Abstract
Gene therapy represents a potential efficient approach of disease prevention and therapy. However, due to their poor in vivo stability, gene molecules need to be associated with delivery systems to overcome extracellular and intracellular barriers and allow access to the site of action. Cationic polymeric nanoparticles are popular carriers for small interfering RNA (siRNA) and DNA-based therapeutics for which efficient and safe delivery are important factors that need to be optimized. Micelle-like nanoparticles (MNP) (half micelles, half polymeric nanoparticles) can overcome some of the disadvantages of such cationic carriers by unifying in one single carrier the best of both delivery systems. In this review, we will discuss how the unique properties of MNP including self-assembly, condensation and protection of nucleic acids, improved cell association and gene transfection, and low toxicity may contribute to the successful application of siRNA- and DNA-based therapeutics into the clinic. Recent developments of MNP involving the addition of stimulus-sensitive functions to respond specifically to pathological or externally applied “triggers” (e.g., temperature, pH or enzymatic catalysis, light, or magnetic fields) will be discussed. Finally, we will overview the use of MNP as two-in-one carriers for the simultaneous delivery of different agents (small molecules, imaging agents) and nucleic acid combinations.
Keywords: siRNA delivery, DNA delivery, gene delivery, complexes, cationic polymers, micelle-like nanoparticles, cationic amphiphiles
1. INTRODUCTION
1.1. Opportunities and Challenges for DNA- and siRNA-Based Therapeutics
Gene therapy holds great promise for treating gene-related disorders. In this therapeutic approach, specific sequences of genes or transcriptional mediators are externally introduced into the patient’s cells in order to replace defective genes or regulate their abnormal expression. Since the first successful clinical trial in a patient with Adenosine Deaminase Deficit (ADA) in 1990,1 the number of gene therapy applications has expanded from rare monogene diseases to more common multifactorial and complex ones, such as infectious or cardiovascular diseases and cancer.2 Depending on the type of nucleotide molecule that is used, gene therapy can be divided into two possible categories: function enhancement [i.e., by using plasmid DNA (pDNA)] and function inhibition [i.e., by using small interfering RNA (siRNA)], respectively, via different mechanisms (Figure S1 in the Supporting Information).
Gene-based drugs offer unique opportunities to fine-tune the expression of genome end products that operate at multiple levels of a given disease pathway. In cancer, most studies target genes involved in apoptotic or proliferative cellular pathways as adjuvant therapies to treat nonresectable tumors or tumors that are resistant to conventional chemotherapy or radiotherapy.3,4 Silencing genes with siRNA targeting antiapoptotic genes (i.e., Bcl-2 siRNA,5 siRNA,6 survivin siRNA7) or pDNA expressing proapoptotic genes (TNF-α gene8 and p53 gene9) are popular strategies. In addition, other targets have been developed from the study of mechanisms related to resistance to chemotherapy or irradiation such as molecules related to DNA repair mechanisms or multidrug resistance (MDR) proteins.10 One of the most studied resistance mechanisms is the reduction of intracellular drug concentration by ATP-binding cassette (ABC) transporter proteins, including P-glycoprotein (P-gp, encoded by the MDR-1 gene), that pump drug out of the cells before they reach their site of action. In tumor tissues, intrinsic or induced overexpression of P-gp after exposure to chemotherapy drugs has been determined to be a major reason for chemotherapy failure in different MDR cancer types.11–13 Recent studies have shown that silencing of the MDR-1/P-gp gene using siRNA can improve the effectiveness of anticancer drugs on MDR tumors.14–17 Research is currently being carried out to evaluate whether treatments in which small anticancer molecules (i.e., doxorubicin, paclitaxel) and nucleic acids simultaneously delivered into cancer cells can act synergistically for a greater anticancer effect.18
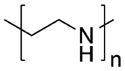
To date, the DNA/RNAi technology has achieved some promising results in cell culture and preclinical animal models. However, only a few products are undergoing clinical trials or are in the market.19 This is due mostly to the difficulties found in delivery of genes to the target site due to their instability, inefficient cell entry, and poor pharmacokinetic profile (i.e., circulatory half-lives of <5 min and low in vivo stability due to a rapid enzymatic degradation within the first minute after administration).20–23 To overcome these problems, various delivery technologies have been developed, including direct introduction of nucleic acids by physical methods,24 recombinant viral vectors,25 and synthetic systems based on the use of cationic lipids or polymers.26–28 Compared to viral vectors, nonviral vectors are attractive alternatives with improved safety and are easier to scale up but they achieve lower levels of gene expression.29 Among cationic carriers, polyethylenimine (PEI) polyamidoamine (PAMAM) dendrimers, polylysine (PLL), and chitosan have been widely used as siRNA/DNA delivery systems with little in vivo success due to low efficiency and toxicity issues.28,30,31
1.2. Hurdles to siRNA/DNA Delivery Using Cationic Carriers
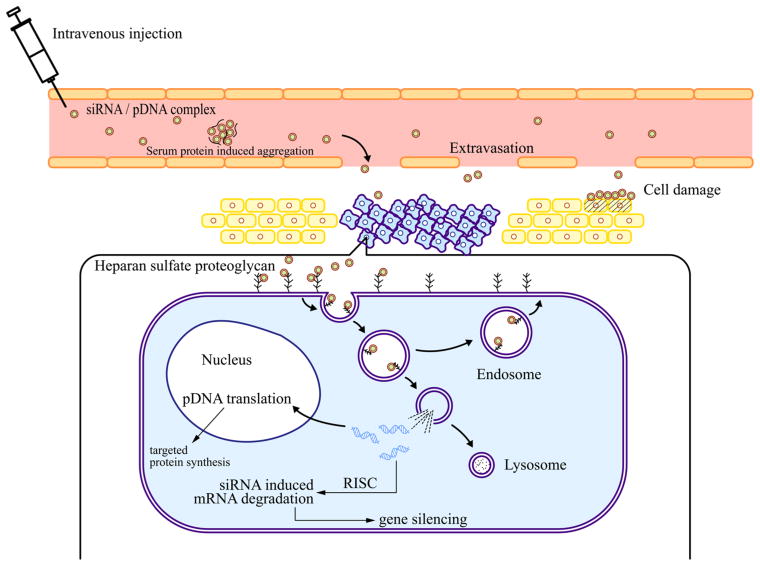
Nucleotide-based molecules must be delivered in the cytoplasm (small interfering RNA) or the nucleus (plasmid DNA) to exert a therapeutic effect. This is not a simple task since there are several barriers that make this process difficult (Figure 1).
Figure 1.
Schematic explanation of the main hurdles in siRNA/pDNA delivery using cationic carriers from the administration site to the target site.
The first requisite for the successful delivery of siRNA or pDNA in cationic assemblies is the formation of highly compacted nanostructures generally termed “complexes”32 that decrease the hydrophilicity, charge, and size of nucleic acids. This increases their cellular tropism and uptake, and protects the nucleic acids by shielding them from enzymatic attack. The formation of complexes is mediated by electrostatic interactions beween the protonated (positively charged) amine groups in the carrier backbone and the negatively charged phosphate groups of the nucleotides. Usually, complexes are prepared in neutral pH buffers by mixing equal volumes of a solution of nucleic acids with a solution of the cationic carrier at different complex N/P ratio (N/P ratio = number of carrier nitrogen/DNA phosphate). Unless targeting moieties are present for receptor specific interaction, the major cell entry for cationic complexes is the nonspecific endocytosis by interaction with heparan sulfate proteoglycans (HSPGs) located in the extracellular matrix.32 Therefore, a slightly overall positive charge is usually preferred to generate stable nanosized complexes and to assist in their interaction with cellular membranes.28,30,33 On the other hand, the net positive charge on the surface of these complexes may induce adverse effects (embolism, hepatotoxicity) due to serum protein induced aggregation after intravenous injection of the complexes and cell damage due to an excessive interaction of the complexes with cell membranes.34 Immediately after cell internalization, siRNA or DNA complexes are confined within the endosomes and lysosomes where acidic enzymatic degradation occurs.35 This is one major limitation for the effective intracellular delivery of gene molecules. Certain polyamine-based polymers, mainly polyethylenimine (PEI), polyamidoamine (PAMAM) dendrimers and chitosan, provide the capacity to escape from the endosomes via the “proton sponge” mechanisim.36 “Proton-sponge” polymers contain inner secondary and tertiary amines that exhibit pKa values between neutral and lysosomal acidic pH can prevent acidification of endosomes, causing the osmotic swelling and rupture of the endosomal membrane and triggering the release of complexes into the cytosol. In recent years, the proton sponge hypothesis has been heavily debated especially as to whether the osmotic stress produced by the proton sponge effect can induce the rupture of the endosomes. 37 Several studies concluded that the “proton sponge” effect is not the dominant mechanism in complex endosomal release and pointed out as one plausible mechanism that protonated polymer amines under the acidic pH might interact with the endosomal membrane, inducing its destabilization and promoting the formation of pores for the escape of the complexes entrapped.38,39
In addition to endosomal entrapment, the nuclear membrane is an additional barrier to DNA delivery. DNA can gain access to the nucleus in actively dividing cells that are undergoing a temporary nuclear envelope breakdown. Neither naked DNA nor DNA carriers can passively diffuse through the 10 nm diameter nuclear pore complex. However, cationic assemblies modified with nuclear localization signals can be specifically recognized by importin proteins and actively transported to the nucleus through the nuclear pore complex.40
1.3. Strategies To Improve Cationic Polymeric Carriers
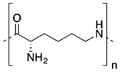
Because of the aforementioned problems, developing a stable and efficient delivery system is a major challenge for gene therapy. Cationic polymers are advantageous in gene delivery due to their (i) high stability, (ii) well-defined size and low polydispersity index, and (iii) great variety of molecular weights, architectures (linear, branched, dendrimeric), and functional groups. At present, polyethylenimine (PEI), poly(L-lysine) (PLL), chitosan, and PAMAM dendrimers, among others, have been developed for effective gene delivery. Their chemical structures are shown in Table 1. However, the balance between the efficacy and toxicity of these systems is still suboptimal. Structure–function relationships showed a correlation between charge density of amine groups on polymer structure and the transfection efficiency of complexes. As outlined in Table 1, high molecular weight (MW) highly charged cationic polymers generate stable complexes and have high transfection efficiencies, but they are toxic. Lower molecular weight polymers have better toxicity profiles but are less efficient.41–43
Table 1.
Properties of Cationic Polymers Commonly Used for Nucleic Acid Delivery: Polylysines (PLL), Chitosan, Polyethylenimine (PEI), and Polyamidoamine (PAMAM) Dendrimers, Polymers with Different MW
| Polymer | Structure | MW | Properties | Ref. |
|---|---|---|---|---|
| PEI |
|
Low MW 2–10 kDa |
|
43, 56–62 |
| High MW > 25 kDa |
|
|||
| PLL |
|
Low MW 4–20 kDa |
|
63–67 |
| High MW > 20 kDa |
|
|||
| PAMAM |
|
G2, G3 2–5 kDa |
|
68–72 |
| G5, G6 20–40 kDa |
|
|||
| G9, G10 > 350 kDa |
|
|||
| Chitosan |
|
Low MW 10 kDa |
|
73–78 |
| High MW |
|
|||
| > 10 kDa |
|
|||
| DDA > 90 % |
|
|||
| DDA 70–90 % |
|
In order to balance cationic polymers’ efficacy and toxicity, different approaches have been investigated. The coating of complexes with hydrophilic polyethylene glycol (PEG) blocks to shield the superficial charge of complexes and hinder their interaction with blood components is a common strategy.44,45 Kissel and co-workers showed that a sufficiently high graft density with at least 2–5 kDa PEG is necessary to achieve a stabilizing effect, prevent opsonization, and avoid rapid clearance.46,47 Another method to produce highly charged but less toxic polymers is to chemically cross-link low MW nontoxic polymers via cleavable reductive or acid labile linkages to enhance their condensation ability and transfection efficacy while reducing their toxicity after cellular uptake.48–50
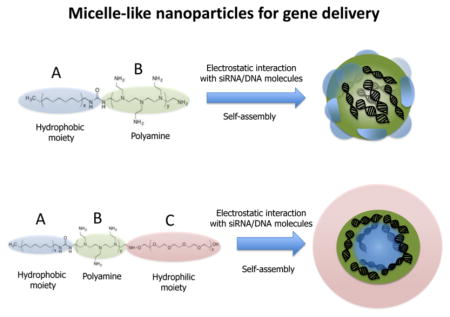
Alternatively, low MW polymers can be modified with hydrophobic moieties including alkanes,51,52 fatty acids,53 and phospholipids.54,55 The resulting cationic amphiphiles can self-assemble in aqueous solution and form micellar structures that, now having clustered cationic groups, possess enough charge density to adsorb nucleic acids into a dense core. In the borderline between micelles and polyplex nanoparticles, these so-called “micelle-like nanoparticles” (MNP) offer unique advantageous features including self-assembly, condensation and protection of nucleic acids, improved cell association and gene transfection, solubilization of hydrophobic and hydrophilic drugs, and safer toxicity profile. In this review, we will discuss the main methods to produce MNP and how the unique properties of MNP may contribute to the successful application of siRNA- and DNA-based therapeutics into the clinic. Recent developments of MNP involving the addition of stimulus-sensitive functions to respond specifically to pathological or externally applied “triggers” (e.g., temperature, pH or enzymatic catalysis, light, or magnetic fields) will be discussed. Finally, we will overview the use of MNP as two-in-one carriers for the simultaneous delivery of different agents (small molecules, imaging agents) and nucleic acid combinations.
2. ASSEMBLY OF MICELLE-LIKE NANOPARTICLES (MNP)
The interaction between nucleic acids and positively charged amphiphiles, either as hydrophobically modified polyamines or as more complex copolymer designs, leads to the formation of micelle-like nanoparticles. (Figure 2). Two driving forces are responsible for MNP formation: (i) the hydrophobic interactions between the hydrophobic segments of the amphiphiles due to the reorganization of the surrounding water and (ii) the attractive electrostatic forces that exist between oppositively charged nucleic acids and cationic amphiphiles. From a thermodynamic perspective, the formation of MNP is an entropically driven process in which hydrophobic and electrostatic contributions are highly cooperative.79–81 The stability of micelles is generally given as their critical micelle concentration (CMC) defined as the concentration of a monomeric amphiphile at which micelles appear. Early studies performed by Ghirlando and co-workers demonstrated that the stability of micellar aggregates had a large influence in the DNA packing process since they can act as counterions of very high valency that interact strongly with DNA and stabilize the system.82 The thermodynamic and structural features of nucleic acid packing in charged micellar aggregates have been studied in detailed in refs 82 and 83.
Figure 2.
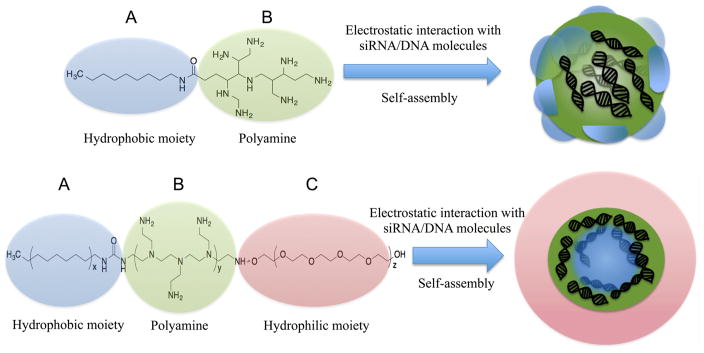
Micelle-like nanoparticles for gene delivery are constructed from amphiphilic diblock AB or triblock ABC copolymers where A counts for the hydrophobic micelle-forming segment, B for the cationic nucleic acid-loading segment, and C for hydrophilic micelle-stabilizer segment.
3. MICELLE-LIKE NANOPARTICLES BASED ON HYDROPHOBICALLY MODIFIED CATIONIC POLYMERS
One major approach to produce MNP is the modification of low MW cationic polymers with hydrophobic moieties to improve their performance as gene carriers while keeping toxicity levels low. As discussed in this section, the attachment of hydrophobic moieties can modify the physicochemical properties of complexes and improve their gene compactation capacity, their binding to the cell surface and cellular uptake, and their escape from the endosomes.
3.1. Hydrophobization of Polyethylenimine (PEI)
High MW PEI is a popular gene transfection agent, both in vitro and in vivo, due to its relatively high efficiency. However, its high toxicity remains a major drawback especially for in vivo applications.84–86 Lower MW PEIs have better toxicity profiles but are far less efficient delivery systems (i.e., less than 5% reduction of gene expression mediated by PEI/siRNA complexes).41,53
The majority of studies are focused on the hydrophobization of low MW PEI (1.8–2 kDa) by the attachment of lipid moieties. This particular MW PEI exhibits great condensation capacity, meaning that only very low N/P ratios = PEI amine/nucleic acid phosphate molar ratios, N/P ~4, are necessary for the complete compaction and adsorption of siRNA/DNA in polyplexes. In addition, it has a very low toxicity. While the IC50 for PEI 25 kDa = 15 μg/mL, PEI 1.8 kDa concentrations of up to 250 μg/mL gave cell viabilities around 80–100%.54
Early studies by Kim and colleagues showed for the first time that the conjugation of PEI with cholesterol improved DNA transfection to mammalian cells.87,88 They demonstrated that cholesterol modification of low MW PEI gave rise to “water-soluble lipopolymers” (WSLP) that greatly increased plasmid DNA transfection compared to PEI 1.8 kDa and self-assembled into micelles (CMC = 1.43 mg/mL, 7.1 × 10−4 M).87,88 Still, the novel lipopolymers showed a discrete improvement of gene expression efficacy corresponding to 15% green fluorescent protein (GFP) positive cells as compared to 0.5% and 5% GFP positive cells for PEI 1.8 and PEI 25 complexes, respectively.
Dewa and co-workers developed three types of polyamine-dialkyl phosphate that were synthesized via the dimerized dialkyl phosphate as synthetic intermediate and evaluated for plasmid DNA transfection.89 Among the polyamine portions that were tested (spermine, spermidine, and PEI 1.8 kDa), the MNP constructed with diacetyl phosphate–PEI showed the highest transfection efficiency. Later, the authors demonstrated that the transgene expression was enhanced by 2–3 orders when the MNP formulation included cholesterol and the phospholipids dioleoylphosphatidylethanolamine (DOPE) or dipalmitoylphosphatidylcholine (DPPC). At a conjugate/cholesterol/DOPE 1:1:1 mol ratio, the transfection efficacy of MNP was 3 times larger than the corresponding conjugate only based MNP. The authors attributed this increment in gene transfection to the bilayer structure of the lipid/conjugate/DNA mixture in the MNP that may favor the interaction with cell membranes and to the fusogenic properties of DOPE that facilitates fusion and destabilization of endosomal membranes. 90
Regarding siRNA delivery, the modification of PEI with different fatty acids or alkane chains was reported to improve gene downregulation efficiency that was dependent mostly on the degree of lipid substitution in the modified PEI and the conjugate-to-siRNA ratio. These features were associated with the siRNA binding affinity and other complex properties such as surface charge, which in turn affects uptake and intracellular trafficking of such complexes.53,91 For example, Schroeder and collaborators performed gel retardation studies showing that the binding affinity of alkylated PEI derivatives to siRNA decreased as the conjugation levels increased and that, due to this reduction of the binding affinity within the complex, the siRNA was readily released into the cytoplasm after cellular internalization.52
Uludag’s group reported on a fatty acid substituted PEI 2 kDa library for DNA92,93 and siRNA delivery.53 They showed that lipid-PEI substitution (1:1) maintains the condensation capacity of PEI and improves the cellular uptake of the complexes. Regarding silencing efficacy, a 30% functional downregulation was observed using caprylic (C8:0) and palmitic (C:16:0)-PEI derivatives and a 60% downregulation using linolenic(C18:1)-PEI derivative. As for their cytotoxicity, fatty acid–PEI conjugates complexed with siRNA (not free polymer) prepared at polymer concentration of 10 μg/mL resulted in cell viabilities of 60–80% depending on the lipid moiety, with higher toxicities for longer unsaturated fatty acids than saturated ones.
In a different study by the same group, computer simulations were used to understand the molecular mechanism of lipid modified PEI and DNA aggregation and condensation.94 It was found that the lipids associated significantly one with another, which linked the lipid modified PEIs and served as a mechanism for aggregating the DNAs and stabilizing the formed MNP. In addition, the molecular dynamics simulations showed that some lipid tails on the lipid modified PEIs stayed at the periphery of the DNA complex. The finding of the external location of the lipids in the complex is quite important since it provides a feasible explanation for the hydrophobic interactions of this kind of carrier and supports previous experimental observations of their better internalization through cellular membranes compared with native PEI complexes.
Our group has also reported on phospholipid-modified PEI.54,55,95–97 Phospholipid conjugation dramatically improved the gene silencing of PEI 1.8 kDa that was otherwise ineffective. We showed that MNP based on the combination of phosphatidylcholine modified PEI (PC-PEI) with PEG and lipids delivered plasmid DNA to a distal tumor in vivo.97 These MNP combined the favorable properties of the low MW PEI 1.8 kDa (nucleic acid condensation, low cytotoxicity,) with those of PEG-stabilized nanocarriers (in vivo stability, prolonged blood circulation) that resulted in MNP tumor accumulation and transgene expression upon intravenous injection. In addition, the MNP were suitable for siRNA delivery, although the gene silencing efficacy of (PC-PEI) MNP was low.98 We optimized this formulation and constructed MNP based on phosphatidylethanolamine modified PEI (DOPE-PEI) that showed higher siRNA silencing transfection than PC-PEI-based ones (75% vs 20% downregulation). The optimized MNP mediated a downregulation of P-glycoprotein (P-gp) expression that overcame doxorubicin (DOX) resistance in breast cancer cells.95 Further evaluation in vivo confirmed the utility of MNP(DOPE-PEI) for systemic delivery of anti-P-gp siRNA to resistant breast tumors.99 They had small particle sizes (<150 nm) compatible with parenteral administration and showed improved colloidal stability when lipidated PEG (PEG-PE) was incorporated in the MNP formulation by hydrophobic interactions. PEGylated MNP showed prolonged circulation and improved siRNA tumoral delivery via enhanced permeability and retention (EPR) effect as compared with non- PEGylated formulations. Regardless of the presence or the absence of PEG, the nanopreparations delivered sufficiently high amounts of siRNA to mediate the specific P-gp downregulation and the sensitization of resistant tumors to noneffective doses of DOX. Simultaneous or sequential administration of the anti-P-gp siRNA formulation and DOX was equally effective in inhibiting tumor growth and was well-tolerated by the animals even after repeated dosing.
In looking for structure/activity relationships for MNP improvement, we synthesized a third conjugate of dipalmitoyl-phosphatidylethanolamine (C16:0)-modified PEI (DPPE-PEI), which differs from DOPE-PEI (DOPE: C18:1) in the absence of unsaturations in the fatty chains of the phospholipid. We compared the PC-PEI, DOPE-PEI, and DPPE-PEI potential as siRNA carriers and the effect of the phospholipid moiety on the in vitro performance.54 Phospholipid conjugation did not change the size, superficial charge, or siRNA compaction within the complexes but had a large impact on their transfection efficacy (60%, 30%, and 5% decrease of GFP expression respectively for DOPE-, DPPE-, and PC-PEI). We attributed these results to the self-assembly of DOPE-PEI and DPPE-PEI into micellar aggregates that significantly improved PEI’s interaction with cell membranes and the siRNA internalization. The CMC values of the conjugates were 97 μg/mL and 75 μg/mL for DOPE-PEI and DPPE-PEI, respectively, indicating a high micellation capacity. For comparison, PEG-PE amphiphiles, which are extensively used in micelle formulation for drug delivery, have CMC values of 43 μg/mL.100 Nonmicellizable PC-PEI-based complexes did not associate with cell membranes and displayed low gene downregulation. Although DPPE-PEI and DOPE-PEI carriers showed similar cell membrane interaction and siRNA uptake, DOPE-PEI displayed a more effective gene silencing. Our mechanistic studies showed differences in their cytosolic trafficking and pointed out the advantage of the DOPE conjugation over other lipidic moieties for improved intracellular trafficking of siRNA complexes due to a greater endosomal escape.100
3.2. Hydrophobization of Polyamidoamine (PAMAM) Dendrimer
Among dendritic polymers, polyamidoamine (PAMAM) dendrimers are the most studied for gene delivery.28 These dendrimers are a relatively new class of synthetic polymers that have a hyperbranched, globular architecture, together with defined monodisperse MW, and numerous accessible terminal groups which can be functionalized for specific delivery purpose along with the ability to encapsulate compounds within cavities. Their synthesis requires an iterative multistep reaction sequence, from which the molecule is built up from the core to the periphery. Each complete reaction sequence results in a new generation with tertiary amines at the branched points and primary amines at the termini that are protonated at physiological pH. As other cationic polymers, PAMAM dendrimers have been shown to be highly effective in transfecting plasmid DNA or siRNA into a variety of cell types.70,101,102 Additionally, in vivo studies suggest that they are not immunogenic or carcinogenic.71,103 The highest dendrimer generations (e.g., G7, G9) are known to be highly effective in vitro and in vivo but present certain toxicities. In contrast, lower generations (<G4) possess a better balance between efficacy and toxicity.28,104 The hydrophobization of noneffective nontoxic dendrimers with lipid moieties, dexamethasone105 or their inclusion into liposomes106,107 are strategies to improve the transfection of these carriers.
The work of Kono and co-workers on alkyl modified dendrimers illustrates how minor changes in the architecture of PAMAM-based MNP can have a great impact in the potency of the carrier. First, they modified G1 to G4 PAMAM dendrimers with two dodecyl chains (C12) and evaluated the resulting amphiphiles as DNA carriers.108 The DNA condensation and transfection activity increased concomitantly with the dendrimer generation and was higher than that of nonmodified dendrimers with the exception of G1, which was ineffective regardless of the C12 modification. Later, they showed that elongation of the alkyl chains from C12 to C18 in modified G3 PAMAM dendrimers led to smaller complexes (hydrodynamic diameter of 2 μm vs 250 nm) bearing highly condensed DNA and high serum resistance.109 Surprisingly, C18 modification of G1 PAMAM dendrimers converted the inefficient G1 into the most potent carrier of the set.110 To further optimize the transfection efficiency, the effect of unsaturated chains in diC18-G1 PAMAM was evaluated.111 Unsaturated C18 chains gave stable and smaller complexes that achieved high endosomal escape through the synergy of a proton sponge effect derived from the tertiary amines of the dendron moiety and a high fusion ability derived from hydrophobic and flexible unsaturated chains. This study and others54,112 suggest the advantages of hydrophilic moieties resembling fusogenic helper lipids (i.e., DOPE, C18:1) for the improvement of MNP as gene carriers.
Liu, Yu, and co-workers also investigated PAMAM-based MNP. They modified a set of dendrimers (G1, G2, G3) with one (mono-C18)86 or two alkyl chains (di-C18).113 Interestingly, mono-C18 G1 was a very ineffective siRNA carrier, in sharp contrast to the powerful di-C18 G1 previously reported.110 The most powerful carriers were those constructed with G3. For instance mono-C18 G3 showed a CMC ~14 μg/mL and formed 100 nm sized complexes that protected siRNA from enzymatic degradation. Such complexes delivered siRNA targeting heat shock protein 27 (Hp27) in vitro to P-13 prostate cells and in vivo to P-13 xenografted tumors with high silencing efficiency and anticancer activity.110
3.3. Hydrophobization of Polylysine (PLL)
Poly-L-lysine (PLL) has been an extensively investigated polymer for the construction of cationic assemblies.63,114–116 PLL has a biocompatible and biodegradable nature as a peptide, which is an advantage for in vivo use. It has the ability to pack nucleotides into complexes at physiological pH (pKa ~10.0). The biological and physicochemical characteristics of PLL assemblies depend on their MW. Inefficient gene transfer was reported with a low MW (~5,000 Da) complex.66,117–119 Their comparatively low transfection efficiency, poor circulatory halflives, high toxicity, and no buffering ability that prevents escape from the endosome are the main reasons for PLL’s insufficient gene transfer.120–122
Early studies showed the advantages of DOPE conjugation for the improvement of noneffective low MW PLL (~3 kDa) transfection efficiency in cultured mammalian cells.112 Also, the modification of PLL (~14 kDa) with D, L-lactic-co-glycolic acid (PLGA) decreased PLL toxicity (IC50 ~100 μg/mL vs 540 μg/mL).123 The PLL-PLGA amphiphile had a CMC of 9.6 μg/mL and self-assembled into micellar aggregates with sizes of 150 nm. Condensation of DNA produced ~200 nm sized MNP with slightly better gene transfer capacity than native PLL. Utilizing the same PLL-PLGA, Blum and co-workers used a double emulsion method (w/o/w) to fabricate nanoparticles acting as a depot for controlled release of DNA. Variations in the preparation methods led to changes in the DNA release profiles from the particles (i.e., burst vs linear). The DNA encapsulated was bioactive after the fabrication process, however, the transfection efficiency of the particles was very low.124
In another study, several endogenous lipids were incorporated into PLL to serve as effective DNA carrier.125 PLL of low and high MW (4 kDa and 25 kDa) were selected to study the influence of the polymer size on the DNA delivery. Endogenous lipids from variable chain lengths (C8 to C18) were used to study the influence of chain length and the degree of substitution. The transfection efficiency of the amphiphiles was positively correlated with the degree of lipid substitution and the size of the PLL. However, no particular trend was observed with regard to the chain length. Lipid-modified high MW PLL demonstrated DNA transfection of bone marrow stromal cells125 and fibroblasts.126
3.4. Hydrophobization of Chitosan
Chitosan is a natural biodegradable polymer obtained by chitin deacetylation. Due to its low immunogenicity and low toxicity, it has received attention in several different fields of pharmaceutical formulation including gene delivery.78,127–132 The major drawbacks affecting the transfection efficiency of chitosan include its insolubility at physiological pH and the deficient release of the cargo in the cytosol due to excessive interaction between protonated amines from chitosan and phosphate groups from nucleic acids.
As has been shown for other cationic polymers, the modification of chitosan with hydrophobic moieties such as lipid chains133 or bile acids134 can enhance the attachment of complexes to cell surfaces, can facilitate endocytic uptake, and, in the case of chitosan, may assist unpacking of DNA from chitosan complexes due to the weakening of electrostatic attractions between DNA and chitosan.135 With this purpose, low MW chitosan was grafted with C18 chains bearing increasing saturations in the chain.136 The CMC of the chitosan derivatives was in the range of 15–60 μg/mL. The lipid modification did not affect the low cytotoxicity of chitosan and significantly improved gene transfection. As reported for other polymers, the presence of 1 or 2 double bounds in the lipid chain grafted to chitosan produced greater transfection efficiencies than saturated chains, whereas higher number of unsaturations produces no improvement.
4. MICELLE-LIKE NANOPARTICLES BASED ON TRIBLOCK COPOLYMERS
The electrostatic interactions between siRNA/DNA molecules and cationic amphiphiles in MNP are inevitably interfered with in vivo due to the abundance of charged biomolecules. Upon injection, charged nanocarriers are opsonized by blood proteins,137 following which they can be recognized by the cells of the MPS and cleared from the circulation.138 The properties that lipid-grafting renders to MNP (improved condensation, protection against nuclease degradation, and stability in serum) are not enough to overcome the in vivo barriers. To compensate for the in vivo low stability, MNP can be produced as complex triblock copolymer designs in which a hydrophilic moiety is added to the cationic and the hydrophobic segment. As discussed in this section, the inclusion of an external hydrophilic layer (i.e., PEG) in the MNP design can provide enhanced colloidal stability and reduce the interaction with serum proteins. In addition, this is a common approach used to transform nanocarriers into stable and long-circulating ones139–142 and to promote their passive accumulation in tumors or inflamed areas.143 Under certain pathological states like inflammation, infarcts, and tumors, the vascular endothelial lining of tends to become more permeable, leading to “gaps” in the lining. Matsumura and Maeda were the first to show that nanoparticles are able to extravasate through these gaps to reach the tumor space and stay there due to the poor lymphatic drainage of tumors.144 This phenomenon was later termed as enhanced permeability and retention (EPR) effect.
MNP were prepared based on ABC triblock copolymers consisting of PEG, poly-ε-caprolactone (PCL), and low MW PEI 2.5 kDa.145 The effect of varying PEG MW (2 kDa, 5 kDa, and 500 Da) and PCL MW (10 kDa, 5 kDa, 2.4 kDa) on the size, stability, and toxicity of the amphiphiles was studied. Increasing the MW of the PCL in the amphiphiles led to larger particles (>100 mn), whereas increasing the MW of PEG chains stabilized the carrier formation and gave smaller micellar structures (~40 nm). As suggested by the lack of aggregation of the particles at high salt concentrations or in the presence of albumin, the PEG block prevented the excessive agglomeration of PCL block. Longer PEG chains (5 kDa) also resulted in reduced toxicity as they produce particles with thicker PEG shells for effective charge shielding and decreased cell interaction. Later, PEG-PLC-PEI particles were coloaded with siRNA and quantum dots (QD) combine nucleic acid delivery and imaging capabilities in a single carrier.146 QD were encapsulated in the core of the MNP by means of the QD’s small size and hydrophobicity. The QD-siRNA-MNP were internalized in the pulmonary epithelium upon intratracheal instillation and mediated gene silencing, MNP with thinner shells (PEG MW 500 Da) being the more effective ones. The fact that thicker PEG shells provided better biocompatibility but thinner PEG shells provided better efficacy brings out the contradictory effect of PEGylation, usually called the “PEG dilemma”,147 and the need to render MNP with stimulus-sensitive detachable protective PEGs (see section 5).
The effect of certain changes in the ABC design was also investigated in PEG-PLL-polyaspartamide triblocks.148 Their stability and in vitro siRNA delivery was compared with that of randomly hydrophobized triblock particles and with non- PEGylated diblock particles. Non-PEGylated diblock MNP showed the best cellular internalization due to the absence of PEG steric hindrance but aggregated fast in the presence of serum. Random addition of hydrophobic moieties to the cationic core of MNP produced particles that did not aggregate immediately but disintegrated overtime and did not retain siRNA in their core. Only a truly ABC designed MNP stably encapsulated siRNA without dissociation or aggregation and achieved the best performance in terms of gene silencing and cytotoxicity.
Our group recently synthesized a triblock copolymeric MNP system, G4 PAMAM- PEG (2 kDa)-DOPE. G4 PAMAM dendrimer was utilized as a cationic source for efficient siRNA condensation; DOPE provided optimum hydrophobicity and compatible cellular interaction for enhanced cell penetration; PEG rendered flexibility to the G(4)-D for easy accessibility of siRNA for condensation.149 The triblock copolymer was mixed with PEG (5 kDa)-DOPE system that improved the micellization of the MNP system from CMC values of 5 × 10−5 to values of 2.5 × 10−5. Such improved MNP formed stable polyplexes with siRNA at low N/P and showed excellent serum stability and a significantly higher cellular uptake of siRNA that resulted in target protein downregulation when compared to the G4 PAMAM dendrimer. Moreover, the mixed micellar system was able to incorporated DOX with high loading efficiency. The combination of dendrimer and polymeric micelles in a single MNP nanocarrier resulted in superior properties in terms of drug loading and siRNA drug codelivery, which could address the challenges of drug and siRNA codelivery for therapeutic purposes, especially in multidrug resistant cancers.
Self-assembled MNP made of amphiphilic ABC copolymers of PEG-PCL-poly(2-aminoethylethylene phosphate) (PPEEA) were evaluated for siRNA delivery in vitro and in vivo.150,151 The MNP were small (50 nm) and negatively charged and produced significant reporter gene silencing and low toxicity in normal144 and cancer cells.145 Systemic administration of such MNP loaded with siRNA targeting acid ceramidase oncogene produced significant apoptosis and growth inhibition in breast cancer xenografts.145 In a different study, a PEG-PCL-PPEEA system was coloaded with apoptotic anti-polokinase-1 siRNA and paclitaxel. The simultaneous delivery of siRNA and chemotherapy in MNP resulted in synergistic anticancer activity toward melanoma cancer in vitro and in vivo. Importantly, it was demonstrated that a physical mixture of anti-polokinase-1 siRNA MNP and paclitaxel MNP could not deliver both drugs to the same cells in the tumor mass and, thus, lesser synergistic effects were possible.152 Alternatively, a MNP system was prepared by mixing PCL-PPEEA and PCL-PEG diblocks to form mixed micelles without the need of difficult synthetic protocols and with easy tuning of size, zeta potential, and PEG density by simple changes in the molar ratio of the two diblock components.153,154 The mixed system was loaded with siRNA targeting hypoxia-inducible factor 1α (HIF-1α) and transfected to prostate cancer cells (PC-3). The treatment suppressed the migration and proliferation of PC-3 cells and prevented VEGF secretion under hypoxia conditions. In vivo, HIF-1α siRNA MNP inhibited tumor growth and sensitized prostate cancer tumors to DOX chemotherapy. Finally, liver targeting of MNP was proposed by N-acetylgalactosamine modification of PCL-PEG diblock (PCL-PEG- Gal).147
5. MICELLE-LIKE NANOPARTICLES WITH STIMULUS SENSITIVITY
MNP can be constructed to release their contents in response to specific pathological “triggers” which are unique to sites of disease (e.g., pH or enzymatic catalysis) or externally applied ones such temperature or magnetic fields.155 The construction of MNP with stimulus-responsive “detachable” PEG shells has been a major approach. As shown in previous sections, the PEG shell in MNP prolongs their blood circulation by reducing their association with plasma proteins and tissues nonspecifically. However, once the target site is reached, the PEG protective function is no longer needed. Moreover, it may prevent the association of the carrier with the cell surface. To deal with this inconvenience, detachable protective coatings are employed. A cleavable PEG coating may provide the prolonged circulation time of MNP, and reconstitute the cellular affinity for such carriers after arriving at the target location by detachment of the protective polymer chains.
To improve the delivery of phospholipid modified PEI-MNP54,95,97 to the relatively acidic tumor microenvironment, 156 two strategies for PEG detachment from MNP were investigated. In the first, MNP were assembled by the mixing of phospholipid modified PEI and phospholipid modified PEG (PEG-PE) diblocks. The resulting particles exhibited a neutral surface charge, resistance to salt-induced aggregation, and good DNA transfection activity in the presence of serum. The use of the low-pH-degradable PEG-hydrazone-PE produced particles with transfection activity sensitive to changes in pH which were proposed for site-specific transfection of acidic tumors.55 In the second strategy, MNP constructed with a cleavable phospholipid- hydrazone-PEI, PEG-PE, and lipids were evaluated for in vitro and in vivo DNA delivery. The pH-cleavable MNP showed higher cellular association at acidic pH and exhibited comparable in vivo stability and tumor accumulation to that of noncleavable MNP.97
Xiong and co-workers developed MNP for colading of siRNA and doxorubicin: DOX that combined passive and active cancer targeting, cell membrane translocation, and pH-triggered drug release.157 MNP were assembled with degradable poly(ethylene oxide)-block-poly(ε-caprolactone) (PEO-b- PCL) block copolymers. The PCL block was used to incorporate short polyamines for complexation with siRNA or to chemically conjugate DOX via a pH-sensitive hydrazone linkage. In addition the MNP were modified with integrin αvβ3-specific ligand for active cancer targeting and a cell-penetrating peptide for enhanced internalization. The MNP simultaneously deliver edDOX and anti-P-gp siRNA to their intracellular targets, leading to the inhibition of P-gp-mediated DOX resistance in vitro and targeting of αvβ3-positive tumors in vivo.
Enzymatic-sensitive MNP were proposed for enhanced tumor cell internalization and synergistic antitumor activity of coloaded siRNA and paclitaxel. A matrix metalloproteinase-2 sensitive (MMP-2) self-assembly copolymer (PEG-pp-PEI-PE) was developed.158,159 The siRNA in PEI corona and paclitaxel was solubilized in the hydrophobic core of the MMP-2 cleavable MNP. Tumor overexpression of MMP-2 is considered a biomarker in many cancer types and has been used as a strategy for tumor targeted delivery via enzymatic-triggered release.155 Upon systemic injection, PEG shielded MNP delivered the dual cargo to A549 MMP-2 expressing tumors via the EPR effect. Once in the tumor site, the MMP-2 mediated cleavage, deshielded PEG, and exposed PEI, leading to the enhanced tumor internalization of the nanoparticles.
6. CONCLUSIONS
Although micelle-like nanoparticles (MNP) have long been used in gene and drug delivery (initial developments were reported in the early 1990s), new challenges in the field have renewed research interest in these carriers. In the borderline between micellar and nanopaticulate systems, MNP contain many important features, i.e., tunable size and superficial charge, effective nucleic acid condensation, enhanced cellular interaction with low cytotoxicity, improved intracellular trafficking via endosomal escape mechanism, and improved pharmacokinetics due to decreased opsonization and clearance. Also, a MNP design based on diblock or triblock copolymers offers endless possibilities for arrangement and modification of different functional segments (micelle forming condensing and stabilizer segments) including stimulus-sensitive or targeting functions. Finally, MNP capacity to be simultaneously loaded with gene molecules and imaging agents or small chemical entities expands the possibilities of these carriers for synergistic combinatorial therapies and theranostic applications.
Supplementary Material
Acknowledgments
The paper was supported in part by NIH Grant 5U54CA151881-012. We also thank Dr. William Hartner for his helpful advice in editing the manuscript.
Footnotes
Notes
The authors declare no competing financial interest.
Schematic representation of the mechanism of plasmid DNA (pDNA) and small interfering RNA (siRNA) action (Figure S1). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Anderson WF, Blaese RM, Culver K. The ADA human gene therapy clinical protocol: Points to Consider response with clinical protocol, July 6, 1990. Hum Gene Ther. 1990;1(3):331–62. doi: 10.1089/hum.1990.1.3-331. [DOI] [PubMed] [Google Scholar]
- 2.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 - an update. J Gene Med. 2013;15(2):65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Patel NR, Pattni BS, Abouzeid AH, Torchilin VP. Nanopreparations to overcome multidrug resistance in cancer. Adv Drug Delivery Rev. 2013;65(13–14):1748–62. doi: 10.1016/j.addr.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura K, Abu Lila AS, Matsunaga M, Doi Y, Ishida T, Kiwada H. A double-modulation strategy in cancer treatment with a chemotherapeutic agent and siRNA. Mol Ther. 2011;19(11):2040–7. doi: 10.1038/mt.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang SH, Cho HJ, Shim G, Lee S, Kim SH, Choi HG, Kim CW, Oh YK. Cationic liposomal co-delivery of small interfering RNA and a MEK inhibitor for enhanced anticancer efficacy. Pharm Res. 2011;28(12):3069–78. doi: 10.1007/s11095-011-0569-4. [DOI] [PubMed] [Google Scholar]
- 7.Salzano G, Riehle R, Navarro G, Perche F, De Rosa G, Torchilin VP. Polymeric micelles containing reversibly phospholipid-modified anti-survivin siRNA: a promising strategy to overcome drug resistance in cancer. Cancer Lett. 2014;343(2):224–31. doi: 10.1016/j.canlet.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su B, Cengizeroglu A, Farkasova K, Viola JR, Anton M, Ellwart JW, Haase R, Wagner E, Ogris M. Systemic TNFalpha gene therapy synergizes with liposomal doxorubicine in the treatment of metastatic cancer. Mol Ther. 2013;21(2):300–8. doi: 10.1038/mt.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Q, Xia Y, Wang CH, Pack DW. Monodisperse double-walled microspheres loaded with chitosan-p53 nanoparticles and doxorubicin for combined gene therapy and chemotherapy. J Controlled Release. 2012;163(2):130–5. doi: 10.1016/j.jconrel.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baguley BC. Multidrug Resistance in Cancer. In: Zhou J, editor. Multi-Drug Resistance in Cancer. Vol. 596. Humana Press; 2010. pp. 1–14. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Hao J, Wang L, Li Y. Coexpression of invasive markers (uPA, CD44) and multiple drug-resistance proteins (MDR1, MRP2) is correlated with epithelial ovarian cancer progression. Br J Cancer. 2009;101(3):432–40. doi: 10.1038/sj.bjc.6605185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee TB, Park JH, Min YD, Kim KJ, Choi CH. Epigenetic mechanisms involved in differential MDR1 mRNA expression between gastric and colon cancer cell lines and rationales for clinical chemotherapy. BMC Gastroenterol. 2008;8:33. doi: 10.1186/1471-230X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonessa F, Clarke R. ATP binding cassette transporters and drug resistance in breast cancer. Endocr-Relat Cancer. 2003;10(1):43–73. doi: 10.1677/erc.0.0100043. [DOI] [PubMed] [Google Scholar]
- 14.Abbasi M, Lavasanifar A, Berthiaume LG, Weinfeld M, Uludag H. Cationic polymer-mediated small interfering RNA delivery for P-glycoprotein down-regulation in tumor cells. Cancer. 2010;116(23):5544–54. doi: 10.1002/cncr.25321. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Zhao G, Liu J, Ma N, Chivukula P, Perelman L, Okada K, Chen Z, Gough D, Yu L. Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J Controlled Release. 2009;140(3):277–83. doi: 10.1016/j.jconrel.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Peng Z, Xiao Z, Wang Y, Liu P, Cai Y, Lu S, Feng W, Han ZC. Reversal of P-glycoprotein-mediated multidrug resistance with small interference RNA (siRNA) in leukemia cells. Cancer Gene Ther. 2004;11(11):707–12. doi: 10.1038/sj.cgt.7700738. [DOI] [PubMed] [Google Scholar]
- 17.Yadav S, van Vlerken LE, Little SR, Amiji MM. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother Pharmacol. 2009;63(4):711–22. doi: 10.1007/s00280-008-0790-y. [DOI] [PubMed] [Google Scholar]
- 18.Tsouris V, Joo MK, Kim SH, Kwon IC, Won YY. Nano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancers. Biotechnol Adv. 2014;32(5):1037–50. doi: 10.1016/j.biotechadv.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Davidson BL, McCray PB., Jr Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;12(5):329–40. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432(7014):173–8. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 21.Malek A, Merkel O, Fink L, Czubayko F, Kissel T, Aigner A. In vivo pharmacokinetics, tissue distribution and underlying mechanisms of various PEI(-PEG)/siRNA complexes. Toxicol Appl Pharmacol. 2009;236(1):97–108. doi: 10.1016/j.taap.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Gao S, Dagnaes-Hansen F, Nielsen EJ, Wengel J, Besenbacher F, Howard KA, Kjems J. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol Ther. 2009;17(7):1225–33. doi: 10.1038/mt.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawabata K, Takakura Y, Hashida M. The Fate of Plasmid DNA after Intravenous-Injection in Mice - Involvement of Scavenger Receptors in Its Hepatic-Uptake. Pharm Res. 1995;12(6):825–830. doi: 10.1023/a:1016248701505. [DOI] [PubMed] [Google Scholar]
- 24.Mellott AJ, Forrest ML, Detamore MS. Physical non-viral gene delivery methods for tissue engineering. Ann Bio Eng. 2013;41(3):446–68. doi: 10.1007/s10439-012-0678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giacca M, Zacchigna S. Virus-mediated gene delivery for human gene therapy. J Controlled Release. 2012;161(2):377–88. doi: 10.1016/j.jconrel.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Tros de Ilarduya C, Sun Y, Duzgunes N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40(3):159–70. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discovery. 2005;4(7):581–93. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 28.Dufes C, Uchegbu IF, Schatzlein AG. Dendrimers in gene delivery. Adv Drug Delivery Rev. 2005;57(15):2177–202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Djurovic S, Iversen N, Jeansson S, Hoover F, Christensen G. Comparison of nonviral transfection and adeno-associated viral transduction on cardiomyocytes. Mol Biotechnol. 2004;28(1):21–31. doi: 10.1385/MB:28:1:21. [DOI] [PubMed] [Google Scholar]
- 30.Kircheis R, Wightman L, Wagner E. Design and gene delivery activity of modified polyethylenimines. Adv Drug Delivery Rev. 2001;53(3):341–58. doi: 10.1016/s0169-409x(01)00202-2. [DOI] [PubMed] [Google Scholar]
- 31.Morille M, Passirani C, Dufort S, Bastiat G, Pitard B, Coll JL, Benoit JP. Tumor transfection after systemic injection of DNA lipid nanocapsules. Biomaterials. 2011;32(9):2327–33. doi: 10.1016/j.biomaterials.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 32.Ruponen M, Ronkko S, Honkakoski P, Pelkonen J, Tammi M, Urtti A. Extracellular glycosaminoglycans modify cellular trafficking of lipoplexes and polyplexes. J Biol Chem. 2001;276(36):33875–80. doi: 10.1074/jbc.M011553200. [DOI] [PubMed] [Google Scholar]
- 33.Lu JJ, Langer R, Chen J. A novel mechanism is involved in cationic lipid-mediated functional siRNA delivery. Mol Pharmaceutics. 2009;6(3):763–71. doi: 10.1021/mp900023v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballarin-Gonzalez B, Howard KA. Polycation-based nanoparticle delivery of RNAi therapeutics: Adverse effects and solutions. Adv Drug Delivery Rev. 2012;64(15):1717–29. doi: 10.1016/j.addr.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, Narang AS, Mahato RI. Subcellular fate and offtarget effects of siRNA, shRNA, and miRNA. Pharm Res. 2011;28(12):2996–3015. doi: 10.1007/s11095-011-0608-1. [DOI] [PubMed] [Google Scholar]
- 36.Behr JP. The proton sponge: A trick to enter cells the viruses did not exploit. Chimia. 1997;51(1–2):34–36. [Google Scholar]
- 37.Won YY, Sharma R, Konieczny SF. Missing pieces in understanding the intracellular trafficking of polycation/DNA complexes. J Controlled Release. 2009;139(2):88–93. doi: 10.1016/j.jconrel.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yue Y, Jin F, Deng R, Cai J, Dai Z, Lin MC, Kung HF, Mattebjerg MA, Andresen TL, Wu C. Revisit complexation between DNA and polyethylenimine–effect of length of free polycationic chains on gene transfection. J Controlled Release. 2011;152(1):143–51. doi: 10.1016/j.jconrel.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther. 2013;21(1):149–57. doi: 10.1038/mt.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam AP, Dean DA. Progress and prospects: nuclear import of nonviral vectors. Gene Ther. 2010;17(4):439–47. doi: 10.1038/gt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grayson AC, Doody AM, Putnam D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm Res. 2006;23(8):1868–76. doi: 10.1007/s11095-006-9009-2. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa M, Takemura S, Takakura Y, Hashida M. Targeted delivery of plasmid DNA to hepatocytes in vivo: optimization of the pharmacokinetics of plasmid DNA/galactosylated poly(L-lysine) complexes by controlling their physicochemical properties. J Pharmacol Exp Ther. 1998;287(1):408–15. [PubMed] [Google Scholar]
- 43.Gebhart CL, Kabanov AV. Evaluation of polyplexes as gene transfer agents. J Controlled Release. 2001;73(2–3):401–16. doi: 10.1016/s0168-3659(01)00357-1. [DOI] [PubMed] [Google Scholar]
- 44.Needham D, McIntosh TJ, Lasic DD. Repulsive interactions and mechanical stability of polymer-grafted lipid membranes. Biochim Biophys Acta. 1992;1108(1):40–8. doi: 10.1016/0005-2736(92)90112-y. [DOI] [PubMed] [Google Scholar]
- 45.Torchilin VP, Omelyanenko VG, Papisov MI, Bogdanov AA, Jr, Trubetskoy VS, Herron JN, Gentry CA. Poly(ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim Biophys Acta. 1994;1195(1):11–20. doi: 10.1016/0005-2736(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 46.Merdan T, Kunath K, Petersen H, Bakowsky U, Voigt KH, Kopecek J, Kissel T. PEGylation of poly(ethylene imine) affects stability of complexes with plasmid DNA under in vivo conditions in a dose-dependent manner after intravenous injection into mice. Bioconjugate Chem. 2005;16(4):785–92. doi: 10.1021/bc049743q. [DOI] [PubMed] [Google Scholar]
- 47.Petersen H, Fechner PM, Martin AL, Kunath K, Stolnik S, Roberts CJ, Fischer D, Davies MC, Kissel T. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjugate Chem. 2002;13(4):845–54. doi: 10.1021/bc025529v. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Jiang X, Xu L, Wang X, Hennink WE, Zhuo R. Novel reduction-responsive cross-linked polyethylenimine derivatives by click chemistry for nonviral gene delivery. Bioconjugate Chem. 2010;21(10):1827–35. doi: 10.1021/bc100191r. [DOI] [PubMed] [Google Scholar]
- 49.Yu ZQ, Yan JJ, You YZ, Zhou QH. Bioreducible and acid-labile poly(amido amine)s for efficient gene delivery. Int J Nanomed. 2012;7:5819–32. doi: 10.2147/IJN.S37334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jere D, Jiang HL, Arote R, Kim YK, Choi YJ, Cho MH, Akaike T, Cho CS. Degradable polyethylenimines as DNA and small interfering RNA carriers. Expert Opin Drug Delivery. 2009;6(8):827–34. doi: 10.1517/17425240903029183. [DOI] [PubMed] [Google Scholar]
- 51.Teo PY, Yang C, Hedrick JL, Engler AC, Coady DJ, Ghaem-Maghami S, George AJ, Yang YY. Hydrophobic modification of low molecular weight polyethylenimine for improved gene transfection. Biomaterials. 2013;34(32):7971–9. doi: 10.1016/j.biomaterials.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Schroeder A, Dahlman JE, Sahay G, Love KT, Jiang S, Eltoukhy AA, Levins CG, Wang Y, Anderson DG. Alkane-modified short polyethyleneimine for siRNA delivery. J Controlled Release. 2012;160(2):172–6. doi: 10.1016/j.jconrel.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aliabadi HM, Landry B, Bahadur RK, Neamnark A, Suwantong O, Uludag H. Impact of lipid substitution on assembly and delivery of siRNA by cationic polymers. Macromol Biosci. 2011;11(5):662–72. doi: 10.1002/mabi.201000402. [DOI] [PubMed] [Google Scholar]
- 54.Navarro G, Essex S, Sawant RR, Biswas S, Nagesha D, Sridhar S, de Ilarduya ICT, Torchilin VP. Phospholipid-modified polyethylenimine-based nanopreparations for siRNA-mediated gene silencing: implications for transfection and the role of lipid components. Nanomedicine. 2014;10(2):411–9. doi: 10.1016/j.nano.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawant RR, Sriraman SK, Navarro G, Biswas S, Dalvi RA, Torchilin VP. Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery. Biomaterials. 2012;33(15):3942–51. doi: 10.1016/j.biomaterials.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bieber T, Elsasser HP. Preparation of a low molecular weight polyethylenimine for efficient cell transfection. BioTechniques. 2001;30(1):74–7. 80–1. doi: 10.2144/01301st03. [DOI] [PubMed] [Google Scholar]
- 57.Dunlap DD, Maggi A, Soria MR, Monaco L. Nanoscopic structure of DNA condensed for gene delivery. Nucleic Acids Res. 1997;25(15):3095–101. doi: 10.1093/nar/25.15.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Werth S, Urban-Klein B, Dai L, Hobel S, Grzelinski M, Bakowsky U, Czubayko F, Aigner A. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J Controlled Release. 2006;112(2):257–70. doi: 10.1016/j.jconrel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Ballarin-Gonzalez B, Howard KA. Polycation-based nanoparticle delivery of RNAi therapeutics: adverse effects and solutions. Adv Drug Delivery Rev. 2012;64(15):1717–29. doi: 10.1016/j.addr.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Wang YQ, Su J, Wu F, Lu P, Yuan LF, Yuan WE, Sheng J, Jin T. Biscarbamate cross-linked polyethylenimine derivative with low molecular weight, low cytotoxicity, and high efficiency for gene delivery. Int J Nanomed. 2012;7:693–704. doi: 10.2147/IJN.S27849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng M, Zhong Y, Meng F, Peng R, Zhong Z. Lipoic acid modified low molecular weight polyethylenimine mediates nontoxic and highly potent in vitro gene transfection. Mol Pharmaceutics. 2011;8(6):2434–43. doi: 10.1021/mp2003797. [DOI] [PubMed] [Google Scholar]
- 62.Kircheis R, Schuller S, Brunner S, Ogris M, Heider KH, Zauner W, Wagner E. Polycation-based DNA complexes for tumor-targeted gene delivery in vivo. J Gene Med. 1999;1(2):111–20. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<111::AID-JGM22>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 63.Kwoh DY, Coffin CC, Lollo CP, Jovenal J, Banaszczyk MG, Mullen P, Phillips A, Amini A, Fabrycki J, Bartholomew RM, Brostoff SW, Carlo DJ. Stabilization of poly-L-lysine/DNA polyplexes for in vivo gene delivery to the liver. Biochim Biophys Acta. 1999;1444(2):171–90. doi: 10.1016/s0167-4781(98)00274-7. [DOI] [PubMed] [Google Scholar]
- 64.Ramsay E, Gumbleton M. Polylysine and polyornithine gene transfer complexes: a study of complex stability and cellular uptake as a basis for their differential in-vitro transfection efficiency. J Drug Targeting. 2002;10(1):1–9. doi: 10.1080/10611860290007487. [DOI] [PubMed] [Google Scholar]
- 65.Ramsay E, Hadgraft J, Birchall J, Gumbleton M. Examination of the biophysical interaction between plasmid DNA and the polycations, polylysine and polyornithine, as a basis for their differential gene transfection in-vitro. Int J Pharm. 2000;210(1–2):97–107. doi: 10.1016/s0378-5173(00)00571-8. [DOI] [PubMed] [Google Scholar]
- 66.Ward CM, Read ML, Seymour LW. Systemic circulation of poly(L-lysine)/DNA vectors is influenced by polycation molecular weight and type of DNA: differential circulation in mice and rats and the implications for human gene therapy. Blood. 2001;97(8):2221–9. doi: 10.1182/blood.v97.8.2221. [DOI] [PubMed] [Google Scholar]
- 67.Wolfert MA, Seymour LW. Atomic force microscopic analysis of the influence of the molecular weight of poly(L)lysine on the size of polyelectrolyte complexes formed with DNA. Gene Ther. 1996;3(3):269–73. [PubMed] [Google Scholar]
- 68.Bielinska AU, Chen C, Johnson J, Baker JR., Jr DNA complexing with polyamidoamine dendrimers: implications for transfection. Bioconjugate Chem. 1999;10(5):843–50. doi: 10.1021/bc990036k. [DOI] [PubMed] [Google Scholar]
- 69.Chen D, Li N, Gu H, Xia X, Xu Q, Ge J, Lu J, Li Y. A novel degradable polymeric carrier for selective release and imaging of magnetic nanoparticles. Chem Commun (Cambridge) 2010;46(36):6708–10. doi: 10.1039/c0cc01857k. [DOI] [PubMed] [Google Scholar]
- 70.Haensler J, Szoka FC., Jr Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjugate Chem. 1993;4(5):372–9. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- 71.Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst dendrimers. J Biomed Mater Res. 1996;30(1):53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 72.Schilrreff P, Mundina-Weilenmann C, Romero EL, Morilla MJ. Selective cytotoxicity of PAMAM G5 core–PAMAM G2.5 shell tecto-dendrimers on melanoma cells. Int J Nanomed. 2012;7:4121–33. doi: 10.2147/IJN.S32785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang M, Khor E, Lim LY. Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm Res. 2004;21(2):344–53. doi: 10.1023/b:pham.0000016249.52831.a5. [DOI] [PubMed] [Google Scholar]
- 74.Huang M, Fong CW, Khor E, Lim LY. Transfection efficiency of chitosan vectors: effect of polymer molecular weight and degree of deacetylation. J Controlled Release. 2005;106(3):391–406. doi: 10.1016/j.jconrel.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Kiang T, Wen J, Lim HW, Leong KW. The effect of the degree of chitosan deacetylation on the efficiency of gene transfection. Biomaterials. 2004;25(22):5293–301. doi: 10.1016/j.biomaterials.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 76.Lavertu M, Methot S, Tran-Khanh N, Buschmann MD. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials. 2006;27(27):4815–24. doi: 10.1016/j.biomaterials.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 77.Xu Q, Wang CH, Pack DW. Polymeric carriers for gene delivery: chitosan and poly(amidoamine) dendrimers. Curr Pharm Des. 2010;16(21):2350–68. doi: 10.2174/138161210791920469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Delivery Rev. 2010;62(1):3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Bronich T, Kabanov AV, Marky LA. A thermodynamic characterization of the interaction of a cationic copolymer with DNA. J Phys Chem B. 2001;105:6042–6050. [Google Scholar]
- 80.Minsky A, Ghirlando R, Gershon H. Structural features of DNA-cationic liposomes complexes and their implication for transfection. In: Barenholz Y, Lasic DD, editors. Handbook of nonmedical applications of liposomes. From gene delivery and Daignostics to Ecology. IV. CRC Press; 1996. pp. 7–30. [Google Scholar]
- 81.Ping SY, Xu Q, Bayer EA, Qian X, Rumbles G, Himmel ME. Bacterial protein complexes applications in nanotecnology. In: Rehm BHA, editor. Microbial bionanotechnology: biological self-assembly systems and biopolymers-based nanostructures. Horizon Bioscience; Great Britain: 2006. [Google Scholar]
- 82.Ghirlando R, Wachtel EJ, Arad T, Minsky A. DNA packaging induced by micellar aggregates: a novel in vitro DNA condensation system. Biochemistry. 1992;31(31):7110–9. doi: 10.1021/bi00146a012. [DOI] [PubMed] [Google Scholar]
- 83.Dias RS, Magno LM, Valente AJ, Das D, Das PK, Maiti S, Miguel MG, Lindman B. Interaction between DNA and cationic surfactants: effect of DNA conformation and surfactant headgroup. J Phys Chem B. 2008;112(46):14446–52. doi: 10.1021/jp8027935. [DOI] [PubMed] [Google Scholar]
- 84.Oskuee RK, Philipp A, Dehshahri A, Wagner E, Ramezani M. The impact of carboxyalkylation of branched polyethylenimine on effectiveness in small interfering RNA delivery. J Gene Med. 2010;12(9):729–38. doi: 10.1002/jgm.1490. [DOI] [PubMed] [Google Scholar]
- 85.Merkel OM, Librizzi D, Pfestroff A, Schurrat T, Buyens K, Sanders NN, De Smedt SC, Behe M, Kissel T. Stability of siRNA polyplexes from poly(ethylenimine) and poly(ethylenimine)-g-poly(ethylene glycol) under in vivo conditions: effects on pharmacokinetics and biodistribution measured by Fluorescence Fluctuation Spectroscopy and Single Photon Emission Computed Tomography (SPECT) imaging. J Controlled Release. 2009;138(2):148–59. doi: 10.1016/j.jconrel.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 86.Navarro G, Maiwald G, Haase R, Rogach AL, Wagner E, de Ilarduya CT, Ogris M. Low generation PAMAM dendrimer and CpG free plasmids allow targeted and extended transgene expression in tumors after systemic delivery. J Controlled Release. 2010;146(1):99–105. doi: 10.1016/j.jconrel.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 87.Han S, Mahato RI, Kim SW. Water-soluble lipopolymer for gene delivery. Bioconjugate Chem. 2001;12(3):337–45. doi: 10.1021/bc000120w. [DOI] [PubMed] [Google Scholar]
- 88.Wang DA, Narang AS, Kotb M, Gaber AO, Miller DD, Kim SW, Mahato RI. Novel branched poly(ethylenimine)- cholesterol water-soluble lipopolymers for gene delivery. Biomacromolecules. 2002;3(6):1197–207. doi: 10.1021/bm025563c. [DOI] [PubMed] [Google Scholar]
- 89.Dewa T, Ieda Y, Morita K, Wang L, MacDonald RC, Iida K, Yamashita K, Oku N, Nango M. Novel polyamine-dialkyl phosphate conjugates for gene carriers. Facile synthetic route via an unprecedented dialkyl phosphate. Bioconjugate Chem. 2004;15(4):824–30. doi: 10.1021/bc049925k. [DOI] [PubMed] [Google Scholar]
- 90.Dewa T, Asai T, Tsunoda Y, Kato K, Baba D, Uchida M, Sumino A, Niwata K, Umemoto T, Iida K, Oku N, Nango M. Liposomal polyamine-dialkyl phosphate conjugates as effective gene carriers: chemical structure, morphology, and gene transfer activity. Bioconjugate Chem. 2010;21(5):844–52. doi: 10.1021/bc900376y. [DOI] [PubMed] [Google Scholar]
- 91.Alshamsan A, Haddadi A, Incani V, Samuel J, Lavasanifar A, Uludag H. Formulation and delivery of siRNA by oleic acid and stearic acid modified polyethylenimine. Mol Pharmaceutics. 2009;6(1):121–33. doi: 10.1021/mp8000815. [DOI] [PubMed] [Google Scholar]
- 92.Neamnark A, Suwantong O, Bahadur RK, Hsu CY, Supaphol P, Uludag H. Aliphatic lipid substitution on 2 kDa polyethylenimine improves plasmid delivery and transgene expression. Mol Pharmaceutics. 2009;6(6):1798–815. doi: 10.1021/mp900074d. [DOI] [PubMed] [Google Scholar]
- 93.Bahadur KC, Landry B, Aliabadi HM, Lavasanifar A, Uludag H. Lipid substitution on low molecular weight (0.6–2.0 kDa) polyethylenimine leads to a higher zeta potential of plasmid DNA and enhances transgene expression. Acta Biomater. 2011;7(5):2209–17. doi: 10.1016/j.actbio.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 94.Sun C, Tang T, Uludag H. A molecular dynamics simulation study on the effect of lipid substitution on polyethylenimine mediated siRNA complexation. Biomaterials. 2013;34(11):2822–33. doi: 10.1016/j.biomaterials.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 95.Navarro G, Sawant RR, Biswas S, Essex S, Tros de Ilarduya C, Torchilin VP. P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells. Nanomedicine (London) 2012;7(1):65–78. doi: 10.2217/nnm.11.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Navarro G, Sawant RR, Essex S, Tros de Ilarduya C, Torchilin VP. Phospholipid-polyethylenimine conjugate-based micelle-like nanoparticles for siRNA delivery. Drug Delivery Transl Res. 2011;1(1):25–33. doi: 10.1007/s13346-010-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ko YT, Kale A, Hartner WC, Papahadjopoulos-Sternberg B, Torchilin VP. Self-assembling micelle-like nanoparticles based on phospholipid-polyethyleneimine conjugates for systemic gene delivery. J Controlled Release. 2009;133(2):132–8. doi: 10.1016/j.jconrel.2008.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Navarro G, Sawant RR, Essex S, Tros de Ilarduya C, Torchilin VP. Phospholipid–polyethylenimine conjugate-based micelle-like nanoparticles for siRNA delivery. Drug Delivery Transl Res. 2011;1(1):25–33. doi: 10.1007/s13346-010-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Essex S, Navarro G, Sabhachandani P, Chordia A, Trivedi M, Movassaghian S, Torchilin VP. Phospholipid-modified PEIbased nanocarriers for in vivo siRNA therapeutics against multidrugresistant tumors. Gene Ther. 2014 doi: 10.1038/gt.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Delivery Rev. 2004;56(9):1273–89. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 101.Bielinska A, Kukowska-Latallo JF, Johnson J, Tomalia DA, Baker JR., Jr Regulation of in vitro gene expression using antisense oligonucleotides or antisense expression plasmids transfected using starburst PAMAM dendrimers. Nucleic Acids Res. 1996;24(11):2176–82. doi: 10.1093/nar/24.11.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kukowska-Latallo JF, Bielinska AU, Johnson J, Spindler R, Tomalia DA, Baker JR., Jr Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc Natl Acad Sci USA. 1996;93(10):4897–902. doi: 10.1073/pnas.93.10.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rajananthanan P, Attard GS, Sheikh NA, Morrow WJ. Evaluation of novel aggregate structures as adjuvants: composition, toxicity studies and humoral responses. Vaccine. 1999;17(7–8):715–30. doi: 10.1016/s0264-410x(98)00256-4. [DOI] [PubMed] [Google Scholar]
- 104.Boas U, Heegaard PM. Dendrimers in drug research. Chem Soc Rev. 2004;33(1):43–63. doi: 10.1039/b309043b. [DOI] [PubMed] [Google Scholar]
- 105.Kim JY, Ryu JH, Hyun H, Kim HA, Choi JS, Yun Lee D, Rhim T, Park JH, Lee M. Dexamethasone conjugation to polyamidoamine dendrimers G1 and G2 for enhanced transfection efficiency with an anti-inflammatory effect. J Drug Targeting. 2012;20(8):667–77. doi: 10.3109/1061186X.2012.712127. [DOI] [PubMed] [Google Scholar]
- 106.Khopade AJ, Shenoy DB, Khopade SA, Jain NK. Phase structures of a hydrated anionic phospholipid composition containing cationic dendrimers and pegylated lipids. Langmuir. 2004;20(18):7368–73. doi: 10.1021/la049682k. [DOI] [PubMed] [Google Scholar]
- 107.Movassaghian S, Moghimi HR, Shirazi FH, Torchilin VP. Dendrosome-dendriplex inside liposomes: as a gene delivery system. J Drug Targeting. 2011;19(10):925–32. doi: 10.3109/1061186X.2011.628396. [DOI] [PubMed] [Google Scholar]
- 108.Takahashi T, Kono K, Itoh T, Emi N, Takagishi T. Synthesis of novel cationic lipids having polyamidoamine dendrons and their transfection activity. Bioconjugate Chem. 2003;14(4):764–73. doi: 10.1021/bc025663f. [DOI] [PubMed] [Google Scholar]
- 109.Takahashi T, Kojima C, Harada A, Kono K. Alkyl chain moieties of polyamidoamine dendron-bearing lipids influence their function as a nonviral gene vector. Bioconjugate Chem. 2007;18(4):1349–54. doi: 10.1021/bc060311k. [DOI] [PubMed] [Google Scholar]
- 110.Kono K, Ikeda R, Tsukamoto K, Yuba E, Kojima C, Harada A. Polyamidoamine dendron-bearing lipids as a nonviral vector: influence of dendron generation. Bioconjugate Chem. 2012;23(4):871–9. doi: 10.1021/bc200368b. [DOI] [PubMed] [Google Scholar]
- 111.Yuba E, Nakajima Y, Tsukamoto K, Iwashita S, Kojima C, Harada A, Kono K. Effect of unsaturated alkyl chains on transfection activity of poly(amidoamine) dendron-bearing lipids. J Controlled Release. 2012;160(3):552–60. doi: 10.1016/j.jconrel.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 112.Zhou XH, Klibanov AL, Huang L. Lipophilic polylysines mediate efficient DNA transfection in mammalian cells. Biochim Biophys Acta. 1991;1065(1):8–14. doi: 10.1016/0005-2736(91)90003-q. [DOI] [PubMed] [Google Scholar]
- 113.Liu X, Zhou J, Yu T, Chen C, Cheng Q, Sengupta K, Huang Y, Li H, Liu C, Wang Y, Posocco P, Wang M, Cui Q, Giorgio S, Fermeglia M, Qu F, Pricl S, Shi Y, Liang Z, Rocchi P, Rossi JJ, Peng L. Adaptive Amphiphilic Dendrimer-Based Nanoassemblies as Robust and Versatile siRNA Delivery Systems. Angew Chem, Int Ed. 2014;53(44):11822–7. doi: 10.1002/anie.201406764. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Park JU, Ishihara T, Kano A, Akaike T, Maruyama A. Preparation of dendritic graft copolymer consisting of poly-(L-lysine) and arabinogalactan as a hepatocyte specific DNA carrier. Prep Biochem Biotechnol. 1999;29(4):353–70. doi: 10.1080/10826069908544934. [DOI] [PubMed] [Google Scholar]
- 115.Wu GY, Wu CH. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem. 1987;262(10):4429–32. [PubMed] [Google Scholar]
- 116.Laemmli UK. Characterization of DNA condensates induced by poly(ethylene oxide) and polylysine. Proc Natl Acad Sci USA. 1975;72(11):4288–92. doi: 10.1073/pnas.72.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ziady AG, Ferkol T, Dawson DV, Perlmutter DH, Davis PB. Chain length of the polylysine in receptor-targeted gene transfer complexes affects duration of reporter gene expression both in vitro and in vivo. J Biol Chem. 1999;274(8):4908–16. doi: 10.1074/jbc.274.8.4908. [DOI] [PubMed] [Google Scholar]
- 118.Kwoh DY, Coffin CC, Lollo CP, Jovenal J, Banaszczyk MG, Mullen P, Phillips A, Amini A, Fabrycki J, Bartholomew RM, Brostoff SW, Carlo DJ. Stabilization of poly-l-lysine/DNA polyplexes for in vivo gene delivery to the liver. Biochim Biophys Acta, Gene Struct Expression. 1999;1444(2):171–190. doi: 10.1016/s0167-4781(98)00274-7. [DOI] [PubMed] [Google Scholar]
- 119.Männistö M, Vanderkerken S, Toncheva V, Elomaa M, Ruponen M, Schacht E, Urtti A. Structure–activity relationships of poly(l-lysines): effects of pegylation and molecular shape on physicochemical and biological properties in gene delivery. J Controlled Release. 2002;83(1):169–182. doi: 10.1016/s0168-3659(02)00178-5. [DOI] [PubMed] [Google Scholar]
- 120.Choi YH, Liu F, Kim JS, Choi YK, Park JS, Kim SW. Polyethylene glycol-grafted poly-L-lysine as polymeric gene carrier. J Controlled Release. 1998;54(1):39–48. doi: 10.1016/s0168-3659(97)00174-0. [DOI] [PubMed] [Google Scholar]
- 121.Wagner E, Ogris M, Zauner W. Polylysine-based transfection systems utilizing receptor-mediated delivery. Adv Drug Delivery Rev. 1998;30(1–3):97–113. doi: 10.1016/s0169-409x(97)00110-5. [DOI] [PubMed] [Google Scholar]
- 122.Plank C, Mechtler K, Szoka FC, Jr, Wagner E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum Gene Ther. 1996;7(12):1437–46. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 123.Jeong JH, Park TG. Poly(L-lysine)-g-poly(D, L-lactic-coglycolic acid) micelles for low cytotoxic biodegradable gene delivery carriers. J Controlled Release. 2002;82(1):159–66. doi: 10.1016/s0168-3659(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 124.Blum JS, Saltzman WM. High loading efficiency and tunable release of plasmid DNA encapsulated in submicron particles fabricated from PLGA conjugated with poly-L-lysine. J Controlled Release. 2008;129(1):66–72. doi: 10.1016/j.jconrel.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Incani V, Lin X, Lavasanifar A, Uludag H. Relationship between the extent of lipid substitution on poly(L-lysine) and the DNA delivery efficiency. ACS Appl Mater Interfaces. 2009;1(4):841–8. doi: 10.1021/am8002445. [DOI] [PubMed] [Google Scholar]
- 126.Abbasi M, Uludag H, Incani V, Hsu CY, Jeffery A. Further investigation of lipid-substituted poly(L-Lysine) polymers for transfection of human skin fibroblasts. Biomacromolecules. 2008;9(6):1618–30. doi: 10.1021/bm800132n. [DOI] [PubMed] [Google Scholar]
- 127.Prabaharan M. Review paper: chitosan derivatives as promising materials for controlled drug delivery. J Biomater Appl. 2008;23(1):5–36. doi: 10.1177/0885328208091562. [DOI] [PubMed] [Google Scholar]
- 128.Mumper RJ, WJ, Claspell JM, Rolland AP. Novel polymeric condensing carriers for gene delivery. Proc Int Symp Controlled Release Bioact Mater. 1995;22:178–179. [Google Scholar]
- 129.Katas H, Alpar HO. Development and characterisation of chitosan nanoparticles for siRNA delivery. J Controlled Release. 2006;115(2):216–25. doi: 10.1016/j.jconrel.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 130.Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MO, Hovgaard MB, Schmitz A, Nyengaard JR, Besenbacher F, Kjems J. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14(4):476–84. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 131.Techaarpornkul S, Wongkupasert S, Opanasopit P, Apirakaramwong A, Nunthanid J, Ruktanonchai U. Chitosan- Mediated siRNA Delivery In Vitro: Effect of Polymer Molecular Weight, Concentration and Salt Forms. AAPS PharmSciTech. 2010;11(1):64–72. doi: 10.1208/s12249-009-9355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jean M, Alameh M, De Jesus D, Thibault M, Lavertu M, Darras V, Nelea M, Buschmann MD, Merzouki A. Chitosan-based therapeutic nanoparticles for combination gene therapy and gene silencing of in vitro cell lines relevant to type 2 diabetes. Eur J Pharm Sci. 2012;45(1–2):138–49. doi: 10.1016/j.ejps.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 133.Du YZ, Lu P, Zhou JP, Yuan H, Hu FQ. Stearic acid grafted chitosan oligosaccharide micelle as a promising vector for gene delivery system: factors affecting the complexation. Int J Pharm. 2010;391(1–2):260–6. doi: 10.1016/j.ijpharm.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 134.Chae SY, Son S, Lee M, Jang MK, Nah JW. Deoxycholic acid-conjugated chitosan oligosaccharide nanoparticles for efficient gene carrier. J Controlled Release. 2005;109(1–3):330–44. doi: 10.1016/j.jconrel.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 135.Kabanov AV, Kabanov VA. DNA Complexes with Polycations for the Delivery of Genetic Material into Cells. Bioconjugate Chem. 1995;6(1):7–20. doi: 10.1021/bc00031a002. [DOI] [PubMed] [Google Scholar]
- 136.Mandke R, Singh J. Effect of acyl chain length and unsaturation on physicochemical properties and transfection efficiency of N-acyl-substituted low-molecular-weight chitosan. J Pharm Sci. 2012;101(1):268–82. doi: 10.1002/jps.22767. [DOI] [PubMed] [Google Scholar]
- 137.Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–57. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 138.Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticleprotein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA. 2007;104(7):2050–5. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sarisozen C, Vural I, Levchenko T, Hincal AA, Torchilin VP. Long-circulating PEG-PE micelles co-loaded with paclitaxel and elacridar (GG918) overcome multidrug resistance. Drug Delivery. 2012;19(8):363–70. doi: 10.3109/10717544.2012.724473. [DOI] [PubMed] [Google Scholar]
- 140.Elbayoumi TA, Torchilin VP. Tumor-specific antinucleosome antibody improves therapeutic efficacy of doxorubicinloaded long-circulating liposomes against primary and metastatic tumor in mice. Mol Pharmaceutics. 2009;6(1):246–54. doi: 10.1021/mp8001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lukyanov AN, Sawant RM, Hartner WC, Torchilin VP. PEGylated dextran as long-circulating pharmaceutical carrier. J Biomater Sci, Polym Ed. 2004;15(5):621–30. doi: 10.1163/156856204323046889. [DOI] [PubMed] [Google Scholar]
- 142.Weissig VV, Babich J, Torchilin VV. Long-circulating gadolinium-loaded liposomes: potential use for magnetic resonance imaging of the blood pool. Colloids Surf, B. 2000;18(3–4):293–9. doi: 10.1016/s0927-7765(99)00155-1. [DOI] [PubMed] [Google Scholar]
- 143.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Delivery Rev. 2011;63(3):131–5. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 144.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12Part 1):6387–92. [PubMed] [Google Scholar]
- 145.Endres TK, Beck-Broichsitter M, Samsonova O, Renette T, Kissel TH. Self-assembled biodegradable amphiphilic PEG-PCL-lPEI triblock copolymers at the borderline between micelles and nanoparticles designed for drug and gene delivery. Biomaterials. 2011;32(30):7721–31. doi: 10.1016/j.biomaterials.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 146.Endres T, Zheng M, Kilic A, Turowska A, Beck-Broichsitter M, Renz H, Merkel OM, Kissel T. Amphiphilic biodegradable PEG-PCL-PEI triblock copolymers for FRET-capable in vitro and in vivo delivery of siRNA and quantum dots. Mol Pharmaceutics. 2014;11(4):1273–81. doi: 10.1021/mp400744a. [DOI] [PubMed] [Google Scholar]
- 147.Wang T, Upponi JR, Torchilin VP. Design of multifunctional non-viral gene vectors to overcome physiological barriers: dilemmas and strategies. Int J Pharm. 2012;427(1):3–20. doi: 10.1016/j.ijpharm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 148.Kim HJ, Miyata K, Nomoto T, Zheng M, Kim A, Liu X, Cabral H, Christie RJ, Nishiyama N, Kataoka K. siRNA delivery from triblock copolymer micelles with spatially-ordered compartments of PEG shell, siRNA-loaded intermediate layer, and hydrophobic core. Biomaterials. 2014;35(15):4548–56. doi: 10.1016/j.biomaterials.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 149.Biswas S, Deshpande PP, Navarro G, Dodwadkar NS, Torchilin VP. Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials. 2013;34(4):1289–301. doi: 10.1016/j.biomaterials.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mao CQ, Du JZ, Sun TM, Yao YD, Zhang PZ, Song EW, Wang J. A biodegradable amphiphilic and cationic triblock copolymer for the delivery of siRNA targeting the acid ceramidase gene for cancer therapy. Biomaterials. 2011;32(11):3124–33. doi: 10.1016/j.biomaterials.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 151.Sun TM, Du JZ, Yan LF, Mao HQ, Wang J. Selfassembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for siRNA delivery. Biomaterials. 2008;29(32):4348–55. doi: 10.1016/j.biomaterials.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 152.Sun TM, Du JZ, Yao YD, Mao CQ, Dou S, Huang SY, Zhang PZ, Leong KW, Song EW, Wang J. Simultaneous delivery of siRNA and paclitaxel via a “two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano. 2011;5(2):1483–94. doi: 10.1021/nn103349h. [DOI] [PubMed] [Google Scholar]
- 153.Wang HX, Xiong MH, Wang YC, Zhu J, Wang J. N-acetylgalactosamine functionalized mixed micellar nanoparticles for targeted delivery of siRNA to liver. J Controlled Release. 2013;166(2):106–14. doi: 10.1016/j.jconrel.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 154.Liu XQ, Xiong MH, Shu XT, Tang RZ, Wang J. Therapeutic delivery of siRNA silencing HIF-1 alpha with micellar nanoparticles inhibits hypoxic tumor growth. Mol Pharmaceutics. 2012;9(10):2863–74. doi: 10.1021/mp300193f. [DOI] [PubMed] [Google Scholar]
- 155.Zhu L, Torchilin VP. Stimulus-responsive nanopreparations for tumor targeting. Integr Biol (Cambridge) 2013;5(1):96–107. doi: 10.1039/c2ib20135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. J Controlled Release. 2008;132(3):164–70. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xiong XB, Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS Nano. 2011;5(6):5202–13. doi: 10.1021/nn2013707. [DOI] [PubMed] [Google Scholar]
- 158.Zhu L, Wang T, Perche F, Taigind A, Torchilin VP. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc Natl Acad Sci USA. 2013;110(42):17047–52. doi: 10.1073/pnas.1304987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhu L, Perche F, Wang T, Torchilin VP. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials. 2014;35(13):4213–22. doi: 10.1016/j.biomaterials.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.