Abstract
Introduction
To describe the basic demographics, analyze the response and survival experience of advanced renal cancer subjects treated in a Phase I trial.
Methods
We conducted a retrospective observational study in 70 renal cancer patients participating in 25 Phase I trials. Descriptive statistics, Kaplan Meier and multivariate Cox proportional hazards analyses were used to examine factors associated with time from study entry to treatment failure (TTF) and survival.
Results
The median age at diagnosis was 56.50 years. 23.19% of the patients had an ECOG performance status of zero; 49.18% had received ≥2 prior lines of systemic therapy. 84.29% patients had ≥2 metastatic sites. A median number of 4.00 cycles of treatment was delivered. Four partial responses (6.25%) and 38 cases of stable disease lasting >4 months (43.75%) were observed. The median TTF was 16.00 weeks. In multivariate analyses, males and patients with lactate dehydrogenase >1.5 times upper limit of normal had a shorter TTF. The median overall survival was 45.57 weeks (319.00 days). In multivariate analysis, factors predicting shorter survival were ECOG ≥1 (P=0.023), age <60 (p=0.015), albumin <3.4 g/dl (P=0.042), and liver metastases (P=0.010).
Conclusion
Advanced renal cancer patients with select clinical characteristics could consider Phase I trials after exhausting standard therapeutic options.
Introduction
Renal cell carcinoma (RCC) accounts for 80-85% of all renal malignancies[1]. In the United States, there are 65,000 new cases of renal cell cancer each year[2]. According to the Surveillance, Epidemiology and End Results (SEER) data from 2006-2010, the median age for RCC at diagnosis was 64 years[3]. The 5-year survival rate of patients with renal cancer is currently estimated to be 71%, due to recent improvements in therapeutic options[4]. Radical nephrectomy with curative intent is the preferred treatment approach for patients with localized disease. Until recently, systemic treatment options for metastatic RCC were limited to cytokine therapy or referral for clinical trials of novel agents. With the advent of targeted therapies in the past decade, transition from cytotoxic treatments to highly selective molecules has radically altered and expanded options for cancer patients. Currently, targeted therapy with tyrosine kinase inhibitors (TKI) and anti-angiogenic agents is the preferred initial treatment approach for most patients with advanced clear cell RCC. There are five such agents approved by The Food and Drug Administration (FDA) as a treatment in the first-line and second-line settings[5-9]. In addition, the inhibitors of mammalian target of rapamycin (mTOR) pathway have important clinical activity and approval for use in both untreated as well as previously treated patients with advanced RCC[10,11].
Treatment planning for patients with progressive disease is challenging because there is little data regarding optimal therapy in patients who have failed above listed treatments. Clinical trials are recommended for such patients with good performance status by National Comprehensive Cancer Network (NCCN) guidelines[12]. The outcomes for patients with advanced stage renal cancer treated on early-phase clinical trials have not been previously reported. This retrospective review reports the general characteristics, time to treatment failure (TTF), prognostic factors, and survival data of advanced renal cancer patients who were enrolled in Phase I trials at our tertiary cancer center.
Methods and Material
Patients and data acquisition
The Cancer Therapy and Research Center (CTRC) is a tertiary care cancer center in San Antonio, Texas. It has a well-established Institute for Drug Development with a particular focus on Phase I clinical trials since the early 1990s. The University of Texas Health Science Center at San Antonio has an informatics data exchange and acquisition program, which serves as a primary research data system. All renal cancer patients participating in Phase I clinical trials at CTRC from January 2002 to December 2012 were identified through this system. All patients completed an informed consent process prior to enrollment onto a trial and all trials were approved by the CTRC Institutional Review Board.
All patients with advanced renal cell cancer of any histological subtype, who were successfully enrolled in a Phase I study, were included. Patients with prior treatment in a Phase I clinical trial and screen failures were excluded from this analysis. Patient's electronic medical records from the initial clinic visit to the time of last visit were reviewed. We extracted demographic data (gender, age, residence); medical information (disease site, tumor histology, date of diagnosis of metastatic disease, number and nature of prior treatments, performance status); details of first Phase I trial (nature of investigational agent, date of consent, date and reason for removal from study); information on clinical outcome, subsequent treatment; and laboratory data (lactate dehydrogenase, albumin, and hemoglobin) from physicians' clinical notes that were dictated at the time of clinic visit. All the data were entered into a password-protected database.
Outcomes
TTF was measured in days from study enrollment to the date patient was removed from the study for any reason. Patients who were still continuing on treatment at the time of last follow-up were censored on that date. Survival was measured in days from the date of study consent until death from any cause. Patients who were still alive at the time of last follow-up were censored on that date.
Statistical Analysis
Descriptive statistics were used to describe patients' demographic and treatment characteristics by outcome (partial response, stable disease, progressive disease), including the Kruskal-Wallis test for continuous outcomes and Fisher's Exact test for categorical outcomes. Survival and time to treatment failure were plotted using Kaplan Meier method. We then used multivariable Cox proportional hazards models to examine the relationship between patient demographic, treatment characteristics and 1) TTF and 2) hazard of death. Each multivariable model was adjusted for age (<60 vs. ≥60), sex (male vs. female), Eastern Cooperative Oncology Group- ECOG status (0 vs. ≥1), tumor histology (clear cell vs. non-clear cell), site of metastatic disease (liver vs. non-liver), lactate dehydrogenase (LDH <1.5 vs. ≥1.5 ULN), albumin above lower limit of normal vs. below lower limit of normal (>LLN vs. <LLN), hemoglobin (<10 vs. ≥10), and time since initial diagnosis (<18 months, 18-36 months, and >36 months). These variables were all measured at the time of informed consent. For patients participating in more than one trial, clinical and laboratory details from the first Phase I study were obtained only. Only patients with complete demographic and clinical information were included in the final multivariable models (n=57). A p-value < 0.05 was considered statistically significant in all analyses. All analyses were performed using R 3.0.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Pre-Enrollment Characteristics
The median age of subjects was 56.50 years (range 44–76) at the time of study enrollment; more men than women were enrolled (65.71% versus 34.29%). A large number of study participants were Hispanic Americans and lived out-of-town. 23.19% of patients had ECOG performance status of zero, 56.52 % were ECOG one, and 20.29% were ECOG two. The most common histological subtypes were clear cell (68.12%), mixed (11.59%), papillary (10.14%), and sarcomatoid (7.25%). 84.29% of patients had ≥2 metastatic sites; 31.43% had lung only, 8.57% had lymph node only, and 17.14% had liver only. Prior nephrectomy rate was 94.03 %. Nearly half of the subjects had received ≥2 prior anticancer therapies for the metastatic disease (49.18%), including a combination of IL-2, TKI, bevacizumab, and chemotherapy. Other baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics.
| Variable | Best treatment outcome | Total N=70 | P-value2 | ||
|---|---|---|---|---|---|
| PR N=4 | SD N=38 | PD N=22 | |||
| Age | 0.01 | ||||
| N | 4 | 38 | 22 | 70 | |
| Median [Q1, Q3] | 67.5 [63.8, 71.8] | 58 [53.2, 65] | 54.5 [52.2, 58.8] | 56.5 [53, 63] | |
| Sex | 0.22 | ||||
| Female | 1 (25) | 11 (28.95) | 11 (50) | 24 (34.29) | |
| Male | 3 (75) | 27 (71.05) | 11 (50) | 46 (65.71) | |
| Town of Residence | 1 | ||||
| Local | 2 (50) | 22 (57.89) | 12 (54.55) | 40 (57.14) | |
| Out of Town | 2 (50) | 16 (42.11) | 10 (45.45) | 30 (42.86) | |
| Ethnicity | 0.11 | ||||
| White | 4 (100) | 26 (68.42) | 10 (45.45) | 41 (58.57) | |
| Hispanic | 0 (0) | 12 (31.58) | 11 (50) | 28 (40) | |
| Other | 0 (0) | 0 (0) | 1 (4.55) | 1 (1.43) | |
| ECOG performance status | 0.02 | ||||
| 0 | 1 (25) | 13 (34.21) | 1 (4.55) | 16 (23.19) | |
| ≥1 | 3 (75) | 25 (65.79) | 21 (95.45) | 53 (76.81) | |
| No of metastatic sites | 0.11 | ||||
| 1 | 1 (25) | 9 (23.68) | 1 (4.55) | 11 (15.71) | |
| ≥2 | 3 (75) | 29 (76.32) | 21 (95.45) | 59 (84.29) | |
| Nephrectomy | 1 | ||||
| Yes | 4 (100) | 35 (94.59) | 19 (95) | 63 (94.03) | |
| No | 0 (0) | 2 (5.41) | 1 (5) | 4 (5.97) | |
| Tumor histology | 0.13 | ||||
| Clear cell | 4 (100) | 27 (72.97) | 12 (54.55) | 47 (68.12) | |
| Non clear cell/Mixed | 0 (0) | 10 (27.03) | 10 (45.45) | 22 (31.88) | |
| No of prior treatment | 0.26 | ||||
| 1 | 2 (50) | 20 (58.82) | 6 (35.29) | 31 (50.82) | |
| ≥2 | 2 (50) | 14 (41.18) | 11 (64.71) | 30 (49.18) | |
| Type of prior treatment | 0.89 | ||||
| TKI | 1 (25) | 5 (13.16) | 3 (14.29) | 9 (13.04) | |
| IL2 | 1 (25) | 8 (21.05) | 2 (9.52) | 14 (20.29) | |
| Bevacizumab | 0 (0) | 1 (2.63) | 2 (9.52) | 3 (4.35) | |
| Chemotherapy | 0 (0) | 4 (10.53) | 4 (19.05) | 8 (11.59) | |
| Clinical trial | 1 (25) | 4 (10.53) | 1 (4.76) | 6 (8.7) | |
| Multiple | 1 (25) | 11 (28.95) | 6 (28.57) | 21 (30.43) | |
| Other | 0 (0) | 5 (13.16) | 3 (14.29) | 8 (11.59) | |
| Type of phase 1 trial | 0.68 | ||||
| Single biological agent | 3 (75) | 27 (72.97) | 12 (60) | 46 (68.66) | |
| Combination therapy | 1 (25) | 9 (24.32) | 6 (30) | 18 (26.87) | |
| Chemotherapy | 0 (0) | 1 (2.7) | 2 (10) | 3 (4.48) | |
| Reason to come off study | 0.23 | ||||
| progression | 3 (75) | 31 (81.58) | 21 (95.45) | 55 (78.57) | |
| Toxicity | 0 (0) | 2 (5.26) | 1 (4.55) | 7 (10) | |
| Patient preference/Other | 1 (25) | 5 (13.16) | 0 (0) | 8 (11.43) | |
| Dose reduction | 0.01 | ||||
| Yes | 1 (25) | 10 (27.03) | 0 (0) | 11 (16.18) | |
| No | 3 (75) | 27 (72.97) | 21 (100) | 57 (83.82) | |
| Site of metastases | < 0.001 | ||||
| Non liver | 4 (100) | 36 (94.74) | 12 (54.55) | 58 (82.86) | |
| Liver | 0 (0) | 2 (5.26) | 10 (45.45) | 12 (17.14) | |
| Treatment on progression | < 0.001 | ||||
| Another trial | 1 (25) | 12 (31.58) | 5 (22.73) | 18 (25.71) | |
| Off trial treatment | 3 (75) | 19 (50) | 3 (13.64) | 28 (40) | |
| No treatment/supportive care | 0 (0) | 1 (2.63) | 12 (54.55) | 14 (20) | |
Six best treatment outcomes on CT were missing.
Kruskal-Wallis test was used for continuous outcomes and Fisher's Exact test for categorical outcomes.
Treatment and trials
In total, 70 patients included in this analysis were treated on 25 Phase I trials; however only 64 were evaluable for a treatment response. Forty-six patients were treated with a single targeted agent, 18 patients with a two-drug targeted therapy combination, and three patients with chemotherapy. Among the 25 Phase I trials, 17 investigated single agents whilst eight evaluated different combinations. Fifteen trials investigated an antibody or small molecule targeting vascular endothelial growth factor receptor, multiple receptor tyrosine kinases, platelet-derived growth factor receptor, fibroblast growth factor receptor, Fms-like tyrosine kinase 3, or human pp60c-Src. Five trials involved agents targeting epidermal growth factors; three investigated agents targeting histone de acetylation and aurora kinases separately; two used chemotherapy agents (gemcitabine and topotecan); and two involved agents with miscellaneous targets (integrin alpha-2 and insulin-like growth factor-I receptor).
Response
Best radiological response was assessed by serial CT or MRI scan using Response Evaluation Criteria In Solid Tumors (RECIST) version 1.0 or 1.1.[13,14] Imaging was performed approximately every two or three cycles depending upon the individual study protocol. In patients with measurable disease, the response was classified as complete, partial, stable or progressive disease.
Of the 64 evaluable patients for tumor response, 4 confirmed partial responses (6.25%) were observed, whereas 28 patients had stable disease (SD) >4 months (43.75%). Another 10 patients (15.63%) achieved SD lasting between 2-4 months. There were eight patients (11.42%) who received treatment for ≥12 months. Twenty-two patients (34.83%) were found to have clinical/radiological progressive disease (PD) before or at the time of first tumor assessment. The total number of patients with partial response or stable disease lasting more than 4 months was 32 (50.00%).
The four patients with PR were white with median age of 67.50 years, had clear cell histology and pulmonary metastases. Two of the four patients had previously received vascular endothelial growth factor (VEGF) –targeted therapy. Two patients with PR received non anti-VEGF agents while the others were treated with TKI. The duration of treatment ranged from 36 to 78 weeks.
Outcome Characteristics, Predictive and Prognostic factors
Among 70 patients, there were 51 deaths. Nine patients were still alive and survival information was missing for 10 patients. The median overall survival from the time of enrollment in a Phase I trial was 319 days (Figure 1). Thirteen subjects lived for more than 24 months after enrolling in a Phase I study. Patients with a PR had better overall survival as compared to patients with SD or PD (Figure 2).
Figure 1. Overall Survival measured from Phase 1 study enrollment until death.
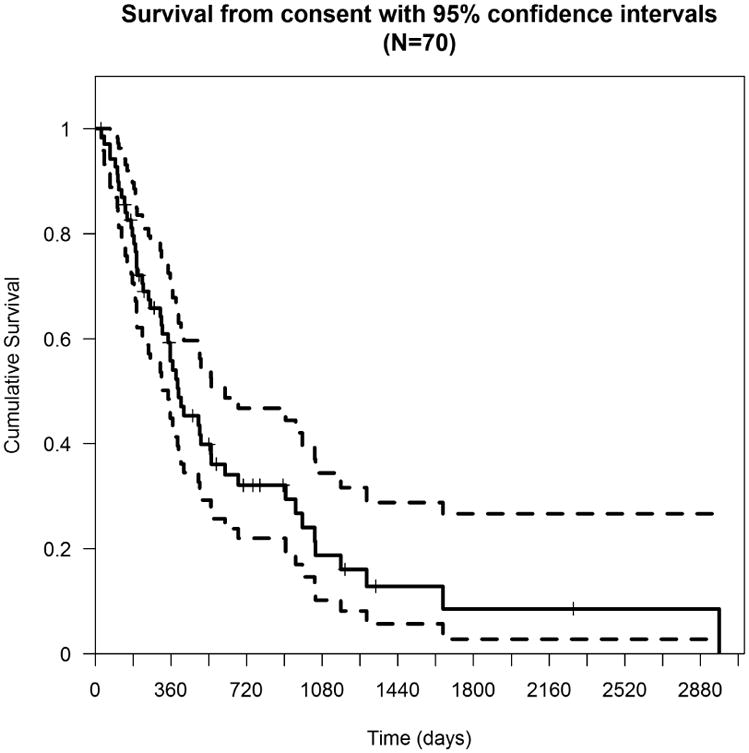
Figure 2. Survival based on treatment response, n=64.
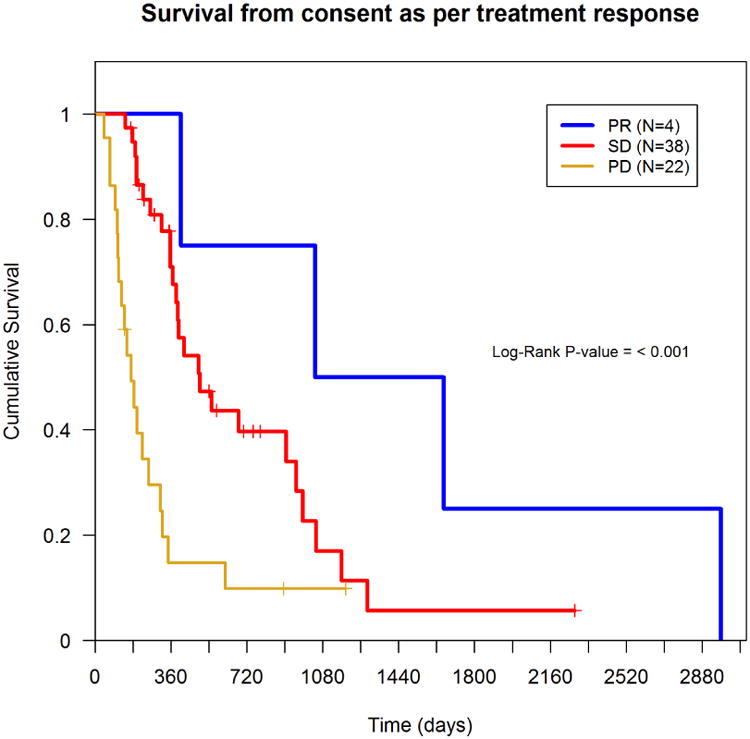
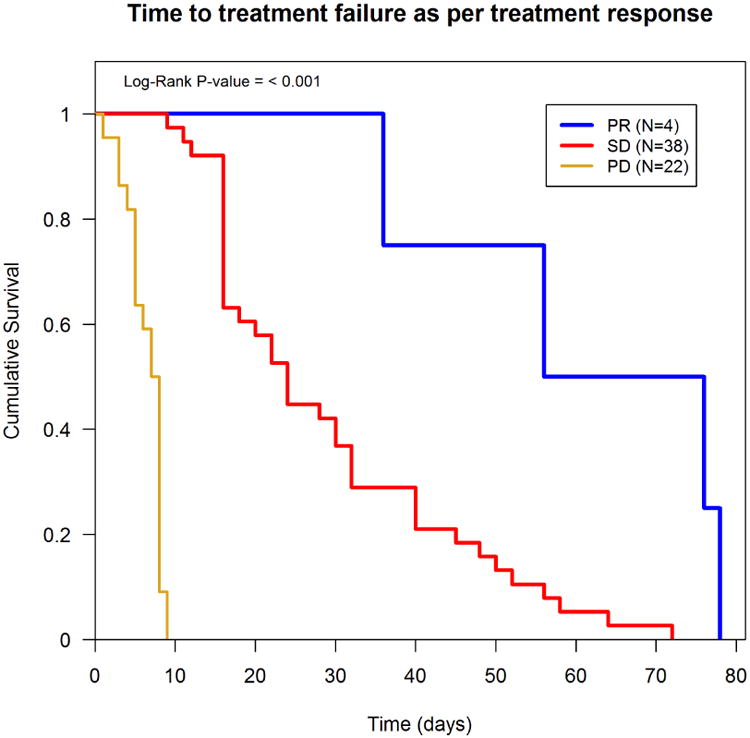
Multivariate analysis using a Cox proportional hazards model identified age <60 (p=0.015), ECOG ≥1 (P=0.023), albumin <3.4 g/dl (P=0.042), and liver metastases (P=0.01) as significant prognostic factors for worse overall survival, (i.e., they had a higher hazard of death) (Table 2). The median time to treatment failure was 16.00 weeks. Factors independently predicting shorter TTF were male gender (P=0.026) and LDH >1.5 ULN (P=0.038) (Table 3). Patients who achieved PR averaged longer TTF as compared to subjects with SD or PD (Figure 3). Also, patients on trials with anti-angiogenic agents had a longer TTF, on average, than those on trials with non-antiangiogenic agents, though the difference was not statistically significant (P=0.22) (data not shown).
Table 2. Overall Survival (Multivariate analysis), n=57.
| Variables | Hazard ratio | Lower 95% CI | Upper 95% CI | P-value |
|---|---|---|---|---|
| Age < 60 | Reference | |||
| Age >= 60 | 0.319 | 0.127 | 0.805 | 0.015 |
| Female | Reference | |||
| Male | 0.997 | 0.494 | 2.014 | 0.994 |
| ECOG performance status 0 | Reference | |||
| ECOG performance status at least 1 | 3.011 | 1.16 | 7.811 | 0.023 |
| Tumor histology clear cell | Reference | |||
| Tumor histology non clear cell | 1.49 | 0.639 | 3.478 | 0.356 |
| Site of metastases (non liver) | Reference | |||
| Site of metastases (liver) | 4.15 | 1.413 | 12.189 | 0.01 |
| Lactate dehydrogenase LDH <1.5 ULN | Reference | |||
| Lactate dehydrogenase LDH >1.5 ULN | 0.5 | 0.169 | 1.479 | 0.21 |
| Albumin > LLN | Reference | |||
| Albumin < LLN | 2.818 | 1.038 | 7.651 | 0.042 |
| Hemoglobin > 10 | Reference | |||
| Hemoglobin < 10 | 0.733 | 0.24 | 2.237 | 0.585 |
| Time since initial diagnosis < 18 months | Reference | |||
| Time since initial diagnosis 18 - 36 months | 2.438 | 0.971 | 6.121 | 0.058 |
| Time since initial diagnosis > 36 months | 1.397 | 0.503 | 3.88 | 0.522 |
Table 3. Time to treatment failure (Multivariate analysis), n=57.
| Variables | Hazard ratio | Lower 95% CI | Upper 95% CI | P-value |
|---|---|---|---|---|
| Age < 60 | Reference | |||
| Age >= 60 | 0.566 | 0.249 | 1.287 | 0.174 |
| Female | Reference | |||
| Male | 2.07 | 1.09 | 3.929 | 0.026 |
| ECOG performance status 0 | Reference | |||
| ECOG performance status at least 1 | 0.875 | 0.443 | 1.728 | 0.7 |
| Tumor histology clear cell | Reference | |||
| Tumor histology non clear cell | 2 | 0.833 | 4.803 | 0.121 |
| Site of metastases (non liver) | Reference | |||
| Site of metastases (liver) | 2.207 | 0.713 | 6.829 | 0.17 |
| Lactate dehydrogenase LDH <1.5 ULN | Reference | |||
| Lactate dehydrogenase LDH >1.5 ULN | 3.326 | 1.07 | 10.341 | 0.038 |
| Albumin > LLN | Reference | |||
| Albumin < LLN | 1.127 | 0.443 | 2.866 | 0.802 |
| Hemoglobin > 10 | Reference | |||
| Hemoglobin < 10 | 1.269 | 0.471 | 3.422 | 0.638 |
| Time since initial diagnosis < 18 months | Reference | |||
| Time since initial diagnosis 18 - 36 months | 1.124 | 0.492 | 2.57 | 0.782 |
| Time since initial diagnosis > 36 months | 1.172 | 0.515 | 2.667 | 0.704 |
Figure 3. TTF as per treatment response, n=64.
Safety and Toxicity
No treatment related death was observed while being treated with an investigational agent. Treatment at the assigned dose level was managed fairly well, dose reduction was only required for 16.18% patients. 10.00% patients came off study because of toxicity reasons. One patient discontinued study due to an immediate anaphylactic reaction. The most common grade 3 and greater toxic effects were drug rash, mucositis, diarrhea, hand foot syndrome, neutropenia, thrombocytopenia and anemia.
Discussion
Phase I clinical trials are conducted to evaluate the safety, pharmacokinetic profile, and pharmacodynamic properties of the experimental agents. The goals of these trials are to obtain sufficient information about the drug's pharmacological effects and establish an optimal dose for Phase 2 efficacy trial. In view of above scientific objectives, the likelihood of direct benefit from classical Phase I trials is considered to be low [15,16].
Patients participating in these trials have usually exhausted conventional treatments and are equally eager to explore therapy using promising newer agents with novel mechanisms of action. It has been commonly assumed that the distance from the dedicated Phase I cancer center is potentially a barrier in patient referral and enrollment. About 42.86 % of subjects in our study were from other cities or states. This reflects a high level of motivation among patients participating in Phase I oncology trials for a possible therapeutic benefit[17].
Historic reviews of Phase I oncology trials have reported a response rate of 4 to 6 percent[18]. In this study, we have analyzed the outcomes of patients with advanced stage renal cancer who were treated in Phase I clinical trials at our center. We discovered that the median TTF was 16.00 weeks (range 1-78 weeks) and median survival was 319.00 days (45.57 weeks). Although the partial response rate was 6.25%, 43.75% of the patients' had stable disease for ≥4 months. These data suggests that a significant number of renal cancer patients derived measurable clinical benefit from their participation in Phase I trials after progression on standard therapy. Our findings are better or at least comparable to a stable disease rate of 34% reported in a meta-analysis of Phase I trials[19]. It is worth noting that among the 25 Phase I agents included in this analysis, only one successfully obtained regulatory approval subsequently for the treatment of renal cell carcinoma.
Based on the retrospective database review, several groups have proposed various prognostic models for overall survival in patients participating in Phase I trials[20,21]. A correlation between these variables and survival outcomes has not been previously reported for advanced renal cancer patients in Phase I trials. The variables studied in our analysis are important elements of the prognostic tool proposed by Motzer et al[22]; however they have not been assessed previously in Phase I patient population. Within our analysis, the independent factors associated with shorter survival were poor ECOG performance status, presence of liver metastases, low albumin and younger age. The influence of age on survival could be due to higher tumor burden, histopathlogical grade and number of prior therapies in the younger group (< 60 years of age). Regarding tumor histology the p-value (0.356) shows that any distinct effect of histology cannot be concluded. Further, we established these variables only at the time of study enrollment and they were not evaluated at each time point.
The present analyses showed that Phase I patients with high LDH had worse subsequent outcomes in terms of TTF than patients with low LDH. The investigators also noted that non-clear histology, liver metastases, low albumin, and hemoglobin did not reach statistical significance for predicting shorter TTF. Women with advanced renal cancer were more likely to have longer TTF compared to men; however this disparity did not translate into survival benefit. Our data also drew attention to the correlation between treatment response and overall outcome. Patients with partial response had statistically significant longer TTF and overall survival as compared to those with progressive disease.
There is a need for more clearly defined treatment strategies for patients in clinical trials with high-risk features as above. The development of new agents that have specific molecular targets may be the best step towards identifying the most effective treatment for specific patients. In the development of a targeted therapy, there is often a biomarker that can potentially identify patients for whom the therapy will most likely be efficacious. With improved understanding of the complex molecular biology of RCC, it is increasingly critical to select a treatment targeted to a particular patient population with a high pretreatment probability of obtaining clinical benefit. As tumor molecular profiling becomes more common and widespread, better patient selection will become a more routine part of cancer drug development, which could further improve clinical outcomes.
As the treatment of RCC continues to evolve, research is currently focusing on immunotherapies with encouraging early results. Novel immunotherapies appear to have the potential to improve outcomes in metastatic RCC, but further analysis of the benefits of these drugs at different stages of development is warranted[19,23-27]. As discussed above, it is desirable that the early phase trials address not only whether the therapy should move forward to Phase 2 evaluation, but also incorporate a biomarker into trial design.
Conclusion
This analysis suggests that Phase I oncology trials in renal cancer patients with select characteristics do offer some potential for clinical benefit and have acceptable toxicity rates. Although this study makes several important contributions, our results must be evaluated within the context of the limitations of its retrospective methodology. A small sample size and reliance on the historical data are some of the constraints on generalizability and utility of our findings. These results and limitations should be seen as critical avenues for future research.
Clinical Practice Points.
The outcomes for patients with advanced stage renal cancer treated on early-phase clinical trials have not been previously reported.
We report here the general characteristics, time to treatment failure (TTF), prognostic factors, and survival data of 70 advanced renal cancer patients who were enrolled in 25 Phase I trials at our tertiary cancer center.
Four partial responses (6.25%) and 38 cases of stable disease lasting >4 months (43.75%) were observed. The median overall survival was 45.57 weeks.
Treatment at the assigned dose level was managed fairly well, dose reduction was only required for 16.18% patients
Participation in an early phase clinical trial is a reasonably safe alternate option for patients who have exhausted standard treatments.
Better patient selection could further improve clinical outcomes.
Acknowledgments
We would like to thank Ms. Susan Beardslee for reviewing the manuscript and providing valuable comments.
Funding: None
Footnotes
Conflict of Interest: All authors have no conflicts of interest
Contributor Information
Laeeq Malik, Institute for Drug Development, Cancer Therapy and Research Center (CTRC), University of Texas Health Science Center, San Antonio, TX.
Helen Parsons, Department of Epidemiology and Biostatistics, University of Texas Health Science Center, San Antonio, TX.
Devalingam Mahalingam, Institute for Drug Development, Cancer Therapy and Research Center (CTRC), University of Texas Health Science Center, San Antonio, TX.
Benjamin Ehler, Department of Epidemiology and Biostatistics, University of Texas Health Science Center, San Antonio, TX.
Martin Goros, Department of Epidemiology and Biostatistics, University of Texas Health Science Center, San Antonio, TX.
Alex Mejia, Institute for Drug Development, Cancer Therapy and Research Center (CTRC), University of Texas Health Science Center, San Antonio, TX.
Andrew Brenner, Institute for Drug Development, Cancer Therapy and Research Center (CTRC), University of Texas Health Science Center, San Antonio, TX.
John Sarantopoulos, Institute for Drug Development, Cancer Therapy and Research Center (CTRC), University of Texas Health Science Center, San Antonio, TX.
References
- 1.Tavani A, La Vecchia C. Epidemiology of renal-cell carcinoma. Journal of nephrology. 1997;10:93–106. [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Surveillance E, and End Results (SEER) 2013 [Google Scholar]
- 4.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611–1623. [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarba JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase iii trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (axis): A randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase iii trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase iii trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 11.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O'Toole T, Lustgarten S, Moore L, Motzer RJ. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 12.National comprehensive cancer network (nccn) NCCN Clinical Practice Guidelines in Oncology Kidney Cancer, 2013. 2013 doi: 10.6004/jnccn.2009.0043. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Shah S, Weitman S, Langevin AM, Bernstein M, Furman W, Pratt C. Phase i therapy trials in children with cancer. J Pediatr Hematol Oncol. 1998;20:431–438. doi: 10.1097/00043426-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Roberts TG, Jr, Goulart BH, Squitieri L, Stallings SC, Halpern EF, Chabner BA, Gazelle GS, Finkelstein SN, Clark JW. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. 2004;292:2130–2140. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- 17.Legge F, Eaton D, Molife R, Ferrandina G, Judson I, de Bono J, Kaye S. Participation of patients with gynecological cancer in phase i clinical trials: Two years experience in a major cancer center. Gynecol Oncol. 2007;104:551–556. doi: 10.1016/j.ygyno.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Estey E, Hoth D, Simon R, Marsoni S, Leyland-Jones B, Wittes R. Therapeutic response in phase i trials of antineoplastic agents. Cancer Treat Rep. 1986;70:1105–1115. [PubMed] [Google Scholar]
- 19.Horstmann E, McCabe MS, Grochow L, Yamamoto S, Rubinstein L, Budd T, Shoemaker D, Emanuel EJ, Grady C. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352:895–904. doi: 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 20.Arkenau HT, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Clinical outcome and prognostic factors for patients treated within the context of a phase i study: The royal marsden hospital experience. Br J Cancer. 2008;98:1029–1033. doi: 10.1038/sj.bjc.6604218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheler J, Tsimberidou AM, Hong D, Naing A, Falchook G, Piha-Paul S, Fu S, Moulder S, Stephen B, Wen S, Kurzrock R. Survival of 1,181 patients in a phase i clinic: The md anderson clinical center for targeted therapy experience. Clin Cancer Res. 2012;18:2922–2929. doi: 10.1158/1078-0432.CCR-11-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 23.Tykodi SS. Progress and potential of immune checkpoint blockade for treating advanced renal cell carcinoma. Immunotherapy. 2013;5:607–619. doi: 10.2217/imt.13.39. [DOI] [PubMed] [Google Scholar]
- 24.Atchison E, Eklund J, Martone B, Wang L, Gidron A, Macvicar G, Rademaker A, Goolsby C, Marszalek L, Kozlowski J, Smith N, Kuzel TM. A pilot study of denileukin diftitox (dd) in combination with high-dose interleukin-2 (il-2) for patients with metastatic renal cell carcinoma (rcc) J Immunother. 2010;33:716–722. doi: 10.1097/CJI.0b013e3181e4752e. [DOI] [PubMed] [Google Scholar]
- 25.McDermott D, Drake C, Sznol M. A phase i study to evaluate safety and antitumor activity of biweekly bms-936558 (anti-pd-1, mdx-1106/ono-4538) in patients with rcc and other advanced refractory malignancies. J Clin Oncol. 2011;29 Abstract 331. [Google Scholar]
- 26.Brignone C, Escudier B, Grygar C, Marcu M, Triebel F. A phase i pharmacokinetic and biological correlative study of imp321, a novel mhc class ii agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res. 2009;15:6225–6231. doi: 10.1158/1078-0432.CCR-09-0068. [DOI] [PubMed] [Google Scholar]
- 27.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, Lowy I, White DE, Rosenberg SA. Ipilimumab (anti-ctla4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]


