, et al. Chronic pelvic pain syndrome/chronic prostatitis affect the acrosome reaction in human spermatozoa. World J Urol. 2006;24:39–44. doi: 10.1007/s00345-005-0038-y. [DOI] [PubMed] [Google Scholar]
- 55.Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, et al. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod. 1998;59:1037–46. doi: 10.1095/biolreprod59.5.1037. [DOI] [PubMed] [Google Scholar]
- 56.Nichi M, Bols PE, Züge RM, Barnabe VH, Goovaerts IG, et al. Seasonal variation in semen quality in Bos indicus and Bos taurus bulls raised under tropical conditions. Theriogenology. 2006;66:822–8. doi: 10.1016/j.theriogenology.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 57.Absalan F, Movahedin M, Mowla SJ. Germ cell apoptosis induced by experimental cryptorchidism is mediated by molecular pathways in mouse testis. Andrologia. 2010;42:5–12. doi: 10.1111/j.1439-0272.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- 58.Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, et al. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem. 2012;287:33048–60. doi: 10.1074/jbc.M112.366690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl. 2014;16:31–8. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]


