Abstract
Evidence is increasing that the integrity of sperm DNA may also be related to implantation failure and recurrent miscarriage (RM). To investigate this, the sperm DNA fragmentation in partners of 35 women with recurrent implantation failure (RIF) following in vitro fertilization, 16 women diagnosed with RM and seven recent fathers (control) were examined. Sperm were examined pre- and post-density centrifugation by the sperm chromatin dispersion (SCD) test and the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. There were no significant differences in the age of either partner or sperm concentration, motility or morphology between three groups. Moreover, there were no obvious differences in sperm DNA fragmentation measured by either test. However, whilst on average sperm DNA fragmentation in all groups was statistically lower in prepared sperm when measured by the SCD test, this was not seen with the results from the TUNEL assay. These results do not support the hypothesis that sperm DNA fragmentation is an important cause of RIF or RM, or that sperm DNA integrity testing has value in such patients. It also highlights significant differences between test methodologies and sperm preparation methods in interpreting the data from sperm DNA fragmentation tests.
Keywords: DNA, recurrent implantation failure, recurrent miscarriage, sperm
INTRODUCTION
Male-factor continues to account for approximately 20% of cases of infertility and in an additional 30%–40% of cases causative factors are identified in both men and women.1 Assessment of male infertility traditionally has depended on the analysis of a semen sample according to World Health Organization (WHO) guidelines.2,3 This analysis is based on a visual estimation of sperm number, motility, and morphology as measured by light microscopy and is difficult to perform reliably.4 Sperm DNA integrity testing has therefore been proposed to be a test with promising potential to compliment the standard semen analysis.5
Many studies have reported an adverse effect of sperm DNA damage on fertility. Sperm DNA fragmentation is associated with failure to conceive,6 longer times to pregnancy,7 poor outcome following stimulated intrauterine insemination,8,9 impaired embryo development,10 higher miscarriage rates11 and increased risk of pregnancy loss after both in vitro fertilization (IVF) and intracytoplasmic sperm injection.12 Sperm DNA damage may, therefore, have far reaching consequences for reproductive outcome.
In spite of the above data, separate reports from the American Society for Reproductive Medicine,13 the European Society for Human Reproduction and Embryology,14 and the British Fertility Society15 have all concluded that at the present time there is insufficient evidence for sperm DNA testing to be introduced as part of clinical practice with the need for further research being identified. However, all three reports conclude that the strongest evidence is currently for a role of sperm DNA testing in cases of implantation failure and recurrent miscarriage (RM). Therefore, the aim of this study was to add to this evidence base and examine the relationship between sperm DNA fragmentation in the male partners of women diagnosed with recurrent implantation failure (RIF) following IVF, RM and in men who had become recent fathers. We aimed to examine the effect of sperm preparation by density centrifugation and to assess sperm DNA fragmentation using two commercially available methods.
MATERIALS AND METHODS
Male partners were recruited from the Jessop Wing of Sheffield Teaching Hospitals NHS Foundation Trust (Sheffield, UK) between January 2010 and January 2011. For the purposes of this study, RIF was defined as the failure to achieve a clinical pregnancy following the transfer of four good quality embryos in a minimum of three fresh and frozen embryo cycles in women aged <40 years. A good quality embryo was defined as having the correct number of cells corresponding to the day of its development, blastomeres of equal size and regular in distribution, even distribution of the cytoplasm without granularity and less than 10% fragmentation.16 RM was defined as three or more consecutive and unexplained pregnancy losses occurring before 20 weeks postmenstruation.17,18,19
All couples (both RIF and RM groups) had normal karyotypes and there was no evidence of risk factors for RIF and RM in the female partners: there were no endocrine disorders, negative testing for antiphospholipid antibodies and lupus antibodies, normal coagulation and absence of uterine structural abnormalities. Male partners had no history of orchitis, testicular trauma, varicocele, testicular torsion, toxic exposure, chronic illness and prior gonadotoxic therapy. All participants (including the control group) were nonsmokers. The control group consisted of men who were of proven fertility without the use of assisted conception and with no history of fertility problems, and these were recruited from posters displayed around the hospital. The study was approved by the South Sheffield Research Ethics Committee (08/H1310/90) and approved by the Sheffield Teaching Hospitals Clinical Governance procedures (STH 15116).
Information leaflets explaining the study were provided to every participant and written informed consent obtained. Semen samples (one per subject) were obtained by masturbation following 3–4 days sexual abstinence. Samples were collected in sterile plastic containers (Sarstedt, Leicester, UK) and allowed to liquefy at 37°C for 30 min before the semen analysis according to WHO methods.2 Following semen analysis, sperm were isolated from seminal plasma using an 80/40% density centrifugation gradient (Cook UK Ltd., Hitchin, UK) and centrifuged at 500 × g for 20 min before being washed in 3 ml Fertilization Medium (Cook UK Ltd., Hitchin, UK) at 500 × g for 5 min, and then finally resuspended in a further 0.3 ml of Fertilization Medium.
Two testing methods were used to measure sperm DNA integrity on both the neat (unprepared) sperm, as well as sperm recovered following density centrifugation (prepared) as described below.
The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed using the Fluorescein FragEL™ DNA Fragmentation Detection Kit (Merck Chemicals Ltd., Nottingham, UK). Briefly, prepared and unprepared samples of semen (see above) were centrifuged at 1000 × g for 10 min and the sperm pellet resuspended in 500 ml tris buffered saline (TBS) (50 mmol l−1 Tris pH 7.9 [VWR, Lutterworth, UK]; 150 mmol l-1 NaCl [Sigma, Poole, UK]) before being centrifuged again at 1000 × g for 5 min and the supernatant again removed carefully without disturbing the pellet. The pellet was then resuspended in a volume of TBS to achieve a final concentration of sperm of approximately 5 × 106 sperm ml−1. Aliquots of 100 ml of this suspension from both prepared and unprepared sperm were pipetted onto poly-lysine coated slides (VWR, Lutterworth, UK) and left to dry overnight before being fixed by being covered with approximately 1 ml of 100% (v/v) methanol (Fisher, Loughborough, UK) for 1 min and allowed to dry. Prior to staining, the outline of the fixed smear on each slide was encircled using a hydrophobic slide marker and then the cells contained within were covered with 50–100 ml of 20 mg ml−1 proteinase K and incubated at room temperature for 5 min. Each slide was then dipped into a beaker of 1 × TBS 2–3 times and excess liquid gently tapped off and then the smear covered with 100 ml of 1 × TdT equilibration buffer supplied by the kit and incubated at room temperature for 30 min. A 60 ml aliquot of TdT labeling reaction mixture was then applied, and the slide incubated in a humidified chamber at 37°C for 1.5 h. After washing each slide twice in 1 × TBS for 1 min at room temperature, a glass coverslip was mounted using Vectashield (Vector Laboratories, Peterborough, UK) with propidium iodide. Labeled nuclei were observed on an Olympus BH2 Fluorescent Microscope using a standard DAPI (4,6-diamidino-2-phenyllndole) filter, 330–380 nm using a ×100 oil immersion lens. A total of 400 sperm were evaluated per slide and the number with a bright green signal (indicating DNA fragmentation) was recorded as a proportion of the number of sperm colored red (i.e., with intact DNA). In each case, control slides provided by the test kit (containing a mixture of normal and apoptotic cells) were also stained as a check to make sure the staining had worked correctly.
The sperm chromatin dispersion (SCD) test20 was performed using a Halosperm Kit (Microm, Bicester, UK) following the manufacturer's instructions. Briefly, this involved adjusting the concentration of prepared and unprepared sperm (see above) with Fertilisation Medium (Cook UK Ltd., Hitchin, UK) to a concentration of 5–10 million ml−1 and then adding 12.5 μl of each sample to 50 μl of melted agarose in a clean Eppendorf tube (Starlabs, Milton Keynes, UK). A 10 μl aliquot of the mixture was then pipetted onto an agarose treated side of the glass slide and covered with a coverslip before being placed in the refrigerator a 2°C for 5 min. The coverslip was removed, and the slide immersed for 7 min in a tray containing 10 ml of distilled water to which 80 μl of the denaturant solution supplied by the kit had been added. The slides were then transferred to the lysis solution for 25 min before being washed in distilled water for 5 min and then passed through an alcohol series of 70% ethanol (2 min), 90% ethanol (2 min) and 100% ethanol (2 min). Slides were then allowed to dry at room temperature and then incubated with the eosin solution, for 6 min followed by a further 6 min in Azur B (blue). The slides were then rinsed briefly in distilled water, air-dried and a coverslip mounted using DPX (Sigma, Poole, Dorset, UK). Two hundred spermatozoa were examined per unprepared and prepared sample using a Leica DM LB Phase Contrast Microscope (Leica, Milton Keynes, UK) at a magnification of ×400. Sperm not demonstrating a halo represented those with fragmented DNA and were expressed as a proportion of the total number of sperm observed.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS Inc. Chicago, IL, USA). The Chi-square test, Fisher's exact test and ANOVA were used for categorical variables, and an independent sample t-test was used for continuous variables that were normally distributed. P < 0.05 was considered as significant.
RESULTS
The partners of 35 women with RIF, 16 women with RM, and 7 controls were recruited to the study and provided semen samples for analysis. There were no significant differences between the three groups in terms of the age of either partner or in sperm concentration, motility and sperm morphology of the ejaculate provided for the study (Table 1).
Table 1.
Age and semen analysis data of the ejaculate provided by the male partners of women with RIF, RM and from recent fathers (control)
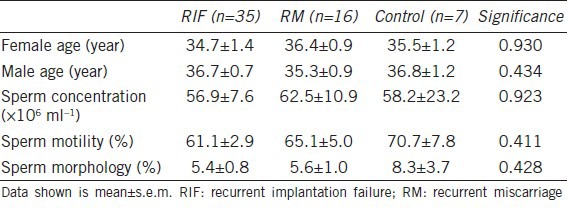
Figure 1 shows the DNA fragmentation measured by the SCD test and TUNEL assay in each of the three groups (RIF, RM and control) of sperm in semen and sperm recovered following density gradient centrifugation (DCG). Briefly, across semen samples, although the mean values obtained for percentage of sperm with fragmented DNA were numerically higher with the SCD test compared with the results of the TUNEL assay, there was only a statistical difference (P < 0.05) between the results obtained on sperm from the recent fathers (cf. Figure 1e and 1f). About sperm obtained following DCG, there were no statistical differences in the percentage of sperm with DNA damage between the three patient groups and the two test methods.
Figure 1.
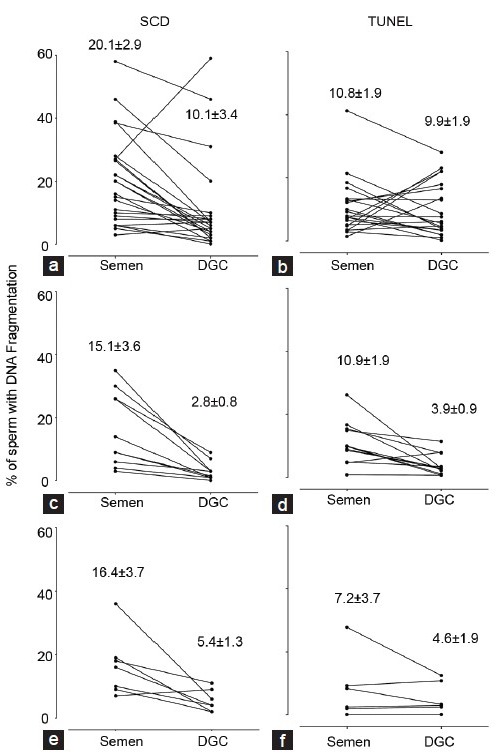
Percentage of sperm with DNA fragmentation in male partners of women with recurrent implantation failure (a and b), recurrent miscarriage (c and d), and in a group of recent fathers (control) (e and f) as measured by sperm chromatin disruption test (left hand graphs) and terminal deoxynucleotidyl transferase dUTP nick end labelling (right hand graphs). All measurements were made on sperm in semen and immediately following density gradient centrifugation. Values show mean ± s.e.m.
In comparing the semen and DCG results from each test, the mean ± s.e.m. for the SCD test were significantly different for the RIF (20.1 ± 2.9 vs 10.6 ± 2.9; P = 0.000), RM 15.1 ± 3.6 vs 2.8 ± 0.8; P = 0.005) and control groups (16.4 ± 3.7 vs 5.4 ± 1.3; P = 0.03) but no such statistical differences were seen between the results of the TUNEL assay. Interestingly, the values for sperm morphology were also not significantly different between sperm observed in semen and those assessed following DCG (data not shown).
DISCUSSION
This study investigated the relationship between sperm DNA fragmentation in the male partners of women diagnosed with RIF following IVF, RM following unassisted conception as well as sperm from men who had become recent fathers as a control group. This study used two commercially available tests and examined both ejaculated sperm as well as sperm recovered following density centrifugation. In summary, although some significant quantitative differences were seen in the results obtained with the two tests and sperm preparation methods across the three groups, there was no convincing evidence that the results could be used to discriminate between the three groups.
Recurrent implantation failure and RM are two types of reproductive failure with different incidences and presentations. A history of three or more consecutive miscarriages (RM) is somewhat rare occurring in approximately 0.5%–3% of women21 whereas following IVF as many as 48% of patients do not achieve a live birth after 3 cycles of treatment and therefore classified as RIF.22 RIF causes considerable distress to women and their partners and poses significant problems for clinicians. Women with RM have a different experience to women with RIF, achieving spontaneous conceptions but repeated pregnancy loss. They experience both joy and excitement at the prospect of a new addition to their family, only to be disappointed at a later time when a spontaneous pregnancy loss is confirmed on ultrasound or the woman experiences vaginal bleeding.
Previous studies to examine sperm DNA fragmentation in RM patients have shown mixed results. For example, the use of acridine orange staining on the sperm from 74 male partners of women with a history of RM found a statistical difference compared to the sperm from 65 recent fathers.23 Similarly, the use of the TUNEL assay on sperm from 24 couples with unexplained recurrent pregnancy loss was significantly different from the sperm obtained from donors of known fertility and unscreened men from the general population.24 In contrast, sperm chromatin integrity was examined on isolated motile sperm from 23 couples with recurrent pregnancy loss and 11 recent fathers to find that the results from the Sperm Chromatin Structure Assay (SCSA) had no weight in the analysis.25 Similarly, Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress were examined as causes of recurrent spontaneous abortion of unknown etiology and it was concluded that sperm DNA fragmentation lacked an adequate predictive power to be employed as a discriminative test of recurrent spontaneous abortion.26 However, across 16 studies involving 2969 patients, a meta-analysis11 showed that there was a significant increase in miscarriage in patients with high DNA damage (risk ratio = 2.16 [1.54, 3.03], P < 0.00001) compared with those with low DNA damage. Moreover, there was significant heterogeneity in the type of test used to generate the DNA damage results (i.e., acridine orange-based assays, the TUNEL assay and the CometAssay). This may be related to the type of DNA damage being detected by these tests, and it is noteworthy that a small study in 20 couples using the alkaline and neutral CometAssay, SCD test and pulsed-field gel electrophoresis suggested that it was double stranded DNA damage that was related to the risk of undergoing a male-factor associated miscarriage.27
In contrast to studies looking at RM, there are relatively few studies that have considered sperm DNA damage in patients with RIF following IVF. For example, 154 embryos from 38 patients undergoing preimplantation diagnoses were examined and it was found that although the sperm samples showed an increased DNA fragmentation after sperm preparation, there was no correlation between DNA fragmentation and the aneuploidy rate in embryos or in fresh or processed sperm samples.28
To conduct this study, two commercially available tests were chosen to measure sperm DNA fragmentation: (i) the SCD test; and (ii) the TUNEL assay. Both are available in kit form and can be used in a busy clinic with only basic laboratory equipment. In spite of their simplicity, these tests measure DNA fragmentation in different ways with the TUNEL assay detecting both single- and double-stranded DNA breaks by labeling the free 3’-OH terminus with modified nucleotides leading to a fluorescent signal.29 In contrast, the SCD test is based on the principle that only sperm with unfragmented DNA will produce a characteristic halo of dispersed DNA loops following denaturation by acid and the removal of nuclear proteins.20
In addition to measuring DNA fragmentation of sperm in the unprocessed ejaculate, the study also examined sperm recovered following density centrifugation. These represent different populations of sperm akin to those that might be deposited in the vagina during coitus (or ejaculated sperm in the case of RM patients) and those that might be used to inseminate oocytes during IVF (and therefore be the important population to consider in cases of the RIF patients recruited). Previous authors have noted that there can be increased levels of DNA fragmentation associated with sperm recovered from DCG30,31,32 presumably associated with increased incubation time and the centrifugation force involved. However, in this study sperm recovered from density centrifugation on average had lower levels of DNA damage, although this was only statistically significant when the SCD test (and not the TUNEL assay) was used. This may reflect known differences with the TUNEL assay33 although may also reflect genuine biological differences in sperm populations that we cannot explain. In this context it is noteworthy that in 8 out of the 35 samples from RIF patients examined using the TUNEL assay (Figure 1b) showed an increase sperm DNA fragmentation following DCG, compared to only 1 out of 35 when the same samples were examined using the SCD test. Interestingly, in the RM and control groups the level of sperm DNA fragmentation in each sample measured by both tests declined in line with the mean results.
This study has some limitations perhaps most notably that for all groups (RIF, RM and control) the sperm examined for DNA fragmentation were not from the ejaculates that led to conception, but from specimens that were produced at a later time for research purposes. We are confident that for RIF and RM patients this was always within 6 weeks at the patient's next follow-up appointment and for men in the control group they were all recruited within the first trimester of pregnancy. Therefore, there are possible differences between groups that are important to acknowledge, as it is known that sperm DNA fragmentation is influenced by environmental and lifestyle factors34 and hence it remains possible that the extent of sperm DNA fragmentation in each man may have changed over time.
In addition, it was also only possible to use two tests of sperm DNA fragmentation and it is plausible that if the SCSA35 or the CometAssay36 had been used, different results may have been obtained. Finally, the groups in this study were unequal and it was easier to recruit to the study the male partners of women who had RIF following IVF than it was the male partners of women who had suffered RM or recent fathers (control). The difficulty in recruiting men to studies concerned with fertility is well-described37 and needs to be acknowledged.
CONCLUSION
Based on the results presented here, it is not possible to support the hypothesis that sperm DNA fragmentation is an important cause of RM or RIF, nor that these tests of DNA fragmentation have predictive value in the prospective identification of women at risk of RM and RIF. However, the paper does provide further insight into the use of commercially available test kits for the measurement of sperm DNA fragmentation and the impact of sperm preparation methods such as density centrifugation.
AUTHOR CONTRIBUTIONS
CC, WL, TL and AAP initiated the study and designed the protocol. CC and TL recruited the patients. The TUNEL assay was performed by SW and the SCD test measurements by HC, RC and JS. CC and AAP wrote the paper. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This research was supported by The University of Sheffield, Jessop Fertility and the Jessop Wing Research Executive Small Grants Scheme. The authors would like to thank Caron Brookfield for technical assistance. The views expressed are those of the authors.
REFERENCES
- 1.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989) Hum Reprod. 1991;6:811–6. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 2.Cambridge, UK: Cambridge University Press; 1999. World Health Organisation. WHO Laboratory Manual for the Examination of Human Semen and Sperm-cervical Mucus Interaction. [Google Scholar]
- 3.5th ed. Geneva: World Health Organisation; 2010. World Health Organisation. WHO Laboratory Manual for the Examination and Processing of Human Semen; p. 271. [Google Scholar]
- 4.Pacey AA. Quality assurance and quality control in the laboratory andrology. Asian J Androl. 2010;12:21–5. doi: 10.1038/aja.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aitken RJ, De Iuliis GN. Origins and consequences of DNA damage in male germ cells. Reprod Biomed Online. 2007;14:727–33. doi: 10.1016/s1472-6483(10)60676-1. [DOI] [PubMed] [Google Scholar]
- 6.Evenson DP, Wixon R. Data analysis of two in vivo fertility studies using Sperm Chromatin Structure Assay-derived DNA fragmentation index vs pregnancy outcome. Fertil Steril. 2008;90:1229–31. doi: 10.1016/j.fertnstert.2007.10.066. [DOI] [PubMed] [Google Scholar]
- 7.Spanò M, Bonde JP, Hjøllund HI, Kolstad HA, Cordelli E, et al. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril. 2000;73:43–50. doi: 10.1016/s0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 8.Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002;17:3122–8. doi: 10.1093/humrep/17.12.3122. [DOI] [PubMed] [Google Scholar]
- 9.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–9. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 10.Morris ID, Ilott S, Dixon L, Brison DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum Reprod. 2002;17:990–8. doi: 10.1093/humrep/17.4.990. [DOI] [PubMed] [Google Scholar]
- 11.Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–17. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 12.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23:2663–8. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 13.Practice Committee of American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing. Fertil Steril. 2008;90:S178–80. doi: 10.1016/j.fertnstert.2008.08.054. [DOI] [PubMed] [Google Scholar]
- 14.Barratt CL, Aitken RJ, Björndahl L, Carrell DT, de Boer P, et al. Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications – A position report. Hum Reprod. 2010;25:824–38. doi: 10.1093/humrep/dep465. [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson M, Lewis S, Morroll D British Fertility Society. Sperm quality and its relationship to natural and assisted conception: British Fertility Society guidelines for practice. Hum Fertil (Camb) 2013;16:175–93. doi: 10.3109/14647273.2013.807522. [DOI] [PubMed] [Google Scholar]
- 16.Cutting R, Morroll D, Roberts SA, Pickering S, Rutherford A, et al. Elective single embryo transfer: guidelines for practice British Fertility Society and Association of Clinical Embryologists. Hum Fertil (Camb) 2008;11:131–46. doi: 10.1080/14647270802302629. [DOI] [PubMed] [Google Scholar]
- 17.Stirrat GM. Recurrent miscarriage. II: clinical associations, causes, and management. Lancet. 1990;336:728–33. doi: 10.1016/0140-6736(90)92215-4. [DOI] [PubMed] [Google Scholar]
- 18.Berry CW, Brambati B, Eskes TK, Exalto N, Fox H, et al. The Euro-Team Early Pregnancy (ETEP) protocol for recurrent miscarriage. Hum Reprod. 1995;10:1516–20. doi: 10.1093/humrep/10.6.1516. [DOI] [PubMed] [Google Scholar]
- 19.Bricker L, Farquharson RG. Types of pregnancy loss in recurrent miscarriage: implications for research and clinical practice. Hum Reprod. 2002;17:1345–50. doi: 10.1093/humrep/17.5.1345. [DOI] [PubMed] [Google Scholar]
- 20.Fernández JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, et al. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 21.Daya S. Evaluation and management of recurrent spontaneous abortion. Curr Opin Obstet Gynecol. 1996;8:188–92. [PubMed] [Google Scholar]
- 22.Gnoth C, Maxrath B, Skonieczny T, Friol K, Godehardt E, et al. Final ART success rates: a 10 years survey. Hum Reprod. 2011;26:2239–46. doi: 10.1093/humrep/der178. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharya SM. Association of various sperm parameters with unexplained repeated early pregnancy loss – Which is most important? Int Urol Nephrol. 2008;40:391–5. doi: 10.1007/s11255-007-9282-y. [DOI] [PubMed] [Google Scholar]
- 24.Carrell DT, Liu L, Peterson CM, Jones KP, Hatasaka HH, et al. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch Androl. 2003;49:49–55. doi: 10.1080/01485010290099390. [DOI] [PubMed] [Google Scholar]
- 25.Gil-Villa AM, Cardona-Maya W, Agarwal A, Sharma R, Cadavid A. Assessment of sperm factors possibly involved in early recurrent pregnancy loss. Fertil Steril. 2010;94:1465–72. doi: 10.1016/j.fertnstert.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 26.Bellver J, Meseguer M, Muriel L, García-Herrero S, Barreto MA, et al. Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology. Hum Reprod. 2010;25:1713–21. doi: 10.1093/humrep/deq098. [DOI] [PubMed] [Google Scholar]
- 27.Ribas-Maynou J, García-Peiró A, Fernandez-Encinas A, Amengual MJ, Prada E, et al. Double stranded sperm DNA breaks, measured by Comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS One. 2012;7:e44679. doi: 10.1371/journal.pone.0044679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bronet F, Martínez E, Gaytán M, Liñán A, Cernuda D, et al. Sperm DNA fragmentation index does not correlate with the sperm or embryo aneuploidy rate in recurrent miscarriage or implantation failure patients. Hum Reprod. 2012;27:1922–9. doi: 10.1093/humrep/des148. [DOI] [PubMed] [Google Scholar]
- 29.Sharma R, Masaki J, Agarwal A. Sperm DNA fragmentation analysis using the TUNEL assay. Methods Mol Biol. 2013;927:121–36. doi: 10.1007/978-1-62703-038-0_12. [DOI] [PubMed] [Google Scholar]
- 30.Zini A, Mak V, Phang D, Jarvi K. Potential adverse effect of semen processing on human sperm deoxyribonucleic acid integrity. Fertil Steril. 1999;72:496–9. doi: 10.1016/s0015-0282(99)00295-2. [DOI] [PubMed] [Google Scholar]
- 31.Zini A, Nam RK, Mak V, Phang D, Jarvi K. Influence of initial semen quality on the integrity of human sperm DNA following semen processing. Fertil Steril. 2000;74:824–7. doi: 10.1016/s0015-0282(00)01495-3. [DOI] [PubMed] [Google Scholar]
- 32.Zini A, Finelli A, Phang D, Jarvi K. Influence of semen processing technique on human sperm DNA integrity. Urology. 2000;56:1081–4. doi: 10.1016/s0090-4295(00)00770-6. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell LA, De Iuliis GN, Aitken RJ. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: development of an improved methodology. Int J Androl. 2011;34:2–13. doi: 10.1111/j.1365-2605.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 34.Pacey AA. Environmental and lifestyle factors associated with sperm DNA damage. Hum Fertil (Camb) 2010;13:189–93. doi: 10.3109/14647273.2010.531883. [DOI] [PubMed] [Google Scholar]
- 35.Evenson DP. Sperm chromatin structure assay (SCSA®) Methods Mol Biol. 2013;927:147–64. doi: 10.1007/978-1-62703-038-0_14. [DOI] [PubMed] [Google Scholar]
- 36.Simon L, Carrell DT. Sperm DNA damage measured by comet assay. Methods Mol Biol. 2013;927:137–46. doi: 10.1007/978-1-62703-038-0_13. [DOI] [PubMed] [Google Scholar]
- 37.Stewart TM, Liu DY, Garrett C, Brown EH, Baker HW. Recruitment bias in studies of semen and other factors affecting pregnancy rates in fertile men. Hum Reprod. 2009;24:2401–8. doi: 10.1093/humrep/dep215. [DOI] [PubMed] [Google Scholar]


