Abstract
Objective
Identify symptom clusters that characterize women’s experiences through the late reproductive stage, the menopausal transition and early postmenopause; and explore the influence of the menopausal transition stages and early postmenopause, compared to the late reproductive stage, on the clusters of symptoms women experience.
Methods
Participants from the Seattle Midlife Women’s Health Study whose symptom calendars were staged for menopausal transition provided data for a total of 6857 occasions. Multilevel latent class analysis was used to identify classes using scores for hot flashes and symptom factors (sleep, cognitive, mood, pain, tension).
Results
Class 1 included observations of low severity levels for all symptoms, whereas class 2 included low severity hot flashes and moderate severity levels for all other symptom factors. Class 3 included high severity hot flashes with lower severity levels of all other symptom factors. During the early and late menopausal transitions stages and early postmenopause, the likelihood of being in class 3 was significantly greater than being in class 1 relative to the late reproductive stage. There were no significant effects of menopausal transition stages on the likelihood of being in class 2.
Conclusions
This effort is the first to examine latent classes or clusters of symptoms over the prolonged period from late reproductive stage through early postmenopause. As such, the data contribute to understanding of symptom experiences beyond our early efforts to characterize the late menopausal transition stage.
Keywords: menopause, symptom clusters, postmenopause, latent class analysis
Introduction
The majority of clinical trials for symptoms during the menopausal transition (MT) and early postmenopause focus on management of hot flashes. Nonetheless, recent studies of symptoms indicate that hot flashes co-occur with mood symptoms1,2,3,4 and sleep disruption5,6 and that the effects of interventions may extend beyond hot flashes7. In addition, complaints of cognitive and pain symptoms often accompany women’s experiences of hot flashes8,9,10, yet these symptoms may not be considered in intervention trials focusing on therapies for hot flashes.
Although investigators have not identified a true “menopausal syndrome” 11, there is evidence that symptoms women experience during the menopausal transition tend to cluster together12,13,14. Symptom clusters are multiple symptoms that co-occur and may be related to one another through a common mechanism or etiology, a common shared variance, or the production of different outcomes than individual symptoms, e.g. pain, nausea, and fatigue15. Although symptom clusters may not be disease-specific, some may constitute a syndrome or characterize a disease. Investigators have differentiated symptom clusters by type of symptom, such as pain and fatigue, as well as by their severity and burden16 and their capacity to interfere with daily living17.
Literature focused on symptom clusters emphasizes the importance of understanding clusters as opposed to individual symptoms as foundational to developing appropriate therapeutics that span multiple symptoms. Moreover, understanding factors associated with a cluster vs. individual symptoms may reveal underlying mechanisms that precipitate or enhance the severity of distress. For example, hot flashes and night-time awakening experienced together may disrupt daytime functioning through difficulty concentrating and irritability8, symptoms which, in turn, interfere with relationships and work roles18. When hot flashes alone are considered as predictors of interference with work and relationships, their effect is negligible, suggesting that their effects may operate through sleep disruption or mood changes18. Although symptom clusters during the menopausal transition have been explored by other researchers12,13, this work has focused on grouping of symptoms that are similar in type, e,g, mood symptoms, vasomotor symptoms. To date, an exploration of the clustering of symptoms of various types and severity (latent classes) has not been accomplished for a period including the late reproductive stage through the postmenopause.
Our earlier investigation of symptom clusters during the late menopausal transition stage, a period of peak prevalence and symptom intensity for many women, revealed four clusters of symptom types and severity based on data from symptom diary ratings of the severity of hot flashes, problem concentrating, joint aches, mood changes, and night-time awakening14. The four clusters were: low symptom severity for all symptoms except moderate severity joint ache (65%), high severity for all symptoms except for moderate hot flashes (13%), high severity hot flashes, night-time awakening, and joint aches (12%), and high severity problem concentrating and joint ache (10%)14. In our efforts to extend our understanding of the types of and stability of latent classes of women beyond just the late MT stage, we undertook analysis of symptom clusters during the late reproductive stage of reproductive aging, the early and late stages of menopausal transition, and the early postmenopause to explore this phenomenon throughout the menopausal transition and postmenopause.
The purposes of the analyses presented here were to:
Identify latent classes that characterize episodes of symptom severity during the late reproductive stage, the menopausal transition and early postmenopause;
Explore the influence of the menopausal transition stages and early postmenopause, compared to the late reproductive stage, on the episodes of symptom severity clusters.
Methods
Sample and design
The current analysis used data from the longitudinal Seattle Midlife Women’s Health Study (SMWHS). The SMWHS database includes participants with up to 20 years of follow-up. In the original study 508 women were enrolled between 1990 and 1992. After completing an initial in-person interview, 390 women consented to provide data annually by questionnaire, daily menstrual calendar, and/or health diary. At the end of 5 years, 243 women consented to continue to participate for an additional 5 years and 170 agreed to provide a first morning urine specimen 8–12 times per year on day 6 of the menstrual cycle19, 20. For the current analyses, the data set contained data from 379 women who provided diary data on at least one occasion.
Eligible participants (N=292/379, 77%) were those who contributed ratings of hot flash, sleep, pain, mood, cognitive and tension symptoms from the health diaries on at least one occasion beginning in 1990 and were in the late reproductive, early or late menopausal transition stages, or early postmenopause during the course of the study. Women were followed until they either dropped out or became ineligible. This length of time varied for each woman. Of the 379 women, 87 (33%) were excluded from the analyses because they could not be staged at any data point in the study. Inability to stage was due to the use of hormones or chemotherapy, as well as being within three months of a pregnancy, or having had a hysterectomy and/or bilateral oophorectomy. Women whose data were available for analysis and were eligible for inclusion were midlife women with a mean age of 46 (SD=5.0) years at the beginning of the study, 15.8 years of education (SD=2.6), and a median family income of $38,800 (SD=$15,000). Most (86%) of the eligible participants were employed, 71% married or partnered, 22% divorced or separated or widowed, and 7% never married or partnered. Eligible women described themselves as follows: 7% African American, 9% Asian American, and 82% White. As seen in Table 1, women who were included in these analyses compared to those who were ineligible were similar with respect to employment status, marital status, age, and education. They differed significantly by income and ethnicity: those who were included in the analyses had higher incomes and were more likely to be White. Data obtained during any occasions of hormone use were not included in the analyses presented here.
Table 1.
Characteristics of Sample at Start of Study (1990–1991): Women whose data were eligible for inclusion in the Latent Class Analysis compared to those whose data were not eligible
| Characteristic |
Eligible Women (n=292) Mean (SD) |
Ineligible Women (n=87) Mean (SD) |
|---|---|---|
|
| ||
| Age (years) | 46 (5.0) | 44.3 (6.7) |
|
| ||
| Years of education | 15.8 (2.6) | 15.3 (2.7) |
|
| ||
| Family income ($) | 38,800 (15,000) | 35,600 (17,400) |
|
| ||
| Characteristic | N (Percent) | N (Percent) |
|
| ||
| Currently employed | ||
| Yes | 250 (85.6) | 76 (87.4) |
| No | 42 (14.4) | 11 (12.6) |
|
| ||
| Race/ethnicity | ||
| African American | 20 (6.8) | 11 (12.6) |
| Asian/Pacific Islander | 27 (9.2) | 6 (6.9) |
| White | 239 (81.8) | 63 (72.4) |
| Other (Hispanic, Mixed) | 6 (2.0) | 7 (8.1) |
|
| ||
| Marital Status | ||
| Married/partnered | 205 (71.0) | 63 (72.4) |
| Divorced/widowed/not partnered | 65 (22.0) | 18 (20.7) |
| Never married/partnered | 22 (7.0) | 6 (6.9) |
Measures
Menopausal transition stages and early postmenopause
Menopausal transition (MT) refers to the period of time leading up to the final menstrual period in which persistent menstrual irregularity occurs21,22,23. Using menstrual calendar data, menstrual patterns for women not taking any hormones were classified according to stages of reproductive aging: late reproductive, early menopausal transition or late menopausal transition based on staging criteria developed by Mitchell, Woods and Mariella24. Early postmenopause (PM) includes the five years after the final menstrual period. The names of stages and the definition of early PM match those recommended at the Stages of Reproductive Aging Workshop (STRAW)25 and validated in follow-up studies by the ReSTAGE Collaboration26,27. The STRAW criteria defines the stages as follows: early transition is characterized by a persistence of a seven or more days difference between two consecutive cycle lengths; late transition is characterized by an interval of amenorrhea of at least sixty days and early postmenopause is defined as the first 5 years since the final menstrual period.27
Symptoms
A 3-day health diary included 47 symptoms that were rated for severity over the previous 24 hours on a 5-point Likert-type scale (0 = not present, 4 = extreme)17. Women completed the diary over three consecutive days each month thru 2000 then completed the diary 4 times per year (quarterly) thereafter. Women filled out the diary on menstrual cycle days 5, 6, and 7 if they were still having periods; if not, on any consistent 3 days each month or each quarter. Ratings were averaged over 3 days.
Data analysis
Multilevel latent class analysis (MLCA) was used to determine empirically whether there were distinct latent classes of symptomatology based on individual diary observations. Traditional latent class analysis is a statistical method used to identify subgroups of related cases that are not directly observed based on categorical or continuous observed variables. This traditional method assumes independence whereas MLCA allows for the nested structure of the diary data28. The diary data are a nested structure because women may have provided diary ratings of symptom severity on multiple occasions within one or more menopausal transition stages. Therefore, the probability that an episode of symptom severity will belong to a high symptom severity class vs. a low symptom severity class may vary significantly across the MT stages.
Prior to performing the MLCA, the following five symptom factors were identified from a principal components analysis (PCA) of 19 symptoms from the SMWHS health diary: sleep (difficulty getting to sleep, night-time awakening, early morning awakening), pain (backache, joint ache, headache), mood (depressed, mood changes, crying, irritable), cognitive (problem concentrating, forgetful, irritable), and tension (panic, nervous, tension) groups29.
Using these five symptom factors identified from the PCA and hot flash as an individual symptom, MLCA (Mplus V. 6) was used to specify 1–6 latent classes. Selection of the optimal number of latent classes (best fitting model) was determined by entropy, the statistical goodness-of-fit measures bootstrap likelihood ratio test (BLRT), and the Bayesian information criterion (BIC) and the underlying logical/substantive interpretation of the resulting classes30. The BIC statistic, one of the most important criteria, balances maximizing the likelihood and model parsimony. Lower BIC values typically reflect a better fit31. The BLRT compares the distribution of the likelihood ratio test statistic from the initial analysis to the likelihood ratio statistics from the bootstrap draws to compute a p-value. A low p-value (<.05) indicates that the current model fits the data as well as or better than the k-1 model32. Entropy is a standardized measure of how accurately subjects, or in this case observations, are classified. Entropy values range from 0 to 1 with > 0.9 seen as a better classification33(Table 2). Once the optimal number of classes was selected, stage was added as a covariate to determine if stage was a significant predictor of class membership. The Mplus program uses an algorithm for multinomial logistic regression to identify latent classes.
Table 2.
Fit Indices of Latent Class Analysis for Symptoms: Late Reproductive Stage through Early Postmenopause
| Model | No of free parameters | BIC | BLRT (p) | Entropy |
|---|---|---|---|---|
| 1 class | 12 | 72669.4 | NA | NA |
| 2 class | 19 | 62670.9 | .000 | .924 |
| 3 class | 32 | 57879.0 | .000 | .944 |
| 4 class | 33 | 56368.3 | .000 | .936 |
| 5 class | 40 | 54877.2 | .000 | .941 |
| 6 class | 47 | 53360.9 | .000 | .948 |
Note: BIC: Bayesian information criterion; BLRT: Bootstrap likelihood ratio test
Results
Using MLCA and fit indices for MLCA, a three class solution was the best fitting model based on the BIC which declined substantially from the one-class to three-class model and then began to stabilize. The class solutions for one to six latent classes are presented in Table 2. The entropy provided additional support for the three-class model. Although the posterior probabilities in the four-class model indicated an adequate model classification, it did not add anything clinically significant to the model. The fourth class represented a slight splitting of an existing class in the three-class model. We chose the three-class model as it most substantially and parsimoniously explained the data in a theoretically meaningful way.
In the three class model seen in Table 3, the class with the largest proportion of observations represented low severity responses and accounted for 74.9% of the sample (Class 1). The other two classes were almost evenly split between responses. Class 2 included observations of low hot flash severity with higher severity ratings for all other symptom groups and accounted for 13% of the observations. Class 3 included observations of high hot flash severity with lower severity for all other symptom groups and accounted for 12% of the observations.
Table 3.
Number of Observations and Mean (SD) Symptom Severity Scores from Late Reproductive Stage through Early Postmenopause According to Latent Classes for 3 Class Model
| Latent Classes | |||
|---|---|---|---|
| 1 – low severity symptoms | 2- low hot flash/moderate others | 3 – high hot flash/moderate others | |
| N (%) in class | 5137(74.9) | 894(13.0) | 826(12.1) |
| Symptom Groups: M (SD) | |||
| Hot flash | 0.121 (.02) | 0.360 (.08) | 2.206 (.09) |
| Sleep group | 0.326 (.03) | 1.185 (.10) | 0.854 (.07) |
| Pain group | 0.609 (.04) | 1.353 (.07) | 0.960 (.09) |
| Mood group | 0.204 (.02) | 1.166 (.06) | 0.373 (.04) |
| Cognitive group | 0.200 (.03) | 1.174 (.09) | 0.557 (.08) |
| Tension group | 0.176 (.02) | 1.114 (.11) | 0.353 (.06) |
After determining the best fitting model, latent class membership was regressed on stage in a multinomial logistic regression, to determine if stage was a significant predictor of class membership. In the regression model the low symptom severity class (class 1) served as the reference group. Relative to being in class 1, being in early and late MT stages increased the likelihood of being in class 2 while being in early postmenopause reduced the likelihood of being in class 2, but the effects were not statistically significant (p > .05).
Relative to being in class 1, being in either the early or the late menopausal transition stage or early postmenopause versus being in the late reproductive stage increased the likelihood of being in class 3 (high severity hot flashes, lower severity other symptoms) (See Table 4 for multinomial regression results). All stage effects (relative to late reproductive stage) were positive, indicating an increased likelihood of being in class three relative to class one during each of these stages. The strongest effect relative to being in the late reproductive stage was being in early postmenopause, which increased the log odds of being in class 3 vs. class 1 by 3.117. In other words, women with a higher propensity for hot flashes vs. those who experienced very low severity symptoms were 3 times more likely to be in early postmenopause.
Table 4.
Effects of stage on Latent Class Membership: Multinomial Logistic Regression Results Comparing Class 2 to Class 1 and Class 3 to Class 1
| Class 2 (n=894) | ||||
|---|---|---|---|---|
| Variables | b-coefficient | Odds Ratio | 95% CI | P-value |
| Stage 3 | 0.146 | 1.157 | −0.301 – 0.592 | 0.591 |
| Stage 4 | 0.251 | 1.286 | −0.306 – 0.808 | 0.458 |
| Stage 5 | −0.451 | 0.637 | −1.188 – 0.286 | 0.314 |
| Class 3 (n=826) | ||||
| Variables | b-coefficient | Odds Ratio | 95% CI | P-value |
| Stage 3 | 1.039 | 2.828 | 0.195 – 1.884 | 0.043 |
| Stage 4 | 2.382 | 10.829 | 1.689 – 3.075 | 0.000 |
| Stage 5 | 3.117 | 22.589 | 2.372 – 3.863 | 0.000 |
Note: Stage 3 – early menopausal transition (MT); Stage 4 – late MT; Stage 5 – early postmenopause. The exposure reference group was late reproductive stage.
Low symptom group (class 1) was used as the referent group (n=5137)
Discussion
During the menopausal transition women experience multiple symptoms, not only hot flashes. Moreover these symptoms cluster together in ways that may inform decisions about therapeutics. In the analyses presented here we determined whether there were empirically derived distinct clusters of symptom severity episodes that women report when data spanning the late reproductive stage through early postmenopause were included in the analyses. We were able to identify three classes of symptom episodes that differed by severity patterns of individual symptom factors (hot flashes, sleep, mood, pain, cognitive, tension) and demonstrated that the likelihood of occurrence of the symptom classes differed according to menopausal transition stages and early postmenopause compared to the late reproductive stage. Class 1 included observations of low severity levels for all symptoms, whereas class 2 included low severity hot flashes and moderate severity levels for all other symptom factors. Class 3 included high severity hot flashes with lower severity levels of all other symptom factors. During the early and late menopausal transition stages and early postmenopause, the likelihood of being in class 3 was significantly greater than being in class 1. There were no significant effects of menopausal transition stages on the likelihood of being in class 2.
This effort is the first to examine latent classes or clusters of symptoms over the prolonged period from late reproductive stage through early postmenopause. As such, the data contribute to an understanding of symptom experiences beyond our early efforts to characterize the late menopausal transition stage14. In the study reported here we included data from 19 symptoms as rated in over 6500 observations for periods of time up to 20 years.
The identification of a large class that included low severity symptoms of each type is consistent with other studies of community-based populations34 including our prior analysis of symptom clusters during the late menopausal transition stage14. Of interest is the symptom cluster (class 3) in which hot flashes constitute the symptom with the highest severity ratings. This cluster suggests that there is a subset of observations characterized by a single symptom with a severity rating that is distinctly higher than all other symptom factors in the cluster. Moreover, membership in this class is uniquely associated with the menopausal transition stages and early postmenopause. Although other symptom factors are rated less severe than hot flashes, the mean severity levels suggest that sleep and pain symptoms are somewhat more severe than the mood, cognitive and tension factor means. These gradations in symptom severity may help account for the observations that some women exhibit what has been labeled a “menopausal syndrome” in which other symptoms are associated with moderate to severe hot flashes. Our findings are consistent with those of Avis and colleagues11 who found no evidence for a universal menopausal syndrome that included vasomotor, psychological, and somatic symptoms. Nonetheless, we did identify a cluster of symptoms that does include elevated sleep and pain symptoms, suggesting that there is a subset of women who experience these symptoms together. Our prior analyses of sleep symptoms during the menopausal transition and early postmenopause identified an elevated severity of night-time awakening during the late menopausal transition stage6 as was seen in the Penn Ovarian Aging Study population5. Moreover, low back pain symptoms increased in severity during the early and late menopausal transition stage8. There is evidence from other studies of midlife women linking sleep symptoms with pain symptoms35,36. Whether sleep disruption and pain symptoms represent a discrete cluster of symptoms that co-occurs with vasomotor symptoms in a subset of women during the menopausal transition and whether the sleep and pain symptoms are related to changing biology during the menopausal transition remains to be determined
The prevalence of observations in class 2, indicating low severity hot flashes coupled with higher severity sleep, pain, mood, cognitive, and tension symptoms would suggest that although some women do not experience severe hot flashes, they do experience other distressing symptoms during the menopausal transition and early postmenopause. The relatively modest severity levels of these symptoms likely indicates increased levels of distress in comparison to that associated with the observations in class 1. Thus it is important to recognize that although women do not complain of hot flashes, they may be experiencing other symptoms which they may perceive as bothersome or which interfere with their everyday lives. This would be consistent with our observations as well as those of others that different types of symptoms may be more bothersome to women17 or may interfere more with different aspects of life, such as relationships or work18.
Limitations of this study include the under-representation of women from lower socioeconomic status and ethnic groups (as seen in Table 1), which may limit the generalizability of these findings to some women. The overall relatively low severity scores on the symptoms as seen in Table 3 (2.2 on hot flashes on a 0–4 scale being the highest) may have impacted the results, again limiting the ability to generalize to a population with more severe symptoms.
Conclusion
In summary, we have identified three latent classes of episodes of symptoms which women experience differentially according to their progression through stages of reproductive aging. The class of symptoms with more severe hot flashes relative to other symptoms (class 3) is more likely to be experienced during the early and late menopausal transition stages and early postmenopause when compared to the largest class with low severity symptoms of each type (class 1). Another class of symptoms represents a more generalized type of distress, with pain, sleep, mood, tension, and cognitive symptoms being more severe than hot flashes (class 2). Because all three classes of symptoms are likely to occur during the menopausal transition and early postmenopause, women will benefit from clinicians attending to the specific groups of symptoms they experience and considering multi-symptom approaches to therapeutics.
Figure 1.
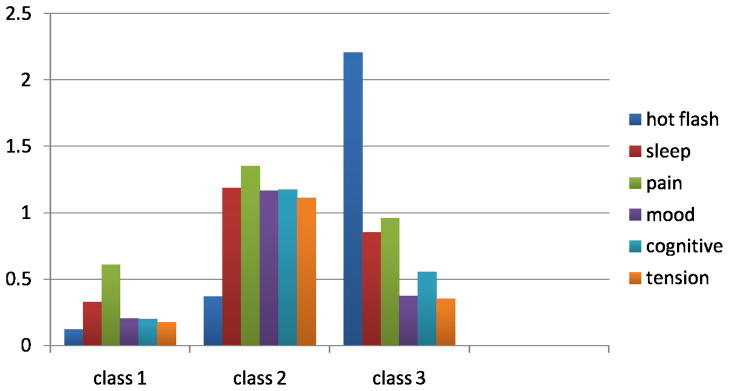
Mean symptom severity level according to class. Means were calculated from symptom severity ratings provided from all occasions during the late reproductive, early and late menopausal transition, and early postmenopause. Each class contains data from all stages.
Acknowledgments
Funding support: This work was supported by grants from the National Institute of Nursing Research (NINR 1R21NR012218-01 Menopause Symptom Clusters: Refocusing Therapeutics; NR 04141 - Menopausal Transition: Biobehavioral Dimensions; P30 NR 04001, P50-NR02323 – Center for Women’s Health and Gender Research).
Footnotes
Conflict of Interests/Disclosures: None
References
- 1.Joffe H, Soares CN, Thurston RC, White DP, Cohen LS, Hall JE. Depression is associated with worse objectively and subjectively measured sleep, but not more frequent awakenings in women with vasomotor symptoms. Menopause. 2009;16:671– 679. doi: 10.1097/gme.0b013e3181957377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman EW, Sammel MD, Lin H. Temporal associations of hot flashes and depression in the transition to menopause. Menopause. 2009;16:728–734. doi: 10.1097/gme.0b013e3181967e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Association between hot flashes, sleep complaints, and psychological functioning among healthy menopausal women. Int J Behav Med. 2006;13:163–172. doi: 10.1207/s15327558ijbm1302_8. [DOI] [PubMed] [Google Scholar]
- 4.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell ES. Depressed mood during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Menopause. 2008;15:223–232. doi: 10.1097/gme.0b013e3181450fc2. [DOI] [PubMed] [Google Scholar]
- 5.Pien GW, Sammel MD, Freeman EW, Lin H, DeBlasis TL. Predictors of sleep quality in women in the menopausal transition. Sleep. 2008;31:991–999. [PMC free article] [PubMed] [Google Scholar]
- 6.Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33:539–549. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman EW, Guthrie KA, Caan B, Sternfeld B, Cohen LS, Joffe H, Carpenter JS, Anderson GL, Larson JC, Ensrud KE, Reed SD, Newton KM, Sherman S, Sammel MD, LaCroix AZ. Efficacy of escitalopram for hot flashes in healthy menopausal women: A randomized controlled trial. JAMA. 2011;305:267–274. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell ES, Woods NF. Cognitive symptoms during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Climacteric. 2011;14:252–261. doi: 10.3109/13697137.2010.516848. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell ES, Woods NF. Pain symptoms during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Climacteric. 2010;13:467–478. doi: 10.3109/13697137.2010.483025. [DOI] [PubMed] [Google Scholar]
- 10.Dennerstein L, Lehert P, Koochaki PE, Graziottin A, Leiblum S, Alexander JL. A symptomatic approach to understanding women’s health experiences: a cross cultural comparison of women aged 20 –70 years. Menopause. 2007;14:688–696. doi: 10.1097/gme.0b013e31802dabf0. [DOI] [PubMed] [Google Scholar]
- 11.Avis NE, Brockwell S, Colvin A. A universal menopausal syndrome? Am J Med. 2005;118 (suppl 12B):S37–46. doi: 10.1016/j.amjmed.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 12.Goldani von Muhlen D, Kritz-Silverstein D, Barrett-Connor E. A community-based study of menopause symptoms and estrogen replacement in older women. Maturitas. 1995;22:71–78. doi: 10.1016/0378-5122(95)00917-a. [DOI] [PubMed] [Google Scholar]
- 13.Kritz-Silverstein D, Goldani von Muhlen D, Barrett-Connor E. Prevalence and clustering of menopausal symptoms in older women by hysterectomy and oophorectomy status. J Womens Health Gend Based Med. 2000;9:747–755. doi: 10.1089/15246090050147727. [DOI] [PubMed] [Google Scholar]
- 14.Cray L, Woods NF, Mitchell ES. Symptom clusters during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Menopause. 2010;17:972–977. doi: 10.1097/gme.0b013e3181dd1f95. [DOI] [PubMed] [Google Scholar]
- 15.Miaksowski C, Aouizerat B, Dodd M, Cooper B. Conceptual issues in symptom clusters research and their Implications for quality-of-life assessment in patients with cancer. J Natl Cancer Inst Monogr. 2007;37:39–46. doi: 10.1093/jncimonographs/lgm003. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland C. Symptom burden: Multiple symptoms and their impact as patient- reported outcomes. J Natl Cancer Inst Monogr. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter JS, Neal JG, Payne J, Kimmick G, Storniolo AM. Cognitive-behavioral interventions for hot flashes. Oncol Nurs Forum. 2007;43:37–42. doi: 10.1188/07.ONF.E1-E8. [DOI] [PubMed] [Google Scholar]
- 18.Woods NF, Mitchell ES. Symptom interference with work and relationships during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Menopause. 2011;18:654–661. doi: 10.1097/gme.0b013e318205bd76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Taylor HJ, Mitchell ES. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: Observations from the Seattle Midlife Women’s Health Study. J Women’s Health. 2007;16(5):667–677. doi: 10.1089/jwh.2006.0138. [DOI] [PubMed] [Google Scholar]
- 20.Wood NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell ES. Depressed mood during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Menopause. 2008;15:223–232. doi: 10.1097/gme.0b013e3181450fc2. [DOI] [PubMed] [Google Scholar]
- 21.Sherman S. Defining the menopausal transition. Am J Med. 2005;118(12B):S3–7. doi: 10.1016/j.amjmed.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Santoro N. The menopausal transition. Am J Med. 2005;118(12B):S8–13. doi: 10.1016/j.amjmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 23.WHO Scientific Group on Research on the Menopause in the 1990s. Research on the menopause in the 1990s: report of WHO Scientific Group. World Health Organization; 1996. [PubMed] [Google Scholar]
- 24.Mitchell ES, Woods NF, Mariella A. Three stages of the menopausal transition: Toward a more precise definition. Menopause. 2000;7:334–49. doi: 10.1097/00042192-200007050-00008. [DOI] [PubMed] [Google Scholar]
- 25.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Stages of Reproductive Aging Workshop (STRAW) J Womens Health Gend Based Med. 2001;10(9):843–48. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- 26.Harlow SD, Mitchell ES, Crawford S, Nan B, Little R, Taffe J. The ReSTAGE Collaboration: defining optimal bleeding criteria for onset of early menopausal transition. Fertil Steril. 2007;89(1):129–40. doi: 10.1016/j.fertnstert.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harlow SD, Cain K, Crawford S, Dennerstein L, Little R, Mitchell ES, et al. Evaluation of four proposed bleeding criteria for the onset of late menopausal transition. J Clin Endocrinol Metab. 2006;91(9):3432–38. doi: 10.1210/jc.2005-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry KL, Muthen B. Multilevel latent class analysis: An application of adolescent smoking typologies with individual and contextual predictors’. Structural Equation Modeling: A Multidisciplinary Journal. 2010;17(2):193–215. doi: 10.1080/10705511003659342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cray L, Woods NF, Mitchell ES. Identifying symptom clusters during the menopausal transition: A principal components analysis. (In progress) [Google Scholar]
- 30.Shevlin M, Murphy J, Dorahy MJ, Adamson G. The distribution of positive psychosis-like symptoms in the population: A latent class analysis of the National Comorbidity Survey. Schizophr Res. 2007;89:101–9. doi: 10.1016/j.schres.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Muthen B, Muthen LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24(6):882–91. [PubMed] [Google Scholar]
- 32.Muthén LK, Muthén BO. Mplus User’s Guide. 5. Los Angeles: Muthén & Muthén; 2007. [Google Scholar]
- 33.Murphy J, Shevlin M, Adamson G. A latent class analysis of positive psychosis symptoms based on the British Psychiatric Morbidity Survey. Pers Individ Dif. 2006;42:1491–1502. [Google Scholar]
- 34.Miaskowski C, Cooper B, Paul S, Dodd M, et al. Subgroups of cancer patients with different symptom experiences and quality-of-life outcomes. ONF. 2006;33:E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 35.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–1592. [PubMed] [Google Scholar]
- 36.Landis CA, Lentz MJ, Rothermel J, Buchwald D, Shaver JL. Decreased sleep spindles and spindle activity in midlife women with fibromyalgia and pain. Sleep. 2004;27:741–750. doi: 10.1093/sleep/27.4.741. [DOI] [PubMed] [Google Scholar]


